Quantitative Proteomic Analysis of Plasma after Remote Ischemic Conditioning in a Rhesus Monkey Ischemic Stroke Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anesthesia and Intraoperative Management
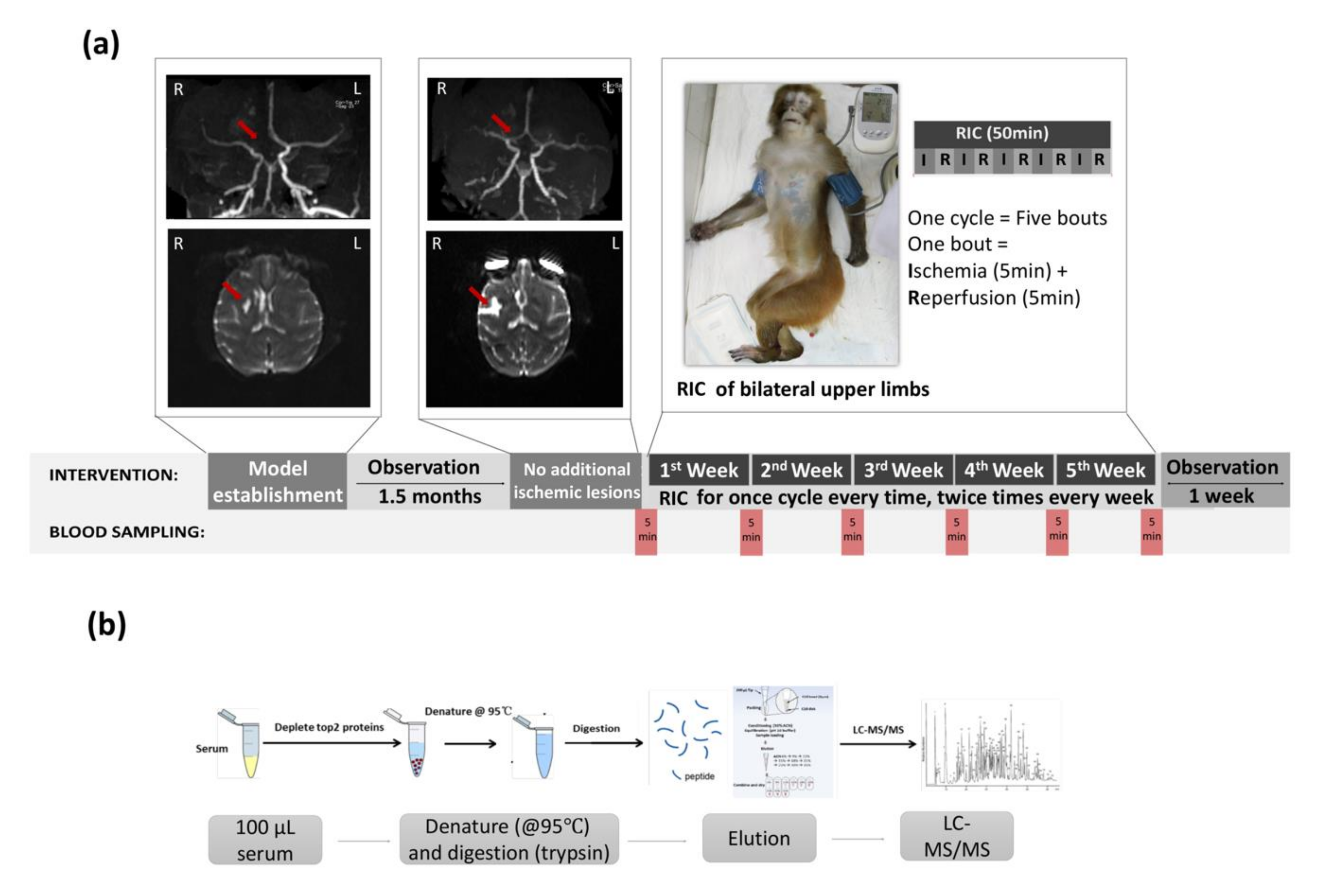
2.3. Stroke Model Establishment
2.4. Imaging Confirmation of Stroke Model
2.5. Limb RIC Intervention
2.6. Blood Sampling
2.7. Safety of RIC
2.8. Sample Treatment
2.9. Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
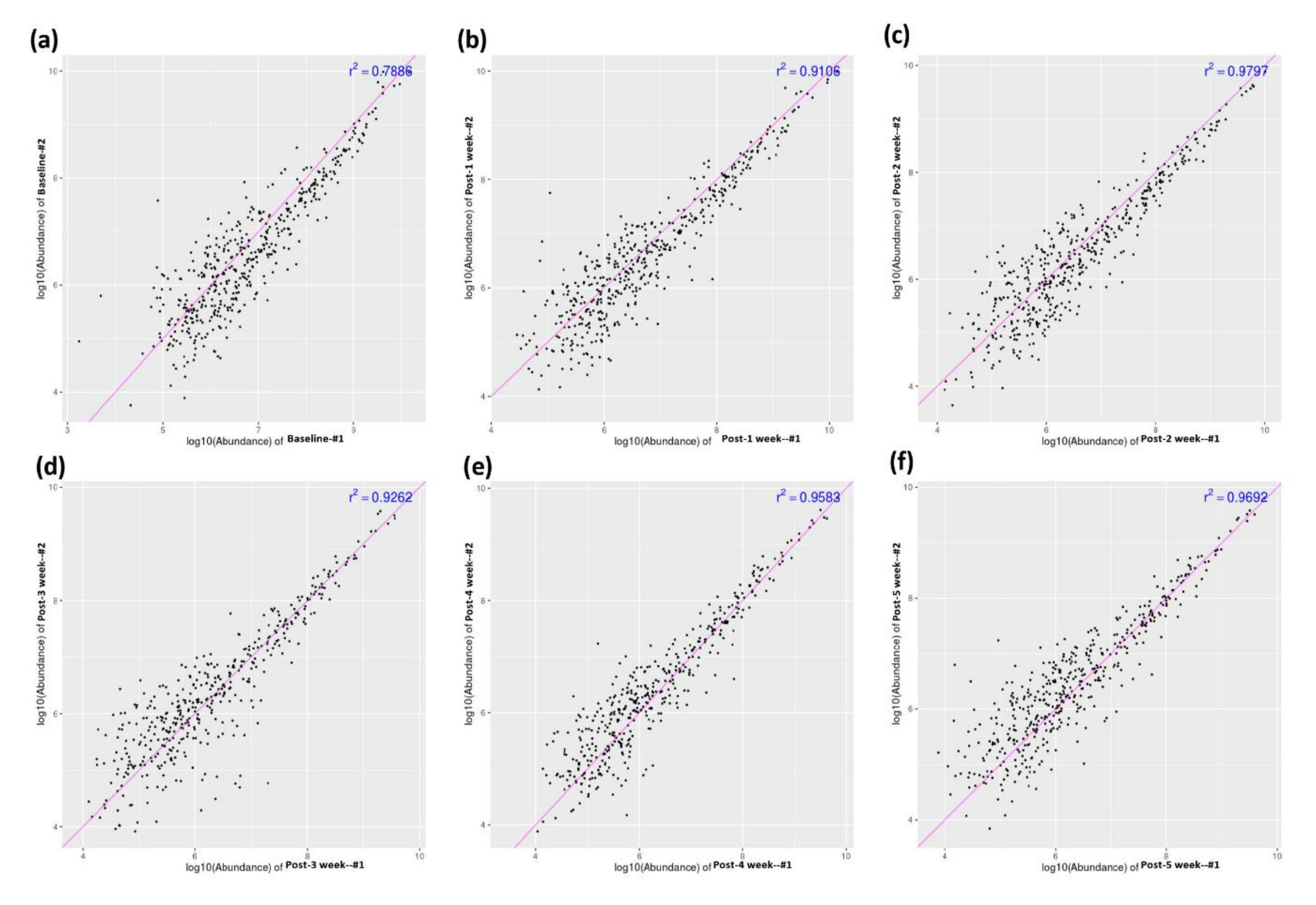
2.10. Proteomics Data Analysis
3. Results
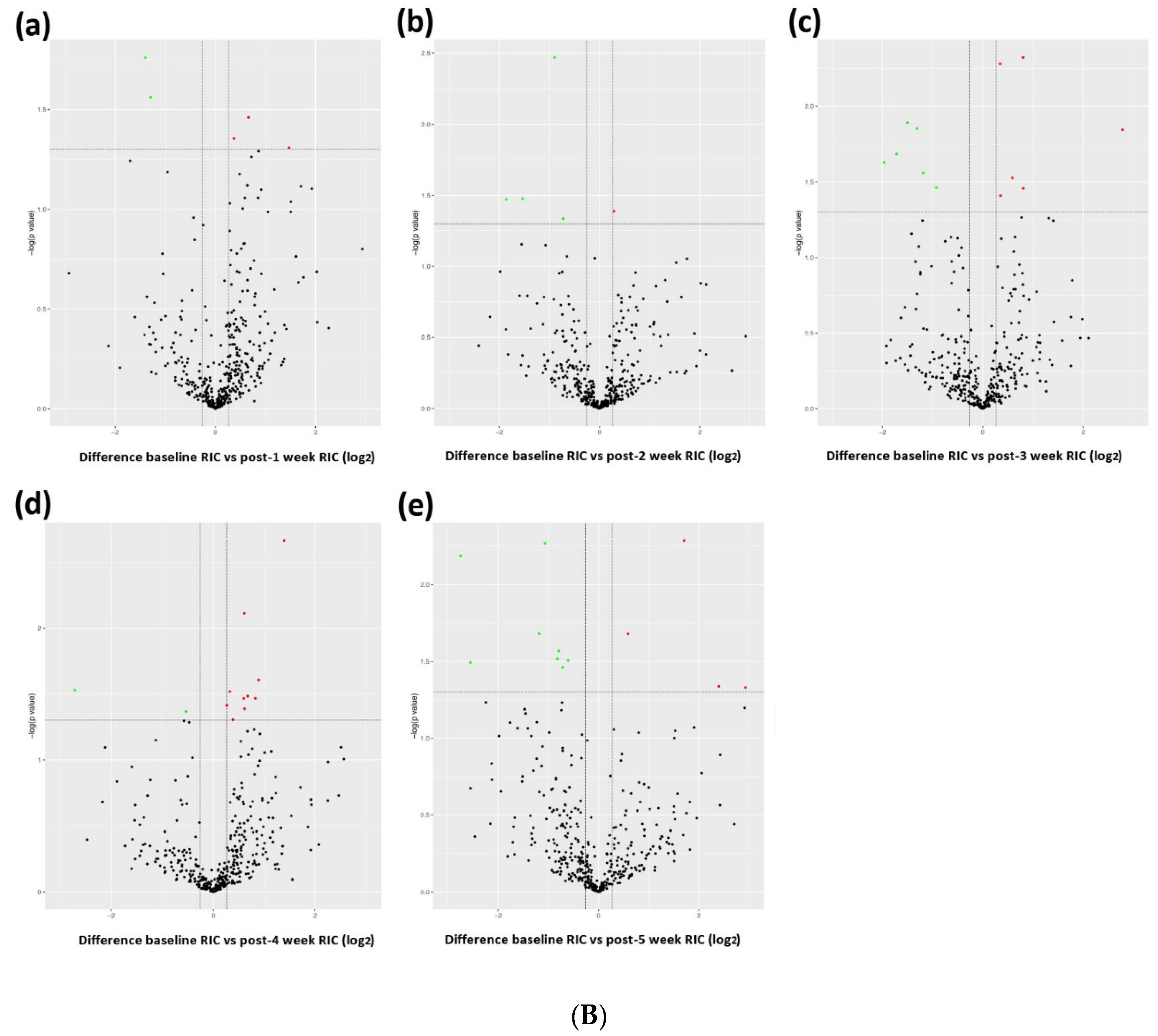
3.1. Profiling Differential Plasma Protein Expression between Baseline and Post-RIC Groups
3.2. Functional Annotation and Classification of the Assembled Unique Sequences
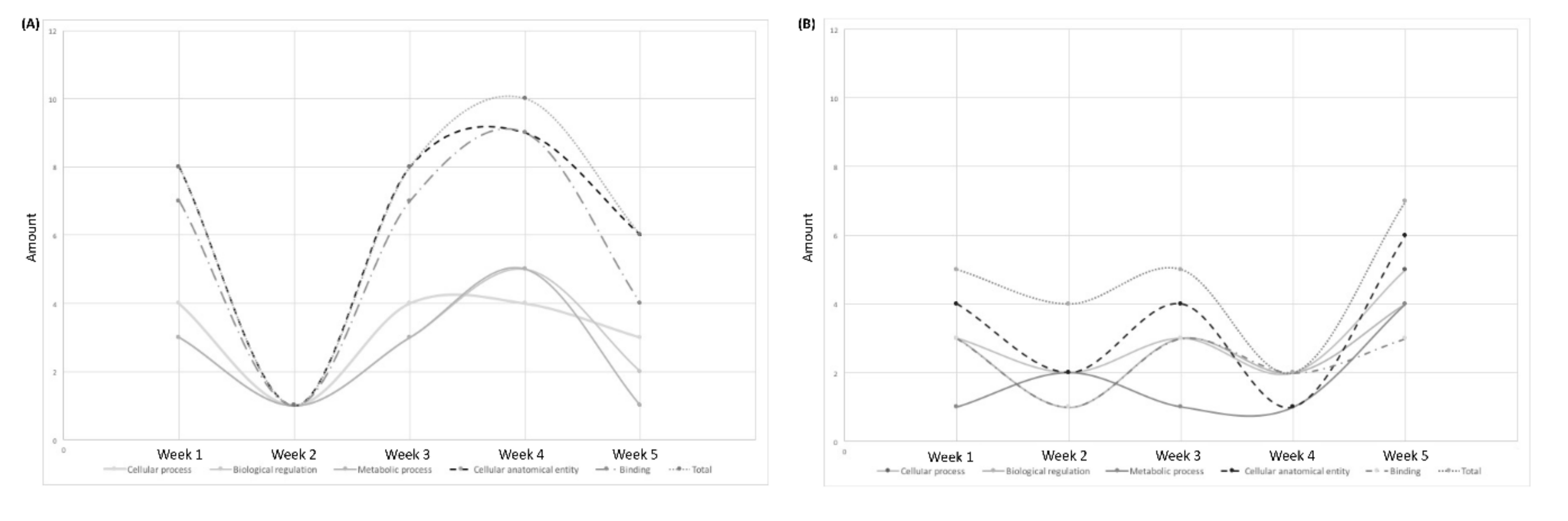
3.2.1. GO Analysis
3.2.2. KOG Analysis
3.2.3. KEGG Analysis
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heusch, G.; Bøtker, H.E.; Przyklenk, K.; Redington, A.; Yellon, D. Remote ischemic conditioning. J. Am. Coll. Cardiol. 2015, 65, 177–195. [Google Scholar] [CrossRef] [Green Version]
- Crowley, L.E.; McIntyre, C.W. Remote ischaemic conditioning—Therapeutic opportunities in renal medicine. Nat. Rev. Nephrol. 2013, 9, 739–746. [Google Scholar] [CrossRef]
- Hess, D.C.; Blauenfeldt, R.A.; Andersen, G.; Hougaard, K.D.; Hoda, M.N.; Ding, Y.; Ji, X. Remote ischaemic conditioning—A new paradigm of self-protection in the brain. Nat. Rev. Neurol. 2015, 11, 698–710. [Google Scholar] [CrossRef]
