Specification and Evaluation of Plasticizer Migration Simulants for Human Blood Products: A Delphi Study
Abstract
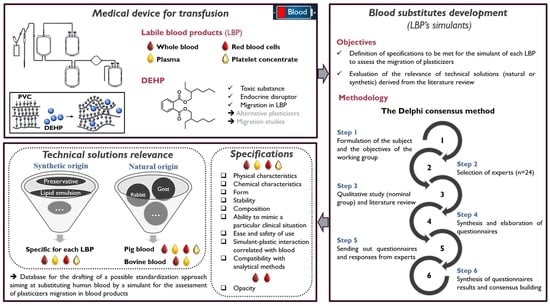
1. Introduction
2. Materials and Methods
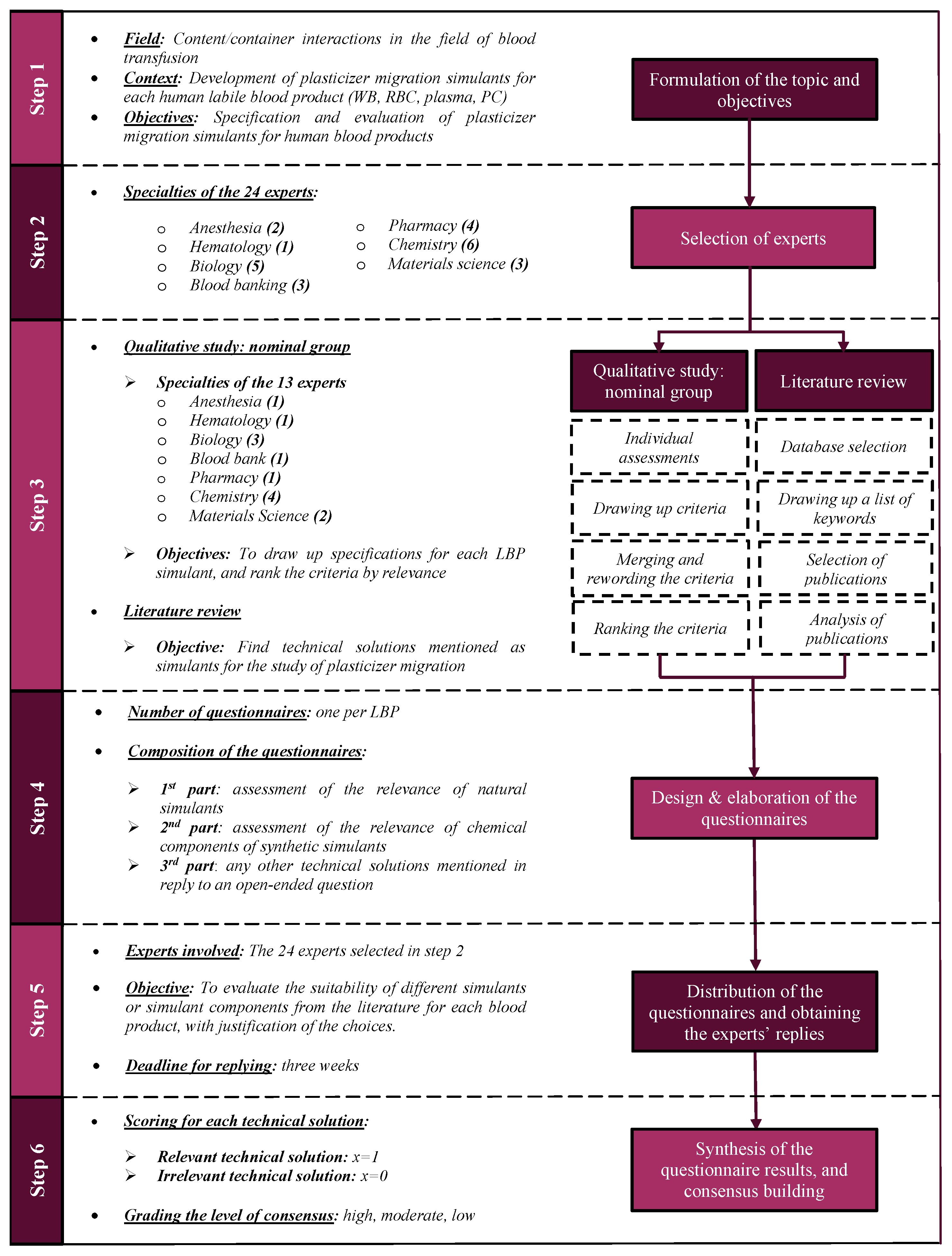
2.1. The Delphi Method
2.2. Study Design
2.2.1. Formulation of the Topic and the Working Group’s Objectives, and Selection of the Experts
2.2.2. Qualitative Study
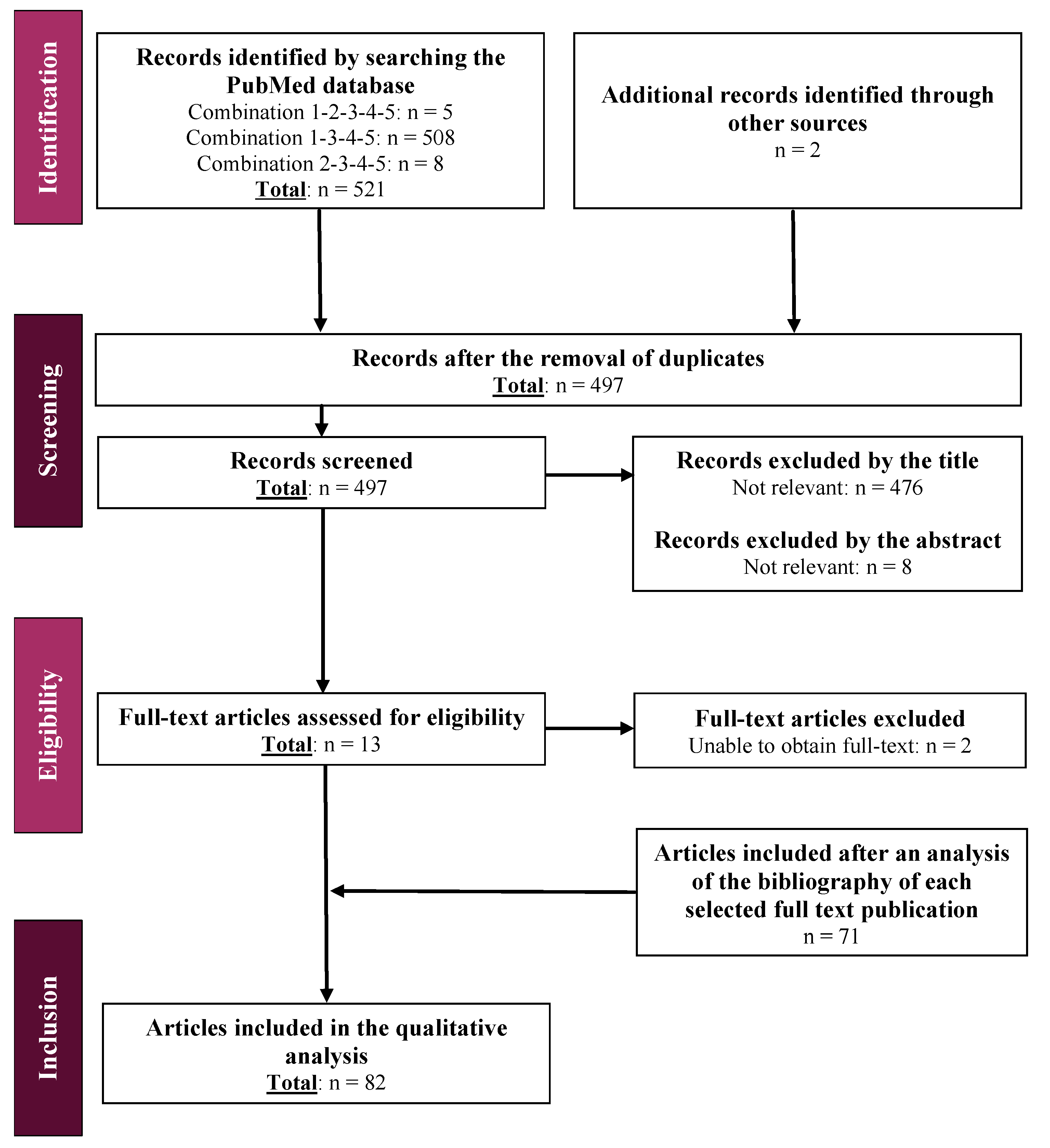
2.2.3. Literature Review
2.2.4. Design and Elaboration of the Questionnaires
2.2.5. Distribution of the Questionnaires
2.2.6. Synthesis of the Questionnaire Results and Consensus Building
3. Results and Discussion
3.1. The Qualitative Study
3.2. Review of the Literature and Elaboration of Questionnaires
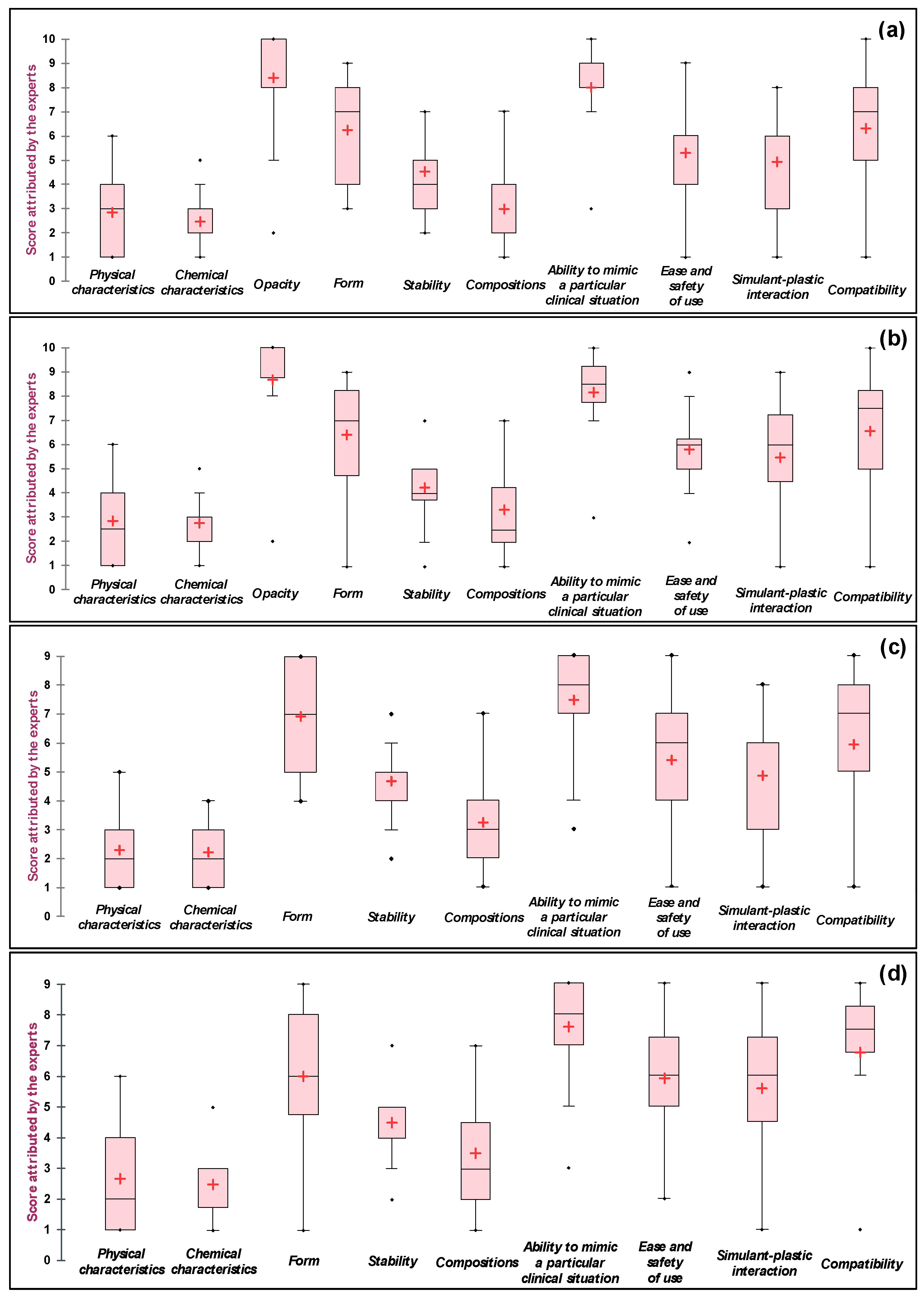
3.3. Questionnaire Results and the Consensus Reached
3.3.1. Technical Solutions of Natural Origin
Technical Solutions of Natural Origin for Whole Blood
Technical Solutions of Natural Origin for Red Blood Cell Concentrate
Technical Solutions of Natural Origin for Plasma
Technical Solutions of Natural Origin for Platelet Concentrate
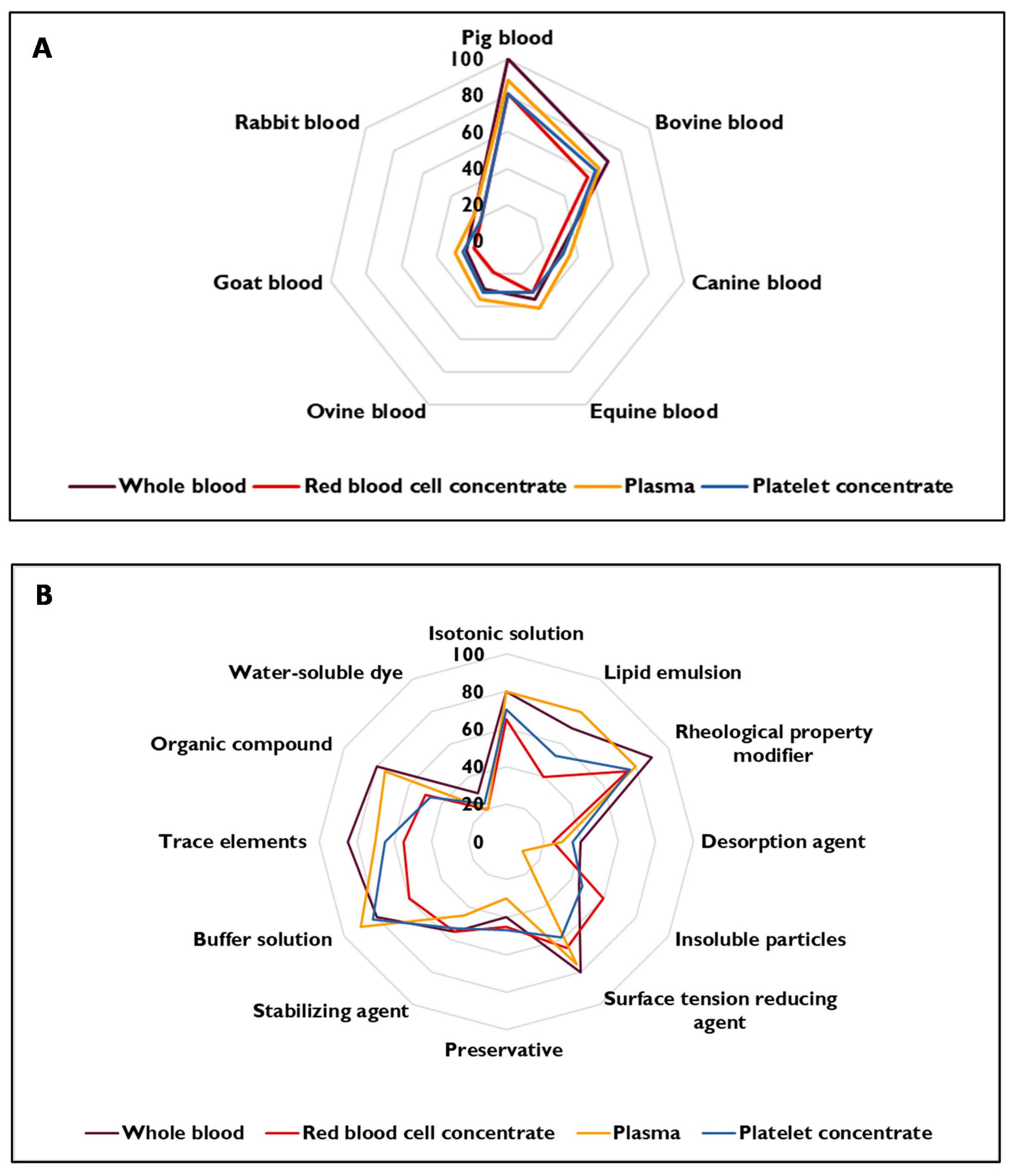
Summary of the Results for Simulants of Natural Origin
3.3.2. Technical Solutions of Synthetic Origin
Technical Solutions of Synthetic Origin for Whole Blood
Technical Solutions of Synthetic Origin for Red Blood Cell Concentrate
Technical Solutions of Synthetic Origin for Plasma
Technical Solutions of Synthetic Origin for Platelet Concentrate
Summary of the Results for Simulants of Synthetic Origin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez-Canal, C.; Pinta, P.-G.; Eljezi, T.; Larbre, V.; Kauffmann, S.; Camilleri, L.; Cosserant, B.; Bernard, L.; Pereira, B.; Constantin, J.-M.; et al. Patients’ Exposure to PVC Plasticizers from ECMO Circuits. Expert. Rev. Med. Devices. 2018, 15, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Prowse, C.V.; de Korte, D.; Hess, J.R.; van der Meer, P.F.; Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Commercially Available Blood Storage Containers. Vox Sang. 2014, 106, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Sandford, B. The Delphi Technique: Making Sense of Consensus. Pract. Assess. Res. Eval. 2019, 12. [Google Scholar] [CrossRef]
- Fink, A.; Kosecoff, J.; Chassin, M.; Brook, R.H. Consensus Methods: Characteristics and Guidelines for Use. Am. J. Public Health 1984, 74, 979–983. [Google Scholar] [CrossRef]
- Jones, J.; Hunter, D. Consensus Methods for Medical and Health Services Research. BMJ 1995, 311, 376–380. [Google Scholar] [CrossRef]
- Visade, F.; Lefebvre, A.; Floret, E.; Decaudin, B.; Puisieux, F.; Delecluse, C.; Beuscart, J.-B. Proposition of a structured list of information items to be transmitted to primary caregivers after in-hospital medication optimization: A qualitative study. Acta Clin. Belg. 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Shekhar, K.P.; Su, H.; Kalaikadal, D.S.; Lorenz, J.N.; Manglik, R.M.; Holland, C.K.; Redington, A.N.; Haworth, K.J. Acoustic Droplet Vaporization-Mediated Dissolved Oxygen Scavenging in Blood-Mimicking Fluids, Plasma, and Blood. Ultrason. Sonochem. 2019, 56, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Cloutier, G.; Pibarot, P.; Durand, L.G. Comparison and Simulation of Different Levels of Erythrocyte Aggregation with Pig, Horse, Sheep, Calf, and Normal Human Blood. Biorheology 1996, 33, 365–377. [Google Scholar] [CrossRef]
