Equivalent Efficacy but Different Safety Profiles of Gemcitabine Plus Nab-Paclitaxel and FOLFIRINOX in Metastatic Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Treatment
2.2. Statistical Analysis
3. Results
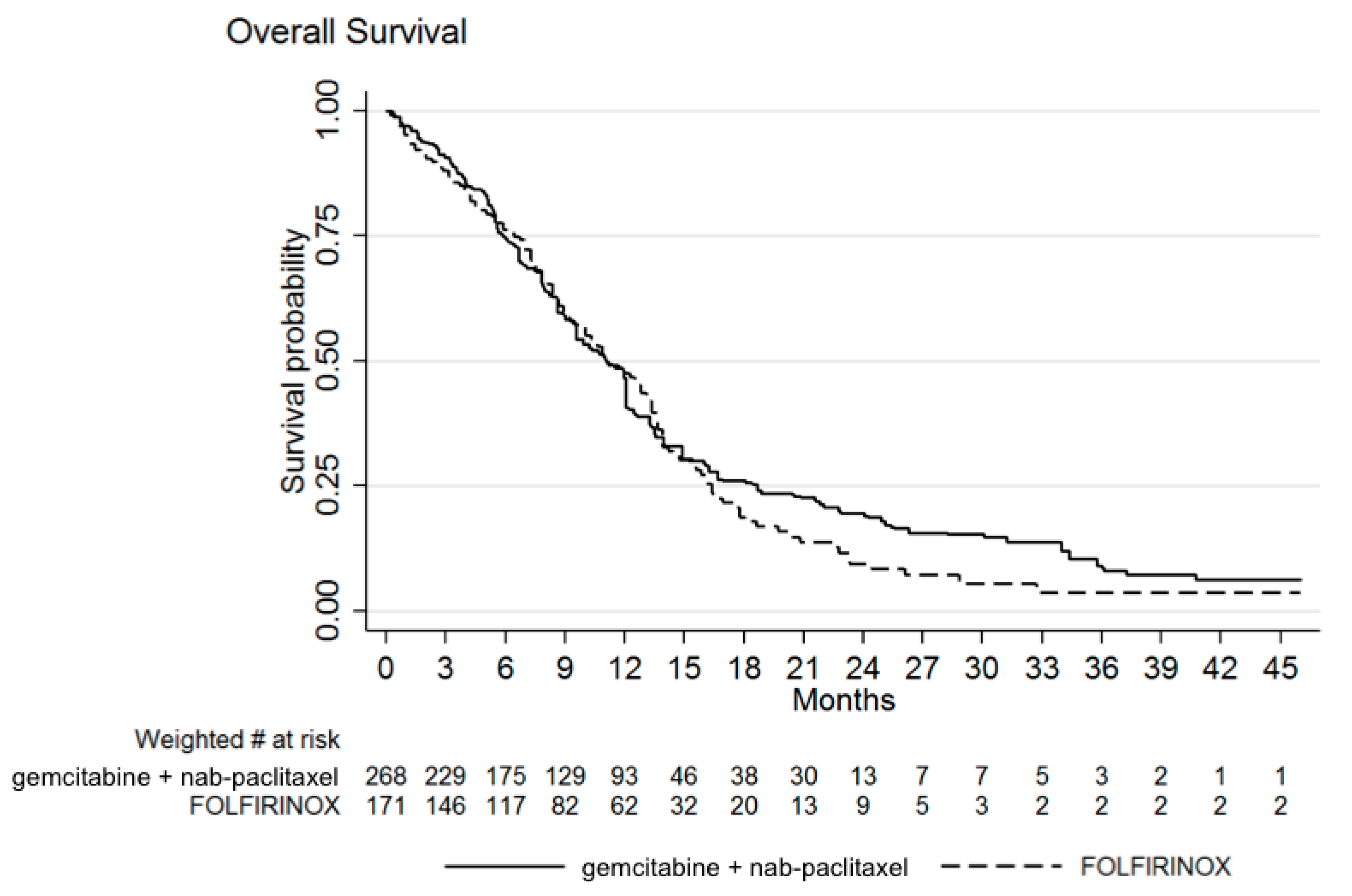
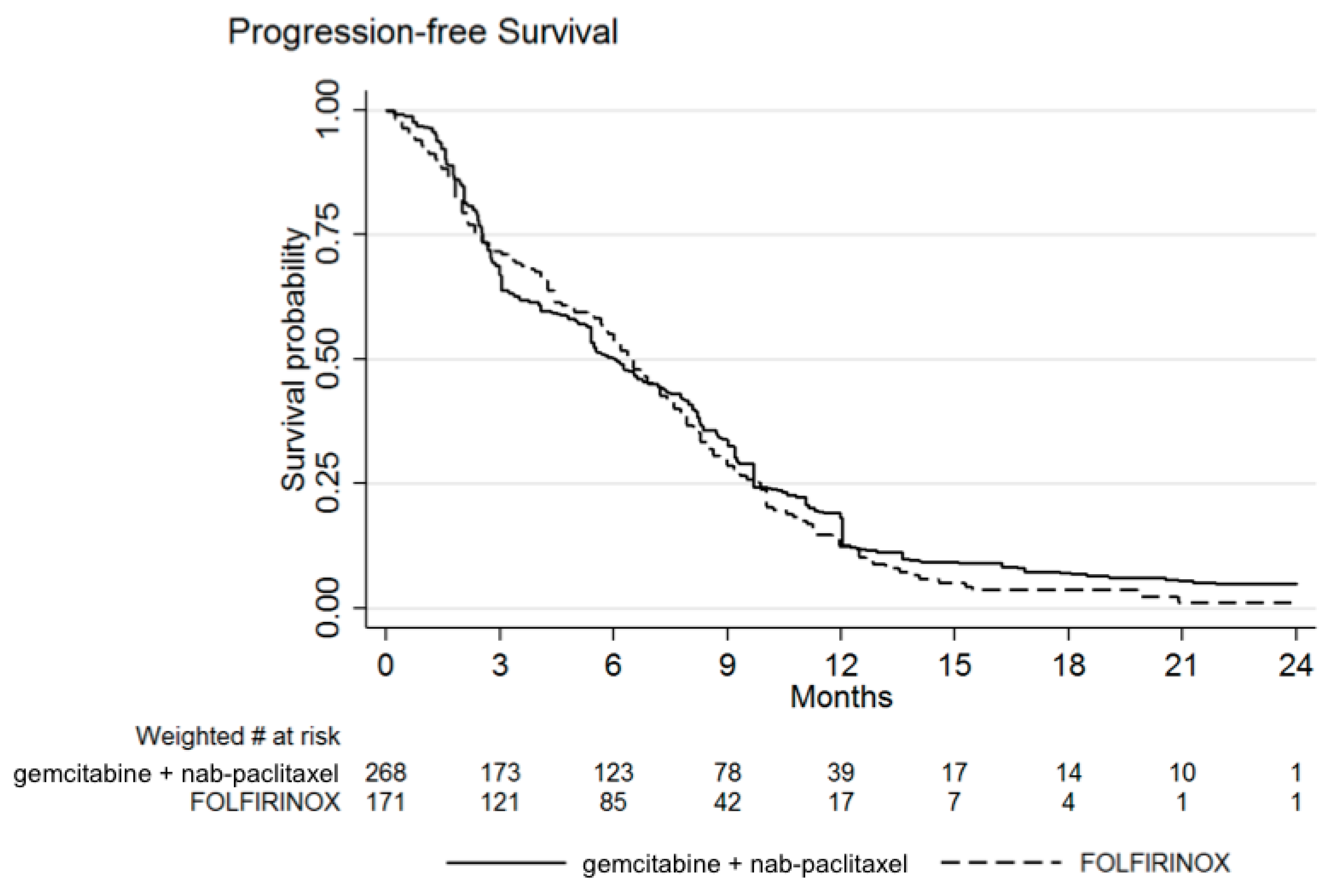
3.1. Efficacy
3.2. Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L. A Glimmer of Hope for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2463–2464. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Reni, M.; Zanon, S.; Peretti, U.; Chiaravalli, M.; Barone, D.; Pircher, C.; Balzano, G.; Macchini, M.; Romi, S.; Gritti, E.; et al. Nab-paclitaxel plus gemcitabine with or without capecitabine and cisplatin in metastatic pancreatic adenocarcinoma (PACT-19): A randomised phase 2 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 691–697. [Google Scholar] [CrossRef]
- Burris, H.A., III; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef]
- Holter, S.; Borgida, A.; Dodd, A.; Grant, R.; Semotiuk, K.; Hedley, D.; Dhani, N.; Narod, S.; Akbari, M.; Moore, M.; et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients with Pancreatic Adenocarcinoma. J. Clin. Oncol. 2015, 33, 3124–3129. [Google Scholar] [CrossRef]
- Wattenberg, M.; Asch, D.; Yu, S.; O’Dwyer, P.J.; Domchek, S.M.; Nathanson, K.L.; Rosen, M.A.; Beatty, G.L.; Siegelman, E.S.; Reiss, K.A. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br. J. Cancer 2020, 122, 333–339. [Google Scholar] [CrossRef]
- Braiteh, F.S.; Patel, M.B.; Parisi, M.; Ni, Q.; Park, S.; Faria, C. Comparative effectiveness and resource utilization of nab-paclitaxel plus gemcitabine vs FOLFIRINOX or gemcitabine for the first-line treatment of metastatic pancreatic adenocarcinoma in a US community setting. Cancer Manag. Res. 2017, ume 9, 141–148. [Google Scholar] [CrossRef]
- Cartwright, T.H.; Parisi, M.; Espirito, J.L.; Wilson, T.W.; Pelletier, C.; Patel, M.; Babiker, H.M. Clinical Outcomes with First-Line Chemotherapy in a Large Retrospective Study of Patients with Metastatic Pancreatic Cancer Treated in a US Community Oncology Setting. Drugs-Real World Outcomes 2018, 5, 149–159. [Google Scholar] [CrossRef]