- Zhou, D.; Ding, J.; Ya, J.; Pan, L.; Wang, Y.; Ji, X.; Meng, R. Remote ischemic conditioning: A promising therapeutic intervention for multi-organ protection. Aging 2018, 10, 1825–1855. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Che, R.; Li, S.; Ren, C.; Li, C.; Wu, C.; Lu, H.; Chen, J.; Duan, J.; Meng, R.; et al. Remote ischemic conditioning for acute stroke patients treated with thrombectomy. Ann. Clin. Transl. Neurol. 2018, 5, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Asmaro, K.; Meng, L.; Liu, Y.; Ma, C.; Xi, C.; Li, G.; Ren, C.; Luo, Y.; Ling, F.; et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 2012, 79, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Ding, Y.; Asmaro, K.; Brogan, D.; Meng, L.; Sui, M.; Shi, J.; Duan, Y.; Sun, Z.; Yu, Y.; et al. Ischemic conditioning is safe and effective for octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics 2015, 12, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Meng, R.; Song, H.; Liu, G.; Hua, Y.; Cui, D.; Zheng, L.; Feng, W.; Liebeskind, D.S.; Fisher, M.; et al. Remote ischemic conditioning may improve outcomes of patients with cerebral small-vessel disease. Stroke 2017, 48, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Hibert, P.; Prunier-Mirebeau, D.; Beseme, O.; Chwastyniak, M.; Tamareille, S.; Pinet, F.; Prunier, F. Modifications