Changes in the Antioxidative Activity and the Content of Phenolics and Iridoids during Fermentation and Aging of Natural Fruit Meads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagent and Standard
2.1.2. Material
2.1.3. Microorganisms
2.2. Methods
2.2.1. Experimental Design
2.2.2. Analytical Methods
Total Polyphenolic Content
Free-Radical-Scavenging Ability Using a DPPH Radical
Free-Radical-Scavenging Ability Using ABTS Radical Cation
Ferric Reducing/Antioxidant Power (FRAP) Assay
Determination of Phenolics and Iridoids Content by HPLC-PDA
Determination of Extract and pH
Carbohydrates, Ethanol, Glycerol, and Acetic Acid Content
Statistical Analysis
3. Results and Discussion
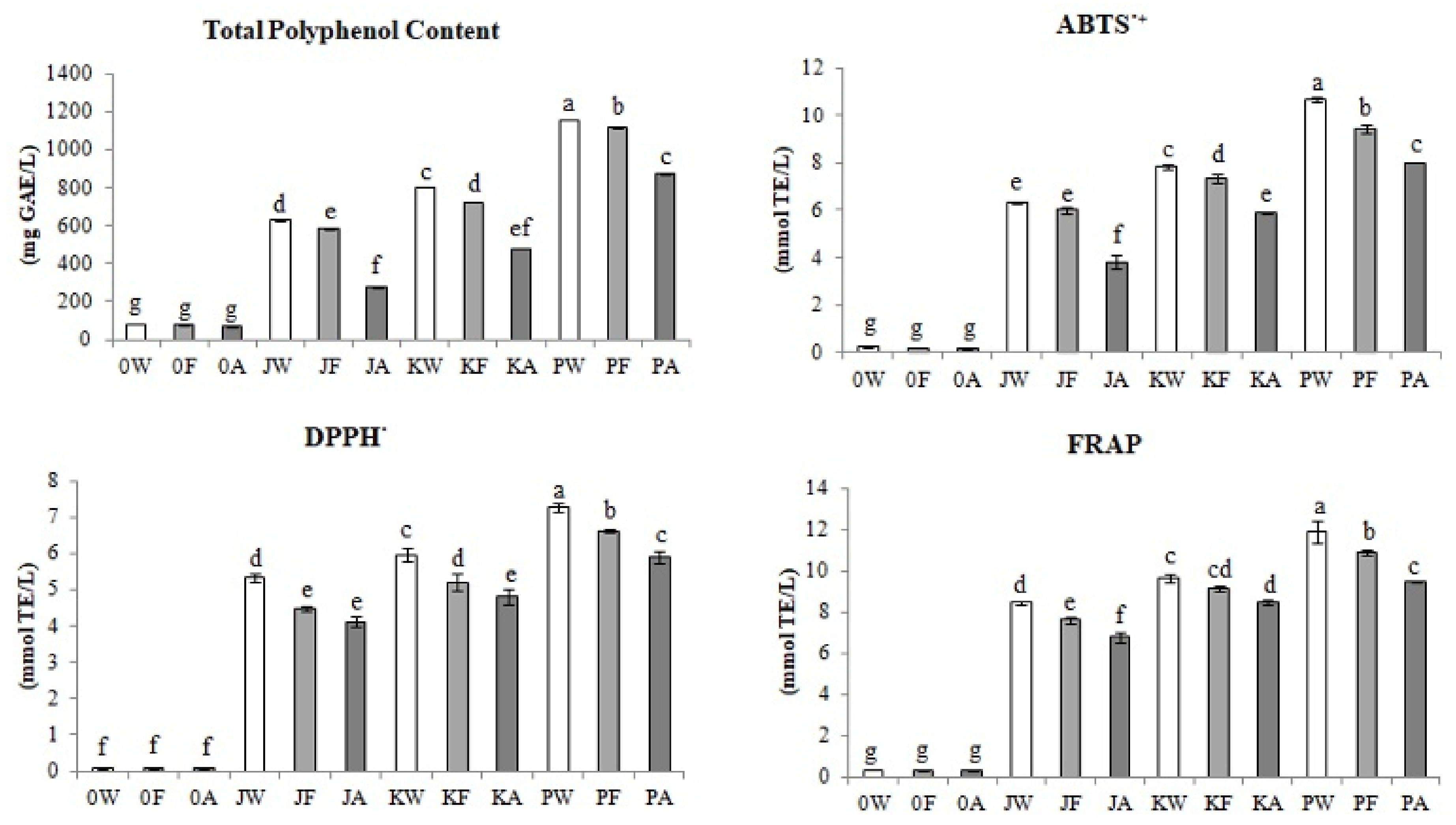
3.1. Total Polyphenols Content and Antioxidative Activity
3.2. Phenolics and Iridoids
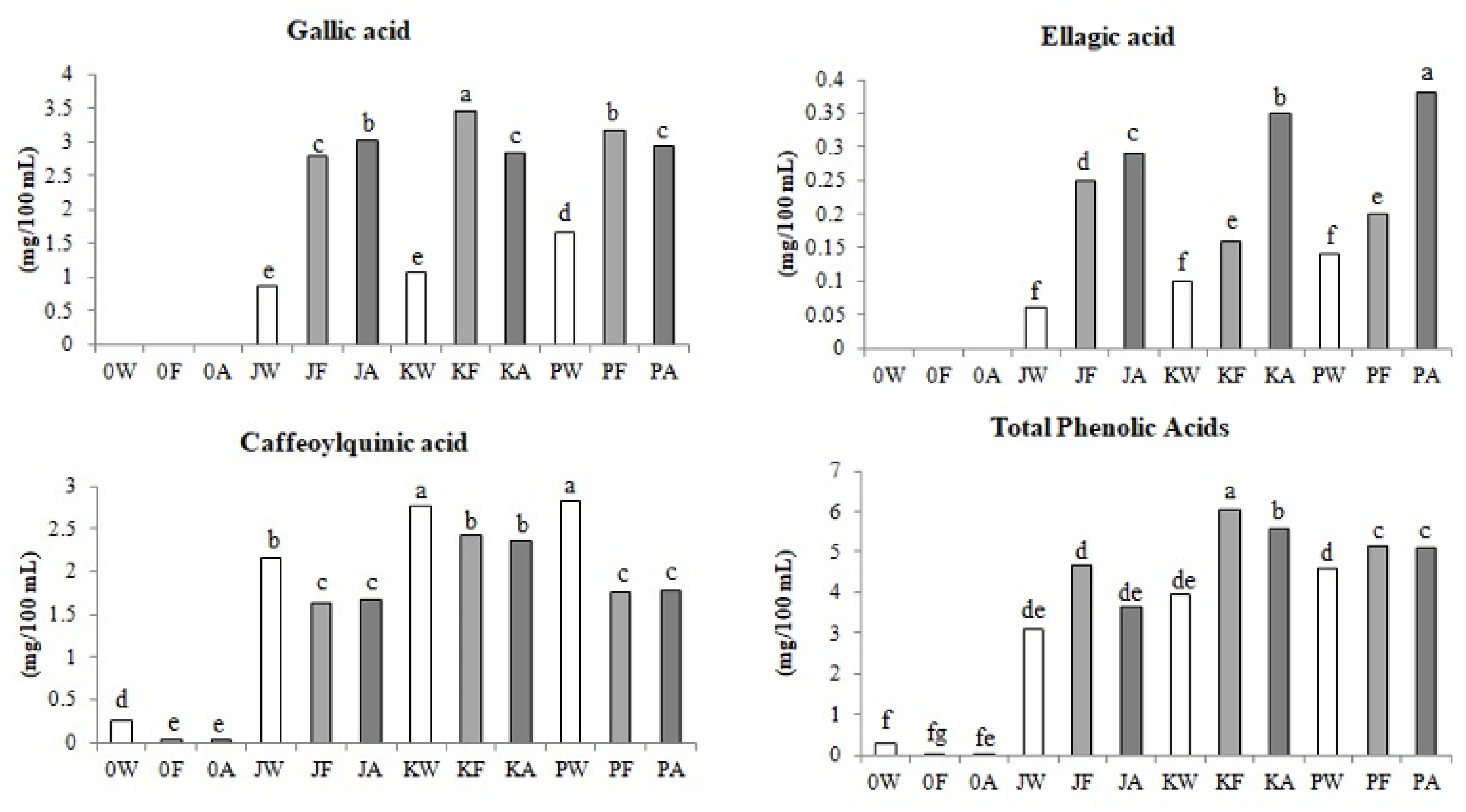
3.2.1. Phenolic Acids
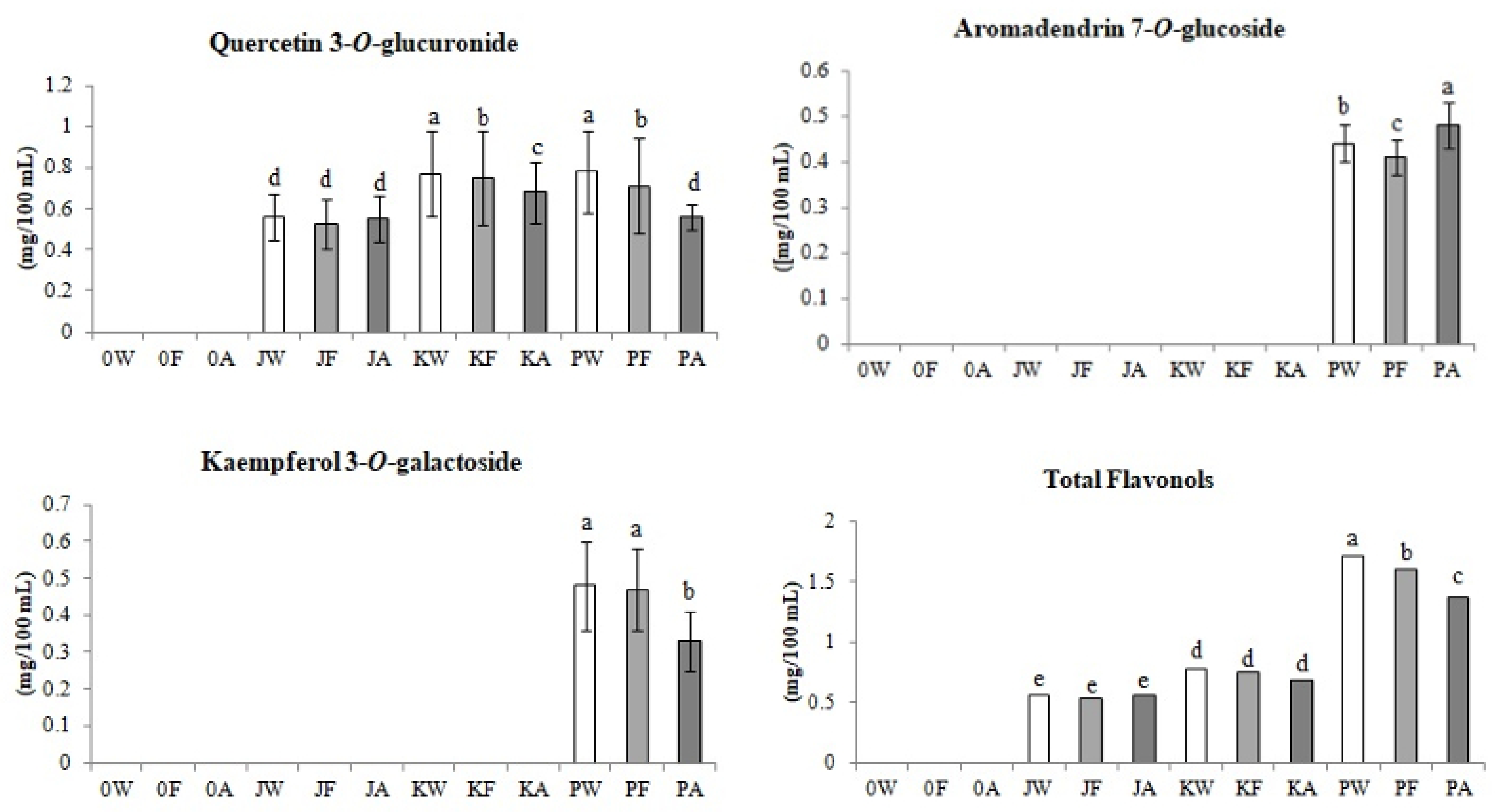
3.2.2. Flavonols
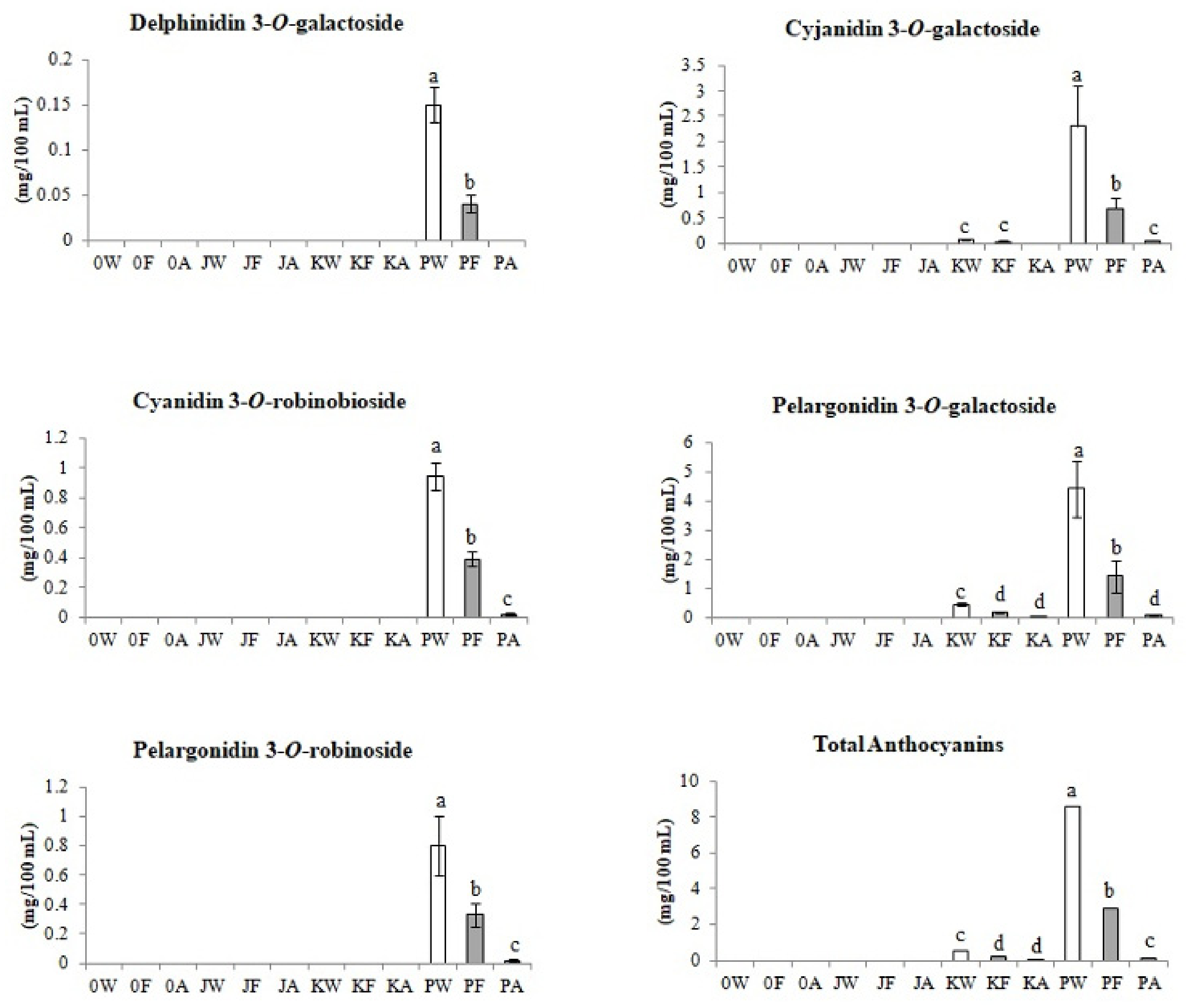
3.2.3. Anthocyanins
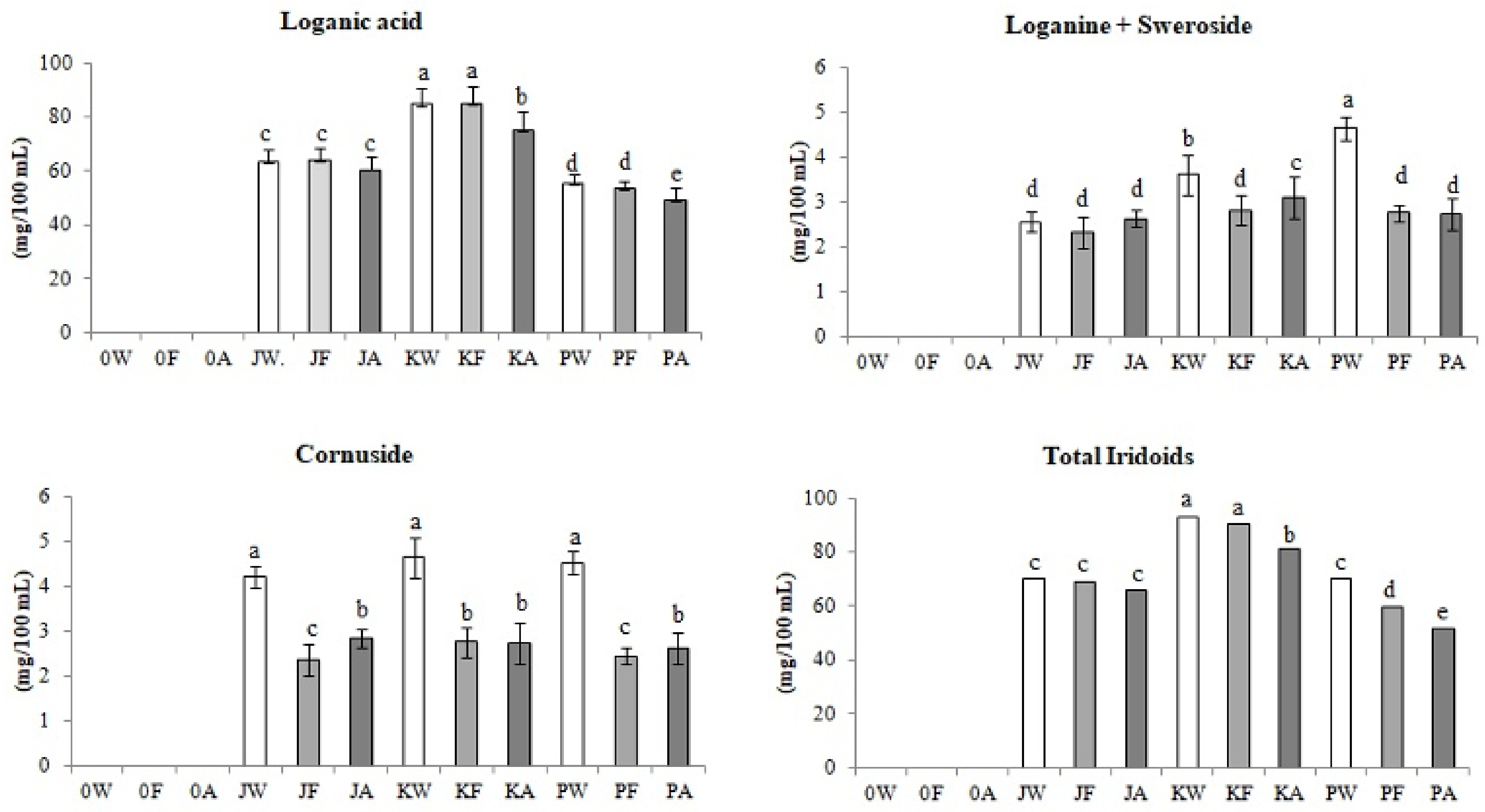
3.2.4. Iridoids
3.3. Basic Physicochemical Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halagarda, M.; Groth, S.; Popek, S.; Rohn, S.; Pedan, V. Antioxidant activity and phenolic profile of selected organic and conventional honeys from Poland. Antioxidants 2020, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Szatkowska, K. Fruit and herbal meads–chemical composition and antioxidant properties. Food Chem. 2019, 283, 19–27. [Google Scholar] [CrossRef]
