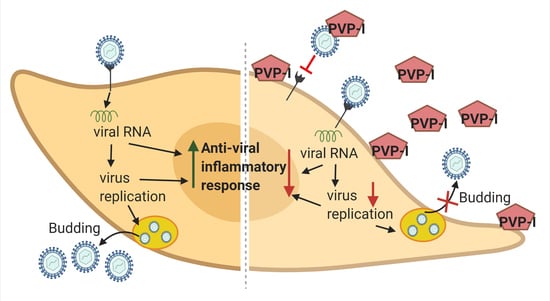
Povidone-Iodine Attenuates Viral Replication in Ocular Cells: Implications for Ocular Transmission of RNA Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Culture Conditions
2.2. Virus Strains and Infection
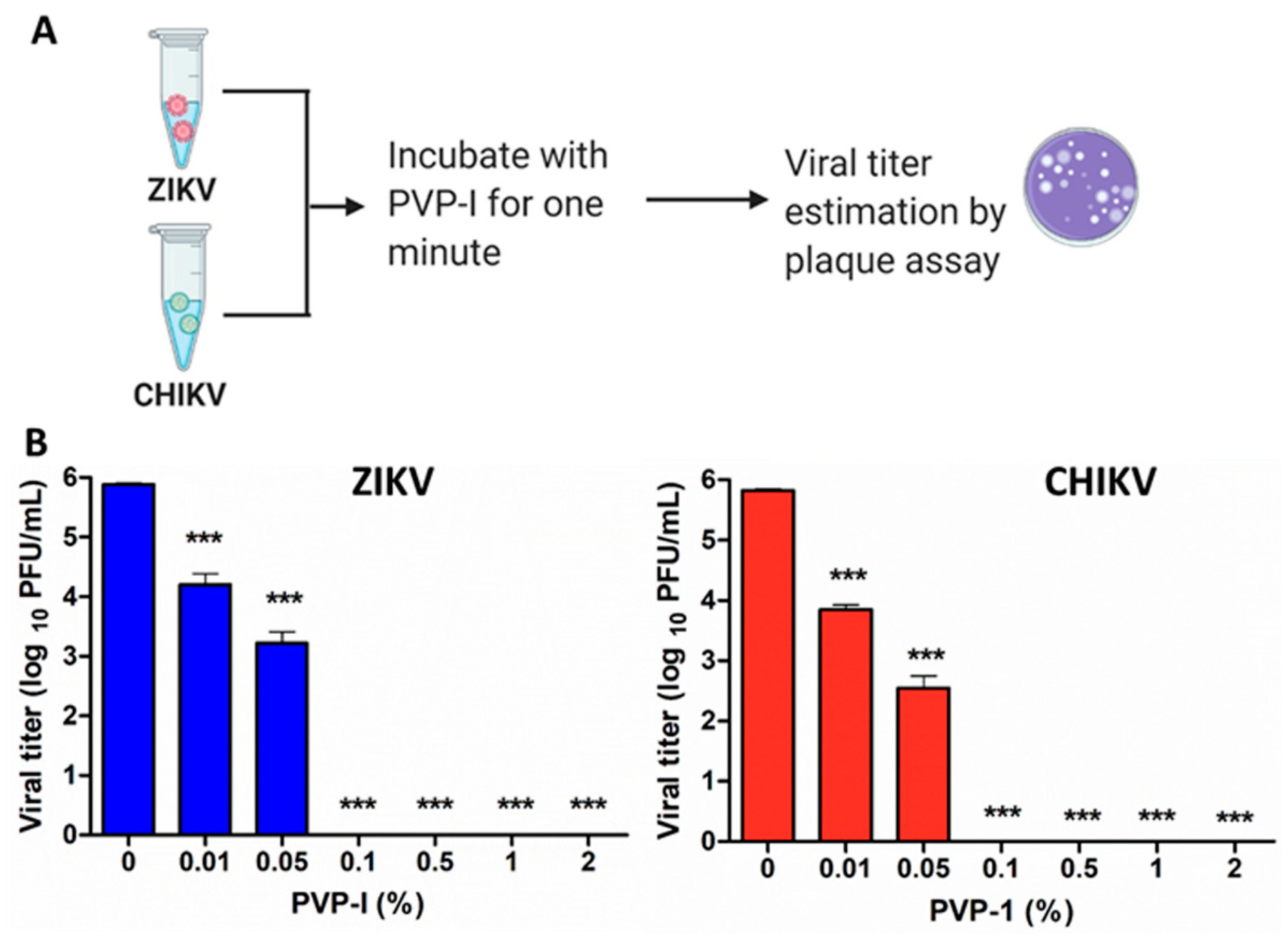
2.3. Plaque Assay
2.4. Immunofluorescence Staining
2.5. Real-Time PCR
2.6. Cellular Toxicity Assay
2.7. Statistical Analysis
3. Results
3.1. PVP-I Directly Inactivates Enveloped RNA Viruses
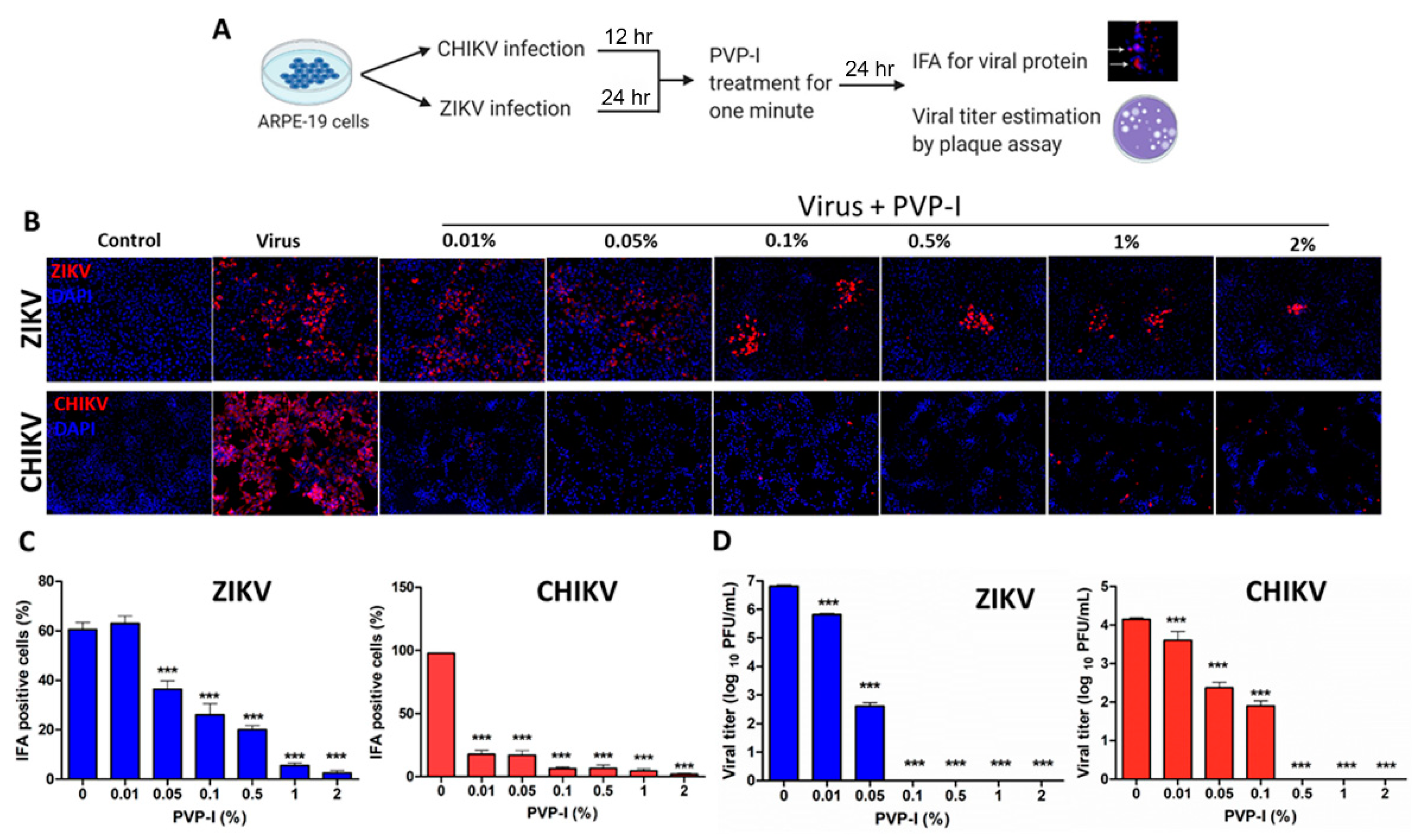
3.2. PVP-I Treatment Attenuates Viral Replication in Human Retinal Pigment Epithelial Cells
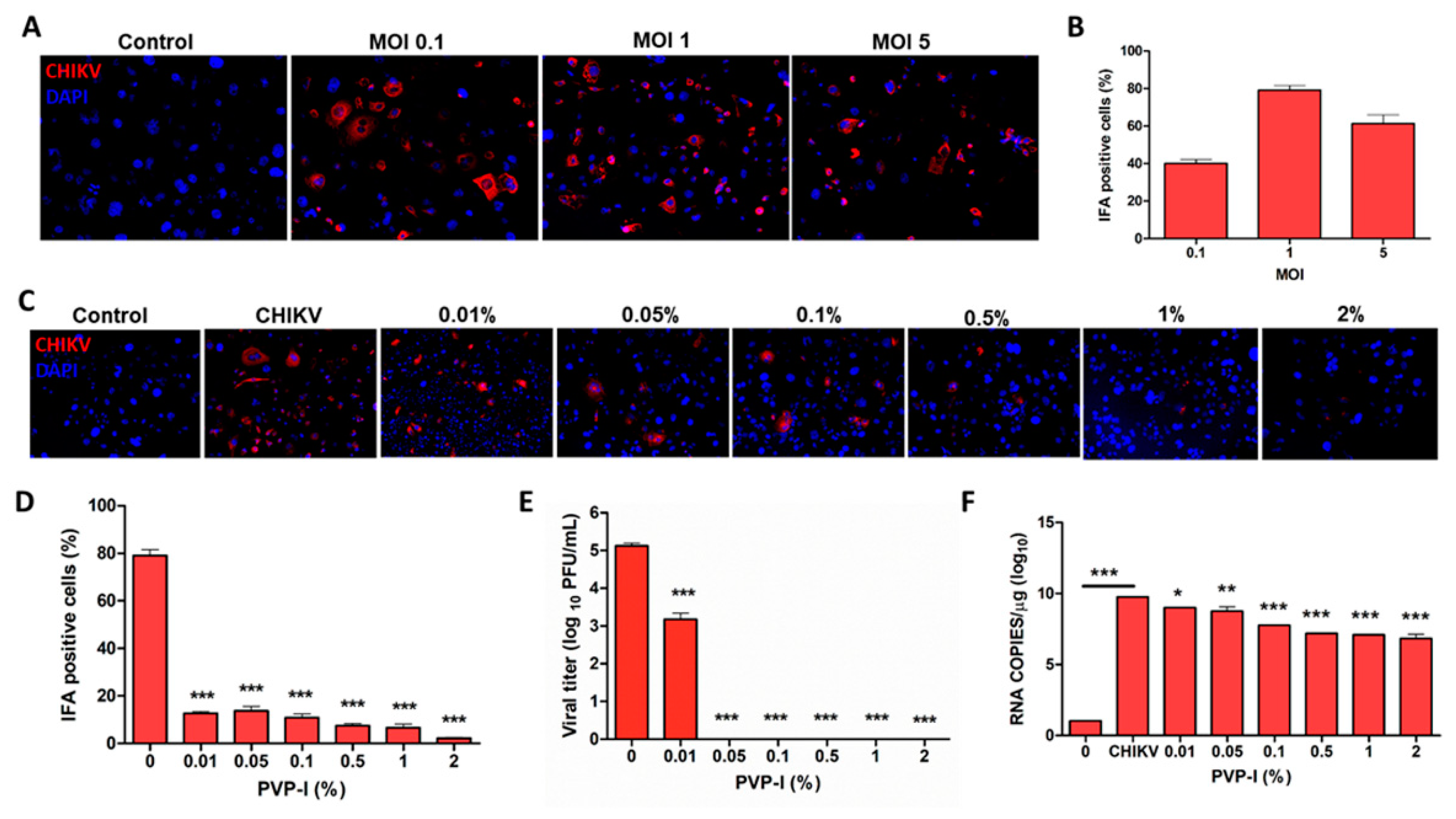
3.3. PVP-I Exposure Attenuated CHIKV Replication in Corneal Epithelial Cells
3.4. PVP-I Attenuates CHIKV-Induced Inflammatory Response in Corneal Epithelial Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Osterholm, M.T.; Moore, K.A.; Kelley, N.S.; Brosseau, L.M.; Wong, G.; Murphy, F.A.; Peters, C.J.; LeDuc, J.W.; Russell, P.K.; Van Herp, M.; et al. Transmission of Ebola viruses: What we know and what we do not know. mBio 2015, 6, e00137. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Dias, J.R.; Ventura, C.V.; Borba, P.D.; de Paula Freitas, B.; Pierroti, L.C.; do Nascimento, A.P.; de Moraes, N.S.B.; Maia, C.; Belfort, R., Jr. Infants with Congenital Zika Syndrome and Ocular Findings from São Paulo, Brazil: Spread of Infection. Retin. Cases Brief Rep. 2018, 12, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Merle, H.; Donnio, A.; Jean-Charles, A.; Guyomarch, J.; Hage, R.; Najioullah, F.; Césaire, R.; Cabié, A. Ocular manifestations of emerging arboviruses: Dengue fever, Chikungunya, Zika virus, West Nile virus, and yellow fever. J. Fr. d’Ophtalmol. 2018, 41, e235–e243. [Google Scholar] [CrossRef]
- Oliver, G.F.; Carr, J.M.; Smith, J.R. Emerging infectious uveitis: Chikungunya, dengue, Zika and Ebola: A review. Clin. Exp. Ophthalmol. 2019, 47, 372–380. [Google Scholar] [CrossRef]