in rat plasma proteome after remote ischemic preconditioning (ripc) stimulus: Identification by a seldi-tof-ms approach. PLoS ONE 2014, 9, e85669. [Google Scholar] [CrossRef] [Green Version]
- Hepponstall, M.; Ignjatovic, V.; Binos, S.; Monagle, P.; Jones, B.; Cheung, M.H.; d’Udekem, Y.; Konstantinov, I.E. Remote ischemic preconditioning (ripc) modifies plasma proteome in humans. PLoS ONE 2012, 7, e48284. [Google Scholar] [CrossRef]
- Nikkola, E.; Laiwalla, A.; Ko, A.; Alvarez, M.; Connolly, M.; Ooi, Y.C.; Hsu, W.; Bui, A.; Pajukanta, P.; Gonzalez, N.R. Remote ischemic conditioning alters methylation and expression of cell cycle genes in aneurysmal subarachnoid hemorrhage. Stroke 2015, 46, 2445–2451. [Google Scholar] [CrossRef] [Green Version]
- Thorne, A.M.; Huang, H.; O’Brien, D.P.; Eijken, M.; Krogstrup, N.V.; Norregaard, R.; Møller, B.; Ploeg, R.J.; Jespersen, B.; Kessler, B.M. Subclinical effects of remote ischaemic conditioning in human kidney transplants revealed by quantitative proteomics. Clin. Proteom. 2020, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, C.D.; Chaturvedi, S.; Gage, K.R.; Herson, P.S.; Hurn, P.D.; Jiménez, M.C.; Kittner, S.J.; Madsen, T.E.; McCullough, L.; McDermott, M.; et al. Sex differences in stroke: Challenges and opportunities. J. Cereb. Blood Flow Metab. 2018, 38, 2179–2191. [Google Scholar] [CrossRef]
