Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview
Abstract
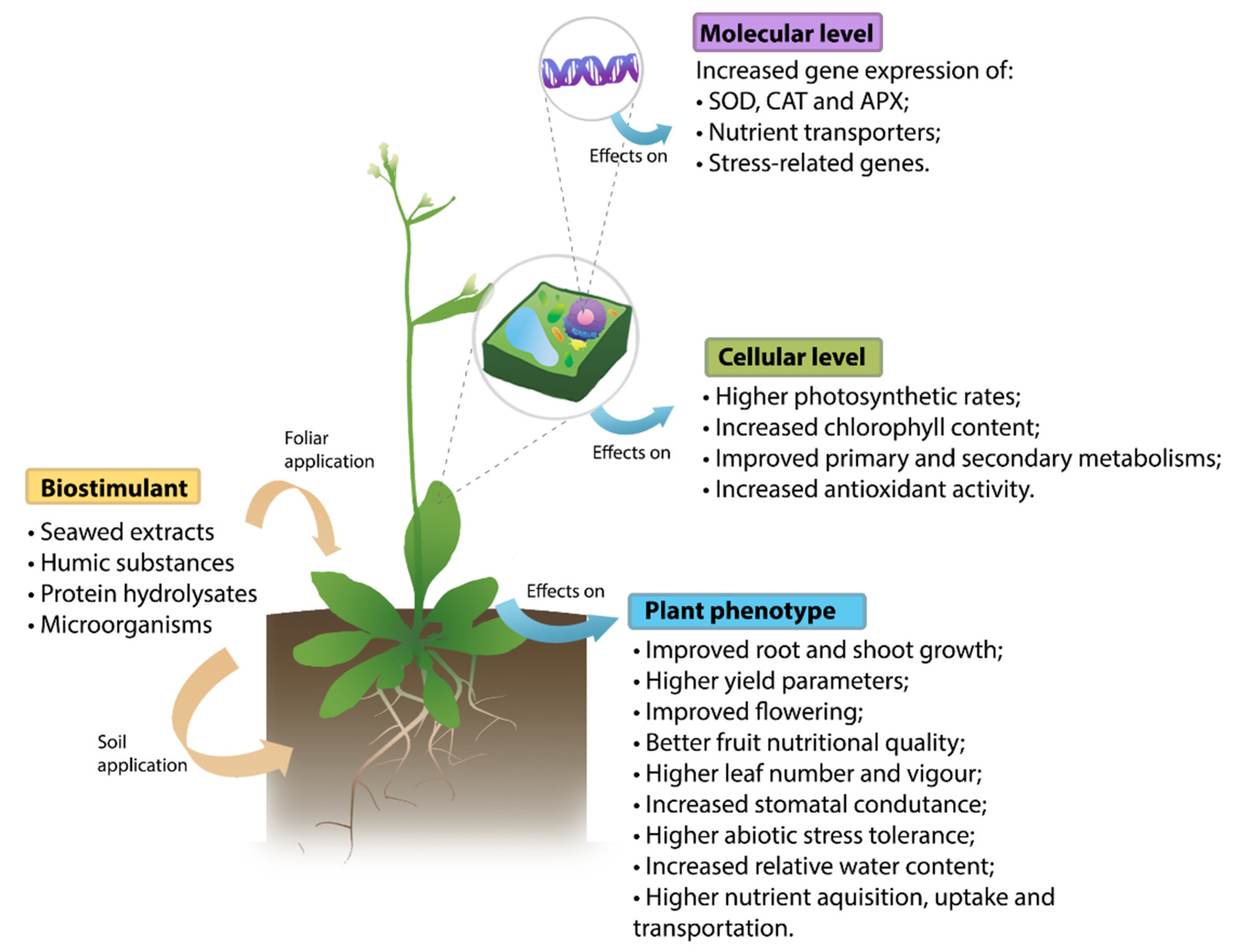
1. Introduction
2. Humic Substances as Biostimulants
2.1. Humic Acids
2.2. Fulvic Acids
3. Protein Hydrolysates
The Molecular Influence of Protein Hydrolysates
4. Seaweed Extracts
4.1. Ascophyllum nodosum
4.2. Ecklonia maxima
4.3. Kappaphycus alvarezii and Gracilaria edulis
5. Microorganism-Based Biostimulants
5.1. PGPB as Biostimulants
5.2. Trichoderma spp.
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alix, A.; Capri, E. Modern Agriculture in Europe and the Role of Pesticides. In Advances in Chemical Pollution, Environmental Management and Protection; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 1–22. ISBN 978-0-12-812866-4. [Google Scholar]
- Rashmi, I.; Roy, T.; Kartika, K.S.; Pal, R.; Coumar, V.; Kala, S.; Shinoji, K.C. Organic and Inorganic Fertilizer Contaminants in Agriculture: Impact on Soil and Water Resources. In Contaminants in Agriculture; Naeem, M., Ansari, A.A., Gill, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–41. ISBN 978-3-030-41551-8. [Google Scholar]
- Verma, B.C.; Pramanik, P.; Bhaduri, D. Organic Fertilizers for Sustainable Soil and Environmental Management. In Nutrient Dynamics for Sustainable Crop Production; Meena, R.S., Ed.; Springer: Singapore, 2020; pp. 289–313. ISBN 9789811386596. [Google Scholar]
- European Biostimulant Industry Council [EBIC] Economic Overview of the European Biostimulants Market. Available online: https://biostimulants.eu/highlights/economic-overview-of-the-european-biostimulants-market/ (accessed on 9 November 2020).
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regula 2019 (EC) No 2003/2003. Off. J. 2019, L107/1, 1–114.
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2015; Volume 130, pp. 141–174. ISBN 978-0-12-802137-8. [Google Scholar]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Carletti, P.; García, A.C.; Silva, C.A.; Merchant, A. Editorial: Towards a Functional Characterization of Plant Biostimulants. Front. Plant Sci. 2021, 12, 677772. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A. Practical Applications of Plant Biostimulants in Greenhouse Vegetable Crop Production. Agronomy 2020, 10, 1569. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant Biostimulants: Innovative Tool for Enhancing Plant Nutrition in Organic Farming. Eur. J. Hortic. Sci. 2018, 82, 277–285. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptistella, J.L.C.; Horz, D.C.; Bortolato, L.M.; Mazzafera, P. Organic Plant Biostimulants and Fruit Quality—A Review. Agronomy 2020, 10, 988. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a Sustainable Agriculture Through Plant Biostimulants: From Experimental Data to Practical Applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Nardi, S.; Carletti, P.; Pizzeghello, D.; Muscolo, A. Biological Activities of Humic Substances. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Senesi, N., Xing, B., Huang, P.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 305–339. ISBN 978-0-470-49495-0. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Chen, Y.; De Nobili, M.; Aviad, T. Stimulatory Effects of Humic Substances on Plant Growth. In Soil Organic Matter in Sustainable Agriculture; Magdoff, F., Weil, R., Eds.; Advances in Agroecology; CRC Press: Boca Raton, FL, USA, 2004; Volume 20042043, ISBN 978-0-8493-1294-6. [Google Scholar]
- Adey, W.H.; Loveland, K. The Input of Organic Energy. In Dynamic Aquaria; Elsevier: Amsterdam, The Netherlands, 2007; pp. 93–100. ISBN 978-0-12-370641-6. [Google Scholar]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A Meta-Analysis and Review of Plant-Growth Response to Humic Substances. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2014; Volume 124, pp. 37–89. ISBN 978-0-12-800138-7. [Google Scholar]
- Olaetxea, M.; Hita, D.D.; Garcia, C.A.; Fuentes, M.; Baigorri, R.; Mora, V.; Garnica, M.; Urrutia, O.; Erro, J.; Zamarreño, A.M.; et al. Hypothetical Framework Integrating the Main Mechanisms Involved in the Promoting Action of Rhizospheric Humic Substances on Plant Root- and Shoot- Growth. Appl. Soil Ecol. 2018, 123, 521–537. [Google Scholar] [CrossRef]
- García-Mina, J.M.; Antolín, M.C.; Sanchez-Diaz, M. Metal-Humic Complexes and Plant Micronutrient Uptake: A Study Based on Different Plant Species Cultivated in Diverse Soil Types. Plant Soil 2004, 258, 57–68. [Google Scholar] [CrossRef]
- García, A.C.; de Souza, L.G.A.; Pereira, M.G.; Castro, R.N.; García-Mina, J.M.; Zonta, E.; Lisboa, F.J.G.; Berbara, R.L.L. Structure-Property-Function Relationship in Humic Substances to Explain the Biological Activity in Plants. Sci. Rep. 2016, 6, 20798. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.G.; Abdel-Baky, Y.R.; El-Awadi, M.E.-S.; Bakhoum, G.S. Enhancement Quality and Quantity of Faba bean Plants Grown under Sandy Soil Conditions by Nicotinamide and/or Humic Acid Application. Bull. Natl. Res. Cent. 2019, 43, 28. [Google Scholar] [CrossRef]
- Gerke, J. Review Article: The Effect of Humic Substances on Phosphate and Iron Acquisition by Higher Plants: Qualitative and Quantitative Aspects. J. Plant Nutr. Soil Sci. 2021, 184, 329–338. [Google Scholar] [CrossRef]
- Nardi, S.; Ertani, A.; Francioso, O. Soil-Root Cross-Talking: The Role of Humic Substances. J. Plant Nutr. Soil Sci. 2017, 180, 5–13. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Ertani, A. Hormone-like Activity of the Soil Organic Matter. Appl. Soil Ecol. 2018, 123, 517–520. [Google Scholar] [CrossRef]
- Zanin, L.; Tomasi, N.; Cesco, S.; Varanini, Z.; Pinton, R. Humic Substances Contribute to Plant Iron Nutrition Acting as Chelators and Biostimulants. Front. Plant Sci. 2019, 10, 675. [Google Scholar] [CrossRef]
- García, A.C.; van Tol de Castro, T.A.; Santos, L.A.; Tavares, O.C.H.; Castro, R.N.; Berbara, R.L.L.; García-Mina, J.M. Structure-Property-Function Relationship of Humic Substances in Modulating the Root Growth of Plants: A Review. J. Environ. Qual. 2019, 48, 1622–1632. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and Fulvic Acids as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Xu, D.-B.; Wang, Q.-J.; Wu, Y.-C.; Yu, G.-H.; Shen, Q.-R.; Huang, Q.-W. Humic-Like Substances from Different Compost Extracts Could Significantly Promote Cucumber Growth. Pedosphere 2012, 22, 815–824. [Google Scholar] [CrossRef]
- Godara, A.; Bakshi, M. Effect Of Application Of Bio Stimulants Like Humic Acid And Vermiwash On The Growth, Quality And Yield Of Plant—A Review. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 22–30. [Google Scholar]
- Bocanegra, M.P.; Lobartini, J.C.; Orioli, G.A. Plant Uptake of Iron Chelated by Humic Acids of Different Molecular Weights. Commun. Soil Sci. Plant Anal. 2006, 37, 239–248. [Google Scholar] [CrossRef]
- Jindo, K.; Olivares, F.L.; da Malcher, D.J.P.; Sánchez-Monedero, M.A.; Kempenaar, C.; Canellas, L.P. From Lab to Field: Role of Humic Substances Under Open-Field and Greenhouse Conditions as Biostimulant and Biocontrol Agent. Front. Plant Sci. 2020, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Zandonadi, D.B.; Santos, M.P.; Busato, J.G.; Peres, L.E.P.; Façanha, A.R. Plant Physiology as Affected by Humified Organic Matter. Theor. Exp. Plant Physiol. 2013, 25, 13–25. [Google Scholar] [CrossRef]
- Scaglia, B.; Pognani, M.; Adani, F. Evaluation of Hormone-like Activity of the Dissolved Organic Matter Fraction (DOM) of Compost and Digestate. Sci. Total Environ. 2015, 514, 314–321. [Google Scholar] [CrossRef]
- Scaglia, B.; Nunes, R.R.; Rezende, M.O.O.; Tambone, F.; Adani, F. Investigating Organic Molecules Responsible of Auxin-like Activity of Humic Acid Fraction Extracted from Vermicompost. Sci. Total Environ. 2016, 562, 289–295. [Google Scholar] [CrossRef]
- Jindo, K.; Canellas, L.; Albacete, A.; Figueiredo dos Santos, L.; Frinhani Rocha, R.; Carvalho Baia, D.; Oliveira Aguiar Canellas, N.; Goron, T.; Olivares, F. Interaction between Humic Substances and Plant Hormones for Phosphorous Acquisition. Agronomy 2020, 10, 640. [Google Scholar] [CrossRef]
- Elmongy, M.S.; Wang, X.; Zhou, H.; Xia, Y. Humic Acid and Auxins Induced Metabolic Changes and Differential Gene Expression during Adventitious Root Development in Azalea Microshoots. HortScience 2020, 55, 926–935. [Google Scholar] [CrossRef]
