Phenolic Composition of Grape Stems from Different Spanish Varieties and Vintages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Determination of the Antioxidant Capacity, Total Phenolic and Total Flavonoid Content of the Grape Stem Extracts
2.3. Identification and Quantification of Phenolic Compounds by HPLC-DAD
2.4. Statistical Analysis
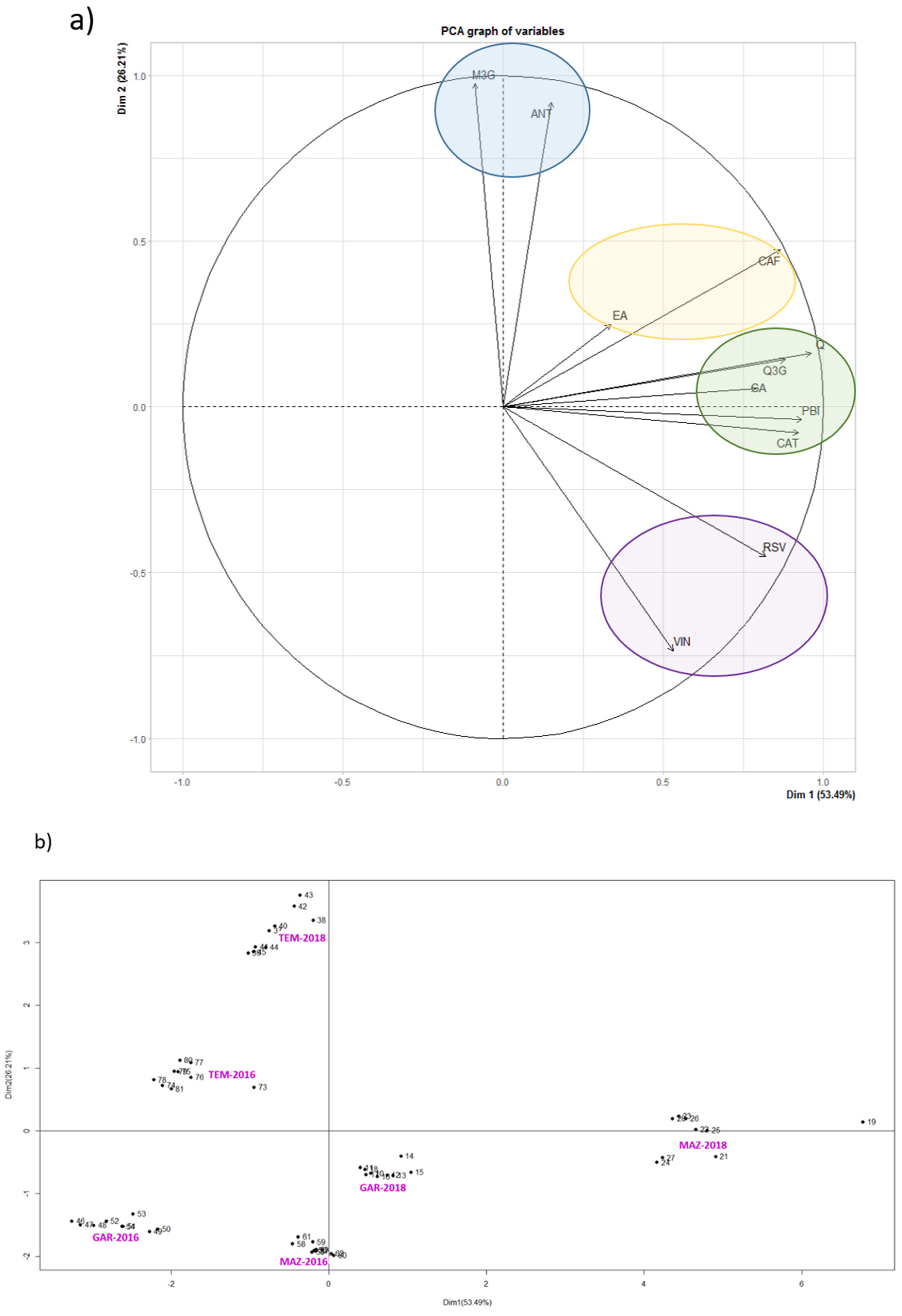
3. Results and Discussion
3.1. Antioxidant Capacity, TF and TPC
3.2. Phenolic Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. El Estado Mundial de La Agricultura y La Alimentación 2012; FAO: Rome, Italy, 2012. [Google Scholar]
- Rajha, H.N.; El Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of Total Phenolic Compounds, Flavonoids, Anthocyanins and Tannins from Grape Byproducts by Response Surface Methodology. Influence of Solid-Liquid Ratio, Particle Size, Time, Temperature and Solvent Mixtures on the Optimization Process. Food Nutr. Sci. 2014, 5, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Cheng, V.J.; Bekhit, A.E.-D.A.; Sedcole, R.; Hamid, N. The Impact of Grape Skin Bioactive Functionality Information on the Acceptability of Tea Infusions Made from Wine By-Products. J. Food Sci. 2010, 75, S167–S172. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Ladas, D.; Mavromatis, A. Potential uses and applications of treated wine waste: A review. Int. J. Food Sci. Technol. 2006, 41, 475–487. [Google Scholar] [CrossRef]
- Pardo, J.E.; Fernández, E.; Rubio, M.; Alvarruiz, A.; Alonso, G.L. Characterization of grape seed oil from different grape varieties (Vitis Vinifera L.). Eur. J. Lipid Sci. Technol. 2009, 111, 188–193. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Vine-shoot waste aqueous extracts for re-use in agricultura obtained by different extraction techniques: Phenolic, volatile and mineral compounds. J. Agric. Food Chem. 2014, 62, 10861–10872. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.-L.; Haroutounian, S.A. Grape stem extracts: Polyphenolic content and assessment of their in vitro antioxidant properties. LWT Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res. Int. 2014, 65, 462–468. [Google Scholar] [CrossRef]
- Blackford, M.; Comby, M.; Zeng, L.; Dienes-Nagy, Á.; Bourdin, G.; Lorenzini, F.; Bach, B. A Review on Stems Composition and Their Impact on Wine Quality. Molecules 2021, 26, 1240. [Google Scholar] [CrossRef] [PubMed]
- Gouvinhas, I.; Queiroz, M.; Rodrigues, M.; Barros, A.I.R.N.A. Evaluation of the phytochemistry and biological activity of grape (Vitis Vinifera L.) stems: Toward a sustainable winery industry. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 381–394. [Google Scholar] [CrossRef]
