Oximes: Novel Therapeutics with Anticancer and Anti-Inflammatory Potential
Abstract
1. Introduction
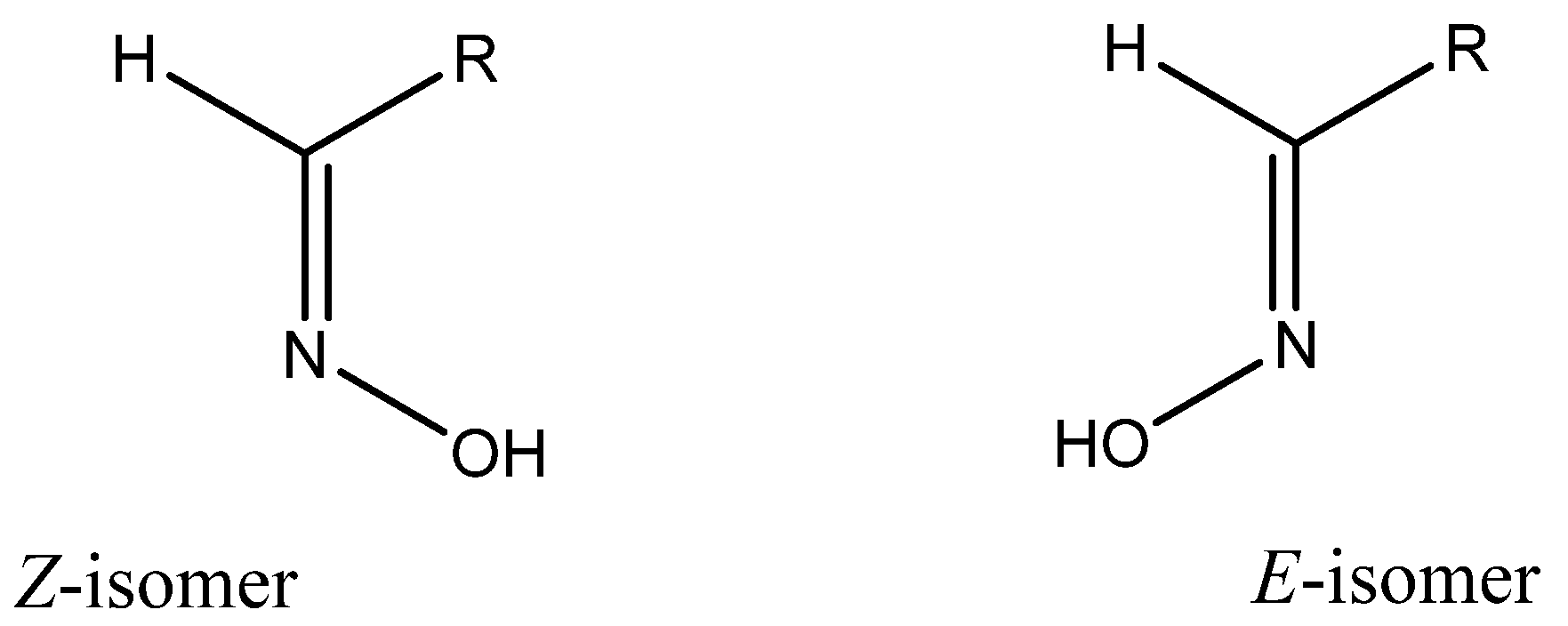
2. Chemical Characterization of Oximes
3. Anticancer Activity of Oximes
4. Anti-Inflammatory Activity of Oximes
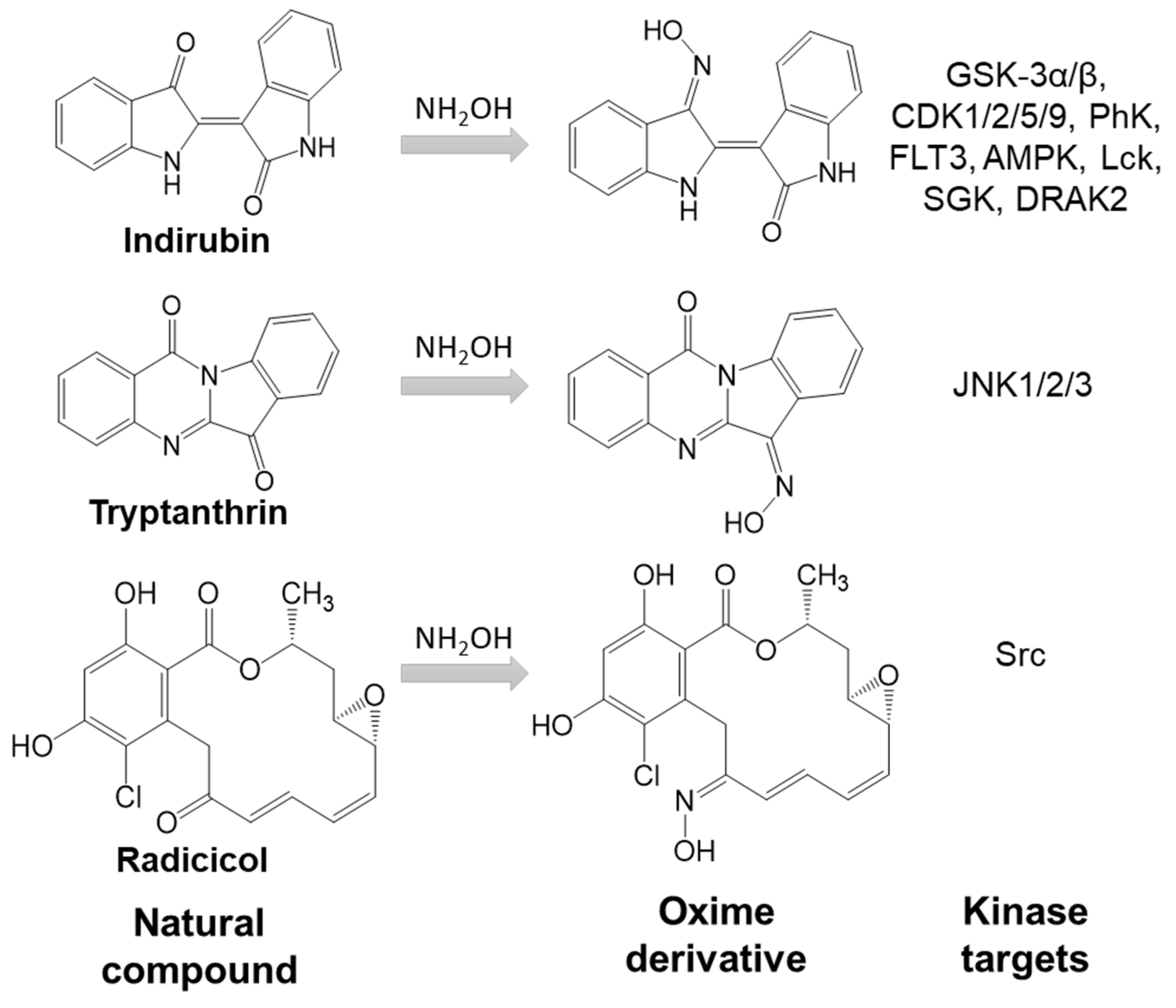
5. Indirubin Oxime-Based Kinase Inhibitors
6. Miscellaneous Oxime Group-Containing Kinase Inhibitors
7. Oximes with Non-kinase Targets
8. Metabolism of Oximes and NO Production
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Kinase Abbreviations | |
| AMPK | AMP-activated protein kinase |
| CDK1/2/5/6/9 | cyclin-dependent kinases |
| Chk1, | checkpoint kinase 1 |
| CK2 | casein kinase 2 |
| DRAK2 | death-associated protein-related apoptotic kinase 2 |
| DYRK | dual-specificity tyrosine-phosphorylated and regulated kinase |
| EGFR | epidermal growth factor receptor tyrosine kinase |
| ERK | extracellular signal-regulated kinase |
| FLT3 | FMS-related receptor tyrosine kinase 3 |
| GSK-3α/β | glycogen synthase kinase 3 |
| IGF1R | receptor of insulin-like growth factor type 1 |
| IRAK4 | interleukin-1 receptor-associated kinase 4 |
| JAK1/2/3 | Janus kinases 1/2/3, tyrosine kinases |
| JNK | c-Jun N-terminal kinase |
| Lck | lymphocyte-specific protein tyrosine kinase |
| Lyn | non-receptor tyrosine-protein kinase |
| PhK | serine/threonine-specific phosphorylase kinase |
| PI3K | phosphatidylinositol 3-kinase |
| PKR | RNA-dependent protein kinase R |
| RSK2 | ribosomal S6 kinase 2 |
| SGK | serine/threonine-protein kinase Sgk1 (serum and glucocorticoid-regulated kinase 1) |
| VEGFR1/2 | vascular endothelial growth factor receptor tyrosine kinase |
| Other Abbreviations | |
| ALP | alkaline phosphatase |
| AP-1 | activator protein 1 |
| ASIC | acid-sensing ion channel |
| ATF-2 | activating transcription factor 2 |
| CAIA | collagen-antibody-induced arthritis |
| CCI | chronic constriction injury |
| CCL | chemokine ligand |
| CCR5 | chemokine receptor 5 |
| CFA | complete Freund’s adjuvant |
| CIA | collagen-induced arthritis |
| COX-2 | cyclooxygenase 2 |
| CysLT | cysteinyl leukotriene |
| DTH | delayed-type hypersensitivity |
| eNOS | endothelial NO synthase |
| FLS | fibroblast-like synoviocytes |
| GluR6 | glutamate receptor 6 |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| HIV | human immunodeficiency virus |
| HNE | human neutrophil elastase |
| HO-1 | heme oxygenase 1 |
| Hsp90 | heat shock protein 90 |
| HUVECs | human umbilical vein endothelial cells |
| IFN | interferon |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| IP-10 | interferon γ-induced protein 10 |
| LO | lipoxygenase |
| LPS | lipopolysaccharide |
| LTB4 | leukotriene B4 |
| MAPK | mitogen-activated protein kinase |
| MCA | middle cerebral artery |
| MCP | monocyte chemoattractant protein |
| MMECs | mouse mammary epithelial cells |
| MMP | matrix metalloproteinase |
| MPO | myeloperoxidase |
| NF-κB | nuclear factor κB |
| NO | nitric oxide |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| OCN | osteocalcin |
| PBMCs | peripheral blood mononuclear cells |
| PDE | phosphodiesterase |
| PGE2 | prostaglandin E2 |
| Pr3 | proteinase 3 |
| PTGS-2 | prostaglandin endoperoxide synthase 2 |
| RANKL | receptor activator of NF-κB ligand |
| RANTES | regulated on activation, normal T cell expressed and secreted |
| ROS | reactive oxygen species |
| Runx2 | runt-related transcription factor 2 |
| S.c. | subcutaneous |
| SOD | superoxide dismutase |
| STAT | signal transducer and activator of transcription |
| TGF-β | transforming growth factor β |
| TLR | Toll-like receptor |
| TNF | tumor necrosis factor |
| TRPA1 | transient receptor potential ankyrin 1 |
| TRPV1 | transient receptor potential vanilloid 1 |
| VDAC, | voltage-dependent anion channel |
| VEGFA | vascular endothelial growth factor A |
| VSMCs | vascular smooth muscle cells |
| Chemical Names | |
| Compound 1 (E231) | indirubin-3′-oxime |
| Compound 2 | indirubin-3′-acetoxime |
| Compound 3 (E804) | indirubin-3′-oxime 2,3-dihydroxypropyl ether |
| Compound 4 (E738) | 5-methoxyindirubin-3′-oxime 1,2-dihydroxyethyl ether |
| Compound 5 | 5′,6′-difluoro-5-methoxy-indirubin-3′-oxime 2-hydroxyethyl ether |
| Compound 6 (LDD970) | 5-[(1-morpholino)carbonyl]indirubin-3′-oxime |
| Compound 7 (AGM130) | 5-nitro-5′-hydroxyindirubin-3′-oxime |
| Compound 8 | 5-(pentanamido)indirubin-3′-oxime |
| Compound 9 (LDD1937) | 5-(methoxycarbonyl)indirubin-3′-oxime 2-(piperazin-1-yl)ethyl ether dihydrochloride |
| Compound 10 | 5-iodoindirubin-3′-oxime |
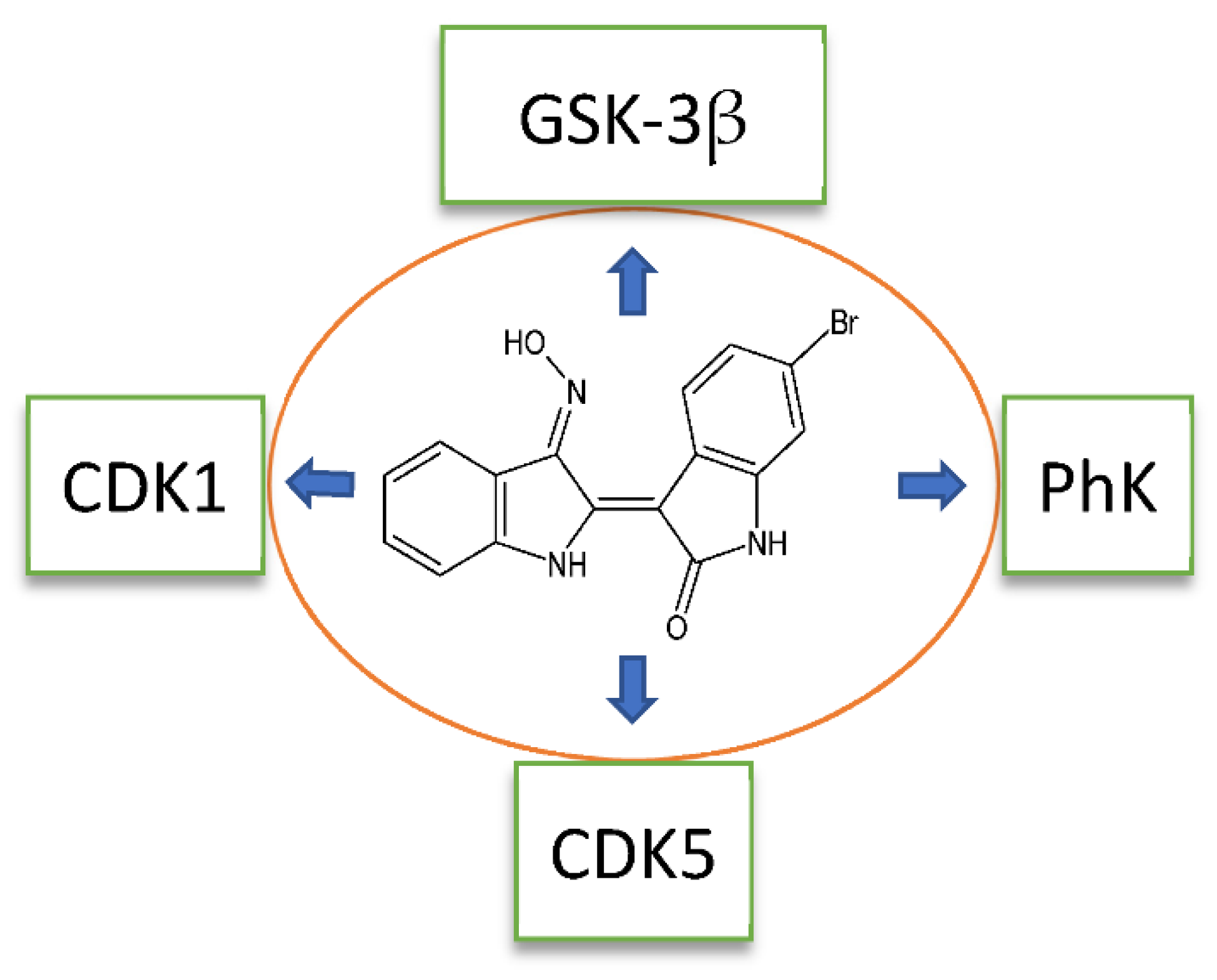
| Compound 11 | 6-bromoindirubin-3′-oxime |
| Compound 12 | 6-bromoindirubin-3′-acetoxime |
| Compound 13 (MLS-2384) | 6-bromoindirubin-3′-oxime 2-(piperazin-1-yl)ethyl ether |
| Compound 14 | 7-bromoindirubin-3′-oxime |
| Compound 15 | 7-bromo-5′-carboxyindirubin-3′-oxime |
| Compound 16 | 5-fluoroindirubin-3′-oxime 2-(piperazin-1-yl)ethyl ether dihydrochloride |
| Compound 17 | radicicol 6-oxime |
| Compound 18 (GDC 0879) | 2,3-dihydro-5-[1-(2-hydroxyethyl)-3-(4-pyridinyl)-1H-pyrazol-4-yl]-1H-inden-1-one oxime |
| Compound 19 (SB 590885) | 5-[2-[4-[2-(dimethylamino)ethoxy]phenyl]-5-(4-pyridinyl)-1H-imidazol-4-yl]-2,3-dihydro-1H-inden-1-one oxime |
| Compound 20 (YM-359445) | (3Z)-3-[6-[(4-methylpiperazin-1-yl)methyl]quinolin-2(1H)-ylidene]-2-oxoindoline-6-carbaldehyde O-(1,3-thiazol-4-ylmethyl)oxime. |
| Compound 21 (JNJ-28871063) | 5E-4-amino-6-(4-benzyloxy-3-chlorophenylamino)pyrimidine-5-carboxaldehyde N-(2-morpholin-4-ylethyl) oxime |
| Compound 22 (JNJ-38158471) | (E)-1-(4-((6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)oxy)-2-chlorophenyl)-3-ethylurea |
| Compound 23 | 1H-indene-1,2,3-trione-2-(phenylhydrazone) 1-oxime |
| Compound 24 | (E)-3-(4-fluorophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde O-(2-fluorobenzyl) oxime |
| Compound 25 | 2,2′-((9-(hydroxyimino)-9H-fluorene-2,7-diyl)bis(oxy))diacetic acid |
| Compound 26 | ((E)-2-(2-(3,4-dichlorophenyl)-2-(hydroxyimino)ethyl)chromeno[4,3-c]pyrazol-4(2H)-one) |
| Compound 27 | (E)-1-(13-isobutyl-4-methyl-6-methylene-2,4,6,7,8,13-hexahydro-1H-indazolo[5,4-a]pyrrolo[3,4-c]carbazol-10-yl)ethan-1-one O-methyl oxime |
| Compound 28 | 2,7-bis(allyloxy)-9H-fluoren-9-one oxime |
| Compound 29 (4-AN) | phenylcyanomethylenequinone oxime-4-(hydroxyimino) cyclohexa-2,5-dien-1-ylidene](phenyl)ethanenitrile |
| Compound 30 (IQ-1) | 11H-indeno[1,2-b]quinoxalin-11-one oxime |
| Compound 31 | tryptanthrin-6-oxime |
| Compound 32 | (E)-4-(N-(2-(1-(hydroxyimino)ethyl)phenyl)sulfamoyl)phenyl pivalate |
| Compound 34 (NS 102) | 6,7,8,9-tetrahydro-5-nitro-1H-benz[g]indole-2,3-dione 3-oxime |
| Compound 35 (SZV-1287) | 3-(4,5-diphenyl-1,3-oxazol-2-yl)propanal oxime |
| Compound 36 (AP 18) | 4-(4-chlorophenyl)-3-methyl-3-buten-2-one oxime |
| Compound 37 (A 967079) | (1E,3E)-1-(4-fluorophenyl)-2-methyl-1-pentene-3-one oxime |
| Compound 38 (NS 383) | 8-ethyl-6,7,8,9-tetrahydro-5-phenyl-1H-pyrrolo[3,2-h]isoquinoline-2,3-dione-3-oxime |
| fMLF | formyl-l-methionyl-l-leucyl-l-phenylalanine |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
References
- Musilek, K.; Dolezal, M.; Gunn-Moore, F.; Kuca, K. Design, evaluation and structure-activity relationship studies of the AChE reactivators against organophosphorus pesticides. Med. Res. Rev. 2011, 31, 548–575. [Google Scholar] [CrossRef]
- Canario, C.; Silvestre, S.; Falcao, A.; Alves, G. Steroidal oximes: Useful compounds with antitumor activities. Curr. Med. Chem. 2018, 25, 660–686. [Google Scholar] [CrossRef]
- Franjesevic, A.J.; Sillart, S.B.; Beck, J.M.; Vyas, S.; Callam, C.S.; Hadad, C.M. Resurrection and reactivation of acetylcholinesterase and butyrylcholinesterase. Chemistry 2019, 25, 5337–5371. [Google Scholar] [CrossRef]
- Sorensen, M.; Neilson, E.H.J.; Moller, B.L. Oximes: Unrecognized chameleons in general and specialized plant metabolism. Mol. Plant 2018, 11, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Fuller, A.T. Antibacterial action of some aromatic amines, amidines, amidoximes, guanidines and diguanides. Biochem. J. 1947, 41, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Varella, E.A.; Nicolaides, D.N. Recent developments in the chemistry and in the biological applications of amidoximes. Curr. Pharm. Des. 2008, 14, 1001–1047. [Google Scholar] [CrossRef]
- Souza, L.G.D.; Almeida, M.C.S.; Lemos, T.L.G.; Ribeiro, P.R.V.; de Brito, E.S.; Silva, V.L.M.; Silva, A.M.S.; Braz, R.; Costa, J.G.M.; Rodrigues, F.F.G.; et al. Synthesis, antibacterial and cytotoxic activities of new biflorin-based hydrazones and oximes. Bioorg. Med. Chem. Lett. 2016, 26, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S.; Kongot, M.; Netalkar, S.P.; Kurjogi, M.M.; Kumar, R.; Avecilla, F.; Kumar, A. Synthesis and evaluation of novel coumarin-oxime ethers as potential anti-tubercular agents: Their DNA cleavage ability and BSA interaction study. Eur. J. Med. Chem. 2018, 150, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Kerrigan, J.E.; Ramachandran, K.; Bender, B.C.; Stanko, J.P.; Jones, S.K.; Patrick, D.A.; Tidwell, R.R. Anti-pneumocystis activities of aromatic diamidoxime prodrugs. Antimicrob. Agents Chemother. 1998, 42, 666–674. [Google Scholar] [CrossRef]