- Nasiri, A.; Sam-Daliri, M.; Shirani-Rad, A.; Mousavi, A.; Jabbari, H. The Response of Growth and Yield of Canola Genotypes to Humic Acid Application in Different Plant Densities. Gesunde Pflanz. 2021, 73, 17–27. [Google Scholar] [CrossRef]
- Delfine, S.; Tognetti, R.; Desiderio, E.; Alvino, A. Effect of Foliar Application of N and Humic Acids on Growth and Yield of Durum wheat. Agron. Sustain. Dev. 2005, 25, 183–191. [Google Scholar] [CrossRef]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Jiao, X.; Zhu, G.; Elsiddig, A.M.I.; Suliman, M.S.E.; Elradi, S.B.M.; Yue, W. Exogenous Jasmonic Acid and Humic Acid Increased Salinity Tolerance of Sorghum. Agron. J. 2020, 112, 871–884. [Google Scholar] [CrossRef]
- Meganid, A.S.; Al-Zahrani, H.S.; El-Metwally, M.S. Effect of Humic Acid Application on Growth and Chlorophyll Contents of Common Bean Plants (Phaseolus vulgaris L.) under Salinity Stress Conditions. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 2651–2660. [Google Scholar]
- Jannin, L.; Arkoun, M.; Ourry, A.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Microarray Analysis of Humic Acid Effects on Brassica napus Growth: Involvement of N, C and S Metabolisms. Plant Soil 2012, 359, 297–319. [Google Scholar] [CrossRef]
- Roomi, S.; Masi, A.; Conselvan, G.B.; Trevisan, S.; Quaggiotti, S.; Pivato, M.; Arrigoni, G.; Yasmin, T.; Carletti, P. Protein Profiling of Arabidopsis Roots Treated With Humic Substances: Insights Into the Metabolic and Interactome Networks. Front. Plant Sci. 2018, 9, 1812. [Google Scholar] [CrossRef] [PubMed]
- Conselvan, G.B.; Fuentes, D.; Merchant, A.; Peggion, C.; Francioso, O.; Carletti, P. Effects of Humic Substances and Indole-3-Acetic Acid on Arabidopsis Sugar and Amino Acid Metabolic Profile. Plant Soil 2018, 426, 17–32. [Google Scholar] [CrossRef]
- Byun, M.Y.; Kim, D.; Youn, U.J.; Lee, S.; Lee, H. Improvement of Moss Photosynthesis by Humic Acids from Antarctic Tundra Soil. Plant Physiol. Biochem. 2021, 159, 37–42. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Sustain. Agric. 2009, 29, 153–188. [Google Scholar]
- Selmar, D.; Kleinwächter, M. Influencing the Product Quality by Deliberately Applying Drought Stress during the Cultivation of Medicinal Plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Khorasaninejad, S.; Ahmadabadi, A.; Hemmati, K. The Effect of Humic Acid on Leaf Morphophysiological and Phytochemical Properties of Echinacea purpurea L. under Water Deficit Stress. Sci. Hortic. 2018, 239, 314–323. [Google Scholar] [CrossRef]
- Qin, K.; Leskovar, D.I. Lignite-Derived Humic Substances Modulate Pepper and Soil-Biota Growth under Water Deficit Stress. J. Plant Nutr. Soil Sci. 2018, 181, 655–663. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Hafez, Y.M.; El-Afry, M.M.; Tantawy, D.S.; Alshaal, T. Effect of Some Osmoregulators on Photosynthesis, Lipid Peroxidation, Antioxidative Capacity, and Productivity of Barley (Hordeum vulgare L.) under Water Deficit Stress. Environ. Sci. Pollut. Res. 2018, 25, 30199–30211. [Google Scholar] [CrossRef]
- Man-hong, Y.; Lei, Z.; Sheng-tao, X.; McLaughlin, N.B.; Jing-hui, L. Effect of Water Soluble Humic Acid Applied to Potato Foliage on Plant Growth, Photosynthesis Characteristics and Fresh Tuber Yield under Different Water Deficits. Sci. Rep. 2020, 10, 7854. [Google Scholar] [CrossRef]
- Lotfi, R.; Kalaji, H.M.; Valizadeh, G.R.; Behrozyar, E.; Hemati, A.; Gharavi-Kochebagh, P.; Ghassemi, A. Effects of Humic Acid on Photosynthetic Efficiency of Rapeseed Plants Growing under Different Watering Conditions. Photosynthetica 2018, 56, 962–970. [Google Scholar] [CrossRef]
- Morsomme, P.; Boutry, M. The Plant Plasma Membrane H+-ATPase: Structure, Function and Regulation. Biochim. Biophys. Acta BBA Biomembr. 2000, 1465, 1–16. [Google Scholar] [CrossRef]
- Singh, R.K.; Deshmukh, R.; Muthamilarasan, M.; Rani, R.; Prasad, M. Versatile Roles of Aquaporin in Physiological Processes and Stress Tolerance in Plants. Plant Physiol. Biochem. 2020, 149, 178–189. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, I.G.; Olivares, F.L.; Ramos, A.C.; Bertolazi, A.A.; Canellas, L.P. Humic Acids and Herbaspirillum seropedicae Change the Extracellular H+ Flux and Gene Expression in Maize Roots Seedlings. Chem. Biol. Technol. Agric. 2019, 6, 8. [Google Scholar] [CrossRef]
- Cha, J.-Y.; Kang, S.-H.; Ali, I.; Lee, S.C.; Ji, M.G.; Jeong, S.Y.; Shin, G.-I.; Kim, M.G.; Jeon, J.-R.; Kim, W.-Y. Humic Acid Enhances Heat Stress Tolerance via Transcriptional Activation of Heat-Shock Proteins in Arabidopsis. Sci. Rep. 2020, 10, 15042. [Google Scholar] [CrossRef]
- Jacob, P.; Hirt, H.; Bendahmane, A. The Heat-shock Protein/Chaperone Network and Multiple Stress Resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef]
- Taspinar, M.S.; Aydin, M.; Sigmaz, B.; Yildirim, N.; Agar, G. Protective Role of Humic Acids against Picloram-Induced Genomic Instability and DNA Methylation in Phaseolus vulgaris. Environ. Sci. Pollut. Res. 2017, 24, 22948–22953. [Google Scholar] [CrossRef]
- Braziene, Z.; Paltanavicius, V.; Avizienytė, D. The Influence of Fulvic Acid on Spring Cereals and Sugar Beets Seed Germination and Plant Productivity. Environ. Res. 2021, 195, 110824. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review Report on the Role of Bioproducts, Biopreparations, Biostimulants and Microbial Inoculants in Organic Production of Fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Capstaff, N.M.; Morrison, F.; Cheema, J.; Brett, P.; Hill, L.; Muñoz-García, J.C.; Khimyak, Y.Z.; Domoney, C.; Miller, A.J. Fulvic Acid Increases Forage Legume Growth Inducing Preferential Up-Regulation of Nodulation and Signalling-Related Genes. J. Exp. Bot. 2020, 71, 5689–5704. [Google Scholar] [CrossRef] [PubMed]
- Che, R.; Huang, L.; Xu, J.-W.; Zhao, P.; Li, T.; Ma, H.; Yu, X. Effect of Fulvic Acid Induction on the Physiology, Metabolism, and Lipid Biosynthesis-Related Gene Transcription of Monoraphidium sp. FXY-10. Bioresour. Technol. 2017, 227, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Priya, B.N.V.; Mahavishnan, K.; Gurumurthy, D.S.; Bindumadhava, H.; Upadhyay, A.P.; Sharma, N.K. Fulvic Acid (FA) for Enhanced Nutrient Uptake and Growth: Insights from Biochemical and Genomic Studies. J. Crop Improv. 2014, 28, 740–757. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as Key Players in Plant Stress Signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Sun, J.; Qiu, C.; Ding, Y.; Wang, Y.; Sun, L.; Fan, K.; Gai, Z.; Dong, G.; Wang, J.; Li, X.; et al. Fulvic Acid Ameliorates Drought Stress-Induced Damage in Tea Plants by Regulating the Ascorbate Metabolism and Flavonoids Biosynthesis. BMC Genomics 2020, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wang, X.; Zhang, X.; Zhao, D.; Tao, J. Effects of Fulvic Acid on the Photosynthetic and Physiological Characteristics of Paeonia ostii under Drought Stress. Plant Signal. Behav. 2020, 15, 1774714. [Google Scholar] [CrossRef]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative Effects of Humic and Fulvic Acids as Biostimulants on Growth, Antioxidant Activity and Nutrient Content of Yarrow (Achillea millefolium L.). Sci. Hortic. 2021, 279, 109912. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing Biostimulants From Agro-Food and Industrial By-Products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef]
- Pasupuleti, V.K.; Braun, S. State of the Art Manufacturing of Protein Hydrolysates. In Protein Hydrolysates in Biotechnology; Pasupuleti, V.K., Demain, A.L., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 11–32. ISBN 978-1-4020-6673-3. [Google Scholar]
- Moreno-Hernández, J.M.; Benítez-García, I.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C.; Sánchez, E. Strategies for Production, Characterization and Application of Protein-Based Biostimulants in Agriculture: A Review. Chil. J. Agric. Res. 2020, 80, 274–289. [Google Scholar] [CrossRef]