- Xu, J.; Qi, Y.; Zhang, J.; Liu, M.; Wei, X.; Fan, M. Effect of reduced glutathione on the quality characteristics of apple wine during alcoholic fermentation. Food Chem. 2019, 300, 125130. [Google Scholar] [CrossRef]
- Šćepanović, R.P.; Wendelin, S.; Raičević, D.; Eder, R. Characterization of the phenolic profile of commercial Montenegrin red and white wines. Eur. Food Res. Technol. 2019, 245, 2233–2245. [Google Scholar] [CrossRef]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.Z. Characteristics of Cornelian cherry sour non-alcoholic beers brewed with the special yeastSaccharomycodes ludwigii. Food Chem. 2020, 312, 125968. [Google Scholar] [CrossRef]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.Z.; Piórecki, N. Characteristics of biologically active compounds in Cornelian cherry meads. Molecules 2018, 23, 2024. [Google Scholar] [CrossRef] [Green Version]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Prorok, P.; Piórecki, N. Physicochemical and antioxidative properties of Cornelian cherry beer. Food Chem. 2019, 281, 147–153. [Google Scholar] [CrossRef]
- Minnaar, P.P.; Du Plessis, H.W.; Jolly, N.P.; Van Der Rijst, M.; Du Toit, M. Non-Saccharomyces yeast and lactic acid bacteria in Co-inoculated fermentations with two Saccharomyces cerevisiae yeast strains: A strategy to improve the phenolic content of Syrah wine. Food Chem. X 2019, 4, 100070. [Google Scholar] [CrossRef] [PubMed]
