Susceptibility of Women to Cardiovascular Disease and the Prevention Potential of Mind–Body Intervention by Changes in Neural Circuits and Cardiovascular Physiology
Abstract
1. Susceptibility of Women to Cardiovascular Disease
1.1. Gender Differences in Mortality from Cardiovascular Disease
1.2. Stress and Cardiovascular Disease
1.3. Relationship between Mental Health and Cardiovascular Health and Its Gender Differences
1.4. Effects of Menopause on Women’s Cardiovascular Health
2. Link between the Brain and Cardiovascular Health
2.1. Sexual Dimorphism in the Connection between the Brain and Cardiovascular Health
2.2. Gender Differences in Stress-Induced HPA Axis Activation
2.3. Gender Differences in Stress-Induced Changes in the Autonomous Nervous System
2.4. Relation of Stress Activation of the HPA Axis and Cardiovascular Health in Women
2.5. Gender Differences in Inflammation and Their Relation with the Cardiovascular System
3. Changes in the Brain and Physiological Responses by Mind–Body Intervention
3.1. Effects of Mind–Body Intervention on Cardiovascular Disease
| Scope | Sub-Scope | Reference | Study Type | Population (n, % Female, Age) | Intervention | Control | Considered Confounders | Outcome |
|---|---|---|---|---|---|---|---|---|
| Brain (psychological) | Depression | Gong et al. [152] | Meta-analysis (six RCTs) | Pregnant women (n = 375, 100% female, age range, 20–40) | Yoga | Usual care or any other physical or mental care | N/A |
|
| Brain (psychological) | PTSD | Van der Kolk et al. [153] | RCT | Women with chronic, treatment-resistant PTSD (n = 64, 100% female, mean age, 43) | Yoga for ten weeks | Health education for ten weeks | Age, race, education, marital status, income, etc. |
|
| Brain (psychological) | Depression, stress, anxiety | Haller et al. [154] | Meta-analysis (ten RCTs) | Women with breast cancer (n = 1709, 100% female) | MBSR, MBCT | Usual care, active comparator (supportive expressive therapy, nutritional education program) | N/A |
|
| Brain (psychological) | Affect (female vs. male) | Kang et al. [155] | RCT | Sixth-grade students (n = 114, 46% female, mean age, 12) | School-based mindfulness training for six weeks | Active control for six weeks | Age, % female, psychological state |
|
| Brain (psychological) | Affect (female vs. male) | Rojiani et al. [156] | A longitudinal study | University students (n = 77, 47% female, mean age, 21) | Meditation for 12 weeks | N/A | Age, affect, mindfulness, self-compassion, placebo effect-like confounders driven by self-selection |
|
| Brain (psychological) | Anxiety, withdrawal symptoms (female vs. male) | Chen et al. [157] | A controlled longitudinal study | Volunteers in the rehabilitation unit of a residential addiction treatment facility (n = 207, 27% female, mean age, 34) | Qigong meditation (relaxation, breathing, guided imagery, inward attention, mindfulness) for two weeks | Stress management and relaxation training for two weeks | Race, % female, employment, education, social perception (religion, general feeling about life, etc.), withdrawal symptoms, etc. |
|
| Brain (structure) | Brain structure (female vs. male) | Luders et al. [158] | A cross-sectional study | Long-term meditators (mean practice time, 20.2 years) vs. meditation-naïve individuals; mean age, 47 years; 50% female; n = 60 | N/A | N/A | Sex, handedness, age |
|
| Brain (psychological) | Depression, anxiety | Wong et al. [159] | RCT | Postmenopausal women with mild to moderate symptoms (n = 197, 100% female, mean age, 52) | MBSR for eight weeks | Menopause education for eight weeks | Age, education, occupation, marital status, religion, family size, income, menopause state |
|
| Cardiovascular | Vasomotor symptoms | Chattha et al. [160] | RCT | Women with menopausal symptoms (n = 120, 100% female, mean age, 48) | Yoga (postures, breathing, meditation) for eight weeks | Exercise (walking, stretching, rest) for eight weeks | Age, occupation, BMI, diet, menopause state |
|
| Cardiovascular | Vasomotor symptoms | Carmody et al. [161] | RCT | Late perimenopausal and early postmenopausal women experiencing moderate or severe hot flushes (including night sweats) (n = 110, 100% female, mean age, 53) | MBSR for three months | Waitlist | Age, race, education, employment, smoking, physical activity, alcohol intake, BMI, QOL, etc. |
|
| Cardiovascular | Blood pressure | Campbell et al. [162] | A waitlist-controlled longitudinal study | Female post-treatment cancer patients (n = 70, 100% female, mean age, 53) | MBSR for eight weeks | Waitlist | Age, SBP, DBP |
|
| ANS, cardiovascular | Blood pressure, HRV | Muthukrishnan et al. [163] | RCT | Pregnant Indian women at 12 weeks gestation (n = 74, 100% female, mean age, 22) | Mindfulness meditation for five weeks | Usual obstetric care for five weeks | SBP, DBP, RR, perceived stress, HRV, cold pressor SBP, cold pressor DBP, etc. |
|
| Cardiovascular | Blood pressure | Rakshani et al. [164] | RCT | Pregnant women at 12 weeks gestation with previous medical history in pregnancy (n = 68, 100% female, mean age, 27) | Yoga (breathing, meditation, yogi postures) for 15 weeks | Standard care plus conventional antenatal exercises (walking) for 15 weeks | Age, education, income, weight, height, BMI, SBP, DBP |
|
| Cardiovascular | Blood pressure | Thornton et al. [165] | RCT | Healthy community-dwelling women (n = 34, 100% female, mean age, 48) | Tai chi for 12 weeks | Control | Age, body weight, body height, blood pressure |
|
| ANS | HRV | Trivedi et al. [166] | RCT | Healthy women (n = 36, 100% female, mean age, 33) | Active meditation (breathing, positive emotions, guided imagery) for 20 min | Control (silence meditation—breathing only) for 20 min | Age, HRV, affect |
|
| ANS | HRV | Praveena et al. [167] | A prospective longitudinal study | Women within five years of menopause (n = 67, 100% female, age range, 45~60) | Yoga for three months | Control | Age, duration of menopause, body fat, resting heart rate, systolic blood pressure, etc. |
|
| ANS | HRV | Audette et al. [168] | RCT | Sedentary women (n = 27, 100% female, mean age, 71) | Tai chi for 12 weeks (RCT) | Brisk walking for 12 weeks (RCT), sedentary life style for 12 weeks (a separate group) | Age, weight, exercise test, HRV, flexibility, single leg balance |
|
| HPA | Cortisol | Field et al. [169] | RCT | Prenatally depressed women at 22 weeks gestation (n = 92, 100% female, mean age, 24) | Yoga for 12 weeks | Social support for 12 weeks | Age, education, SES, ethnicity, marital status |
|
| HPA | Cortisol slope, stress, QOL | Carlson et al. [170] | RCT | Distressed survivor women of stage I to III breast cancer (n = 271, 100% female, mean age, 55) | MBCR for eight weeks | SET for 12 weeks, control (one-day stress management) | Age, cancer severity, time since diagnosis, alcohol, nicotine intake, quality of sleep, diet |
|
| HPA | Cortisol | Daubenmier et al. [171] | RCT | Overweight/obese women (n = 47, 100% female, mean age, 41) | A four-month mindfulness program for stress eating | Waitlist | Age, weight, BMI, waist circumference, psychological state, CAR response, eating behavior |
|
| HPA, immune | Cortisol, cytokine | Witek–Janusek et al. [172] | A longitudinal study | Women newly diagnosed with early-stage breast cancer (n = 66, 100% female, mean age, 55), women without cancer (mean age = 55, n = 30) | MBSR for eight weeks | Non-MBSR, cancer-free group | Age, assessment time of the day |
|
| Immune | Cytokine | Robins et al. [173] | RCT | Women with high CVD risk (n = 63, 100% female, 35–50 years) | Tai chi for eight weeks | Waitlist | Age, waist circumference |
|
| Immune | Cytokine | Harkess et al. [174] | RCT | A subsample (n = 28, mean age, 41) from a population of women reporting psychological distress (n = 116, 100% female) | Yoga for eight weeks | Waitlist | Age, weight-to-height ratio |
|
| Immune | Cytokine | Gallegos et al. [175] | A longitudinal study | Trauma-exposed women (n = 50, 100% female, mean age, 44) | MBSR for eight weeks | N/A | Age, race, employment status, income |
|
3.2. Changes in Mental Health Caused by Mind–Body Intervention and Findings in Women
3.3. Changes in Brain Structures and Functions by Mind–Body Intervention
3.3.1. Structural Changes in the Prefrontal Cortex by Mind–Body Intervention
3.3.2. Functional Changes in the Prefrontal Cortex by Mind–Body Intervention
3.3.3. Changes in Functional Connectivity between the Prefrontal Cortex and the Amygdala by Mind–Body Intervention
3.3.4. Gender Differences in Brain Structural Changes by Mind–Body Intervention
3.4. Changes in Cortisol Secretion by Mind–Body Intervention
3.5. Changes in Blood Pressure, Heart Rate and Heart Rate Variability by Mind–Body Intervention
3.5.1. Resting Systolic Blood Pressure
3.5.2. Resting Diastolic Blood Pressure
3.5.3. Ambulatory Systolic Blood Pressure
3.5.4. Ambulatory Diastolic Blood Pressure
3.5.5. Heart Rate
3.5.6. Heart Rate Variability, Respiration Rate, Arterial Pressure
3.6. The Effects of Mind–Body Intervention on Lipid Profile and Blood Glucose
3.7. Changes in Inflammatory Response Levels by Mind–Body Intervention
3.8. The Effects of Mind–Body Intervention on Menopausal Symptoms Related with Cardiovascular Health
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shaw, L.J.; Bugiardini, R.; Merz, C.N. Women and ischemic heart disease: Evolving knowledge. J. Am. Coll. Cardiol. 2009, 54, 1561–1575. [Google Scholar] [CrossRef]
- Wilmot, K.A.; O’Flaherty, M.; Capewell, S.; Ford, E.S.; Vaccarino, V. Coronary Heart Disease Mortality Declines in the United States from 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation 2015, 132, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Mahajan, A.M.; Roe, M.T.; Hellkamp, A.S.; Chiswell, K.; Gulati, M.; Reynolds, H.R. Mortality of Myocardial Infarction by Sex, Age, and Obstructive Coronary Artery Disease Status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get with the Guidelines). Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003443. [Google Scholar] [CrossRef]