- Pecha, J.; Fürst, T.; Kolomazník, K.; Friebrová, V.; Svoboda, P. Protein Biostimulant Foliar Uptake Modeling: The Impact of Climatic Conditions. AIChE J. 2012, 58, 2010–2019. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein Hydrolysates as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Apone, F.; Tito, A.; Carola, A.; Arciello, S.; Tortora, A.; Filippini, L.; Monoli, I.; Cucchiara, M.; Gibertoni, S.; Chrispeels, M.J.; et al. A Mixture of Peptides and Sugars Derived from Plant Cell Walls Increases Plant Defense Responses to Stress and Attenuates Ageing-Associated Molecular Changes in Cultured Skin Cells. J. Biotechnol. 2010, 145, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant Action of a Plant-Derived Protein Hydrolysate Produced through Enzymatic Hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant Activity of Two Protein Hydrolyzates in the Growth and Nitrogen Metabolism of Maize Seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- García-Martínez, A.M.; Díaz, A.; Tejada, M.; Bautista, J.; Rodríguez, B.; Santa María, C.; Revilla, E.; Parrado, J. Enzymatic Production of an Organic Soil Biostimulant from Wheat-Condensed Distiller Solubles: Effects on Soil Biochemistry and Biodiversity. Process Biochem. 2010, 45, 1127–1133. [Google Scholar] [CrossRef]
- Luziatelli, F.; Ficca, A.G.; Colla, G.; Baldassarre Švecová, E.; Ruzzi, M. Foliar Application of Vegetal-Derived Bioactive Compounds Stimulates the Growth of Beneficial Bacteria and Enhances Microbiome Biodiversity in Lettuce. Front. Plant Sci. 2019, 10, 60. [Google Scholar] [CrossRef]
- Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Rosati, A.; Di Vaio, C.; Famiani, F. A Biostimulant Based on Protein Hydrolysates Promotes the Growth of Young Olive Trees. Agriculture 2020, 10, 618. [Google Scholar] [CrossRef]
- Caruso, G.; El-Nakhel, C.; Rouphael, Y.; Comite, E.; Lombardi, N.; Cuciniello, A.; Woo, S.L. Diplotaxis tenuifolia (L.) DC. Yield and Quality as Influenced by Cropping Season, Protein Hydrolysates, and Trichoderma Applications. Plants 2020, 9, 697. [Google Scholar] [CrossRef]
- Kałużewicz, A.; Krzesiński, W.; Spiżewski, T.; Zaworska, A. Effect of Biostimulants on Several Physiological Characteristics and Chlorophyll Content in Broccoli under Drought Stress and Re-Watering. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 197–202. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-Wide Identification of Differentially Expressed Genes in Solanum lycopersicon L. in Response to an Alfalfa-Protein Hydrolysate Using Microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef]
- Tsouvaltzis, P.; Koukounaras, A.; Siomos, A.S. Application of Amino Acids Improves Lettuce Crop Uniformity and Inhibits Nitrate Accumulation Induced by the Supplemental Inorganic Nitrogen Fertilization. Int. J. Agric. Biol. 2014, 16, 951–955. [Google Scholar]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein Hydrolysate Stimulates Growth in Tomato Coupled with N-Dependent Gene Expression Involved in N Assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, S.; Manoli, A.; Quaggiotti, S. A Novel Biostimulant, Belonging to Protein Hydrolysates, Mitigates Abiotic Stress Effects on Maize Seedlings Grown in Hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef]
- Guan, L.M.; Scandalios, J.G. Catalase Transcript Accumulation in Response to Dehydration and Osmotic Stress in Leaves of Maize Viviparous Mutants. Redox Rep. 2000, 5, 377–383. [Google Scholar] [CrossRef][Green Version]
- Rentel, M.C.; Lecourieux, D.; Ouaked, F.; Usher, S.L.; Petersen, L.; Okamoto, H.; Knight, H.; Peck, S.C.; Grierson, C.S.; Hirt, H. OXI1 Kinase Is Necessary for Oxidative Burst-Mediated Signalling in Arabidopsis. Nature 2004, 427, 858–861. [Google Scholar] [CrossRef]
- Shah, J. The Salicylic Acid Loop in Plant Defense. Curr. Opin. Plant Biol. 2003, 6, 365–371. [Google Scholar] [CrossRef]
- Seo, P.J.; Lee, A.-K.; Xiang, F.; Park, C.-M. Molecular and Functional Profiling of Arabidopsis Pathogenesis-Related Genes: Insights into Their Roles in Salt Response of Seed Germination. Plant Cell Physiol. 2008, 49, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A Combined Phenotypic and Metabolomic Approach for Elucidating the Biostimulant Action of a Plant-Derived Protein Hydrolysate on Tomato Grown Under Limited Water Availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef]
- Casadesús, A.; Polo, J.; Munné-Bosch, S. Hormonal Effects of an Enzymatically Hydrolyzed Animal Protein-Based Biostimulant (Pepton) in Water-Stressed Tomato Plants. Front. Plant Sci. 2019, 10, 758. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth Stimulatory Effects and Genome-Wide Transcriptional Changes Produced by Protein Hydrolysates in Maize Seedlings. Front. Plant Sci. 2017, 8, 433. [Google Scholar] [CrossRef]
- Lucini, L.; Miras-Moreno, B.; Rouphael, Y.; Cardarelli, M.; Colla, G. Combining Molecular Weight Fractionation and Metabolomics to Elucidate the Bioactivity of Vegetal Protein Hydrolysates in Tomato Plants. Front. Plant Sci. 2020, 11, 976. [Google Scholar] [CrossRef]
- Ebinezer, L.B.; Franchin, C.; Trentin, A.R.; Carletti, P.; Trevisan, S.; Agrawal, G.K.; Rakwal, R.; Quaggiotti, S.; Arrigoni, G.; Masi, A. Quantitative Proteomics of Maize Roots Treated with a Protein Hydrolysate: A Comparative Study with Transcriptomics Highlights the Molecular Mechanisms Responsive to Biostimulants. J. Agric. Food Chem. 2020, 68, 7541–7553. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Rengasamy, K.R.R.; Kulkarni, M.G.; Staden, J. Plant Biostimulants from Seaweed: An Overview. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 31–55. ISBN 978-1-119-35719-3. [Google Scholar]
- EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Guinan, K.J.; Sujeeth, N.; Copeland, R.B.; Jones, P.W.; O’Brien, N.M.; Sharma, H.S.S.; Prouteau, P.F.J.; O’Sullivan, J.T. Discrete Roles for Extracts of Ascophyllum nodosum in Enhancing Plant Growth and Tolerance to Abiotic and Biotic Stresses. Acta Hortic. 2012, 127–135. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed Extracts as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Corrado, G.; Chiaiese, P.; Rouphael, Y. Enhancing Sustainability by Improving Plant Salt Tolerance through Macro- and Micro-Algal Biostimulants. Biology 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Ryu, C.-M. Algae as New Kids in the Beneficial Plant Microbiome. Front. Plant Sci. 2021, 12, 599742. [Google Scholar] [CrossRef]
- Pohl, A.; Kalisz, A.; Sękara, A. Seaweed Extracts’ Multifactorial Action: Influence on Physiological and Biochemical Status of Solanaceae Plants. Acta Agrobot 2019, 72, 12. [Google Scholar] [CrossRef]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Seaweed-Based Compounds and Products for Sustainable Protection against Plant Pathogens. Mar. Drugs 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.C. Gibberellins as Endogenous Growth Regulators in Green and Brown Algae. Planta 1968, 80, 34–42. [Google Scholar] [CrossRef]
- Brain, K.R.; Chalopin, M.C.; Turner, T.D.; Blunden, G.; Wildgoose, P.B. Cytokinin Activity of Commercial Aqueous Seaweed Extract. Plant Sci. Lett. 1973, 1, 241–245. [Google Scholar] [CrossRef]
- Hussain, A.; Boney, A.D. Hydrophilic Growth Inhibitors from Laminaria and Ascophyllum. New Phytol. 1973, 72, 403–410. [Google Scholar] [CrossRef]
- Blunden, G.; Wildgoose, P.B. The Effects of Aqueous Seaweed Extract and Kinetin on Potato Yields. J. Sci. Food Agric. 1977, 28, 121–125. [Google Scholar] [CrossRef]
- Kingman, A.R.; Moore, J. Isolation, Purification and Quantitation of Several Growth Regulating Substances in Ascophyllum nodosum (Phaeophyta). Bot. Mar. 1982, 25, 149–154. [Google Scholar] [CrossRef]
- Featonby-Smith, B.C.; Van Staden, J. The Effect of Seaweed Concentrate and Fertilizer on the Growth of Beta vulgaris. Z. Für Pflanzenphysiol. 1983, 112, 155–162. [Google Scholar] [CrossRef]
- Finnie, J.F.; van Staden, J. Effect of Seaweed Concentrate and Applied Hormones on In Vitro Cultured Tomato Roots. J. Plant Physiol. 1985, 120, 215–222. [Google Scholar] [CrossRef]
- Tay, S.A.B.; Macleod, J.K.; Palni, L.M.S.; Letham, D.S. Detection of Cytokinins in a Seaweed Extract. Phytochemistry 1985, 24, 2611–2614. [Google Scholar] [CrossRef]
- Sanderson, K.J.; Jameson, P.E. The Cytokinins In A Liquid Seaweed Extract: Could They Be The Active Ingredients? In Proceedings of the Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 1986; Volume 179, pp. 113–116. [Google Scholar]
- Sanderson, K.J.; Jameson, P.E.; Zabkiewicz, J.A. Auxin in a Seaweed Extract: Identification and Quantitation of Indole-3-Acetic Acid by Gas Chromatography-Mass Spectrometry. J. Plant Physiol. 1987, 129, 363–367. [Google Scholar] [CrossRef]
- Tay, S.A.B.; Palni, L.M.S.; MacLeod, J.K. Identification of Cytokinin Glucosides in a Seaweed Extract. J. Plant Growth Regul. 1987, 5, 133–138. [Google Scholar] [CrossRef]