- Ruiz-Moreno, M.J.; Raposo, R.; Cayuela, J.M.; Zafrilla, P.; Piñeiro, Z.; Rojas, J.M.M.; Mulero, J.; Puertas, B.; Girón, F.; Guerrero, R.F.; et al. Valorization of grape stems. Ind. Crops Prod. 2015, 63, 152–157. [Google Scholar] [CrossRef]
- Barros, A.; Gironés-Vilaplana, A.; Teixeira, A.; Collado-González, J.; Moreno-Fernández, D.; Gil-Izquierdo, A.; Rosa, E.; Domínguez-Perles, R. Evaluation of grape (Vitis vinifera L.) stems from Portuguese varieties as a resource of (poly)phenolic compounds: A comparative study. Food Res. Int. 2014, 65, 375–384. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Guedes, A.; Queiroz, M.; Silva, A.M.; Barros, A.I.R.N.A. Oxidative stress prevention and an-ti-apoptosis activity of grape (Vitis vinifera L.) stems in human keratinocytes. Food Res. Int. 2016, 87, 92–102. [Google Scholar] [CrossRef]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A. Potential application of grape (Vitis vinifera L.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Dias, C.; Domínguez-Perles, R.; Aires, A.; Teixeira, A.; Rosa, E.; Barros, A.; Saavedra, M.J. Phytochemistry and activity against digestive pathogens of grape (Vitis vinifera L.) stem’s (poly)phenolic extracts. LWT Food Sci. Technol. 2015, 61, 25–32. [Google Scholar] [CrossRef]
- Poveda, J.M.; Loarce, L.; Alarcón, M.; Díaz-Maroto, M.C.; Alañón, M.E. Revalorization of winery by-products as source of natural preservatives obtained by means of green extraction techniques. Ind. Crops Prod. 2018, 112, 617–625. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Pinto, R.; Santos, R.A.; Saavedra, M.J.; Barros, A. Enhanced phytochemical composition and biological activities of grape (Vitis vinifera L.) Stems growing in low altitude regions. Sci. Hortic. 2020, 265, 109248. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quero, J.; Jiménez-Moreno, N.; Esparza, I.; Osada, J.; Cerrada, E.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M. Grape Stem Extracts with Potential Anticancer and Antioxidant Properties. Antioxidants 2021, 10, 243. [Google Scholar] [CrossRef]