- Cloutier, G.; Shung, K.K. Cyclic Variation of Doppler Backscattering Power from Porcine Blood in a Pulsatile Flow Model. In Proceedings of the IEEE 1991 Ultrasonics Symposium, Orlando, FL, USA, 8–11 December 1991; Volume 2, pp. 1301–1304. [Google Scholar] [CrossRef]
- Wu, S.J.; Shung, K.K. Cyclic Variation of Doppler Power from Whole Blood under Pulsatile Flow. Ultrasound Med. Biol. 1996, 22, 883–894. [Google Scholar] [CrossRef]
- Brookshier, K.K.; Tarbell, J.M. Effect of Hematocrit on Wall Shear Rate in Oscillatory Flow: Do the Elastic Properties of Blood Play a Role? Biorheology 1991, 28, 569–587. [Google Scholar] [CrossRef]
- Allard, L.; Cloutier, G.; Durand, L. Effect of the Insonification Angle on the Doppler Backscattered Power under Red Blood Cell Aggregation Conditions. IEEE T. Ultrason. Ferr. 1996, 43, 211–219. [Google Scholar] [CrossRef]
- Cloutier, G.; Shung, K.K.; Durand, L.G. Experimental Evaluation of Intrinsic and Nonstationary Ultrasonic Doppler Spectral Broadening in Steady and Pulsatile Flow Loop Models. IEEE Trans. Ultrason. Ferr. 1993, 40, 786–795. [Google Scholar] [CrossRef]
- Badimon, L.; Padro, T.; Vilahur, G. Extracorporeal Assays of Thrombosis. Methods Mol. Biol. 2012, 788, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.Y.; Shung, K.K. High Frequency Ultrasonic Backscatter from Erythrocyte Suspension. IEEE Trans. Biomed. Eng. 1994, 41, 29–34. [Google Scholar] [CrossRef]
- Badimon, J.J.; Lettino, M.; Toschi, V.; Fuster, V.; Berrozpe, M.; Chesebro, J.H.; Badimon, L. Local Inhibition of Tissue Factor Reduces the Thrombogenicity of Disrupted Human Atherosclerotic Plaques: Effects of Tissue Factor Pathway Inhibitor on Plaque Thrombogenicity under Flow Conditions. Circulation 1999, 99, 1780–1787. [Google Scholar] [CrossRef]
- Mo, L.Y.; Yip, G.; Cobbold, R.S.; Gutt, C.; Joy, M.; Santyr, G.; Shung, K.K. Non-Newtonian Behavior of Whole Blood in a Large Diameter Tube. Biorheology 1991, 28, 421–427. [Google Scholar] [CrossRef]
- Greaby, R.; Zderic, V.; Vaezy, S. Pulsatile Flow Phantom for Ultrasound Image-Guided HIFU Treatment of Vascular Injuries. Ultrasound Med. Biol. 2007, 33, 1269–1276. [Google Scholar] [CrossRef][Green Version]
- Cloutier, G.; Qin, Z. Shear Rate Dependence of Normal, Hypo-, and Hyper-Aggregating Erythrocytes Studied with Power Doppler Ultrasound. In Acoustical Imaging; Lees, S., Ferrari, L.A., Eds.; Springer: Boston, MA, USA, 1997; Volume 23, pp. 291–296. [Google Scholar]
- Cloutier, G.; Shung, K.K. Study of Red Cell Aggregation in Pulsatile Flow from Ultrasonic Doppler Power Measurements. Biorheology 1993, 30, 443–461. [Google Scholar] [CrossRef]
- Cloutier, G.; Shung, K.K. The Effect of Turbulence on the Variation of the Ultrasonic Doppler Amplitude within the Cardiac Cycle. In Proceedings of the 14th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Paris, France, 29 October–1 November 1992; pp. 2118–2119. [Google Scholar] [CrossRef]
- Shung, K.K.; Cloutier, G.; Lim, C.C. The Effects of Hematocrit, Shear Rate, and Turbulence on Ultrasonic Doppler Spectrum from Blood. IEEE Trans. Biomed. Eng. 1992, 39, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.W.; Shung, K.K. Ultrasonic Backscatter from Flowing Whole Blood. I: Dependence on Shear Rate and Hematocrit. J. Acoust. Soc. Am. 1988, 84, 52–58. [Google Scholar] [CrossRef]
- Yuan, Y.W.; Shung, K.K. Ultrasonic Backscatter from Flowing Whole Blood. II: Dependence on Frequency and Fibrinogen Concentration. J. Acoust. Soc. Am. 1988, 84, 1195–1200. [Google Scholar] [CrossRef]
