Cholesteryl Ester Promotes Mammary Tumor Growth in MMTV-PyMT Mice and Activates Akt-mTOR Pathway in Tumor Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. MMTV-PyMT Mice and Diets
2.2. Drugs and Reagents
2.3. Clinical Data Collection and Analysis
2.4. Lipidomics and CE Analysis
2.5. Cell Culture
2.6. Cell Counting
2.7. Flow Cytometry Analysis of Cell Cycle
2.8. Western Blot
2.9. Statistical Analysis
3. Results
3.1. Intratumor Cholesteryl Ester Level Is Elevated in Tumor Tissues
3.2. Exogenous Cholesteryl Ester Decreases Tumor-Free Survival
3.3. Endogenous Cholesteryl Ester Promotes Tumor Growth
3.4. CE Synthase Inhibitor Reduces AKT and mTOR Activation and Suppresses Tumor Cell Proliferation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tosi, M.R.; Tugnoli, V. Cholesteryl esters in malignancy. Clin. Chim. Acta 2005, 359, 27–45. [Google Scholar] [CrossRef]
- Gonen, A.; Miller, Y.I. From Inert Storage to Biological Activity—In Search of Identity for Oxidized Cholesteryl Esters. Front. Endocrinol. 2020, 11, 602252. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo-Calvo, D.; López-Vilaró, L.; Nasarre, L.; Perez-Olabarria, M.; Vázquez, T.; Escuin, D.; Badimon, L.; Barnadas, A.; Lerma, E.; Llorente-Cortés, V. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: A molecular and clinicopathological study. BMC Cancer 2015, 15, 460. [Google Scholar] [CrossRef]
- Yue, S.; Li, J.; Lee, S.-Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl Ester Accumulation Induced by PTEN Loss and PI3K/AKT Activation Underlies Human Prostate Cancer Aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, A.; Zhao, Y.; Ying, W.; Sun, H.; Yang, X.; Xing, B.; Sun, W.; Ren, L.; Hu, B.; et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature 2019, 567, 257–261. [Google Scholar] [CrossRef]