- Sartor, S.; Burin, V.M.; Ferreira-Lima, N.E.; Caliari, V.; Bordignon-Luiz, M.T. Polyphenolic profiling, browning, and glutathione content of sparkling wines produced with nontraditional grape varieties: Indicator of quality during the biological aging. J. Food Sci. 2019, 84, 3546–3554. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Foddai, M.S. Phenolics Profile and Antioxidant Activity of Special Beers. Molecules 2020, 25, 2466. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef]
- Czabaj, S.; Kawa-Rygielska, J.; Kucharska, A.Z.; Kliks, J. Effects of mead wort heat treatment on the mead fermentation process and antioxidant activity. Molecules 2017, 22, 803. [Google Scholar] [CrossRef] [PubMed]
- Sroka, P.; Satora, P. The influence of hydrocolloids on mead wort fermentation. Food Hydrocoll. 2017, 63, 233–239. [Google Scholar] [CrossRef]
- Pereira, A.P.; Mendes-Ferreira, A.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. High-cell-density fermentation of Saccharomyces cerevisiae for the optimisation of mead production. Food Microbiol. 2013, 33, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Akalın, H.; Bayram, M.; Anlı, R.E. Determination of some individual phenolic compounds and antioxidant capacity of mead produced from different types of honey. J. Inst. Brew. 2017, 123, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Kahoun, D.; Řezková, S.; Královský, J. Effect of heat treatment and storage conditions on mead composition. Food Chem. 2017, 219, 357–363. [Google Scholar] [CrossRef]
