The Combination of Paraformaldehyde and Glutaraldehyde Is a Potential Fixative for Mitochondria
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Chemical Fixation
2.3. Confocal Imaging
2.4. RNA Isolation
2.5. Quantitative RT-PCR
2.6. Protein Isolation
2.7. Western Blot
2.8. Immunofluorescent Labeling
2.9. Mitochondrial Image Analysis
2.10. Statistical Analysis
3. Results
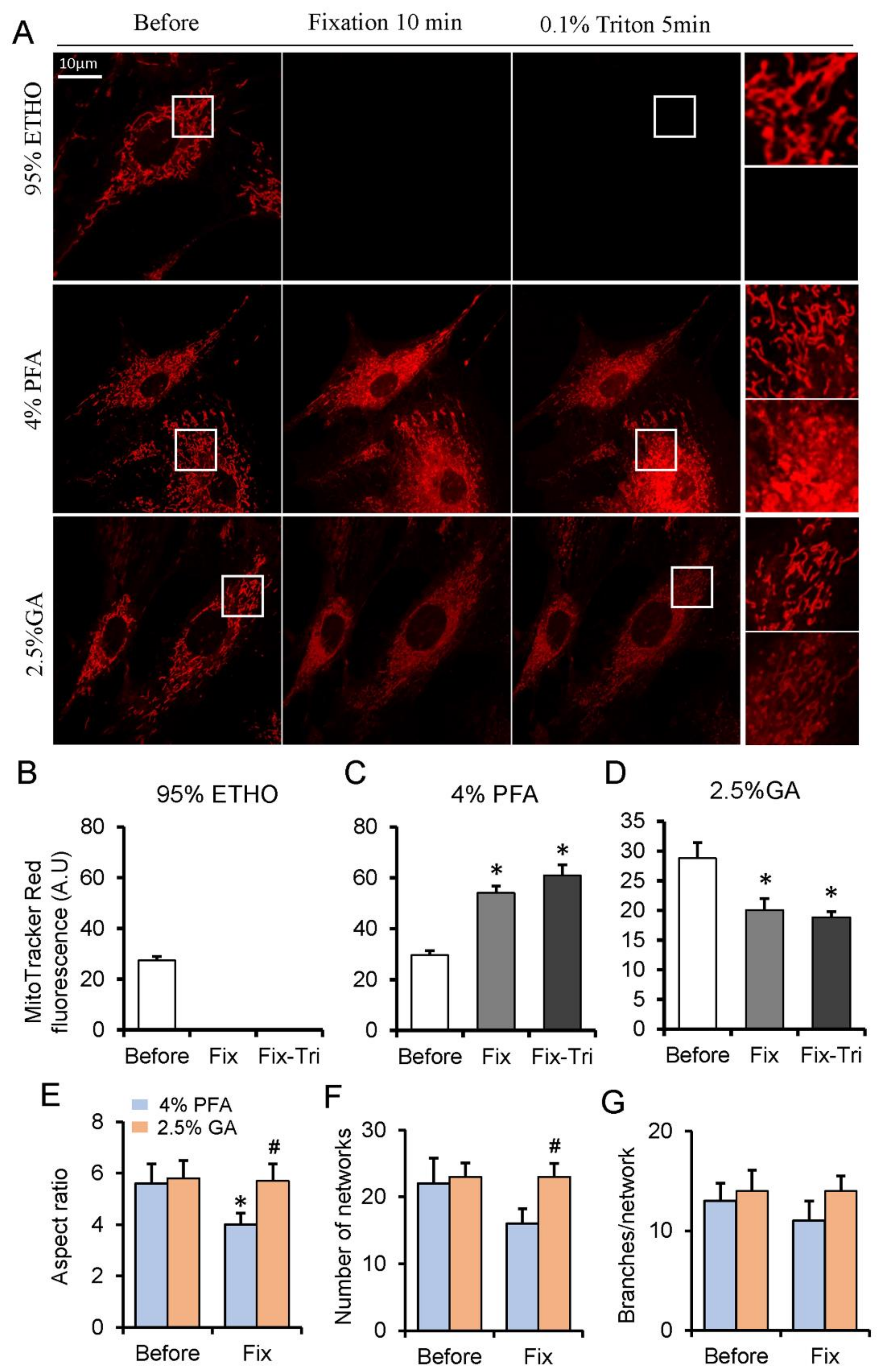
3.1. The Effect of Fixatives on Cells and Mitochondrial Indicators
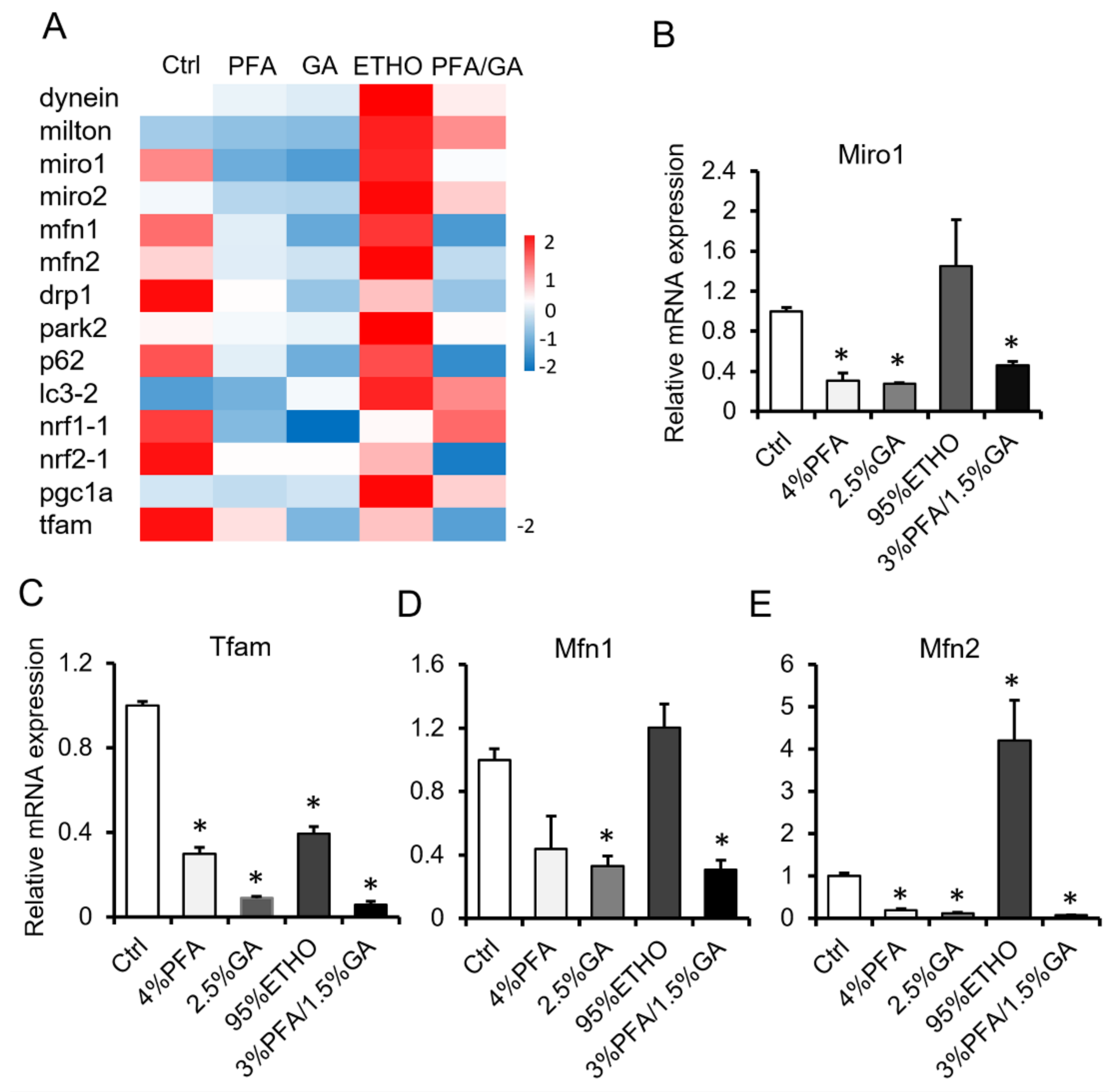
3.2. The Effect of Fixative on Cross-Linking Proteins
3.3. The Screening of More Suitable Fixative for Mitochondria
3.4. mRNA Level After Fixation
3.5. Immunostaining After Fixation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soubannier, V.; McBride, H.M. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2009, 1793, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, A.; Liu, B.; Jiang, W.; Gao, M.; Tian, X.; Gong, G. Mitochondrial fusion mediated by fusion promotion and fission inhibition directs adult mouse heart function toward a different direction. FASEB J. 2020, 34, 663–675. [Google Scholar] [CrossRef] [PubMed]
- El-Hattab, A.W.; Suleiman, J.; Almannai, M.; Scaglia, F. Mitochondrial dynamics: Biological roles, molecular machinery, and related diseases. Mol. Genet. Metab. 2018, 125, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gao, M.; Jiang, W.; Qin, Y.; Gong, G. Mitochondrial Dynamics in Adult Cardiomyocytes and Heart Diseases. Front. Cell Dev. Biol. 2020, 8, 584800. [Google Scholar] [CrossRef]
- Tepp, K.; Shevchuk, I.; Chekulayev, V.; Timohhina, N.; Kuznetsov, A.V.; Guzun, R.; Saks, V.; Kaambre, T. High efficiency of energy flux controls within mitochondrial interactosome in cardiac intracellular energetic units. Biochim. Biophys. Acta (BBA) Bioenerg. 2011, 1807, 1549–1561. [Google Scholar] [CrossRef]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- Dorn Ii, G.W. Mitochondrial dynamism and heart disease: Changing shape and shaping change. EMBO Mol. Med. 2015, 7, 865–877. [Google Scholar] [CrossRef]
- Dorn, G.W. Mitochondrial dynamics in heart disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 233–241. [Google Scholar] [CrossRef]