- Esparza, I.; Martínez-Inda, B.; Cimminelli, M.J.; Jimeno-Mendoza, M.C.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Reducing SO2 Doses in Red Wines by Using Grape Stem Extracts as Antioxidants. Biomolecules 2020, 10, 1369. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Svarc-Gajic, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Poten-tial of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Moreno, N.; Volpe, F.; Moler, J.A.; Esparza, I.; Ancín-Azpilicueta, C. Impact of Extraction Conditions on the Phenolic Composition and Antioxidant Capacity of Grape Stem Extracts. Antioxidants 2019, 8, 597. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, N.; Mateus, N.; Freitas, V.; Oliveira, J. Wine industry by-product: Full polyphenolic characterization of grape stalks. Food Chem. 2018, 268, 110–117. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Roselló, C.; Teissedre, P.L. Proanthocyanidin composition and antioxidant potential of the stem winemaking byproducts from 10 different grape varieties (Vitis vinifera L.). J. Agric. Food Chem. 2012, 60, 11850–11858. [Google Scholar] [CrossRef]
- Souquet, J.-M.; Labarbe, B.; Le Guernevé, C.; Cheynier, V.; Moutounet, M. Phenolic Composition of Grape Stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxi-dants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1998, 299, 152–178. [Google Scholar]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evid. Based Complement. Altern. Med. 2014, 4, 253875. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 22 July 2021).
- Lenth, R.V. Least-Squares Means: The R Package Ismeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Schain, K.M. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2004, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Gerós, H. Berry Phenolics of Grapevine under Challenging Environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribereau-Gayon, P.; Dubordieu, D.; Donèche, B.; Lonvaud, A. Tratado de Enología 1. Microbiología del Vino, Vinificaciones; Mundi-Prensa: Madrid, Spain, 2003; p. 342. [Google Scholar]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.-C.; Escribano-Bailon, M.T. Influence of climatic conditions on the phenolic composition of Vitis vinifera L. cv. Graciano. Anal. Chim. Acta 2012, 732, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora, F. Elaboración y Crianza del Vino Tinto: Aspectos Científicos y Prácticos; Mundi-Prensa: Madrid, Spain, 2003; pp. 71–72. [Google Scholar]
- Marchante, L.; Gómez Alonso, S.; Alañón, M.E.; Pérez-Coello, M.S.; Díaz-Maroto, M.C. Natural extracts from fresh and ov-en-dried winemaking by-products as valuable source of antioxidant compounds. Food Sci. Nutr. 2018, 6, 1564–1574. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.; Pereira, J.; Amaral, J.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control. 2018, 92, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Blancquaert, E.H.; Oberholster, A.; Ricardo-Da-Silva, J.M.; Deloire, A.J. Grape Flavonoid Evolution and Composition Under Altered Light and Temperature Conditions in Cabernet Sauvignon (Vitis vinifera L.). Front. Plant Sci. 2019, 10, 1062. [Google Scholar] [CrossRef] [Green Version]