- Socha, R.; Pająk, P.; Fortuna, T.; Buksa, K. Phenolic profile and antioxidant activity of Polish meads. Int. J. Food Prop. 2015, 18, 2713–2725. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Quiles, J.L.; Gil, E.; Bompadre, S.; Simal-Gandara, J.; Battino, M.; Giampieri, F. The influence of in vitro gastrointestinal digestion on the anticancer activity of manuka honey. Antioxidants 2020, 9, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascoe, H.M.; Ames, J.M.; Chandra, S. Critical stages of the brewing process for changes in antioxidant activity and levels of phenolic compounds in ale. J. Am. Soc. Brew. Chem. 2003, 61, 203–209. [Google Scholar] [CrossRef]
- Szwajgier, D.; Pielecki, J.; Targoński, Z. Changes of free ferulic and coumaric acid contents during malting of barley grain. Pol. J. Food Nutr. Sci. 2005, 14, 423–429. [Google Scholar]
- Przybylska, D.; Kucharska, A.Z.; Cybulska, I.; Sozański, T.; Piórecki, N.; Fecka, I. Cornus mas L. Stones: A Valuable by-product as an ellagitannin source with high antioxidant potential. Molecules 2020, 25, 4646. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Cantatore, V.; Polo, A.; Celano, G.; Martinovic, A.; Cavoski, I.; Gobbetti, M. Design of potential probiotic yeast starters tailored for making a cornelian cherry (Cornus mas L.) functional beverage. Int. J. Food Microbiol. 2020, 323, 108591. [Google Scholar] [CrossRef]