- Papanicolaou, K.N.; Khairallah, R.J.; Ngoh, G.A.; Chikando, A.; Luptak, I.; Shea, K.M.; Riley, D.D.; Lugus, J.J.; Colucci, W.S.; Lederer, W.J.; et al. Mitofusin-2 Maintains Mitochondrial Structure and Contributes to Stress-Induced Permeability Transition in Cardiac Myocytes. Mol. Cell. Biol. 2011, 31, 1309. [Google Scholar] [CrossRef]
- Rambold, A.S.; Pearce, E.L. Mitochondrial Dynamics at the Interface of Immune Cell Metabolism and Function. Trends Immunol. 2018, 39, 6–18. [Google Scholar] [CrossRef]
- Gong, G.; Song, M.; Csordas, G.; Kelly, D.P.; Matkovich, S.J.; Dorn, G.W., II. Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science 2015, 350, aad2459. [Google Scholar] [CrossRef]
- Qin, Y.; Gao, M.; Li, A.; Sun, J.; Liu, B.; Gong, G. Mitoflash lights single mitochondrial dynamics events in mature cardiomyocytes. Biochem. Biophys. Res. Commun. 2018, 503, 729–736. [Google Scholar] [CrossRef]
- Chen, Y.; Dorn, G.W., II. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 2013, 340, 471–475. [Google Scholar] [CrossRef]
- Song, J.; Lee, C.; Lin, C.-H.; Chen, L.B. Electron microscopic studies of the ER in whole-mount cultured cells fixed with potassium permanganate. J. Struct. Biol. 1991, 107, 106–115. [Google Scholar] [CrossRef]
- Paavilainen, L.; Edvinsson, Å.; Asplund, A.; Hober, S.; Kampf, C.; Pontén, F.; Wester, K. The Impact of Tissue Fixatives on Morphology and Antibody-based Protein Profiling in Tissues and Cells. J. Histochem. Cytochem. 2009, 58, 237–246. [Google Scholar] [CrossRef]
- St-Laurent, J.; Boulay, M.-E.; Prince, P.; Bissonnette, E.; Boulet, L.-P. Comparison of cell fixation methods of induced sputum specimens: An immunocytochemical analysis. J. Immunol. Methods. 2006, 308, 36–42. [Google Scholar] [CrossRef]
- Tanaka, K.A.K.; Suzuki, K.G.N.; Shirai, Y.M.; Shibutani, S.T.; Miyahara, M.S.H.; Tsuboi, H.; Yahara, M.; Yoshimura, A.; Mayor, S.; Fujiwara, T.K.; et al. Membrane molecules mobile even after chemical fixation. Nat. Methods 2010, 7, 865–866. [Google Scholar] [CrossRef]
- Karnovsky, M. A Formaldehyde-Glutaraldehyde Fixative of High Osmolality for Use in Electron Microscopy. J. Cell Biol. 1964, 27, 1A–149A. [Google Scholar]
- Kiernan, J.A. Formaldehyde, Formalin, Paraformaldehyde and glutaraldehyde: What they are and what they do. Microsc. Today 2000, 8, 8–13. [Google Scholar] [CrossRef]