- Mo, L.Y.; Kuo, I.Y.; Shung, K.K.; Ceresne, L.; Cobbold, R.S. Ultrasound Scattering from Blood with Hematocrits up to 100%. IEEE Trans. Biomed. Eng. 1994, 41, 91–95. [Google Scholar] [CrossRef]
- Embree, P.M.; O’Brien, W.R. Volumetric Blood Flow via Time-Domain Correlation: Experimental Verification. IEEE T. Ultrason. Ferr. 1990, 37, 176–189. [Google Scholar] [CrossRef]
- Cloutier, G.; Allard, L.; Durand, L.G. Changes in Ultrasonic Doppler Backscattered Power Downstream of Concentric and Eccentric Stenoses under Pulsatile Flow. Ultrasound Med. Biol. 1995, 21, 59–70. [Google Scholar] [CrossRef]
- Cloutier, G.; Allard, L.; Durand, L.G. Characterization of Blood Flow Turbulence with Pulsed-Wave and Power Doppler Ultrasound Imaging. J. Biomech. Eng. 1996, 118, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Shung, K.K.; Yuan, Y.W.; Fei, D.Y.; Tarbell, J.M. Effect of Flow Disturbance on Ultrasonic Backscatter from Blood. J. Acoust. Soc. Am. 1984, 75, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.E.; Tarbell, J.M. Flow of Non-Newtonian Blood Analog Fluids in Rigid Curved and Straight Artery Models. Biorheology 1990, 27, 711–733. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.W.; Shung, K.K. Further Studies on Ultrasonic Backscatter from Blood. Ultrason. Symp. 1984, 666–668. [Google Scholar] [CrossRef]
- Lucas, R.J.; Twersky, V. Inversion of Ultrasonic Scattering Data for Red Blood Cell Suspensions under Different Flow Conditions. J. Acoust. Soc. Am. 1987, 82, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Berger, N.E.; Lucas, R.J.; Twersky, V. Polydisperse Scattering Theory and Comparisons with Data for Red Blood Cells. J. Acoust. Soc. Am. 1991, 89, 1394–1401. [Google Scholar] [CrossRef]
- Fayz, S.; Herbert, R.; Martin, A.M. The Release of Plasticizer from Polyvinyl Chloride Haemodialysis Tubing. J. Pharm. Pharmacol. 1977, 29, 407–410. [Google Scholar] [CrossRef]
- Kripfgans, O.D.; Fowlkes, J.B.; Miller, D.L.; Eldevik, O.P.; Carson, P.L. Acoustic Droplet Vaporization for Therapeutic and Diagnostic Applications. Ultrasound Med. Biol. 2000, 26, 1177–1189. [Google Scholar] [CrossRef]
- Yamada, E.G.; Fitzgerald, P.J.; Sudhir, K.; Hargrave, V.K.; Yock, P.G. Intravascular Ultrasound Imaging of Blood: The Effect of Hematocrit and Flow on Backscatter. J. Am. Soc. Echocardiogr. 1992, 5, 385–392. [Google Scholar] [CrossRef]
- Borders, S.E.; Fronek, A.; Kemper, W.S.; Franklin, D. Ultrasonic Energy Backscattered from Blood. An Experimental Determination of the Variation of Sound Energy with Hematocrit. Ann. Biomed. Eng. 1978, 6, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Pajek, D.; Burgess, A.; Huang, Y.; Hynynen, K. High-Intensity Focused Ultrasound Sonothrombolysis: The Use of Perfluorocarbon Droplets to Achieve Clot Lysis at Reduced Acoustic Power. Ultrasound Med. Biol. 2014, 40, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Mazur, H.I.; Stennett, D.J.; Egging, P.K. Extraction of Diethylhexylphthalate from Total Nutrient Solution-Containing Polyvinyl Chloride Bags. J. Parenter. Enter. Nutr. 1989, 13, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Nandy, S.; Tarbell, J.M. Flush-Mounted Hot Film Anemometer Accuracy in Pulsatile Flow. J. Biomech. Eng. 1986, 108, 228–231. [Google Scholar] [CrossRef]
- Yongchareon, W.; Young, D.F. Initiation of Turbulence in Models of Arterial Stenoses. J. Biomech. 1979, 12, 185–196. [Google Scholar] [CrossRef]
- Landwehr, P.; Schindler, R.; Heinrich, U.; Dölken, W.; Krahe, T.; Lackner, K. Quantification of Vascular Stenosis with Color Doppler Flow Imaging: In Vitro Investigations. Radiology 1991, 178, 701–704. [Google Scholar] [CrossRef]