- Ciucure, C.T.; Geană, E.I. Phenolic compounds profile and biochemical properties of honeys in relationship to the honey floral sources. Phytochem. Anal. 2019, 30, 481–492. [Google Scholar] [CrossRef]
- De Biaggi, M.; Donno, D.; Mellano, M.G.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. Cornus mas (L.) fruit as a potential source of natural health-promoting compounds: Physico-chemical characterisation of bioactive components. Plant Foods Hum. Nutr. 2018, 73, 89–94. [Google Scholar] [CrossRef]
- Ou, J.; Wang, M.; Zheng, J.; Ou, S. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019, 284, 90–99. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, phenolic compounds and antioxidant activity of edible honeysuckle berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oszmiański, J.; Kucharska, A.Z. Effect of pre-treatment of blue honeysuckle berries on bioactive iridoid content. Food Chem. 2018, 240, 1087–1091. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2019, 113, 859–871. [Google Scholar] [CrossRef]
- Jensen, H.D.; Krogfelt, K.A.; Cornett, C.; Hansen, S.H.; Christensen, S.B. Hydrophilic carboxylic acids and iridoid glycosides in the juice of American and European cranberries (Vaccinium macrocarpon and V. oxycoccos), lingonberries (V. vitis-idaea), and blueberries (V. myrtillus). J. Agric. Food Chem. 2002, 50, 6871–6874. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; West, B.J.; Jensen, C.J. UPLC-TOF-MS characterization and identification of bioactive iridoids in Cornus mas fruit. J. Anal. Methods Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sozański, T.; Kucharska, A.Z.; Szumny, D.; Magdalan, J.; Merwid-Ląd, A.; Nowak, B.; Piórecki, N.; Dzimira, S.; Jodkowska, A.; Szeląg, A.; et al. Cornelian cherry consumption increases the L-arginine/ADMA ratio, lowers ADMA and SDMA levels in the plasma, and enhances the aorta glutathione level in rabbits fed a high-cholesterol diet. J. Funct. Foods 2017, 34, 189–196. [Google Scholar] [CrossRef]
- Wei, S.; Chi, H.; Kodama, H.; Chen, G. Anti-inflammatory effect of three iridoids in human neutrophils. Nat. Prod. Res. 2013, 27, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyżowska, A.; Kucharska, A.Z.; Fecka, I. Composition and Antibacterial Activity of Aronia melanocarpa (Michx.) Elliot, Cornus mas L. and Chaenomeles superba Lindl. Leaf Extracts. Molecules 2020, 25, 2011. [Google Scholar] [CrossRef]
- Hu, L.; Wu, Z.; Jiang, Y.; Wang, X.; He, A.; Song, J.; Zhou, S.; Zhao, Y.; Xu, J. Recent advances in catalytic and autocatalytic production of biomass-derived 5-hydroxymethylfurfural. Renew. Sustain. Energy Rev. 2020, 134, 110317. [Google Scholar] [CrossRef]
- Gomes, T.; Dias, T.; Cadavez, V.; Verdial, J.; Morais, J.S.; Ramalhosa, E.; Estevinho, L.M. Influence of sweetness and ethanol content on mead acceptability. Pol. J. Food Nutr. Sci. 2015, 65, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Niemes, J.P.; Kolc, C.S.M.; Torres, Y.R.; Felsner, M.L.; da