- Hopwood, D. Theoretical and practical aspects of glutaraldehyde fixation. Histochem. J. 1972, 4, 267–303. [Google Scholar] [CrossRef]
- Huebinger, J.; Spindler, J.; Holl, K.J.; Koos, B. Quantification of protein mobility and associated reshuffling of cytoplasm during chemical fixation. Sci. Rep. 2018, 8, 17756. [Google Scholar] [CrossRef] [PubMed]
- Suthipintawong, C.; Fcap, A.; Vinyuvat, S. Immunostaining of Cell Preparations: A Comparative Evaluation of Common Fixatives and Protocols. Diagn. Cytopathol. 1996, 15, 167–174. [Google Scholar] [CrossRef]
- Richter, K.N.; Revelo, N.H.; Seitz, K.J.; Helm, M.S.; Sarkar, D.; Saleeb, R.S.; D’Este, E.; Eberle, J.; Wagner, E.; Vogl, C.; et al. Glyoxal as an alternative fixative to formaldehyde in immunostaining and super-resolution microscopy. EMBO J. 2018, 37, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, M.A.; Alshammari, T.K.; Laezza, F. Improved Methods for Fluorescence Microscopy Detection of Macromolecules at the Axon Initial Segment. Front. Cell. Neurosci. 2016, 10, 5. [Google Scholar] [CrossRef]
- Vekemans, K.; Rosseel, L.; Wisse, E.; Braet, F. Immuno-localization of Fas and FasL in rat hepatic endothelial cells: Influence of different fixation protocols. Micron 2004, 35, 303–306. [Google Scholar] [CrossRef]
- Celie, J.W.A.M.; Beelen, R.H.J.; van den Born, J. Effect of fixation protocols on in situ detection of L-selectin ligands. J. Immunol. Methods 2005, 298, 155–159. [Google Scholar] [CrossRef]
- Jamur, M.; Oliver, C. Cell Fixatives for Immunostaining. Methods Mol. Biol. 2010, 588, 55–61. [Google Scholar]
- Melan, M. Overview of Cell Fixatives and Cell Membrane Permeants. Methods Mol. Biol. 1999, 115, 45–55. [Google Scholar]
- Connelly, M.; Chakraborty, U.; Brooks, H. Cell fixative and method of analyzing virally infected cells. Biotechnol. Adv. 1997, 15, 716. [Google Scholar]
- Li, A.; Qin, Y.; Gao, M.; Jiang, W.; Liu, B.; Tian, X.; Gong, G. Protocol for Imaging of Mitoflashes in Live Cardiomyocytes. STAR Protoc. 2020, 1, 100101. [Google Scholar] [CrossRef]
- Rocha, A.G.; Franco, A.; Krezel, A.M.; Rumsey, J.M.; Alberti, J.M.; Knight, W.C.; Biris, N.; Zacharioudakis, E.; Janetka, J.W.; Baloh, R.H.; et al. MFN2 agonists reverse mitochondrial defects in preclinical models of Charcot-Marie-Tooth disease type 2A. Science 2018, 360, 336–341. [Google Scholar] [CrossRef]