- Singh, S.; Farr, D.; Kumar, A. Ocular Manifestations of Emerging Flaviviruses and the Blood-Retinal Barrier. Viruses 2018, 10, 530. [Google Scholar] [CrossRef]
- Singh, P.K.; Khatri, I.; Jha, A.; Pretto, C.D.; Spindler, K.R.; Arumugaswami, V.; Giri, S.; Kumar, A.; Bhasin, M.K. Determination of system level alterations in host transcriptome due to Zika virus (ZIKV) Infection in retinal pigment epithelium. Sci. Rep. 2018, 8, 11209. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Kasetti, R.B.; Zode, G.S.; Goyal, A.; Juzych, M.S.; Kumar, A. Zika Virus Infects Trabecular Meshwork and Causes Trabeculitis and Glaucomatous Pathology in Mouse Eyes. mSphere 2019, 4, e00173-19. [Google Scholar] [CrossRef]
- Singh, P.K.; Guest, J.-M.; Kanwar, M.; Boss, J.; Gao, N.; Juzych, M.S.; Abrams, G.W.; Yu, F.-S.; Kumar, A. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight 2017, 2, e92340. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, S.; Farr, D.; Kumar, A. Interferon-stimulated gene 15 (ISG15) restricts Zika virus replication in primary human corneal epithelial cells. Ocul. Surf. 2019, 17, 551–559. [Google Scholar] [CrossRef]
- Couderc, T.; Gangneux, N.; Caro, V.; Ducloux, B.; Tolou, H.; Chrétien, F.; Le Luong, T.; Lecuit, M.; Grandadam, M. Chikungunya Virus Infection of Corneal Grafts. J. Infect. Dis. 2012, 206, 851–859. [Google Scholar] [CrossRef]
- Hayek, S.; Rousseau, A.; Bouthry, E.; Prat, C.M.; Labetoulle, M. Chikungunya Virus Infection and Bilateral Stromal Keratouveitis. JAMA Ophthalmol. 2015, 133, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Mahendradas, P.; Ranganna, S.K.; Shetty, R.; Balu, R.; Narayana, K.M.; Babu, R.B.; Shetty, B.K. Ocular Manifestations Associated with Chikungunya. Ophthalmology 2008, 115, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Mahendradas, P.; Shetty, R.; Malathi, J.; Madhavan, H.N. Chikungunya virus iridocyclitis in Fuchs′ heterochromic iridocyclitis. Indian J. Ophthalmol. 