Clinically Applicable Cyclotron-Produced Gallium-68 Gives High-Yield Radiolabeling of DOTA-Based Tracers
Abstract
1. Introduction
2. Materials and Methods
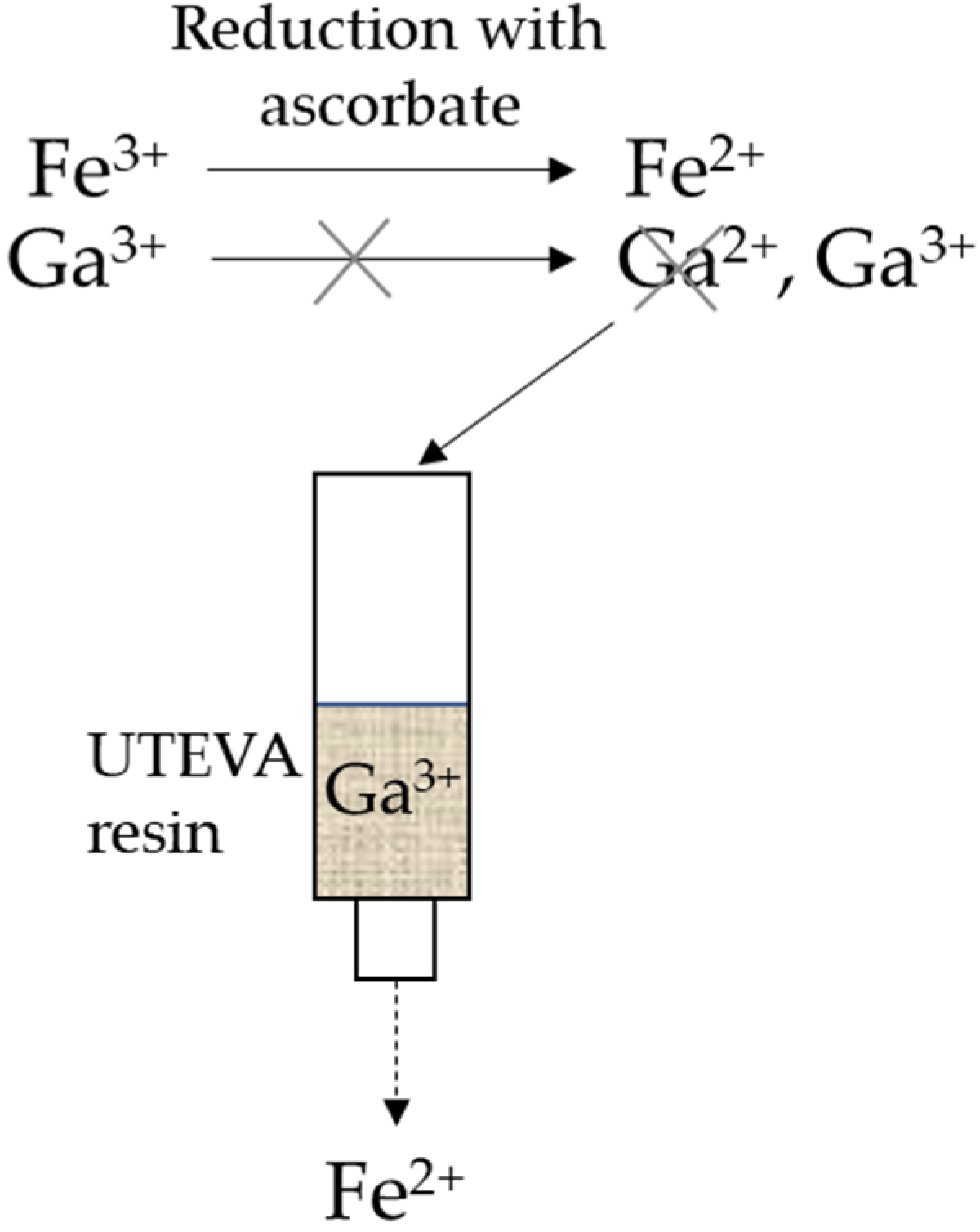
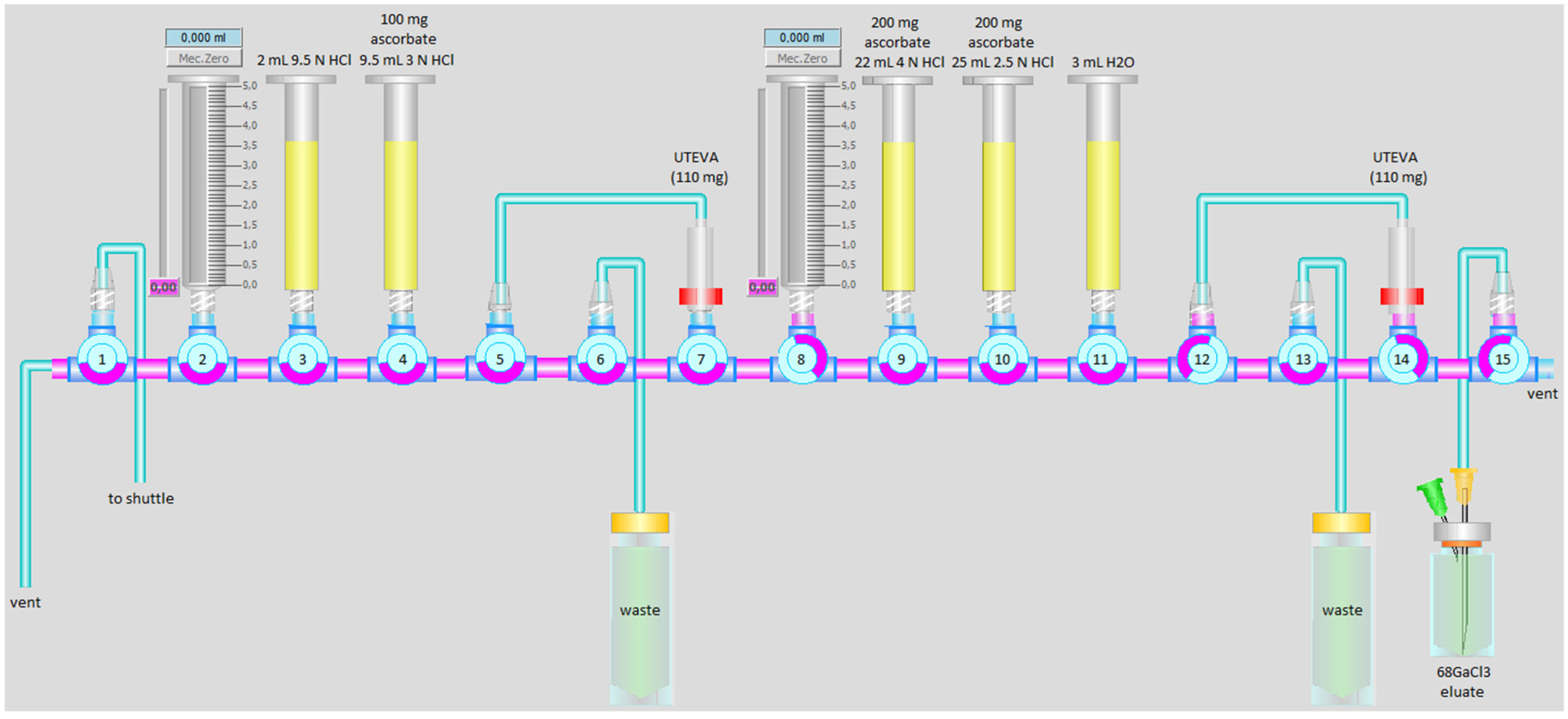
2.1. 68Ga Production and Purification Modification with Added Ascorbate
2.2. Colorimetric Test of Iron Content and ICP-MS Measurements