- Wu, D.; Chen, J.; Wang, B.; Zhang, M.; Shi, J.; Ma, Y.; Zhu, Z.; Yan, F.; He, X.; Li, S.; et al. Endovascular ischemic stroke models of adult rhesus monkeys: A comparison of two endovascular methods. Sci. Rep. 2016, 6, 31608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, R.L.; Jiang, Q.; Ding, G.; Chopp, M.; Zhang, Z.G. Focal embolic cerebral ischemia in the rat. Nat. Protoc. 2015, 10, 539–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M.; Goto, S. Kegg: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The cog database: An updated version includes eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Kei, A.A.; Filippatos, T.D.; Tsimihodimos, V.; Elisaf, M.S. A review of the role of apolipoprotein C-II in lipoprotein metabolism and cardiovascular disease. Metabolism 2012, 61, 906–921. [Google Scholar] [CrossRef]
- Wolska, A.; Dunbar, R.L.; Freeman, L.A.; Ueda, M.; Amar, M.J.; Sviridov, D.O.; Remaley, A.T. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 2017, 267, 49–60. [Google Scholar] [CrossRef]
- Rousset, X.; Vaisman, B.; Amar, M.; Sethi, A.A.; Remaley, A.T. Lecithin: Cholesterol acyltransferase—From biochemistry to role in cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Chaithra, V.H.; Jacob, S.P.; Lakshmikanth, C.L.; Sumanth, M.S.; Abhilasha, K.V.; Chen, C.H.; Thyagarajan, A.; Sahu, R.P.; Traver, J.B.; McIntyre, T.M.; et al. Modulation