2010, 58, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Sawant, O.B.; Singh, S.; Wright, R.E., III; Jones, K.M.; Titus, M.S.; Dennis, E.; Hicks, E.; Majmudar, P.A.; Kumar, A.; Mian, S.I. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul. Surf. 2020, 19, 322–329. [Google Scholar] [CrossRef]
- Feng, Y.; Armenti, S.T.; Mian, S.I. COVID-19 and the Eye: A Comprehensive Review of the Literature. Int. Ophthalmol. Clin. 2021, 61, 1–14. [Google Scholar] [CrossRef]
- Gopinathan, U.; Reddy, M.K.; Nadkarni, M.S.; Dasari, S.; Rao, G.N. Antimicrobial Effect of Ciprofloxacin, Povidone—Iodine, and Gentamicin in the Decontamination of Human Donor Globes. Cornea 1998, 17, 57. [Google Scholar] [CrossRef]
- Sperling, S.; Sørensen, I.G. Decontamination of Cadaver Corneas. Acta Ophthalmol. 2009, 59, 126–133. [Google Scholar] [CrossRef]
- Pels, E.; Vrensen, G.F.J.M. Microbial decontamination of human donor eyes with povidone-iodine: Penetration, toxicity, and effectiveness. Br. J. Ophthalmol. 1999, 83, 1019–1026. [Google Scholar] [CrossRef]
- Atum, M.; Boz, A.A.E.; Cakir, B.; Karabay, O.; Koroglu, M.; Ogutlu, A.; Alagöz, G. Evaluation of Conjunctival Swab PCR Results in Patients with SARS-CoV-2 Infection. Ocul. Immunol. Inflamm. 2020, 28, 745–748. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; El-harakeh, A.; Bognanni, A.; Lotfi, T.; Loeb, M.; et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Güemes-Villahoz, N.; Burgos-Blasco, B.; Arribi-Vilela, A.; Arriola-Villalobos, P.; Vidal-Villegas, B.; Mendez-Fernandez, R.; Delgado-Iribarren, A.; Garcia-Feijoo, J. SARS-CoV-2 RNA detection in tears and conjunctival secretions of COVID-19 patients with conjunctivitis. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Arabi, A.; Shahraki, T.; Safi, S. Detection of severe acute respiratory syndrome Coronavirus-2 in the tears of patients with Coronavirus disease 2019. Eye 2020, 34, 1220–1223. [Google Scholar] [CrossRef]