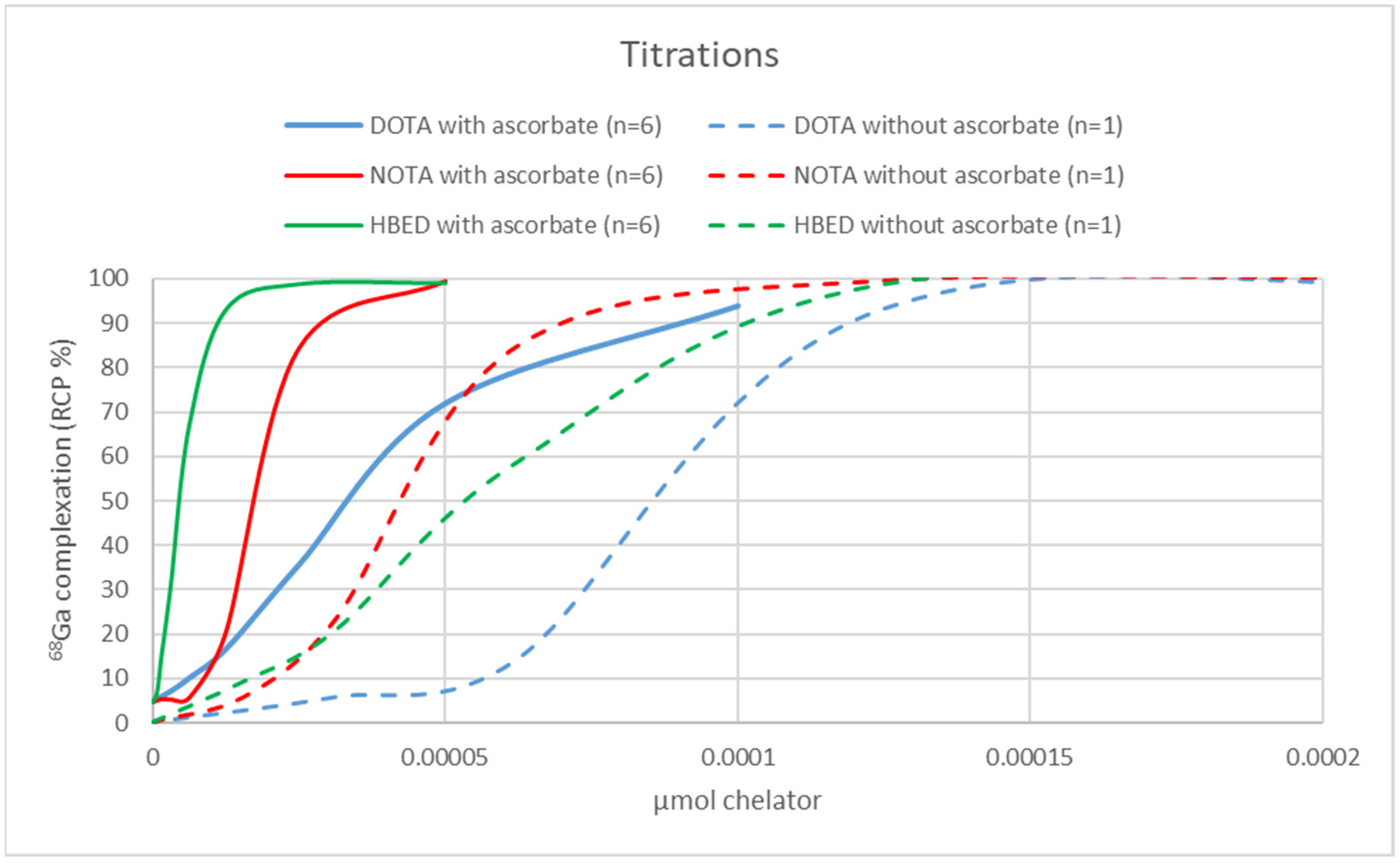
2.3. Chelator Titrations and AMA Determination
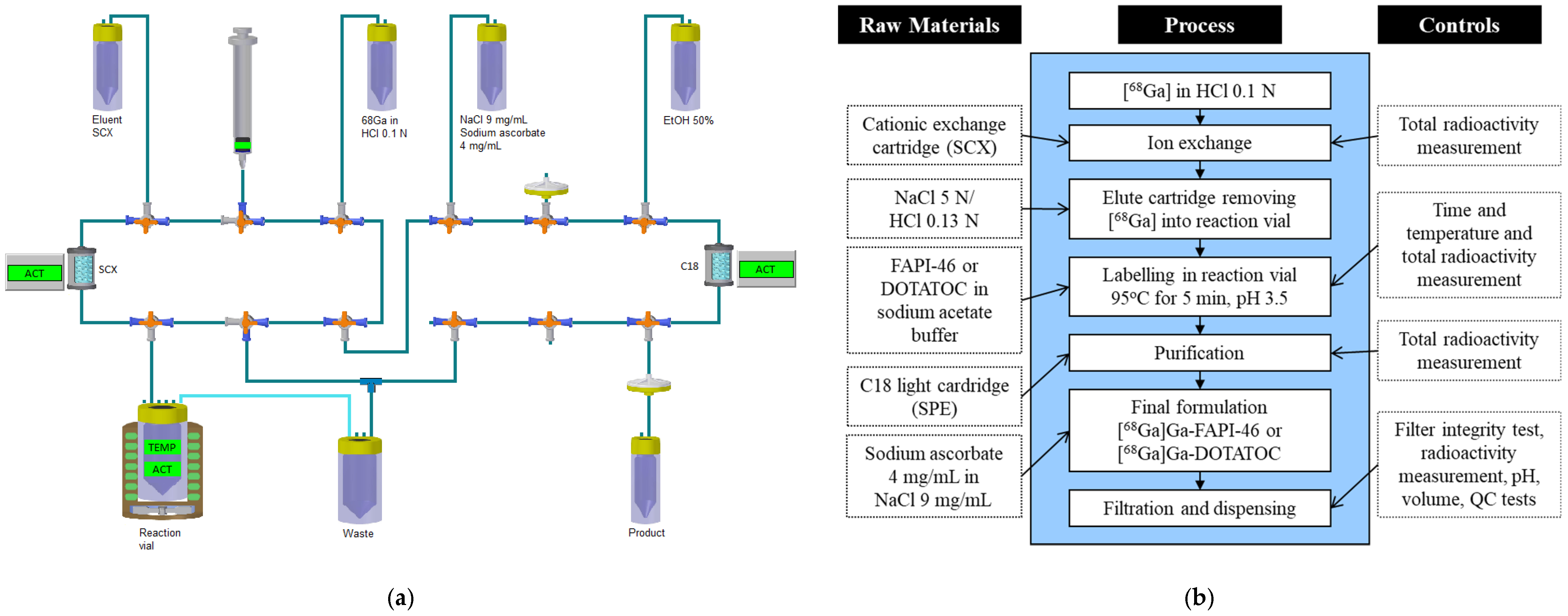
2.4. Synthesis of [68Ga]Ga-FAPI-46 and [68Ga]Ga-DOTATOC
2.5. Quality Control of [68Ga]Ga-FAPI-46 and [68Ga]Ga-DOTATOC
3. Results
3.1. 68Ga Production and Radionuclidic Purity
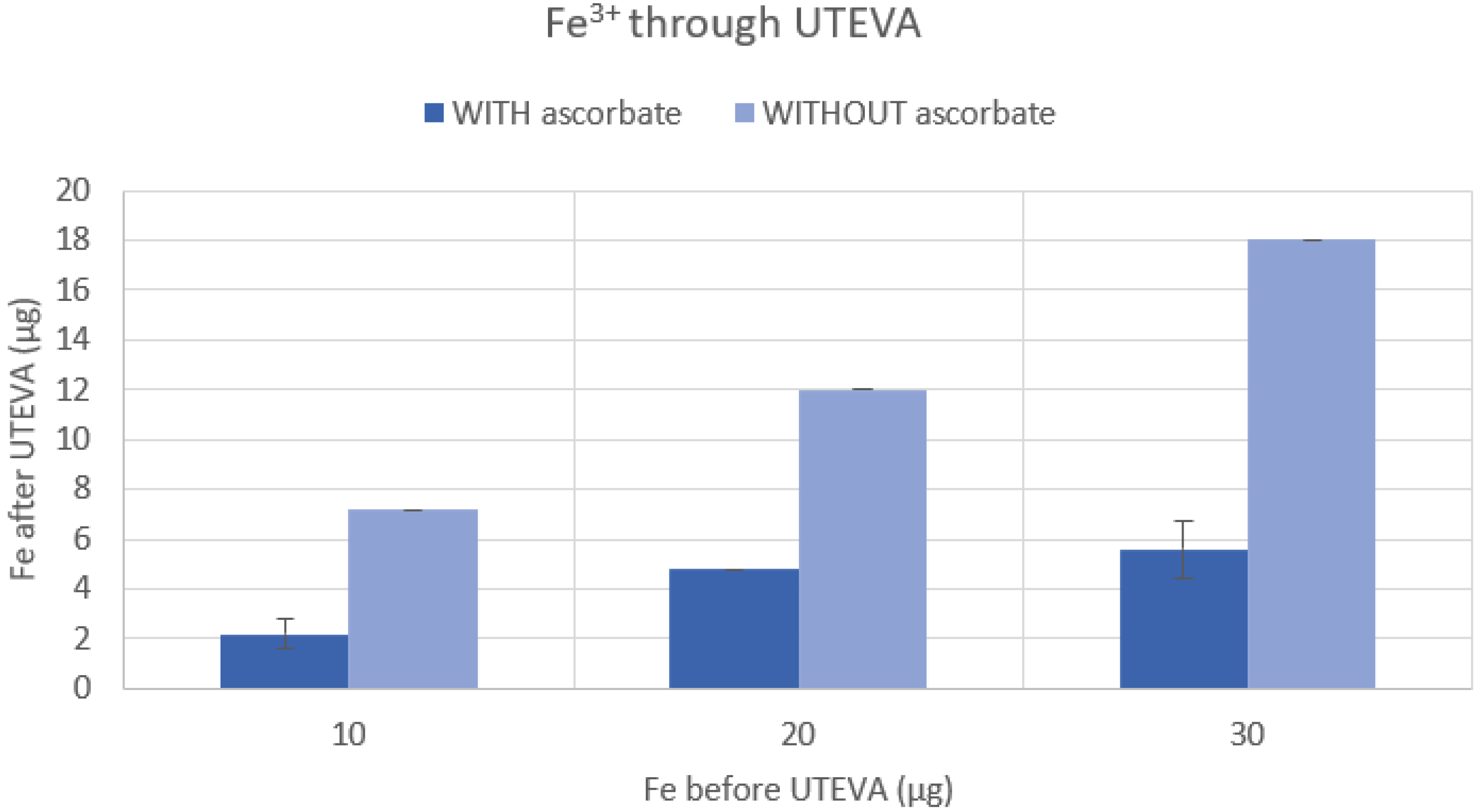
3.2. Verification of Iron Content after Addition of Ascorbate
3.3. Titrations with DOTA, NOTA, and HBED
3.4. Synthesis and Quality Control of [68Ga]Ga-FAPI-46 and [68Ga]Ga-DOTATOC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martiniova, L.; Palatis, L.; Etchebehere, E.; Ravizzini, G. Gallium-68 in Medical Imaging. Curr. Radiopharm. 2016, 9, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Roesch, F.; Riss, P.J. The renaissance of the (6)(8)Ge/(6)(8)Ga radionuclide generator initiates new developments in (6)(8)Ga radiopharmaceutical chemistry. Curr. Top. Med. Chem. 2010, 10, 1633–1668. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. 68Ga-Based radiopharmaceuticals: Production and application relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K. The Current Status of the Production and Supply of Gallium-68. Cancer Biother Radiopharm. 2020, 35, 163–166. [Google Scholar] [CrossRef] [PubMed]
- AuntMinnie.com. FDA Clears Cyclotron Ga-68 DOTATOC Production Process. Available online: https://www.auntminnie.com/index.aspx?sec=ser&sub=def&pag=dis&ItemID=130589 (accessed on 21 June 2021).
