Understanding the Therapeutic Potential of Ascorbic Acid in the Battle to Overcome Cancer
Abstract
1. Introduction
1.1. Natural Sources
1.2. Dietary Supplements
2. Metabolism
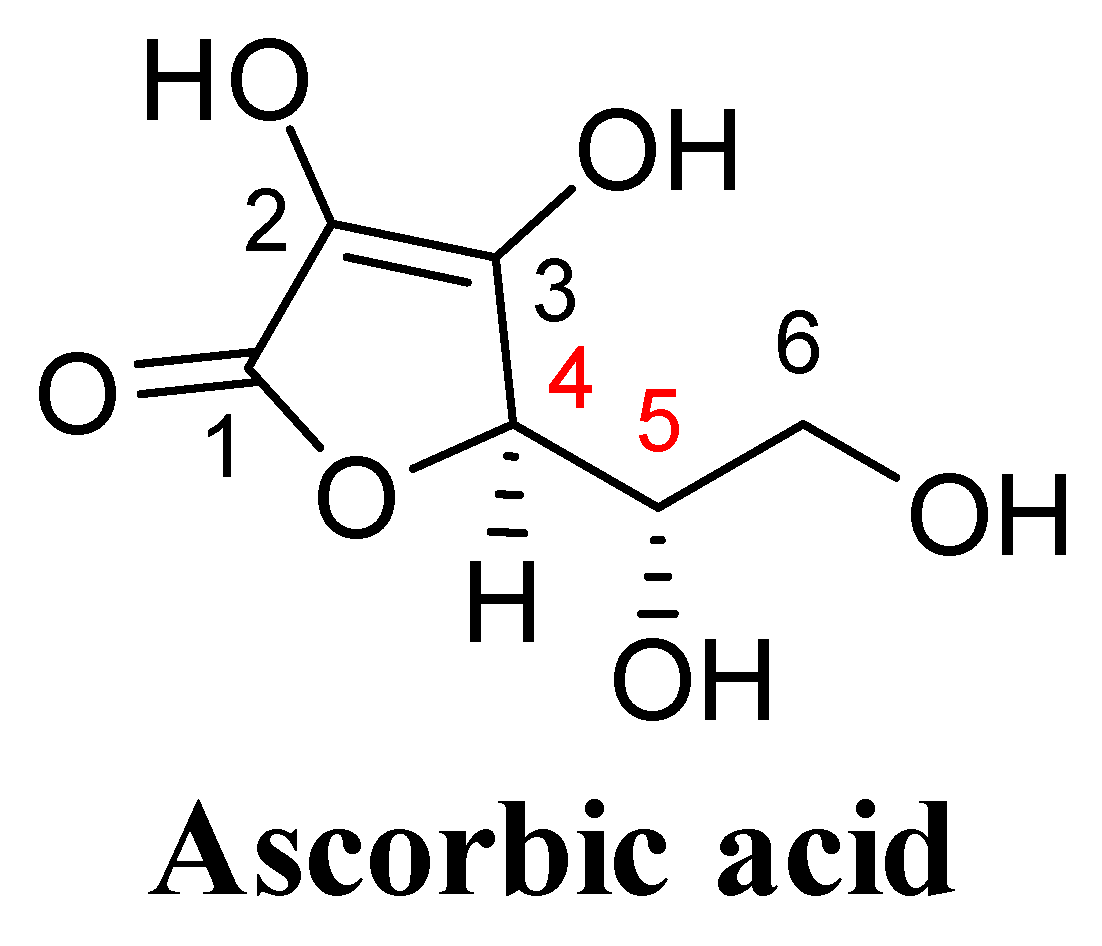
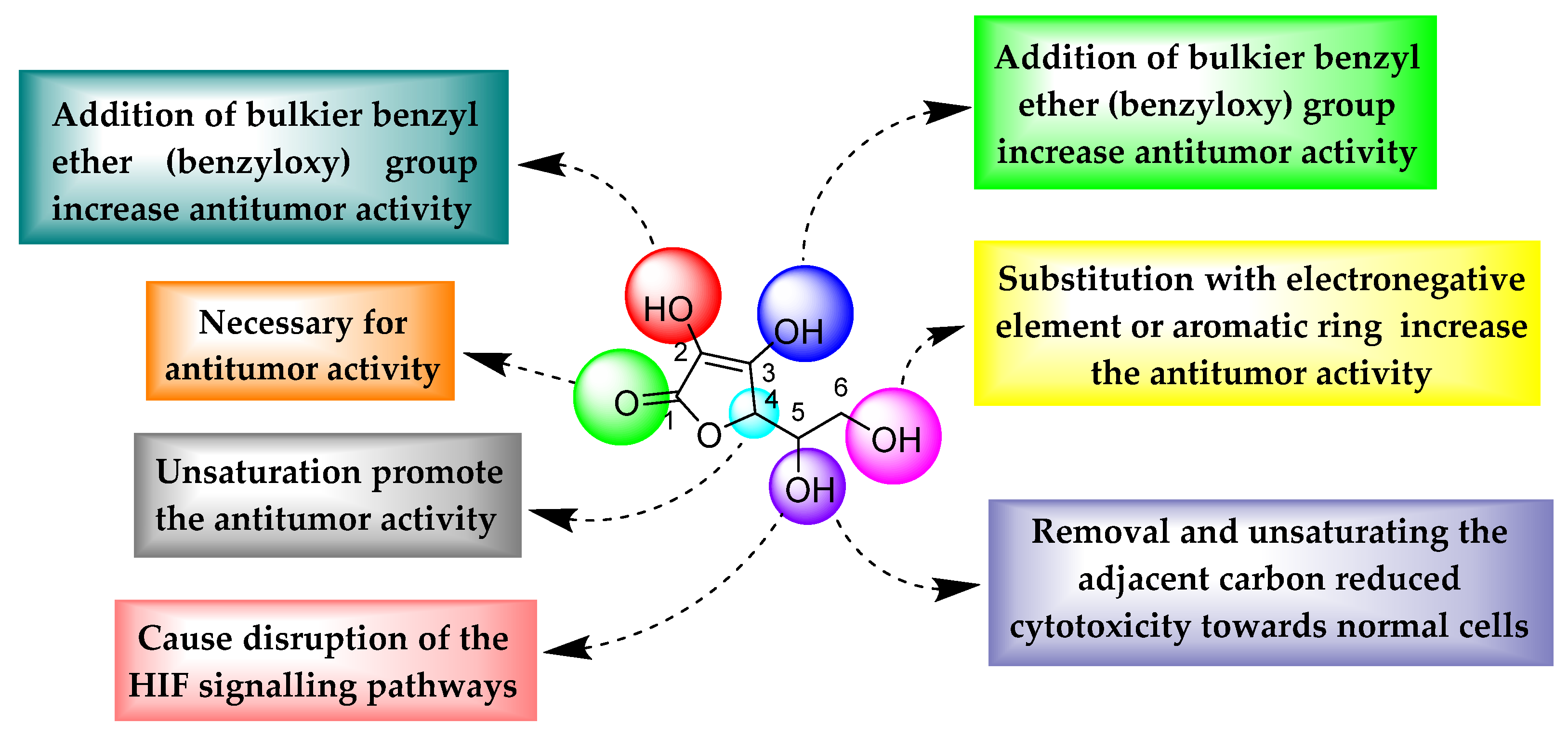
3. Structural Features
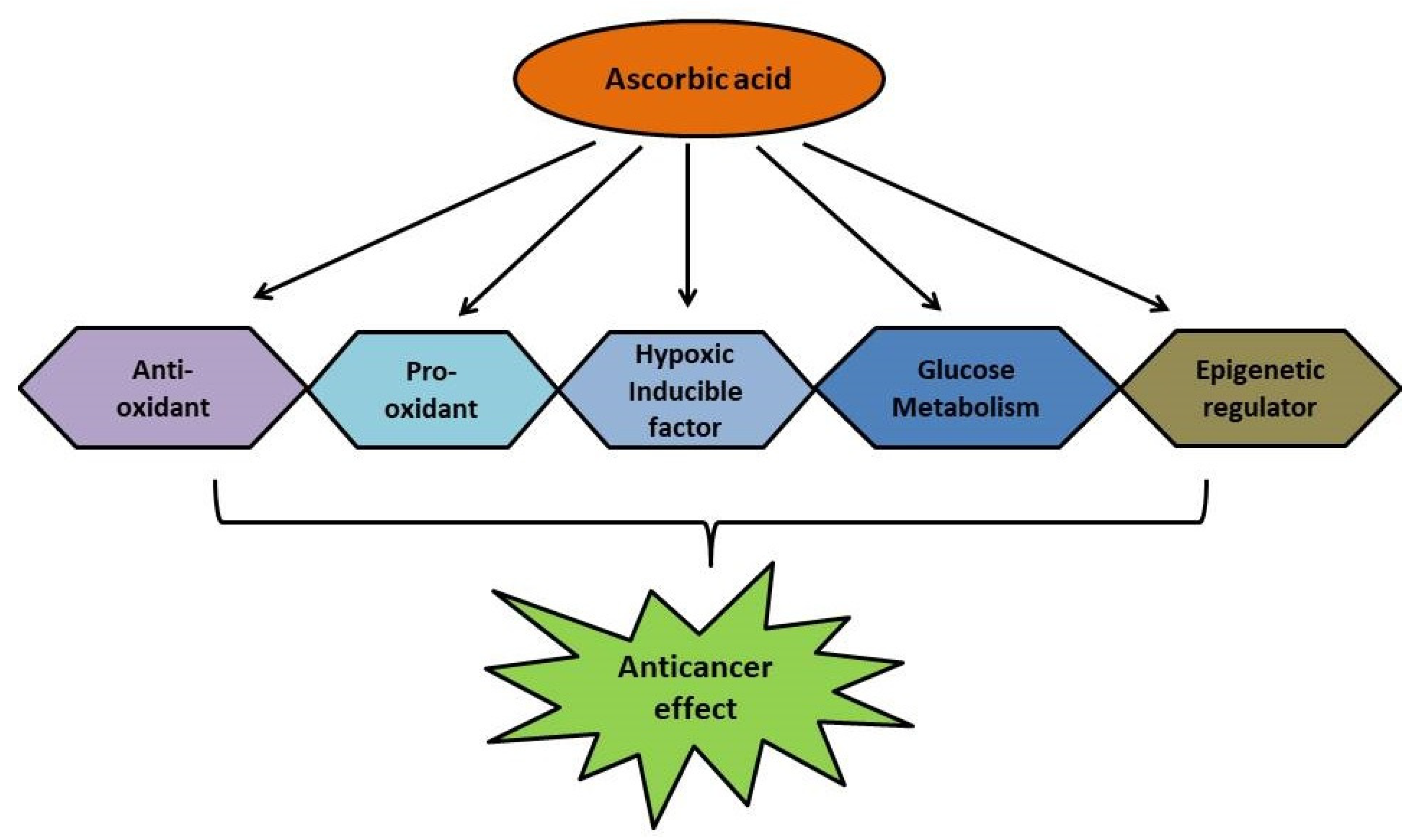
4. Possible Interventions in Cancer Treatment
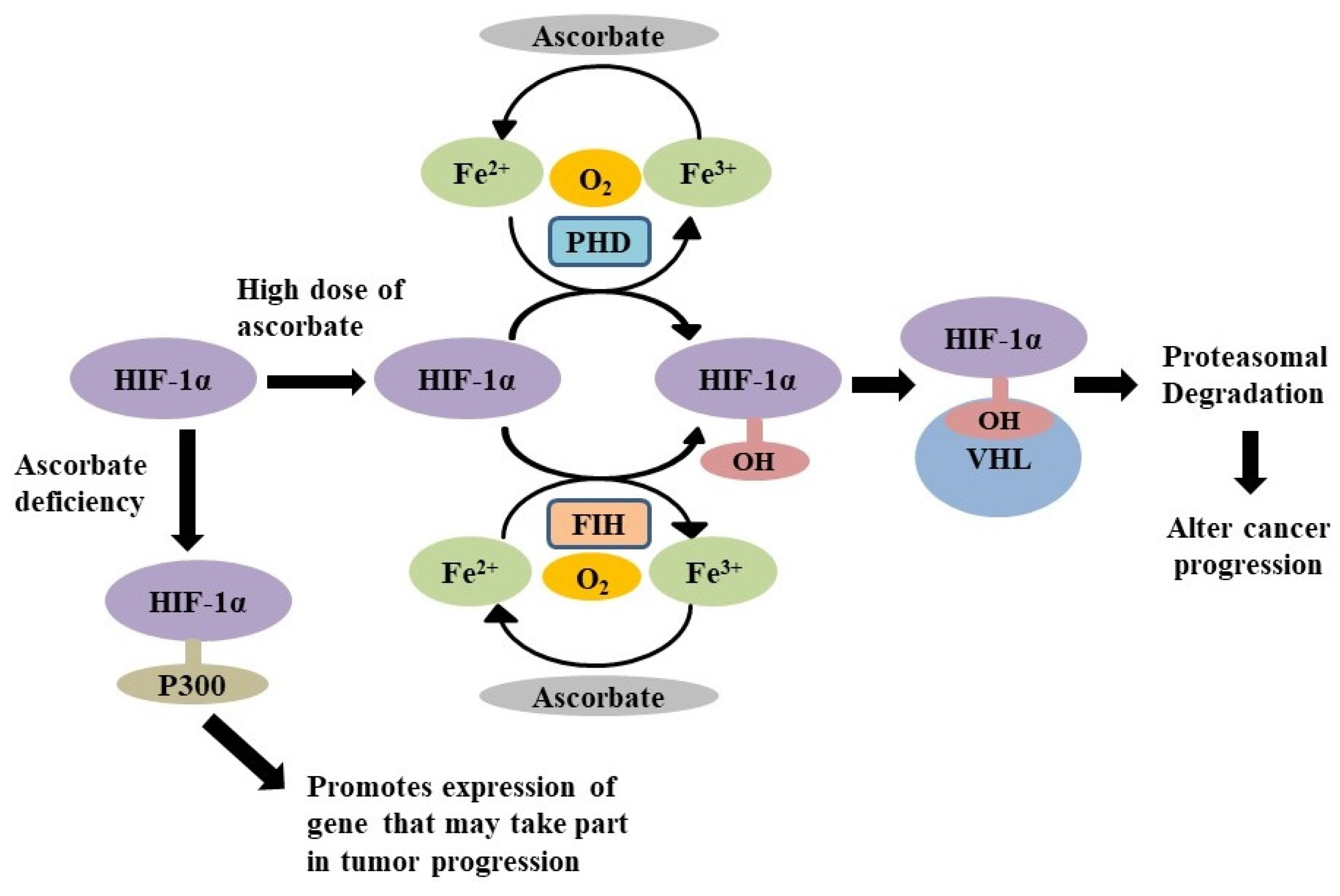
4.1. As the Down Regulator of Hypoxic Inducible Factors
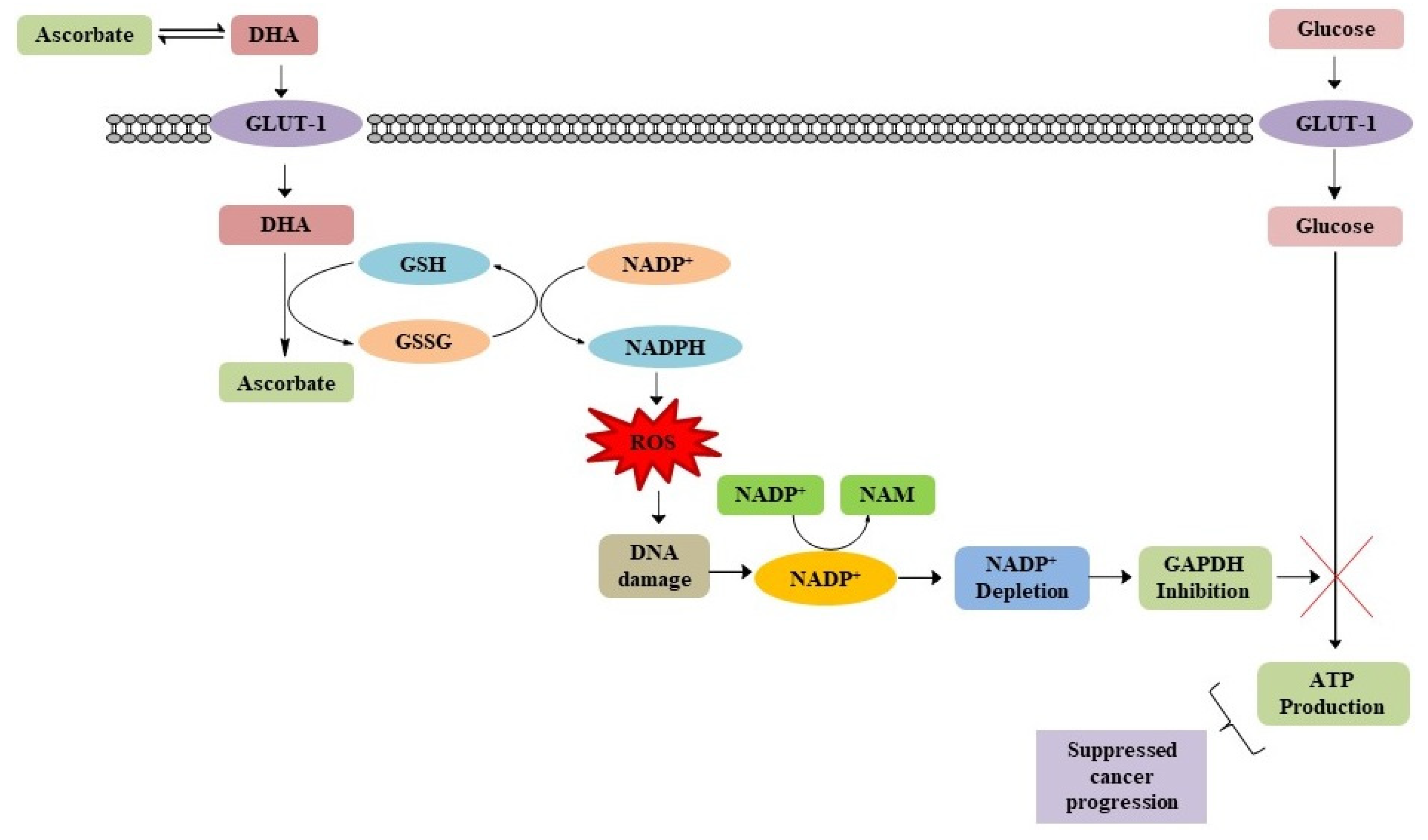
4.2. Impairing Glucose Metabolism in Cancer Cells
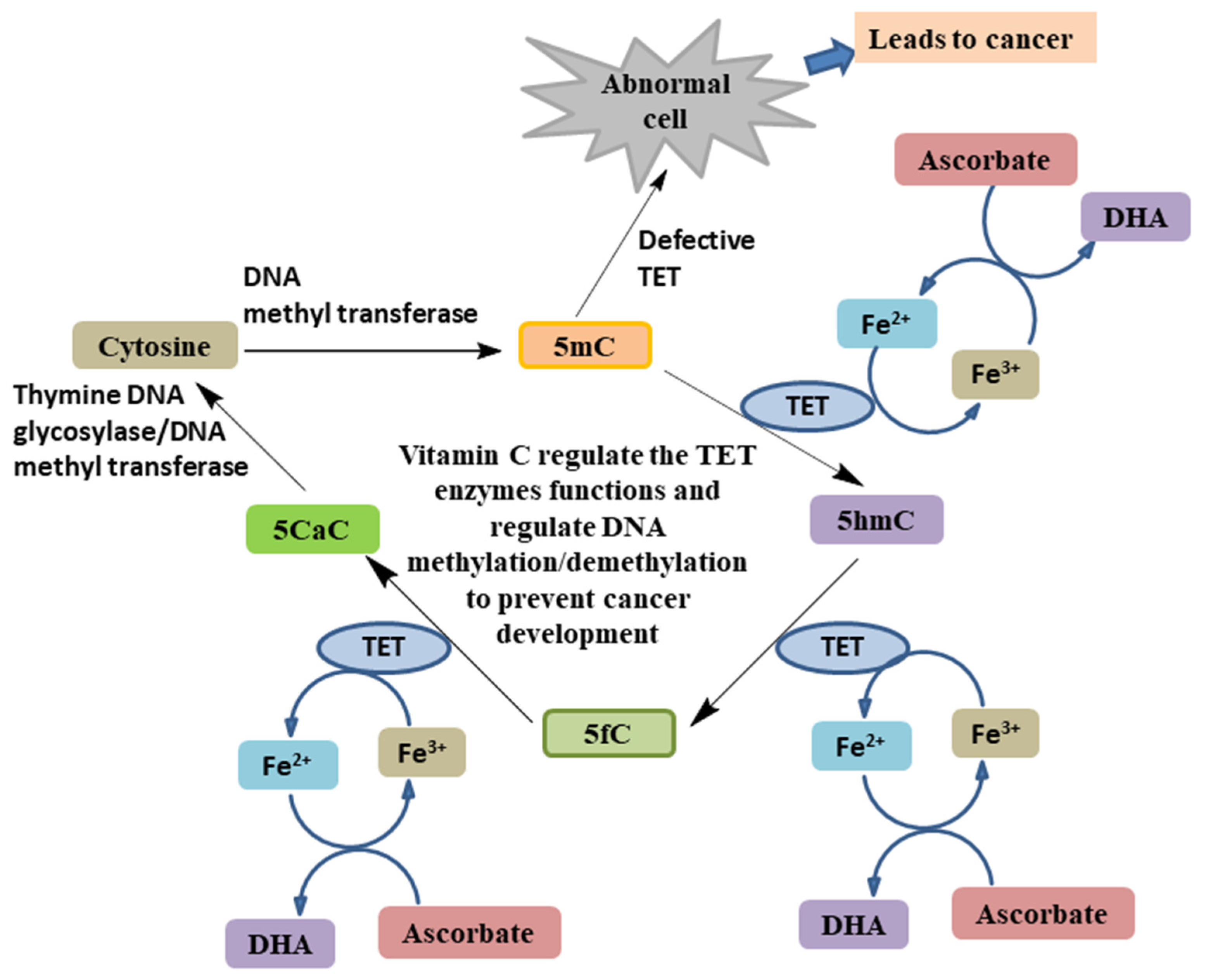
4.3. As an Epigenetic Regulator
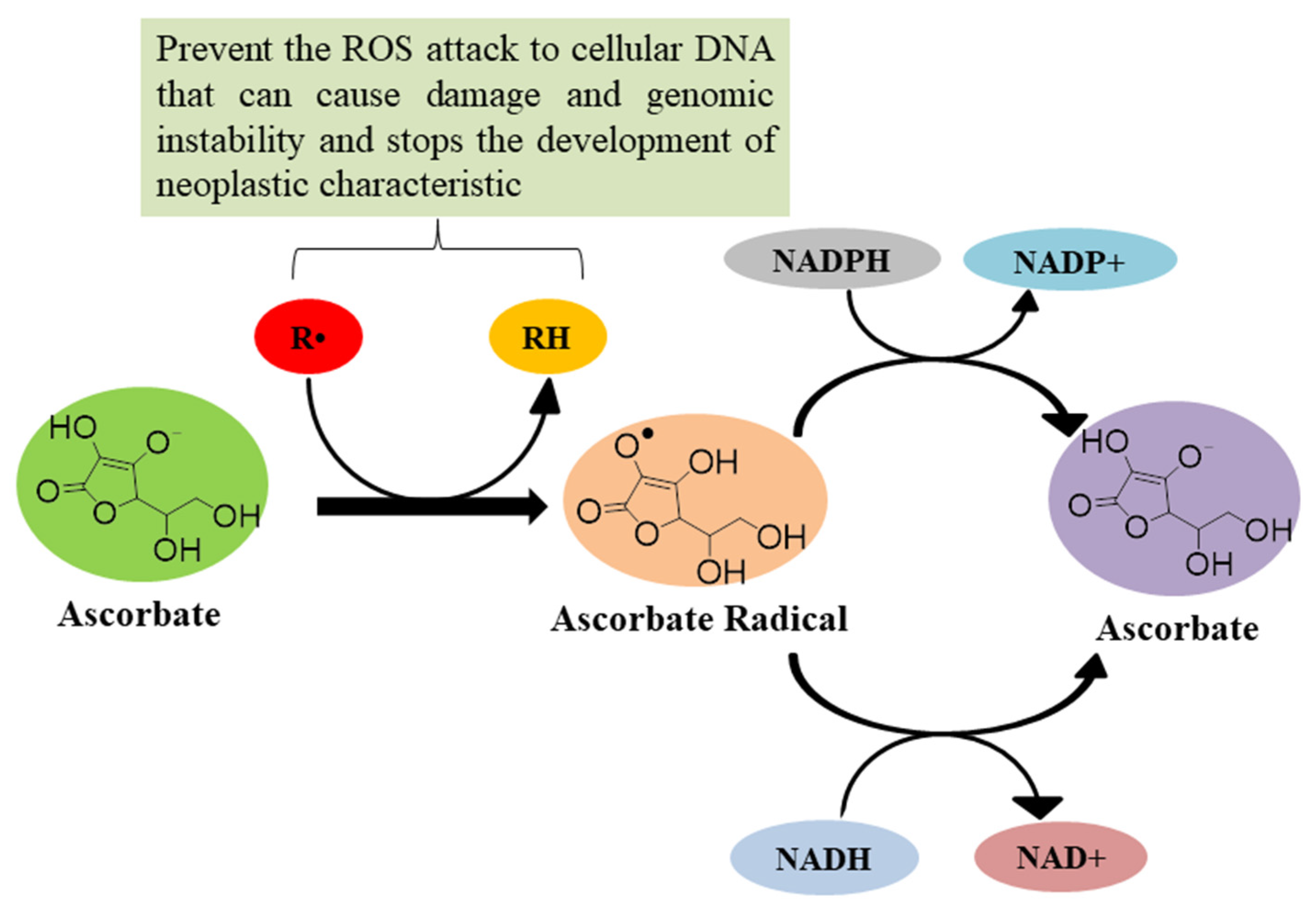
4.4. As an Antioxidant Agent
4.5. Pro-Oxidant Role
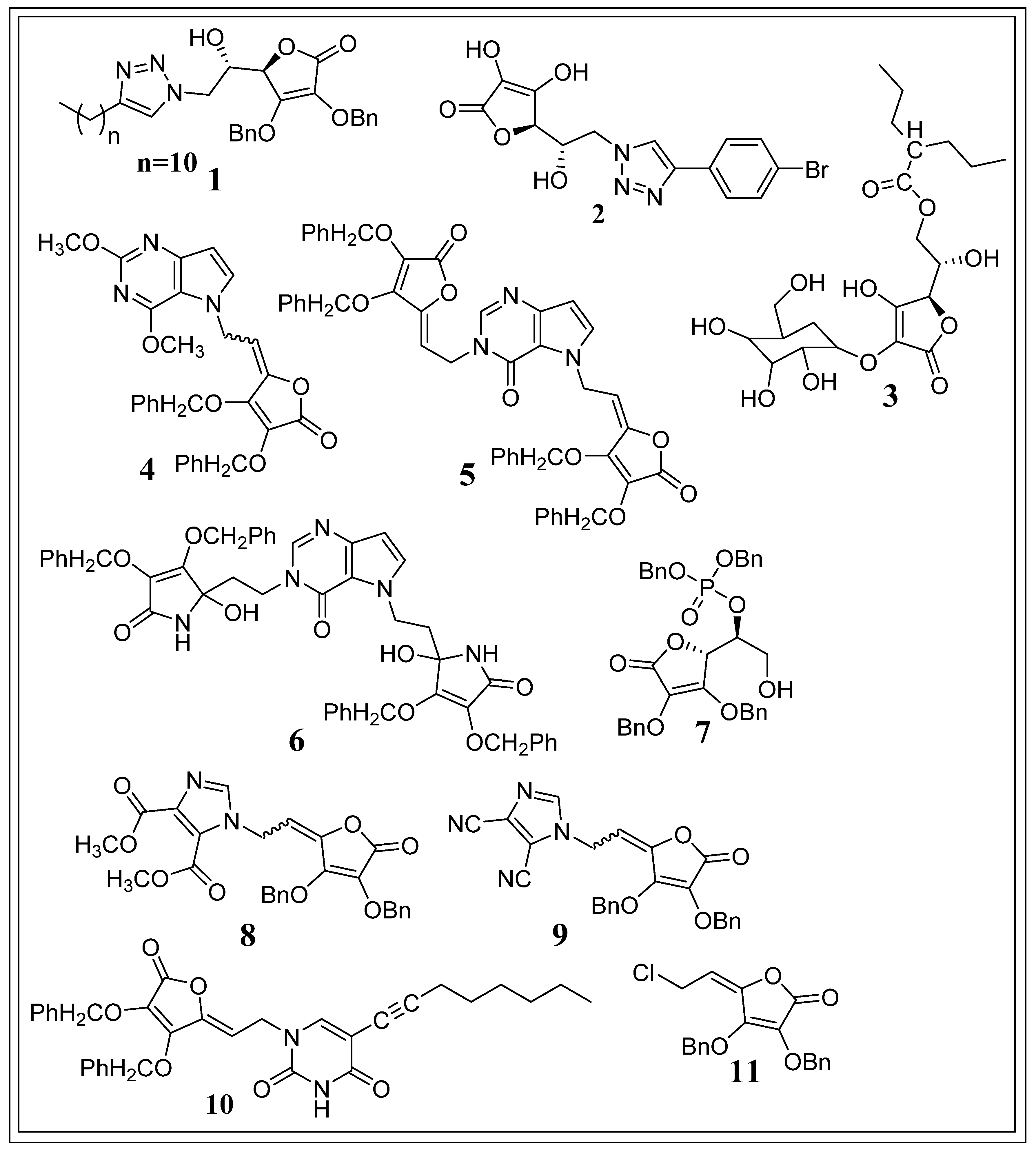
5. Potent Synthetic Derivatives of Ascorbic Acid against Cancer
6. Emerging Trends in Cancer Therapy
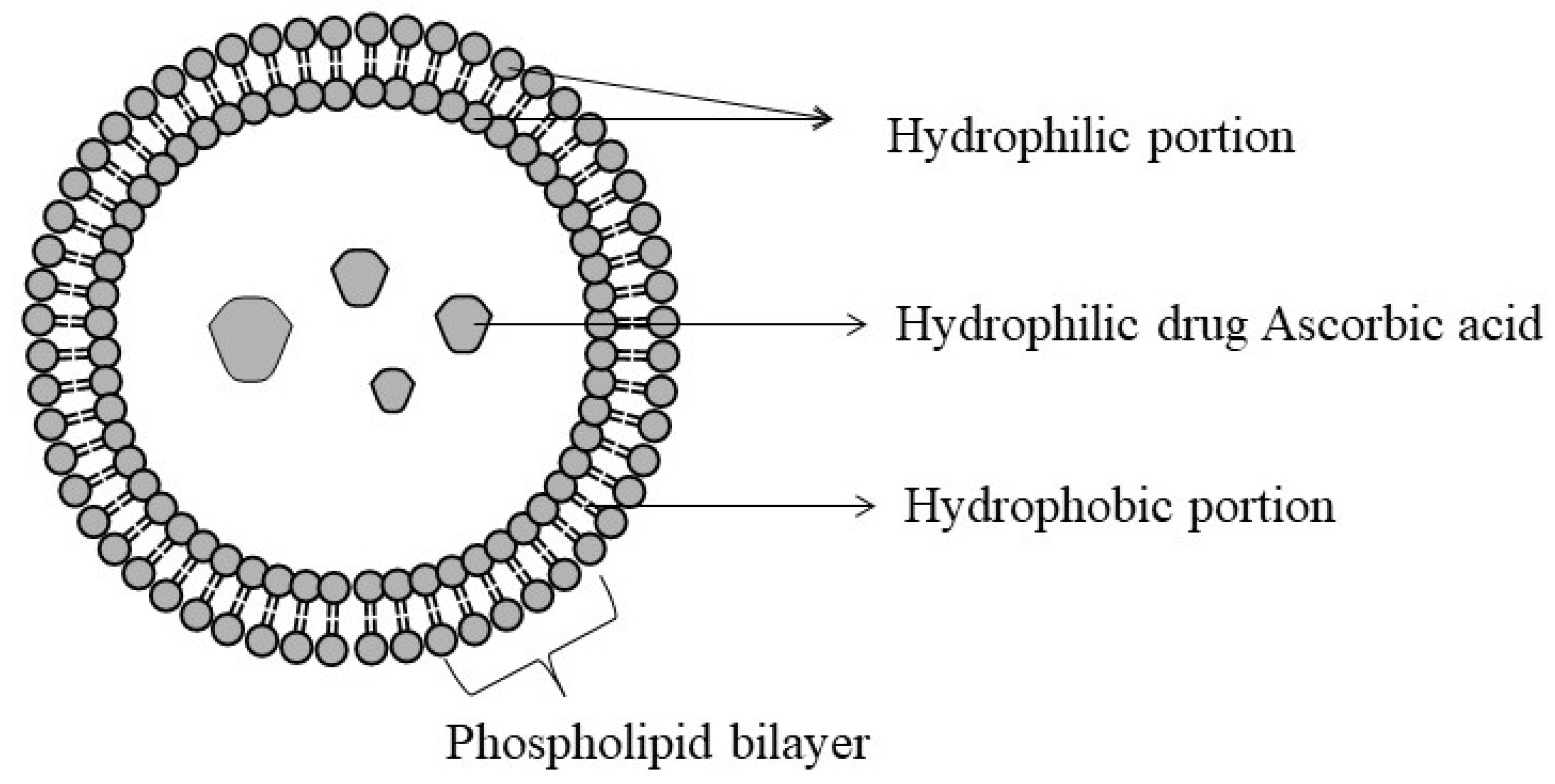
6.1. Liposomes
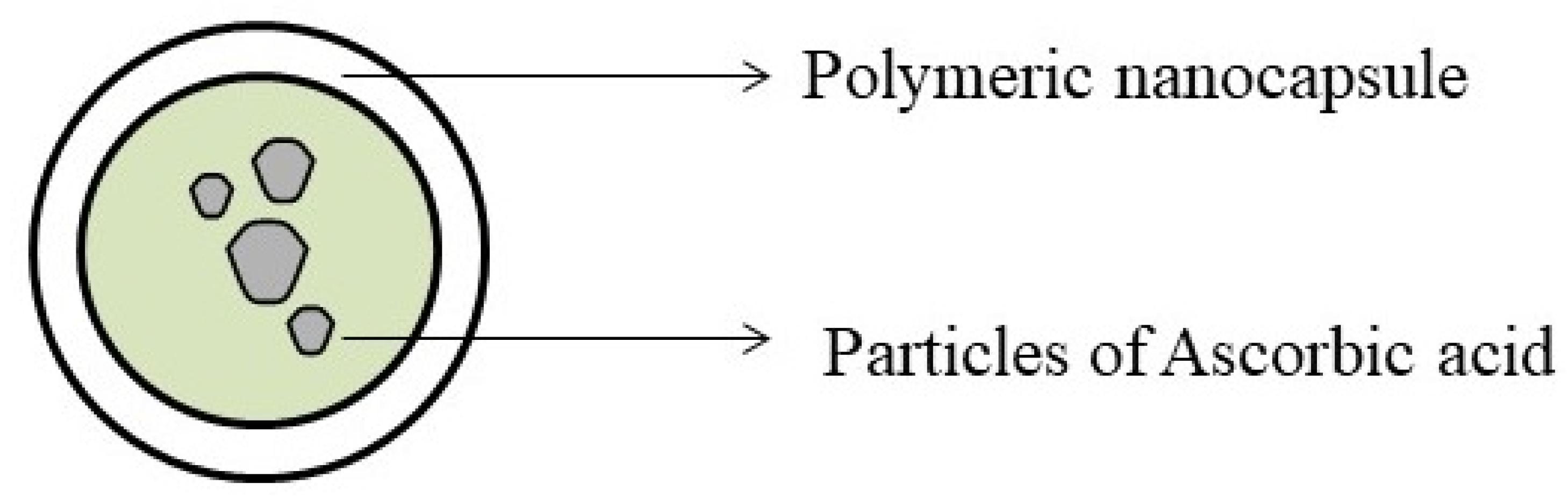
6.2. Nanoparticles
7. Patents as an Anticancer Agent
8. Challenges and Current Status
9. Future Prospects and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GLUT | Glucose transporter |
| HIF | Hypoxia-inducible factor |
| TET | Ten-eleven translocation |
| FIH | Factor Inhibiting HIF |
| PHD | Prolyl hydroxylase domain enzyme |
| VHL | von Hippel-Lindau |