| Phenolic Compound | λ (nm) | Concentration Range (µg/mL) | Rt (min) | R2 | LLOD (µg/mL) | LLOQ (µg/mL) |
|---|---|---|---|---|---|---|
| Gallic Acid | 272 | 5.1–168.7 | 6.5 ± 0.2 | >0.999 | 1.1 | 5.1 |
| Ellagic Acid | 367 | 0.5–36.3 | 28.7 ± 0.4 | >0.999 | 0.4 | 0.5 |
| Caftaric Acid | 329 | 0.5–36.5 | 15.4 ± 0.3 | >0.998 | 0.8 | 0.5 |
| Catechin | 279 | 40.0–133.2 | 17.4 ± 0.4 | >0.991 | 1.4 | 40.0 |
| trans-Resveratrol | 306 | 0.5–42.6 | 38.8 ± 0.3 | >0.997 | 0.9 | 0.5 |
| ε-Viniferin | 324 | 2.1–21.1 | 44.6 ± 0.1 | >0.993 | 0.5 | 2.1 |
| Quercetin | 369 | 0.4–17.6 | 42.2 ± 0.2 | >0.994 | 0.8 | 0.4 |
| Qurecetin-3-derivative | 354 | 7.8–78.2 | 30.0 ± 0.4 | >0.995 | 2.1 | 7.8 |
| Procyanidin B1 | 279 | 6.8–136.5 | 14.7 ± 0.3 | >0.997 | 6.3 | 6.8 |
| Malvidin-3-glucoside | 526 | 0.4–34.6 | 23.3 ± 0.1 | >0.998 | 1.1 | 0.4 |
| Variety | Antioxidant Activity 1 | Total Polyphenol Content 2 | Total Flavonoid Content 3 | ||
|---|---|---|---|---|---|
| ABTS | FRAP | DPPH | |||
| Vintage 2016 | |||||
| GAR | 0.33 ± 0.05 a | 0.16 ± 0.02 a | 0.24 ± 0.02 a | 34 ± 5 a | 1.5 ± 0.2 a |
| MAZ | 0.80 ± 0.05 b | 0.36 ± 0.03 b | 0.48 ± 0.03 b | 82 ± 1 b | 2.5 ± 0.1 b |
| TEM | 0.81 ± 0.05 b | 0.46 ± 0.03 c | 0.60 ± 0.01 c | 87 ± 3 b | 2.2 ± 0.1 b |
| GRA | 0.92 ± 0.06 b | 0.42 ± 0.03 bc | 0.58 ± 0.02 c | 90 ± 5 b | 2.2 ± 0.1 b |
| Vintage 2018 | |||||
| GAR | 0.84 ± 0.01 a | 0.44 ± 0.02 a | 0.54 ± 0.01 a | 86 ± 1 a | 2.3 ± 0.1 b |
| MAZ | 1.51 ± 0.17 c | 0.80 ± 0.09 b | 0.81 ± 0.08 b | 172 ± 6 c | 2.8 ± 0.1 c |
| TEM | 1.12 ± 0.03 b | 0.57 ± 0.04 a | 0.63 ± 0.01 a | 135 ± 7 b | 3.0 ± 0.1 d |
| CHA | 0.78 ± 0.02 a | 0.43 ± 0.02 a | 0.57 ± 0.02 a | 84 ± 2 a | 2.0 ± 0.1 a |
| CS | 0.92 ± 0.05 ab | 0.50 ± 0.02 a | 0.64 ± 0.03 a | 95 ± 4 a | 1.9 ± 0.1 a |
| Gallic Acid | Ellagic Acid | Caftaric Acid | Catechin | trans-Resveratrol | ε-Viniferin | Quercetin | Quercetin-3-Derivative | Procyanidin B1 | Malvidin-3-Glucoside | Unknown Anthocyanin | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vintage 2016 | |||||||||||
| GAR | 0.12 ± 0.03 a | 0.031 ± 0.007 a | 0.05 ± 0.01 a | 1.0 ± 0.2 a | 0.06 ± 0.01 a | 0.33 ± 0.05 ab | 0.014 ± 0.002 a | 0.24 ± 0.06 a | 0.4 ± 0.1 a | 0.03 ± 0.01 a | <LD a |
