Post-Surgical Peritoneal Scarring and Key Molecular Mechanisms
Abstract
1. Introduction
2. Serosal Repair and Adhesion Formation
2.1. Serosal Repair
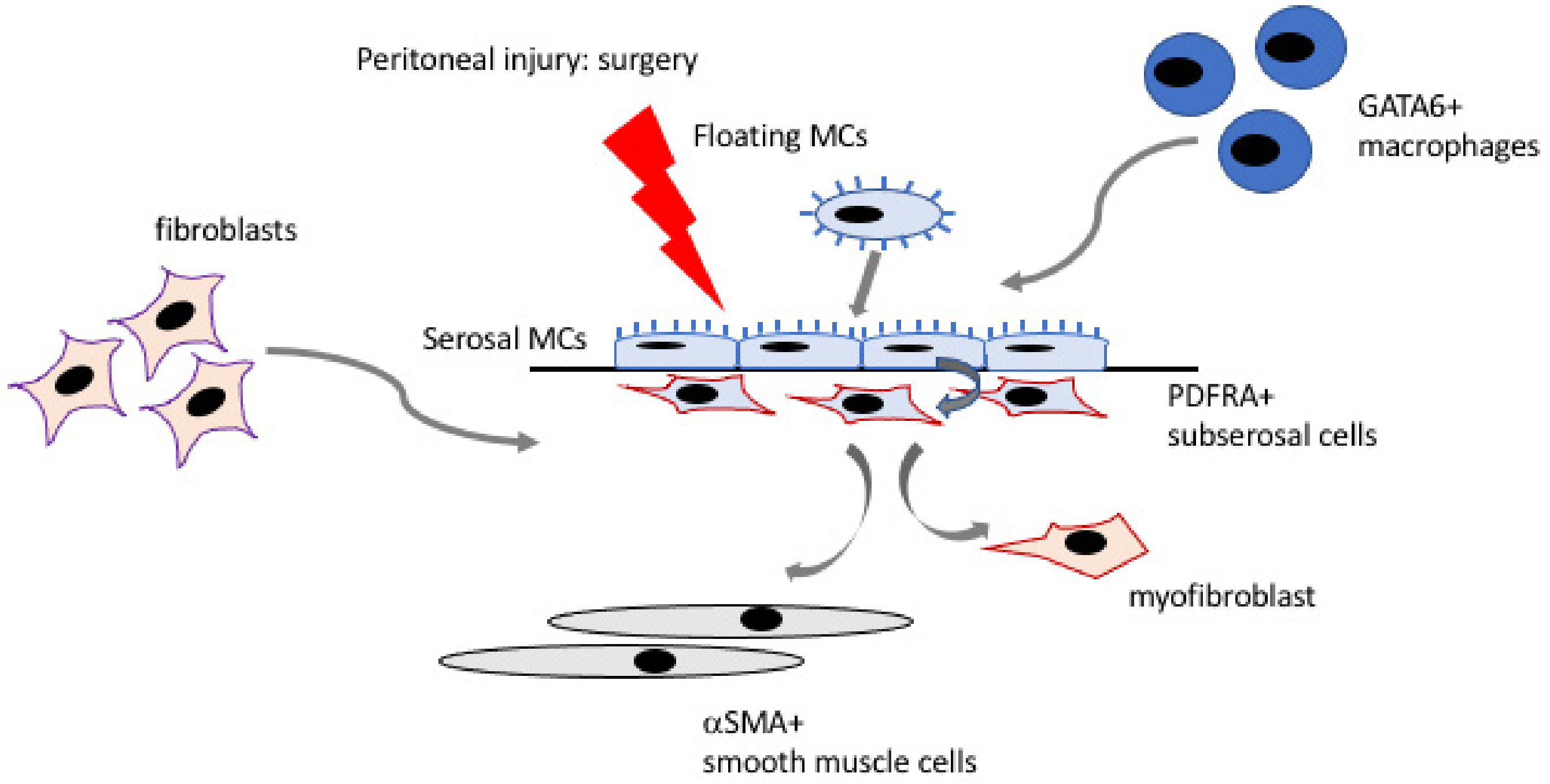
2.2. Mechanism of Adhesion Formation
2.3. Cellular Contribution to Adhesions
3. The Developmental Origin of Mesothelium
Molecular Markers
4. Further Discussion and Areas of Future Research
Funding
Conflicts of Interest
References
- Herrick, S.E.; Mutsaers, S.E.; Ozua, P.; Sulaiman, H.; Omer, A.; Boulos, P.; Foster, M.L.; Laurent, G.J. Human peritoneal adhesions are highly cellular, innervated, and vascularized. J. Pathol. 2000, 192, 67–72. [Google Scholar] [CrossRef]
- Epstein, J.C.; Wilson, M.S.; Wilkosz, S.; Ireland, G.; O’Dwyer, S.T.; Herrick, S.E. Human peritoneal adhesions show evidence of tissue remodeling and markers of angiogenesis. Dis. Colon Rectum 2006, 49, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Binnebösel, M.; Rosch, R.; Junge, K.; Lynen-Jansen, P.; Schumpelick, V.; Klinge, U. Macrophage and T-lymphocyte infiltrates in human peritoneal adhesions indicate a chronic inflammatory disease. World J. Surg. 2008, 32, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xiang, Z.; Bernards, M.T.; Chen, S. Peritoneal adhesions: Occurrence, prevention and experimental models. Acta Biomater. 2020, 116, 84–104. [Google Scholar] [CrossRef]
- Diamond, M.P.; Freeman, M.L. Clinical implications of postsurgical adhesions. Hum. Reprod. Update 2001, 7, 567–576. [Google Scholar] [CrossRef]
- Stommel, M.W.J.; Ten Broek, R.P.G.; Strik, C.; Slooter, G.D.; Verhoef, C.; Grünhagen, D.J.; van Duijvendijk, P.; Bemelmans, M.H.A.; den Dulk, M.; Sietses, C.; et al. Multicenter Observational Study of Adhesion Formation After Open-and Laparoscopic Surgery for Colorectal Cancer. Ann. Surg. 2018, 267, 743–748. [Google Scholar] [CrossRef]
- Ellis, H.; Moran, B.J.; Thompson, J.N.; Parker, M.C.; Wilson, M.S.; Menzies, D.; McGuire, A.; Lower, A.M.; Hawthorn, R.J.; O’Brien, F.; et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: A retrospective cohort study. Lancet 1999, 353, 1476–1480. [Google Scholar] [CrossRef]
- Parker, M.C.; Ellis, H.; Moran, B.J.; Thompson, J.N.; Wilson, M.S.; Menzies, D.; McGuire, A.; Lower, A.M.; Hawthorn, R.J.; O’Briena, F.; et al. Postoperative adhesions: Ten-year follow-up of 12,584 patients undergoing lower abdominal surgery. Dis. Colon Rectum 2001, 44, 822–829, discussion 829–830. [Google Scholar] [CrossRef]
- Lower, A.M.; Hawthorn, R.J.; Ellis, H.; O’Brien, F.; Buchan, S.; Crowe, A.M. The impact of adhesions on hospital readmissions over ten years after 8849 open gynaecological operations: An assessment from the Surgical and Clinical Adhesions Research Study. BJOG 2000, 107, 855–862. [Google Scholar] [CrossRef]