| DHA | Dehydroascorbate |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| GSH | Glutathione |
| GSSG | Glutathione disulphide |
| NADP | Nicotinamide adenine dinucleotide phosphate |
| PARP | Poly (ADP- ribose) polymerase |
| NAD | Nicotinamide adenine dinucleotide |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| NAM | Nicotinamide |
| 5mC | 5-methylcytosine |
| 5hmC | 5-hydroxymethylcytosine |
| 5fC | 5-formylcytosine |
| 5CaC | 5-carboxylcytosine |
| AML | Acute myeloid leukaemia |
| CMML | Chronic myelomonocytic leukaemia |
| Fe2+/α-KGDD | Iron/α-ketoglutarate-dependent dioxygenases |
| ROS | Reactive oxygen species |
| HNE | 4-hydroxynonenal |
| AscH− | Ascorbate |
| Asc⋅− | Ascorbate free radical |
| H2O2 | Hydrogen peroxide |
| HO⋅ | Hydroxyl peroxide radical |
| MTX | Mitoxantrone |
| PA | Palmitoyl ascorbate |
| DOX | Doxorubicin |
| DC | Docetaxel |
| EPI | Epirubicin |
| PTX | Paclitaxel |
| TEM | Transmission electronic microscopy |
| SLN | Solid lipid nanoparticle |
| DLS | Dynamic light scattering |
| DHC | trans-dehydrocrotonin |
| AAS | L-ascorbic acid 6-stearate |
| 5-FU | 5-fluorouracil |
References
- World Health Organization (WHO). Cancer Overview. Available online: https://www.who.int/health-topics/cancer#tab=tab_3 (accessed on 31 March 2021).