- Chahed, N.; Péronneau, P.; Delouche, A.; Diebold, B. Velocity Profiles and Streamlines of a Revolution Post-Stenotic Flow. Biorheology 1991, 28, 383–400. [Google Scholar] [CrossRef]
- Walburn, F.J.; Stein, P.D. Velocity Profiles in Symmetrically Branched Tubes Simulating the Aortic Bifurcation. J. Biomech. 1981, 14, 601–611. [Google Scholar] [CrossRef]
- McIlhenny, S.E. Blood and Bone Marrow Simulant. US20140243181A1, 28 August 2014. [Google Scholar]
- Loff, S.; Hannmann, T.; Subotic, U.; Reinecke, F.-M.; Wischmann, H.; Brade, J. Extraction of Diethylhexylphthalate by Home Total Parenteral Nutrition from Polyvinyl Chloride Infusion Lines Commonly Used in the Home. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Loff, S.; Kabs, F.; Subotic, U.; Schaible, T.; Reinecke, F.; Langbein, M. Kinetics of Diethylhexyl-Phthalate Extraction From Polyvinylchloride-Infusion Lines. J. Parenter. Enteral. Nutr. 2002, 26, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Faessler, D.; McCombie, G.; Biedermann, M.; Felder, F.; Subotic, U. Leaching of Plasticizers from Polyvinylchloride Perfusion Lines by Different Lipid Emulsions for Premature Infants under Clinical Conditions. Int. J. Pharm. 2017, 520, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Loff, S.; Kabs, F.; Witt, K.; Sartoris, J.; Mandl, B.; Niessen, K.H.; Waag, K.L. Polyvinylchloride Infusion Lines Expose Infants to Large Amounts of Toxic Plasticizers. J. Pediatr. Surg. 2000, 35, 1775–1781. [Google Scholar] [CrossRef]
- Hoskins, P.R.; Loupas, T.; McDicken, W.N. A Comparison of the Doppler Spectra from Human Blood and Artificial Blood Used in a Flow Phantom. Ultrasound Med. Biol. 1990, 16, 141–147. [Google Scholar] [CrossRef]
- Hoskins, P.R.; Anderson, T.; McDicken, W.N. A Computer Controlled Flow Phantom for Generation of Physiological Doppler Waveforms. Phys. Med. Biol. 1989, 34, 1709–1717. [Google Scholar] [CrossRef]
- Hein, I.A.; O’Brien, W.D. A Flexible Blood Flow Phantom Capable of Independently Producing Constant and Pulsatile Flow with a Predictable Spatial Flow Profile for Ultrasound Flow Measurement Validations. IEEE Trans. Biomed. Eng. 1992, 39, 1111–1122. [Google Scholar] [CrossRef]
- Dabrowski, W.; Dunmore-Buyze, J.; Cardinal, H.N.; Fenster, A. A Real Vessel Phantom for Flow Imaging: 3-D Doppler Ultrasound of Steady Flow. Ultrasound Med. Biol. 2001, 27, 135–141. [Google Scholar] [CrossRef]
- McDicken, W.N. A Versatile Test-Object for the Calibration of Ultrasonic Doppler Flow Instruments. Ultrasound Med. Biol. 1986, 12, 245–249. [Google Scholar] [CrossRef]
- Rickey, D.W.; Picot, P.A.; Christopher, D.A.; Fenster, A. A Wall-Less Vessel Phantom for Doppler Ultrasound Studies. Ultrasound Med. Biol. 1995, 21, 1163–1176. [Google Scholar] [CrossRef]
- Samavat, H.; Evans, J.A. An Ideal Blood Mimicking Fluid for Doppler Ultrasound Phantoms. J. Med. Phys. 2006, 31, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Douville, Y.; Johnston, K.W.; Kassam, M.; Zuech, P.; Cobbold, R.S.; Jares, A. An in Vitro Model and Its Application for the Study of Carotid Doppler Spectral Broadening. Ultrasound Med. Biol. 1983, 9, 347–356. [Google Scholar] [CrossRef]
- Poepping, T.L.; Nikolov, H.N.; Rankin, R.N.; Lee, M.; Holdsworth, D.W. An in Vitro System for Doppler Ultrasound Flow Studies in the Stenosed Carotid Artery Bifurcation. Ultrasound Med. Biol. 2002, 28, 495–506. [Google Scholar] [CrossRef]
- Lubbers, J. Application of a New Blood-Mimicking Fluid in a Flow Doppler Test Object. Eur. J. Ultrasound 1999, 9, 267–276. [Google Scholar] [CrossRef]