- Geng, F.; Cheng, X.; Wu, X.; Yoo, J.Y.; Cheng, C.; Guo, J.Y.; Mo, X.; Ru, P.; Hurwitz, B.; Kim, S.-H.; et al. Inhibition of SOAT1 Suppresses Glioblastoma Growth via Blocking SREBP-1–Mediated Lipogenesis. Clin. Cancer Res. 2016, 22, 5337–5348. [Google Scholar] [CrossRef]
- Li, J.; Gu, D.; Lee, S.S.-Y.; Song, B.; Bandyopadhyay, S.; Chen, S.; Konieczny, S.F.; Ratliff, T.L.; Liu, X.; Xie, J.; et al. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene 2016, 35, 6378–6388. [Google Scholar] [CrossRef]
- Antalis, C.; Uchida, A.; Buhman, K.; Siddiqui, R.A. Migration of MDA-MB-231 breast cancer cells depends on the availability of exogenous lipids and cholesterol esterification. Clin. Exp. Metastasis 2011, 28, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhou, S.; Tang, Q.; Xia, H.; Bi, F. Cholesterol metabolism: New functions and therapeutic approaches in cancer. Biochim. Biophys. Acta Bioenerg. 2020, 1874, 188394. [Google Scholar] [CrossRef]
- Chang, C.C.; Lee, C.-Y.G.; Chang, E.T.; Cruz, J.C.; Levesque, M.C.; Chang, T.-Y. Recombinant Acyl-CoA:cholesterol Acyltransferase-1 (ACAT-1) Purified to Essential Homogeneity Utilizes Cholesterol in Mixed Micelles or in Vesicles in a Highly Cooperative Manner. J. Biol. Chem. 1998, 273, 35132–35141. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Chang, A.C.C.Y.; Cheng, D. Acyl-coenzyme A:cholesterol acyltransferase. Annu. Rev. Biochem. 1997, 66, 613–638. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Chang, C.C.; Ohgami, N.; Yamauchi, Y. Cholesterol Sensing, Trafficking, and Esterification. Annu. Rev. Cell Dev. Biol. 2006, 22, 129–157. [Google Scholar] [CrossRef]