- World Health Organization (WHO). Cancer Tomorrow, International Agency for Research on Cancer. Available online: https://gco.iarc.fr/tomorrow/graphic-isotype (accessed on 31 March 2021).
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020, 188, 112016. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Sharma, D.; Sharma, A.; Bhagat, M.; Ola, M.; Thakur, V.K.; Bhardwaj, J.K.; Goyal, R.K. Recent advances in microbial toxin-related strategies to combat cancer. Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Sharma, D.; Sharma, A.; Bansal, K.K.; Rajak, H.; Sharma, S.; Thakur, V.K. New horizons in benzothiazole scaffold for cancer therapy: Advances in bioactivity, functionality, and chemistry. Appl. Mater. Today 2020, 20, 100783. [Google Scholar] [CrossRef]
- Side Effects of Anti-Cancer Drugs, University of Iowa Hospitals & Clinics. Available online: https://uihc.org/health-topics/side-effects-anti-cancer-drugs (accessed on 31 March 2021).
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Wilson, M.K.; Baguley, B.C.; Wall, C.; Jameson, M.B.; Findlay, M.P. Review of high-dose intravenous vitamin C as an anticancer agent. Asia-Pac. J. Clin. Oncol. 2014, 10, 22–37. [Google Scholar] [CrossRef]