- Crouch, I.J.; Smith, M.T.; van Staden, J.; Lewis, M.J.; Hoad, G.V. Identification of Auxins in a Commercial Seaweed Concentrate. J. Plant Physiol. 1992, 139, 590–594. [Google Scholar] [CrossRef]
- Crouch, I.J.; Van Staden, J. Commercial Seaweed Products as Biostimulants in Horticulture. J. Home Consum. Hortic. 1993, 1, 19–76. [Google Scholar] [CrossRef]
- Stirk, W.A.; Van Staden, J. Comparison of Cytokinin- and Auxin-like Activity in Some Commercially Used Seaweed Extracts. J. Appl. Phycol. 1996, 8, 503–508. [Google Scholar] [CrossRef]
- Stirk, W.A.; Van Staden, J. Isolation and Identification of Cytokinins in a New Commercial Seaweed Product Made from Fucus serratus L. J. Appl. Phycol. 1997, 9, 327. [Google Scholar] [CrossRef]
- Stirk, W.A.; Arthur, G.D.; Lourens, A.F.; Novák, O.; Strnad, M.; van Staden, J. Changes in Cytokinin and Auxin Concentrations in Seaweed Concentrates When Stored at an Elevated Temperature. J. Appl. Phycol. 2004, 16, 31. [Google Scholar] [CrossRef]
- Stirk, W.A.; Tarkowská, D.; Turečová, V.; Strnad, M.; Van Staden, J. Abscisic Acid, Gibberellins and Brassinosteroids in Kelpak®, a Commercial Seaweed Extract Made from Ecklonia maxima. J. Appl. Phycol. 2014, 26, 561–567. [Google Scholar] [CrossRef]
- Górka, B.; Wieczorek, P.P. Simultaneous Determination of Nine Phytohormones in Seaweed and Algae Extracts by HPLC-PDA. J. Chromatogr. B 2017, 1057, 32–39. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of Seaweed Extracts From Laminaria and Ascophyllum nodosum spp. as Biostimulants in Zea mays L. Using a Combination of Chemical, Biochemical and Morphological Approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef]
- Taylor, I.E.P.; Wilkinson, A.J. The Occurrence of Gibberellins and Gibberellin-like Substances in Algae. Phycologia 1977, 16, 37–42. [Google Scholar] [CrossRef]
- Stirk, W.A.; Novák, O.; Hradecká, V.; Pĕnčík, A.; Rolčík, J.; Strnad, M.; Van Staden, J. Endogenous Cytokinins, Auxins and Abscisic Acid in Ulva fasciata (Chlorophyta) and Dictyota humifusa (Phaeophyta): Towards Understanding Their Biosynthesis and Homoeostasis. Eur. J. Phycol. 2009, 44, 231–240. [Google Scholar] [CrossRef]
- Wally, O.S.D.; Critchley, A.T.; Hiltz, D.; Craigie, J.S.; Han, X.; Zaharia, L.I.; Abrams, S.R.; Prithiviraj, B. Regulation of Phytohormone Biosynthesis and Accumulation in Arabidopsis Following Treatment with Commercial Extract from the Marine Macroalga Ascophyllum nodosum. J. Plant Growth Regul. 2013, 32, 324–339. [Google Scholar] [CrossRef]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the Molecular Understanding of the Action Mechanism of Ascophyllum nodosum Extracts on Plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Rayorath, P.; Jithesh, M.N.; Farid, A.; Khan, W.; Palanisamy, R.; Hankins, S.D.; Critchley, A.T.; Prithiviraj, B. Rapid Bioassays to Evaluate the Plant Growth Promoting Activity of Ascophyllum nodosum (L.) Le Jol. Using a Model Plant, Arabidopsis thaliana (L.) Heynh. J. Appl. Phycol. 2008, 20, 423–429. [Google Scholar] [CrossRef]
- Khan, W.; Hiltz, D.; Critchley, A.T.; Prithiviraj, B. Bioassay to Detect Ascophyllum nodosum Extract-Induced Cytokinin-like Activity in Arabidopsis thaliana. J. Appl. Phycol. 2011, 23, 409–414. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed Extract Stimuli in Plant Science and Agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Ghaderiardakani, F.; Collas, E.; Damiano, D.K.; Tagg, K.; Graham, N.S.; Coates, J.C. Effects of Green Seaweed Extract on Arabidopsis Early Development Suggest Roles for Hormone Signalling in Plant Responses to Algal Fertilisers. Sci. Rep. 2019, 9, 1983. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.H.S.; Lyons, G.; McRoberts, C.; McCall, D.; Carmichael, E.; Andrews, F.; McCormack, R. Brown Seaweed Species from Strangford Lough: Compositional Analyses of Seaweed Species and Biostimulant Formulations by Rapid Instrumental Methods. J. Appl. Phycol. 2012, 24, 1141–1157. [Google Scholar] [CrossRef]
- Omidbakhshfard, M.A.; Sujeeth, N.; Gupta, S.; Omranian, N.; Guinan, K.J.; Brotman, Y.; Nikoloski, Z.; Fernie, A.R.; Mueller-Roeber, B.; Gechev, T.S. A Biostimulant Obtained from the Seaweed Ascophyllum nodosum Protects Arabidopsis thaliana from Severe Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 474. [Google Scholar] [CrossRef] [PubMed]
- Staykov, N.S.; Angelov, M.; Petrov, V.; Minkov, P.; Kanojia, A.; Guinan, K.J.; Alseekh, S.; Fernie, A.R.; Sujeeth, N.; Gechev, T.S. An Ascophyllum nodosum-Derived Biostimulant Protects Model and Crop Plants from Oxidative Stress. Metabolites 2021, 11, 24. [Google Scholar] [CrossRef]
- Rasul, F.; Gupta, S.; Olas, J.J.; Gechev, T.; Sujeeth, N.; Mueller-Roeber, B. Priming with a Seaweed Extract Strongly Improves Drought Tolerance in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 1469. [Google Scholar] [CrossRef]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A Concise Review of the Brown Macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Critchley, A.T. A Review of Multiple Biostimulant and Bioeffector Benefits of AMPEP, an Extract of the Brown Alga Ascophyllum nodosum, as Applied to the Enhanced Cultivation and Micropropagation of the Commercially Important Red Algal Carrageenophyte Kappaphycus alvarezii and Its Selected Cultivars. J. Appl. Phycol. 2018, 30, 2859–2873. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant- and Seaweed-Based Extracts Increase Yield but Differentially Modulate Nutritional Quality of Greenhouse Spinach through Biostimulant Action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Taskos, D.; Stamatiadis, S.; Yvin, J.-C.; Jamois, F. Effects of an Ascophyllum nodosum (L.) Le Jol. Extract on Grapevine Yield and Berry Composition of a Merlot Vineyard. Sci. Hortic. 2019, 250, 27–32. [Google Scholar] [CrossRef]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a Biostimulant Derived from the Brown Seaweed Ascophyllum nodosum on Ripening Dynamics and Fruit Quality of Grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- Gonçalves, B.; Morais, M.C.; Sequeira, A.; Ribeiro, C.; Guedes, F.; Silva, A.P.; Aires, A. Quality Preservation of Sweet Cherry Cv. “staccato” by Using Glycine-Betaine or Ascophyllum nodosum. Food Chem. 2020, 322, 126713. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Queirós, F.; Ferreira, H.; Morais, M.C.; Afonso, S.; Silva, A.P.; Gonçalves, B. Foliar Application of Calcium and Growth Regulators Modulate Sweet Cherry (Prunus avium L.) Tree Performance. Plants 2020, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Santos, M.; Glińska, S.; Gapińska, M.; Matos, M.; Carnide, V.; Schouten, R.; Silva, A.P.; Gonçalves, B. Effects of Exogenous Compound Sprays on Cherry Cracking: Skin Properties and Gene Expression. J. Sci. Food Agric. 2020, 100, 2911–2921. [Google Scholar] [CrossRef]
- Pohl, A.; Grabowska, A.; Kalisz, A.; Sękara, A. Biostimulant Application Enhances Fruit Setting in Eggplant—An Insight into the Biology of Flowering. Agronomy 2019, 9, 482. [Google Scholar] [CrossRef]
- Shukla, P.S.; Prithiviraj, B. Ascophyllum Nodosum Biostimulant Improves the Growth of Zea mays Grown Under Phosphorus Impoverished Conditions. Front. Plant Sci. 2021, 11, 601843. [Google Scholar] [CrossRef] [PubMed]
- Carmody, N.; Goñi, O.; Łangowski, Ł.; O’Connell, S. Ascophyllum nodosum Extract Biostimulant Processing and Its Impact on Enhancing Heat Stress Tolerance During Tomato Fruit Set. Front. Plant Sci. 2020, 11, 807. [Google Scholar] [CrossRef] [PubMed]
- do Rosário Rosa, V.; Farias dos Santos, A.L.; Alves da Silva, A.; Peduti Vicentini Sab, M.; Germino, G.H.; Barcellos Cardoso, F.; de Almeida Silva, M. Increased Soybean Tolerance to Water Deficiency through Biostimulant Based on Fulvic Acids and Ascophyllum nodosum (L.) Seaweed Extract. Plant Physiol. Biochem. 2021, 158, 228–243. [Google Scholar] [CrossRef]
- Cabo, S.; Morais, M.C.; Aires, A.; Carvalho, R.; Pascual-Seva, N.; Silva, A.P.; Gonçalves, B. Kaolin and Seaweed-based Extracts Can Be Used as Middle and Long-term Strategy to Mitigate Negative Effects of Climate Change in Physiological Performance of Hazelnut Tree. J. Agron. Crop Sci. 2020, 206, 28–42. [Google Scholar] [CrossRef]
- Cabo, S.; Aires, A.; Carvalho, R.; Vilela, A.; Pascual-Seva, N.; Silva, A.P.; Gonçalves, B. Kaolin, Ascophyllum nodosum and Salicylic Acid Mitigate Effects of Summer Stress Improving Hazelnut Quality. J. Sci. Food Agric. 2021, 101, 459–475. [Google Scholar] [CrossRef]