- Otten, A.M.; Maas, A.H.; Ottervanger, J.P.; Kloosterman, A.; van ‘t Hof, A.W.; Dambrink, J.H.; Gosselink, A.T.; Hoorntje, J.C.; Suryapranata, H.; de Boer, M.J.; et al. Is the difference in outcome between men and women treated by primary percutaneous coronary intervention age dependent? Gender difference in STEMI stratified on age. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 334–341. [Google Scholar] [CrossRef]
- Vaccarino, V.; Parsons, L.; Every, N.R.; Barron, H.V.; Krumholz, H.M. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N. Engl. J. Med. 1999, 341, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.; Cruz, S.D.; Mehta, P.K.; Merz, C.N. Coronary microvascular dysfunction: Sex-specific risk, diagnosis, and therapy. Nat. Rev. Cardiol. 2015, 12, 406–414. [Google Scholar] [CrossRef]
- Gebhard, C.; Bengs, S.; Haider, A.; Fiechter, M. The Neuro-Inflammatory-Vascular Circuit: Evidence for a Sex-Dependent Interrelation? Front. Neurosci. 2020, 14, 614345. [Google Scholar] [CrossRef]
- Maredziak, M.; Bengs, S.; Portmann, A.; Haider, A.; Wijnen, W.J.; Warnock, G.I.; Etter, D.; Froehlich, S.; Fiechter, M.; Meisel, A.; et al. Microvascular dysfunction and sympathetic hyperactivity in women with supra-normal left ventricular ejection fraction (snLVEF). Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3094–3106. [Google Scholar] [CrossRef]
- Kivimaki, M.; Kawachi, I. Work Stress as a Risk Factor for Cardiovascular Disease. Curr. Cardiol. Rep. 2015, 17, 630. [Google Scholar] [CrossRef]
- Dragano, N.; Siegrist, J.; Nyberg, S.T.; Lunau, T.; Fransson, E.I.; Alfredsson, L.; Bjorner, J.B.; Borritz, M.; Burr, H.; Erbel, R.; et al. Effort-Reward Imbalance at Work and Incident Coronary Heart Disease: A Multicohort Study of 90,164 Individuals. Epidemiology 2017, 28, 619–626. [Google Scholar] [CrossRef]
- Kivimaki, M.; Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 2018, 15, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, S.; Hua, J.; Zhu, D.; Liu, C.; Hu, Y.; Liu, T.; Xu, D. Association between job strain and risk of incident stroke: A meta-analysis. Neurology 2015, 85, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Carey, I.M.; Shah, S.M.; DeWilde, S.; Harris, T.; Victor, C.R.; Cook, D.G. Increased risk of acute cardiovascular events after partner bereavement: A matched cohort study. JAMA Intern. Med. 2014, 174, 598–605. [Google Scholar] [CrossRef]
- Smyth, A.; O’Donnell, M.; Lamelas, P.; Teo, K.; Rangarajan, S.; Yusuf, S.; Investigators, I. Physical Activity and Anger or Emotional Upset as Triggers of Acute Myocardial Infarction: The INTERHEART Study. Circulation 2016, 134, 1059–1067. [Google Scholar] [CrossRef]
- Mostofsky, E.; Penner, E.A.; Mittleman, M.A. Outbursts of anger as a trigger of acute cardiovascular events: A systematic review and meta-analysis. Eur. Heart J. 2014, 35, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Chin, S.L.; Rangarajan, S.; Xavier, D.; Liu, L.; Zhang, H.; Rao-Melacini, P.; Zhang, X.; Pais, P.; Agapay, S.; et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): A case-control study. Lancet 2016, 388, 761–775. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Loerbroks, A.; Angerer, P.; Siegrist, J. Work stress and the risk of recurrent coronary heart disease events: A systematic review and meta-analysis. Int. J. Occup. Med. Environ. Health 2015, 28, 8–19. [Google Scholar] [CrossRef]
- Arnold, S.V.; Smolderen, K.G.; Buchanan, D.M.; Li, Y.; Spertus, J.A. Perceived stress in myocardial infarction: Long-term mortality and health status outcomes. J. Am. Coll. Cardiol. 2012, 60, 1756–1763. [Google Scholar] [CrossRef]
- Wilbert-Lampen, U.; Leistner, D.; Greven, S.; Pohl, T.; Sper, S.; Volker, C.; Guthlin, D.; Plasse, A.; Knez, A.; Kuchenhoff, H.; et al. Cardiovascular events during World Cup soccer. N. Engl. J. Med. 2008, 358, 475–483. [Google Scholar] [CrossRef]
- Stewart, R.A.H.; Colquhoun, D.M.; Marschner, S.L.; Kirby, A.C.; Simes, J.; Nestel, P.J.; Glozier, N.; O’Neil, A.; Oldenburg, B.; White, H.D.; et al. Persistent psychological distress and mortality in patients with stable coronary artery disease. Heart 2017, 103, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Rooks, C.; Ramadan, R.; Shah, A.J.; Bremner, J.D.; Quyyumi, A.A.; Kutner, M.; Vaccarino, V. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am. J. Cardiol. 2014, 114, 187–192. [Google Scholar] [CrossRef]
- Oken, B.S.; Chamine, I.; Wakeland, W. A systems approach to stress, stressors and resilience in humans. Behav. Brain Res. 2015, 282, 144–154. [Google Scholar] [CrossRef]
- Iwata, M.; Ota, K.T.; Duman, R.S. The inflammasome: Pathways linking psychological stress, depression, and systemic illnesses. Brain. Behav. Immun. 2013, 31, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Ventriglio, A.; Gentile, A.; Baldessarini, R.J.; Bellomo, A. Early-life stress and psychiatric disorders: Epidemiology, neurobiology and innovative pharmacological targets. Curr. Pharm. Des. 2015, 21, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Hammen, C.L. Stress and depression: Old questions, new approaches. Curr. Opin. Psychol. 2015, 4, 80–85. [Google Scholar] [CrossRef]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. WHO/MSD/MER/2017.2. Available online: https://apps.who.int/iris/handle/10665/254610 (accessed on 10 February 2021).
- Albert, P.R. Why is depression more prevalent in women? J. Psychiatry Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef]
- Kuehner, C. Why is depression more common among women than among men? Lancet Psychiatry 2017, 4, 146–158. [Google Scholar] [CrossRef]
- Dhar, A.K.; Barton, D.A. Depression and the Link with Cardiovascular Disease. Front. Psychiatry 2016, 7, 33. [Google Scholar] [CrossRef]
- Bucciarelli, V.; Caterino, A.L.; Bianco, F.; Caputi, C.G.; Salerni, S.; Sciomer, S.; Maffei, S.; Gallina, S. Depression and cardiovascular disease: The deep blue sea of women’s Heart. Trends Cardiovasc. Med. 2020, 30, 170–176. [Google Scholar] [CrossRef]
- Goldstein, B.I.; Carnethon, M.R.; Matthews, K.A.; McIntyre, R.S.; Miller, G.E.; Raghuveer, G.; Stoney, C.M.; Wasiak, H.; McCrindle, B.W.; American Heart Association, A.; et al. Major Depressive Disorder and Bipolar Disorder Predispose Youth to Accelerated Atherosclerosis and Early Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2015, 132, 965–986. [Google Scholar] [CrossRef]
- Lichtman, J.H.; Froelicher, E.S.; Blumenthal, J.A.; Carney, R.M.; Doering, L.V.; Frasure-Smith, N.; Freedland, K.E.; Jaffe, A.S.; Leifheit-Limson, E.C.; Sheps, D.S.; et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: Systematic review and recommendations: A scientific statement from the American Heart Association. Circulation 2014, 129, 1350–1369. [Google Scholar] [CrossRef]
- Gafarov, V.V.; Panov, D.O.; Gromova, E.A.; Gagulin, I.V.; Gafarova, A.V. The influence of depression on risk development of acute cardiovascular diseases in the female population aged 25-64 in Russia. Int. J. Circumpolar. Health 2013, 72. [Google Scholar] [CrossRef]
- McLean, C.P.; Asnaani, A.; Litz, B.T.; Hofmann, S.G. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011, 45, 1027–1035. [Google Scholar] [CrossRef]
- Allgulander, C. Anxiety as a risk factor in cardiovascular disease. Curr. Opin. Psychiatry 2016, 29, 13–17. [Google Scholar] [CrossRef]
- Templin, C.; Hanggi, J.; Klein, C.; Topka, M.S.; Hiestand, T.; Levinson, R.A.; Jurisic, S.; Luscher, T.F.; Ghadri, J.R.; Jancke, L. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur. Heart J. 2019, 40, 1183–1187. [Google Scholar] [CrossRef]
- Ueyama, T.; Kasamatsu, K.; Hano, T.; Tsuruo, Y.; Ishikura, F. Catecholamines and estrogen are involved in the pathogenesis of emotional stress-induced acute heart attack. Ann. N. Y. Acad. Sci. 2008, 1148, 479–485. [Google Scholar] [CrossRef]
- Deshmukh, A.; Kumar, G.; Pant, S.; Rihal, C.; Murugiah, K.; Mehta, J.L. Prevalence of Takotsubo cardiomyopathy in the United States. Am. Heart J. 2012, 164, 66–71. [Google Scholar] [CrossRef]
- Samad, Z.; Boyle, S.; Ersboll, M.; Vora, A.N.; Zhang, Y.; Becker, R.C.; Williams, R.; Kuhn, C.; Ortel, T.L.; Rogers, J.G.; et al. Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: Insights from the REMIT study. J. Am. Coll. Cardiol. 2014, 64, 1669–1678. [Google Scholar] [CrossRef]
- Wassertheil-Smoller, S.; Shumaker, S.; Ockene, J.; Talavera, G.A.; Greenland, P.; Cochrane, B.; Robbins, J.; Aragaki, A.; Dunbar-Jacob, J. Depression and cardiovascular sequelae in postmenopausal women. The Women’s Health Initiative (WHI). Arch. Intern. Med. 2004, 164, 289–298. [Google Scholar] [CrossRef]
- Zerriaa, O.; Moula, O.; Ben Saadi, S.; Ghachem, J.R. Benefits of antidepressant treatment after a stroke. Eur. Psychiatry 2017, 41, S315. [Google Scholar] [CrossRef]
- Vaccarino, V.; Sullivan, S.; Hammadah, M.; Wilmot, K.; Al Mheid, I.; Ramadan, R.; Elon, L.; Pimple, P.M.; Garcia, E.V.; Nye, J.; et al. Mental Stress-Induced-Myocardial Ischemia in Young Patients with Recent Myocardial Infarction: Sex Differences and Mechanisms. Circulation 2018, 137, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Tepper, P.G.; Randolph, J.F., Jr.; McConnell, D.S.; Crawford, S.L.; El Khoudary, S.R.; Joffe, H.; Gold, E.B.; Zheng, H.; Bromberger, J.T.; Sutton-Tyrrell, K. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health across the Nation (SWAN). J. Clin. Endocrinol. Metab. 2012, 97, 2872–2880. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.P.; Reckelhoff, J.F. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 133–138. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Thurston, R.C. Cardiovascular Implications of the Menopause Transition: Endogenous Sex Hormones and Vasomotor Symptoms. Obstet. Gynecol. Clin. N. Am. 2018, 45, 641–661. [Google Scholar] [CrossRef]