- Clement, B.; Burenheide, A.; Rieckert, W.; Schwarz, J. Diacetyldiamidoximeester of pentamidine, a prodrug for treatment of protozoal diseases: Synthesis, in vitro and in vivo biotransformation. ChemMedChem 2006, 1, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, J.P.; Chen, L.Z.; Wang, J.Q.; Zhou, H.P.; Tang, W.J.; Xue, W.; Liu, X.H. New pentadienone oxime ester derivatives: Synthesis and anti-inflammatory activity. J. Enzym. Inhib. Med. Chem. 2017, 33, 130–138. [Google Scholar] [CrossRef]
- Liu, C.; Tang, X.; Zhang, W.; Li, G.; Chen, Y.; Guo, A.; Hu, C. 6-bromoindirubin-3′-oxime suppresses LPS-induced inflammation via inhibition of the TLR4/NF-κB and TLR4/MAPK signaling pathways. Inflammation 2019, 42, 2192–2204. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Yoon, C.H.; Lee, S.W.; Park, Y.B.; Lee, S.K.; Park, M.C. Inhibition of glycogen synthase kinase-3β suppresses inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes and collagen-induced arthritis. Jt. Bone Spine 2014, 81, 240–246. [Google Scholar] [CrossRef]
- Payrits, M.; Saghy, E.; Matyus, P.; Czompa, A.; Ludmerczki, R.; Deme, R.; Sandor, Z.; Helyes, Z.; Szoke, E. A novel 3-(4,5-diphenyl-1,3-oxazol-2-yl)propanal oxime compound is a potent transient receptor potential ankyrin 1 and vanilloid 1 (TRPA1 and V1) receptor antagonist. Neuroscience 2016, 324, 151–162. [Google Scholar] [CrossRef]
- Hwang, T.L.; Wang, W.H.; Wang, T.Y.; Yu, H.P.; Hsieh, P.W. Synthesis and pharmacological characterization of 2-aminobenzaldehyde oxime analogs as dual inhibitors of neutrophil elastase and proteinase 3. Bioorg. Med. Chem. 2015, 23, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Komai, T.; Yagi, R.; Suzuki-Sunagawa, H.; Ishikawa, Y.; Kasuya, A.; Miyamoto, S.; Handa, H.; Nishigaki, T. Inhibition of HIV-1 protease by oxim derivatives. Biochem. Biophys. Res. Commun. 1997, 230, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Heredia, A.; Davis, C.; Bamba, D.; Le, N.; Gwarzo, M.Y.; Sadowska, M.; Gallo, R.C.; Redfield, R.R. Indirubin-3 ‘-monoxime, a derivative of a chinese antileukemia medicine, inhibits P-TEFb function and HIV-1 replication. AIDS 2005, 19, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Chaubal, R.; Mujumdar, A.M.; Misar, A.; Deshpande, V.H.; Deshpande, N.R. Structure-activity relationship study of androstene steroids with respect to local anti-inflammatory activity. Arzneimittelforschung 2006, 56, 394–398. [Google Scholar] [CrossRef]
- Antoniadou-Vyza, E.; Avramidis, N.; Kourounakis, A.; Hadjipetrou, L. Anti-inflammatory properties of new adamantane derivatives. Design, synthesis, and biological evaluation. Arch. Pharm. 1998, 331, 72–78. [Google Scholar] [CrossRef]
- Zeferino-Diaz, R.; Olivera-Castillo, L.; Davalos, A.; Grant, G.; Kantun-Moreno, N.; Rodriguez-Canul, R.; Bernes, S.; Sandoval-Ramirez, J.; Fernandez-Herrera, M.A. 22-oxocholestane oximes as potential anti-inflammatory drug candidates. Eur. J. Med. Chem. 2019, 168, 78–86. [Google Scholar] [CrossRef]
- Shen, S.; Xu, N.; Klamer, G.; Ko, K.H.; Khoo, M.; Ma, D.; Moore, J.; O’Brien, T.A.; Dolnikov, A. Small-molecule inhibitor of glycogen synthase kinase 3β 6-bromoindirubin-3-oxime inhibits hematopoietic regeneration in stem cell recipient mice. Stem. Cells Dev. 2015, 24, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Castanotto, D.; Nam, S.; Horne, D.; Stein, C. 6bio enhances oligonucleotide activity in cells: A potential combinatorial anti-androgen receptor therapy in prostate cancer cells. Mol. Ther. 2017, 25, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.E.; Huang, R.Z.; Yao, G.Y.; Li, J.L.; Ye, M.Y.; Wang, H.S.; Liu, L. Synthesis and pharmacological evaluation of novel bisindole derivatives bearing oximes moiety: Identification of novel proapoptotic agents. Eur. J. Med. Chem. 2015, 95, 400–415. [Google Scholar] [CrossRef]
- Chiou, C.T.; Lee, W.C.; Liao, J.H.; Cheng, J.J.; Lin, L.C.; Chen, C.Y.; Song, J.S.; Wu, M.H.; Shia, K.S.; Li, W.T. Synthesis and evaluation of 3-ylideneoxindole acetamides as potent anticancer agents. Eur. J. Med. Chem. 2015, 98, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Blazevic, T.; Heiss, E.H.; Atanasov, A.G.; Breuss, J.M.; Dirsch, V.M.; Uhrin, P. Indirubin and indirubin derivatives for counteracting proliferative diseases. Evid. Based Complement. Alternat. Med. 2015, 2015, 654098. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Chen, S.; Zhu, P.; Huang, M.; Gao, W.; Zhu, R.; Qian, J.; Peng, Y.; Zhang, Y.; Dai, H.; et al. Design, synthesis, and biological evaluation of novel thiazolyl substituted bis-pyrazole oxime derivatives with potent antitumor activities by selectively inducing apoptosis and ROS in cancer cells. Med. Chem. 2019, 15, 743–754. [Google Scholar] [CrossRef]
- Galmozzi, E.; Facchetti, F.; La Porta, C.A. Cancer stem cells and therapeutic perspectives. Curr. Med. Chem. 2006, 13, 603–607. [Google Scholar] [CrossRef]
- Avrahami, L.; Farfara, D.; Shaham-Kol, M.; Vassar, R.; Frenkel, D.; Eldar-Finkelman, H. Inhibition of glycogen synthase kinase-3 ameliorates β-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the alzheimer disease mouse model: In vivo and in vitro studies. J. Biol. Chem. 2013, 288, 1295–1306. [Google Scholar] [CrossRef]
- Sathiya Priya, C.; Vidhya, R.; Kalpana, K.; Anuradha, C.V. Indirubin-3′-monoxime prevents aberrant activation of gsk-3beta/nf-kappab and alleviates high fat-high fructose induced abeta-aggregation, gliosis and apoptosis in mice brain. Int. Immunopharmacol. 2019, 70, 396–407. [Google Scholar] [CrossRef]
- Yuskaitis, C.J.; Jope, R.S. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell. Signal. 2009, 21, 264–273. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Wang, K.L.; Liu, Y.X.; Li, Y.Q.; Wang, Q.M. Synthesis and antiviral, insecticidal, and fungicidal activities of gossypol derivatives containing alkylimine, oxime or hydrazine moiety. Bioorg. Med. Chem. 2016, 24, 474–483. [Google Scholar] [CrossRef]
- Hong, S.; Shin, Y.; Jung, M.; Ha, M.W.; Park, Y.; Lee, Y.J.; Shin, J.; Oh, K.B.; Lee, S.K.; Park, H.G. Efficient synthesis and biological activity of psammaplin a and its analogues as antitumor agents. Eur. J. Med. Chem. 2015, 96, 218–230. [Google Scholar] [CrossRef]
- Soga, S.; Neckers, L.M.; Schulte, T.W.; Shiotsu, Y.; Akasaka, K.; Narumi, H.; Agatsuma, T.; Ikuina, Y.; Murakata, C.; Tamaoki, T.; et al. KF25706, a novel oxime derivative of radicicol, exhibits in vivo antitumor activity via selective depletion of Hsp90 binding signaling molecules. Cancer Res. 1999, 59, 2931–2938. [Google Scholar] [PubMed]
- Ikuina, Y.; Amishiro, N.; Miyata, M.; Narumi, H.; Ogawa, H.; Akiyama, T.; Shiotsu, Y.; Akinaga, S.; Murakata, C. Synthesis and antitumor activity of novel O-carbamoylmethyloxime derivatives of radicicol. J. Med. Chem. 2003, 46, 2534–2541. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk-Cwynar, B.; Zaprutko, L. Recent advances in synthesis and biological activity of triterpenic acylated oximes. Phytochem. Rev. 2015, 14, 203–231. [Google Scholar] [CrossRef] [PubMed]
- Vougogiannopoulou, K.; Skaltsounis, A.L. From tyrian purple to kinase modulators: Naturally halogenated indirubins and synthetic analogues. Planta Med. 2012, 78, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, S.; Garnier, M.; Hoessel, R.; Marko, D.; Bibb, J.A.; Snyder, G.L.; Greengard, P.; Biernat, J.; Wu, Y.Z.; Mandelkow, E.M.; et al. Indirubins inhibit glycogen synthase kinase-3β and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in alzheimer’s disease—A property common to most cycline-dependent kinase inhibitors? J. Biol. Chem. 2001, 276, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Khlebnikov, A.I.; Potapov, A.S.; Kovrizhina, A.R.; Matveevskaya, V.V.; Belyanin, M.L.; Atochin, D.N.; Zanoza, S.O.; Gaidarzhy, N.M.; Lyakhov, S.A.; et al. Synthesis, biological evaluation, and molecular modeling of 11H-indeno[1,2-b]quinoxalin-11-one derivatives and tryptanthrin-6-oxime as c-Jun N-terminal kinase inhibitors. Eur. J. Med. Chem. 2019, 161, 179–191. [Google Scholar] [CrossRef]
- Lu, L.; Sha, S.; Wang, K.; Zhang, Y.H.; Liu, Y.D.; Ju, G.D.; Wang, B.; Zhu, H.L. Discovery of chromeno[4,3-c]pyrazol-4(2H)-one containing carbonyl or oxime derivatives as potential, selective inhibitors PI3Kα. Chem. Pharm. Bull. 2016, 64, 1576–1581. [Google Scholar] [CrossRef]
- Begum, J.; Skamnaki, V.T.; Moffatt, C.; Bischler, N.; Sarrou, J.; Skaltsounis, A.L.; Leonidas, D.D.; Oikonomakos, N.G.; Hayes, J.M. An evaluation of indirubin analogues as phosphorylase kinase inhibitors. J. Mol. Graph. Model. 2015, 61, 231–242. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Hanks, T.S.; Kochetkova, I.; Pascual, D.W.; Jutila, M.A.; Quinn, M.T. Identification and characterization of a novel class of c-Jun N-terminal kinase inhibitors. Mol. Pharmacol. 2012, 81, 832–845. [Google Scholar] [CrossRef]
- Nam, S.; Scuto, A.; Yang, F.; Chen, W.; Park, S.; Yoo, H.S.; Konig, H.; Bhatia, R.; Cheng, X.; Merz, K.H.; et al. Indirubin derivatives induce apoptosis of chronic myelogenous leukemia cells involving inhibition of STAT5 signaling. Mol. Oncol. 2012, 6, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Hoessel, R.; Leclerc, S.; Endicott, J.A.; Nobel, M.E.; Lawrie, A.; Tunnah, P.; Leost, M.; Damiens, E.; Marie, D.; Marko, D.; et al. Indirubin, the active constituent of a chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999, 1, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.; Skaltsounis, A.L.; Magiatis, P.; Polychronopoulos, P.; Knockaert, M.; Leost, M.; Ryan, X.P.; Vonica, C.A.; Brivanlou, A.; Dajani, R.; et al. GSK-3-selective inhibitors derived from tyrian purple indirubins. Chem. Biol. 2003, 10, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.K.; Kwok, H.H.; Chan, L.S.; Leung, K.S.; Shi, J.; Mak, N.K.; Wong, R.N.; Yue, P.Y. An indirubin derivative, E804, exhibits potent angiosuppressive activity. Biochem. Pharmacol. 2012, 83, 598–607. [Google Scholar] [CrossRef]