| MAZ | 0.23 ± 0.01 b | 0.028 ± 0.001 a | 0.13 ± 0.00 b | 1.2 ± 0.1 ab | 0.24 ± 0.02 b | 0.69 ± 0.03 c | 0.043 ± 0.002 c | 0.99 ± 0.03 d | 0.4 ± 0.0 a | 0.09 ± 0.00 a | 0.10 ± 0.00 b |
| TEM | 0.14 ± 0.02 a | 0.043 ± 0.009 a | 0.12 ± 0.01 b | 1.0 ± 0.2 a | 0.04 ± 0.01 a | 0.20 ± 0.06 a | 0.029 ± 0.008 b | 0.70 ± 0.06 c | 0.5 ± 0.1 a | 0.48 ± 0.04 b | 0.22 ± 0.02 c |
| GRA | 0.18 ± 0.00 ab | 0.042 ± 0.002 a | 0.14 ± 0.01 b | 1.6 ± 0.0 b | 0.37 ± 0.03 c | 0.45 ± 0.03 b | 0.013 ± 0.001 a | 0.52 ± 0.02 b | 1.1 ± 0.1 b | 0.61 ± 0.03 c | 0.37 ± 0.03 d |
| Vintage 2018 | |||||||||||
| GAR | 1.29 ± 0.06 c | 0.097 ± 0.004 a | 0.14 ± 0.01 a | 1.3 ± 0.1 a | 0.13 ± 0.01 c | 0.46 ± 0.04 b | 0.073 ± 0.003 b | 0.62 ± 0.03 ab | 0.9 ± 0.1 a | 0.12 ± 0.00 b | 0.03 ± 0.00 a |
| MAZ | 1.18 ± 0.09 c | 0.054 ± 0.005 a | 0.33 ± 0.03 b | 3.5 ± 0.3 b | 0.30 ± 0.04 d | 0.55 ± 0.08 b | 0.108 ± 0.011 c | 1.50 ± 0.14 c | 2.5 ± 0.2 c | 0.22 ± 0.04 b | 0.24 ± 0.04 b |
| TEM | 0.52 ± 0.04 b | 0.060 ± 0.004 a | 0.23 ± 0.02 ab | 0.9 ± 0.1 a | 0.04 ± 0.00 ab | 0.15 ± 0.03 a | 0.056 ± 0.004 b | 0.79 ± 0.05 b | 0.4 ± 0.0 a | 0.80 ± 0.06 c | 0.55 ± 0.07 c |
| CHA | 0.15 ± 0.02 a | 0.049 ± 0.006 a | 0.20 ± 0.02 a | 1.0 ± 0.2 a | 0.07 ± 0.01 b | 0.42 ± 0.08 b | 0.013 ± 0.001 a | 0.39 ± 0.05 a | 0.7 ± 0.1 a | nd a | nd a |
| CS | 0.25 ± 0.04 a | 0.089 ± 0.015 a | 0.26 ± 0.03 ab | 2.6 ± 0.6 b | 0.01 ± 0.00 a | 0.28 ± 0.06 ab | 0.013 ±0.002 a | 0.75 ± 0.07 ab | 1.8 ± 0.4 b | 0.07 ± 0.01 ab | 0.01 ± 0.00 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esparza, I.; Moler, J.A.; Arteta, M.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Phenolic Composition of Grape Stems from Different Spanish Varieties and Vintages. Biomolecules 2021, 11, 1221. https://doi.org/10.3390/biom11081221
Esparza I, Moler JA, Arteta M, Jiménez-Moreno N, Ancín-Azpilicueta C. Phenolic Composition of Grape Stems from Different Spanish Varieties and Vintages. Biomolecules. 2021; 11(8):1221. https://doi.org/10.3390/biom11081221
Chicago/Turabian StyleEsparza, Irene, José Antonio Moler, Maite Arteta, Nerea Jiménez-Moreno, and Carmen Ancín-Azpilicueta. 2021. "Phenolic Composition of Grape Stems from Different Spanish Varieties and Vintages" Biomolecules 11, no. 8: 1221. https://doi.org/10.3390/biom11081221
APA StyleEsparza, I., Moler, J. A., Arteta, M., Jiménez-Moreno, N., & Ancín-Azpilicueta, C. (2021). Phenolic Composition of Grape Stems from Different Spanish Varieties and Vintages. Biomolecules, 11(8), 1221. https://doi.org/10.3390/biom11081221









