Full-Length Recombinant hSP-D Binds and Inhibits SARS-CoV-2
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Determination of hSP-D Levels in Bronchoalveolar Lavage of COVID-19 Patients
2.3. SARS-CoV-2 Spike-Protein and rhSP-D ELISA Binding Assay
2.4. Protein-Bridge (Aggregation) Assay
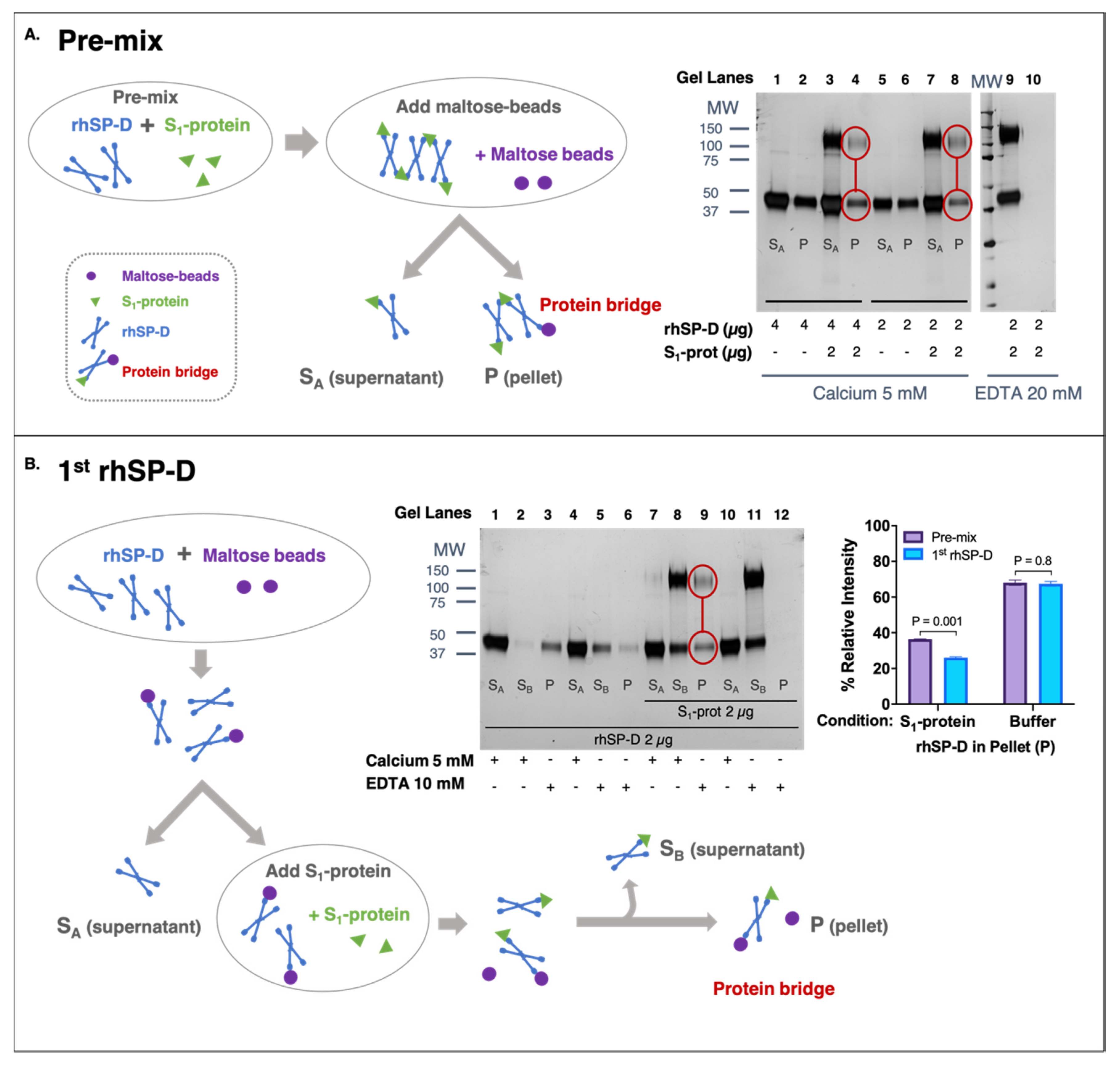
2.4.1. rhSP-D First Approach
2.4.2. Pre-Mix Approach
2.5. Competition of SARS-CoV-2 Spike-Protein Binding to ACE2 Protein by rhSP-D