- Nam, S.; Buettner, R.; Turkson, J.; Kim, D.; Cheng, J.Q.; Muehlbeyer, S.; Hippe, F.; Vatter, S.; Merz, K.H.; Eisenbrand, G.; et al. Indirubin derivatives inhibit STAT3 signaling and induce apoptosis in human cancer cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5998–6003. [Google Scholar] [CrossRef]
- Nam, S.; Wen, W.; Schroeder, A.; Herrmann, A.; Yu, H.; Cheng, X.; Merz, K.H.; Eisenbrand, G.; Li, H.; Yuan, Y.C.; et al. Dual inhibition of janus and src family kinases by novel indirubin derivative blocks constitutively-activated STAT3 signaling associated with apoptosis of human pancreatic cancer cells. Mol. Oncol. 2013, 7, 369–378. [Google Scholar] [CrossRef]
- Cheng, X.; Merz, K.H.; Vatter, S.; Christ, J.; Wolfl, S.; Eisenbrand, G. 7,7′-diazaindirubin--a small molecule inhibitor of casein kinase 2 in vitro and in cells. Bioorg. Med. Chem. 2014, 22, 247–255. [Google Scholar] [CrossRef]
- Choi, S.J.; Moon, M.J.; Lee, S.D.; Choi, S.U.; Han, S.Y.; Kim, Y.C. Indirubin derivatives as potent FLT3 inhibitors with anti-proliferative activity of acute myeloid leukemic cells. Bioorg. Med. Chem. Lett. 2010, 20, 2033–2037. [Google Scholar] [CrossRef]
- Bain, J.; McLauchlan, H.; Elliott, M.; Cohen, P. The specificities of protein kinase inhibitors: An update. Biochem. J. 2003, 371, 199–204. [Google Scholar] [CrossRef]
- Jung, M.E.; Byun, B.J.; Kim, H.M.; Lee, J.Y.; Park, J.H.; Lee, N.; Son, Y.H.; Choi, S.U.; Yang, K.M.; Kim, S.J.; et al. Discovery of indirubin derivatives as new class of DRAK2 inhibitors from high throughput screening. Bioorg. Med. Chem. Lett. 2016, 26, 2719–2723. [Google Scholar] [CrossRef]
- Cheng, X.L.; Merz, K.H.; Vatter, S.; Zeller, J.; Muehlbeyer, S.; Thommet, A.; Christ, J.; Wolfl, S.; Eisenbrand, G. Identification of a water-soluble indirubin derivative as potent inhibitor of insulin-like growth factor 1 receptor through structural modification of the parent natural molecule. J. Med. Chem. 2017, 60, 4949–4962. [Google Scholar] [CrossRef]
- Yan, L.; Lai, F.F.; Chen, X.G.; Xiao, Z.Y. Discovery of novel indirubin-3 ‘-monoxime derivatives as potent inhibitors against CDK2 and CDK9. Bioorg. Med. Chem. Lett. 2015, 25, 2447–2451. [Google Scholar] [CrossRef]
- Ndolo, K.M.; Park, K.R.; Lee, H.J.; Bin Yoon, K.; Kim, Y.C.; Han, S.Y. Characterization of the indirubin derivative LDD970 as a small molecule aurora kinase a inhibitor in human colorectal cancer cells. Immune. Netw. 2017, 17, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Lee, J.E.; Jeong, S.Y.; Im, I.; Lee, S.D.; Lee, E.J.; Lee, S.K.; Kwon, S.M.; Ahn, S.G.; Yoon, J.H.; et al. 5,5′-Substituted indirubin-3′-oxime derivatives as potent cyclin-dependent kinase inhibitors with anticancer activity. J. Med. Chem. 2010, 53, 3696–3706. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.; Jeong, P.; Choi, J.; Baek, J.; Ahn, S.J.; Moon, Y.; Heo, J.D.; Choi, Y.H.; Chin, Y.W.; et al. Discovery of a FLT3 inhibitor LDD1937 as an anti-leukemic agent for acute myeloid leukemia. Oncotarget 2018, 9, 924–936. [Google Scholar] [CrossRef]
- Myrianthopoulos, V.; Magiatis, P.; Ferandin, Y.; Skaltsounis, A.L.; Meijer, L.; Mikros, E. An integrated computational approach to the phenomenon of potent and selective inhibition of aurora kinases B and C by a series of 7-substituted indirubins. J. Med. Chem. 2007, 50, 4027–4037. [Google Scholar] [CrossRef] [PubMed]
- Myrianthopoulos, V.; Kritsanida, M.; Gaboriaud-Kolar, N.; Magiatis, P.; Ferandin, Y.; Durieu, E.; Lozach, O.; Cappel, D.; Soundararajan, M.; Filippakopoulos, P.; et al. Novel inverse binding mode of indirubin derivatives yields improved selectivity for DYRK kinases. ACS Med. Chem. Lett. 2013, 4, 22–26. [Google Scholar] [CrossRef]
- Vougogiannopoulou, K.; Ferandin, Y.; Bettayeb, K.; Myrianthopoulos, V.; Lozach, O.; Fan, Y.; Johnson, C.H.; Magiatis, P.; Skaltsounis, A.L.; Mikros, E.; et al. Soluble 3‘,6-substituted indirubins with enhanced selectivity toward glycogen synthase kinase-3 alter circadian period. J. Med. Chem. 2008, 51, 6421–6431. [Google Scholar] [CrossRef] [PubMed]
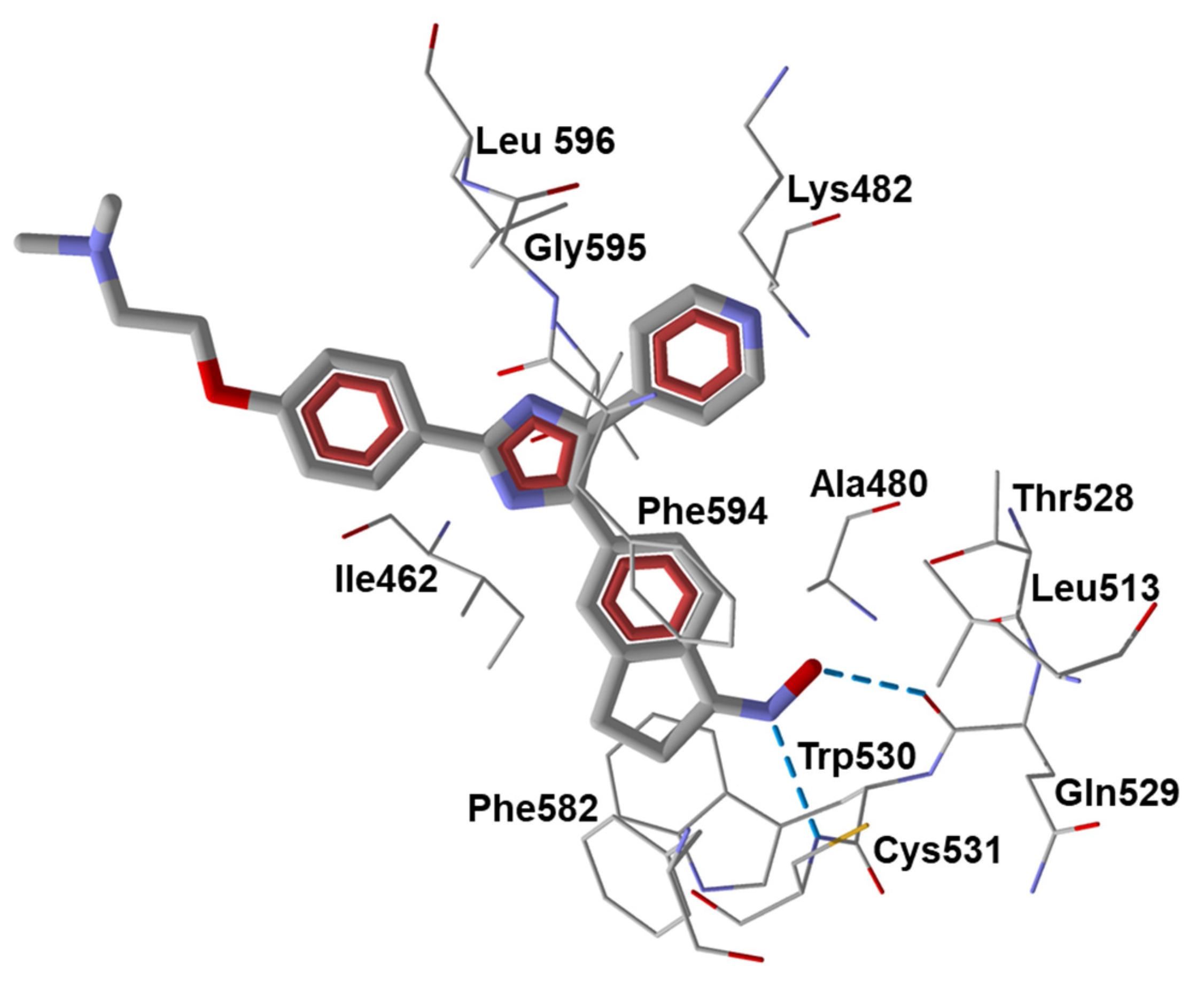
- Liu, L.; Gaboriaud, N.; Vougogianopoulou, K.; Tian, Y.; Wu, J.; Wen, W.; Skaltsounis, L.; Jove, R. MLS-2384, a new 6-bromoindirubin derivative with dual JAK/Src kinase inhibitory activity, suppresses growth of diverse cancer cells. Cancer Biol. Ther. 2014, 15, 178–184. [Google Scholar] [CrossRef]
- Jeong, P.; Moon, Y.; Lee, J.H.; Lee, S.D.; Park, J.; Lee, J.; Kim, J.; Lee, H.J.; Kim, N.Y.; Choi, J.; et al. Discovery of orally active indirubin-3 ‘-oxime derivatives as potent type 1 FLT3 inhibitors for acute myeloid leukemia. Eur. J. Med. Chem. 2020, 195, 112205. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.D.; Grina, J.; Newhouse, B.; Welch, M.; Topalov, G.; Littman, N.; Callejo, M.; Gloor, S.; Martinson, M.; Laird, E.; et al. Potent and selective pyrazole-based inhibitors of b-raf kinase. Bioorg. Med. Chem. Lett. 2008, 18, 4692–4695. [Google Scholar] [CrossRef] [PubMed]
- Takle, A.K.; Brown, M.J.B.; Davies, S.; Dean, D.K.; Francis, G.; Gaiba, A.; Hird, A.W.; King, F.D.; Lovell, P.J.; Naylor, A.; et al. The identification of potent and selective imidazole-based inhibitors of B-Raf kinase. Bioorg. Med. Chem. Lett. 2006, 16, 378–381. [Google Scholar] [CrossRef]
- Amino, N.; Ideyama, Y.; Yamano, M.; Kuromitsu, S.; Tajinda, K.; Samizu, K.; Hisamichi, H.; Matsuhisa, A.; Shirasuna, K.; Kudoh, M.; et al. YM-359445, an orally bioavailable vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor, has highly potent antitumor activity against established tumors. Clin. Cancer Res. 2006, 12, 1630–1638. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Emanuel, S.L.; Hughes, T.V.; Adams, M.; Rugg, C.A.; Fuentes-Pesquera, A.; Connolly, P.J.; Pandey, N.; Moreno-Mazza, S.; Butler, J.; Borowski, V.; et al. Cellular and in vivo activity of JNJ-28871063, a nonquinazoline pan-ErbB kinase inhibitor that crosses the blood-brain barrier and displays efficacy against intracranial tumors. Mol. Pharmacol. 2008, 73, 338–348. [Google Scholar] [CrossRef]
- LaMontagne, K.R.; Butler, J.; Borowski, V.B.; Fuentes-Pesquera, A.R.; Blevitt, J.M.; Huang, S.L.; Li, R.H.; Connolly, P.J.; Greenberger, L.M. A highly selective, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor has potent activity in vitro and in vivo. Angiogenesis 2009, 12, 287–296. [Google Scholar] [CrossRef]
- Cavasotto, C.N.; Ortiz, M.A.; Abagyan, R.A.; Piedrafita, F.J. In silico identification of novel EGFR inhibitors with antiproliferative activity against cancer cells. Bioorg. Med. Chem. Lett. 2006, 16, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.H.; Li, Q.S.; Ren, Z.L.; Chu, M.J.; Sun, J.; Zhang, X.; Xing, M.; Zhu, H.L.; Cao, H.Q. (E)-1,3-diphenyl-1H-pyrazole derivatives containing O-benzyl oxime moiety as potential immunosuppressive agents: Design, synthesis, molecular docking and biological evaluation. Eur. J. Med. Chem. 2016, 108, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Foloppe, N.; Fisher, L.M.; Howes, R.; Potter, A.; Robertson, A.G.S.; Surgenor, A.E. Identification of chemically diverse Chk1 inhibitors by receptor-based virtual screening. Biorg. Med. Chem. 2006, 14, 4792–4802. [Google Scholar] [CrossRef]
- Dandu, R.; Zulli, A.L.; Bacon, E.R.; Underiner, T.; Robinson, C.; Chang, H.; Miknyoczki, S.; Grobelny, J.; Ruggeri, B.A.; Yang, S.; et al. Design and synthesis of dihydroindazolo[5,4-a] pyrrolo[3,4-c] carbazole oximes as potent dual inhibitors of TIE-2 and VEGF-R2 receptor tyrosine kinases. Bioorg. Med. Chem. Lett. 2008, 18, 1916–1921. [Google Scholar] [CrossRef]
- Maslyk, M.; Janeczko, M.; Demchuk, O.M.; Boguszewska-Czubara, A.; Golczyk, H.; Sieroslawska, A.; Rymuszka, A.; Martyna, A.; Kubinski, K. A representative of arylcyanomethylenequinone oximes effectively inhibits growth and formation of hyphae in Candida albicans and influences the activity of protein kinases in vitro. Saudi Pharm. J. 2018, 26, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ansideri, F.; Dammann, M.; Boeckler, F.M.; Koch, P. Fluorescence polarization-based competition binding assay for c-Jun N-terminal kinases 1 and 2. Anal. Biochem. 2017, 532, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Karabatsos, G.J.; Taller, R.A. Structural studies by nuclear magnetic resonance XV. Conformations and configurations of oximes. Tetrahedron 1968, 24, 3347–3360. [Google Scholar] [CrossRef]
- Claassen, V.; Davies, J.E.; Hertting, G.; Placheta, P. Fluvoxamine, a specific 5-hydroxytryptamine uptake inhibitor. Br. J. Pharmacol. 1977, 60, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Bohle, D.S.; Chua, Z.; Perepichka, I.; Rosadiuk, K. E/Z oxime isomerism in PhC(NOH)CN. Chem. Eur. J. 2013, 19, 4223–4229. [Google Scholar] [CrossRef] [PubMed]