- Krielen, P.; van den Beukel, B.A.; Stommel, M.W.J.; van Goor, H.; Strik, C.; Ten Broek, R.P.G. In-hospital costs of an admission for adhesive small bowel obstruction. World J. Emerg. Surg. 2016, 11, 49–57. [Google Scholar] [CrossRef]
- Pilpel, Y.; Pines, G.; Birkenfeld, A.; Bornstein, S.R.; Miller, R. Metabolic Syndrome is a Risk Factor for Post-Operative Adhesions: Need for Novel Treatment Strategies. Horm. Metab. Res. 2019, 51, 35–41. [Google Scholar] [CrossRef]
- Parker, M.C.; Wilson, M.S.; van Goor, H.; Moran, B.J.; Jeekel, J.; Duron, J.J.; Menzies, D.; Wexner, S.D.; Ellis, H. Adhesions and colorectal surgery—Call for action. Colorectal Dis. 2007, 9, 66–72. [Google Scholar] [CrossRef]
- Strik, C.; Stommel, M.W.J.; Hol, J.C.; van Goor, H.; Ten Broek, R.P.G. Quality of life, functional status and adhesiolysis during elective abdominal surgery. Am. J. Surg. 2018, 215, 104–112. [Google Scholar] [CrossRef]
- Tabibian, N.; Swehli, E.; Boyd, A.; Umbreen, A.; Tabibian, J.H. Abdominal adhesions: A practical review of an often overlooked entity. Ann. Med. Surg. 2017, 15, 9–13. [Google Scholar] [CrossRef]
- Ten Broek, R.P.; Issa, Y.; van Santbrink, E.J.; Bouvy, N.D.; Kruitwagen, R.F.; Jeekel, J.; Bakkum, E.A.; Rovers, M.M.; van Goor, H. Burden of adhesions in abdominal and pelvic surgery: Systematic review and met-analysis. BMJ 2013, 347, 5588–5603. [Google Scholar] [CrossRef]
- Ten Broek, R.P.; Bakkum, E.A.; Laarhoven, C.J.; van Goor, H. Epidemiology and Prevention of Postsurgical Adhesions Revisited. Ann. Surg. 2016, 263, 12–19. [Google Scholar] [CrossRef]
- Lundorff, P.; Brölmann, H.; Koninckx, P.R.; Mara, M.; Wattiez, A.; Wallwiener, M.; Trew, G.; Crowe, A.M.; De Wilde, R.L.; Anti-Adhesions in Gynaecology Expert Panel. Predicting formation of adhesions after gynaecological surgery: Development of a risk score. Arch. Gynecol. Obstet. 2015, 292, 931–938. [Google Scholar] [CrossRef]
- Dawood, A.S.; Elgergawy, A.E. Incidence and sites of pelvic adhesions in women with post-caesarean infertility. J. Obstet. Gynaecol. 2018, 38, 1158–1163. [Google Scholar] [CrossRef]
- Krielen, P.; Stommel, M.W.J.; Pargmae, P.; Bouvy, N.D.; Bakkum, E.A.; Ellis, H.; Parker, M.C.; Griffiths, E.A.; van Goor, H.; Ten Broek, R.P.G. Adhesion-related readmissions after open and laparoscopic surgery: A retrospective cohort study (SCAR update). Lancet 2020, 395, 33–41. [Google Scholar] [CrossRef]