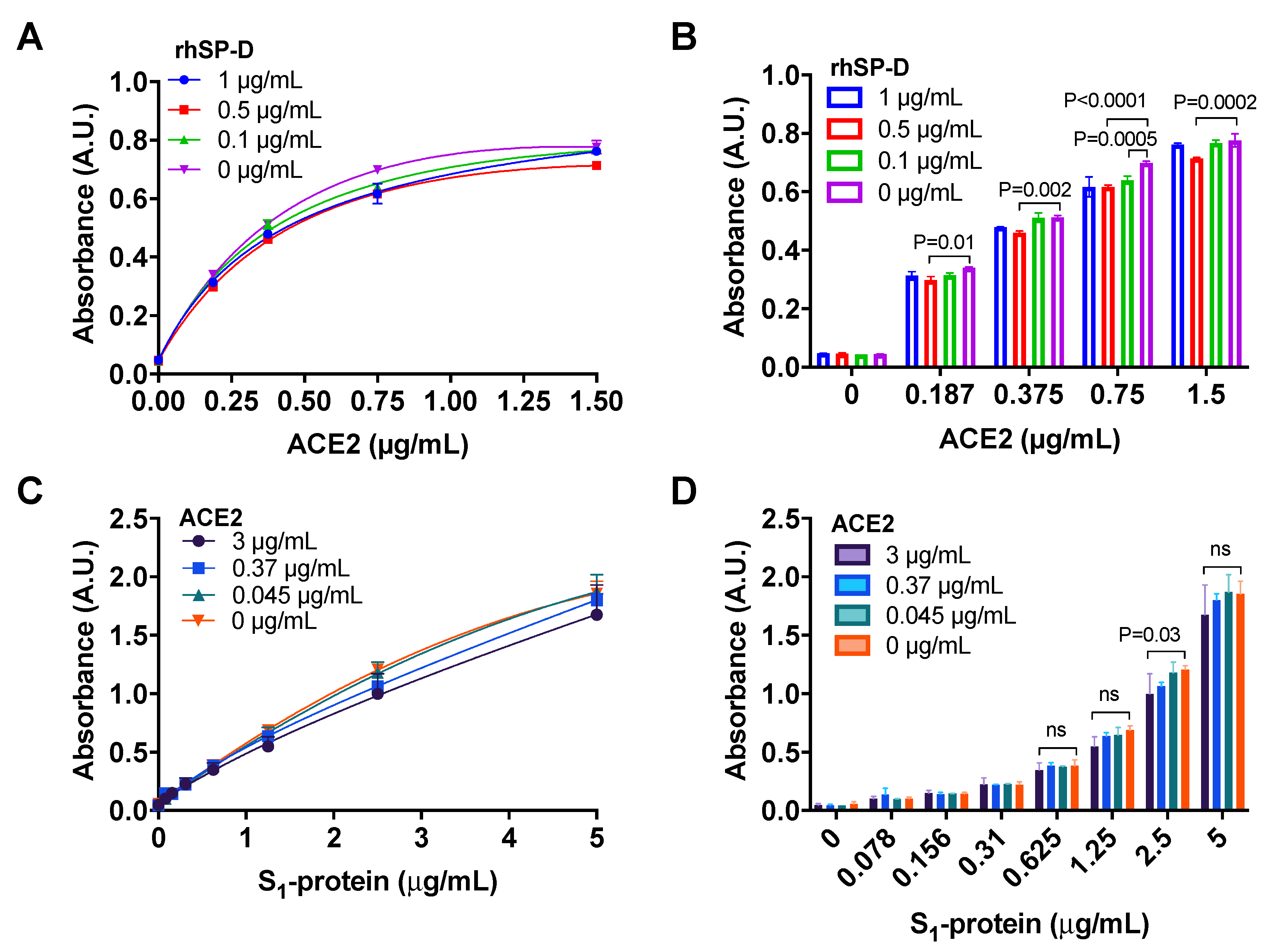
2.5.1. Binding of ACE2 to S1-Protein in the Presence of rhSP-D
2.5.2. Binding of S1-Protein to rhSP-D in the Presence of ACE2
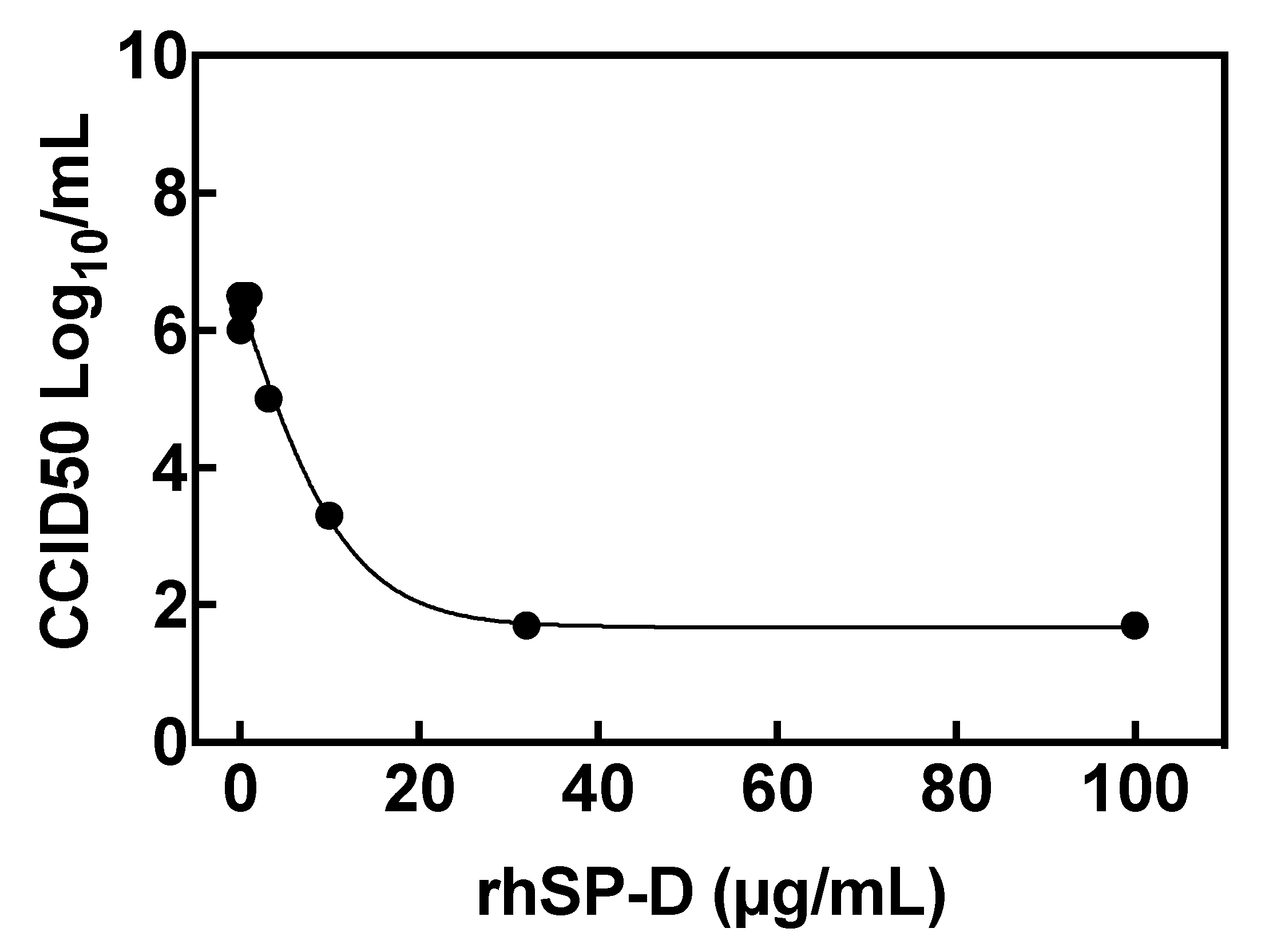
2.6. Inhibition of Viral Replication: Reduction of Virus Yield (VYR) Assay
2.7. Statistics Analysis
3. Results
3.1. COVID-19 Patients Show Low Concentrations of Pulmonary SP-D
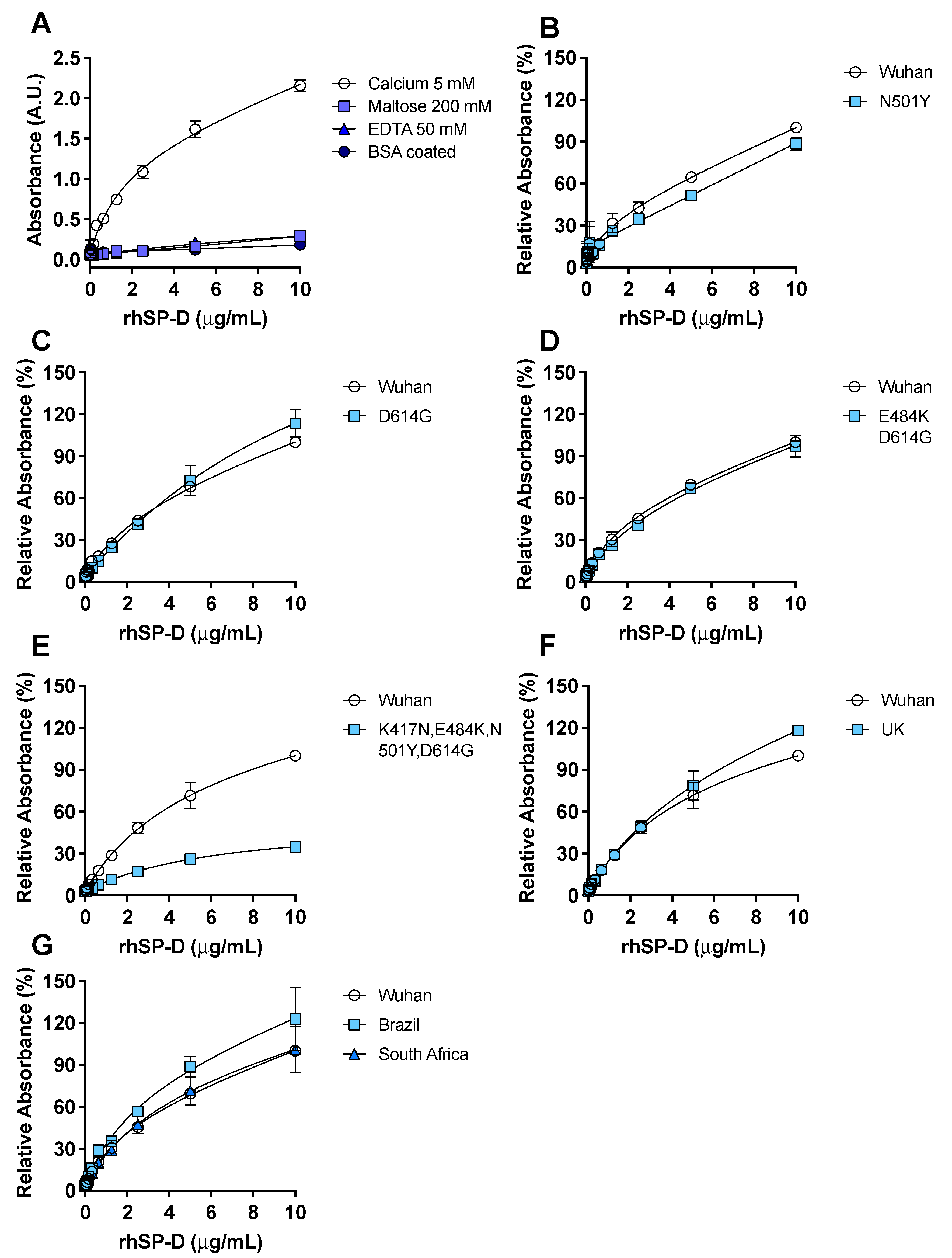
3.2. Recombinant hSP-D Binds to the S-Protein of SARS-CoV-2
3.3. rhSP-D Forms Protein Bridges with the S-Protein of SARS-CoV-2
3.4. ACE2 Receptor Does Not Interfere in the Interaction between S-Protein and rhSP-D
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA 2020, 323, 1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Preliminary Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2020, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Emergence of a SARS-CoV-2 variant of concern with mutations in spike glycoprotein. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.L.N.; Pybus, O.; Barclay, W.; Barrett, J.; Carabelli, A.; Connor, T.; Peacock, T.; Robertson, D.L.; Volz, E. Preliminary Genomic Characterisation of an Emergent SARS-CoV-2 Lineage in the UK Defined by a Novel Set of Spike Mutations. 2020. Available online: https://virological.org/t/preliminary-genomiccharacterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-anovel-set-of-spike-mutations/563 (accessed on 12 May 2021).