- Dell’Aversana, E.; Cirillo, V.; Van Oosten, M.J.; Di Stasio, E.; Saiano, K.; Woodrow, P.; Ciarmiello, L.F.; Maggio, A.; Carillo, P. Ascophyllum nodosum Based Extracts Counteract Salinity Stress in Tomato by Remodeling Leaf Nitrogen Metabolism. Plants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Kerchev, P.; van der Meer, T.; Sujeeth, N.; Verlee, A.; Stevens, C.V.; Van Breusegem, F.; Gechev, T. Molecular Priming as an Approach to Induce Tolerance against Abiotic and Oxidative Stresses in Crop Plants. Biotechnol. Adv. 2020, 40, 107503. [Google Scholar] [CrossRef]
- Di Stasio, E.; Cirillo, V.; Raimondi, G.; Giordano, M.; Esposito, M.; Maggio, A. Osmo-Priming with Seaweed Extracts Enhances Yield of Salt-Stressed Tomato Plants. Agronomy 2020, 10, 1559. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Hara, P.; Treder, K.; Findura, P.; Bartoš, P.; Filip, M. Biochemical and Economical Effect of Application Biostimulants Containing Seaweed Extracts and Amino Acids as an Element of Agroecological Management of Bean Cultivation. Sci. Rep. 2020, 10, 17759. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Rengasamy, K.R.R.; Pendota, S.C.; Gruz, J.; Plačková, L.; Novák, O.; Doležal, K.; Van Staden, J. Bioactive Molecules Derived from Smoke and Seaweed Ecklonia maxima Showing Phytohormone-like Activity in Spinacia oleracea L. New Biotechnol. 2019, 48, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; El-Nakhel, C.; Leone, V.; Mori, M. Effect of Seaweed (Ecklonia maxima) Extract and Legume-Derived Protein Hydrolysate Biostimulants on Baby Leaf Lettuce Grown on Optimal Doses of Nitrogen under Greenhouse Conditions. Aust. J. Crop Sci. 2020, 1456–1464. [Google Scholar] [CrossRef]
- Rouphael, Y.; De Micco, V.; Arena, C.; Raimondi, G.; Colla, G.; De Pascale, S. Effect of Ecklonia maxima Seaweed Extract on Yield, Mineral Composition, Gas Exchange, and Leaf Anatomy of Zucchini Squash Grown under Saline Conditions. J. Appl. Phycol. 2017, 29, 459–470. [Google Scholar] [CrossRef]
- Bindu, M.S.; Levine, I.A. The Commercial Red Seaweed Kappaphycus alvarezii—An Overview on Farming and Environment. J. Appl. Phycol. 2011, 23, 789–796. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Getachew, A.T.; Chun, B.-S. Characteristics of Functional Materials Recovered from Solomon Islands Red Seaweed (Kappaphycus alvarezii) Using Pressurized Hot Water Extraction. J. Appl. Phycol. 2017, 29, 1609–1621. [Google Scholar] [CrossRef]
- Rudke, A.R.; de Andrade, C.J.; Ferreira, S.R.S. Kappaphycus alvarezii Macroalgae: An Unexplored and Valuable Biomass for Green Biorefinery Conversion. Trends Food Sci. Technol. 2020, 103, 214–224. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.K.; Pal, S.K.; Trivedi, K.; Yesuraj, D.; Singh, C.S.; Anand, K.G.V.; Chandramohan, M.; Patidar, R.; Kubavat, D.; et al. Sustainable Enhancement in Yield and Quality of Rain-Fed Maize through Gracilaria edulis and Kappaphycus alvarezii Seaweed Sap. J. Appl. Phycol. 2016, 28, 2099–2112. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Shanmugam, M. The Effect of Potassium-Rich Biostimulant from Seaweed Kappaphycus alvarezii on Yield and Quality of Cane and Cane Juice of Sugarcane Var. Co 86032 under Plantation and Ratoon Crops. J. Appl. Phycol. 2017, 29, 3245–3252. [Google Scholar] [CrossRef]
- Trivedi, K.; Vijay Anand, K.G.; Kubavat, D.; Patidar, R.; Ghosh, A. Drought Alleviatory Potential of Kappaphycus Seaweed Extract and the Role of the Quaternary Ammonium Compounds as Its Constituents towards Imparting Drought Tolerance in Zea mays L. J. Appl. Phycol. 2018, 30, 2001–2015. [Google Scholar] [CrossRef]
- Trivedi, K.; Vijay Anand, K.G.; Vaghela, P.; Ghosh, A. Differential Growth, Yield and Biochemical Responses of Maize to the Exogenous Application of Kappaphycus alvarezii Seaweed Extract, at Grain-Filling Stage under Normal and Drought Conditions. Algal Res. 2018, 35, 236–244. [Google Scholar] [CrossRef]
- Basavaraja, P.K.; Yogendra, N.D.; Zodape, S.T.; Prakash, R.; Ghosh, A. Effect of Seaweed Sap as Foliar Spray on Growth and Yield of Hybrid Maize. J. Plant Nutr. 2018, 41, 1851–1861. [Google Scholar] [CrossRef]
- Sharma, L.; Banerjee, M.; Malik, G.C.; Gopalakrishnan, V.A.K.; Zodape, S.T.; Ghosh, A. Sustainable Agro-Technology for Enhancement of Rice Production in the Red and Lateritic Soils Using Seaweed Based Biostimulants. J. Clean. Prod. 2017, 149, 968–975. [Google Scholar] [CrossRef]
- Layek, J.; Das, A.; Idapuganti, R.G.; Sarkar, D.; Ghosh, A.; Zodape, S.T.; Lal, R.; Yadav, G.S.; Panwar, A.S.; Ngachan, S.; et al. Seaweed Extract as Organic Bio-Stimulant Improves Productivity and Quality of Rice in Eastern Himalayas. J. Appl. Phycol. 2018, 30, 547–558. [Google Scholar] [CrossRef]
- Pramanick, B.; Brahmachari, K.; Mahapatra, B.S.; Ghosh, A.; Ghosh, D.; Kar, S. Growth, Yield and Quality Improvement of Potato Tubers through the Application of Seaweed Sap Derived from the Marine Alga Kappaphycus alvarezii. J. Appl. Phycol. 2017, 29, 3253–3260. [Google Scholar] [CrossRef]
- Garai, S.; Brahmachari, K.; Sarkar, S.; Mondal, M.; Banerjee, H.; Nanda, M.K.; Chakravarty, K. Impact of Seaweed Sap Foliar Application on Growth, Yield, and Tuber Quality of Potato (Solanum tuberosum L.). J. Appl. Phycol. 2021, 33, 1893–1904. [Google Scholar] [CrossRef]
- Patel, K.; Agarwal, P.; Agarwal, P.K. Kappaphycus alvarezii Sap Mitigates Abiotic-Induced Stress in Triticum durum by Modulating Metabolic Coordination and Improves Growth and Yield. J. Appl. Phycol. 2018, 30, 2659–2673. [Google Scholar] [CrossRef]
- Kumar, R.; Trivedi, K.; Anand, K.G.V.; Ghosh, A. Science behind Biostimulant Action of Seaweed Extract on Growth and Crop Yield: Insights into Transcriptional Changes in Roots of Maize Treated with Kappaphycus alvarezii Seaweed Extract under Soil Moisture Stressed Conditions. J. Appl. Phycol. 2020, 32, 599–613. [Google Scholar] [CrossRef]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for Beneficial Microorganisms Inocula Used as Biofertilizers. Sci. World J. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Malusá, E.; Vassilev, N. A Contribution to Set a Legal Framework for Biofertilisers. Appl. Microbiol. Biotechnol. 2014, 98, 6599–6607. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V. Biofertilizers and Biopesticides in Sustainable Agriculture. In Role of Rhizospheric Microbes in Soil; Meena, V.S., Ed.; Springer: Singapore, 2018; pp. 377–398. ISBN 978-981-10-8401-0. [Google Scholar]
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2020; Volume 162, pp. 31–87. ISBN 978-0-12-820767-3. [Google Scholar]
- Nuti, M.; Giovannetti, G. Borderline Products between Bio-Fertilizers/ Bio-Effectors and Plant Protectants: The Role of Microbial Consortia. J. Agric. Sci. Technol. A 2015, 5, 305–315. [Google Scholar] [CrossRef]
- Mickan, B.S.; Alsharmani, A.R.; Solaiman, Z.M.; Leopold, M.; Abbott, L.K. Plant-Dependent Soil Bacterial Responses Following Amendment With a Multispecies Microbial Biostimulant Compared to Rock Mineral and Chemical Fertilizers. Front. Plant Sci. 2021, 11, 550169. [Google Scholar] [CrossRef] [PubMed]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The Importance of the Microbiome of the Plant Holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Jha, C.K.; Saraf, M. Plant Growth Promoting Rhizobacteria (PGPR): A Review. J. Agric. Res. Dev. 2015, 5, 108–119. [Google Scholar]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant Growth Promoting Bacteria in Agriculture: Two Sides of a Coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant Growth-Promoting Rhizobacteria Act as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, H.; Zhao, D.; Zhu, X.; Wang, Y.; Duan, Y.; Xuan, Y.; Chen, L. Isolation and Identification of Bacteria from Rhizosphere Soil and Their Effect on Plant Growth Promotion and Root-Knot Nematode Disease. Biol. Control 2018, 119, 12–19. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Barros-Rodríguez, A.; Rangseekaew, P.; Lasudee, K.; Pathom-aree, W.; Manzanera, M. Regulatory Risks Associated with Bacteria as Biostimulants and Biofertilizers in the Frame of the European Regulation (EU) 2019/1009. Sci. Total Environ. 2020, 740, 140239. [Google Scholar] [CrossRef]
- Jacobsen, B.J.; Zidack, N.K.; Larson, B.J. The Role of Bacillus -Based Biological Control Agents in Integrated Pest Management Systems: Plant Diseases. Phytopathology® 2004, 94, 1272–1275. [Google Scholar] [CrossRef]