- Valente, A.J.; Maddalena, L.A.; Robb, E.L.; Moradi, F.; Stuart, J.A. A simple ImageJ macro tool for analyzing mitochondrial network morphology in mammalian cell culture. Acta Histochem. 2017, 119, 315–326. [Google Scholar] [CrossRef]
- Presley, A.D.; Fuller, K.M.; Arriaga, E.A. MitoTracker Green labeling of mitochondrial proteins and their subsequent analysis by capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. B 2003, 793, 141–150. [Google Scholar] [CrossRef]
- Caro, A.A.; Bell, M.; Ejiofor, S.; Zurcher, G.; Petersen, D.R.; Ronis, M.J.J. N-acetylcysteine inhibits the up-regulation of mitochondrial biogenesis genes in livers from rats fed ethanol chronically. Alcohol Clin. Exp. Res. 2014, 38, 2896–2906. [Google Scholar] [CrossRef]
- Brock, R.; Hamelers, I.H.L.; Jovin, T.M. Comparison of fixation protocols for adherent cultured cells applied to a GFP fusion protein of the epidermal growth factor receptor. Cytometry 1999, 35, 353–362. [Google Scholar] [CrossRef]
- Fox, C.H.; Johnson, F.B.; Whiting, J.; Roller, P.P. Formaldehyde fixation. J. Histochem. Cytochem. 1985, 33, 845–853. [Google Scholar] [CrossRef]
- Thavarajah, R.; Mudimbaimannar, V.; Elizabeth, J.; Rao, U.; Ranganathan, K. Chemical and physical basics of routine formaldehyde fixation. J. Oral Maxillofac. Pathol. 2012, 16, 400–405. [Google Scholar] [CrossRef]
- Okuda, K.; Urabe, I.; Yamada, Y.; Okada, H. Reaction of glutaraldehyde with amino and thiol compounds. J. Ferment. Bioeng. 1991, 71, 100–105. [Google Scholar] [CrossRef]
- Smith, J.E.; Reese, T.S. Use of Aldehyde Fixatives to Determine the Rate of Synaptic Transmitter Release. J. Exp. Biol. 1980, 89, 19. [Google Scholar] [CrossRef]
- Sabatini, D.; Bensch, K.; Barrnett, R. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J. Cell Biol. 1963, 17, 19–58. [Google Scholar] [CrossRef]
- Stanly, T.A.; Fritzsche, M.; Banerji, S.; García, E.; Bernardino de la Serna, J.; Jackson, D.G.; Eggeling, C. Critical importance of appropriate fixation conditions for faithful imaging of receptor microclusters. Biol. Open 2016, 5, 1343–1350. [Google Scholar] [CrossRef]