- Chan, K.K.W.; Guo, H.; Cheng, S.; Beca, J.M.; Redmond-Misner, R.; Isaranuwatchai, W.; Qiao, L.; Earle, C.; Berry, S.R.; Biagi, J.J.; et al. Real-world outcomes of FOLFIRINOX vs gemcitabine and nab-paclitaxel in advanced pancreatic cancer: A population-based propensity score-weighted analysis. Cancer Med. 2019, 9, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Abrams, T.A.; Meyer, G.; Meyerhardt, J.A.; Wolpin, B.M.; Schrag, D.; Fuchs, C.S. Patterns of Chemotherapy Use in a U.S.-Based Cohort of Patients with Metastatic Pancreatic Cancer. Oncologist 2017, 22, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Signorovitch, J.E.; Sikirica, V.; Erder, M.H.; Xie, J.; Lu, M.; Hodgkins, P.S.; Betts, K.A.; Wu, E.Q. Matching-Adjusted Indirect Comparisons: A New Tool for Timely Comparative Effectiveness Research. Value Health 2012, 15, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Guyot, P.; Ades, A.E.; Ouwens, M.J.N.M.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef]
- Franco, F.; Camara, J.C.; Martín-Valadés, J.I.; López-Alfonso, A.; Marrupe, D.; Gutiérrez-Abad, D.; Martínez-Amores, B.; León, A.; Juez, I.; Pérez, M.; et al. Clinical outcomes of FOLFIRINOX and gemcitabine–nab paclitaxel for metastatic pancreatic cancer in the real world setting. Clin. Transl. Oncol. 2021, 23, 812–819. [Google Scholar] [CrossRef]
- Kieler, M.; Unseld, M.; Bianconi, D.; Schindl, M.; Kornek, G.V.; Scheithauer, W.; Prager, G.W. Impact of New Chemotherapy Regimens on the Treatment Landscape and Survival of Locally Advanced and Metastatic Pancreatic Cancer Patients. J. Clin. Med. 2020, 9, 648. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, I.; Yoo, C.; Kim, K.-P.; Jeong, J.H.; Chang, H.-M.; Lee, S.S.; Park, D.H.; Song, T.J.; Seo, D.W.; et al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: Retrospective analysis. Investig. New Drugs 2018, 36, 732–741. [Google Scholar] [CrossRef]
- Kim, S.; Signorovitch, J.E.; Yang, H.; Patterson-Lomba, O.; Xiang, C.Q.; Ung, B.; Parisi, M.; Marshall, J.L. Comparative Effectiveness of nab-Paclitaxel Plus Gemcitabine vs FOLFIRINOX in Metastatic Pancreatic Cancer: A Retrospective Nationwide Chart Review in the United States. Adv. Ther. 2018, 35, 1564–1577. [Google Scholar] [CrossRef]
- Lee, J.-C.; Woo, S.M.; Shin, D.W.; Kim, J.; Yang, S.Y.; Kim, M.J.; Kim, J.W.; Lee, W.J.; Cha, H.S.; Park, P.; et al. Comparison of FOLFIRINOX and Gemcitabine Plus Nab-paclitaxel for Treatment of Metastatic Pancreatic Cancer. Am. J. Clin. Oncol. 2020, 43, 654–659. [Google Scholar] [CrossRef]
- Papneja, N.; Zaidi, A.; Chalchal, H.; Moser, M.; Tan, K.; Olson, C.; Haider, K.; Shaw, J.; Ahmed, S. Comparisons of Out-comes of Real-World Patients with Advanced Pancreatic Cancer Treated with FOLFIRINOX Versus Gemcitabine and Nab-Paclitaxel: A Population-Based Cohort Study. Pancreas 2019, 48, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Chiorean, E.G.; Cheung, W.Y.; Giordano, G.; Kim, G.; Al-Batran, S.-E. Real-world comparative effectiveness of nab-paclitaxel plus gemcitabine versus FOLFIRINOX in advanced pancreatic cancer: A systematic review. Ther. Adv. Med. Oncol. 2019, 11, 11. [Google Scholar] [CrossRef]