- Randolph, J.F., Jr.; Zheng, H.; Sowers, M.R.; Crandall, C.; Crawford, S.; Gold, E.B.; Vuga, M. Change in follicle-stimulating hormone and estradiol across the menopausal transition: Effect of age at the final menstrual period. J. Clin. Endocrinol. Metab. 2011, 96, 746–754. [Google Scholar] [CrossRef]
- Burger, H.G.; Dudley, E.C.; Hopper, J.L.; Groome, N.; Guthrie, J.R.; Green, A.; Dennerstein, L. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J. Clin. Endocrinol. Metab. 1999, 84, 4025–4030. [Google Scholar] [CrossRef]
- Celestino Catao Da Silva, D.; Nogueira De Almeida Vasconcelos, A.; Cleto Maria Cerqueira, J.; De Oliveira Cipriano Torres, D.; Oliveira Dos Santos, A.C.; De Lima Ferreira Fernandes Costa, H.; Bregieiro Fernandes Costa, L.O. Endogenous sex hormones are not associated with subclinical atherosclerosis in menopausal women. Minerva Ginecol. 2013, 65, 297–302. [Google Scholar]
- Moreau, K.L.; Hildreth, K.L.; Meditz, A.L.; Deane, K.D.; Kohrt, W.M. Endothelial function is impaired across the stages of the menopause transition in healthy women. J. Clin. Endocrinol. Metab. 2012, 97, 4692–4700. [Google Scholar] [CrossRef] [PubMed]
- Munir, J.A.; Wu, H.; Bauer, K.; Bindeman, J.; Byrd, C.; Feuerstein, I.M.; Villines, T.C.; Taylor, A.J. The perimenopausal atherosclerosis transition: Relationships between calcified and noncalcified coronary, aortic, and carotid atherosclerosis and risk factors and hormone levels. Menopause 2012, 19, 10–15. [Google Scholar] [CrossRef]
- Derby, C.A.; Crawford, S.L.; Pasternak, R.C.; Sowers, M.; Sternfeld, B.; Matthews, K.A. Lipid changes during the menopause transition in relation to age and weight: The Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2009, 169, 1352–1361. [Google Scholar] [CrossRef]
- De Kat, A.C.; Dam, V.; Onland-Moret, N.C.; Eijkemans, M.J.; Broekmans, F.J.; van der Schouw, Y.T. Unraveling the associations of age and menopause with cardiovascular risk factors in a large population-based study. BMC Med. 2017, 15, 2. [Google Scholar] [CrossRef]
- Matthews, K.A.; Crawford, S.L.; Chae, C.U.; Everson-Rose, S.A.; Sowers, M.F.; Sternfeld, B.; Sutton-Tyrrell, K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J. Am. Coll. Cardiol. 2009, 54, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; El Khoudary, S.R.; Brooks, M.M.; Derby, C.A.; Harlow, S.D.; Barinas-Mitchell, E.J.; Thurston, R.C. Lipid Changes Around the Final Menstrual Period Predict Carotid Subclinical Disease in Postmenopausal Women. Stroke 2017, 48, 70–76. [Google Scholar] [CrossRef]
- Wildman, R.P.; Colvin, A.B.; Powell, L.H.; Matthews, K.A.; Everson-Rose, S.A.; Hollenberg, S.; Johnston, J.M.; Sutton-Tyrrell, K. Associations of endogenous sex hormones with the vasculature in menopausal women: The Study of Women’s Health Across the Nation (SWAN). Menopause 2008, 15, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.D.; Dwyer, K.M.; Stanczyk, F.Z.; Bittner, V.; Berga, S.L.; Braunstein, G.D.; Azziz, R.; Yang, Y.; Hale, G.E.; Bairey Merz, C.N. The relationship of menopausal status and rapid menopausal transition with carotid intima-media thickness progression in women: A report from the Los Angeles Atherosclerosis Study. J. Clin. Endocrinol. Metab. 2010, 95, 4432–4440. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Wildman, R.P.; Matthews, K.; Thurston, R.C.; Bromberger, J.T.; Sutton-Tyrrell, K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause 2013, 20, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Wofford, M.; Reckelhoff, J.F. Hypertension in postmenopausal women. Curr. Hypertens. Rep. 2012, 14, 254–260. [Google Scholar] [CrossRef]
- Strain, W.D.; Paldanius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, M.; Liu, Y.; Sun, X.; Wang, B.; Zhao, Y.; Liu, D.; Liu, X.; Zhang, D.; Liu, F.; et al. Association of menopause and type 2 diabetes mellitus. Menopause 2019, 26, 325–330. [Google Scholar] [CrossRef]
- Atsma, F.; Bartelink, M.L.; Grobbee, D.E.; van der Schouw, Y.T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause 2006, 13, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Oliver-Williams, C.; Kunutsor, S.; Laven, J.S.; Fauser, B.C.; Chowdhury, R.; Kavousi, M.; Franco, O.H. Association of Age at Onset of Menopause and Time Since Onset of Menopause with Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol. 2016, 1, 767–776. [Google Scholar] [CrossRef]
- Tao, X.Y.; Zuo, A.Z.; Wang, J.Q.; Tao, F.B. Effect of primary ovarian insufficiency and early natural menopause on mortality: A meta-analysis. Climacteric 2016, 19, 27–36. [Google Scholar] [CrossRef]
- Zhu, D.; Chung, H.F.; Dobson, A.J.; Pandeya, N.; Giles, G.G.; Bruinsma, F.; Brunner, E.J.; Kuh, D.; Hardy, R.; Avis, N.E.; et al. Age at natural menopause and risk of incident cardiovascular disease: A pooled analysis of individual patient data. Lancet Public Health 2019, 4, e553–e564. [Google Scholar] [CrossRef]
- Brotman, D.J.; Golden, S.H.; Wittstein, I.S. The cardiovascular toll of stress. Lancet 2007, 370, 1089–1100. [Google Scholar] [CrossRef]
- Vaccarino, V.; Bremner, J.D. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci. Biobehav. Rev. 2017, 74, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F. Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat. Neurosci. 2015, 18, 1376–1385. [Google Scholar] [CrossRef]
- Fleshner, M.; Crane, C.R. Exosomes, DAMPs and miRNA: Features of Stress Physiology and Immune Homeostasis. Trends Immunol. 2017, 38, 768–776. [Google Scholar] [CrossRef]
- Lima, B.B.; Hammadah, M.; Kim, J.H.; Uphoff, I.; Shah, A.; Levantsevych, O.; Almuwaqqat, Z.; Moazzami, K.; Sullivan, S.; Ward, L.; et al. Association of Transient Endothelial Dysfunction Induced by Mental Stress with Major Adverse Cardiovascular Events in Men and Women with Coronary Artery Disease. JAMA Cardiol. 2019, 4, 988–996. [Google Scholar] [CrossRef]
- Mehta, P.K.; Lima, B.B.; Nelson, M.D.; Bairey Merz, C.N. Adverse cardiovascular outcomes in women: Blame the amygdala? Eur. Heart J. Cardiovasc. Imaging 2019, 20, 633–635. [Google Scholar] [CrossRef]
- Garcia, R.G.; Mareckova, K.; Holsen, L.M.; Cohen, J.E.; Whitfield-Gabrieli, S.; Napadow, V.; Barbieri, R.; Goldstein, J.M. Impact of sex and depressed mood on the central regulation of cardiac autonomic function. Neuropsychopharmacology 2020, 45, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Mareckova, K.; Holsen, L.; Admon, R.; Whitfield-Gabrieli, S.; Seidman, L.J.; Buka, S.L.; Klibanski, A.; Goldstein, J.M. Neural—Hormonal responses to negative affective stimuli: Impact of dysphoric mood and sex. J. Affect. Disord. 2017, 222, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Fiechter, M.; Haider, A.; Bengs, S.; Maredziak, M.; Burger, I.A.; Roggo, A.; Portmann, A.; Warnock, G.I.; Schade, K.; Treyer, V.; et al. Sex Differences in the Association between Inflammation and Ischemic Heart Disease. Thromb. Haemost. 2019, 119, 1471–1480. [Google Scholar] [CrossRef]
- Fiechter, M.; Haider, A.; Bengs, S.; Maredziak, M.; Burger, I.A.; Roggo, A.; Portmann, A.; Schade, K.; Warnock, G.I.; Treyer, V.; et al. Sex-dependent association between inflammation, neural stress responses, and impaired myocardial function. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2010–2015. [Google Scholar] [CrossRef]
- Kogler, L.; Muller, V.I.; Seidel, E.M.; Boubela, R.; Kalcher, K.; Moser, E.; Habel, U.; Gur, R.C.; Eickhoff, S.B.; Derntl, B. Sex differences in the functional connectivity of the amygdalae in association with cortisol. Neuroimage 2016, 134, 410–423. [Google Scholar] [CrossRef]
- Tawakol, A.; Ishai, A.; Takx, R.A.; Figueroa, A.L.; Ali, A.; Kaiser, Y.; Truong, Q.A.; Solomon, C.J.; Calcagno, C.; Mani, V.; et al. Relation between resting amygdalar activity and cardiovascular events: A longitudinal and cohort study. Lancet 2017, 389, 834–845. [Google Scholar] [CrossRef]
- Gianaros, P.J.; Hariri, A.R.; Sheu, L.K.; Muldoon, M.F.; Sutton-Tyrrell, K.; Manuck, S.B. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol. Psychiatry 2009, 65, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog. Brain. Res. 2000, 126, 413–431. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Tsigos, C.; Chrousos, G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Salim, S.; Chugh, G.; Asghar, M. Inflammation in anxiety. Adv. Protein. Chem. Struct. Biol. 2012, 88, 1–25. [Google Scholar] [CrossRef]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef]
- Miller, D.B.; O’Callaghan, J.P. Neuroendocrine aspects of the response to stress. Metabolism 2002, 51, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, M.C.; Crewther, S.G.; Carey, L.M.; Crewther, D.P. Inflammation and depression: Why poststroke depression may be the norm and not the exception. Int. J. Stroke 2011, 6, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Bangasser, D.A.; Valentino, R.J. Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Front. Neuroendocrinol. 2014, 35, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Kudielka, B.M.; Kirschbaum, C. Sex differences in HPA axis responses to stress: A review. Biol. Psychol. 2005, 69, 113–132. [Google Scholar] [CrossRef]
- Bale, T.L.; Epperson, C.N. Sex differences and stress across the lifespan. Nat. Neurosci. 2015, 18, 1413–1420. [Google Scholar] [CrossRef]
- Heim, C.; Nemeroff, C.B. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol. Psychiatry 1999, 46, 1509–1522. [Google Scholar] [CrossRef]
- Romeo, R.D. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front. Neuroendocrinol. 2010, 31, 232–240. [Google Scholar] [CrossRef]
- Seeman, T.E.; Singer, B.; Wilkinson, C.W.; McEwen, B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology 2001, 26, 225–240. [Google Scholar] [CrossRef]
- Uhart, M.; Chong, R.Y.; Oswald, L.; Lin, P.I.; Wand, G.S. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology 2006, 31, 642–652. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Kudielka, B.M.; Gaab, J.; Schommer, N.C.; Hellhammer, D.H. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999, 61, 154–162. [Google Scholar] [CrossRef]