- Lee, S.S.-Y.; Li, J.; Tai, J.N.; Ratliff, T.L.; Park, K.; Cheng, J.-X. Avasimibe Encapsulated in Human Serum Albumin Blocks Cholesterol Esterification for Selective Cancer Treatment. ACS Nano 2015, 9, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, Y.; Wei, J.; Cun, X.; Lu, Z.; Qiu, Y.; Zhang, Z.; He, Q. Enhanced chemo-immunotherapy against melanoma by inhibition of cholesterol esterification in CD8+ T cells. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.T.; Cardiff, R.D.; Muller, W.J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol. Cell Biol. 1992, 12, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Fluck, M.M.; Schaffhausen, B.S. Lessons in Signaling and Tumorigenesis from Polyomavirus Middle T Antigen. Microbiol. Mol. Biol. Rev. 2009, 73, 542–563. [Google Scholar] [CrossRef]
- Berquin, I.M.; Min, Y.; Wu, R.; Wu, J.; Perry, D.; Cline, J.M.; Thomas, M.J.; Thornburg, T.; Kulik, G.; Smith, A.; et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J. Clin. Investig. 2007, 117, 1866–1875. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, J.; Chen, Y.Q. Multi-dimensional, comprehensive sample extraction combined with LC-GC/MS analysis for complex biological samples: Application in the metabolomics study of acute pancreatitis. RSC Adv. 2016, 6, 25837–25849. [Google Scholar] [CrossRef]
- Debnath, A.; Calvet, C.M.; Jennings, G.; Zhou, W.; Aksenov, A.; Luth, M.R.; Abagyan, R.; Nes, W.D.; McKerrow, J.H.; Podust, L.M. CYP51 is an essential drug target for the treatment of primary amoebic meningoencephalitis (PAM). PLoS Negl. Trop. Dis. 2017, 11, e0006104. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef]