- Chan, K.; Shah, K.; Lien, K.; Coyle, D.; Lam, H.; Ko, Y.-J. A Bayesian Meta-Analysis of Multiple Treatment Comparisons of Systemic Regimens for Advanced Pancreatic Cancer. PLoS ONE 2014, 9, e108749. [Google Scholar] [CrossRef]
- Gresham, G.K.; Wells, G.A.; Gill, S.; Cameron, C.; Jonker, D.J. Chemotherapy regimens for advanced pancreatic cancer: A systematic review and network meta-analysis. BMC Cancer 2014, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, S.; Ghidini, M.; Torchio, M.; Corti, F.; Tomasello, G.; Niger, M.; Prinzi, N.; Nichetti, F.; Coinu, A.; Di Bartolomeo, M.; et al. Comparative Effectiveness of Gemcitabine plus nab-Paclitaxel and FOLFIRINOX in the First-Line Setting of Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 484. [Google Scholar] [CrossRef]
- Peixoto, R.D.; Ho, M.; Renouf, D.J.; Lim, H.J.; Gill, S.; Ruan, J.Y.; Cheung, W.Y. Eligibility of Metastatic Pancreatic Cancer Pa-tients for First-Line Palliative Intent NAB-Paclitaxel Plus Gemcitabine Versus FOLFIRINOX. Am. J. Clin. Oncol. 2017, 40, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Camateros, P.; Cheung, W.Y. A Real-World Comparison of FOLFIRINOX, Gemcitabine Plus nab-Paclitaxel, and Gemcitabine in Advanced Pancreatic Cancers. J. Gastrointest. Cancer 2017, 50, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Shirakawa, T.; Shimokawa, M.; Koga, F.; Kawaguchi, Y.; Ueda, Y.; Nakazawa, J.; Komori, A.; Otsu, S.; Arima, S.; et al. A multicenter propensity score analysis of FOLFIRINOX vs gemcitabine plus nab-paclitaxel administered to patients with metastatic pancreatic cancer: Results from the NAPOLEON study. Int. J. Clin. Oncol. 2021, 26, 941–950. [Google Scholar] [CrossRef]
- Williet, N.; Saint, A.; Pointet, A.-L.; Tougeron, D.; Pernot, S.; Pozet, A.; Bechade, D.; Trouilloud, I.; Lourenco, N.; Hautefeuille, V.; et al. Folfirinox versus gemcitabine/nab-paclitaxel as first-line therapy in patients with metastatic pancreatic cancer: A comparative propensity score study. Ther. Adv. Gastroenterol. 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Glatzer, M.; Horber, D.; Montemurro, M.; Winterhalder, R.; Inauen, R.; Berger, M.; Pestalozzi, B.; Pederiva, S.; Pless, M.; Putora, P. Choice of first line systemic treatment in pancreatic cancer among national experts. Pancreatology 2020, 20, 686–690. [Google Scholar] [CrossRef]