- Pandey, M.K.; Byrne, J.F.; Jiang, H.; Packard, A.B.; DeGrado, T.R. Cyclotron production of (68)Ga via the (68)Zn(p,n)(68)Ga reaction in aqueous solution. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 303–310. [Google Scholar]
- Rodnick, M.E.; Sollert, C.; Stark, D.; Clark, M.; Katsifis, A.; Hockley, B.G.; Parr, D.C.; Frigell, J.; Henderson, B.D.; Abghari-Gerst, M.; et al. Cyclotron-based production of (68)Ga, [(68)Ga]GaCl3, and [(68)Ga]Ga-PSMA-11 from a liquid target. EJNMMI Radiopharm. Chem. 2020, 5, 25. [Google Scholar] [CrossRef]
- Lin, M.; Waligorski, G.J.; Lepera, C.G. Production of curie quantities of (68)Ga with a medical cyclotron via the (68)Zn(p,n)(68)Ga reaction. Appl. Radiat. Isot. 2018, 133, 1–3. [Google Scholar] [CrossRef]
- Tieu, W.; Hollis, C.A.; Kuan, K.K.W.; Takhar, P.; Stuckings, M.; Spooner, N.; Malinconico, M. Rapid and automated production of [(68)Ga]gallium chloride and [(68)Ga]Ga-DOTA-TATE on a medical cyclotron. Nucl. Med. Biol. 2019, 74–75, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.B.; Wilson, J.; Richter, S.; Duke, M.J.M.; Wuest, M.; Wuest, F. Taking cyclotron (68)Ga production to the next level: Expeditious solid target production of (68)Ga for preparation of radiotracers. Nucl. Med. Biol. 2020, 80–81, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Thisgaard, H.; Kumlin, J.; Langkjaer, N.; Chua, J.; Hook, B.; Jensen, M.; Kassaian, A.; Zeisler, S.; Borjian, S.; Cross, M.; et al. Multi-curie production of gallium-68 on a biomedical cyclotron and automated radiolabelling of PSMA-11 and DOTATATE. EJNMMI Radiopharm. Chem. 2021, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.V.; Chernysheva, M.; Barnhart, T.E.; Gagnon, K.; Engle, J.W. A review of accelerator-produced Ga-68 with solid targets. Curr. Radiopharm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Siikanen, J.; Jussing, E.; Milton, S.; Steiger, C.; Ulin, J.; Jonsson, C.; Samén, E.; Tran, T.A. Cyclotron-produced 68Ga from enriched 68Zn foils. Appl. Radiat. Isot. 2021, 109825. [Google Scholar] [CrossRef] [PubMed]
- Martell, A.E.; Motekaitis, R.J.; Chen, D.; Hancock, R.D.; McManus, D. Selection of new Fe(III)/Fe(II) chelating agents as catalysts for the oxidation of hydrogen sulfide to sulfur by air. Can. J. Chem. 1996, 74, 1872–1879. [Google Scholar] [CrossRef]
- Martell, A.E.; Motekaitis, R.J.; Clarke, E.T.; Delgado, R.; Sun, Y.; Ma, R. Stability constants of metal complexes of macrocyclic ligands with pendant donor groups. Supramol. Chem. 1996, 6, 353–363. [Google Scholar] [CrossRef]
- EDQM Council of Europe. European Pharmacopoeia. Gallium (68Ga) chloride (accelerator-produced) solution for radiolabelling Monograph PA/PH/Exp. 14/T (18) 13 ANP: 3109; EDQM Council of Europe: Strasbourg, France, 2020. [Google Scholar]
- Marinov, G.M.; Marinova, A.P.; Medvedev, D.V.; Dadakhanov, J.A.; Milanova, M.M.; Happel, S.; Radchenko, V.I.; Filosofov, D.V. Determination of distribution coefficients (Kd) of various radionuclides on UTEVA resin. Radiochim. Acta 2016, 104, 735–742. [Google Scholar] [CrossRef]