- Bahrami, A.; Khazaei, M.; Shahidsales, S.; Hassanian, S.M.; Hasanzadeh, M.; Maftouh, M.; Ferns, G.A.; Avan, A. The Therapeutic Potential of PI3K/Akt/mTOR Inhibitors in Breast Cancer: Rational and Progress. J. Cell. Biochem. 2018, 119, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.-S.; Seo, K.-I. Lupiwighteone induces caspase-dependent and -independent apoptosis on human breast cancer cells via inhibiting PI3K/Akt/mTOR pathway. Food Chem. Toxicol. 2020, 135, 110863. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, E.; Cardoso, F.; De Castro, G., Jr.; Colozza, M.; Mano, M.S.; Durbecq, V.; Sotiriou, C.; Larsimont, D.; Piccart-Gebhart, M.; Paesmans, M. Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12,155 patients. Br. J. Cancer 2007, 96, 1504–1513. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2020. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Tian, J.; Zhang, J.-T.; Cheng, J.-X. Cholesterol esterification inhibition and gemcitabine synergistically suppress pancreatic ductal adenocarcinoma proliferation. PLoS ONE 2018, 13, e0193318. [Google Scholar] [CrossRef]
- Antalis, C.J.; Arnold, T.; Rasool, T.; Lee, B.; Buhman, K.; Siddiqui, R.A. High ACAT1 expression in estrogen receptor negative basal-like breast cancer cells is associated with LDL-induced proliferation. Breast Cancer Res. Treat. 2009, 122, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Fu, W.-Q.; Zheng, X.-J.; Li, W.; Ren, L.-W.; Wang, J.-H.; Yang, C.; Du, G.-H. Avasimibe exerts anticancer effects on human glioblastoma cells via inducing cell apoptosis and cell cycle arrest. Acta Pharmacol. Sin. 2021, 42, 97–107. [Google Scholar] [CrossRef]