- Taieb, J.; Prager, G.W.; Melisi, D.; Westphalen, C.B.; D’Esquermes, N.; Ferreras, A.; Carrato, A.; Macarulla, T. First-line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: A retrospective, observational chart review study. ESMO Open 2020, 5, e000587. [Google Scholar] [CrossRef]
- Martín, A.M.; Hidalgo, M.; Alvarez, R.; Arrazubi, V.; Martínez-Galán, J.; Salgado, M.; Macarulla, T.; Carrato, A. From First Line to Sequential Treatment in the Management of Metastatic Pancreatic Cancer. J. Cancer 2018, 9, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.; Jeong, H.; Cheon, J.; Chon, H.J.; Ryu, H.; Kim, I.-H.; Kang, M.J.; Jeong, J.H.; Ryoo, B.-Y.; Kim, K.-P.; et al. Efficacy and safety of second-line nab-paclitaxel plus gemcitabine after progression on FOLFIRINOX for unresectable or metastatic pancreatic ductal adenocarcinoma: Multicenter retrospective analysis. Ther. Adv. Med. Oncol. 2020, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mita, N.; Iwashita, T.; Uemura, S.; Yoshida, K.; Iwasa, Y.; Ando, N.; Iwata, K.; Okuno, M.; Mukai, T.; Shimizu, M. Second-Line Gemcitabine Plus Nab-Paclitaxel for Patients with Unresectable Advanced Pancreatic Cancer after First-Line FOLFIRINOX Failure. J. Clin. Med. 2019, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Kalyan, A.; Beasley, H.S.; Singhi, A.D.; Sun, W.; Zeh, H.J.; Normolle, D.; Bahary, N. Gemcitabine/nab-paclitaxel as second-line therapy following FOLFIRINOX in metastatic/advanced pancreatic cancer—retrospective analysis of response. J. Gastrointest. Oncol. 2017, 8, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Portal, A.; Pernot, S.; Tougeron, D.; Arbaud, C.; Bidault, A.T.; De La Fouchardière, C.; Hammel, P.; Lecomte, T.; Dréanic, J.; Coriat, R.; et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: An AGEO prospective multicentre cohort. Br. J. Cancer 2015, 113, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Vogl, U.M.; Andalibi, H.; Klaus, A.; Vormittag, L.; Schima, W.; Heinrich, B.; Kafka, A.; Winkler, T.; Öhler, L. Nab-paclitaxel and gemcitabine or FOLFIRINOX as first-line treatment in patients with unresectable adenocarcinoma of the pancreas: Does sequence matter? BMC Cancer 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Sawada, M.; Kasuga, A.; Mie, T.; Furukawa, T.; Taniguchi, T.; Fukuda, K.; Yamada, Y.; Takeda, T.; Kanata, R.; Matsuyama, M.; et al. Modified FOLFIRINOX as a second-line therapy following gemcitabine plus nab-paclitaxel therapy in metastatic pancreatic cancer. BMC Cancer 2020, 20, 449. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Feeney, K.; Lee, M.A.; Muñoz, A.; Gracián, A.C.; Lonardi, S.; Ryoo, B.-Y.; Hung, A.; Lin, Y.; Bendell, J.; et al. Meta-analysis examining overall survival in patients with pancreatic cancer treated with second-line 5-fluorouracil and oxaliplatin-based therapy after failing first-line gemcitabine-containing therapy: Effect of performance status and comparison with other regimens. BMC Cancer 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Hubner, R.A.; Siveke, J.T.; Von Hoff, D.D.; Belanger, B.; de Jong, F.A.; Mirakhur, B.; Chen, L.-T. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer 2019, 108, 78–87. [Google Scholar] [CrossRef]
- Hegewisch-Becker, S.; Aldaoud, A.; Wolf, T.; Krammer-Steiner, B.; Linde, H.; Scheiner-Sparna, R.; Hamm, D.; Jänicke, M.; Marschner, N.; The TPK-Group (Tumour Registry Pancreatic Cancer). Results from the prospective German TPK clinical cohort study: Treatment algorithms and survival of 1174 patients with locally advanced, inoperable, or metastatic pancreatic ductal adenocarcinoma. Int. J. Cancer 2018, 144, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Coyle, D.; Ko, Y.-J.; Coyle, K.; Saluja, R.; Shah, K.; Lien, K.; Lam, H.; Chan, K.K. Cost-Effectiveness Analysis of Systemic Therapies in Advanced Pancreatic Cancer in the Canadian Health Care System. Value Health 2017, 20, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, X.; Qu, S.; Wang, L. Cost-effectiveness analysis of nab-paclitaxel plus gemcitabine versus folfirinox in the treatment of metastatic pancreatic cancer in china. Expert Rev. Pharm. Outcomes Res. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.P.; Parisi, M.F.; Patel, M.B.; Pelletier, C.L.; Belk, K.W. Comparison of treatment patterns, resource utilization, and cost of care in patients with metastatic pancreatic cancer treated with first-linenab-paclitaxel plus gemcitabine or FOLFIRINOX. Expert Rev. Clin. Pharmacol. 2017, 10, 559–565. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.; Bonafede, M.; Cai, Q.; Princic, N.; Tran, O.; Pelletier, C.; Parisi, M.; Patel, M. Comparison of treatment patterns and economic outcomes among metastatic pancreatic cancer patients initiated on nab-paclitaxel plus gemcitabine versus FOLFIRINOX. Expert Rev. Clin. Pharmacol. 