- Mahmood, F.; Shahid, M.; Hussain, S.; Shahzad, T.; Tahir, M.; Ijaz, M.; Hussain, A.; Mahmood, K.; Imran, M.; Babar, S.A.K. Potential Plant Growth-Promoting Strain Bacillus Sp. SR-2-1/1 Decolorized Azo Dyes through NADH-Ubiquinone:Oxidoreductase Activity. Bioresour. Technol. 2017, 235, 176–184. [Google Scholar] [CrossRef]
- Caulier, S.; Gillis, A.; Colau, G.; Licciardi, F.; Liépin, M.; Desoignies, N.; Modrie, P.; Legrève, A.; Mahillon, J.; Bragard, C. Versatile Antagonistic Activities of Soil-Borne Bacillus spp. and Pseudomonas spp. against Phytophthora Infestans and Other Potato Pathogens. Front. Microbiol. 2018, 9, 143. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- He, Y.; Pantigoso, H.A.; Wu, Z.; Vivanco, J.M. Co-inoculation of Bacillus sp. and Pseudomonas putida at Different Development Stages Acts as a Biostimulant to Promote Growth, Yield and Nutrient Uptake of Tomato. J. Appl. Microbiol. 2019, 127, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.L.; Glaes, J.; Spaepen, S.; Bodson, B.; du Jardin, P.; Delaplace, P. Biostimulant Effects of Bacillus Strains on Wheat from in Vitro towards Field Conditions Are Modulated by Nitrogen Supply. J. Plant Nutr. Soil Sci. 2019, 182, 325–334. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, J.; Liu, Y.; Chen, S. Isolation and Identification of Nitrogen-Fixing Bacilli from Plant Rhizospheres in Beijing Region. J. Appl. Microbiol. 2005, 99, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Kuan, K.B.; Othman, R.; Abdul Rahim, K.; Shamsuddin, Z.H. Plant Growth-Promoting Rhizobacteria Inoculation to Enhance Vegetative Growth, Nitrogen Fixation and Nitrogen Remobilisation of Maize under Greenhouse Conditions. PLoS ONE 2016, 11, e0152478. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.L.; Spaepen, S.; du Jardin, P.; Delaplace, P. Biostimulant Effects of Rhizobacteria on Wheat Growth and Nutrient Uptake Depend on Nitrogen Application and Plant Development. Arch. Agron. Soil Sci. 2019, 65, 58–73. [Google Scholar] [CrossRef]
- Mantelin, S.; Touraine, B. Plant Growth-Promoting Bacteria and Nitrate Availability: Impacts on Root Development and Nitrate Uptake. J. Exp. Bot. 2004, 55, 27–34. [Google Scholar] [CrossRef]
- Xie, S.-S.; Wu, H.-J.; Zang, H.-Y.; Wu, L.-M.; Zhu, Q.-Q.; Gao, X.-W. Plant Growth Promotion by Spermidine-Producing Bacillus subtilis OKB105. Mol. Plant-Microbe Interact. ® 2014, 27, 655–663. [Google Scholar] [CrossRef]
- Kim, M.-J.; Radhakrishnan, R.; Kang, S.-M.; You, Y.-H.; Jeong, E.-J.; Kim, J.-G.; Lee, I.-J. Plant Growth Promoting Effect of Bacillus amyloliquefaciens H-2-5 on Crop Plants and Influence on Physiological Changes in Soybean under Soil Salinity. Physiol. Mol. Biol. Plants 2017, 23, 571–580. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wu, G.; Veronican Njeri, K.; Shen, Q.; Zhang, N.; Zhang, R. Induced Maize Salt Tolerance by Rhizosphere Inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016, 158, 34–44. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of High Salinity Stress Damage by Plant Growth-Promoting Bacterial Endophytes That Contain ACC Deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef]
- Santoro, M.V.; Cappellari, L.R.; Giordano, W.; Banchio, E. Plant Growth-Promoting Effects of Native Pseudomonas Strains on Mentha piperita (Peppermint): An in Vitro Study. Plant Biol. 2015, 17, 1218–1226. [Google Scholar] [CrossRef]
- Patel, T.; Saraf, M. Biosynthesis of Phytohormones from Novel Rhizobacterial Isolates and Their in Vitro Plant Growth-Promoting Efficacy. J. Plant Interact. 2017, 12, 480–487. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Novinscak, A.; Filion, M. Pseudomonas fluorescens LBUM677 Differentially Increases Plant Biomass, Total Oil Content and Lipid Composition in Three Oilseed Crops. J. Appl. Microbiol. 2020, 128, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Ngoroyemoto, N.; Kulkarni, M.G.; Stirk, W.A.; Gupta, S.; Finnie, J.F.; Staden, J. van Interactions Between Microorganisms and a Seaweed-Derived Biostimulant on the Growth and Biochemical Composition of Amaranthus hybridus L. Nat. Prod. Commun. 2020, 15, 1934578X20934228. [Google Scholar] [CrossRef]
- Al-Garni, S.M.S.; Khan, M.M.A.; Bahieldin, A. Plant Growth-Promoting Bacteria and Silicon Fertilizer Enhance Plant Growth and Salinity Tolerance in Coriandrum sativum. J. Plant Interact. 2019, 14, 386–396. [Google Scholar] [CrossRef]
- Fan, D.; Subramanian, S.; Smith, D.L. Plant Endophytes Promote Growth and Alleviate Salt Stress in Arabidopsis thaliana. Sci. Rep. 2020, 10, 12740. [Google Scholar] [CrossRef] [PubMed]
- Amrutha, R.N.; Pallaval Veera Bramhachari; Prakasham, R.S. Quorum-Sensing Mechanism in Rhizobium sp.: Revealing Complexity in a Molecular Dialogue. In Implication of Quorum Sensing System in Biofilm Formation and Virulence; Pallaval Veera Bramhachari, Ed.; Springer: Singapore, 2018; pp. 249–258. ISBN 9789811324284. [Google Scholar]
- Rafique, M.; Naveed, M.; Mustafa, A.; Akhtar, S.; Munawar, M.; Kaukab, S.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z.M. The Combined Effects of Gibberellic Acid and Rhizobium on Growth, Yield and Nutritional Status in Chickpea (Cicer arietinum L.). Agronomy 2021, 11, 105. [Google Scholar] [CrossRef]
- Widawati, S. Suliasih Comprehensive Test of Rhizobacteria as Biostimulant, Vesicular Arbuscular Mycorhizza (VAM) and Graded Dose of NPK Fertilizer on the Growth of Bok Choy ( Brassica rapa L.). IOP Conf. Ser. Earth Environ. Sci. 2020, 572, 012023. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; De Pascale, S.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Stand-Alone and Combinatorial Effects of Plant-Based Biostimulants on the Production and Leaf Quality of Perennial Wall Rocket. Plants 2020, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Singh, A.; Dwivedi, D.H.; Arora, N.K. Zinc and Phosphate Solubilizing Rhizobium radiobacter (LB2) for Enhancing Quality and Yield of Loose Leaf Lettuce in Saline Soil. Environ. Sustain. 2020, 3, 209–218. [Google Scholar] [CrossRef]
- Ochieno, D.M.W.; Karoney, E.M.; Muge, E.K.; Nyaboga, E.N.; Baraza, D.L.; Shibairo, S.I.; Naluyange, V. Rhizobium-Linked Nutritional and Phytochemical Changes Under Multitrophic Functional Contexts in Sustainable Food Systems. Front. Sustain. Food Syst. 2021, 4, 283. [Google Scholar] [CrossRef]
- Hernández-Soberano, C.; Ruíz-Herrera, L.F.; Valencia-Cantero, E. Endophytic Bacteria Arthrobacter agilis UMCV2 and Bacillus methylotrophicus M4-96 Stimulate Achene Germination, in Vitro Growth, and Greenhouse Yield of Strawberry (Fragaria × ananassa). Sci. Hortic. 2020, 261, 109005. [Google Scholar] [CrossRef]
- Hernández-Calderón, E.; Aviles-Garcia, M.E.; Castulo-Rubio, D.Y.; Macías-Rodríguez, L.; Ramírez, V.M.; Santoyo, G.; López-Bucio, J.; Valencia-Cantero, E. Volatile Compounds from Beneficial or Pathogenic Bacteria Differentially Regulate Root Exudation, Transcription of Iron Transporters, and Defense Signaling Pathways in Sorghum bicolor. Plant Mol. Biol. 2018, 96, 291–304. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as Biostimulant: Exploiting the Multilevel Properties of a Plant Beneficial Fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-Beneficial Effects of Trichoderma and of Its Genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-Based Products and Their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L. Trichoderma: A Multi-Purpose Tool for Integrated Pest Management. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 345–353. ISBN 978-3-319-08574-6. [Google Scholar]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-Based Biostimulants Optimize N Use Efficiency and Stimulate Growth of Leafy Vegetables in Greenhouse Intensive Cropping Systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef]
- Marra, R.; Lombardi, N.; d’Errico, G.; Troisi, J.; Scala, G.; Vinale, F.; Woo, S.L.; Bonanomi, G.; Lorito, M. Application of Trichoderma Strains and Metabolites Enhances Soybean Productivity and Nutrient Content. J. Agric. Food Chem. 2019, 67, 1814–1822. [Google Scholar] [CrossRef]