- Seah, I.; Agrawal, R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocul. Immunol. Inflamm. 2020, 28, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Vogt, P.M.; Hauser, J.; Mueller, S.; Bosse, B.; Hopp, M. Efficacy of Conventional and Liposomal Povidone–Iodine in Infected Mesh Skin Grafts: An Exploratory Study. Infect. Dis. Ther. 2017, 6, 545–555. [Google Scholar] [CrossRef]
- Eggers, M.; Koburger-Janssen, T.; Eickmann, M.; Zorn, J. In Vitro Bactericidal and Virucidal Efficacy of Povidone-Iodine Gargle/Mouthwash against Respiratory and Oral Tract Pathogens. Infect. Dis. Ther. 2018, 7, 249–259. [Google Scholar] [CrossRef]
- Vergara-Buenaventura, A.; Castro-Ruiz, C. Use of mouthwashes against COVID-19 in dentistry. Br. J. Oral Maxillofac. Surg. 2020, 58, 924–927. [Google Scholar] [CrossRef]
- Pelletier, J.S.; Tessema, B.; Frank, S.; Westover, J.B.; Brown, S.M.; Capriotti, J.A. Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2). Ear Nose Throat J. 2020, 100, 192S–196S. [Google Scholar] [CrossRef]
- Kawana, R.; Kitamura, T.; Nakagomi, O.; Matsumoto, I.; Arita, M.; Yoshihara, N.; Yanagi, K.; Yamada, A.; Morita, O.; Yoshida, Y.; et al. Inactivation of Human Viruses by Povidone-Iodine in Comparison with Other Antiseptics. Dermatology 1997, 195, 29–35. [Google Scholar] [CrossRef]
- Kariwa, H.; Fujii, N.; Takashima, I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology 2006, 212 (Suppl. 1), 119–123. [Google Scholar] [CrossRef]
- Eggers, M.; Eickmann, M.; Zorn, J. Rapid and Effective Virucidal Activity of Povidone-Iodine Products Against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA). Infect. Dis. Ther. 2015, 4, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Sivalingam, V.; Kang, A.E.; Ananthanarayanan, A.; Arumugam, H.; Jenkins, T.M.; Hadjiat, Y.; Eggers, M. Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease. Infect. Dis. Ther. 2020, 9, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Bidra, A.S.; Pelletier, J.S.; Westover, J.B.; Frank, S.; Brown, S.M.; Tessema, B. Comparison of In Vitro Inactivation of SARS CoV-2 with Hydrogen Peroxide and Povidone-Iodine Oral Antiseptic Rinses. J. Prosthodont. 2020, 29, 599–603. [Google Scholar] [CrossRef]
- Liang, B.; Yuan, X.; Wei, G.; Wang, W.; Zhang, M.; Peng, H.; Javer, A.; Mendenhall, M.; Julander, J.; Huang, S.; et al. In-Vivo Toxicity Studies and In-Vitro Inactivation of SARS-CoV-2 by Povidone-iodine In-situ Gel Forming Formulations. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wutzler, P.; Sauerbrei, A.; Klöcking, R.; Burkhardt, J.; Schacke, M.; Thust, R.; Fleischer, W.; Reimer, K. Virucidal and Chlamydicidal Activities of Eye Drops with Povidone-Iodine Liposome Complex. Ophthalmic Res. 2000, 32, 118–125. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, C.N.; Glybina, I.V.; Mahmoud, T.H.; Yu, F.S. Toll-like receptor 2 ligand-induced protection against bacterial endophthalmitis. J. Infect. Dis. 2010, 201, 255–263. [Google Scholar] [CrossRef]
- Kumar, M.V.; Nagineni, C.N.; Chin, M.S.; Hooks, J.J.; Detrick, B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J. Neuroimmunol. 2004, 153, 7–15. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.K.; Suhail, H.; Arumugaswami, V.; Pellett, P.E.; Giri, S.; Kumar, A. AMP-Activated Protein Kinase Restricts Zika Virus Replication in Endothelial Cells by Potentiating Innate Antiviral Responses and Inhibiting Glycolysis. J. Immunol. 2020, 204, 1810–1824. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.; Singh, S.; Nair, S.R.; Hulyalkar, N.V.; Surendran, A.; Jaleel, A.; Sreekumar, E. Nucleophosmin (NPM1)/B23 in the Proteome of Human Astrocytic Cells Restricts Chikungunya Virus Replication. J. Proteome Res. 2017, 16, 4144–4155. [Google Scholar] [CrossRef]