Rosa, M.R. Development an in house validation for 5-hydroxy-2-methyl-furfuraldehyde (HMF) analysis in fermented beverages produced from honey, cane syrup and corn syrup by HPLC-UV. Orbital: Electron. J. Chem. 2018, 10, 156–163. [Google Scholar] [CrossRef]
| Sample | Glucose | Fructose | Alcohol | Glycerol | Acetic Acid | HMF | pH |
|---|---|---|---|---|---|---|---|
| g/L | mg/100 mL | ||||||
| 0W | 134.34 ± 0.23 a 1 | 83.33 ± 0.44 a | na 2 | nd 3 | nd | 1.62 ± 0.23 c | 3.69 ± 0.01 a |
| 0F | 15.80 ± 0.46 c | 37.35 ± 0.12 c | 96.90 ± 0.75 d | 7.65 ± 0.14 d | 1.44 ± 0.00 a | nd | 3.44 ± 0.00 b |
| 0A | 5.40 ± 0.54 d | 15.88 ± 1.46 d | 115.65 ± 0.65 a | 8.15 ± 0.18 c | 1.44 ± 0.01 a | 0.33 ± 0.01 e | 3.38 ± 0.00 c |
| JW | 149.66 ± 0.10 a | 95.70 ± 0.03 a | na | nd | nd | 1.99 ± 0.09 b | 3.46 ± 0.02 b |
| JF | 28.20 ± 0.38 b | 64.20 ± 0.22 b | 92.90 ± 0.23 e | 7.75 ± 0.09 d | 1.06 ± 0.01 c | nd | 3.25 ± 0.01 c |
| JA | 25.80 ± 0.69 b | 63.10 ± 0.61 b | 103.20 ± 0.48 c | 9.15 ± 0.36 b | 1.10 ± 0.03 bc | 1.21 ± 0.65 d | 3.17 ± 0.00 cd |
| KW | 157.77 ± 1.37 a | 99.66 ± 1.04 a | na | nd | nd | 1.98 ± 0.25 b | 3.55 ± 0.01 ab |
| KF | 30.00 ± 0.03 b | 65.85 ± 0.46 b | 91.60 ± 0.46 e | 8.45 ± 0.44 c | 1.05 ± 0.02 c | nd | 3.35 ± 0.02 c |
| KA | 27.40 ± 0.89 b | 64.30 ± 0.02 b | 104.50 ± 0.99 c | 9.10 ± 0.32 b | 1.13 ± 0.12 b | 1.11 ± 0.66 d | 3.32 ± 0.00 c |