- Silletti, S.; Di Stasio, E.; Van Oosten, M.J.; Ventorino, V.; Pepe, O.; Napolitano, M.; Marra, R.; Woo, S.L.; Cirillo, V.; Maggio, A. Biostimulant Activity of Azotobacter chroococcum and Trichoderma harzianum in Durum Wheat under Water and Nitrogen Deficiency. Agronomy 2021, 11, 380. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-Based Biostimulants Modulate Rhizosphere Microbial Populations and Improve N Uptake Efficiency, Yield, and Nutritional Quality of Leafy Vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.; Milagrosa, S.; Francisco, C.; Francisco, M. Biostimulant Activity of Trichoderma saturnisporum in Melon (Cucumis melo). HortScience 2018, 53, 810–815. [Google Scholar] [CrossRef]
- Lombardi, N.; Caira, S.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Salzano, A.M.; Lorito, M.; Woo, S.L. Trichoderma Applications on Strawberry Plants Modulate the Physiological Processes Positively Affecting Fruit Production and Quality. Front. Microbiol. 2020, 11, 1364. [Google Scholar] [CrossRef]
- Devi, S.S.; Sreenivasulu, Y.; Rao, K.V. Protective Role of Trichoderma logibrachiatum (WT2) on Lead Induced Oxidative Stress in Helianthus annus L. Indian J. Exp. Biol. 2017, 55, 235–241. [Google Scholar]
- Abou-Shanab, R.A.; Ghanem, K.; Ghanem, N.; Al-Kolaibe, A. The Role of Bacteria on Heavy-Metal Extraction and Uptake by Plants Growing on Multi-Metal-Contaminated Soils. World J. Microbiol. Biotechnol. 2008, 24, 253–262. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The Effect of Trichoderma harzianum in Mitigating Low Temperature Stress in Tomato (Solanum lycopersicum L.) Plants. Sci. Hortic. 2018, 230, 134–141. [Google Scholar] [CrossRef]
- Oljira, A.M.; Hussain, T.; Waghmode, T.R.; Zhao, H.; Sun, H.; Liu, X.; Wang, X.; Liu, B. Trichoderma Enhances Net Photosynthesis, Water Use Efficiency, and Growth of Wheat (Triticum aestivum L.) under Salt Stress. Microorganisms 2020, 8, 1565. [Google Scholar] [CrossRef] [PubMed]
| Biostimulant | Plant Species | Plant Response | References |
|---|---|---|---|
| Humic acids | Achillea millefolium L. | Improved growth parameters: increased photosynthetic pigments, total phenols, total flavonoids and antioxidant activity of the leaves and flowers were increased significantly. | [69] |
| Arabidopsis thaliana | Enhanced thermotolerance by upregulation of heat-shock protein genes under heat stress; Increased concentrations of proteins related to cell wall and energy metabolism, respiration, protein synthesis, protein folding, protein degradation, response to inorganic substances and heat and cell trafficking and division; decreased concentration of carbohydrates and amino acids. | [44,45,58] | |
| Brassica napus | Increase in yield, chlorophyll content; improved oil quality, plant net photosynthesis, gas exchange rate and electron transport flux; decrease in soluble carbohydrates, linolenic and erucic acid. | [39,54] | |
| Capsicum annuum L. | Improved root development and increased plant biomass under drought stress while rapidly decreased leaf stomatal conductance and transpiration rates; increased chlorophyll content leading to improved net photosynthesis. | [51] | |
| Echinacea purpurea L. | Improved plant growth under drought stress; increased flavonoid, phenolic and proline concentration; increased relative water content and photosynthetic pigments concentration. | [50] | |
| Hordeum vulgare L. | Increase in photosynthetic pigment concentration and NPK levels; improved plant growth and yield parameters under drought stress. | [52] | |
| Phaseolus vulgaris L. | Protective effects against DNA hypomethylation and damage; alterations in the expression of stress-related genes. | [60] | |
| Rhododendron | Increase in phenolic content, flavonoids, soluble carbohydrates, starch and soluble proteins; upregulation of peroxidase genes (POD1). | [38] | |
| Solanum tuberosum | Increase in tuber yield and plant biomass; improved plant growth, nutrient transport and photosynthetic parameters under drought stress. | [53] | |
| Zea mays | Improved water and nitrogen efficiency; upregulation of genes related to water transport, nutrient absorption and nitrate transporters. | [57] | |
| Fulvic acids | Achillea millefolium L. | Improved growth parameters: increased photosynthetic pigments, total phenols, total flavonoids and antioxidant activity of the leaves and flowers were increased significantly. | [69] |
| Beta vulgaris | Improved germination parameters; increased root size, yield and soluble sugar content. | [61] | |
| Camellia sinensis L. | Upregulation of genes related to metabolism of ascorbate and glutathione, and biosynthesis of flavonoids improving the antioxidant defense under water stress; increased leaf water content and chlorophyll content; reduction in accumulation of ROS. | [67] | |
| Hordeum vulgare | Improved germination parameters. | [61] | |
| Medicago sativa | Upregulation of genes related to early nodulation signaling, N metabolism, nutrient transporters and hydrolases; increased total yield and plant biomass. | [63] | |
| Monoraphidium sp. | Upregulation of lipid biosynthesis genes; increased protein concentration and chlorophyll content. | [64] | |
| Paeonia ostii | Amelioration of drought stress effects; increased plants’ RWC; increased activity of antioxidant enzymes (SOD, CAT and POD) leading to lower ROS concentration; increased photosynthetic parameters; maintained the integrity of mesophyll cell ultrastructure and chloroplasts; increased expression of drought-tolerance genes. | [68] | |
| Triticum aestivum L. | Improved germination parameters; increased yield and grain quality. | [61] | |
| Protein hydrolysates | Brassica oleracea | Improved photosynthetic rate and stomatal conductance under drought stress; amelioration of the negative effects of stress in gas exchange and transpiration rates. | [84] |
| Diplotaxis tenuifolia L. | Increased plant biomass, yield and chlorophyll biosynthesis; improved photosynthetic rate and leaf antioxidant activity; increased nutrient and organic acid concentration. | [83] | |
| Lactuca sativa L. | Stimulated the growth of plant growth promoting bacteria leading to increases in leaf chlorophyll and plant biomass. | [81] | |
| Olea europaea | Improved photosynthetic rate and stomatal conductance; increased plant growth and biomass; had a lasting positive effect in the sink/source ratio. | [82] | |
| Solanum lycopersicon L. | Increased plant biomass, chlorophyll and phenolic content and soluble sugars concentration; improved photosynthetic rate, root growth and upregulation of genes related to antioxidant activity, photosynthesis, nutrient uptake and primary metabolisms. Increased phenylpropanoids, terpenes, nitrogen-containing compounds, glucosinates and alkaloids. Under different N regimes, improved photosynthetic rates and N content in leaves; upregulation of genes related to amino acid and N transport. Under drought stress, increased plant biomass, transpiration rates and stomatal conductance; improved redox status of treated plants and tolerance to ROS-mediated oxidative imbalance; increased antioxidant protection, IAA, cytokinins and jasmonic acid concentrations. | [85,87,93,94,96] | |
| Zea mays | Enhanced plant stress response; improved root growth; increased expression of nitrate transporters and ROS response genes; increased transport and root accumulation of nutrients; upregulation of genes involved in nutrient transport, hormone metabolism, transport and cytoskeletal reorganization; induced changes at the transcriptomic and proteomic level. | [88,95,97] | |
| Seaweed extracts (Ascophyllum nodosum) | Arabidopsis thaliana | Oxidative and drought stress tolerance, reduced accumulation of ROS and cell damage; downregulation of genes related to growth impairment during stress; upregulation of ROS scavengers, cell cycle and cell division genes. | [135,136,137] |
| Corylus avellana | Increased plant RWC and CO2 assimilation; improved plant water use efficiency; reduced electrolyte leakage, membrane lipid peroxidation, antioxidant enzymes and proline content; increased fruit biometric parameters, antioxidant activity vitamin E, soluble sugars and phenolics content. | [150,151] | |
| Glycine max L. | Under drought stress, increased stomatal conductance, photosynthetic activity and efficiency, chlorophyll content and antioxidant activity; improved root growth and photoassimilates production. | [149] | |
| Lycopersicon esculentum | Under normal and high temperatures, improved thermo tolerance, pollen viability and photosynthetic parameters; increased fruit number and chlorophyll content; upregulation and downregulation of HSP genes; in addition, improved fruit yield components under normal and salt stress conditions | [148,154] | |