- Henson, D.E.; Block, G.; Levine, M. Ascorbic Acid: Biologic Functions and Relation to Cancer. JNCI 1991, 83, 547–550. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho Melo-Cavalcante, A.A.; da Rocha Sousa, L.; Alencar, M.V.O.B.; de Oliveira Santos, J.V.; da Mata, A.M.O.; Paz, M.F.C.J.; de Carvalho, R.M.; Nunes, N.M.F.; Islam, M.T.; Mendes, A.N.; et al. Retinol palmitate and ascorbic acid: Role in oncological prevention and therapy. Biomed. Pharmacother. 2019, 109, 1394–1405. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Kumar, D.; Rizvi, S.I. Significance of vitamin C in human health and disease. Ann. Phytomed. 2012, 1, 9–13. [Google Scholar]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef]
- Souyoul, S.A.; Saussy, K.P.; Lupo, M.P. Nutraceuticals: A Review. Dermatol. Ther. 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Find Drugs (Ascorbic Acid). Available online: https://www.mims.com/india (accessed on 31 March 2021).
- Vitamin C-Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/ (accessed on 31 March 2021).
- González, M.J.; Miranda-Massari, J.R.; Mora, E.M.; Guzmán, A.; Riordan, N.H.; Riordan, H.D.; Casciari, J.J.; Jackson, J.A.; Román-Franco, A. Orthomolecular oncology review: Ascorbic acid and cancer 25 years later. Integr. Cancer Ther. 2005, 4, 32–44. [Google Scholar] [CrossRef]