- Voloch, C.M.; da Silva Francisco, R., Jr.; de Almeida, L.G.P.; Cardoso, C.C.; Brustolini, O.J.; Gerber, A.L.; Guimaraes, A.P.C.; Mariani, D.; da Costa, R.M.; Ferreira, O.C., Jr.; et al. Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. J. Virol. 2021, 95. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Plante, K.S.; Plante, J.A.; Xie, X.; Zhang, X.; Ku, Z.; An, Z.; Scharton, D.; Schindewolf, C.; et al. The N501Y spike substitution enhances SARS-CoV-2 transmission. bioRxiv 2021. [Google Scholar] [CrossRef]
- Rees-Spear, C.; Muir, L.; Griffith, S.A.; Heaney, J.; Aldon, Y.; Snitselaar, J.L.; Thomas, P.; Graham, C.; Seow, J.; Lee, N.; et al. The effect of spike mutations on SARS-CoV-2 neutralization. Cell Rep. 2021, 34, 108890. [Google Scholar] [CrossRef]
- Wright, J.R. Immunoregulatory functions of surfactant proteins. Nat. Rev. Immunol. 2005, 5, 58–68. [Google Scholar] [CrossRef]
- Kingma, P.S.; Whitsett, J.A. In defense of the lung: Surfactant protein A and surfactant protein D. Curr. Opin. Pharm. 2006, 6, 277–283. [Google Scholar] [CrossRef]
- Arroyo, R.; Echaide, M.; Moreno-Herrero, F.; Perez-Gil, J.; Kingma, P.S. Functional characterization of the different oligomeric forms of human surfactant protein SP-D. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140436. [Google Scholar] [CrossRef] [PubMed]
- LeVine, A.M.; Elliott, J.; Whitsett, J.A.; Srikiatkhachorn, A.; Crouch, E.; DeSilva, N.; Korfhagen, T. Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 2004, 31, 193–199. [Google Scholar] [CrossRef]
- Ikegami, M.; Carter, K.; Bishop, K.; Yadav, A.; Masterjohn, E.; Brondyk, W.; Scheule, R.K.; Whitsett, J.A. Intratracheal recombinant surfactant protein D prevents endotoxin shock in the newborn preterm lamb. Am. J. Respir. Crit. Care Med. 2006, 173, 1342–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikegami, M.; Scoville, E.A.; Grant, S.; Korfhagen, T.; Brondyk, W.; Scheule, R.K.; Whitsett, J.A. Surfactant protein-D and surfactant inhibit endotoxin-induced pulmonary inflammation. Chest 2007, 132, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Whitsett, J.A.; Scheule, R.K.; Ikegami, M. Surfactant protein-D inhibits lung inflammation caused by ventilation in premature newborn lambs. Am. J. Respir. Crit. Care Med. 2010, 181, 1098–1105. [Google Scholar] [CrossRef] [Green Version]
- King, B.A.; Kingma, P.S. Surfactant protein D deficiency increases lung injury during endotoxemia. Am. J. Respir. Cell Mol. Biol. 2011, 44, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, R.; Khan, M.A.; Echaide, M.; Perez-Gil, J.; Palaniyar, N. SP-D attenuates LPS-induced formation of human neutrophil extracellular traps (NETs), protecting pulmonary surfactant inactivation by NETs. Commun. Biol. 2019, 2, 470. [Google Scholar] [CrossRef]
- Greene, K.E.; Wright, J.R.; Steinberg, K.P.; Ruzinski, J.T.; Caldwell, E.; Wong, W.B.; Hull, W.; Whitsett, J.A.; Akino, T.; Kuroki, Y.; et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am. J. Respir. Crit. Care Med. 1999, 160, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Hartshorn, K.; Chang, D.; Rust, K.; White, M.; Heuser, J.; Crouch, E. Interactions of recombinant human pulmonary surfactant protein D and SP-D multimers with influenza A. Am. J. Physiol. 1996, 271, L753–L762. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, R.; Martin-Gonzalez, A.; Echaide, M.; Jain, A.; Brondyk, W.H.; Rosenbaum, J.; Moreno-Herrero, F.; Perez-Gil, J. Supramolecular Assembly of Human Pulmonary Surfactant Protein SP-D. J. Mol. Biol. 2018, 430, 1495–1509. [Google Scholar] [CrossRef]