- Rohleder, N.; Schommer, N.C.; Hellhammer, D.H.; Engel, R.; Kirschbaum, C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom. Med. 2001, 63, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Juster, R.P.; Raymond, C.; Desrochers, A.B.; Bourdon, O.; Durand, N.; Wan, N.; Pruessner, J.C.; Lupien, S.J. Sex hormones adjust “sex-specific” reactive and diurnal cortisol profiles. Psychoneuroendocrinology 2016, 63, 282–290. [Google Scholar] [CrossRef]
- LeDoux, J.E.; Iwata, J.; Cicchetti, P.; Reis, D.J. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 1988, 8, 2517–2529. [Google Scholar] [CrossRef]
- Curtis, B.M.; O’Keefe, J.H., Jr. Autonomic tone as a cardiovascular risk factor: The dangers of chronic fight or flight. Mayo Clin. Proc. 2002, 77, 45–54. [Google Scholar] [CrossRef]
- Hage, M.P.; Azar, S.T. The Link between Thyroid Function and Depression. J. Thyroid Res. 2012, 2012, 590648. [Google Scholar] [CrossRef]
- Voss, A.; Boettger, M.K.; Schulz, S.; Gross, K.; Bar, K.J. Gender-dependent impact of major depression on autonomic cardiovascular modulation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Di Monaco, A.; Bruno, I.; Calcagni, M.L.; Nerla, R.; Lamendola, P.; Barone, L.; Scalone, G.; Mollo, R.; Coviello, I.; Bagnato, A.; et al. Cardiac adrenergic nerve function in patients with cardiac syndrome X. J. Cardiovasc. Med. 2010, 11, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef]
- Ubrich, R.; Barthel, P.; Haller, B.; Hnatkova, K.; Huster, K.M.; Steger, A.; Muller, A.; Malik, M.; Schmidt, G. Sex differences in long-term mortality among acute myocardial infarction patients: Results from the ISAR-RISK and ART studies. PLoS ONE 2017, 12, e0186783. [Google Scholar] [CrossRef]
- Hogarth, A.J.; Graham, L.N.; Mary, D.A.; Greenwood, J.P. Gender differences in sympathetic neural activation following uncomplicated acute myocardial infarction. Eur. Heart J. 2009, 30, 1764–1770. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Bigger, J.T., Jr.; Marcus, F.I.; Mortara, A.; Schwartz, P.J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998, 351, 478–484. [Google Scholar] [CrossRef]
- Mitoff, P.R.; Gam, D.; Ivanov, J.; Al-hesayen, A.; Azevedo, E.R.; Newton, G.E.; Parker, J.D.; Mak, S. Cardiac-specific sympathetic activation in men and women with and without heart failure. Heart 2011, 97, 382–387. [Google Scholar] [CrossRef]
- McEwen, B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef]
- Tomfohr, L.M.; Martin, T.M.; Miller, G.E. Symptoms of depression and impaired endothelial function in healthy adolescent women. J. Behav. Med. 2008, 31, 137–143. [Google Scholar] [CrossRef]
- Hiteshi, A.K.; Li, D.; Gao, Y.; Chen, A.; Flores, F.; Mao, S.S.; Budoff, M.J. Gender differences in coronary artery diameter are not related to body habitus or left ventricular mass. Clin. Cardiol. 2014, 37, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.L.; Lee, B.C.; Sitek, A.; Naya, M.; Moody, J.; Polavarapu, V.; Ficaro, E.P.; Di Carli, M.F. Comparison and prognostic validation of multiple methods of quantification of myocardial blood flow with 82Rb PET. J. Nucl. Med. 2014, 55, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Bengs, S.; Maredziak, M.; Messerli, M.; Fiechter, M.; Giannopoulos, A.A.; Treyer, V.; Schwyzer, M.; Kamani, C.H.; Patriki, D.; et al. Heart rate reserve during pharmacological stress is a significant negative predictor of impaired coronary flow reserve in women. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Bui, L.P.; Kirkeeide, R.L.; Gould, K.L. Imaging Microvascular Dysfunction and Mechanisms for Female-Male Differences in CAD. JACC Cardiovasc. Imaging 2016, 9, 465–482. [Google Scholar] [CrossRef]
- Magkos, F.; Patterson, B.W.; Mohammed, B.S.; Klein, S.; Mittendorfer, B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J. Clin. Endocrinol. Metab. 2007, 92, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, K.N.; Boon, W.C.; Murata, Y.; Jones, M.E.; Simpson, E.R. The aromatase knockout mouse presents with a sexually dimorphic disruption to cholesterol homeostasis. Endocrinology 2003, 144, 3895–3903. [Google Scholar] [CrossRef]
- Muka, T.; Oliver-Williams, C.; Colpani, V.; Kunutsor, S.; Chowdhury, S.; Chowdhury, R.; Kavousi, M.; Franco, O.H. Association of Vasomotor and Other Menopausal Symptoms with Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0157417. [Google Scholar] [CrossRef] [PubMed]
- Herber-Gast, G.; Brown, W.J.; Mishra, G.D. Hot flushes and night sweats are associated with coronary heart disease risk in midlife: A longitudinal study. BJOG 2015, 122, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.J.; Park, H.T.; Kwon, D.H.; Yang, K.S.; Kim, Y.J.; Yi, K.W.; Shin, J.H.; Hur, J.Y.; Kim, T. Vasomotor symptoms and metabolic syndrome in Korean postmenopausal women. Menopause 2015, 22, 1239–1245. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Muscatell, K.A.; Dedovic, K.; Slavich, G.M.; Jarcho, M.R.; Breen, E.C.; Bower, J.E.; Irwin, M.R.; Eisenberger, N.I. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain. Behav. Immun. 2015, 43, 46–53. [Google Scholar] [CrossRef]
- Huang, Q.H.; Takaki, A.; Arimura, A. Central noradrenergic system modulates plasma interleukin-6 production by peripheral interleukin-1. Am. J. Physiol. 1997, 273, R731–R738. [Google Scholar] [CrossRef]
- Goebel, M.U.; Mills, P.J.; Irwin, M.R.; Ziegler, M.G. Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: Differential effects and pathways. Psychosom. Med. 2000, 62, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Wolf, J.; Andrassy, M.; Rohleder, N.; Humpert, P.M.; Petrov, D.; Ferstl, R.; von Eynatten, M.; Wendt, T.; Rudofsky, G.; et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. USA 2003, 100, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- DeRijk, R.H.; Boelen, A.; Tilders, F.J.; Berkenbosch, F. Induction of plasma interleukin-6 by circulating adrenaline in the rat. Psychoneuroendocrinology 1994, 19, 155–163. [Google Scholar] [CrossRef]
- Kop, W.J.; Weissman, N.J.; Zhu, J.; Bonsall, R.W.; Doyle, M.; Stretch, M.R.; Glaes, S.B.; Krantz, D.S.; Gottdiener, J.S.; Tracy, R.P. Effects of acute mental stress and exercise on inflammatory markers in patients with coronary artery disease and healthy controls. Am. J. Cardiol. 2008, 101, 767–773. [Google Scholar] [CrossRef]
- Smyth, G.P.; Stapleton, P.P.; Freeman, T.A.; Concannon, E.M.; Mestre, J.R.; Duff, M.; Maddali, S.; Daly, J.M. Glucocorticoid pretreatment induces cytokine overexpression and nuclear factor-kappaB activation in macrophages. J. Surg. Res. 2004, 116, 253–261. [Google Scholar] [CrossRef]
- Yeager, M.P.; Pioli, P.A.; Guyre, P.M. Cortisol exerts bi-phasic regulation of inflammation in humans. Dose Response 2011, 9, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Kinlein, S.A.; Wilson, C.D.; Karatsoreos, I.N. Dysregulated hypothalamic-pituitary-adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Front. Psychiatry 2015, 6, 31. [Google Scholar] [CrossRef]
- Markle, J.G.; Fish, E.N. SeXX matters in immunity. Trends Immunol. 2014, 35, 97–104. [Google Scholar] [CrossRef]
- Casimir, G.J.; Lefevre, N.; Corazza, F.; Duchateau, J.; Chamekh, M. The Acid-Base Balance and Gender in Inflammation: A Mini-Review. Front. Immunol. 2018, 9, 475. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef]
- Casimir, G.J.; Lefevre, N.; Corazza, F.; Duchateau, J. Sex and inflammation in respiratory diseases: A clinical viewpoint. Biol. Sex Differ. 2013, 4, 16. [Google Scholar] [CrossRef]
- Rosenfeld, M.; Davis, R.; FitzSimmons, S.; Pepe, M.; Ramsey, B. Gender gap in cystic fibrosis mortality. Am. J. Epidemiol. 1997, 145, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Wong, N.D.; Pio, J.; Valencia, R.; Thakal, G. Distribution of C-reactive protein and its relation to risk factors and coronary heart disease risk estimation in the National Health and Nutrition Examination Survey (NHANES) III. Prev. Cardiol. 2001, 4, 109–114. [Google Scholar] [CrossRef]
- Qasim, A.N.; Budharaju, V.; Mehta, N.N.; St Clair, C.; Farouk, S.; Braunstein, S.; Schutta, M.; Iqbal, N.; Rader, D.J.; Reilly, M.P. Gender differences in the association of C-reactive protein with coronary artery calcium in type-2 diabetes. Clin. Endocrinol. 2011, 74, 44–50. [Google Scholar] [CrossRef]
- Pruijm, M.; Vollenweider, P.; Mooser, V.; Paccaud, F.; Preisig, M.; Waeber, G.; Marques-Vidal, P.; Burnier, M.; Bochud, M. Inflammatory markers and blood pressure: Sex differences and the effect of fat mass in the CoLaus Study. J. Hum. Hypertens. 2013, 27, 169–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shanahan, L.; Freeman, J.; Bauldry, S. Is very high C-reactive protein in young adults associated with indicators of chronic disease risk? Psychoneuroendocrinology 2014, 40, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Hammadah, M.; Wilmot, K.; Ramadan, R.; Pearce, B.D.; Shah, A.; Kaseer, B.; Gafeer, M.M.; Lima, B.B.; Kim, J.H.; et al. Young Women with Coronary Artery Disease Exhibit Higher Concentrations of Interleukin-6 at Baseline and in Response to Mental Stress. J. Am. Heart Assoc. 2018, 7, e010329. [Google Scholar] [CrossRef]
- Kushner, R.F.; Sorensen, K.W. Lifestyle medicine: The future of chronic disease management. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 389–395. [Google Scholar] [CrossRef]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Doughty, K.N.; Del Pilar, N.X.; Audette, A.; Katz, D.L. Lifestyle Medicine and the Management of Cardiovascular Disease. Curr. Cardiol. Rep. 2017, 19, 116. [Google Scholar] [CrossRef]
- Barbaresko, J.; Rienks, J.; Nothlings, U. Lifestyle Indices and Cardiovascular Disease Risk: A Meta-analysis. Am. J. Prev. Med. 2018, 55, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Singh, S.; Sibinga, E.M.; Gould, N.F.; Rowland-Seymour, A.; Sharma, R.; Berger, Z.; Sleicher, D.; Maron, D.D.; Shihab, H.M.; et al. Meditation programs for psychological stress and well-being: A systematic review and meta-analysis. JAMA Intern. Med. 2014, 174, 357–368. [Google Scholar] [CrossRef]