- Kumar, S.; Jaffar-Bandjee, M.-C.; Giry, C.; De Kerillis, L.C.; Merits, A.; Gasque, P.; Hoarau, J.-J. Mouse macrophage innate immune response to chikungunya virus infection. Virol. J. 2012, 9, 313. [Google Scholar] [CrossRef]
- Lee, J.H.; Agarwal, A.; Mahendradas, P.; Lee, C.S.; Gupta, V.; Pavesio, C.E.; Agrawal, R. Viral posterior uveitis. Surv. Ophthalmol. 2017, 62, 404–445. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, G.C.; Ventura, C.V.; Mello Filho, P.A.; Maia, M.; Vianello, S.; Rodrigues, E.B. Arboviruses and the eye. Int. J. Retina Vitr. 2017, 3, 4. [Google Scholar] [CrossRef]
- Roehrich, H.; Yuan, C.; Hou, J.H. Immunohistochemical Study of SARS-CoV-2 Viral Entry Factors in the Cornea and Ocular Surface. Cornea 2020, 39, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Schreier, H.; Erdos, G.; Reimer, K.; König, B.; König, W.; Fleischer, W. Molecular Effects of Povidone-Iodine on Relevant Microorganisms: An Electron-Microscopic and Biochemical Study. Dermatology 1997, 195, 111–116. [Google Scholar] [CrossRef]
- Siddharta, A.; Pfaender, S.; Vielle, N.J.; Dijkman, R.; Friesland, M.; Becker, B.; Yang, J.; Engelmann, M.; Todt, D.; Windisch, M.P.; et al. Virucidal Activity of World Health Organization–Recommended Formulations Against Enveloped Viruses, Including Zika, Ebola, and Emerging Coronaviruses. J. Infect. Dis. 2017, 215, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Brown, S.M.; Capriotti, J.A.; Westover, J.B.; Pelletier, J.S.; Tessema, B. In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 1054–1058. [Google Scholar] [CrossRef]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 1–6. [Google Scholar] [CrossRef]
- Sriwilaijaroen, N.; Wilairat, P.; Hiramatsu, H.; Takahashi, T.; Suzuki, T.; Ito, M.; Ito, Y.; Tashiro, M.; Suzuki, Y. Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: Its effects on hemagglutination and sialidase activities. Virol. J. 2009, 6, 124. [Google Scholar] [CrossRef]
- Eggers, M. Correction to: Infectious Disease Management and Control with Povidone Iodine. Infect. Dis. Ther. 2019, 8, 595. [Google Scholar] [CrossRef]