- Khalifa, A.M.; Giddens, D.P. Characterization and Evolution Poststenotic Flow Disturbances. J. Biomech. 1981, 14, 279–296. [Google Scholar] [CrossRef]
- Winkler, A.J.; Wu, J. Correction of Intrinsic Spectral Broadening Errors in Doppler Peak Velocity Measurements Made with Phased Sector and Linear Array Transducers. Ultrasound Med. Biol. 1995, 21, 1029–1035. [Google Scholar] [CrossRef]
- Liu, Y.; Maruvada, S.; King, R.L.; Herman, B.A.; Wear, K.A. Development and Characterization of a Blood Mimicking Fluid for High Intensity Focused Ultrasound. J. Acoust. Soc. Am. 2008, 124, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Teirlinck, C.J.; Bezemer, R.A.; Kollmann, C.; Lubbers, J.; Hoskins, P.R.; Ramnarine, K.V.; Fish, P.; Fredeldt, K.E.; Schaarschmidt, U.G. Development of an Example Flow Test Object and Comparison of Five of These Test Objects, Constructed in Various Laboratories. Ultrasonics 1998, 36, 653–660. [Google Scholar] [CrossRef]
- Ramnarine, K.V.; Hoskins, P.R.; Routh, H.F.; Davidson, F. Doppler Backscatter Properties of a Blood-Mimicking Fluid for Doppler Performance Assessment. Ultrasound Med. Biol. 1999, 25, 105–110. [Google Scholar] [CrossRef]
- Hutchison, K.J. Doppler Ultrasound Spectral Shape in the Poststenotic Velocity Field. Ultrasound Med. Biol. 1993, 19, 649–659. [Google Scholar] [CrossRef]
- Seeley, B.D.; Young, D.F. Effect of Geometry on Pressure Losses across Models of Arterial Stenoses. J. Biomech. 1976, 9, 439–448. [Google Scholar] [CrossRef]
- Solzbach, U.; Wollschläger, H.; Zeiher, A.; Just, H. Effect of Stenotic Geometry on Flow Behaviour across Stenotic Models. Med. Biol. Eng. Comput. 1987, 25, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Fei, D.Y.; Billian, C.; Rittgers, S.E. Flow Dynamics in a Stenosed Carotid Bifurcation Model--Part I: Basic Velocity Measurements. Ultrasound Med. Biol. 1988, 14, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Tortoli, P.; Thompson, R.S.; Berti, P.; Guidi, F. Flow Imaging with Pulsed Doppler Ultrasound and Flow Phantoms. IEEE Trans. Ultrason. Ferr. 1999, 46, 1591–1596. [Google Scholar] [CrossRef]
- Moravec, S.; Liepsch, D. Flow Investigations in a Model of a Three-Dimensional Human Artery with Newtonian and Non-Newtonian Fluids. Part, I. Biorheology 1983, 20, 745–759. [Google Scholar] [CrossRef]
- Tortoli, P.; Guidi, G.; Newhouse, V.L. Improved Blood Velocity Estimation Using the Maximum Doppler Frequency. Ultrasound Med. Biol. 1995, 21, 527–532. [Google Scholar] [CrossRef]
- Hariharan, P.; Aycock, K.I.; Buesen, M.; Day, S.W.; Good, B.C.; Herbertson, L.H.; Steinseifer, U.; Manning, K.B.; Craven, B.A.; Malinauskas, R.A. Inter-Laboratory Characterization of the Velocity Field in the FDA Blood Pump Model Using Particle Image Velocimetry (PIV). Cardiovasc. Eng. Technol. 2018, 9, 623–640. [Google Scholar] [CrossRef]
- Feinberg, D.A.; Crooks, L.E.; Sheldon, P.; Hoenninger, J.; Watts, J.; Arakawa, M. Magnetic Resonance Imaging the Velocity Vector Components of Fluid Flow. Magn. Reson. Med. 1985, 2, 555–566. [Google Scholar] [CrossRef]
- Kraft, K.A.; Fatouros, P.P.; Fei, D.Y.; Rittgers, S.E.; Kishore, P.R. MR Imaging of Model Fluid Velocity Profiles. Magn Reson Imaging 1989, 7, 69–77. [Google Scholar] [CrossRef]
- Boote, E.J.; Zagzebski, J.A. Performance Tests of Doppler Ultrasound Equipment with a Tissue and Blood-Mimicking Phantom. J. Ultrasound Med. 1988, 7, 137–147. [Google Scholar] [CrossRef]