- Gallegos, A.M.; Crean, H.F.; Pigeon, W.R.; Heffner, K.L. Meditation and yoga for posttraumatic stress disorder: A meta-analytic review of randomized controlled trials. Clin. Psychol. Rev. 2017, 58, 115–124. [Google Scholar] [CrossRef]
- Katterman, S.N.; Kleinman, B.M.; Hood, M.M.; Nackers, L.M.; Corsica, J.A. Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: A systematic review. Eat. Behav. 2014, 15, 197–204. [Google Scholar] [CrossRef]
- Davis, J.M.; Goldberg, S.B.; Anderson, M.C.; Manley, A.R.; Smith, S.S.; Baker, T.B. Randomized trial on mindfulness training for smokers targeted to a disadvantaged population. Subst. Use Misuse 2014, 49, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.S.; Knobf, T.; Oh, B.; Funk, M. Physical and Psychological Health Outcomes of Qigong Exercise in Older Adults: A Systematic Review and Meta-Analysis. Am. J. Chin. Med. 2019, 47, 301–322. [Google Scholar] [CrossRef]
- Strowger, M.; Kiken, L.G.; Ramcharran, K. Mindfulness meditation and physical activity: Evidence from 2012 National Health Interview Survey. Health Psychol. 2018, 37, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Lange, R.A.; Bairey-Merz, C.N.; Davidson, R.J.; Jamerson, K.; Mehta, P.K.; Michos, E.D.; Norris, K.; Ray, I.B.; Saban, K.L.; et al. Meditation and Cardiovascular Risk Reduction: A Scientific Statement from the American Heart Association. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, M.C.; Thompson, D.R.; Ski, C.F. Meditation and Endocrine Health and Wellbeing. Trends Endocrinol. Metab. 2020, 31, 469–477. [Google Scholar] [CrossRef]
- Krittanawong, C.; Kumar, A.; Wang, Z.; Narasimhan, B.; Jneid, H.; Virani, S.S.; Levine, G.N. Meditation and Cardiovascular Health in the US. Am. J. Cardiol. 2020, 131, 23–26. [Google Scholar] [CrossRef]
- Gong, H.; Ni, C.; Shen, X.; Wu, T.; Jiang, C. Yoga for prenatal depression: A systematic review and meta-analysis. BMC Psychiatry 2015, 15, 14. [Google Scholar] [CrossRef]
- Van der Kolk, B.A.; Stone, L.; West, J.; Rhodes, A.; Emerson, D.; Suvak, M.; Spinazzola, J. Yoga as an adjunctive treatment for posttraumatic stress disorder: A randomized controlled trial. J. Clin. Psychiatry 2014, 75, e559–e565. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Winkler, M.M.; Klose, P.; Dobos, G.; Kummel, S.; Cramer, H. Mindfulness-based interventions for women with breast cancer: An updated systematic review and meta-analysis. Acta. Oncol. 2017, 56, 1665–1676. [Google Scholar] [CrossRef]
- Kang, Y.; Rahrig, H.; Eichel, K.; Niles, H.F.; Rocha, T.; Lepp, N.E.; Gold, J.; Britton, W.B. Gender differences in response to a school-based mindfulness training intervention for early adolescents. J. Sch. Psychol. 2018, 68, 163–176. [Google Scholar] [CrossRef]
- Rojiani, R.; Santoyo, J.F.; Rahrig, H.; Roth, H.D.; Britton, W.B. Women Benefit More Than Men in Response to College-based Meditation Training. Front. Psychol. 2017, 8, 551. [Google Scholar] [CrossRef]
- Chen, K.W.; Comerford, A.; Shinnick, P.; Ziedonis, D.M. Introducing qigong meditation into residential addiction treatment: A pilot study where gender makes a difference. J. Altern. Complement. Med. 2010, 16, 875–882. [Google Scholar] [CrossRef]
- Luders, E.; Thompson, P.M.; Kurth, F. Larger hippocampal dimensions in meditation practitioners: Differential effects in women and men. Front. Psychol. 2015, 6, 186. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Yip, B.H.; Gao, T.; Lam, K.Y.Y.; Woo, D.M.S.; Yip, A.L.K.; Chin, C.Y.; Tang, W.P.Y.; Choy, M.M.T.; Tsang, K.W.K.; et al. Mindfulness-Based Stress Reduction (MBSR) or Psychoeducation for the Reduction of Menopausal Symptoms: A Randomized, Controlled Clinical Trial. Sci. Rep. 2018, 8, 6609. [Google Scholar] [CrossRef] [PubMed]
- Chattha, R.; Raghuram, N.; Venkatram, P.; Hongasandra, N.R. Treating the climacteric symptoms in Indian women with an integrated approach to yoga therapy: A randomized control study. Menopause 2008, 15, 862–870. [Google Scholar] [CrossRef]
- Carmody, J.F.; Crawford, S.; Salmoirago-Blotcher, E.; Leung, K.; Churchill, L.; Olendzki, N. Mindfulness training for coping with hot flashes: Results of a randomized trial. Menopause 2011, 18, 611–620. [Google Scholar] [CrossRef]
- Campbell, T.S.; Labelle, L.E.; Bacon, S.L.; Faris, P.; Carlson, L.E. Impact of Mindfulness-Based Stress Reduction (MBSR) on attention, rumination and resting blood pressure in women with cancer: A waitlist-controlled study. J. Behav. Med. 2012, 35, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.; Jain, R.; Kohli, S.; Batra, S. Effect of Mindfulness Meditation on Perceived Stress Scores and Autonomic Function Tests of Pregnant Indian Women. J. Clin. Diagn. Res. 2016, 10, CC05–CC08. [Google Scholar] [CrossRef] [PubMed]
- Rakhshani, A.; Nagarathna, R.; Mhaskar, R.; Mhaskar, A.; Thomas, A.; Gunasheela, S. The effects of yoga in prevention of pregnancy complications in high-risk pregnancies: A randomized controlled trial. Prev. Med. 2012, 55, 333–340. [Google Scholar] [CrossRef]
- Thornton, E.W.; Sykes, K.S.; Tang, W.K. Health benefits of Tai Chi exercise: Improved balance and blood pressure in middle-aged women. Health Promot. Int. 2004, 19, 33–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trivedi, G.Y.; Patel, V.; Shah, M.H.; Dhok, M.J.; Bhoyania, K. Comparative Study of the Impact of Active Meditation Protocol and Silence Meditation on Heart Rate Variability and Mood in Women. Int. J. Yoga 2020, 13, 255–260. [Google Scholar] [CrossRef]
- Praveena, S.M.; Asha, G.; Sunita, M.; Anju, J.; Ratna, B. Yoga Offers Cardiovascular Protection in Early Postmenopausal Women. Int. J. Yoga 2018, 11, 37–43. [Google Scholar] [CrossRef]
- Audette, J.F.; Jin, Y.S.; Newcomer, R.; Stein, L.; Duncan, G.; Frontera, W.R. Tai Chi versus brisk walking in elderly women. Age Ageing 2006, 35, 388–393. [Google Scholar] [CrossRef]
- Field, T.; Diego, M.; Delgado, J.; Medina, L. Yoga and social support reduce prenatal depression, anxiety and cortisol. J. Bodyw. Mov. Ther. 2013, 17, 397–403. [Google Scholar] [CrossRef]
- Carlson, L.E.; Doll, R.; Stephen, J.; Faris, P.; Tamagawa, R.; Drysdale, E.; Speca, M. Randomized controlled trial of Mindfulness-based cancer recovery versus supportive expressive group therapy for distressed survivors of breast cancer. J. Clin. Oncol. 2013, 31, 3119–3126. [Google Scholar] [CrossRef]
- Daubenmier, J.; Kristeller, J.; Hecht, F.M.; Maninger, N.; Kuwata, M.; Jhaveri, K.; Lustig, R.H.; Kemeny, M.; Karan, L.; Epel, E. Mindfulness Intervention for Stress Eating to Reduce Cortisol and Abdominal Fat among Overweight and Obese Women: An Exploratory Randomized Controlled Study. J. Obes. 2011, 2011, 651936. [Google Scholar] [CrossRef]
- Witek-Janusek, L.; Albuquerque, K.; Chroniak, K.R.; Chroniak, C.; Durazo-Arvizu, R.; Mathews, H.L. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain. Behav. Immun. 2008, 22, 969–981. [Google Scholar] [CrossRef]
- Robins, J.L.; Elswick, R.K., Jr.; Sturgill, J.; McCain, N.L. The Effects of Tai Chi on Cardiovascular Risk in Women. Am. J. Health Promot. 2016, 30, 613–622. [Google Scholar] [CrossRef]
- Harkess, K.N.; Ryan, J.; Delfabbro, P.H.; Cohen-Woods, S. Preliminary indications of the effect of a brief yoga intervention on markers of inflammation and DNA methylation in chronically stressed women. Transl. Psychiatry 2016, 6, e965. [Google Scholar] [CrossRef]
- Gallegos, A.M.; Lytle, M.C.; Moynihan, J.A.; Talbot, N.L. Mindfulness-based stress reduction to enhance psychological functioning and improve inflammatory biomarkers in trauma-exposed women: A pilot study. Psychol. Trauma 2015, 7, 525–532. [Google Scholar] [CrossRef]
- Breedvelt, J.J.F.; Amanvermez, Y.; Harrer, M.; Karyotaki, E.; Gilbody, S.; Bockting, C.L.H.; Cuijpers, P.; Ebert, D.D. The Effects of Meditation, Yoga, and Mindfulness on Depression, Anxiety, and Stress in Tertiary Education Students: A Meta-Analysis. Front. Psychiatry 2019, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Brinsley, J.; Schuch, F.; Lederman, O.; Girard, D.; Smout, M.; Immink, M.A.; Stubbs, B.; Firth, J.; Davison, K.; Rosenbaum, S. Effects of yoga on depressive symptoms in people with mental disorders: A systematic review and meta-analysis. Br. J. Sports Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- So, W.W.Y.; Cai, S.; Yau, S.Y.; Tsang, H.W.H. The Neurophysiological and Psychological Mechanisms of Qigong as a Treatment for Depression: A Systematic Review and Meta-Analysis. Front. Psychiatry 2019, 10, 820. [Google Scholar] [CrossRef]
- Reangsing, C.; Rittiwong, T.; Schneider, J.K. Effects of mindfulness meditation interventions on depression in older adults: A meta-analysis. Aging Ment. Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Yeung, A.; Li, C.; Wei, G.X.; Chen, K.W.; Kinser, P.A.; Chan, J.S.M.; Ren, Z. Effects of Meditative Movements on Major Depressive Disorder: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2018, 7, 195. [Google Scholar] [CrossRef]
- Gonzalez-Valero, G.; Zurita-Ortega, F.; Ubago-Jimenez, J.L.; Puertas-Molero, P. Use of Meditation and Cognitive Behavioral Therapies for the Treatment of Stress, Depression and Anxiety in Students. A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 4394. [Google Scholar] [CrossRef]
- Cramer, H.; Peng, W.; Lauche, R. Yoga for menopausal symptoms-A systematic review and meta-analysis. Maturitas 2018, 109, 13–25. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, K.; Wang, Y.; Zhang, L.; Liang, H. Could yoga practice improve treatment-related side effects and quality of life for women with breast cancer? A systematic review and meta-analysis. Asia Pac. J. Clin. Oncol. 2017, 13, e79–e95. [Google Scholar] [CrossRef]