of inflammatory platelet-activating factor (paf) receptor by the acyl analogue of paf. J. Lipid Res. 2018, 59, 2063–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noto, H.; Hara, M.; Karasawa, K.; Iso, O.N.; Satoh, H.; Togo, M.; Hashimoto, Y.; Yamada, Y.; Kosada, T.; Kawamura, M.; et al. Human plasma platelet-activating factor acetylhydrolase binds to all the murine lipoproteins, conferring protection against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.C.; Beaulieu, L.M.; Huntington, J.A.; Church, F.C. Serpins in thrombosis, hemostasis and fibrinolysis. J. Thromb. Haemost. 2007, 5, 102–115. [Google Scholar] [CrossRef]
- Teoh, N.; Field, J.; Farrell, G. Interleukin-6 is a key mediator of the hepatoprotective and pro-proliferative effects of ischaemic preconditioning in mice. J. Hepatol. 2006, 45, 20–27. [Google Scholar] [CrossRef]
- Cai, Z.P.; Parajuli, N.; Zheng, X.; Becker, L. Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic Res. Cardiol. 2012, 107, 277. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.J.; Chen, C.; Li, X.R.; Ran, Y.Y.; Xu, T.; Zhang, Y.; Geng, X.-K.; Zhang, Y.; Du, H.-S.; Leak, R.K.; et al. Remote ischemic preconditioning-mediated neuroprotection against stroke is associated with significant alterations in peripheral immune responses. CNS Neurosci. Ther. 2016, 22, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchand, M.; Monnot, C.; Muller, L.; Germain, S. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin. Cell Dev. Biol. 2019, 89, 147–156. [Google Scholar] [CrossRef]