- McAlister, D.R.; Philip Horwitz, E. Automated two column generator systems for medical radionuclides. Appl. Radiat. Isot. 2009, 67, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Lee, K.-J.; Oh, Y.-J. Solvent Extraction Equilibria of FeCl3 from Hydrochloric Acid Solution with Alamine336. Mater. Trans. 2004, 45, 2364–2368. [Google Scholar] [CrossRef][Green Version]
- Ilbert, M.; Bonnefoy, V. Insight into the evolution of the iron oxidation pathways. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1827, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Lee, C.-W. Electrochemistry of Gallium. J. Electrochem. Sci. Technol. 2013, 4, 1–18. [Google Scholar] [CrossRef]
- Borsook, H.; Keighley, G. Oxidation-Reduction Potential of Ascorbic Acid (Vitamin C). Proc. Natl. Acad. Sci. USA 1933, 19, 875–878. [Google Scholar] [CrossRef]
- IAEA-TECDOC-1863. Gallium-68 Cyclotron Production; International Atomic Energy Agency: Vienna, Austria, 2019. [Google Scholar]
- Liu, S.; Ellars, C.E.; Edwards, D.S. Ascorbic acid: Useful as a buffer agent and radiolytic stabilizer for metalloradiopharmaceuticals. Bioconjug. Chem. 2003, 14, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, T.; Nakao, R.; Yamaguchi, M.; Suzuki, K. Stability of 11C-labeled PET radiopharmaceuticals. Appl. Radiat. Isot. 2004, 61, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- EDQM Council of Europe. European Pharmacopoeia. Gallium (68Ga) Edotreotide Injection PA/PH/Exp. 14/T (07) 12 COM ANP: 2482; EDQM Council of Europe: Strasbourg, France, 2011. [Google Scholar]
- Lin, M.; Paolillo, V.; Ta, R.T.; Damasco, J.; Rojo, R.D.; Carl, J.C.; Melancon, M.P.; Ravizzini, G.C.; Le, D.B.; Santos, E.B. Fully automated preparation of (68)Ga-PSMA-11atcurie level quantity using cyclotron-produced (68)Ga for clinical applications. Appl. Radiat. Isot. 2020, 155, 108936. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Crespo, A.; Jussing, E.; Bjorklund, A.C.; Pokrovskaja Tamm, K. Hallmarks in prostate cancer imaging with Ga68-PSMA-11-PET/CT with reference to detection limits and quantitative properties. EJNMMI Res. 2018, 8, 27. [Google Scholar] [CrossRef]
- Von Hacht, J.L.; Erdmann, S.; Niederstadt, L.; Prasad, S.; Wagener, A.; Exner, S.; Beindorff, N.; Brenner, W.; Grotzinger, C. Increasing molar activity by HPLC purification improves 68Ga-DOTA-NAPamide tumor accumulation in a B16/F1 melanoma xenograft model. PLoS ONE 2019, 14, e0217883. [Google Scholar] [CrossRef]
| 68GaCl3 from Generator * | 68GaCl3 from Cyclotron | ||
|---|---|---|---|
| No Ascorbate (n = 3) | No Ascorbate (n = 1) | with Ascorbate (n = 6) | |
| Chelator | AMA (GBq/µmol) | AMA (GBq/µmol) | AMA (GBq/µmol) |
| DOTA | 10 ± 3 | 209 | 491 ± 204 |
| NOTA | Not analyzed | 314 | 993 ± 405 |
| HBED | Not analyzed | 280 | 4480 ± 3060 |
| Parameter | Product Specification | Generator-Produced (n = 4 ± SD) | Cyclotron-Produced (n = 3 ± SD) |
|---|---|---|---|
| Start activity (GBq) | Not specified | 0.99 ± 0.16 | 9.8 ± 0.26 |
| Precursor mass (µg) | Not specified | 50 | 50 |
| Product activity/batch (GBq) | Not specified | 0.58 ± 0.09 | 5.58 ± 0.35 |
| Activity concentration (MBq/mL) | Not specified | 60.5 ± 10.5 | 602 ± 45 |
| Non-decay-corrected RCY (%) | Not specified | 58.2 ± 3.2 | 57.0 ± 2.5 |
| AMA (GBq/µmol) | Not specified | 10.0 ± 1.7 | 98.8 ± 6.2 |