- Liepsch, D.; Moravec, S. Pulsatile Flow of Non-Newtonian Fluid in Distensible Models of Human Arteries. Biorheology 1984, 21, 571–586. [Google Scholar] [CrossRef]
- Brown, T.D.; Gabel, R.H.; Pedersen, D.R.; Bell, L.D.; Blair, W.F. Some Characteristics of Laminar Flow Velocity Spectra Detected by a 20 MHz Pulsed Ultrasound Doppler. J. Biomech. 1985, 18, 927–938. [Google Scholar] [CrossRef]
- Steinman, A.H.; Tavakkoli, J.; Myers, J.G.; Cobbold, R.S.; Johnston, K.W. Sources of Error in Maximum Velocity Estimation Using Linear Phased-Array Doppler Systems with Steady Flow. Ultrasound Med. Biol. 2001, 27, 655–664. [Google Scholar] [CrossRef]
- Siouffi, M.; Pelissier, R.; Farahifar, D.; Rieu, R. The Effect of Unsteadiness on the Flow through Stenoses and Bifurcations. J. Biomech. 1984, 17, 299–315. [Google Scholar] [CrossRef]
- Ku, D.N.; Liepsch, D. The Effects of Non-Newtonian Viscoelasticity and Wall Elasticity on Flow at a 90 Degrees Bifurcation. Biorheology 1986, 23, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Fenster, A. Three-Dimensional Power Doppler Imaging: A Phantom Study to Quantify Vessel Stenosis. Ultrasound Med. Biol. 1996, 22, 1059–1069. [Google Scholar] [CrossRef]
- Oates, C.P. Towards an Ideal Blood Analogue for Doppler Ultrasound Phantoms. Phys. Med. Biol. 1991, 36, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Giddens, D.P.; Khalifa, A.M. Turbulence Measurements with Pulsed Doppler Ultrasound Employing a Frequency Tracking Method. Ultrasound Med. Biol. 1982, 8, 427–437. [Google Scholar] [CrossRef]
- Ramnarine, K.V.; Nassiri, D.K.; Hoskins, P.R.; Lubbers, J. Validation of a New Blood-Mimicking Fluid for Use in Doppler Flow Test Objects. Ultrasound Med. Biol. 1998, 24, 451–459. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Giddens, D.P. Velocity Measurements in Steady Flow through Axisymmetric Stenoses at Moderate Reynolds Numbers. J. Biomech. 1983, 16, 505–516. [Google Scholar] [CrossRef]
- Hood, W.H.; Spears, M.J.; Worley, W.J. Menstrual Fluid Simulant Containing Blood Product, Gelatin, Polyacrylamide and Buffer. US7659372B2, 9 February 2010. [Google Scholar]
- Bernard, L.; Cueff, R.; Breysse, C.; Décaudin, B.; Sautou, V.; Armed Study Group. Migrability of PVC Plasticizers from Medical Devices into a Simulant of Infused Solutions. Int. J. Pharm. 2015, 485, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhang, J.; Sluyter, R.; Zhao, Q.; Yan, S.; Alici, G.; Li, W. Continuous Plasma Extraction under Viscoelastic Fluid in a Straight Channel with Asymmetrical Expansion-Contraction Cavity Arrays. Lab Chip 2016, 16, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, G.; Shung, K.K. Cyclic Variation of the Power of Ultrasonic Doppler Signals Backscattered by Polystyrene Microspheres and Porcine Erythrocyte Suspensions. IEEE Trans. Biomed. Eng. 1993, 40, 953–962. [Google Scholar] [CrossRef] [PubMed]
| List Number | Keywords |
|---|---|
| 1 | Containers OR Bags OR Kits OR Pouch OR Packs OR Medical devices OR Medical grade OR Blood bags system |
| 2 | Material * OR Phthalate * OR “non-DEHP” OR Plasticiz * OR Di(2-ethylhexyl)phthalate OR (DEHP AND free) OR Polyvinylchloride OR Ester * OR PVC OR Plasticis * OR Bis(2-ethylhexyl) phthalate OR Di(2-ethylhexyl) phthalate OR BIS(2-ETHYLHEXYL)PHTHALATE OR Di-2-ethylhexyl phthalate OR Polyvinyl chloride |
| 3 | Blood OR Plasma OR Platelets OR Erythrocytes OR Hematology OR Transfusion OR Thrombocyte OR Intravenous * OR Parenteral * |
| 4 | Simulant * OR Mimic * |
| 5 | Leach * OR Extract * OR Release * OR Diffus * OR Deliv * |
| Score (%) | Degree of Consensus |