- Cimmino, L.; Neel, B.G.; Aifantis, I. Vitamin C in Stem Cell Reprogramming and Cancer. Trends Cell Biol. 2018, 28, 698–708. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral. Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Riggi, N.; Aguet, M.; Stamenkovic, I. Cancer Metastasis: A reappraisal of its underlying mechanisms and their relevance to treatment. Annu. Rev. Pathol. 2018, 13, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Mikirova, N.A.; Ichim, T.E.; Riordan, N.H. Anti-angiogenic effect of high doses of ascorbic acid. J. Transl. Med. 2008, 6, 50. [Google Scholar] [CrossRef]
- Devaki, S.J.; Raveendran, R.L. Vitamin C: Sources, Functions, Sensing and Analysis. 2017. Available online: https://www.intechopen.com/books/vitamin-c/vitamin-c-sources-functions-sensing-and-analysis (accessed on 31 March 2021).
- Lee, W.J. The prospects of vitamin C in cancer therapy. Immune Netw. 2009, 9, 147–152. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Linowiecka, K.; Foksinski, M.; Brożyna, A.A. Vitamin C Transporters and Their Implications in Carcinogenesis. Nutrients 2020, 12, 3869. [Google Scholar] [CrossRef] [PubMed]
- Olabisi, A.O. The Chemistry of L-Ascorbic Acid Derivatives in the Asymmetric Synthesis of C2- and C3- Substituted Aldono-γ-lactones. Ph.D. Thesis, Wichita State University. Available online: http://hdl.handle.net/10057/540 (accessed on 31 March 2021).