2017, 10, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Kim, D.U.; Seol, Y.M.; Kim, S.; Lee, N.K.; Hong, S.B.; Seo, H. Il Comparison of gemcitabine plus nab-paclitaxel and FOLFIRINOX in metastatic pancreatic cancer. World J. Clin. Cases 2020, 8, 3718–3729. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Yu, X. Selecting chemotherapy for pancreatic cancer: Far away or so close? Semin. Oncol. 2019, 46, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Vivaldi, C.; Crucitta, S.; Catanese, S.; Cucchiara, F.; Arrigoni, E.; Pecora, I.; Rofi, E.; Fornaro, L.; Salani, F.; Massa, V.; et al. Comprehensive pharmacogenetic analysis of DPYD, UGT, CDA, and ABCB1 polymorphisms in pancreatic cancer patients receiving mFOLFIRINOX or gemcitabine plus nab-paclitaxel. Pharm. J. 2021, 21, 233–242. [Google Scholar] [CrossRef]
- Vaccaro, V.; Sperduti, I.; Vari, S.; Bria, E.; Melisi, D.; Garufi, C.; Nuzzo, C.; Scarpa, A.; Tortora, G.; Cognetti, F.; et al. Metastatic pancreatic cancer: Is there a light at the end of the tunnel? World J. Gastroenterol. 2015, 21, 4788–4801. [Google Scholar] [CrossRef]
| Before Adjustment | After Adjustment on Subgroup PRODIGE Trial | |||
|---|---|---|---|---|
| Gemcitabine + nab-Paclitaxel (n = 268) | Gemcitabine + nab-Paclitaxel (n = 268) | FOLFIRINOX (n = 171) | d Value | |
| Age (years) | 0.007 | |||
| Median | 69 | 62 | 61 | |
| Range | 33–76 | 33–76 | 34–75 | |
| Sex | 0.001 | |||
| Male | 141 (52.6%) | 166 (61.9%) | 106 (62.0%) | |
| Female | 127 (47.4%) | 102 (38.1%) | 65 (38.0%) | |
| ECOG PS score | ||||
| 0 | 128 (47.8%) | 101 (37.7%) | 64 (37.4%) | 0.006 |
| 1 | 131 (48.9%) | 165 (61.6%) | 106 (61.9%) | 0.007 |
| 2 | 9 (3.4%) | 2 (0.7%) | 1 (0.6%) | 0.005 |
| Tumor Location | ||||
| Head | 127 (47.4%) | 105 (39.3%) | 67 (39.2%) | 0.002 |
| Body | 77 (28.7%) | 83 (31.0%) | 53 (31.0%) | 0.001 |
| Tail | 61 (22.8%) | 70 (26.1%) | 45 (26.3%) | 0.004 |
| Multicentric | 3 (1.1%) | 9 (3.4%) | 6 (3.5%) | 0.008 |
| Biliary Stent | 0.004 | |||
| Yes | 53 (19.8%) | 42 (15.7%) | 27 (15.8%) | |
| No | 215 (80.2%) | 226 (84.3%) | 144 (84.2%) | |
| Level of CA 19-9 | ||||
| Normal | 50 (18.7%) | 39 (14.6%) | 24/164 (14.6%) | 0.001 |
| Elevated, <59 × ULN | 112 (41.8%) | 118 (44.0%) | 72/164 (43.9%) | 0.003 |
| Elevated, ≥59 × ULN | 106 (39.6%) | 111 (41.4%) | 68/164 (41.5%) | 0.002 |
| Metastatic Sites | ||||
| Liver | 199 (74.3%) | 234 (87.3%) | 149/170 (87.6%) | 0.009 |
| Lymph node | 96 (35.8%) | 77 (28.7%) | 49/170 (52.9%) | 0.002 |
| Lung | 59 (22.0%) | 52 (19.4%) | 33/170 (19.4%) | 0.001 |