- Arroyo, R.; Echaide, M.; Wilmanowski, R.M.; Martin-Gonzalez, A.; Batllori, E.; Galindo, A.; Rosenbaum, J.S.; Moreno-Herrero, F.; Kingma, P.S.; Perez-Gil, J. Structure and activity of human surfactant protein sp-d from different natural sources. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L148–L158. [Google Scholar] [CrossRef]
- Pandolfi, L.; Fossali, T.; Frangipane, V.; Bozzini, S.; Morosini, M.; D’Amato, M.; Lettieri, S.; Urtis, M.; Di Toro, A.; Saracino, L.; et al. Broncho-alveolar inflammation in COVID-19 patients: A correlation with clinical outcome. BMC Pulm. Med. 2020, 20, 301. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Sorensen, G.L.; Husby, S.; Holmskov, U. Surfactant protein A and surfactant protein D variation in pulmonary disease. Immunobiology 2007, 212, 381–416. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; McLellan, J.S.; Crispin, M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020, 369, 330–333. [Google Scholar] [CrossRef]
- Honda, Y.; Kuroki, Y.; Matsuura, E.; Nagae, H.; Takahashi, H.; Akino, T.; Abe, S. Pulmonary surfactant protein D in sera and bronchoalveolar lavage fluids. Am. J. Respir. Crit. Care Med. 1995, 152, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Bernard, A. Lung epithelium-specific proteins: Characteristics and potential applications as markers. Am. J. Respir. Crit. Care Med. 1999, 159, 646–678. [Google Scholar] [CrossRef]
- Winkler, C.; Atochina-Vasserman, E.N.; Holz, O.; Beers, M.F.; Erpenbeck, V.J.; Krug, N.; Roepcke, S.; Lauer, G.; Elmlinger, M.; Hohlfeld, J.M. Comprehensive characterisation of pulmonary and serum surfactant protein D in COPD. Respir. Res. 2011, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Hartshorn, K.L.; Crouch, E.; White, M.R.; Colamussi, M.L.; Kakkanatt, A.; Tauber, B.; Shepherd, V.; Sastry, K.N. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. Am. J. Physiol. 1998, 274, L958–L969. [Google Scholar] [CrossRef]
- LeVine, A.M.; Whitsett, J.A.; Hartshorn, K.L.; Crouch, E.C.; Korfhagen, T.R. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J. Immunol. 2001, 167, 5868–5873. [Google Scholar] [CrossRef]
- Leth-Larsen, R.; Zhong, F.; Chow, V.T.; Holmskov, U.; Lu, J. The SARS coronavirus spike glycoprotein is selectively recognized by lung surfactant protein D and activates macrophages. Immunobiology 2007, 212, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Madan, T.; Biswas, B.; Varghese, P.M.; Subedi, R.; Pandit, H.; Idicula-Thomas, S.; Kundu, I.; Rooge, S.; Agarwal, R.; Tripathi, D.M.; et al. A Recombinant Fragment of Human Surfactant Protein D Binds Spike Protein and Inhibits Infectivity and Replication of SARS-CoV-2 in Clinical Samples. Am. J. Respir. Cell Mol. Biol. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Dejnirattisai, W.; Supasa, P.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Tuekprakhon, A.; Nutalai, R.; et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 2021, 184, 2348–2361.e6. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, R.; Grant, S.N.; Gouwens, K.R.; Miller, D.M.; Kingma, P.S. Evaluation of recombinant human SP-D in the rat premature lung model. Ann. Anat. 2021, 235, 151670. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Beirag, N.; Murugaiah, V.; Chou, Y.C.; Kuo, W.S.; Kao, H.F.; Madan, T.; Kishore, U.; Wang, J.Y. Human Surfactant Protein D Binds Spike Protein and Acts as an Entry Inhibitor of SARS-CoV-2 Pseudotyped Viral Particles. Front. Immunol. 2021, 12, 641360. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardai, S.J.; Xiao, Y.Q.; Dickinson, M.; Nick, J.A.; Voelker, D.R.; Greene, K.E.; Henson, P.M. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 2003, 115, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Ohya, M.; Nishitani, C.; Sano, H.; Yamada, C.; Mitsuzawa, H.; Shimizu, T.; Saito, T.; Smith, K.; Crouch, E.; Kuroki, Y. Human pulmonary surfactant protein D binds the extracellular domains of Toll-like receptors 2 and 4 through the carbohydrate recognition domain by a mechanism different from its binding to phosphatidylinositol and lipopolysaccharide. Biochemistry 2006, 45, 8657–8664. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, M.; Nishitani, C.; Takahashi, M.; Katoh, T.; Ariki, S.; Shimizu, T.; Mitsuzawa, H.; Sawada, K.; Voelker, D.R.; Takahashi, H.; et al. Pulmonary surfactant protein D inhibits lipopolysaccharide (LPS)-induced inflammatory cell responses by altering LPS binding to its receptors. J. Biol. Chem. 2008, 283, 35878–35888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingma, P.S.; Zhang, L.; Ikegami, M.; Hartshorn, K.; McCormack, F.X.; Whitsett, J.A. Correction of pulmonary abnormalities in Sftpd−/− mice requires the collagenous domain of surfactant protein D. J. Biol. Chem. 2006, 281, 24496–24505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Age | Sex | BMI | BALF SP-D | Collection of BAL Sample at | Comorbidities and/or Smoking | |
|---|---|---|---|---|---|---|
| Hospitalization Day | Intubation Day | |||||
| 28 | F | 26.6 | 55 | 9 | 3 | N |
| 40 | M | 44.2 | 83 | 11 | 10 | N |
| 46 | F | 29.4 | 7 | 14 | 14 | N |
| 50 | M | 34.6 | 107 | 3 | 3 | Y (HIV) |
| 53 | M | 26.2 | 32 | 11 | 9 | Y (smoker) |
| 55 | M | 24.8 | 479 | 24 | 13 | Y (smoker, CV, cancer) |
| 60 | M | - | 752 | 4 | 3 | Y (CV) |
| 61 | M | 21.6 | 8 | 6 | 5 | Y (cancer) |
| 64 | M | 32.8 | 1145 | 6 | 6 | Y (CV) |
| 68 | F | 23.4 | 217 | 9 | 9 | N |
| 68 | M | 25.2 | 45 | 50 | 50 | N |
| 73 | M | 29.4 | 7 | 4 | 3 | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arroyo, R.; Grant, S.N.; Colombo, M.; Salvioni, L.; Corsi, F.; Truffi, M.; Ottolina, D.; Hurst, B.; Salzberg, M.; Prosperi, D.; et al. Full-Length Recombinant hSP-D Binds and Inhibits SARS-CoV-2. Biomolecules 2021, 11, 1114. https://doi.org/10.3390/biom11081114
Arroyo R, Grant SN, Colombo M, Salvioni L, Corsi F, Truffi M, Ottolina D, Hurst B, Salzberg M, Prosperi D, et al. Full-Length Recombinant hSP-D Binds and Inhibits SARS-CoV-2. Biomolecules. 2021; 11(8):1114. https://doi.org/10.3390/biom11081114
Chicago/Turabian StyleArroyo, Raquel, Shawn N. Grant, Miriam Colombo, Lucia Salvioni, Fabio Corsi, Marta Truffi, Davide Ottolina, Brett Hurst, Marc Salzberg, Davide Prosperi, and et al. 2021. "Full-Length Recombinant hSP-D Binds and Inhibits SARS-CoV-2" Biomolecules 11, no. 8: 1114. https://doi.org/10.3390/biom11081114
APA StyleArroyo, R., Grant, S. N., Colombo, M., Salvioni, L., Corsi, F., Truffi, M., Ottolina, D., Hurst, B., Salzberg, M., Prosperi, D., & Kingma, P. S. (2021). Full-Length Recombinant hSP-D Binds and Inhibits SARS-CoV-2. Biomolecules, 11(8), 1114. https://doi.org/10.3390/biom11081114