- Wylie, B.B.; Isaacson, E.I.; Delgado, J.N. Synthesis of oxime esters and ethers as potential psychotropic aegents. J. Pharmaceut. Sci. 1965, 54, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Hatem, J.; Henriet-Bernard, C.; Grimaldi, J.; Maurin, R. Radical Cyclization of β-allenic oxime ethers. Tetrahedron Lett. 1992, 3, 1057–1058. [Google Scholar] [CrossRef]
- Kurtz, A.P.; D’Silva, T.D. Estimation of dissociation constants (pKa’s) of oximes from proton chemical shifts in dimethyl sulfoxide solution. J. Pharm. Sci. 1987, 76, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Musil, K.; Florianova, V.; Bucek, P.; Dohnal, V.; Kuca, K.; Musilek, K. Development and validation of a FIA/UV-vis method for pK(a) determination of oxime based acetylcholinesterase reactivators. J. Pharmaceut. Biomed. Anal. 2016, 117, 240–246. [Google Scholar] [CrossRef]
- Sano, M.; Ichimaru, Y.; Kurita, M.; Hayashi, E.; Homma, T.; Saito, H.; Masuda, S.; Nemoto, N.; Hemmi, A.; Suzuki, T.; et al. Induction of cell death in pancreatic ductal adenocarcinoma by indirubin 3 ‘-oxime and 5-methoxyindirubin 3 ‘-oxime in vitro and in vivo. Cancer Lett. 2017, 397, 72–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, L.; Li, J.; Zhang, Y.; Lu, X.; Zhang, B. Inhibitory effects of indirubin-3′-monoxime against human osteosarcoma. IUBMB Life 2019, 71, 1465–1474. [Google Scholar] [CrossRef]
- Lee, M.Y.; Li, Y.Z.; Huang, K.J.; Huang, H.C.; Lin, C.Y.; Lee, Y.R. Indirubin-3′-oxime suppresses human cholangiocarcinoma through cell-cycle arrest and apoptosis. Eur. J. Pharmacol. 2018, 839, 57–65. [Google Scholar] [CrossRef]
- Nicolaou, K.A.; Liapis, V.; Evdokiou, A.; Constantinou, C.; Magiatis, P.; Skaltsounis, A.L.; Koumas, L.; Costeas, P.A.; Constantinou, A.I. Induction of discrete apoptotic pathways by bromo-substituted indirubin derivatives in invasive breast cancer cells. Biochem. Bioph. Res. Commun. 2012, 425, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Broecker-Preuss, M.; Becher-Boveleth, N.; Gall, S.; Rehmann, K.; Schenke, S.; Mann, K. Induction of atypical cell death in thyroid carcinoma cells by the indirubin derivative 7-bromoindirubin-3′-oxime (7BIO). Cancer Cell Int. 2015, 15, 97. [Google Scholar] [CrossRef][Green Version]
- Ribas, J.; Bettayeb, K.; Ferandin, Y.; Knockaert, M.; Garrofe-Ochoa, X.; Totzke, F.; Schachtele, C.; Mester, J.; Polychronopoulos, P.; Magiatis, P.; et al. 7-bromoindirubin-3′-oxime induces caspase-independent cell death. Oncogene 2006, 25, 6304–6318. [Google Scholar] [CrossRef]
- Fu, B.; Yin, G.; Song, K.; Mu, X.; Xu, B.; Zhang, X. Indirubin-3′-oxime (IDR3O) inhibits proliferation of osteosarcoma cells in vitro and tumor growth in vivo through AMPK-activation and PGC-1α/TFAM up-regulation. Dokl. Biochem. Biophys. 2020, 495, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Brighi, N.; Conteduca, V.; Lolli, C.; Gurioli, G.; Schepisi, G.; Palleschi, M.; Mariotti, M.; Casadei, C.; De Giorgi, U. The cyclin-dependent kinases pathway as a target for prostate cancer treatment: Rationale and future perspectives. Crit. Rev. Oncol. Hematol. 2021, 157, 103199. [Google Scholar] [CrossRef]
- Augello, G.; Emma, M.R.; Cusimano, A.; Azzolina, A.; Montalto, G.; McCubrey, J.A.; Cervello, M. The role of GSK-3 in cancer immunotherapy: GSK-3 inhibitors as a new frontier in cancer treatment. Cells 2020, 9, 1427. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.; Eturi, A.; De Souza, A.; Pamarthy, S.; Tavora, F.; Giles, F.J.; Carneiro, B.A. Glycogen synthase kinase-3β inhibitors as novel cancer treatments and modulators of antitumor immune responses. Cancer Biol. Ther. 2019, 20, 1047–1056. [Google Scholar] [CrossRef]
- Qi, G.; Liu, J.; Mi, S.; Tsunematsu, T.; Jin, S.; Shao, W.; Liu, T.; Ishimaru, N.; Tang, B.; Kudo, Y. Aurora kinase inhibitors in head and neck cancer. Curr. Top. Med. Chem. 2018, 18, 199–213. [Google Scholar] [CrossRef]
- Falchook, G.S.; Bastida, C.C.; Kurzrock, R. Aurora kinase inhibitors in oncology clinical trials: Current state of the progress. Semin. Oncol. 2015, 42, 832–848. [Google Scholar] [CrossRef]
- Yuan, T.; Qi, B.; Jiang, Z.; Dong, W.; Zhong, L.; Bai, L.; Tong, R.; Yu, J.; Shi, J. Dual FLT3 inhibitors: Against the drug resistance of acute myeloid leukemia in recent decade. Eur. J. Med. Chem. 2019, 178, 468–483. [Google Scholar] [CrossRef]
- Alim, K.; Bruyere, A.; Lescoat, A.; Jouan, E.; Lecureur, V.; Le Vee, M.; Fardel, O. Interactions of janus kinase inhibitors with drug transporters and consequences for pharmacokinetics and toxicity. Expert Opin. Drug Metab. Toxicol. 2021, 1–13. [Google Scholar] [CrossRef]
- Abbassi, R.; Johns, T.G.; Kassiou, M.; Munoz, L. DYRK1A in neurodegeneration and cancer: Molecular basis and clinical implications. Pharmacol. Therapeut. 2015, 151, 87–98. [Google Scholar] [CrossRef]
- Guo, T.; Ma, S. Recent advances in the discovery of multitargeted tyrosine kinase inhibitors as anticancer agents. ChemMedChem 2021, 16, 600–620. [Google Scholar] [CrossRef] [PubMed]
- Gerritse, S.L.; Janssen, J.B.E.; Labots, M.; de Vries, R.; Rudek, M.; Carducci, M.; van Erp, N.P.; Verheul, H.M.W. High-dose administration of tyrosine kinase inhibitors to improve clinical benefit: A systematic review. Cancer Treat. Rev. 2021, 97, 102171. [Google Scholar] [CrossRef]
- Yumura, M.; Nagano, T.; Nishimura, Y. Novel multitarget therapies for lung cancer and respiratory disease. Molecules 2020, 25, 3987. [Google Scholar] [CrossRef] [PubMed]
- Sola, A.M.; Johnson, D.E.; Grandis, J.R. Investigational multitargeted kinase inhibitors in development for head and neck neoplasms. Expert Opin. Investig. Drugs 2019, 28, 351–363. [Google Scholar] [CrossRef]
- Basolo, A.; Matrone, A.; Elisei, R.; Santini, F. Effects of tyrosine kinase inhibitors on thyroid function and thyroid hormone metabolism. Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef]
- Sundar, V.; Vimal, S.; Mithlesh, M.S.S.; Dutta, A.; Tamizhselvi, R.; Manickam, V. Transcriptional cyclin-dependent kinases as the mediators of inflammation—A review. Gene 2021, 769, 145200. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Rehani, K.; Jope, R.S.; Michalek, S.M. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005, 6, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Michalek, S.M.; Jope, R.S. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010, 31, 24–31. [Google Scholar] [CrossRef]
- Maity, A.; Sen, D.; Kandar, C.C. Anti-inflammatory potential of GSK-3 inhibitors. Curr. Drug Targets 2021. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.; Jain, P.; Jadhav, H.R. Glycogen synthase kinase 3 (GSK3): Its role and inhibitors. Curr. Top. Med. Chem. 2020, 20, 1522–1534. [Google Scholar] [CrossRef]
- Simon, L.S.; Taylor, P.C.; Choy, E.H.; Sebba, A.; Quebe, A.; Knopp, K.L.; Porreca, F. The JAK/STAT pathway: A focus on pain in rheumatoid arthritis. Semin. Arthritis. Rheum. 2021, 51, 278–284. [Google Scholar] [CrossRef]
- Choudhary, S.A.; Bora, N.; Banerjee, D.; Arora, L.; Das, A.S.; Yadav, R.; Klotz, K.N.; Pal, D.; Jha, A.N.; Dasgupta, S. A novel small molecule A2A adenosine receptor agonist, indirubin-3′-monoxime, alleviates lipid-induced inflammation and insulin resistance in 3T3-l1 adipocytes. Biochem. J. 2019, 476, 2371–2391. [Google Scholar] [CrossRef] [PubMed]
- Udumula, M.P.; Medapi, B.; Dhar, I.; Bhat, A.; Desai, K.; Sriram, D.; Dhar, A. The small molecule indirubin-3′-oxime inhibits protein kinase R: Antiapoptotic and antioxidant effect in rat cardiac myocytes. Pharmacology 2016, 97, 25–30. [Google Scholar] [CrossRef]
- Jung, H.J.; Nam, K.N.; Son, M.S.; Kang, H.; Hong, J.W.; Kim, J.W.; Lee, E.H. Indirubin-3′-oxime inhibits inflammatory activation of rat brain microglia. Neurosci. Lett. 2011, 487, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Blazevic, T.; Schaible, A.M.; Weinhaupl, K.; Schachner, D.; Nikels, F.; Weinigel, C.; Barz, D.; Atanasov, A.G.; Pergola, C.; Werz, O.; et al. Indirubin-3′-monoxime exerts a dual mode of inhibition towards leukotriene-mediated vascular smooth muscle cell migration. Cardiovasc. Res. 2014, 101, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.K.; Kang, S.S.; Chan, R.W.; Yue, P.Y.; Mak, N.K.; Poon, L.L.; Wong, R.N.; Peiris, J.S.; Chan, M.C. Anti-inflammatory and antiviral effects of indirubin derivatives in influenza a (H5N1) virus infected primary human peripheral blood-derived macrophages and alveolar epithelial cells. Antiviral. Res. 2014, 106, 95–104. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, J.; Lin, J.; Jin, L.; Yu, R.; Mak, S.; Hu, S.; Sun, H.; Wu, X.; Zhang, Z.; et al. Indirubin-3-oxime prevents H2O2-induced neuronal apoptosis via concurrently inhibiting GSK3β and the ERK pathway. Cell. Mol. Neurobiol. 2017, 37, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, G.M. Indirubin-3-monoxime exhibits anti-inflammatory properties by down-regulating NF-κB and JNK signaling pathways in lipopolysaccharide-treated RAW264.7 cells. Inflamm. Res. 2012, 61, 319–325. [Google Scholar] [CrossRef]