- Fox, K.C.; Nijeboer, S.; Dixon, M.L.; Floman, J.L.; Ellamil, M.; Rumak, S.P.; Sedlmeier, P.; Christoff, K. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci. Biobehav. Rev. 2014, 43, 48–73. [Google Scholar] [CrossRef]
- Price, J.L.; Amaral, D.G. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J. Neurosci. 1981, 1, 1242–1259. [Google Scholar] [CrossRef]
- Price, J.L.; Carmichael, S.T.; Drevets, W.C. Networks related to the orbital and medial prefrontal cortex; a substrate for emotional behavior? Prog. Brain Res. 1996, 107, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaei, H.T.; Barbas, H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 2002, 115, 1261–1279. [Google Scholar] [CrossRef]
- Debiec, J.; LeDoux, J.E. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: Treatment implications for PTSD. Ann. N. Y. Acad. Sci. 2006, 1071, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.W.; Kerr, C.E.; Wasserman, R.H.; Gray, J.R.; Greve, D.N.; Treadway, M.T.; McGarvey, M.; Quinn, B.T.; Dusek, J.A.; Benson, H.; et al. Meditation experience is associated with increased cortical thickness. Neuroreport 2005, 16, 1893–1897. [Google Scholar] [CrossRef]
- Kang, D.H.; Jo, H.J.; Jung, W.H.; Kim, S.H.; Jung, Y.H.; Choi, C.H.; Lee, U.S.; An, S.C.; Jang, J.H.; Kwon, J.S. The effect of meditation on brain structure: Cortical thickness mapping and diffusion tensor Imaging. Soc. Cogn. Affect. Neurosci. 2013, 8, 27–33. [Google Scholar] [CrossRef]
- Vestergaard-Poulsen, P.; van Beek, M.; Skewes, J.; Bjarkam, C.R.; Stubberup, M.; Bertelsen, J.; Roepstorff, A. Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport 2009, 20, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.A.; Duerden, E.G.; Courtemanche, J.; Cherkasova, M.; Duncan, G.H.; Rainville, P. Cortical thickness, mental absorption and meditative practice: Possible implications for disorders of attention. Biol. Psychol. 2013, 92, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Guleria, A.; Kishan, S.S.; Khetrapal, C.L. Effect of SOHAM meditation on human brain: A voxel-based morphometry study. J. NeuroImaging 2014, 24, 187–190. [Google Scholar] [CrossRef]
- Fox, K.C.; Dixon, M.L.; Nijeboer, S.; Girn, M.; Floman, J.L.; Lifshitz, M.; Ellamil, M.; Sedlmeier, P.; Christoff, K. Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations. Neurosci. Biobehav. Rev. 2016, 65, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Brefczynski-Lewis, J.A.; Lutz, A.; Schaefer, H.S.; Levinson, D.B.; Davidson, R.J. Neural correlates of attentional expertise in long-term meditation practitioners. Proc. Natl. Acad. Sci. USA 2007, 104, 11483–11488. [Google Scholar] [CrossRef]
- Manna, A.; Raffone, A.; Perrucci, M.G.; Nardo, D.; Ferretti, A.; Tartaro, A.; Londei, A.; Del Gratta, C.; Belardinelli, M.O.; Romani, G.L. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res. Bull. 2010, 82, 46–56. [Google Scholar] [CrossRef]
- Brewer, J.A.; Worhunsky, P.D.; Gray, J.R.; Tang, Y.Y.; Weber, J.; Kober, H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl. Acad. Sci. USA 2011, 108, 20254–20259. [Google Scholar] [CrossRef] [PubMed]
- Hasenkamp, W.; Wilson-Mendenhall, C.D.; Duncan, E.; Barsalou, L.W. Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. Neuroimage 2012, 59, 750–760. [Google Scholar] [CrossRef]
- Lee, T.M.; Leung, M.K.; Hou, W.K.; Tang, J.C.; Yin, J.; So, K.F.; Lee, C.F.; Chan, C.C. Distinct neural activity associated with focused-attention meditation and loving-kindness meditation. PLoS ONE 2012, 7, e40054. [Google Scholar] [CrossRef]
- Dickenson, J.; Berkman, E.T.; Arch, J.; Lieberman, M.D. Neural correlates of focused attention during a brief mindfulness induction. Soc. Cogn. Affect. Neurosci. 2013, 8, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Vik, A.; Groote, I.R.; Lagopoulos, J.; Holen, A.; Ellingsen, O.; Haberg, A.K.; Davanger, S. Nondirective meditation activates default mode network and areas associated with memory retrieval and emotional processing. Front. Hum. Neurosci. 2014, 8, 86. [Google Scholar] [CrossRef]
- Lazar, S.W.; Bush, G.; Gollub, R.L.; Fricchione, G.L.; Khalsa, G.; Benson, H. Functional brain mapping of the relaxation response and meditation. Neuroreport 2000, 11, 1581–1585. [Google Scholar] [CrossRef]
- Shimomura, T.; Fujiki, M.; Akiyoshi, J.; Yoshida, T.; Tabata, M.; Kabasawa, H.; Kobayashi, H. Functional brain mapping during recitation of Buddhist scriptures and repetition of the Namu Amida Butsu: A study in experienced Japanese monks. Turk. Neurosurg. 2008, 18, 134–141. [Google Scholar]
- Davanger, S.; Ellingsen, O.; Holen, A.; Hugdahl, K. Meditation-specific prefrontal cortical activation during acem meditation: An fMRI study. Percept. Mot. Skills 2010, 111, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, M.; Pihlsgard, J.; Lundberg, P.; Soderfeldt, B. Functional magnetic resonance imaging of hippocampal activation during silent mantra meditation. J. Altern. Complement. Med. 2010, 16, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, B.G.; Venkatasubramanian, G.; Arasappa, R.; Rao, N.P.; Kalmady, S.V.; Behere, R.V.; Rao, H.; Vasudev, M.K.; Gangadhar, B.N. Neurohemodynamic correlates of “OM” chanting: A pilot functional magnetic resonance imaging study. Int. J. Yoga 2011, 4, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.J.; Rao, H.; Korczykowski, M.; Wintering, N.; Pluta, J.; Khalsa, D.S.; Newberg, A.B. Cerebral blood flow changes associated with different meditation practices and perceived depth of meditation. Psychiatry Res. 2011, 191, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Guleria, A.; Kumar, U.; Kishan, S.S.; Khetrapal, C.L. Effect of “SOHAM” meditation on the human brain: An fMRI study. Psychiatry Res. 2013, 214, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.C.; Kjaer, T.W.; Friberg, L.; Wildschiodtz, G.; Holm, S.; Nowak, M. A 15O-H2O PET study of meditation and the resting state of normal consciousness. Hum. Brain Mapp. 1999, 7, 98–105. [Google Scholar] [CrossRef]
- Farb, N.A.; Segal, Z.V.; Mayberg, H.; Bean, J.; McKeon, D.; Fatima, Z.; Anderson, A.K. Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Soc. Cogn. Affect. Neurosci. 2007, 2, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ives-Deliperi, V.L.; Solms, M.; Meintjes, E.M. The neural substrates of mindfulness: An fMRI investigation. Soc. Neurosci. 2011, 6, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Taylor, V.A.; Grant, J.; Daneault, V.; Scavone, G.; Breton, E.; Roffe-Vidal, S.; Courtemanche, J.; Lavarenne, A.S.; Beauregard, M. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. Neuroimage 2011, 57, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Gard, T.; Holzel, B.K.; Sack, A.T.; Hempel, H.; Lazar, S.W.; Vaitl, D.; Ott, U. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb. Cortex. 2012, 22, 2692–2702. [Google Scholar] [CrossRef] [PubMed]
- Farb, N.A.; Segal, Z.V.; Anderson, A.K. Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb. Cortex. 2013, 23, 114–126. [Google Scholar] [CrossRef]
- Lutz, J.; Herwig, U.; Opialla, S.; Hittmeyer, A.; Jancke, L.; Rufer, M.; Grosse Holtforth, M.; Bruhl, A.B. Mindfulness and emotion regulation--an fMRI study. Soc. Cogn. Affect. Neurosci. 2014, 9, 776–785. [Google Scholar] [CrossRef]
- Lutz, A.; Brefczynski-Lewis, J.; Johnstone, T.; Davidson, R.J. Regulation of the neural circuitry of emotion by compassion meditation: Effects of meditative expertise. PLoS ONE 2008, 3, e1897. [Google Scholar] [CrossRef]
- Lutz, A.; Greischar, L.L.; Perlman, D.M.; Davidson, R.J. BOLD signal in insula is differentially related to cardiac function during compassion meditation in experts vs. novices. Neuroimage 2009, 47, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.Y.; Fox, A.S.; Shackman, A.J.; Stodola, D.E.; Caldwell, J.Z.; Olson, M.C.; Rogers, G.M.; Davidson, R.J. Compassion training alters altruism and neural responses to suffering. Psychol. Sci. 2013, 24, 1171–1180. [Google Scholar] [CrossRef]
- Wager, T.D.; Davidson, M.L.; Hughes, B.L.; Lindquist, M.A.; Ochsner, K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 2008, 59, 1037–1050. [Google Scholar] [CrossRef]
- Lieberman, M.D.; Eisenberger, N.I.; Crockett, M.J.; Tom, S.M.; Pfeifer, J.H.; Way, B.M. Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli. Psychol. Sci. 2007, 18, 421–428. [Google Scholar] [CrossRef]
- Ray, R.D.; Zald, D.H. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev. 2012, 36, 479–501. [Google Scholar] [CrossRef]
- Kim, M.J.; Gee, D.G.; Loucks, R.A.; Davis, F.C.; Whalen, P.J. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb. Cortex. 2011, 21, 1667–1673. [Google Scholar] [CrossRef]
- Gotink, R.A.; Meijboom, R.; Vernooij, M.W.; Smits, M.; Hunink, M.G. 8-week Mindfulness Based Stress Reduction induces brain changes similar to traditional long-term meditation practice—A systematic review. Brain. Cogn. 2016, 108, 32–41. [Google Scholar] [CrossRef]
- Kral, T.R.A.; Schuyler, B.S.; Mumford, J.A.; Rosenkranz, M.A.; Lutz, A.; Davidson, R.J. Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. Neuroimage 2018, 181, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Doll, A.; Holzel, B.K.; Mulej Bratec, S.; Boucard, C.C.; Xie, X.; Wohlschlager, A.M.; Sorg, C. Mindful attention to breath regulates emotions via increased amygdala-prefrontal cortex connectivity. Neuroimage 2016, 134, 305–313. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.C.; Chen, K.L.; Cheng, Y. Atypical Anxiety-Related Amygdala Reactivity and Functional Connectivity in Sant Mat Meditation. Front. Behav. Neurosci. 2018, 12, 298. [Google Scholar] [CrossRef]
- Black, D.S.; Peng, C.; Sleight, A.G.; Nguyen, N.; Lenz, H.J.; Figueiredo, J.C. Mindfulness practice reduces cortisol blunting during chemotherapy: A randomized controlled study of colorectal cancer patients. Cancer 2017, 123, 3088–3096. [Google Scholar] [CrossRef]
- Carlson, L.E.; Speca, M.; Faris, P.; Patel, K.D. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav. Immun. 2007, 21, 1038–1049. [Google Scholar] [CrossRef]