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1976, 73, 3685–3689. [Google Scholar] [CrossRef]
- Semenza, G.L. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim. Biophys. Acta 2016, 1863, 382–391. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Semenza, G.L. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 2012, 92, 967–1003. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.C.; Rathore, A.; Younas, H.; Gilkes, D.; Polotsky, V.Y. Hypoxia-Inducible Factors and Cancer. Curr. Sleep Med. Rep. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Akanji, M.A.; Rotimi, D.; Adeyemi, O.S. Hypoxia-Inducible Factors as an Alternative Source of Treatment Strategy for Cancer. Oxid. Med. Cell. Longev. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef]
- Unwith, S.; Zhao, H.; Hennah, L.; Ma, D. The potential role of HIF on tumour progression and dissemination. Int. J. Cancer 2015, 136, 2491–2503. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.; Das, A.B. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Front. Physiol. 2018, 9, 809. [Google Scholar] [CrossRef]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef]
- Wilkes, J.G.; O’Leary, B.R.; Du, J.; Klinger, A.R.; Sibenaller, Z.A.; Doskey, C.M.; Gibson-Corley, K.N.; Alexander, M.S.; Tsai, S.; Buettner, G.R.; et al. Pharmacologic ascorbate (P-AscH-) suppresses hypoxia-inducible Factor-1α (HIF-1α) in pancreatic adenocarcinoma. Clin. Exp. Metastasis 2018, 35, 37–51. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, H.; Dinavahi, R.; Li, F.; Xiang, Y.; Raman, V.; Bhujwalla, Z.M.; Felsher, D.W.; Cheng, L.; Pevsner, J.; et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 2007, 12, 230–238. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Zhang, H. HIF-1 suppresses lipid catabolism to promote cancer progression. Mol. Cell Oncol. 2015, 2, e980184. [Google Scholar] [CrossRef]
- Marchiq, I.; Pouysségur, J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J. Mol. Med. 2016, 94, 155–171. [Google Scholar] [CrossRef]
- Yun, J.; Rago, C.; Cheong, I.; Pagliarini, R.; Angenendt, P.; Rajagopalan, H.; Schmidt, K.; Willson, J.K.; Markowitz, S.; Zhou, S.; et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 2009, 325, 1555–1559. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Martel, F. Targeting Glucose Transporters for Breast Cancer Therapy: The Effect of Natural and Synthetic Compounds. Cancers 2020, 12, 154. [Google Scholar] [CrossRef]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef]
- Tu, H.; Wang, Y.; Li, H.; Brinster, L.R.; Levine, M. Chemical Transport Knockout for Oxidized Vitamin C, Dehydroascorbic Acid, Reveals Its Functions in vivo. EBioMedicine 2017, 23, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.X.; Wu, Q.N.; Chen, D.L.; Chen, L.Z.; Wang, Z.X.; Ren, C.; Mo, H.Y.; Chen, Y.; Sheng, H.; Wang, Y.N.; et al. Pharmacological Ascorbate Suppresses Growth of Gastric Cancer Cells with GLUT1 Overexpression and Enhances the Efficacy of Oxaliplatin Through Redox Modulation. Theranostics 2018, 8, 1312–1326. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Y.; Xu, Y.; Guo, X.; Wang, B.; Sun, L.; Liu, L.; Cui, F.; Zhuang, Q.; Bao, X.; et al. The hypoxia-inducible factor renders cancer cells more sensitive to vitamin C-induced toxicity. J. Biol. Chem. 2014, 289, 3339–3351. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Jones, P.A. Epigenetic determinants of cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef]
- Pawlowska, E.; Szczepanska, J.; Blasiak, J. Pro- And antioxidant effects of Vitamin C in cancer in correspondence to its dietary and pharmacological concentrations. Oxid. Med. Cell. Longev. 2019, 2019, 7286737. [Google Scholar] [CrossRef]
- Ehrlich, M.; Lacey, M. DNA hypomethylation and hemimethylation in cancer. Adv. Exp. Med. Biol. 2013, 754, 31–56. [Google Scholar]
- Baylin, S.B.; Jones, P.A. A decade of exploring the cancer epigenome—Biological and translational implications. Nat. Rev. Cancer 2011, 11, 726–734. [Google Scholar] [CrossRef]
- Rasmussen, K.D.; Helin, K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016, 30, 733–750. [Google Scholar] [CrossRef]
- Cimmino, L.; Dolgalev, I.; Wang, Y.; Yoshimi, A.; Martin, G.H.; Wang, J.; Ng, V.; Xia, B.; Witkowski, M.T.; Mitchell-Flack, M.; et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell 2017, 170, 1079–1095.e20. [Google Scholar] [CrossRef]
- Shenoy, N.; Bhagat, T.; Nieves, E.; Stenson, M.; Lawson, J.; Choudhary, G.S.; Habermann, T.; Nowakowski, G.; Singh, R.; Wu, X.; et al. Upregulation of TET activity with ascorbic acid induces epigenetic modulation of lymphoma cells. Blood Cancer J. 2017, 7, e587. [Google Scholar] [CrossRef]
- He, Y.F.; Li, B.Z.; Li, Z.; Liu, P.; Wang, Y.; Tang, Q.; Ding, J.; Jia, Y.; Chen, Z.; Li, L.; et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011, 333, 1303–1307. [Google Scholar] [CrossRef]
- Ito, S.; Shen, L.; Dai, Q.; Wu, S.C.; Collins, L.B.; Swenberg, J.A.; He, C.; Zhang, Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011, 333, 1300–1303. [Google Scholar] [CrossRef]
- Santiago, M.; Antunes, C.; Guedes, M.; Sousa, N.; Marques, C.J. TET enzymes and DNA hydroxymethylation in neural development and function—How critical are they? Genomics 2014, 104, 334–340. [Google Scholar] [CrossRef]
- Hore, T.A.; Von Meyenn, F.; Ravichandran, M.; Bachman, M.; Ficz, G.; Oxley, D.; Santos, F.; Balasubramanian, S.; Jurkowski, T.P.; Reik, W. Retinol and ascorbate drive erasure of Epigenetic memory and enhance reprogramming to naïve pluripotency by complementary mechanisms. Proc. Natl. Acad. Sci. USA 2016, 113, 12202–12207. [Google Scholar] [CrossRef]
- An, J.; Rao, A.; Ko, M. TET family dioxygenases and DNA demethylation in stem cells and cancers. Exp. Mol. Med. 2017, 49, e323. [Google Scholar] [CrossRef]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y. Do Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants—A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Bruno, R.S.; Leonard, S.W.; Atkinson, J.; Montine, T.J.; Ramakrishnan, R.; Bray, T.M.; Traber, M.G. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic. Biol. Med. 2006, 40, 689–697. [Google Scholar] [CrossRef]
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic toxicity: A systematic review of the evidence from randomized controlled trials. Int. J. Cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef]
- Suh, J.; Zhu, B.Z.; Frei, B. Ascorbate does not act as a pro-oxidant towards lipids and proteins in human plasma exposed to redox-active transition metal ions and hydrogen peroxide. Free Radic. Biol. Med. 2003, 34, 1306–1314. [Google Scholar] [CrossRef]
- Chen, K.; Suh, J.; Carr, A.C.; Morrow, J.D.; Zeind, J.; Frei, B. Vitamin C suppresses oxidative lipid damage in vivo, even in the presence of iron overload. Am. J. Physiol. Endocrinol. Metab. 2000, 279, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, D.; Pelosi, E.; Castelli, G.; Lo-Coco, F.; Testa, U. Mechanisms of anti-cancer effects of ascorbate: Cytotoxic activity and epigenetic modulation. Blood Cells, Mol. Dis. 2018, 69, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C Pharmacokinetics: Implications for Oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–538. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Lee, J.H.; Krishna, M.C.; Shacter, E.; Choyke, P.L.; Pooput, C.; Kirk, K.L.; Buettner, G.R.; et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 8749–8754. [Google Scholar] [CrossRef]
- Kanno, T.; Nakamura, K.; Ikai, H.; Kikuchi, K.; Sasaki, K.; Niwano, Y. Literature review of the role of hydroxyl radicals in chemically-induced mutagenicity and carcinogenicity for the risk assessment of a disinfection system utilizing photolysis of hydrogen peroxide. J. Clin. Biochem. Nutr. 2012, 51, 9–14. [Google Scholar] [CrossRef][Green Version]
- Baronzio, G.; Schwartz, L.; Kiselevsky, M.; Guais, A.; Sanders, E.; Milanesi, G.; Baronzio, M.; Freitas, I. Tumor interstitial fluid as modulator of cancer inflammation, thrombosis, immunity and angiogenesis. Anticancer Res. 2012, 32, 405–414. [Google Scholar]
- Cieslak, J.; Cullen, J. Treatment of Pancreatic Cancer with Pharmacological Ascorbate. Curr. Pharm. Biotechnol. 2015, 16, 759–770. [Google Scholar] [CrossRef]
- Cater, M.A.; Haupt, Y. Clioquinol induces cytoplasmic clearance of the X-linked inhibitor of apoptosis protein (XIAP): Therapeutic indication for prostate cancer. Biochem. J. 2011, 436, 481–491. [Google Scholar] [CrossRef]
- Safi, R.; Nelson, E.R.; Chitneni, S.K.; Franz, K.J.; George, D.J.; Zalutsky, M.R.; McDonnell, D.P. Copper signaling axis as a target for prostate cancer therapeutics. Cancer Res. 2014, 74, 5819–5831. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Pearson, H.B.; Clatworthy, S.A.S.; Smith, Z.M.; Francis, P.S.; Llanos, R.M.; Volitakis, I.; Phillips, W.A.; Meggyesy, P.M.; Masaldan, S.; et al. Copper as a target for prostate cancer therapeutics: Copper-ionophore pharmacology and altering systemic copper distribution. Oncotarget 2016, 7, 37064–37080. [Google Scholar] [CrossRef]