- Park, D.W.; Jiang, S.; Liu, Y.; Siegal, G.P.; Inoki, K.; Abraham, E.; Zmijewski, J.W. GSK3β-dependent inhibition of AMPK potentiates activation of neutrophils and macrophages and enhances severity of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L735–L745. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, M.B.; Aliev, O.I.; Shamanaev, A.Y.; Sidekhmenova, A.V.; Anishchenko, A.M.; Fomina, T.I.; Rydchenko, V.S.; Khlebnikov, A.I.; Anfinogenova, Y.J.; Schepetkin, I.A.; et al. Antihypertensive activity of a new c-Jun N-terminal kinase inhibitor in spontaneously hypertensive rats. Hypertens. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Xia, X.; Zhao, Y.; Zhang, S.; Zhang, Y.; Wang, J. JNK selective inhibitor, IQ-1S, protects the mice against lipopolysaccharides-induced sepsis. Bioorg. Med. Chem. 2021, 30, 115945. [Google Scholar] [CrossRef]
- Kirpotina, L.N.; Schepetkin, I.A.; Hammaker, D.; Kuhs, A.; Khlebnikov, A.I.; Quinn, M.T. Therapeutic effects of tryptanthrin and tryptanthrin-6-oxime in models of rheumatoid arthritis. Front. Pharmacol. 2020, 11, 1145. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Chen, C.L.; Tsai, C.C.; Huang, W.C.; Tseng, P.C.; Lin, Y.S.; Chen, S.H.; Wong, T.W.; Choi, P.C.; Lin, C.F. Inhibiting glycogen synthase kinase-3 decreases 12-o-tetradecanoylphorbol-13-acetate-induced interferon-γ-mediated skin inflammation. J. Pharmacol. Exp. Ther. 2012, 343, 125–133. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Z.; Yu, Z.; Wu, X.; Li, R.; Tang, X. Bio alleviates inflammation through inhibition of GSK-3β in a rat model of intracerebral hemorrhage. J. Neurosurg. 2019, 1–9. [Google Scholar] [CrossRef]
- Jean LeBlanc, N.; Menet, R.; Picard, K.; Parent, G.; Tremblay, M.E.; ElAli, A. Canonical wnt pathway maintains blood-brain barrier integrity upon ischemic stroke and its activation ameliorates tissue plasminogen activator therapy. Mol. Neurobiol. 2019, 56, 6521–6538. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, Y.; Zhang, S.; Wang, B.; Shang, L.; Shao, J.; Lin, M.; Cui, Y.; Sun, S.; Ge, S. 6-bromoindirubin-3′-oxime promotes osteogenic differentiation of periodontal ligament stem cells and facilitates bone regeneration in a mouse periodontitis model. ACS Biomater. Sci. Eng. 2021, 7, 232–241. [Google Scholar] [CrossRef]
- Guo, D.; Shen, Y.; Li, W.; Li, Q.; Zhao, Y.; Pan, C.; Chen, B.; Zhong, Y.; Miao, Y. 6-bromoindirubin-3′-oxime (6BIO) suppresses the mtor pathway, promotes autophagy, and exerts anti-aging effects in rodent liver. Front. Pharmacol. 2019, 10, 320. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Hammaker, D.; Kochetkova, I.; Khlebnikov, A.I.; Lyakhov, S.A.; Firestein, G.S.; Quinn, M.T. Anti-inflammatory effects and joint protection in collagen-induced arthritis after treatment with IQ-1S, a selective c-Jun N-terminal kinase inhibitor. J. Pharmacol. Exp. Ther. 2015, 353, 505–516. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Chernysheva, G.A.; Smolyakova, V.I.; Aliev, O.I.; Trofimova, E.S.; Sherstoboev, E.Y.; Osipenko, A.N.; Khlebnikov, A.I.; Anfinogenova, Y.J.; Schepetkin, I.A.; et al. Neuroprotective effects of a novel inhibitor of c-Jun N-terminal kinase in the rat model of transient focal cerebral ischemia. Cells 2020, 9, 1860. [Google Scholar] [CrossRef]
- Marko, D.; Schatzle, S.; Friedel, A.; Genzlinger, A.; Zankl, H.; Meijer, L.; Eisenbrand, G. Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br. J. Cancer 2001, 84, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Hao, Y.; Liu, B.; Qian, L. Indirubin and meisoindigo in the treatment of chronic myelogenous leukemia in china. Leuk. Lymphoma 2002, 43, 1763–1768. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Oishi, Y.; Nikiforov, N.G.; Zhelankin, A.V.; Dubrovsky, L.; Sobenin, I.A.; Kel, A.; Stelmashenko, D.; Makeev, V.J.; Foxx, K.; et al. Modified LDL particles activate inflammatory pathways in monocyte-derived macrophages: Transcriptome analysis. Curr. Pharm. Des. 2018, 24, 3143–3151. [Google Scholar] [CrossRef] [PubMed]
- Back, M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc. Drugs Ther. 2009, 23, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hlawaty, H.; Jacob, M.P.; Louedec, L.; Letourneur, D.; Brink, C.; Michel, J.B.; Feldman, L.; Back, M. Leukotriene receptor antagonism and the prevention of extracellular matrix degradation during atherosclerosis and in-stent stenosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Walter, L.; Neumann, H. Role of microglia in neuronal degeneration and regeneration. Semin. Immunopathol. 2009, 31, 513–525. [Google Scholar] [CrossRef]
- Zhang, S.G.; Wang, X.S.; Zhang, Y.D.; Di, Q.; Shi, J.P.; Qian, M.; Xu, L.G.; Lin, X.J.; Lu, J. Indirubin-3′-monoxime suppresses amyloid-β-induced apoptosis by inhibiting tau hyperphosphorylation. Neural. Regen. Res. 2016, 11, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Konda, V.R.; Desai, A.; Darland, G.; Bland, J.S.; Tripp, M.L. Rho iso-alpha acids from hops inhibit the GSK-3/NF-κB pathway and reduce inflammatory markers associated with bone and cartilage degradation. J. Inflamm. 2009, 6, 26. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Hayes, J.M.; Skamnaki, V.T.; Archontis, G.; Lamprakis, C.; Sarrou, J.; Bischler, N.; Skaltsounis, A.L.; Zographos, S.E.; Oikonomakos, N.G. Kinetics, in silico docking, molecular dynamics, and MM-GBSA binding studies on prototype indirubins, KT5720, and staurosporine as phosphorylase kinase ATP-binding site inhibitors: The role of water molecules examined. Proteins 2011, 79, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Scobie, M.R.; Houke, H.R.; Rice, C.D. Modulation of glioma-inflammation crosstalk profiles in human glioblastoma cells by indirubin-3′-(2,3 dihydroxypropyl)-oximether (E804) and 7-bromoindirubin-3′-oxime (7BIO). Chem. Biol. Interact. 2019, 312, 108816. [Google Scholar] [CrossRef] [PubMed]
- Czapka, A.; Konig, S.; Pergola, C.; Grune, C.; Vougogiannopoulou, K.; Skaltsounis, A.L.; Fischer, D.; Werz, O. The indirubin derivative 6-bromoindirubin-3′-glycerol-oxime ether (6BIGOE) potently modulates inflammatory cytokine and prostaglandin release from human monocytes through GSK-3 interference. Biochem. Pharmacol. 2020, 180, 114170. [Google Scholar] [CrossRef] [PubMed]
- Freyberg, Z.; Ferrando, S.J.; Javitch, J.A. Roles of the AKT/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am. J. Psychiatry 2010, 167, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Kim, T.H.; Kwon, S.M.; Yoon, H.E.; Kim, H.S.; Kim, J.I.; Kim, Y.C.; Kang, K.W.; Ahn, S.G.; Yoon, J.H. 5-nitro-5′-hydroxy-indirubin-3′-oxime (AGM130), an indirubin-3′-oxime derivative, inhibits tumor growth by inducing apoptosis against non-small cell lung cancer in vitro and in vivo. Eur. J. Pharm. Sci. 2015, 79, 122–131. [Google Scholar] [CrossRef]
- Moon, M.J.; Lee, S.K.; Lee, J.W.; Song, W.K.; Kim, S.W.; Kim, J.I.; Cho, C.; Choi, S.J.; Kim, Y.C. Synthesis and structure-activity relationships of novel indirubin derivatives as potent anti-proliferative agents with CDK2 inhibitory activities. Biorg. Med. Chem. 2006, 14, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Polychronopoulos, P.; Magiatis, P.; Skaltsounis, A.L.; Myrianthopoulos, V.; Mikros, E.; Tarricone, A.; Musacchio, A.; Roe, S.M.; Pearl, L.; Leost, M.; et al. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J. Med. Chem. 2004, 47, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Nisha, C.M.; Kumar, A.; Vimal, A.; Bai, B.M.; Pal, D.; Kumar, A. Docking and ADMET prediction of few GSK-3 inhibitors divulges 6-bromoindirubin-3-oxime as a potential inhibitor. J. Mol. Graph. Model. 2016, 65, 100–107. [Google Scholar] [CrossRef]
- Sandoval, K.E.; Witt, K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008, 32, 200–219. [Google Scholar] [CrossRef]
- Brouns, R.; Wauters, A.; De Surgeloose, D.; Marien, P.; De Deyn, P.P. Biochemical markers for blood-brain barrier dysfunction in acute ischemic stroke correlate with evolution and outcome. Eur. Neurol. 2011, 65, 23–31. [Google Scholar] [CrossRef]
- Sussman, E.S.; Connolly, E.S., Jr. Hemorrhagic transformation: A review of the rate of hemorrhage in the major clinical trials of acute ischemic stroke. Front. Neurol. 2013, 4, 69. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Corada, M.; Bangsow, T.; Babbage, J.; Taddei, A.; Czupalla, C.J.; Reis, M.; Felici, A.; Wolburg, H.; Fruttiger, M.; et al. Wnt/β-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 2008, 183, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Creedon, H.; Brunton, V.G. Src kinase inhibitors: Promising cancer therapeutics? Crit. Rev. Oncog. 2012, 17, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Chavda, J.; Bhatt, H. Systemic review on B-RafV600E mutation as potential therapeutic target for the treatment of cancer. Eur. J. Med. Chem. 2020, 206. [Google Scholar] [CrossRef]
- Hoeflich, K.P.; Herter, S.; Tien, J.; Wong, L.; Berry, L.; Chan, J.; O’Brien, C.; Modrusan, Z.; Seshagiri, S.; Lackner, M.; et al. Antitumor efficacy of the novel RAF inhibitor GDC-0879 is predicted by BRAFV600E mutational status and sustained extracellular signal-regulated kinase/mitogen-activated protein kinase pathway suppression. Cancer Res. 2009, 69, 3042–3051. [Google Scholar] [CrossRef]