- Manzaneque, J.M.; Vera, F.M.; Ramos, N.S.; Godoy, Y.A.; Rodriguez, F.M.; Blanca, M.J.; Fernandez, A.; Enguix, A. Psychobiological modulation in anxious and depressed patients after a mindfulness meditation programme: A pilot study. Stress Health 2011, 27, 216–222. [Google Scholar] [CrossRef]
- Vandana, B.; Vaidyanathan, K.; Saraswathy, L.A.; Sundaram, K.R.; Kumar, H. Impact of integrated amrita meditation technique on adrenaline and cortisol levels in healthy volunteers. Evid. Based Complement. Altern. Med. 2011, 2011, 379645. [Google Scholar] [CrossRef]
- Jevning, R.; Wilson, A.F.; Davidson, J.M. Adrenocortical activity during meditation. Horm. Behav. 1978, 10, 54–60. [Google Scholar] [CrossRef]
- Pascoe, M.C.; Thompson, D.R.; Jenkins, Z.M.; Ski, C.F. Mindfulness mediates the physiological markers of stress: Systematic review and meta-analysis. J. Psychiatr. Res. 2017, 95, 156–178. [Google Scholar] [CrossRef] [PubMed]
- Koncz, A.; Demetrovics, Z.; Takacs, Z.K. Meditation interventions efficiently reduce cortisol levels of at-risk samples: A meta-analysis. Health Psychol. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Heckenberg, R.A.; Eddy, P.; Kent, S.; Wright, B.J. Do workplace-based mindfulness meditation programs improve physiological indices of stress? A systematic review and meta-analysis. J. Psychosom. Res. 2018, 114, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Meland, A.; Ishimatsu, K.; Pensgaard, A.M.; Wagstaff, A.; Fonne, V.; Garde, A.H.; Harris, A. Impact of Mindfulness Training on Physiological Measures of Stress and Objective Measures of Attention Control in a Military Helicopter Unit. Int. J. Aviat. Psychol. 2015, 25, 191–208. [Google Scholar] [CrossRef]
- Roeser, R.W.; Schonert-Reichi, K.A.; Jha, A.; Cullen, M.; Wallace, L.; Wilensky, R.; Oberle, E.; Thomson, K.; Taylor, C.; Harrison, J. Mindfulness training and reductions in teacher stress and burnout: Results from two randomized, waitlist-control field trials. J. Educ. Psychol. 2013, 105, 787–804. [Google Scholar] [CrossRef]
- Flook, L.; Goldberg, S.B.; Pinger, L.; Bonus, K.; Davidson, R.J. Mindfulness for teachers: A pilot study to assess effects on stress, burnout and teaching efficacy. Mind Brain Educ. 2013, 7. [Google Scholar] [CrossRef]
- Malarkey, W.B.; Jarjoura, D.; Klatt, M. Workplace based mindfulness practice and inflammation: A randomized trial. Brain. Behav. Immun. 2013, 27, 145–154. [Google Scholar] [CrossRef]
- Pascoe, M.C.; Thompson, D.R.; Ski, C.F. Yoga, mindfulness-based stress reduction and stress-related physiological measures: A meta-analysis. Psychoneuroendocrinology 2017, 86, 152–168. [Google Scholar] [CrossRef]
- Bower, J.E.; Greendale, G.; Crosswell, A.D.; Garet, D.; Sternlieb, B.; Ganz, P.A.; Irwin, M.R.; Olmstead, R.; Arevalo, J.; Cole, S.W. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Psychoneuroendocrinology 2014, 43, 20–29. [Google Scholar] [CrossRef]
- Corey, S.M.; Epel, E.; Schembri, M.; Pawlowsky, S.B.; Cole, R.J.; Araneta, M.R.; Barrett-Connor, E.; Kanaya, A.M. Effect of restorative yoga vs. stretching on diurnal cortisol dynamics and psychosocial outcomes in individuals with the metabolic syndrome: The PRYSMS randomized controlled trial. Psychoneuroendocrinology 2014, 49, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Sieverdes, J.C.; Mueller, M.; Gregoski, M.J.; Brunner-Jackson, B.; McQuade, L.; Matthews, C.; Treiber, F.A. Effects of Hatha yoga on blood pressure, salivary alpha-amylase, and cortisol function among normotensive and prehypertensive youth. J. Altern. Complement. Med. 2014, 20, 241–250. [Google Scholar] [CrossRef]
- Vadiraja, H.S.; Raghavendra, R.M.; Nagarathna, R.; Nagendra, H.R.; Rekha, M.; Vanitha, N.; Gopinath, K.S.; Srinath, B.S.; Vishweshwara, M.S.; Madhavi, Y.S.; et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: A randomized controlled trial. Integr. Cancer Ther. 2009, 8, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gothe, N.P.; Keswani, R.K.; McAuley, E. Yoga practice improves executive function by attenuating stress levels. Biol. Psychol. 2016, 121, 109–116. [Google Scholar] [CrossRef]
- Granath, J.; Ingvarsson, S.; von Thiele, U.; Lundberg, U. Stress management: A randomized study of cognitive behavioural therapy and yoga. Cogn. Behav. Ther. 2006, 35, 3–10. [Google Scholar] [CrossRef]
- Carrive, P. Dual activation of cardiac sympathetic and parasympathetic components during conditioned fear to context in the rat. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1251–1254. [Google Scholar] [CrossRef]
- Nesvold, A.; Fagerland, M.W.; Davanger, S.; Ellingsen, O.; Solberg, E.E.; Holen, A.; Sevre, K.; Atar, D. Increased heart rate variability during nondirective meditation. Eur. J. Prev. Cardiol. 2012, 19, 773–780. [Google Scholar] [CrossRef]
- Barnes, V.A.; Treiber, F.A.; Davis, H. Impact of Transcendental Meditation on cardiovascular function at rest and during acute stress in adolescents with high normal blood pressure. J. Psychosom. Res. 2001, 51, 597–605. [Google Scholar] [CrossRef]
- Patel, C.; North, W.R. Randomised controlled trial of yoga and bio-feedback in management of hypertension. Lancet 1975, 2, 93–95. [Google Scholar] [CrossRef]
- Paul-Labrador, M.; Polk, D.; Dwyer, J.H.; Velasquez, I.; Nidich, S.; Rainforth, M.; Schneider, R.; Merz, C.N. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Arch. Intern. Med. 2006, 166, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Curiati, J.A.; Bocchi, E.; Freire, J.O.; Arantes, A.C.; Braga, M.; Garcia, Y.; Guimaraes, G.; Fo, W.J. Meditation reduces sympathetic activation and improves the quality of life in elderly patients with optimally treated heart failure: A prospective randomized study. J. Altern. Complement. Med. 2005, 11, 465–472. [Google Scholar] [CrossRef]
- Harinath, K.; Malhotra, A.S.; Pal, K.; Prasad, R.; Kumar, R.; Kain, T.C.; Rai, L.; Sawhney, R.C. Effects of Hatha yoga and Omkar meditation on cardiorespiratory performance, psychologic profile, and melatonin secretion. J. Altern. Complement. Med. 2004, 10, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Prakhinkit, S.; Suppapitiporn, S.; Tanaka, H.; Suksom, D. Effects of Buddhism walking meditation on depression, functional fitness, and endothelium-dependent vasodilation in depressed elderly. J. Altern. Complement. Med. 2014, 20, 411–416. [Google Scholar] [CrossRef]
- Barnes, V.A.; Davis, H.C.; Murzynowski, J.B.; Treiber, F.A. Impact of meditation on resting and ambulatory blood pressure and heart rate in youth. Psychosom. Med. 2004, 66, 909–914. [Google Scholar] [CrossRef]
- Daubenmier, J.; Moran, P.J.; Kristeller, J.; Acree, M.; Bacchetti, P.; Kemeny, M.E.; Dallman, M.; Lustig, R.H.; Grunfeld, C.; Nixon, D.F.; et al. Effects of a mindfulness-based weight loss intervention in adults with obesity: A randomized clinical trial. Obesity 2016, 24, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Kingston, J.; Chadwick, P.; Meron, D.; Skinner, T.C. A pilot randomized control trial investigating the effect of mindfulness practice on pain tolerance, psychological well-being, and physiological activity. J. Psychosom. Res. 2007, 62, 297–300. [Google Scholar] [CrossRef]
- Palta, P.; Page, G.; Piferi, R.L.; Gill, J.M.; Hayat, M.J.; Connolly, A.B.; Szanton, S.L. Evaluation of a mindfulness-based intervention program to decrease blood pressure in low-income African-American older adults. J. Urban Health 2012, 89, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Parswani, M.J.; Sharma, M.P.; Iyengar, S. Mindfulness-based stress reduction program in coronary heart disease: A randomized control trial. Int. J. Yoga 2013, 6, 111–117. [Google Scholar] [CrossRef]
- Babbar, S.; Hill, J.B.; Williams, K.B.; Pinon, M.; Chauhan, S.P.; Maulik, D. Acute feTal behavioral Response to prenatal Yoga: A single, blinded, randomized controlled trial (TRY yoga). Am. J. Obstet. Gynecol. 2016, 214, 399 e391–e398. [Google Scholar] [CrossRef]
- Cohen, D.L.; Bloedon, L.T.; Rothman, R.L.; Farrar, J.T.; Galantino, M.L.; Volger, S.; Mayor, C.; Szapary, P.O.; Townsend, R.R. Iyengar Yoga versus Enhanced Usual Care on Blood Pressure in Patients with Prehypertension to Stage I Hypertension: A Randomized Controlled Trial. Evid. Based Complement. Altern. Med. 2011, 2011, 546428. [Google Scholar] [CrossRef] [PubMed]
- Ebnezar, J.; Nagarathna, R.; Yogitha, B.; Nagendra, H.R. Effect of integrated yoga therapy on pain, morning stiffness and anxiety in osteoarthritis of the knee joint: A randomized control study. Int. J. Yoga 2012, 5, 28–36. [Google Scholar] [CrossRef]
- Hughes, J.W.; Fresco, D.M.; Myerscough, R.; van Dulmen, M.H.; Carlson, L.E.; Josephson, R. Randomized controlled trial of mindfulness-based stress reduction for prehypertension. Psychosom. Med. 2013, 75, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Innes, K.E.; Selfe, T.K. The Effects of a Gentle Yoga Program on Sleep, Mood, and Blood Pressure in Older Women with Restless Legs Syndrome (RLS): A Preliminary Randomized Controlled Trial. Evid. Based Complement. Altern. Med. 2012, 2012, 294058. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, A.M.; Araneta, M.R.; Pawlowsky, S.B.; Barrett-Connor, E.; Grady, D.; Vittinghoff, E.; Schembri, M.; Chang, A.; Carrion-Petersen, M.L.; Coggins, T.; et al. Restorative yoga and metabolic risk factors: The Practicing Restorative Yoga vs. Stretching for the Metabolic Syndrome (PRYSMS) randomized trial. J. Diabetes Complicat. 2014, 28, 406–412. [Google Scholar] [CrossRef]
- Patil, S.G.; Aithala, M.R.; Das, K.K. Effect of yoga on arterial stiffness in elderly subjects with increased pulse pressure: A randomized controlled study. Complement. Ther. Med. 2015, 23, 562–569. [Google Scholar] [CrossRef]