| PW | 154.14 ± 0.98 a | 95.62 ± 0.40 a | na | nd | nd | 2.17 ± 0.09 a | 3.51 ± 0.00 ab |
| PF | 32.25 ± 0.20 b | 68.00 ± 1.30 b | 89.90 ± 0.31 e | 8.65 ± 0.09 c | 1.07 ± 0.08 c | nd | 3.34 ± 0.00 c |
| PA | 27.80 ± 1.01 b | 65.00 ± 0.89 b | 107.30 ± 0.24 b | 9.60 ± 0.13 a | 1.17 ± 0.11 b | 0.96 ± 0.19 b | 3.23 ± 0.01 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.Z.; Głowacki, A.; Piórecki, N. Changes in the Antioxidative Activity and the Content of Phenolics and Iridoids during Fermentation and Aging of Natural Fruit Meads. Biomolecules 2021, 11, 1113. https://doi.org/10.3390/biom11081113
Adamenko K, Kawa-Rygielska J, Kucharska AZ, Głowacki A, Piórecki N. Changes in the Antioxidative Activity and the Content of Phenolics and Iridoids during Fermentation and Aging of Natural Fruit Meads. Biomolecules. 2021; 11(8):1113. https://doi.org/10.3390/biom11081113
Chicago/Turabian StyleAdamenko, Kinga, Joanna Kawa-Rygielska, Alicja Z. Kucharska, Adam Głowacki, and Narcyz Piórecki. 2021. "Changes in the Antioxidative Activity and the Content of Phenolics and Iridoids during Fermentation and Aging of Natural Fruit Meads" Biomolecules 11, no. 8: 1113. https://doi.org/10.3390/biom11081113
APA StyleAdamenko, K., Kawa-Rygielska, J., Kucharska, A. Z., Głowacki, A., & Piórecki, N. (2021). Changes in the Antioxidative Activity and the Content of Phenolics and Iridoids during Fermentation and Aging of Natural Fruit Meads. Biomolecules, 11(8), 1113. https://doi.org/10.3390/biom11081113