- Choo, E.F.; Driscoll, J.P.; Feng, J.; Liederer, B.; Plise, E.; Randolph, N.; Shin, Y.; Wong, S.; Ran, Y. Disposition of GDC-0879, a B-Raf kinase inhibitor in preclinical species. Xenobiotica 2009, 39, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Boudny, M.; Trbusek, M. ATR-CHK1 pathway as a therapeutic target for acute and chronic leukemias. Cancer Treat. Rev. 2020, 88. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, G.; Barra, F.; Moioli, M.; Sala, P.; Stigliani, S.; Gustavino, C.; Costantini, S.; Ferrero, S. Prexasertib: An investigational checkpoint kinase inhibitor for the treatment of high-grade serous ovarian cancer. Expert Opin. Investig. Drug 2020, 29, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Bonello, M.; Sims, A.H.; Langdon, S.P. Human epidermal growth factor receptor targeted inhibitors for the treatment of ovarian cancer. Cancer Biol. Med. 2018, 15, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, K.; Jokar, M.H.; Sedighi, S.; Moradzadeh, M. Therapeutic potency of PI3K pharmacological inhibitors of gastrointestinal cancer. Middle East J. Dig. Dis. 2019, 11, 5–16. [Google Scholar] [CrossRef]
- Husain, K.; Williamson, T.T.; Nelson, N.; Ghansah, T. Protein kinase 2 (CK2): A potential regulator of immune cell development and function in cancer. Immunol. Med. 2020, 1–16. [Google Scholar] [CrossRef]
- Bogoyevitch, M.A.; Boehm, I.; Oakley, A.; Ketterman, A.J.; Barr, R.K. Targeting the JNK MAPK cascade for inhibition: Basic science and therapeutic potential. Biochim. Biophys. Acta 2004, 1697, 89–101. [Google Scholar] [CrossRef]
- Bhagwat, S.S. Map kinase inhibitors in inflammation and autoimmune disorders. Annu. Rep. Med. Chem. 2007, 42, 265–278. [Google Scholar] [CrossRef]
- Shvedova, M.; Anfinogenova, Y.; Atochina-Vasserman, E.N.; Schepetkin, I.A.; Atochin, D.N. C-Jun N-terminal kinases (JNKs) in myocardial and cerebral ischemia/reperfusion injury. Front. Pharmacol. 2018, 9, 715. [Google Scholar] [CrossRef]
- Atochin, D.N.; Schepetkin, I.A.; Khlebnikov, A.I.; Seledtsov, V.I.; Swanson, H.; Quinn, M.T.; Huang, P.L. A novel dual no-donating oxime and c-Jun N-terminal kinase inhibitor protects against cerebral ischemia-reperfusion injury in mice. Neurosci. Lett. 2016, 618, 45–49. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Chernysheva, G.A.; Aliev, O.I.; Smol’iakova, V.I.; Fomina, T.I.; Osipenko, A.N.; Rydchenko, V.S.; Anfinogenova, Y.J.; Khlebnikov, A.I.; Schepetkin, I.A.; et al. Protective effects of a new c-Jun N-terminal kinase inhibitor in the model of global cerebral ischemia in rats. Molecules 2019, 24, 1722. [Google Scholar] [CrossRef]
- Pergola, C.; Gaboriaud-Kolar, N.; Jestadt, N.; Konig, S.; Kritsanida, M.; Schaible, A.M.; Li, H.K.; Garscha, U.; Weinigel, C.; Barz, D.; et al. Indirubin core structure of glycogen synthase kinase-3 inhibitors as novel chemotype for intervention with 5-lipoxygenase. J. Med. Chem. 2014, 57, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuzniak, V.; Bednarczyk-Cwynar, B.; Paluszczak, J.; Szaefer, H.; Narozna, M.; Zaprutko, L.; Baer-Dubowska, W. Oleanolic acid oxime derivatives and their conjugates with aspirin modulate the NF-κB-mediated transcription in HEPG2 hepatoma cells. Bioorg. Chem. 2019, 93, 103326. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.N.; Jin, M.H.; Han, B.; Feng, L.; Han, Y.H.; Shen, G.N.; Yu, Y.Z.; Jin, C.H.; Lian, Z.X.; Lee, D.S.; et al. 16α,17α-epoxypregnenolone-20-oxime prevent LPS-induced NO production and iNOS expression in BV-2 microglial cells by inhibiting JNK phosphorylation. Biol. Pharm. Bull. 2014, 37, 1096–1102. [Google Scholar] [CrossRef]
- Sun, H.N.; Han, Y.H.; Feng, L.; Jin, C.H.; Han, B.; Liu, L.; Lee, D.S.; Kwon, T.H.; Li, L.G.; Ge, W.Z.; et al. 16α,17α-epoxypregnenolone-20-oxime inhibits NO and IL-6 production in LPS-treated RAW264.7 cells. Mol. Med. Rep. 2016, 13, 4927–4933. [Google Scholar] [CrossRef][Green Version]
- Kolsi, L.E.; Leal, A.S.; Yli-Kauhaluoma, J.; Liby, K.T.; Moreira, V.M. Dehydroabietic oximes halt pancreatic cancer cell growth in the G1 phase through induction of p27 and downregulation of cyclin D1. Sci. Rep. 2018, 8, 15923. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhao, Y.L.; Lu, C.M.; Tzeng, C.C.; Wang, J.P. Synthesis, cytotoxicity, and anti-inflammatory evaluation of 2-(furan-2-yl)-4-(phenoxy)quinoline derivatives. Part 4. Bioorg. Med. Chem. 2006, 14, 4373–4378. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Chen, I.L.; Lu, C.M.; Tzeng, C.C.; Tsao, L.T.; Wang, J.P. Synthesis and anti-inflammatory evaluation of 4-anilinofuro[2,3-b]quinoline and 4-phenoxyfuro[2,3-b]quinoline derivatives. Part 3. Bioorg. Med. Chem. 2004, 12, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Strizki, J.M.; Xu, S.; Wagner, N.E.; Wojcik, L.; Liu, J.; Hou, Y.; Endres, M.; Palani, A.; Shapiro, S.; Clader, J.W.; et al. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 12718–12723. [Google Scholar] [CrossRef]
- Tsamis, F.; Gavrilov, S.; Kajumo, F.; Seibert, C.; Kuhmann, S.; Ketas, T.; Trkola, A.; Palani, A.; Clader, J.W.; Tagat, J.R.; et al. Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry. J. Virol. 2003, 77, 5201–5208. [Google Scholar] [CrossRef]
- Johansen, T.H.; Drejer, J.; Watjen, F.; Nielsen, E.O. A novel non-NMDA receptor antagonist shows selective displacement of low-affinity [H-3] kainate binding. Eur. J. Pharm. Molec. Pharmacol. 1993, 246, 195–204. [Google Scholar] [CrossRef]
- Guo, W.; Zou, S.P.; Tal, M.; Ren, K. Activation of spinal kainate receptors after inflammation: Behavioral hyperalgesia and subunit gene expression. Eur. J. Pharmacol. 2002, 452, 309–318. [Google Scholar] [CrossRef]
- Petrus, M.; Peier, A.M.; Bandell, M.; Hwang, S.W.; Huynh, T.; Olney, N.; Jegla, T.; Patapoutian, A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol. Pain 2007, 3, 40. [Google Scholar] [CrossRef]
- McGaraughty, S.; Chu, K.L.; Perner, R.J.; Didomenico, S.; Kort, M.E.; Kym, P.R. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Mol. Pain 2010, 6, 14. [Google Scholar] [CrossRef]
- Munro, G.; Christensen, J.K.; Erichsen, H.K.; Dyhring, T.; Demnitz, J.; Dam, E.; Ahring, P.K. NS383 selectively inhibits acid-sensing ion channels containing 1a and 3 subunits to reverse inflammatory and neuropathic hyperalgesia in rats. CNS Neurosci. Ther. 2016, 22, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Bordet, T.; Buisson, B.; Michaud, M.; Drouot, C.; Galea, P.; Delaage, P.; Akentieva, N.P.; Evers, A.S.; Covey, D.F.; Ostuni, M.A.; et al. Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J. Pharmacol. Exp. Ther. 2007, 322, 709–720. [Google Scholar] [CrossRef]
- Kurebayashi, J.; Otsuki, T.; Kurosumi, M.; Soga, S.; Akinaga, S.; Sonoo, H. A radicicol derivative, KF58333, inhibits expression of hypoxia-inducible factor-1α and vascular endothelial growth factor, angiogenesis and growth of human breast cancer xenografts. Jpn. J. Cancer Res. 2001, 92, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.D.; Rathod, P.D.; Franklin, P.X.; Padh, H.; Vasu, K.K.; Sudarsanam, V. Design, synthesis, and sar studies of some 5-aliphatic oximino esters of thiophene as potential anti-inflammatory leads: Comparative biological activity profile of aliphatic oximes vs aromatic oximes. Biochem. Biophys. Res. Commun. 2004, 317, 1067–1074. [Google Scholar] [CrossRef]
- Yu, X.; Park, E.J.; Kondratyuk, T.P.; Pezzuto, J.M.; Sun, D. Synthesis of 2-arylindole derivatives and evaluation as nitric oxide synthase and NF-κB inhibitors. Org. Biomol. Chem. 2012, 10, 8835–8847. [Google Scholar] [CrossRef] [PubMed]
- Franklin, P.X.; Pillai, A.D.; Rathod, P.D.; Yerande, S.; Nivsarkar, M.; Padh, H.; Vasu, K.K.; Sudarsanam, V. 2-amino-5-thiazolyl motif: A novel scaffold for designing anti-inflammatory agents of diverse structures. Eur. J. Med. Chem. 2008, 43, 129–134. [Google Scholar] [CrossRef]
- Bagdanoff, J.T.; Donoviel, M.S.; Nouraldeen, A.; Carlsen, M.; Jessop, T.C.; Tarver, J.; Aleem, S.; Dong, L.; Zhang, H.; Boteju, L.; et al. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: Discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932). J. Med. Chem. 2010, 53, 8650–8662. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Frohlich, L.; Sixt, M.; Lammermann, T.; Pfister, H.; Bateman, A.; Belaaouaj, A.; Ring, J.; Ollert, M.; Fassler, R.; et al. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J. Clin. Investig. 2008, 118, 2438–2447. [Google Scholar] [CrossRef]
- Crocetti, L.; Quinn, M.T.; Schepetkin, I.A.; Giovannoni, M.P. A patenting perspective on human neutrophil elastase (HNE) inhibitors (2014–2018) and their therapeutic applications. Expert Opin. Ther. Pat. 2019, 29, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Katabami, K.; Wada, T.; Sugano, M.; Hoshino, H.; Sawamura, A.; Gando, S. Sivelestat (selective neutrophil elastase inhibitor) improves the mortality rate of sepsis associated with both acute respiratory distress syndrome and disseminated intravascular coagulation patients. Shock 2010, 33, 14–18. [Google Scholar] [CrossRef]
- Essayan, D.M. Cyclic nucleotide phosphodiesterase (PDE) inhibitors and immunomodulation. Biochem. Pharmacol. 1999, 57, 965–973. [Google Scholar] [CrossRef]
- Yoo, E.S.; Son, H.J.; Park, J.S.; Kim, A.R.; Baik, K.U.; Park, M.H.; Cho, J.Y. Effects of dialkoxylphenyl compounds with oxime group on macrophage function and the proliferation of lymphocytes. J. Pharm. Pharmacol. 2004, 56, 503–512. [Google Scholar] [CrossRef]
- Larm, J.A.; Cheung, N.S.; Beart, P.M. (S)-5-fluorowillardiine-mediated neurotoxicity in cultured murine cortical neurones occurs via AMPA and kainate receptors. Eur. J. Pharmacol. 1996, 314, 249–254. [Google Scholar] [CrossRef]
- El-Sherief, H.A.M.; Youssif, B.G.M.; Bukhari, S.N.A.; Abdelazeem, A.H.; Abdel-Aziz, M.; Abdel-Rahman, H.M. Synthesis, anticancer activity and molecular modeling studies of 1,2,4-triazole derivatives as EGFR inhibitors. Eur. J. Med. Chem. 2018, 156, 774–789. [Google Scholar] [CrossRef]