- Ruby, M.; Repka, C.P.; Arciero, P.J. Comparison of Protein-Pacing Alone or with Yoga/Stretching and Resistance Training on Glycemia, Total and Regional Body Composition, and Aerobic Fitness in Overweight Women. J. Phys. Act. Health 2016, 13, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Saptharishi, L.; Soudarssanane, M.; Thiruselvakumar, D.; Navasakthi, D.; Mathanraj, S.; Karthigeyan, M.; Sahai, A. Community-based Randomized Controlled Trial of Non-pharmacological Interventions in Prevention and Control of Hypertension among Young Adults. Indian J. Community Med. 2009, 34, 329–334. [Google Scholar] [CrossRef]
- Thiyagarajan, R.; Pal, P.; Pal, G.K.; Subramanian, S.K.; Trakroo, M.; Bobby, Z.; Das, A.K. Additional benefit of yoga to standard lifestyle modification on blood pressure in prehypertensive subjects: A randomized controlled study. Hypertens. Res. 2015, 38, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Bernardo, L.M.; Sereika, S.M.; Conroy, M.B.; Balk, J.; Burke, L.E. Utilization of 3-month yoga program for adults at high risk for type 2 diabetes: A pilot study. Evid. Based Complement. Altern. Med. 2011, 2011, 257891. [Google Scholar] [CrossRef]
- Li, J.; Gao, X.; Hao, X.; Kantas, D.; Mohamed, E.A.; Zheng, X.; Xu, H.; Zhang, L. Yoga for secondary prevention of coronary heart disease: A systematic review and meta-analysis. Complement. Ther. Med. 2020, 57, 102643. [Google Scholar] [CrossRef]
- Pal, A.; Srivastava, N.; Tiwari, S.; Verma, N.S.; Narain, V.S.; Agrawal, G.G.; Natu, S.M.; Kumar, K. Effect of yogic practices on lipid profile and body fat composition in patients of coronary artery disease. Complement. Ther. Med. 2011, 19, 122–127. [Google Scholar] [CrossRef]
- Tillin, T.; Tuson, C.; Sowa, B.; Chattopadhyay, K.; Sattar, N.; Welsh, P.; Roberts, I.; Ebrahim, S.; Kinra, S.; Hughes, A.; et al. Yoga and Cardiovascular Health Trial (YACHT): A UK-based randomised mechanistic study of a yoga intervention plus usual care versus usual care alone following an acute coronary event. BMJ Open 2019, 9, e030119. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, S.; Singh, K.; Pai, P. Effect of yoga regimen on lung functions including diffusion capacity in coronary artery disease patients: A randomized controlled study. Int. J. Yoga 2015, 8, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Luo, S.; Chen, X.; Lu, Y.; Liu, Z.; Wei, L. Effects of Tai Chi exercise on cardiovascular disease risk factors and quality of life in adults with essential hypertension: A meta-analysis. Heart Lung 2020, 49, 353–363. [Google Scholar] [CrossRef]
- Scott-Sheldon, L.A.J.; Gathright, E.C.; Donahue, M.L.; Balletto, B.; Feulner, M.M.; DeCosta, J.; Cruess, D.G.; Wing, R.R.; Carey, M.P.; Salmoirago-Blotcher, E. Mindfulness-Based Interventions for Adults with Cardiovascular Disease: A Systematic Review and Meta-Analysis. Ann. Behav. Med. 2020, 54, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Blom, K.; Baker, B.; How, M.; Dai, M.; Irvine, J.; Abbey, S.; Abramson, B.L.; Myers, M.G.; Kiss, A.; Perkins, N.J.; et al. Hypertension analysis of stress reduction using mindfulness meditation and yoga: Results from the HARMONY randomized controlled trial. Am. J. Hypertens. 2014, 27, 122–129. [Google Scholar] [CrossRef]
- Delui, M.H.; Yari, M.; Khouyinezhad, G.; Amini, M.; Bayazi, M.H. Comparison of cardiac rehabilitation programs combined with relaxation and meditation techniques on reduction of depression and anxiety of cardiovascular patients. Open. Cardiovasc. Med. J. 2013, 7, 99–103. [Google Scholar] [CrossRef]
- Momeni, J.; Omidi, A.; Raygan, F.; Akbari, H. The effects of mindfulness-based stress reduction on cardiac patients’ blood pressure, perceived stress, and anger: A single-blind randomized controlled trial. J. Am. Soc. Hypertens. 2016, 10, 763–771. [Google Scholar] [CrossRef]
- Younge, J.O.; Wery, M.F.; Gotink, R.A.; Utens, E.M.; Michels, M.; Rizopoulos, D.; van Rossum, E.F.; Hunink, M.G.; Roos-Hesselink, J.W. Web-Based Mindfulness Intervention in Heart Disease: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0143843. [Google Scholar] [CrossRef]
- Barnes, V.A.; Treiber, F.A.; Johnson, M.H. Impact of transcendental meditation on ambulatory blood pressure in African-American adolescents. Am. J. Hypertens. 2004, 17, 366–369. [Google Scholar] [CrossRef]
- Barnes, V.A.; Pendergrast, R.A.; Harshfield, G.A.; Treiber, F.A. Impact of breathing awareness meditation on ambulatory blood pressure and sodium handling in prehypertensive African American adolescents. Ethn. Dis. 2008, 18, 1–5. [Google Scholar]
- Gregoski, M.J.; Barnes, V.A.; Tingen, M.S.; Harshfield, G.A.; Treiber, F.A. Breathing awareness meditation and LifeSkills Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J. Adolesc. Health 2011, 48, 59–64. [Google Scholar] [CrossRef]
- Cohen, D.L.; Boudhar, S.; Bowler, A.; Townsend, R.R. Blood Pressure Effects of Yoga, Alone or in Combination with Lifestyle Measures: Results of the Lifestyle Modification and Blood Pressure Study (LIMBS). J. Clin. Hypertens. 2016, 18, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Hagins, M.; Rundle, A.; Consedine, N.S.; Khalsa, S.B. A randomized controlled trial comparing the effects of yoga with an active control on ambulatory blood pressure in individuals with prehypertension and stage 1 hypertension. J. Clin. Hypertens. 2014, 16, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ziv, A.; Vogel, O.; Keret, D.; Pintov, S.; Bodenstein, E.; Wolkomir, K.; Doenyas, K.; Mirovski, Y.; Efrati, S. Comprehensive Approach to Lower Blood Pressure (CALM-BP): A randomized controlled trial of a multifactorial lifestyle intervention. J. Hum. Hypertens. 2013, 27, 594–600. [Google Scholar] [CrossRef]
- Wenneberg, S.R.; Schneider, R.H.; Walton, K.G.; Maclean, C.R.; Levitsky, D.K.; Salerno, J.W.; Wallace, R.K.; Mandarino, J.V.; Rainforth, M.V.; Waziri, R. A controlled study of the effects of the Transcendental Meditation program on cardiovascular reactivity and ambulatory blood pressure. Int. J. Neurosci. 1997, 89, 15–28. [Google Scholar] [CrossRef]
- Wielgosz, J.; Schuyler, B.S.; Lutz, A.; Davidson, R.J. Long-term mindfulness training is associated with reliable differences in resting respiration rate. Sci. Rep. 2016, 6, 27533. [Google Scholar] [CrossRef]
- Nidhi, R.; Padmalatha, V.; Nagarathna, R.; Ram, A. Effect of a yoga program on glucose metabolism and blood lipid levels in adolescent girls with polycystic ovary syndrome. Int. J. Gynaecol. Obstet. 2012, 118, 37–41. [Google Scholar] [CrossRef]
- Singh, A.K.; Kaur, N.; Kaushal, S.; Tyagi, R.; Mathur, D.; Sivapuram, M.S.; Metri, K.; Bammidi, S.; Podder, V.; Modgil, S.; et al. Partitioning of radiological, stress and biochemical changes in pre-diabetic women subjected to Diabetic Yoga Protocol. Diabetes Metab. Syndr. 2019, 13, 2705–2713. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Mattina, G.F.; Van Lieshout, R.J.; Steiner, M. Inflammation, depression and cardiovascular disease in women: The role of the immune system across critical reproductive events. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719851950. [Google Scholar] [CrossRef]
- Black, D.S.; Slavich, G.M. Mindfulness meditation and the immune system: A systematic review of randomized controlled trials. Ann. N. Y. Acad. Sci. 2016, 1373, 13–24. [Google Scholar] [CrossRef]
- Pace, T.W.; Mletzko, T.C.; Alagbe, O.; Musselman, D.L.; Nemeroff, C.B.; Miller, A.H.; Heim, C.M. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 2006, 163, 1630–1633. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.L.; Miller, G.E.; Wrosch, C. Conscientiousness and stress exposure and reactivity: A prospective study of adolescent females. J. Behav. Med. 2013, 36, 153–164. [Google Scholar] [CrossRef]
- Bower, J.E.; Crosswell, A.D.; Stanton, A.L.; Crespi, C.M.; Winston, D.; Arevalo, J.; Ma, J.; Cole, S.W.; Ganz, P.A. Mindfulness meditation for younger breast cancer survivors: A randomized controlled trial. Cancer 2015, 121, 1231–1240. [Google Scholar] [CrossRef]
- Creswell, J.D.; Irwin, M.R.; Burklund, L.J.; Lieberman, M.D.; Arevalo, J.M.; Ma, J.; Breen, E.C.; Cole, S.W. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: A small randomized controlled trial. Brain. Behav. Immun. 2012, 26, 1095–1101. [Google Scholar] [CrossRef]
- Black, D.S.; O’Reilly, G.A.; Olmstead, R.; Breen, E.C.; Irwin, M.R. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 494–501. [Google Scholar] [CrossRef]
- Jedel, S.; Hoffman, A.; Merriman, P.; Swanson, B.; Voigt, R.; Rajan, K.B.; Shaikh, M.; Li, H.; Keshavarzian, A. A randomized controlled trial of mindfulness-based stress reduction to prevent flare-up in patients with inactive ulcerative colitis. Digestion 2014, 89, 142–155. [Google Scholar] [CrossRef]
- Ray, I.B.; Menezes, A.R.; Malur, P.; Hiltbold, A.E.; Reilly, J.P.; Lavie, C.J. Meditation and coronary heart disease: A review of the current clinical evidence. Ochsner J. 2014, 14, 696–703. [Google Scholar]
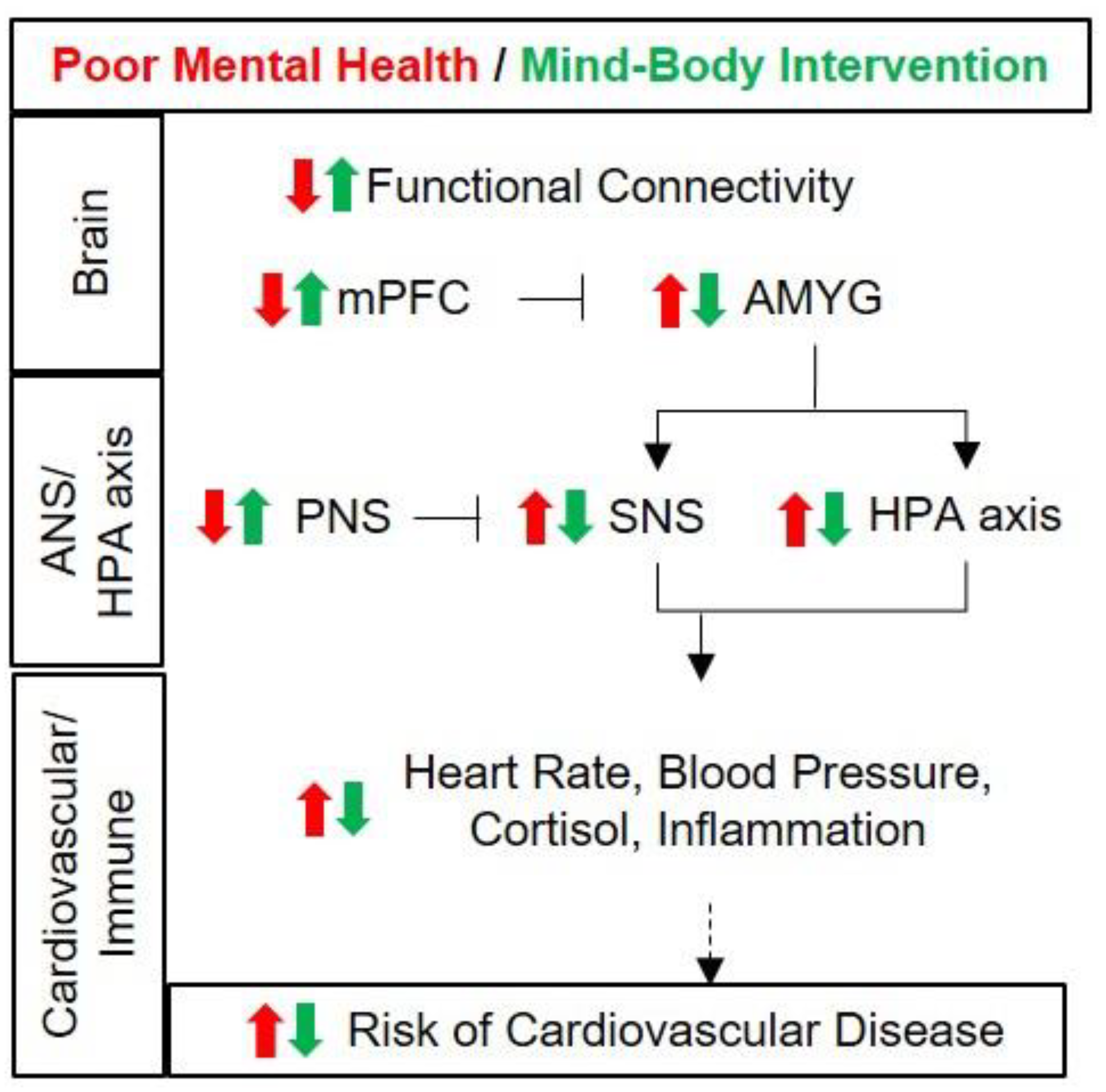
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-J.; Koh, E.; Kang, Y. Susceptibility of Women to Cardiovascular Disease and the Prevention Potential of Mind–Body Intervention by Changes in Neural Circuits and Cardiovascular Physiology. Biomolecules 2021, 11, 708. https://doi.org/10.3390/biom11050708
Yang H-J, Koh E, Kang Y. Susceptibility of Women to Cardiovascular Disease and the Prevention Potential of Mind–Body Intervention by Changes in Neural Circuits and Cardiovascular Physiology. Biomolecules. 2021; 11(5):708. https://doi.org/10.3390/biom11050708
Chicago/Turabian StyleYang, Hyun-Jeong, Eugene Koh, and Yunjeong Kang. 2021. "Susceptibility of Women to Cardiovascular Disease and the Prevention Potential of Mind–Body Intervention by Changes in Neural Circuits and Cardiovascular Physiology" Biomolecules 11, no. 5: 708. https://doi.org/10.3390/biom11050708
APA StyleYang, H.-J., Koh, E., & Kang, Y. (2021). Susceptibility of Women to Cardiovascular Disease and the Prevention Potential of Mind–Body Intervention by Changes in Neural Circuits and Cardiovascular Physiology. Biomolecules, 11(5), 708. https://doi.org/10.3390/biom11050708