- Ullah, M.F.; Khan, H.Y.; Zubair, H.; Shamim, U.; Hadi, S.M. The antioxidant ascorbic acid mobilizes nuclear copper leading to a prooxidant breakage of cellular DNA: Implications for chemotherapeutic action against cancer. Cancer Chemother. Pharmacol. 2011, 67, 103–110. [Google Scholar] [CrossRef]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum ferritin: Past, present and future. Biochim. Biophys. Acta Gen. Subj. 2010, 1800, 760–769. [Google Scholar] [CrossRef]
- Baek, M.W.; Cho, H.S.; Kim, S.H.; Kim, W.J.; Jung, J.Y. Ascorbic Acid Induces Necrosis in Human Laryngeal Squamous Cell Carcinoma via ROS, PKC, and Calcium Signaling. J. Cell. Physiol. 2017, 232, 417–425. [Google Scholar] [CrossRef]
- Takemura, Y.; Satoh, M.; Satoh, K.; Hamada, H.; Sekido, Y.; Kubota, S. High dose of ascorbic acid induces cell death in mesothelioma cells. Biochem. Biophys. Res. Commun. 2010, 394, 249–253. [Google Scholar] [CrossRef]
- Andrews, G.C.; Crawford, T. Recent Advances in the Derivatization of L-Ascorbic Acid. In Ascorbic Acid: Chemistry, Metabolism, and Uses; Seib, P.A., Tolbert, B.M., Eds.; American Chemical Society: Washington, DC, USA, 1982; Volume 200, Chapter 3; pp. 59–79. [Google Scholar]
- Tsao, C.S. Vitamin C in health and disease. Antioxids. Health Dis. 1997, 5, 25–58. [Google Scholar]
- Macan, A.M.; Harej, A.; Cazin, I.; Klobučar, M.; Stepanić, V.; Pavelić, K.; Pavelić, S.K.; Schols, D.; Snoeck, R.; Andrei, G.; et al. Antitumor and antiviral activities of 4-substituted 1,2,3-triazolyl-2,3-dibenzyl-L-ascorbic acid derivatives. Eur. J. Med. Chem. 2019, 184, 111739. [Google Scholar] [CrossRef]
- Harej, A.; Meščić Macan, A.; Stepanić, V.; Klobučar, M.; Pavelić, K.; Pavelić, S.K.; Raić-Malić, S. The antioxidant and antiproliferative activities of 1,2,3-triazolyl-L-ascorbic acid derivatives. Int. J. Mol. Sci. 2019, 20, 4735. [Google Scholar] [CrossRef]
- Miura, K.; Haraguchi, M.; Ito, H.; Tai, A. Potential antitumor activity of 2-O-α-D-glucopyranosyl-6-O-(2-pentylheptanoyl)-L-ascorbic acid. Int. J. Mol. Sci. 2018, 19, 535. [Google Scholar] [CrossRef] [PubMed]
- Stipkovic Babic, M.; Makuc, D.; Plavec, J.; Martinovic, T.; Pavelic, S.K.; Pavelic, K.; Snoeck, R.; Andrei, G.; Schols, D.; Wittine, K.; et al. Novel halogenated 3-deazapurine, 7-deazapurine and alkylated 9-deazapurine derivatives of L-ascorbic or imino-L-ascorbic acid: Synthesis, antitumour and antiviral activity evaluations. Eur. J. Med. Chem. 2015, 102, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, B.; Chiron, J.; Fontés, M. Ascorbic acid derivatives as a new class of antiproliferative molecules. Cancer Lett. 2013, 338, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Wittine, K.; Stipković Babić, M.; Makuc, D.; Plavec, J.; Kraljević Pavelić, S.; Sedić, M.; Pavelić, K.; Leyssen, P.; Neyts, J.; Balzarini, J.; et al. Novel 1,2,4-triazole and imidazole derivatives of l-ascorbic and imino-ascorbic acid: Synthesis, anti-HCV and antitumor activity evaluations. Bioorganic Med. Chem. 2012, 20, 3675–3685. [Google Scholar] [CrossRef]
- Gazivoda, T.; Sÿokcevic, M.; Kralj, M.; Sÿuman, L.; Pavelic, K.; De Clercq, E.; Andrei, G.; Snoeck, R.; Balzarini, J.; Mintas, M.; et al. Synthesis and Antiviral and Cytostatic Evaluations of the New C-5 Substituted Pyrimidine and Furo[2,3-d]pyrimidine 4′,5′-Didehydro-L-ascorbic acid derivatives. J. Med. Chem. 2007, 50, 4105–4112. [Google Scholar] [CrossRef] [PubMed]
- Gazivoda, T.; Wittine, K.; Lovrić, I.; Makuc, D.; Plavec, J.; Cetina, M.; Mrvoš-Sermek, D.; Šuman, L.; Kralj, M.; Pavelić, K.; et al. Synthesis, structural studies, and cytostatic evaluation of 5,6-di-O-modified L-ascorbic acid derivatives. Carbohydr. Res. 2006, 341, 433–442. [Google Scholar] [CrossRef]
- Fong, J.F.Y.; Ng, Y.H.; Ng, S.M. Chapter 7—Carbon dots as a new class of light emitters for biomedical diagnostics and therapeutic applications. In Fullerens, Graphenes and Nanotubes: A Pharmaceutical Approach; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 227–295. ISBN 9780128136911. [Google Scholar]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Let. 2013, 8, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, A. Chapter 15—Drug Delivery Aspects of Herbal Medicines. In Japanese Kampo Medicines for the Treatment of Common Diseases—Focus on Inflammation; Arumugam, S., Watanabe, K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 143–164. ISBN 978-0-12-809398-6. [Google Scholar]
- Azanza, J.R.; Sádada, B.; Reis, J. Liposomal formulations of amphotericin B: Differences according to the scientific evidence. Rev. Esp. Quimioter. 2015, 28, 275–281. [Google Scholar]
- Filipczak, N.; Jaromin, A.; Piwoni, A.; Mahmud, M.; Sarisozen, C.; Torchilin, V.; Gubernator, J. Triple Co-Delivery Liposomal Carrier That Enhances Apoptosis via an Intrinsic Pathway in Melanoma Cells. Cancers 2019, 11, 1982. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, X.; Liu, Q.; Dai, Y.; Zhu, X.; Wen, Y.; Xu, J.; Lu, Y.; Zhao, D.; Chen, X.; et al. Palmitoyl ascorbate and doxorubicin co-encapsulated liposome for synergistic anticancer therapy. Eur. J. Pharm. Sci. 2017, 105, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, C.; Feng, F.; Fan, A.; Dai, Y.; Li, N.; Zhao, D.; Chen, X.; Lu, Y. Co-delivery of docetaxel and palmitoyl ascorbate by liposome for enhanced synergistic antitumor efficacy. Sci. Rep. 2016, 6, 38787. [Google Scholar] [CrossRef] [PubMed]
- Lipka, D.; Gubernator, J.; Filipczak, N.; Barnert, S.; Süss, R.; Legut, M.; Kozubek, A. Vitamin C-driven epirubicin loading into liposomes. Int. J. Nanomed. 2013, 8, 3573–3585. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterization, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Lemieux, P.; Vinogradov, S.; Alakhov, V. Pluronic® block copolymers: Novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 2002, 54, 223–233. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and He. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef]
- Zhou, M.; Li, X.; Li, Y.; Yao, Q.; Ming, Y.; Li, Z.; Lu, L.; Shi, S. Ascorbyl palmitate-incorporated paclitaxel-loaded composite nanoparticles for synergistic anti-tumoral therapy. Drug Deliv. 2017, 24, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Güney, G.; Kutlu, H.M.; Genç, L. Preparation and characterization of ascorbic acid loaded solid lipid nanoparticles and investigation of their apoptotic effects. Colloids Surf. B Biointerfaces 2014, 121, 270–280. [Google Scholar] [CrossRef]
- Sawant, R.R.; Vaze, O.S.; Rockwell, K.; Torchilin, V.P. Palmitoyl ascorbate-modified liposomes as nanoparticle platform for ascorbate-mediated cytotoxicity and paclitaxel co-delivery. Eur. J. Pharm. Biopharm. 2010, 75, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Frungillo, L.; Anazzetti, M.C.; Melo, P.S.; Durán, N. Antitumoral activity of L-ascorbic acid-poly-D,L-(lactide-co-glycolide) nanoparticles containing violacein. Int. J. Nanomed. 2010, 5, 77–85. [Google Scholar] [CrossRef]
- Frungillo, L.; Martins, D.; Teixeira, S.; Anazetti, M.C.; Melo, P.D.S.; Durán, N. Targeted antitumoral dehydrocrotonin nanoparticles with L-ascorbic acid 6-stearate. J. Pharm. Sci. 2010, 98, 4796–4807. [Google Scholar] [CrossRef]
- Medasani, M.; Kumar, P. Novel Anti-Viral and Anti-Cancer Molecule. International Patent Application WO 2018/092107A1, 24 May 2018. [Google Scholar]
- Hu, S.; Luan, L. 3-O-(para-methanesulfonate benzyl)-Ascorbic Acid with Anti-Cancer Activity and Preparation Method. Patent Application CN201710195165A, 18 August 2017. [Google Scholar]
- Tai, A.; Miura, K.; Wada, A. Antitumor Agent. Patent Application JP 2016/138087A, 4 August 2016. [Google Scholar]
- Lee, B.R. Anticancer Therapy—Aiding Composition Comprising Polyphenol, and Ascorbic Acid or the Derivatives. International Patent Application WO 2005/063235A1, 14 July 2005. [Google Scholar]
- Itoh, S.; Miwa, N.; Ogata, E.; Suzuki, M.; Tsuchiya, T.; Tsuzuki, T. Pharmaceutical Preparation of Ascorbic Acid Derivatives for Medical Treatment of Cancer. European Patent Application EP 0875246A1, 11 April 1998. [Google Scholar]
- Honore, P.M.; Spapen, H.D.; Marik, P.; Boer, W.; Oudemans-van Straaten, H. Dosing vitamin C in critically ill patients with special attention to renal replacement therapy: A narrative review. Ann. Intensive Care 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.V.; Turin, C.G.; McCormick, D.W.; Haas, C.; Constantine, G. Ascorbic acid-induced oxalate nephropathy: A case report and discussion of pathologic mechanisms. CEN Case Rep. 2019, 8, 67–70. [Google Scholar] [CrossRef] [PubMed]
- VITAMIN C. Available online: https://www.rxlist.com/vitamin_c/supplements.htm (accessed on 21 July 2021).