- Sanchez-Pavon, E.; Lopez-Monteon, A.; Hernandez-Romero, D.; de la Soledad Lagunes-Castro, M.; Zanatta-Garcia, D.Y.; Ramos-Ligonio, A. Design and synthesis of IMR-23, an oxime derived from nitroimidazole as an immunomodulatory molecule. Curr. Drug Deliv. 2020, 17, 324–332. [Google Scholar] [CrossRef]
- Androniklion, V.; Boucher, J.L.; Delaforge, M.; Henry, Y.; Mansuy, D. Formation of nitric-oxide by cytochrome P450-catalyzed oxidation of aromatic amidoximes. Biochem. Biophys. Res. Commun. 1992, 185, 452–458. [Google Scholar] [CrossRef]
- Caro, A.A.; Cederbaum, A.L.; Stoyanovsky, D.A. Oxidation of the ketoxime acetoxime to nitric oxide by oxygen radical-generating systems. Nitric Oxide Biol. Chem. 2001, 5, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Veras, R.C.; Rodrigues, K.G.; Alustau, M.D.; Araujo, I.G.A.; de Barros, A.L.B.; Alves, R.J.; Nakao, L.S.; Braga, V.A.; Silva, D.F.; de Medeiros, I.A. Participation of nitric oxide pathway in the relaxation response induced by E-cinnamaldehyde oxime in superior mesenteric artery isolated from rats. J. Cardiovasc. Pharmacol. 2013, 62, 58–66. [Google Scholar] [CrossRef]
- Sahyoun, T.; Arrault, A.; Schneider, R. Amidoximes and oximes: Synthesis, structure, and their key role as NO donors. Molecules 2019, 24, 2470. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, D.; Boucher, J.L.; Clement, B. On the mechanism of nitric oxide formation upon oxidative cleavage of C=N(OH) bonds by no-synthases and cytochromes P450. Biochimie 1995, 77, 661–667. [Google Scholar] [CrossRef]
- Volkel, W.; Wolf, N.; Derelanko, M.; Dekant, W. Slow oxidation of acetoxime and methylethyl ketoxime to the corresponding nitronates and hydroxy nitronates by liver microsomes from rats, mice, and humans. Toxicol. Sci. 1999, 47, 144–150. [Google Scholar] [CrossRef][Green Version]
- Jousserandot, A.; Boucher, J.L.; Henry, Y.; Niklaus, B.; Clement, B.; Mansuy, D. Microsomal cytochrome p450 dependent oxidation of N-hydroxyguanidines, amidoximes, and ketoximes: Mechanism of the oxidative cleavage of their C=N(OH) bond with formation of nitrogen oxides. Biochemistry 1998, 37, 17179–17191. [Google Scholar] [CrossRef]
- Vetrovsky, P.; Boucher, J.L.; Schott, C.; Beranova, P.; Chalupsky, K.; Callizot, N.; Muller, B.; Entlicher, G.; Mansuy, D.; Stoclet, J.C. Involvement of NO in the endothelium-independent relaxing effects of N-omega-hydroxy-L-arginine and other compounds bearing a C=NOH function in the rat aorta. J. Pharmacol. Exp. Ther. 2002, 303, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Chalupsky, K.I.L.; Nepveu, F.I.G.; Beranova, P.; Entlicher, G.; Stoclet, J.C.; Muller, B. Relaxant effect of oxime derivatives in isolated rat aorta: Role of nitric oxide (NO) formation in smooth muscle. Biochem. Pharmacol. 2004, 67, 1203–1214. [Google Scholar] [CrossRef]
- Jaros, F.; Straka, T.; Dobesova, Z.; Pinterova, M.; Chalupsky, K.; Kunes, J.; Entlicher, G.; Zicha, J. Vasorelaxant activity of some oxime derivatives. Eur. J. Pharmacol. 2007, 575, 122–126. [Google Scholar] [CrossRef]
- Hassan, G.S.; Hegazy, G.H.; Ibrahim, N.M.; Fahim, S.H. New ibuprofen derivatives as H2S and NO donors as safer anti-inflammatory agents. Future Med. Chem. 2019, 11, 3029–3045. [Google Scholar] [CrossRef]
- Mauge, L.; Fotopoulou, T.; Deemasure, S.; Dutartre, P.; Koufaki, M.; Connat, J.L. In vitro inflammatory/anti-inflammatory effects of nitrate esters of purines. Eur. J. Pharmacol. 2014, 730, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, K.; Ryann, Y.; Qiao, L.L.; Williams, J.L.; Chen, J.; Del Soldato, P.; Traganos, F.; Rigas, B. Nitric oxide-donating nonsteroidal anti-inflammatory drugs inhibit the growth of various cultured human cancer cells: Evidence of a tissue type-independent effect. J. Pharmacol. Exp. Ther. 2002, 303, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, B.; Boydens, C.; Brouckaert, P.; Van de Voorde, J. Oximes induce erection and are resistant to oxidative stress. J. Sex. Med. 2015, 12, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, B.; Boydens, C.; Decaluwe, K.; Van de Voorde, J. NO-donating oximes relax corpora cavernosa through mechanisms other than those involved in arterial relaxation. J. Sex. Med. 2014, 11, 1664–1674. [Google Scholar] [CrossRef][Green Version]
- Rehse, K.; Bade, S.; Harsdorf, A.; Clement, B. New NO-donors with antithrombotic and vasodilating activities, Part 17—Arylazomidoximes and 3-arylazo-1,2,4-oxadiazol-5-ones. Arch. Pharm. 1997, 330, 392–398. [Google Scholar] [CrossRef]
- Shahid, M.; Martorana, M.G.; Cottney, J.E.; Marshall, R.J. Pharmacological and biochemical effects of the cardiotonic agent ORG10325 in isolated cardiac and vascular tissue preparations. Br. J. Pharmacol. 1990, 100, 735–742. [Google Scholar] [CrossRef]
- Rehse, K.; Brehme, F. New NO donors with antithrombotic and vasodilating activities, Part 27: Azide oximes and 1-hydroxytetrazoles. Arch. Pharm. 2000, 333, 157–161. [Google Scholar] [CrossRef]
- Dantas, B.P.V.; Ribeiro, T.P.; Assis, V.L.; Furtado, F.F.; Assis, K.S.; Alves, J.S.; Silva, T.M.S.; Camara, C.A.; Franca-Silva, M.S.; Veras, R.C.; et al. Vasorelaxation induced by a new naphthoquinone-oxime is mediated by NO-SGC-CGMP pathway. Molecules 2014, 19, 9773–9785. [Google Scholar] [CrossRef]
- Oresmaa, L.; Kotikoski, H.; Haukka, M.; Oksala, O.; Pohjala, E.; Vapaatalo, H.; Moilanen, E.; Vainiotalo, P.; Aulaskari, P. Synthesis, ocular effects, and nitric oxide donation of imidazole amidoximes. Eur. J. Med. Chem. 2006, 41, 1073–1079. [Google Scholar] [CrossRef]
- Abuo-Rahma, G.E.D.A.A.; Abdel-Aziz, M.; Beshr, E.A.M.; Ali, T.F.S. 1,2,4-triazole/oxime hybrids as new strategy for nitric oxide donors: Synthesis, anti-inflammatory, ulceroginicity and antiproliferative activities. Eur. J. Med. Chem. 2014, 71, 185–198. [Google Scholar] [CrossRef]
- Gaboriaud-Kolar, N.; Vougogiannopoulou, K.; Skaltsounis, A.L. Indirubin derivatives: A patent review (2010-present). Expert Opin. Ther. Pat. 2015, 25, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Tchoumtchoua, J.; Halabalaki, M.; Gikas, E.; Tsarbopoulos, A.; Fotaki, N.; Liu, L.; Nam, S.; Jove, R.; Skaltsounis, L.A. Preliminary pharmacokinetic study of the anticancer 6BIO in mice using an UHPLC-MS/MS approach. J. Pharm. Biomed. Anal. 2019, 164, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D.E.; Kalasz, H.; Petroianu, G.A.; Tekes, K. Entry of oximes into the brain: A review. Curr. Med. Chem. 2008, 15, 743–753. [Google Scholar] [CrossRef]
- Kobrlova, T.; Korabecny, J.; Soukup, O. Current approaches to enhancing oxime reactivator delivery into the brain. Toxicology 2019, 423, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Thomas, T.P.; Leroueil, P.; Kotlyar, A.; Van Der Spek, A.F.; Baker, J.R., Jr. Specific and cooperative interactions between oximes and pamam dendrimers as demonstrated by 1H NMR study. J. Phys. Chem. B 2012, 116, 10387–10397. [Google Scholar] [CrossRef]
- Baell, J.B. Screening-based translation of public research encounters painful problems. ACS Med. Chem. Lett. 2015, 6, 229–234. [Google Scholar] [CrossRef]
- Dahlin, J.L.; Walters, M.A. How to triage PAINS-full research. Assay Drug Dev. Technol. 2016, 14, 168–174. [Google Scholar] [CrossRef]

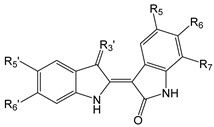
| Compound | R5 | R6 | R7 | R3′ | R5′ | R6′ |
|---|---|---|---|---|---|---|
| 1 | H | H | H | =N-OH | H | H |
| 2 | H | H | H | =N-OAc | H | H |
| 3 | H | H | H | =N-OCH2CHOHCH2OH | H | H |
| 4 | OCH3 | H | H | =N-O-CHOH-CH2OH | H | H |
| 5 | OCH3 | H | H | =N-O-(CH2)2OH | F | F |
| 6 | 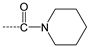 | H | H | =N-OH | H | H |
| 7 | NO2 | H | H | =N-OH | OH | H |
| 8 | NHC(O)Bu | H | H | =N-OH | H | H |
| 9 | C(O)OCH3 | H | H | 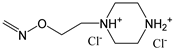 | H | H |
| 10 | I | H | H | =N-OH | H | H |
| 11 | H | Br | H | =N-OH | H | H |
| 12 | H | Br | H | =N-OAc | H | H |
| 13 | H | Br | H | 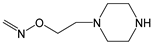 | H | H |
| 14 | H | H | Br | =N-OH | H | H |
| 15 | H | H | Br | =N-OH | COOH | H |
| 16 | F | H | H | 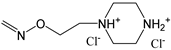 | H | H |
| Compound | Kinase target (IC50, μM) | Ref. | ||||
| 1 | GSK-3α/β (0.022), CDK1 (0.18), CDK2 (0.7), CDK5 (0.1), CDK9 (2.4), PhK (0.21), FLT3 (0.033), AMPK (0.22), Lck (0.3), SGK (0.38), DRAK2 (0.71) | [37,40,43,44,49,50,51] | ||||
| 2 | CDK1 (1.2), CD5 (0.7), PhK (0.17), GSK-3α/β (0.2) | [40] | ||||
| 3 | CDK2 (0.23), Src (0.43), CDK6, CDK16, GSK-3β | [47,52] | ||||
| 4 | CDK2 (0.043), JAK1 (0.01), JAK2 (0.074), Tyk2 (0.001), c-Src (0.011), Lyn (0.03), Hck (0.264), Aurora A, c-Kit, GSK-3β, IGF1R, VEGFR2, ABL | [47,52] | ||||
| 5 | CDK2 (0.4), CDK9 (0.3) | [53] | ||||
| 6 | Aurora A (0.37) | [54] | ||||
| 7 | CDK2 (0.002) | [55] | ||||
| 8 | DRAK2 | [51] | ||||
| 9 | FLT3 (0.003), JAK2 (0.52), JAK3 (0.69), cMET (0.24), IRAK4 (0.3) | [56] | ||||
| 10 | GSK-3α/β, CDK1, CDK5 | [37] | ||||
| 11 | GSK-3β (0.005), CDK1 (0.32), CDK5 (0.083), PhK, Aurora A (0.6), Aurora B (0.9), Aurora C (0.2), DYRK1a (1.7), DYRK2 (2.1) | [40,44,57,58] | ||||
| 12 | CDK5 (2.4), GSK-3α/β (0.01), PhK (0.33) | [40,44] | ||||
| 13 | c-Src (0.0002), JAK1 (0.6), JAK2 (0.03), TYK2 (0.05), GSK-3β (0.003) | [59,60] | ||||