- Ochs, M.; Mühlfeld, C. Quantitative microscopy of the lung: A problem-based approach. Part 1: Basic principles of lung stereology. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L15–L22. [Google Scholar] [CrossRef]
- Li, Y.; Almassalha, L.M.; Chandler, J.E.; Zhou, X.; Stypula-Cyrus, Y.E.; Hujsak, K.A.; Roth, E.W.; Bleher, R.; Subramanian, H.; Szleifer, I.; et al. The effects of chemical fixation on the cellular nanostructure. Exp. Cell Res. 2017, 358, 253–259. [Google Scholar] [CrossRef]
- Jalali, M.; Saldanha, F.; Jalali, M. Basic Science Methods for Clinical Researchers; Academic Press: Cambridge, MA, USA, 2017; Volume 119. [Google Scholar]
- Dimitriadis, G.J. Effect of detergents on antibody-antigen interaction. Anal. Biochem. 1979, 98, 445–451. [Google Scholar] [CrossRef]
- Jamur, M.C.; Oliver, C. Permeabilization of cell membranes immunocytochemical methods and protocols. Methods Mol. Biol. 2009, 588, 63–66. [Google Scholar]
- Koley, D.; Bard, A.J. Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM). Proc. Natl. Acad. Sci. USA 2010, 107, 16783. [Google Scholar] [CrossRef]
- Schnell, U.; Dijk, F.; Sjollema, K.A.; Giepmans, B.N.G. Immunolabeling artifacts and the need for live-cell imaging. Nat. Methods 2012, 9, 152–158. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Jiang, W.; Li, A.; Gao, M.; Liu, H.; Gao, Y.; Tian, X.; Gong, G. The Combination of Paraformaldehyde and Glutaraldehyde Is a Potential Fixative for Mitochondria. Biomolecules 2021, 11, 711. https://doi.org/10.3390/biom11050711
Qin Y, Jiang W, Li A, Gao M, Liu H, Gao Y, Tian X, Gong G. The Combination of Paraformaldehyde and Glutaraldehyde Is a Potential Fixative for Mitochondria. Biomolecules. 2021; 11(5):711. https://doi.org/10.3390/biom11050711
Chicago/Turabian StyleQin, Yuan, Wenting Jiang, Anqi Li, Meng Gao, Hanyu Liu, Yufei Gao, Xiangang Tian, and Guohua Gong. 2021. "The Combination of Paraformaldehyde and Glutaraldehyde Is a Potential Fixative for Mitochondria" Biomolecules 11, no. 5: 711. https://doi.org/10.3390/biom11050711
APA StyleQin, Y., Jiang, W., Li, A., Gao, M., Liu, H., Gao, Y., Tian, X., & Gong, G. (2021). The Combination of Paraformaldehyde and Glutaraldehyde Is a Potential Fixative for Mitochondria. Biomolecules, 11(5), 711. https://doi.org/10.3390/biom11050711