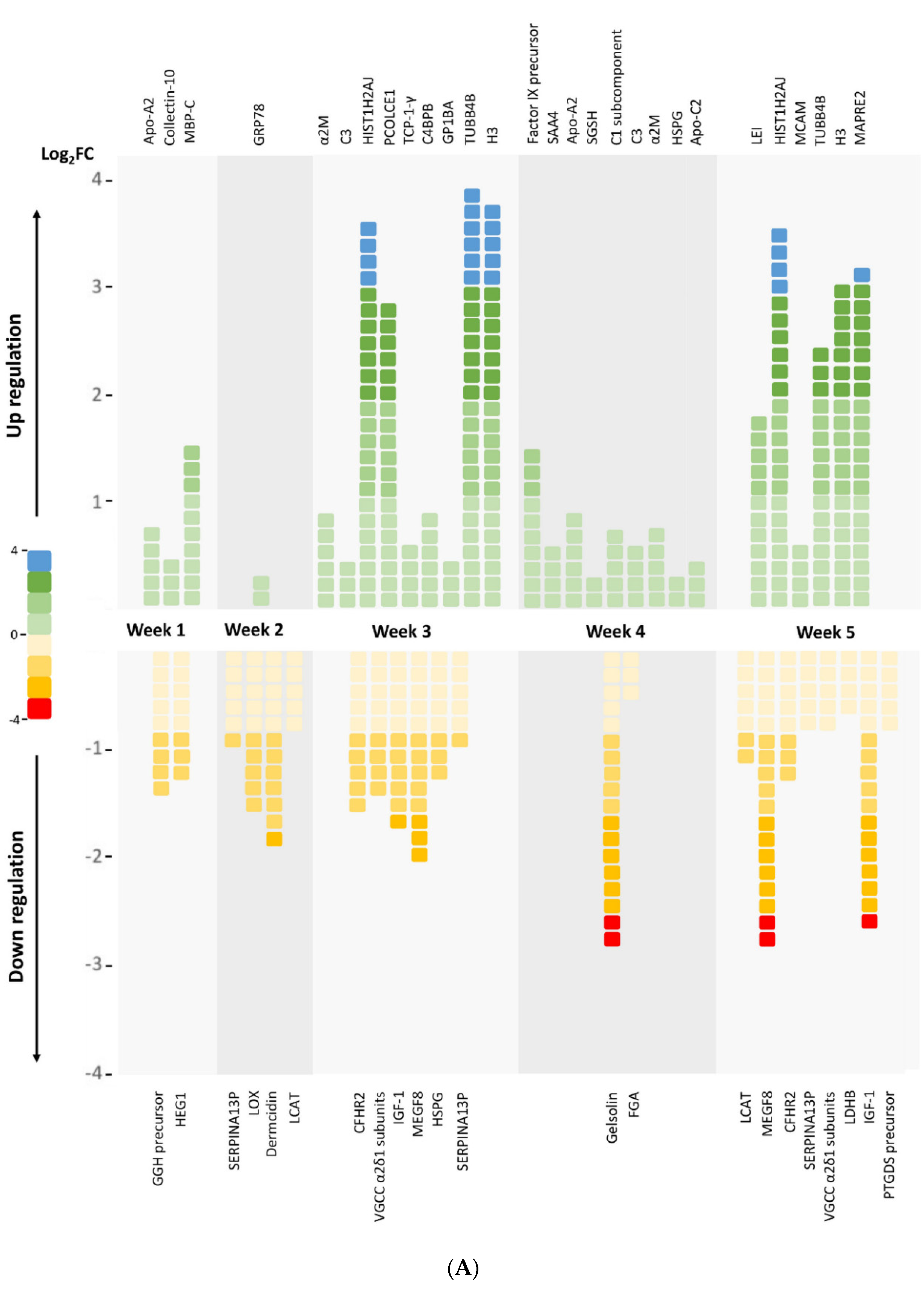
| Protein Name | Modulation | Main Function | Major Changed Time Post-RIC |
|---|---|---|---|
| Apolipoprotein A-II | Upregulated | Lipid metabolism, antioxidative, and antiatherogenesis | Week 1; Week 4 |
| Apolipoprotein C-II isoform X1 | Upregulated | Lipid metabolism | Week 4 |
| Complement C3 | Upregulated | Humoral immunity | Week 3; Week 4 |
| C4b-binding protein beta chain isoform X2 | Upregulated | Humoral immunity | Week 3 |
| Complement C1s subcomponent | Upregulated | Humoral immunity | Week 4 |
| Histone cluster 1, H2aj | Upregulated | Chromatin structure and dynamics | Week 3; Week 5 |
| Histone H3.1t-like | Upregulated | Chromatin structure and dynamics | Week 3; Week 5 |
| Fibrinogen alpha chain | Downregulated | Hemostasis, platelet aggregation, and proinflammation | Week 4 |
| Factor IX precursor | Upregulated | Hemostasis | Week 4 |
| Alpha-2-macroglobulin | Upregulated | Fibrinolysis inhibition, anticoagulation, and carrier protein | Week 3; Week 4 |
| Glucose-regulated protein precursor | Upregulated | Signaling, proliferation, invasion, apoptosis, inflammation, and immunity | Week 2 |
| Insulin-like growth factor I isoform X3 | Downregulated | Growth-promoting and DNA synthesis regulation | Week 3; Week 5 |