| Prunus avium L. | Increased plant yield, RWC, photosynthetic pigments, soluble sugars and protein concentration; improved gas exchange and water use efficiency; reduced fruit cracking; increased fruit size, soluble solids content, polyphenols, vitamin C and antioxidant potential; improved fruit quality, acidity, color parameters and ripening process; up-regulation of genes related to cell-wall and cuticular waxes. | [143,144,145] | |
| Solanum melongena L. | Increased the number of pollen tubes and fertilized ovules; improved flowering and fruiting of the plants. | [146] | |
| Spinacia oleracea L. | Increase plant biomass, protein and nutrient content and concentration of phenolic compounds in the leaves; improved chlorophyll synthesis and photosynthetic rate; enhanced nutritional value. | [140] | |
| Vitis vinifera L. | Increased plant biomass, yield, N and soluble sugar concentration; increased berry number, anthocyanins and phenolics concentration, without negatively affecting their quality. | [141,142] | |
| Zea mays | Under limited phosphorus conditions, improved plant growth; increased plant biomass, NPK, photosynthetic pigments, total soluble sugars, phenolic compounds, flavonoids and amino acids content; diminished oxidative damage and electrolyte leakage; positively affected the expression of genes related to P homeostasis. | [147] | |
| Seaweed extracts (Ecklonia maxima) | Cucurbita pepo L. | Under salt-stress, increased yield and plant biomass; improved fruit quality and nutritional status, photosynthetic parameters and pigment synthesis; decreased oxidative stress. | [158] |
| Lactuca sativa L. | Under sub-optimal N concentration in the soil, increased yield, chlorophyll and carotenoids content; enhanced photosynthetic parameters and antioxidant activity. | [157] | |
| Phaseolus vulgaris L. | Increased yield and antioxidant activity; increased biosynthesis of phenolics, flavonoids and anthocyanins; improved nutritional quality of the seeds. | [155] | |
| Spinacia oleracea L. | Improved plant growth, yield and nutritional quality; increased concentration of chlorophyll, carotenoids, protein content and phytohormones; promoted activity of enzymes related to compound biosynthesis. | [156] | |
| Seaweed extracts (Kappaphycus alvarezii) | Oryza sativa | Increased yield parameters, grain number, protein and nutrient content in the grain, plant biomass and chlorophyll content; improved germination, seedling vigor and root growth. | [167,168] |
| Saccharum officinarum | Increased plant yield and brix content of the juice; improved plant growth. | [163] | |
| Solanum tuberosum L. | Improved plant growth parameters; increased yield, yield quality, nutrient concentration and ascorbic acid and soluble sugar content. | [169,170] | |
| Triticum durum | Increased plant growth, root growth, photosynthetic pigments content, RWC, proline, amino acids and soluble sugars content; improved plant stress tolerance; upregulation of stress response genes (WCK-1, TaWRKY10, TdCAT and TdSOD). | [171] | |
| Zea mays | Under drought stress, increased yield parameters, photosynthetic pigments, antioxidants and grain quality and protein content; decreased photosystem damage and lipid peroxidation. Under optimal conditions, increased yield parameters and quality, nutrient uptake; improved plant growth, antioxidant activity; decreased lipid peroxidation and accumulation of ROS; upregulation of genes related to fatty acid metabolism, starch synthesis, nutrient transport and metabolism, cell cycle and division. | [164,165,166,172] | |
| Microorganisms (Plant growth promoting bacteria) | Amaranthus hybridus L. | Increased leaves nutrient concentration increase; improved nutritional quality, plant growth and photosynthetic pigments under certain circumstances. | [204] |
| Arabidopsis thaliana | Under optimal and salt-stress, improved plant growth and biomass; increased antioxidant activity, and proline and chlorophyll content. | [206] | |
| Brassica napus | Improved plant growth, plant biomass, yield parameters and seed fatty acid concentration. | [203] | |
| Brassica rapa L. | Increased plant biomass, number of leaves and root length, total content of IAA, P and N. | [209] | |
| Buglossoides arvensis | Improved plant growth, plant biomass, yield parameters and seed fatty acid concentration. | [203] | |
| Cicer arietinum L. | Increased plant biomass and yield, chlorophyll and NPK content; improved seeds nutritional content. | [208] | |
| Coriandrum sativum | Under optimal conditions, increased RWC, photosynthetic pigments concentration; improved plant growth. Under salt-stress, improved plant growth; increased RWC and photosynthetic pigments concentration. | [205] | |
| Fragaria × ananassa | Increased plant yield; improved achene germination and germination rate. | [213] | |
| Glycine max L. | Improved plant growth, plant biomass, yield parameters and seed fatty acid concentration. Under salt stress, increased plant biomass and gibberellin and abscisic acid concentrations; improved plant growth and development. | [198,203] | |
| Solanum lycopersicum L. | Increased plant biomass, RWC, healthy fruit yield, fruit micro- and micronutrient content. | [191] | |
| Sorghum bicolor | Increased plant growth and chlorophyll pigments; upregulation of genes related to iron absorption and transport (IRT1, IRT2, YS1 and YS2) | [214] | |
| Triticum aestivum L. | Improved early plant development and nutrient uptake; increased plant macro- and micronutrients concentration and grain yield. Under low N content in the soil, ameliorated negative effects on root growth and yield parameters. | [192,195] | |
| Zea mays | Under salt stress, improved root growth; increased chlorophyll and soluble sugar content; decreased lipid peroxidation; improved POD and CAT activity; upregulation of RuBisCO, NHX1, NHX7, H+-PPase and HKT1 genes. | [199] | |
| Microorganisms (Trichoderma spp.) | Cucumis melon | Increased plant vigor, biomass and yield; improved germination and fruit quality. | [223] |
| Eruca sativa Mill. | Increased plant biomass, yield, phenols content and antioxidant activity; improved nitrogen usage efficiency and uptake. | [219,222] | |
| Fragaria x ananassa Duch. | Increased biomass, yield, nutrient uptake, anthocyanins and antioxidants content, concentration of proteins involved in carbohydrate metabolism, glycolysis and alcoholic fermentation, higher concentrations of NADH dehydrogenase components and defense related machinery components. | [224] | |
| Glycine max L. | Increased plant growth and biomass, nutrient uptake and fatty acid and mineral content in the seeds. | [220] | |
| Helianthus annus L. | Under lead stress, increased antioxidant activity; enhanced heavy metal stress tolerance. | [225] | |
| Lactuca sativa L. | Increased plant biomass, yield, phenols content and antioxidant activity; improved nitrogen usage efficiency and uptake. | [219,222] | |
| Solanum lycopersicum L. | Under chilling stress, increased biomass, RWC and proline content; improved photosynthetic rate; decreased lipid peroxidation and electrolyte leakage; upregulation of genes related to osmoregulators and hormone biosynthesis. | [227] | |
| Triticum aestivum L. | Under salt stress, increased plant biomass, proline and IAA content; improved photosynthetic parameters and water use efficiency. | [228] | |
| Triticum durum | Under normal and drought-stress conditions, increased plant growth and biomass, plant yield; upregulation of genes related to drought stress response. | [221] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules 2021, 11, 1096. https://doi.org/10.3390/biom11081096
Baltazar M, Correia S, Guinan KJ, Sujeeth N, Bragança R, Gonçalves B. Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules. 2021; 11(8):1096. https://doi.org/10.3390/biom11081096
Chicago/Turabian StyleBaltazar, Miguel, Sofia Correia, Kieran J. Guinan, Neerakkal Sujeeth, Radek Bragança, and Berta Gonçalves. 2021. "Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview" Biomolecules 11, no. 8: 1096. https://doi.org/10.3390/biom11081096
APA StyleBaltazar, M., Correia, S., Guinan, K. J., Sujeeth, N., Bragança, R., & Gonçalves, B. (2021). Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules, 11(8), 1096. https://doi.org/10.3390/biom11081096