| 14 | Aurora B (4.6), Aurora C (0.7), DYRK1a (1.9), DYRK2 (1.3) | [57,58] | ||||
| 15 | DYRK1a (0.21), DYRK2 (0.13) | [58] | ||||
| 16 | FLT3 (0.001) | [61] | ||||
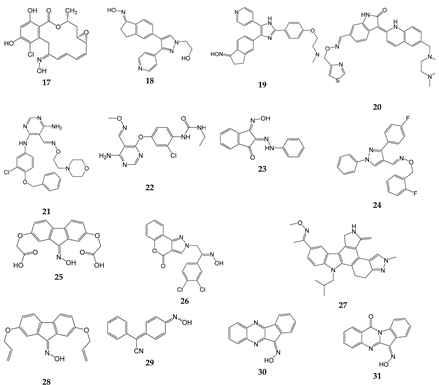
| Compound | Kinase target (IC50, µM) | Ref. |
|---|---|---|
| 17 | Src (0.056) | [34] |
| 18 | B-Raf (0.0001) | [62] |
| 19 | B-Raf (Ki = 0.0002), c-Raf (Ki = 0.0017) | [63] |
| 20 | VEGFR2 (0.009) | [64] |
| 21 | ErbB1 (0.022), ErbB2 (0.038), ErbB4 (0.021) | [65] |
| 22 | VEGFR-2 (0.04), Ret (0.18), Kit (0.5 | [66] |
| 23 | EGFR (50.3% at 100 µM) | [67] |
| 24 | PI3Kγ (1.3) | [68] |
| 25 | Chk1 (13.4) | [69] |
| 26 | PI3Kα (0.012), PI3Kβ (0.187), PI3Kγ (0.293), PI3Kσ (0.219) | [39] |
| 27 | VEGF-R1 (0.008), VEGF-R3 (0.01), TIE-2 (0.03) | [70] |
| 28 | EGFR (55.3% at 100 µM) | [67] |
| 29 | CK2 | [71] |
| 30 | JNK1/2/3 | [41,72] |
| 31 | JNK1/2/3 | [38] |
| Compound | Cells | Concentration range (µM) | Effect/Mechanism a | Ref. |
|---|---|---|---|---|
| 1 | Pancreatic ductal adenocarcinoma cells | 1–10 | ↓ p-CDK1/cyclinB1 | [80] |
| MG63 and U2-OS osteosarcoma | 1–10 | ↓ CDK2/4, FAK | [81] | |
| Cholangiocarcinoma linesNOZ, HuCCT1, OCUG-1, and OZ | 1–60 | [82] | ||
| 11, 14 | MDA-MB-231-TXSA breast cancer | 10–50 | ↑ Caspase-3 | [83] |
| 14 | Thyroid carcinoma | 1–10 | ↑ Caspase-3 | [84] |
| Neuroblastoma SH-SY5Y | 10–100 | [85] | ||
| 16 | MG63 and Saos-2 osteosarcoma | 1–30 | ↑ AMPK | [86] |
| MV4-11 and FLT3/D835Y expressed MOLM14 | IC50 = 0.001 (toward FLT3) | ↓ FLT3 | [61] | |
| 17 | 3Y1-B, SR-3Y1, NRK,KNRK5.2 cells | IC50 = 0.025 (toward v-Src) | ↓ v-Src activity; ↓ Raf-1 expression | [34] |
| 26 | Human colorectal carcinoma HCT-116, human lung cancer A549, human liver carcinoma Huh7, human leukemia HL60 | 0.1–1 | Inhibitor of PI3Kα, PI3Kβ, PI3Kγ and PI3Kδ | [39] |
| Compound | Model | Treatment | Ref. |
|---|---|---|---|
| 1 | Pancreatic ductal adenocarcinoma cells, inoculated s.c. | 10–40 mg/kg, i.p., daily for 4 days | [80] |
| 16 | MG63 osteosarcoma cells, inoculated s.c. | 5 mg/kg, i.p. daily for 45 days | [86] |
| MV-4-11 B-myelomonocytic leukemia cells, inoculated s.c. | 20 mg/kg, orally, daily for 21 days | [61] | |
| 20 | Lung cancer A549 cells, inoculated s.c. | 4 mg/kg, orally, daily for 14 day | [64] |
| 22 | A431 epidermoid carcinoma cells, HCT116 colorectal carcinoma cells, A375 skin melanoma cells; all cells inoculated s.c. | 10, 50, 100 and 200 mg/kg, intragastically, daily for 35 days, | [66] |
| 27 | A375 skin melanoma cells, inoculated s.c. | 10 mg/kg, orally, for 22 days | [70] |
| Compound | Cell Culture | Model | Concentration Range (µM) | Effect/Mechanism a | Ref. |
|---|---|---|---|---|---|
| 1 | Adipocytes | Saturated free fatty acid-induced inflammation | 2–10 | ↑ Cell viability; ↑ mRNA for IL-4, IL-10, IL-13, TGF-β; ↓ mRNA for TNF, IL-1β, IL-6 | [106] |
| H9C2 rat cardiac myocyte cells | Incubation of cells with high glucose | 3–30 | ↓ PKR protein and mRNA; ↓ JNK and NF-κB mRNA; ↓ Caspase-3 mRNA; ↓ ROS | [107] | |
| Cultured rat brain microglia, hippocampal slice cultures | LPS stimulation | 0.5–4 | ↓ NF-κB activation; ↓ TNF, IL-1β, PGE2, ROS; ↓ Hippocampal cell death | [108] | |
| Mouse microglia BV-2 cells, hippocampal slice cultures | LPS stimulation | 10 | ↓ Migration; ↓ iNOS expression; ↓ IL-6 and NO production | [30] | |
| Human neutrophils, monocytes, VSMCs | LTB4, CysLT and LT-enriched medium | 0.3–10 | ↓ LT-induced VSMC migration; ↑ HO-1 induction; ↓ 5-LO in monocytes and neutrophils | [109] | |
| Human macrophages, primary type-I like pneumocytes | Influenza virus H5N1 infection | 10 | ↓ IP-10, IL-1β, RANTES, IFN-β, TNF; ↑ Delay of virus replication | [110] | |
| SH-SY5Y cells, primary cerebellar granule neurons | H2O2-induced apoptosis | 0.1–3 | ↑ Cell viability; ↓ p-Akt and p-GSK-3β | [111] | |
| 11 | Human FLS | TNF stimulation | 0.050 | ↓ mRNA for IL-1, IL-6, CCL-2, CCL-7, COX-2, MMP-9; ↓ IL-1, IL-6, CCL-2, CCL-7, COX-2, MMP-9; ↓ NF-κB, p-JNK, p-c-Jun, p-ATF-2, p-p38 | [13] |
| RAW264.7 macrophages | LPS stimulation | 2.5–20 | ↓ NO, PGE2; ↓ iNOS mRNA, COX-2;↓ IL-1β, IL-6; ↓ p-JNK, p-IκB-α; ↑ IκB-α | [112] | |
| Neutrophils, RAW264.7 macrophages | LPS stimulation | 5 | ↓ TNF; ↑ IκB-α | [113] | |
| Mouse mammary epithelial cells | LPS stimulation | 5–50 | ↓ mRNA for IL-1β, IL-6, IL-10, TNF; ↓ IL-1β, IL-6, TNF; ↑ IL-10; ↓ TLR4/NF-κB and TLR4/MAPK expression and phosphorylation | [12] | |
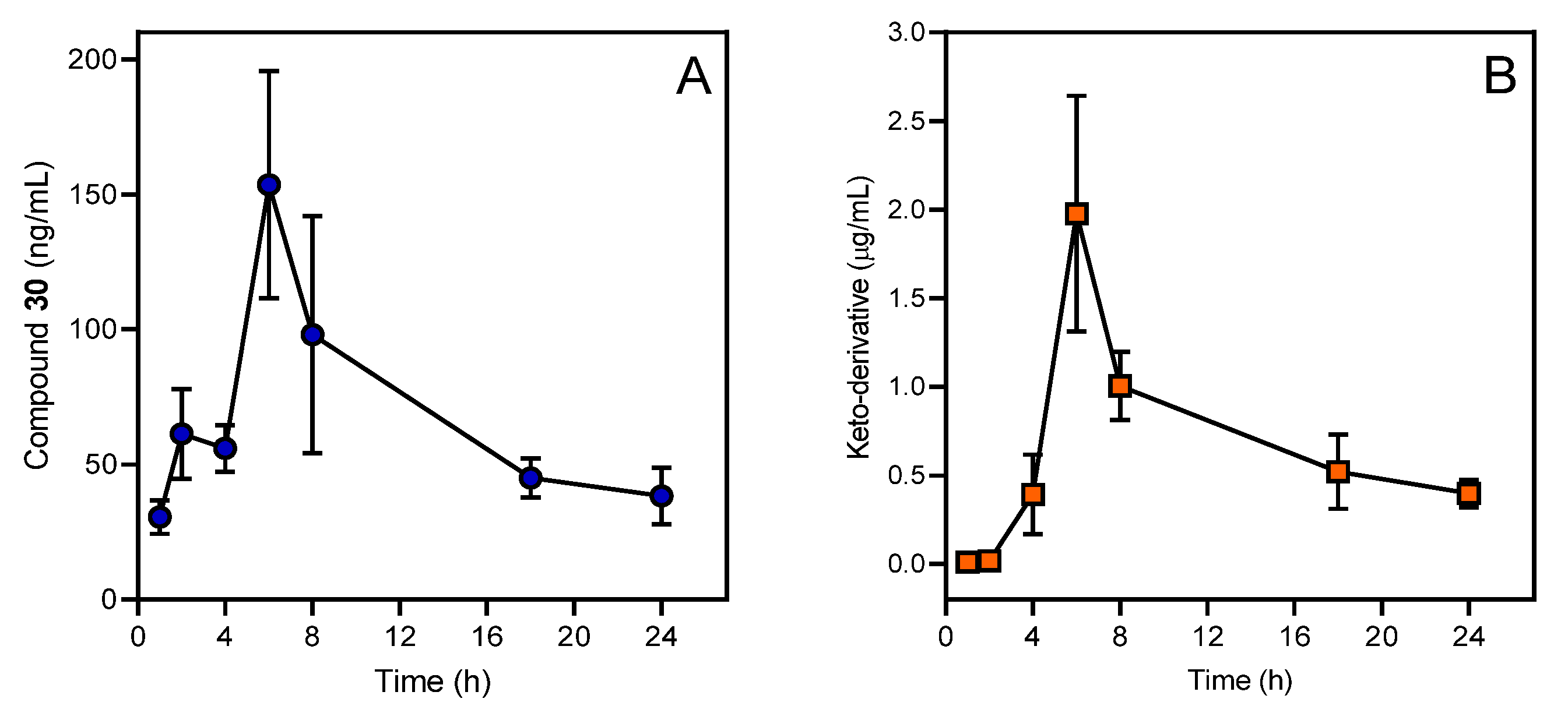
| 30 | PBMCs, MonoMac-6, J774.A1 cells | LPS stimulation | 0.2–30 | ↓ IL-1α, IL-1β, IL-6, TNF, IFN-γ, GM-CSF, NO production by human and murine monocyte/macrophages. | [41] |
| HUVECs | 0.3–10 | ↓ Endothelin-1 secretion | [114] | ||
| Macrophages, T-cells | LPS stimulation | 1 | ↓ TNF, IL-6, IL-1β; ↓ p-JNK2, p-p38, p-IκBα, p-IKKβ; ↓ IL-6 mRNA, TNF, iNOS | [115] | |
| 31 | Human FLS, synovial SW982 cells, HUVECs, monocytic THP-1 cells | IL-1β stimulation | 1–25 | ↓MMP-3 gene expression; ↓ MMP-1/3 and IL-6 secretion | [116] |
| 32 | Human neutrophils | fMLF stimulation | 0.03–20 | ↓ HNE and Pr3 activities; ↓ ROS generation, HNE release | [15] |
| Compound | Animal | Model | Dose | Effect/Mechanism a | Ref. |
|---|---|---|---|---|---|
| 1 | Swiss albino mice | High fat-high fructose diet-induced neuropathological changes | 0.4 mg/kg for 7 days | ↓ Area occupied by dark neurons; ↓ Amyloid spots in hippocampus ↓ NF-κB; ↓ TNF, IL-6 ↓ Bax and caspase-3; ↑ Bcl-2 | [29] |
| 11 | C57BL/6 mice | TPA-induced ear skin inflammation | 1.5 µg/ear | ↓ GSK-3β activity; ↓ IFN-γ production; ↓ Ear skin edema, epidermis hyperproliferation and dermis angiogenesis | [117] |
| Rats | Intracerebral hemorrhage | 10, 20, 40, 60, 80, & 100 µg/kg | ↓ NF-κB, COX-2, GSK-3β phosphorylation; ↑ Brain-derived neurotrophic factor; ↓ IL-1β and IL-6, ↑ IL-10; ↓ Microglia activation and cell apoptosis | [118] | |
| C57BL6/J mice | Transient occlusion of the MCA | 1 mg/kg i.p., 3 and 6 h after occlusion | ↑ Wnt/β-catenin pathway activation ;↓ Brain edema, IgG extravasation, perivascular petechial bleeding; ↓ Hemorrhagic transformation after ischemic stroke | [119] | |
| C57BL/6 mice | Ligature + LPS-induced periodontitis | 0.5−5 μg in 1 mL hydrogel | ↓ Inflammatory cell infiltration; ↑ Expression of ALP, and Runx2 | [120] | |
| Mice | Aging | 1 mg/kg, i.p. during 2 weeks | ↓ IL-6 in liver and serum; ↑ SOD and GSH in liver; ↓ Total cholesterol and triglycerides in liver & serum | [121] | |
| Mice | Arthritis (collagen + complete Freund’s adjuvant) | 1 and 10 mg/kg | ↓ Synovial hyperplasia, infiltration of inflammatory cells, cartilage destruction, and bone erosion; ↓ TNF, IL-1, IL-6, and IFN-γ in serum | [13] | |
| 30 | Mice | Ovalbumin-specific DTH response | Every 12 h with 12.5 mg/kg, i.p., 5 injections | ↓Ear thickness | [41] |
| Mice | Acute lung inflammation (LPS plus D-galactosamine) | 200 µg/mouse, i.p. | ↓ Lethality and lung inflammation; ↓ TNF, IL-6 and IL-1β; ↓ p-JNK2, p-p38, p-IκBα & p-IKKβ; ↓ mRNA for IL-6, TNF and iNOS | [115] | |
| Mice | CIA | 5, 20, 30 and 50 mg/kg, daily, i.p. | ↓CIA and CAIA severity; ↓Cartilage erosion; ↓ Collagen II-specific antibody | [122] | |
| Rats | Focal cerebral ischemia/reperfusion | 5 and 25 mg/kg, i.p. | ↓ p-c-Jun | [123] | |
| 31 | Mice | CIA and CAIA | 30 mg/kg i.p., daily, 34 days | ↓ CIA and CAIA severity; ↓ Cartilage erosion; ↓ IL-17A, GM-CSF, RANKL | [116] |
| 32 | Mice | HNE-induced paw edema | 50–100 mg/kg, i.p. | ↓ Paw edema | [15] |
| LPS-induced acute lung injury | 100 mg/kg, i.p. | ↓ MPO; ↓ Edematous changes, alveolar thickening, leukocyte infiltration, and lung tissue destruction |
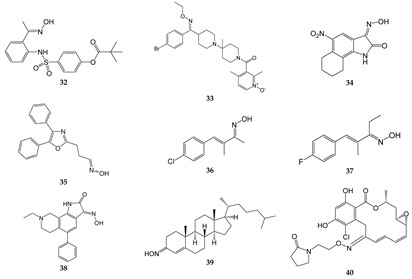
| Compound | Molecular Target/Mechanism | Ref. |
|---|---|---|
| 32 | Dual inhibitor of HNE and Pr3 | [15] |
| 33 | CCR5 antagonist | [169,170] |
| 34 | GluR6 antagonist, amelioration of inflammatory hyperalgesia | [171,172] |
| 35 | TRPA1 and TRPV1 antagonist | [14] |
| 36 | TRPA1 antagonist | [173,174] |
| 37 | TRPA1 antagonist | [173,174] |
| 38 | ASIC blocker, attenuation of pathophysiological nociceptive behaviors in CFA-inflamed and CCI rats | [175] |
| 39 | Binds directly to two components of the mitochondrial permeability pore, the VDAC, and translocator protein; inhibits MPTP opening | [176] |
| 40 | Binds to Hsp90 and provides a significant decrease in HIF-1α expression | [177] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schepetkin, I.A.; Plotnikov, M.B.; Khlebnikov, A.I.; Plotnikova, T.M.; Quinn, M.T. Oximes: Novel Therapeutics with Anticancer and Anti-Inflammatory Potential. Biomolecules 2021, 11, 777. https://doi.org/10.3390/biom11060777
Schepetkin IA, Plotnikov MB, Khlebnikov AI, Plotnikova TM, Quinn MT. Oximes: Novel Therapeutics with Anticancer and Anti-Inflammatory Potential. Biomolecules. 2021; 11(6):777. https://doi.org/10.3390/biom11060777
Chicago/Turabian StyleSchepetkin, Igor A., Mark B. Plotnikov, Andrei I. Khlebnikov, Tatiana M. Plotnikova, and Mark T. Quinn. 2021. "Oximes: Novel Therapeutics with Anticancer and Anti-Inflammatory Potential" Biomolecules 11, no. 6: 777. https://doi.org/10.3390/biom11060777
APA StyleSchepetkin, I. A., Plotnikov, M. B., Khlebnikov, A. I., Plotnikova, T. M., & Quinn, M. T. (2021). Oximes: Novel Therapeutics with Anticancer and Anti-Inflammatory Potential. Biomolecules, 11(6), 777. https://doi.org/10.3390/biom11060777