- Bai, T.; Zhu, B.; Shao, D.; Lian, Z.; Liu, P.; Shi, J.; Kong, J. Blocking ACAT-1 Activity for Tumor Therapy with Fluorescent Hyperstar Polymer-Encapsulated Avasimible. Macromol. Biosci. 2020, 20, e1900438. [Google Scholar] [CrossRef]
- Lei, J.; Wang, H.; Zhu, D.-M.; Wan, Y.; Yin, L. Combined effects of avasimibe immunotherapy, doxorubicin chemotherapy, and metal–organic frameworks nanoparticles on breast cancer. J. Cell. Physiol. 2020, 235, 4814–4823. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpande, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pander, A.; Chinnaiyan, A.M. ONCOMINE: A Cancer Microarray Database and Integrated Data-Mining Platform. Neoplasia 2004, 6, 1–6. [Google Scholar] [CrossRef]
- Llaverías, G.; Laguna, J.C.; Alegret, M. Pharmacology of the ACAT inhibitor avasimibe (CI-1011). Cardiovasc. Drug Rev. 2003, 21, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Zotano, A.; Mayer, I.A.; Arteaga, C.L. PI3K/AKT/mTOR: Role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016, 35, 515–524. [Google Scholar] [CrossRef] [PubMed]
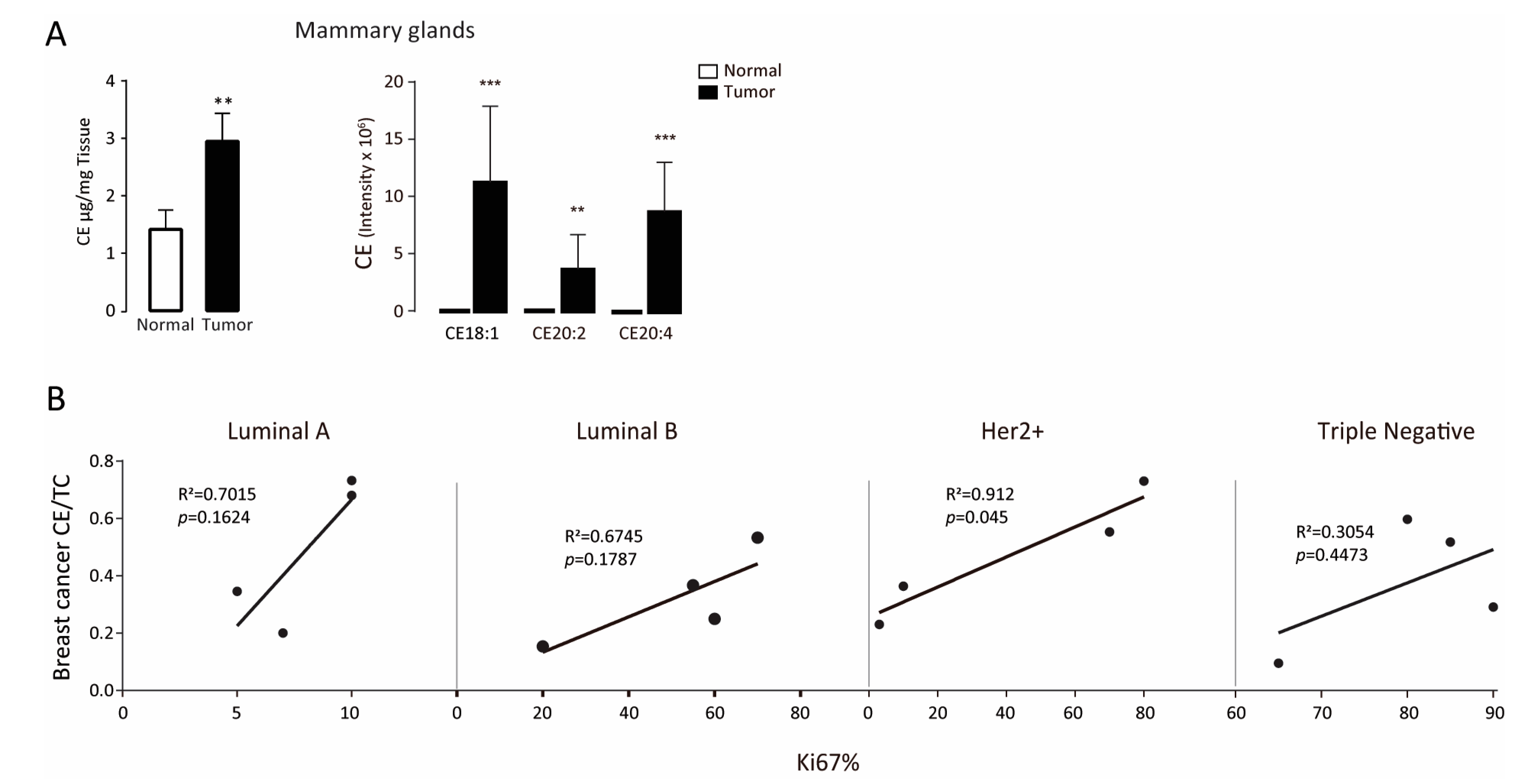
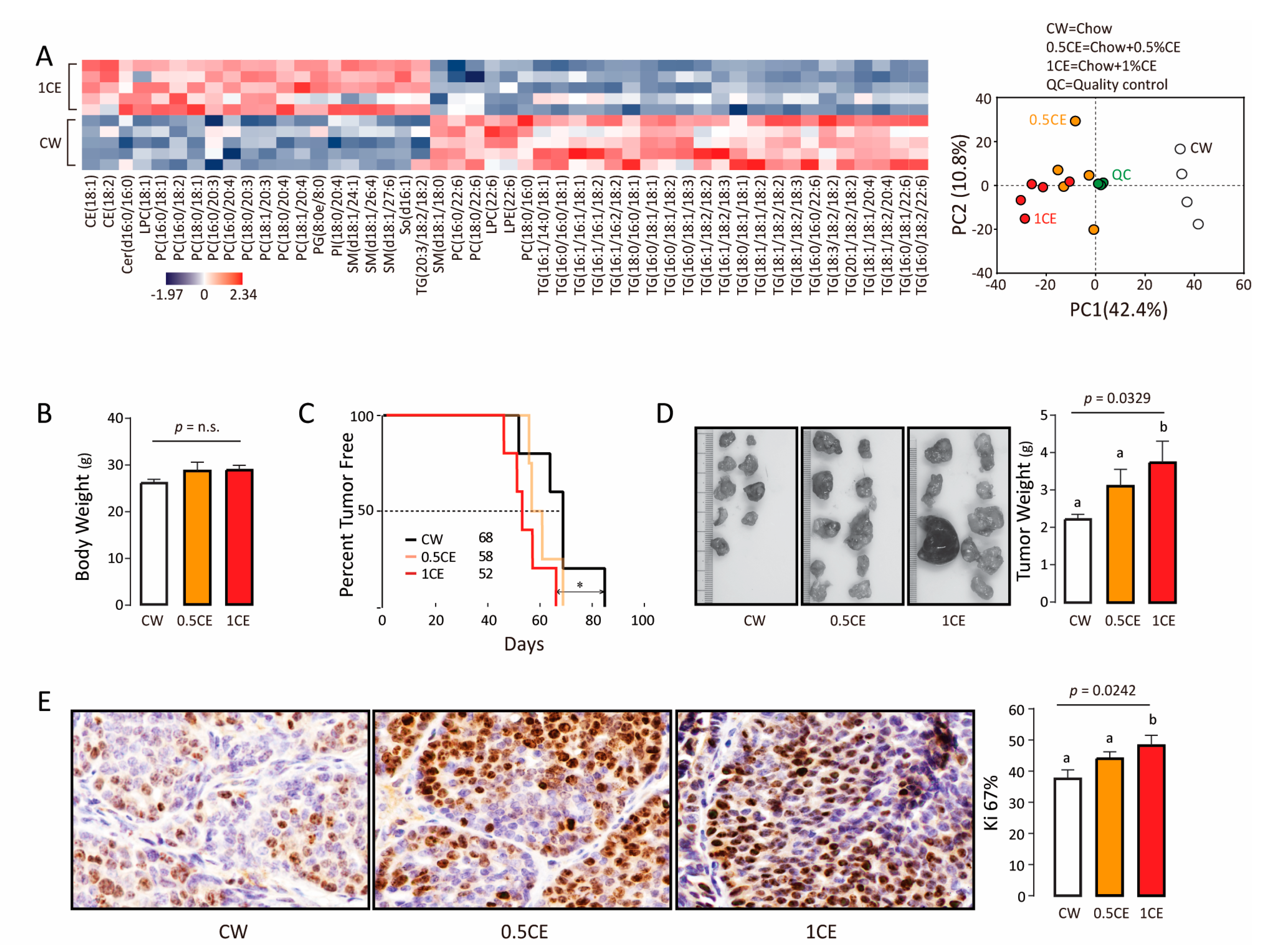

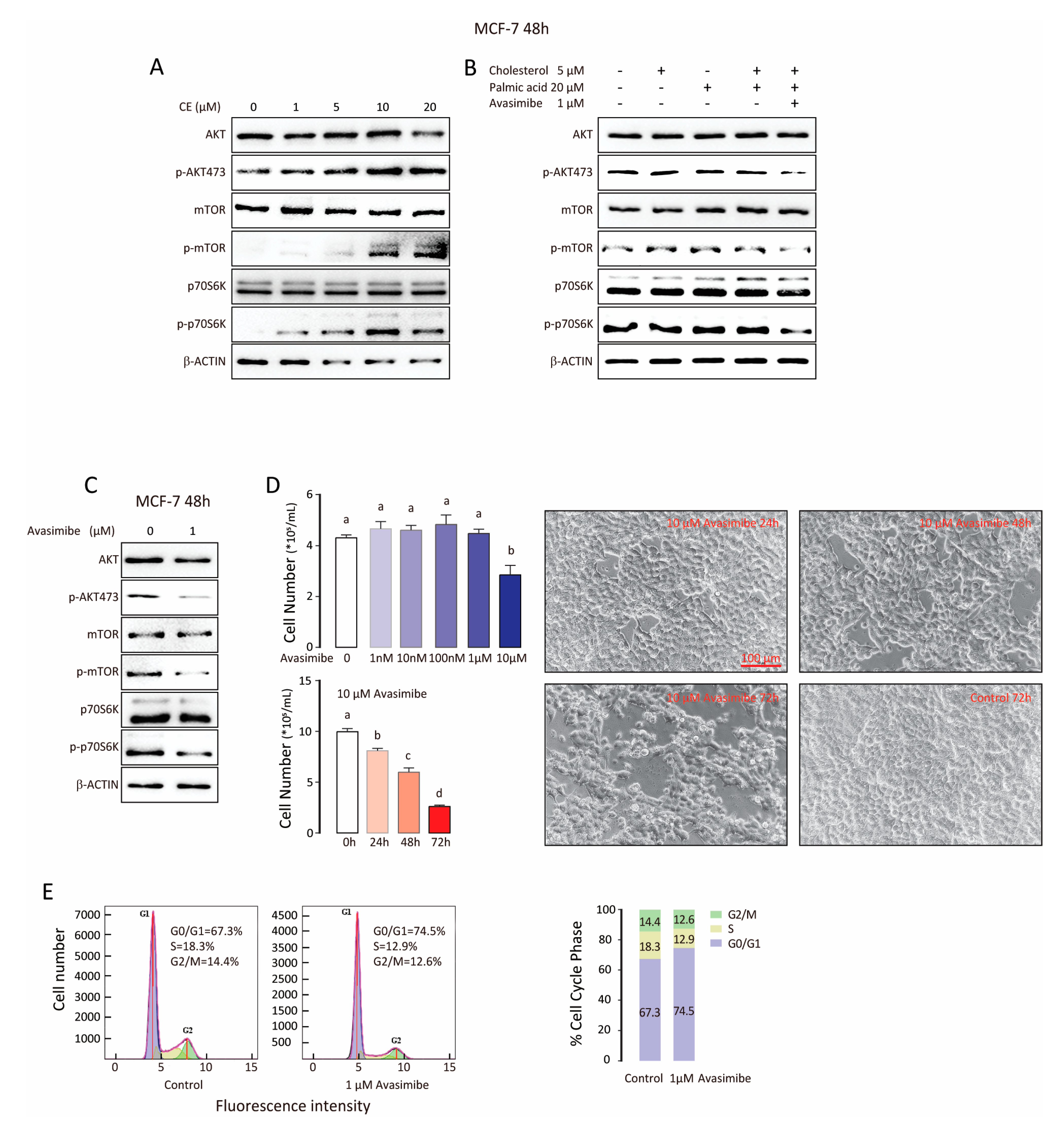
| BCa Type | Patient Number | ER | PR | Her-2 | Ki-67 | Lymph Node Affected | Tumor Size cm | TNM Stage | Age | Menopause |
|---|---|---|---|---|---|---|---|---|---|---|
| LB | 14176 | + | − | + | 70 | 5/17 | 2.5 × 2.5 | T2N2M0 | 38 | |
| 07648 | + | − | ++ | 60 | 5/28 | 2.8 × 2 × 2 | T2N2M0 | 65 | Menopause | |
| 03074 | + | − | ++ | 20 | 0/18 | 4 × 4 × 2 | T1N0M0 | 63 | Menopause | |
| 07857 | + | − | ++ | 50–60 | 0/13 | 2.5 × 1.5 | T2N0M0 | 60 | Menopause | |
| LA | 13673 | ++ | ++ | − | 5–10 | 0/19 | 2.5 × 2.5 | T2N0M0 | 42 | |
| 03075 | + | + | − | 5 | 0/20 | 1.5 × 1 × 1 | T1N0M0 | 52 | ||