| Putative serpin A13 | Downregulated | Serine-type endopeptidase inhibitor activity | Week 2; Week 3 |
| Protein Name | KOG ID | Functional Categories | Function Class Definition | Major Changed Time Post-RIC |
|---|---|---|---|---|
| Serpin | KOG2392 | Cellular process and signaling | Defense mechanisms | Week 2; Week 3; Week 5 |
| Basement membrane-specific heparan sulfate proteoglycan (HSPG) core protein | KOG3509 | Cellular process and signaling | Posttranslational modification, protein turnover, chaperones | Week 3; Week 4 |
| Histone 2A | KOG1756 | Information storage and processing | Chromatin structure and dynamics | Week 3; Week 5 |
| Histones H3 and H4 | KOG1745 | Information storage and processing | Chromatin structure and dynamics | Week 3; Week 5 |
| Lecithin:cholesterol acyltransferase (LCAT)/Acyl-ceramide synthase | KOG2369 | Metabolism | Lipid transport and metabolism | Week 2; Week 5 |
| C-type lectin | KOG4297 | Cellular process and signaling | Immunity and homeostasis | Week 1; Week 3 |
| Attractin and platelet-activating factor acetylhydrolase | KOG1388 | Metabolism | Lipid metabolism | Week 3; Week 5 |
| Pathway Name | Pathway ID | Major Changed Time Post-RIC |
|---|---|---|
| Cholesterol metabolism | mcc04979 | Week 1; Week 2; Week 4 |
| Glycerophospholipid metabolism | mcc00564 | Week 2; Week 5 |
| Long-term depression | mcc04730 | Week 3; Week 5 |