| Peritoneal | 79 (29.5%) | 52 (19.4%) | 33/170 (19.4%) | 0.001 |
| Other | 27 (10.1%) | 28 (10.4%) | 18/170 (10.6%) | 0.005 |
| Second-Line Therapy | 141 (52.6%) | 126 (47.0%) | 80 (46.8%) | 0.005 |
| Before Adjustment | After Adjustment on Subgroup PRODIGE Trial | |||
|---|---|---|---|---|
| Gemcitabine + nab-Paclitaxel (n = 268) | Gemcitabine + nab-Paclitaxel (n = 268) | FOLFIRINOX (n = 171) | p Value | |
| Hematologic | ||||
| Neutropenia | 108 (40.3%) | 115 (42.9%) | 75/164 (45.7%) | 0.618 |
| Febrile neutropenia | 9 (3.4%) | 13 (4.9%) | 9/166 (5.4%) | 0.824 |
| Thrombocytopenia | 19 (7.1%) | 26 (9.7%) | 15/165 (9.1%) | 0.868 |
| Anemia | 39 (14.6%) | 47 (17.6%) | 13/166 (7.8%) | 0.004 |
| Non-hematologic | ||||
| Fatigue | 48 (17.9%) | 60 (22.4%) | 39/165 (23.6%) | 0.275 |
| Vomiting | 9 (3.4%) | 8 (3.0%) | 24/166 (14.5%) | 0.001 |
| Diarrhea | 13 (4.9%) | 15 (5.6%) | 21/165 (12.7%) | 0.012 |
| Neuropathy | 25 (9.3%) | 25 (9.3%) | 15/166 (9.0%) | 0.742 |
| Thromboembolism | 25 (9.3%) | 26 (9.7%) | 11/166 (6.6%) | 0.294 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapposelli, I.G.; Casadei-Gardini, A.; Vivaldi, C.; Bartolini, G.; Bernardini, L.; Passardi, A.; Frassineti, G.L.; Massa, V.; Cucchetti, A. Equivalent Efficacy but Different Safety Profiles of Gemcitabine Plus Nab-Paclitaxel and FOLFIRINOX in Metastatic Pancreatic Cancer. Biomolecules 2021, 11, 780. https://doi.org/10.3390/biom11060780
Rapposelli IG, Casadei-Gardini A, Vivaldi C, Bartolini G, Bernardini L, Passardi A, Frassineti GL, Massa V, Cucchetti A. Equivalent Efficacy but Different Safety Profiles of Gemcitabine Plus Nab-Paclitaxel and FOLFIRINOX in Metastatic Pancreatic Cancer. Biomolecules. 2021; 11(6):780. https://doi.org/10.3390/biom11060780
Chicago/Turabian StyleRapposelli, Ilario Giovanni, Andrea Casadei-Gardini, Caterina Vivaldi, Giulia Bartolini, Laura Bernardini, Alessandro Passardi, Giovanni Luca Frassineti, Valentina Massa, and Alessandro Cucchetti. 2021. "Equivalent Efficacy but Different Safety Profiles of Gemcitabine Plus Nab-Paclitaxel and FOLFIRINOX in Metastatic Pancreatic Cancer" Biomolecules 11, no. 6: 780. https://doi.org/10.3390/biom11060780
APA StyleRapposelli, I. G., Casadei-Gardini, A., Vivaldi, C., Bartolini, G., Bernardini, L., Passardi, A., Frassineti, G. L., Massa, V., & Cucchetti, A. (2021). Equivalent Efficacy but Different Safety Profiles of Gemcitabine Plus Nab-Paclitaxel and FOLFIRINOX in Metastatic Pancreatic Cancer. Biomolecules, 11(6), 780. https://doi.org/10.3390/biom11060780