|---|---|
| x ≥ 85 | High degree of relevance |
| 75 ≤ x ≤ 84 | Moderate degree of relevance |
| 65 ≤ x ≤ 74 | Low degree of relevance |
| 35 < x < 65 | Lack of consensus |
| 26 ≤ x ≤ 35 | Low degree of irrelevance |
| 16 ≤ x ≤ 25 | Moderate degree of irrelevance |
| x ≤ 15 | High degree of irrelevance |
| Criterion | Abbreviation | Labile Blood Product | |||
|---|---|---|---|---|---|
| WB | RBC | P | PC | ||
| Physical characteristics (viscosity, surface tension, thermolability, and density (centrifugation behaviour)) | Physical characteristics 1 | √ | X | X | X |
| Physical characteristics (viscosity, surface tension, thermolability, and density) | Physical characteristics 2 | X | √ | √ | √ |
| Chemical characteristics (hydrophilicity, lipophilicity, pH, surface tension, osmolarity, ionic forces, polarity, and strong/weak bond interactions) | Chemical characteristics | √ | √ | √ | √ |
| Product opacity | Opacity | √ | √ | X | X |
| Form (size/shape of cells/particles, suspension/solution) | Form | √ | √ | √ | √ |
| Stability during preparation, storage and use (time, temperature, light, pressure, centrifugation, and processing of the blood product) | Stability | √ | √ | √ | √ |
| Organic/inorganic chemical composition (sugar (glucose), proteins (transport proteins and albumin), phospholipids, cell phase/lipid phase ratio, absence of plasticizer, liposomes, bile acids, presence of anticoagulant or preservative solutions in the LBP, and the presence of substances that can clot) | Composition 1 | √ | √ | X | √ |
| Organic/inorganic chemical compositions (sugar (glucose), proteins (transport proteins and albumin), phospholipids, absence of plasticizer, liposomes, bile acids, presence of anticoagulant or preservative solutions in the LBP, and the presence of substances that can clot) | Composition 2 | X | X | √ | X |
| Ability to mimic a particular clinical situation (hyperuricaemia, hyperlactatemia, hyperglycemia, acidosis, hemolysis, and the heterogeneity of patients’ blood) | Ability to mimic a particular clinical situation | √ | √ | √ | √ |
| Ease and safety of use: cost, ease of supply, ease of disposal, inter-batch reproducibility, and ability to combine products | Ease and safety of use | √ | √ | √ | √ |
| Blood-correlated simulant-plastic interaction: ability to dissolve in the simulant, and structural change with plasticizers | Simulant-plastic interaction | √ | √ | √ | √ |
| Compatibility with extraction and analysis method | Compatibility | √ | √ | √ | √ |
| Number of criteria | 10 | 10 | 9 | 9 | |
| Criteria | LBP | |||
|---|---|---|---|---|
| WB | RBC | P | PC | |
| Important | “Physical characteristics” “Chemical characteristics” “Composition” | |||
| Moderately important | “Stability” “Ease and safety of use” “Simulant–plastic interaction” | “Form” “Stability” “Ease and safety of use” “Simulant–plastic interaction” | ||
| Less important | “Opacity” “Form” “Ability to mimic a particular clinical situation” “Compatibility” | “Form” “Ability to mimic a particular clinical situation” “Compatibility” | “Ability to mimic a particular clinical situation” “Compatibility” | |
| Criterion | WB | RBC | P | PC | ||||
|---|---|---|---|---|---|---|---|---|
| Ranking | Score | Ranking | Score | Ranking | Score | Ranking | Score | |
| Chemical characteristics | 1 | 32 | 1 | 33 | 1 | 29 | 1 | 30 |
| Physical characteristics | 2 | 37 | 2 | 34 | 2 | 30 | 2 | 32 |
| Compositions | 3 | 39 | 3 | 40 | 3 | 42 | 3 | 42 |
| Stability | 4 | 59 | 4 | 51 | 4 | 61 | 4 | 54 |