- Beukelman, C.; Berg, A.V.D.; Hoekstra, M.; Uhl, R.; Reimer, K.; Mueller, S. Anti-inflammatory properties of a liposomal hydrogel with povidone-iodine (Repithel®) for wound healing in vitro. Burns 2008, 34, 845–855. [Google Scholar] [CrossRef]
- Bilal, M.Y.; Dambaeva, S.; Kwak-Kim, J.; Gilman-Sachs, A.; Beaman, K.D. A Role for Iodide and Thyroglobulin in Modulating the Function of Human Immune Cells. Front. Immunol. 2017, 8, 1573. [Google Scholar] [CrossRef] [PubMed]
- König, B.; Reimer, K.; Fleischer, W.; König, W. Effects of Betaisodona® on Parameters of Host Defense. Dermatology 1997, 195, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Thomas, A.; Harding, K.G. Iodine released from the wound dressing Iodosorb modulates the secretion of cytokines by human macrophages responding to bacterial lipopolysaccharide. Int. J. Biochem. Cell Biol. 1997, 29, 163–171. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Sawant, O.B.; Mian, S.I.; Kumar, A. Povidone-Iodine Attenuates Viral Replication in Ocular Cells: Implications for Ocular Transmission of RNA Viruses. Biomolecules 2021, 11, 753. https://doi.org/10.3390/biom11050753
Singh S, Sawant OB, Mian SI, Kumar A. Povidone-Iodine Attenuates Viral Replication in Ocular Cells: Implications for Ocular Transmission of RNA Viruses. Biomolecules. 2021; 11(5):753. https://doi.org/10.3390/biom11050753
Chicago/Turabian StyleSingh, Sneha, Onkar B. Sawant, Shahzad I. Mian, and Ashok Kumar. 2021. "Povidone-Iodine Attenuates Viral Replication in Ocular Cells: Implications for Ocular Transmission of RNA Viruses" Biomolecules 11, no. 5: 753. https://doi.org/10.3390/biom11050753
APA StyleSingh, S., Sawant, O. B., Mian, S. I., & Kumar, A. (2021). Povidone-Iodine Attenuates Viral Replication in Ocular Cells: Implications for Ocular Transmission of RNA Viruses. Biomolecules, 11(5), 753. https://doi.org/10.3390/biom11050753