| 09276 | ++ | ++ | − | 10 | 12/15 | 3.8 × 2.8 × 2 | T2N3M0 | 44 | ||
| 08041 | ++ | ++ | − | 10 | 0/15 | 3 × 3 | T2N0M0 | 43 | ||
| TN | 11713 | − | − | − | 60–70 | 0/5 | 2.5 × 2 × 1 | T2N0M0 | 50 | |
| 08223 | − | − | − | 80 | 0/15 | 1.8 × 1.8 | T2N0M0 | 61 | Menopause | |
| 13887 | − | − | − | 90 | 0/16 | 3 × 2 × 1.5 | T2N0M0 | 46 | ||
| 02587 | − | − | − | 85 | 1/31 | 2.5 × 1.5 × 1.5 | T2N1M0 | 47 | ||
| Her-2 | 11540 | − | − | +++ | 3 | 0/6 | 3 × 2 × 1.5 | T2N0M0 | 66 | Menopause |
| 05831 | − | − | ++ | 80 | 0/25 | T2N0M0 | 66 | Menopause | ||
| 04004 | − | − | ++ | 70 | 0/9 | 4.5 × 1 × 1 | T2N0M0 | 49 | ||
| 07933 | − | − | ++ | 10 | 4/24 | 2.5 × 2 | T2N2M0 | 37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Lu, X.; Weng, S.; Zhu, S.; Chen, Y. Cholesteryl Ester Promotes Mammary Tumor Growth in MMTV-PyMT Mice and Activates Akt-mTOR Pathway in Tumor Cells. Biomolecules 2021, 11, 853. https://doi.org/10.3390/biom11060853
Wei L, Lu X, Weng S, Zhu S, Chen Y. Cholesteryl Ester Promotes Mammary Tumor Growth in MMTV-PyMT Mice and Activates Akt-mTOR Pathway in Tumor Cells. Biomolecules. 2021; 11(6):853. https://doi.org/10.3390/biom11060853
Chicago/Turabian StyleWei, Lengyun, Xuyang Lu, Shengmei Weng, Shenglong Zhu, and Yongquan Chen. 2021. "Cholesteryl Ester Promotes Mammary Tumor Growth in MMTV-PyMT Mice and Activates Akt-mTOR Pathway in Tumor Cells" Biomolecules 11, no. 6: 853. https://doi.org/10.3390/biom11060853
APA StyleWei, L., Lu, X., Weng, S., Zhu, S., & Chen, Y. (2021). Cholesteryl Ester Promotes Mammary Tumor Growth in MMTV-PyMT Mice and Activates Akt-mTOR Pathway in Tumor Cells. Biomolecules, 11(6), 853. https://doi.org/10.3390/biom11060853