| Appearance | Clear or slightly yellow. Free of particles | Conforms | Conforms |
| pH | 4.0–8.0 | 5.3 ± 0 | 5.3 ± 0.3 |
| Product identity [68Ga]Ga-FAPI-46 | |RtRD –RtUV| < 60 s | 40 ± 9.8 | 31 ± 9.5 |
| Total chemical purity (µg/mL) | ≤10 µg/mL | ≤10 | ≤10 |
| Radiochemical impurity, B (%) | ≤3% | 0.2 ± 0.4 | 0.26 ± 0.05 |
| Total radiochemical purity (%) RCPTot = (100 − B) × T | ≥91% | 98.3 ± 0.01 | 9 7.4 ± 0.81 |
| Filter integrity (bar) | ≥3.5 bar | 4.2 ± 0.0 * | 4.1 ± 0.06 |
| Bacterial endotoxins (EU/mL) | <17.5 EU/mL | <5.0 | <5.0 |
| Ethanol (%) | <10% | 6.4 ± 0.45 | 6.8 ± 0.26 |
| Sterility | Sterile, 0 CFU | Sterile | Sterile ** |
| Radiochemical stability **** | RCPTot ≥ 91% | 95 ± 0.02 | 96 ± 1.5 *** |
| Parameter | Product Specification | Generator-Produced (n = 86 * ± SD) | Cyclotron-Produced (n = 3 ± SD) |
|---|---|---|---|
| Start activity (GBq) | Not specified | 1.0 ± 0.2 | 9.3 ± 1.4 |
| Precursor mass (µg) | Not specified | 40 | 40 |
| Product activity (GBq) | Not specified | 0.6 ± 0.2 | 6.1 ± 1.3 |
| Activity concentration (MBq/mL) | Not specified | 70.7 ± 0.2 | 650 ± 124 |
| Non-corrected RCY (%) | Not specified | 60.9 ± 7.8 | 64.4 ± 4.7 |
| AMA (GBq/µmol) | Not specified | 21.7 ± 5.6 | 215.1 ± 44.8 |
| Appearance | Clear or slightly yellow. Free of particles | Conforms | Conforms |
| pH | 4.0–8.0 | 5.8 ± 0.4 | 5.5 ± 0.3 |
| Product identity | |RtRD–RtUV| < 120 s | 83 ± 7 | 42 ± 6 |
| [68Ga] gallium ion on HPLC | ≤2% | Not detected | Not detected |
| Edotreotide plus [68Ga] 68Ga-DOTATOC | ≤5 µg/mL | ≤5 | ≤5 |
| Radiochemical impurity, B (%) | ≤3% | 0.81 ± 0.61 | 0.18 ± 0.16 |
| Total radiochemical purity (%) RCPTot = (100 − B) × T | ≥91% | 98.6 ± 3.6 | 99.8 ± 0.2 |
| Filter integrity (bar) | ≥3.5 bar | 4.0 ± 0.1 ** | 4.1 ± 0.2 |
| Bacterial endotoxins (EU/mL) | <17.5 EU/mL | <5 ** | <5 |
| Ethanol (%) | <10% | 6.49 ± 0.32 ** | 6.4 ± 0.2 |
| Sterility | Sterile, 0 CFU | Sterile ** | Sterile |
| Radiochemical stability (%) *** | RCPTot ≥ 91% | 97.7 | 99.2 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jussing, E.; Milton, S.; Samén, E.; Moein, M.M.; Bylund, L.; Axelsson, R.; Siikanen, J.; Tran, T.A. Clinically Applicable Cyclotron-Produced Gallium-68 Gives High-Yield Radiolabeling of DOTA-Based Tracers. Biomolecules 2021, 11, 1118. https://doi.org/10.3390/biom11081118
Jussing E, Milton S, Samén E, Moein MM, Bylund L, Axelsson R, Siikanen J, Tran TA. Clinically Applicable Cyclotron-Produced Gallium-68 Gives High-Yield Radiolabeling of DOTA-Based Tracers. Biomolecules. 2021; 11(8):1118. https://doi.org/10.3390/biom11081118
Chicago/Turabian StyleJussing, Emma, Stefan Milton, Erik Samén, Mohammad Mahdi Moein, Lovisa Bylund, Rimma Axelsson, Jonathan Siikanen, and Thuy A. Tran. 2021. "Clinically Applicable Cyclotron-Produced Gallium-68 Gives High-Yield Radiolabeling of DOTA-Based Tracers" Biomolecules 11, no. 8: 1118. https://doi.org/10.3390/biom11081118
APA StyleJussing, E., Milton, S., Samén, E., Moein, M. M., Bylund, L., Axelsson, R., Siikanen, J., & Tran, T. A. (2021). Clinically Applicable Cyclotron-Produced Gallium-68 Gives High-Yield Radiolabeling of DOTA-Based Tracers. Biomolecules, 11(8), 1118. https://doi.org/10.3390/biom11081118