- Knight, J.; Madduma-Liyanage, K.; Mobley, J.A.; Assimos, D.G.; Holmes, R.P. Ascorbic acid intake and oxalate synthesis. Urolithiasis 2016, 44, 289–297. [Google Scholar] [CrossRef]
- Ascorbic Acid Market 2021 Global Industry Current Trends, Size, Share, Growth Factors, Application Development, Top Companies and Gross Margin Analysis Forecast to 2027. Available online: https://www.marketwatch.com/press-release/ascorbic-acid-market-2021-global-industry-current-trends-size-share-growth-factors-application-development-top-companies-and-gross-margin-analysis-forecast-to-2027-2021-05-05 (accessed on 21 July 2021).
- Share Survey 2021–2025 with Top Countries Data Industry Trends, Share, Size, Demand, Growth Opportunities, Industry Revenue, Future and Business Analysis by Forecast. Available online: https://www.marketwatch.com/press-release/vitamin-c-ascorbic-acid-market-share-survey-2021-2025-with-top-countries-data-industry-trends-share-size-demand-growth-opportunities-industry-revenue-future-and-business-analysis-by-forecast-2021-07-07 (accessed on 21 July 2021).
- Darwiche, W.; Gomila, C.; Ouled-Haddou, H.; Naudot, M.; Doualle, C.; Morel, P.; Nguyen-Khac, F.; Garçon, L.; Marolleau, J.P.; Ghamlouch, H. Ascorbic acid (vitamin C) synergistically enhances the therapeutic effect of targeted therapy in chronic lymphocytic leukemia. J. Exp. Clin. Cancer Res. 2020, 39, 1–17. [Google Scholar] [CrossRef]
- Carr, A.C.; Cook, J. Intravenous vitamin C for cancer therapy—Identifying the current gaps in our knowledge. Front. Physiol. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Camarena, V.; Wang, G. The epigenetic role of vitamin C in health and disease. Cell. Mol. Life Sci. 2016, 73, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, N.; Creagan, E.; Witzig, T.; Levine, M. Ascorbic Acid in Cancer Treatment: Let the Phoenix Fly. Cancer Cell 2018, 34, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; He, M.M.; Wang, Z.X.; Li, S.; Jin, Y.; Ren, C.; Shi, S.M.; Bi, B.T.; Chen, S.Z.; Lv, Z.-D.; et al. Phase I study of high-dose ascorbic acid with mFOLFOX6 or FOLFIRI in patients with metastatic colorectal cancer or gastric cancer. BMC Cancer 2019, 19, 460. [Google Scholar] [CrossRef]
- Lv, H.; Wang, C.; Fang, T.; Li, T.; Lv, G.; Han, Q.; Yang, W.; Wang, H. Vitamin C preferentially kills cancer stem cells in hepatocellular carcinoma via SVCT-2. NPJ Precis. Oncol. 2018, 2, 1–13. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, H.; Huang, J.; Zhu, Y.; Hong, M.; Zhu, H.; Zhang, J.; Li, S.; Yang, L.; Lian, Y.; et al. The synergy of Vitamin C with decitabine activates TET2 in leukemic cells and significantly improves overall survival in elderly patients with acute myeloid leukemia. Leuk. Res. 2018, 66, 1–7. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Sibenaller, Z.A.; Mapuskar, K.A.; Wagner, B.A.; Cramer-morales, K.L.; Furqan, M.; Sandhu, S.; Carlisle, T.L.; Smith, M.C.; Hejleh, T.A.; et al. O2•− and H2O2-Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer Cell 2018, 31, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Mustafi, S.; Camarena, V.; Volmar, C.H.; Huff, T.C.; Sant, D.W.; Brothers, S.P.; Liu, Z.J.; Wahlestedt, C.; Wang, G. Vitamin C sensitizes melanoma to BET inhibitors. Cancer Res. 2018, 78, 572–583. [Google Scholar] [CrossRef]
- Du, J.; Cieslak, J.A., 3rd; Welsh, J.L.; Sibenaller, Z.A.; Allen, B.G.; Wagner, B.A.; Kalen, A.L.; Doskey, C.M.; Strother, R.K.; Button, A.M.; et al. Pharmacological ascorbate radiosensitizes pancreatic cancer. Cancer Res. 2015, 75, 3314–3326. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. Cancer: High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014, 6, 222ra18. [Google Scholar] [CrossRef]

| S. No | Invention Disclosed | Patent Application Number | References |
|---|---|---|---|
| 1 | A patent was filed to present an invention comprising nucleobase or its derivatives or its analogues and an ascorbic acid molecule or its derivatives or its analogues, attached together with or without oxygen bond in-between to form a single molecule. Once administered, it is expected to exert antiviral and anticancer effect | PCT/IB20 17/057270 | [117] |
| 2 | A patent was filed for the preparation method of novel 3-O-(p-mesylate benzyl)-ascorbic acid as an anticancer agent | CN201710195165A | [118] |
| 3 | An acylation derivative of L-ascorbic acid, wherein hydroxyl functionality bound to second place carbon of L-ascorbic acid is substituted by a substituent capable of being degraded in vivo and being converted into a hydroxyl group or is unsubstituted, and a hydroxyl group bound to the position 6 of L-ascorbic acid is acylated by an acyl group with a branch | JP2015152439A | [119] |
| 4 | The patent was filed for a composition of polyphenol and ascorbic acid or its derivatives for aiding in anticancer therapy. Polyphenol with an amount of 50.0–99.9 parts by weight and ascorbic acid or its derivatives in an amount of 0.1–50.0 parts by weight, extraordinarily improved anticancer effects of conventional anticancer agents in contrast to the individual use alone | PCT/KR2004/003478 | [120] |
| 5 | A patent was filed to present the invention of a pharmaceutical preparation comprising L-ascorbic acid derivative with anti-malignant tumour agent, which can be used to treat cancer | EP98106276A | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reang, J.; Sharma, P.C.; Thakur, V.K.; Majeed, J. Understanding the Therapeutic Potential of Ascorbic Acid in the Battle to Overcome Cancer. Biomolecules 2021, 11, 1130. https://doi.org/10.3390/biom11081130
Reang J, Sharma PC, Thakur VK, Majeed J. Understanding the Therapeutic Potential of Ascorbic Acid in the Battle to Overcome Cancer. Biomolecules. 2021; 11(8):1130. https://doi.org/10.3390/biom11081130
Chicago/Turabian StyleReang, Jurnal, Prabodh Chander Sharma, Vijay Kumar Thakur, and Jaseela Majeed. 2021. "Understanding the Therapeutic Potential of Ascorbic Acid in the Battle to Overcome Cancer" Biomolecules 11, no. 8: 1130. https://doi.org/10.3390/biom11081130
APA StyleReang, J., Sharma, P. C., Thakur, V. K., & Majeed, J. (2021). Understanding the Therapeutic Potential of Ascorbic Acid in the Battle to Overcome Cancer. Biomolecules, 11(8), 1130. https://doi.org/10.3390/biom11081130