| MAPK signaling pathway | mcc04010 | Week 3; Week 5 |
| Complement and coagulation cascades | mcc04610 | Week 3; Week 4 |
| Phagosome | mcc04145 | Week 3; Week 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Guo, L.; Wu, D.; Shi, J.; Duan, Y.; He, X.; Liu, Y.; Ding, Y.; Ji, X.; Meng, R. Quantitative Proteomic Analysis of Plasma after Remote Ischemic Conditioning in a Rhesus Monkey Ischemic Stroke Model. Biomolecules 2021, 11, 1164. https://doi.org/10.3390/biom11081164
Song S, Guo L, Wu D, Shi J, Duan Y, He X, Liu Y, Ding Y, Ji X, Meng R. Quantitative Proteomic Analysis of Plasma after Remote Ischemic Conditioning in a Rhesus Monkey Ischemic Stroke Model. Biomolecules. 2021; 11(8):1164. https://doi.org/10.3390/biom11081164
Chicago/Turabian StyleSong, Siying, Linlin Guo, Di Wu, Jingfei Shi, Yunxia Duan, Xiaoduo He, Yunhuan Liu, Yuchuan Ding, Xunming Ji, and Ran Meng. 2021. "Quantitative Proteomic Analysis of Plasma after Remote Ischemic Conditioning in a Rhesus Monkey Ischemic Stroke Model" Biomolecules 11, no. 8: 1164. https://doi.org/10.3390/biom11081164
APA StyleSong, S., Guo, L., Wu, D., Shi, J., Duan, Y., He, X., Liu, Y., Ding, Y., Ji, X., & Meng, R. (2021). Quantitative Proteomic Analysis of Plasma after Remote Ischemic Conditioning in a Rhesus Monkey Ischemic Stroke Model. Biomolecules, 11(8), 1164. https://doi.org/10.3390/biom11081164