| Simulant–plastic interaction | 5 | 64 | 5 | 66 | 5 | 63 | 5 | 67 |
| Ease and safety of use | 6 | 69 | 6 | 70 | 6 | 70 | 6 | 71 |
| Forms | 7 | 81 | 7 | 77 | 8 | 90 | 7 | 72 |
| Compatibility | 8 | 82 | 8 | 79 | 7 | 77 | 8 | 81 |
| Ability to mimic a particular clinical situation | 9 | 104 | 9 | 98 | 9 | 97 | 9 | 91 |
| Opacity | 10 | 109 | 10 | 104 | - | - | - | - |
| Technical Solution | References | |
|---|---|---|
| Origin | Suggestion | |
| Natural | Pig’s blood | [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] |
| Bovine blood | [8,12,19,22,24,27,28,29,30,31,32,33,34] | |
| Canine blood | [35,36,37] | |
| Equine blood | [8] | |
| Ovine blood | [8] | |
| Goat blood | [37] | |
| Rabbit blood | [38] | |
| Synthetic | Isotonic solutions: physiological saline solutions, NaCl 0.9%, etc. | [8,9,12,15,19,21,25,27,34,39,40,41,42,43,44,45] |
| Lipid emulsions: parenteral nutrition products, etc. | [39,46,47,48,49] | |
| Rheological property modifiers: glycerol, gelatine, gellan gum, cellulose and its derivatives, dextran, albumin, silicone oil, polyacrylamide, cutting fluid, etc. | [9,12,13,26,27,30,40,41,42,43,44,45,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86] | |
| Desorption agents: solvents, silicone oil, etc. | [45,87] | |
| Insoluble particles: carbon fibres, nylon, polyethene and polystyrene microspheres, polyamide particles, silicon carbides, etc. | [9,12,53,55,56,58,59,62,76,78,81,82,83,84,88,89] | |
| Surface tension reducing agents: alcohols, glycoproteins, fatty acids, etc. | [45,87] | |
| Preservatives: copper sulfate, potassium sorbate, antifungal agents, sodium azide, etc. | [59,73] | |
| Stabilizing agents: starch, xanthan gum, gellan gum, surfactants like lecithin, etc. | [30,45,58,62,63,64,84,85] | |
| Buffer solutions: potassium phosphate, etc. | [45,86] | |
| Trace elements: potassium chloride, sodium iodide, magnesium sulfate, calcium gluceptate, etc. | [39,72] | |
| Organic compounds: amino acids, dextrose, etc. | [9,13,27,39] | |
| Water-soluble dyes: beetroot juice, etc. | [45] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thelliez, A.; Hénard, G.; Delorme, B.; Chatellier, S.; Danel, C.; Ducoroy, L.; Dupont, A.; Garrigue, D.; Genay, S.; Goossens, J.-F.; et al. Specification and Evaluation of Plasticizer Migration Simulants for Human Blood Products: A Delphi Study. Biomolecules 2021, 11, 1081. https://doi.org/10.3390/biom11081081
Thelliez A, Hénard G, Delorme B, Chatellier S, Danel C, Ducoroy L, Dupont A, Garrigue D, Genay S, Goossens J-F, et al. Specification and Evaluation of Plasticizer Migration Simulants for Human Blood Products: A Delphi Study. Biomolecules. 2021; 11(8):1081. https://doi.org/10.3390/biom11081081
Chicago/Turabian StyleThelliez, Aurélie, Grégory Hénard, Bruno Delorme, Sonia Chatellier, Cécile Danel, Laurent Ducoroy, Annabelle Dupont, Delphine Garrigue, Stéphanie Genay, Jean-François Goossens, and et al. 2021. "Specification and Evaluation of Plasticizer Migration Simulants for Human Blood Products: A Delphi Study" Biomolecules 11, no. 8: 1081. https://doi.org/10.3390/biom11081081
APA StyleThelliez, A., Hénard, G., Delorme, B., Chatellier, S., Danel, C., Ducoroy, L., Dupont, A., Garrigue, D., Genay, S., Goossens, J.-F., Goossens, L., Havet, C., Hecq, J.-D., Maeght, C., Mendel, I., Najdovski, T., Odou, P., Saint-Lorant, G., Ung, A., ... Décaudin, B. (2021). Specification and Evaluation of Plasticizer Migration Simulants for Human Blood Products: A Delphi Study. Biomolecules, 11(8), 1081. https://doi.org/10.3390/biom11081081