- Van den Beukel, B.A.; de Ree, R.; van Leuven, S.; Bakkum, E.A.; Strik, C.; van Goor, H.; Ten Broek, R.P.G. Surgical treatment of adhesion-related chronic abdominal and pelvic pain after gynaecological and general surgery: A systematic review and meta-analysis. Hum. Reprod. Update 2017, 23, 276–288. [Google Scholar] [CrossRef]
- Sulaiman, H.; Gabella, G.; Davis MSc, C.; Mutsaers, S.E.; Boulos, P.; Laurent, G.J.; Herrick, S.E. Presence and distribution of sensory nerve fibers in human peritoneal adhesions. Ann. Surg. 2001, 234, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Demco, L. Pain mapping of adhesions. J. Am. Assoc. Gynecol. Laparosc. 2004, 11, 181–183. [Google Scholar] [CrossRef]
- Beyene, R.T.; Kavalukas, S.L.; Barbul, A. Intra-abdominal adhesions: Anatomy, physiology, pathophysiology, and treatment. Curr. Probl. Surg. 2015, 52, 271–319. [Google Scholar] [CrossRef] [PubMed]
- Van Steensel, S.; van den Hil, L.C.L.; Schreinemacher, M.H.F.; Ten Broek, R.P.G.; van Goor, H.; Bouvy, N.D. Adhesion awareness in 2016: An update of the national survey of surgeons. PLoS ONE 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.P.; Wexner, S.D.; diZereg, G.S.; Korell, M.; Zmora, O.; Van Goor, H.; Kamar, M. Adhesion prevention and reduction: Current status and future recommendations of a multinational interdisciplinary consensus conference. Surg. Innov. 2010, 17, 183–188. [Google Scholar] [CrossRef]
- Van Baal, J.O.; Van de Vijver, K.K.; Nieuwland, R.; van Noorden, C.J.; van Driel, W.J.; Sturk, A.; Kenter, G.G.; Rikkert, L.G.; Lok, C.A. The histophysiology and pathophysiology of the peritoneum. Tissue Cell 2017, 49, 95–105. [Google Scholar] [CrossRef]
- Gazvani, R.; Templeton, A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction 2002, 123, 217–226. [Google Scholar] [CrossRef]
- Mutsaers, S.E. The mesothelial cell. Int. J. Biochem. Cell Biol. 2004, 36, 9–16. [Google Scholar] [CrossRef]
- Connell, N.D.; Rheinwald, J.G. Regulation of the cytoskeleton in mesothelial cells: Reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell 1983, 34, 245–253. [Google Scholar] [CrossRef]
- Kanamori-Katayama, M.; Kaiho, A.; Ishizu, Y.; Okamura-Oho, Y.; Hino, O.; Abe, M.; Kishimoto, T.; Sekihara, H.; Nakamura, Y.; Suzuki, H.; et al. LRRN4 and UPK3B are markers of primary mesothelial cells. PLoS ONE 2011, 6, 1–8. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Mori, T.; Sahoo, D.; Xu, P.X.; Bermingham, J.R.; Weissman, I.L. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat. Cell Biol. 2012, 14, 1251–1260. [Google Scholar] [CrossRef]
- Lua, I.; Li, Y.; Pappoe, L.S.; Asahina, K. Myofibroblastic Conversion and Regeneration of Mesothelial Cells in Peritoneal and Liver Fibrosis. Am. J. Pathol. 2015, 185, 3258–3273. [Google Scholar] [CrossRef]
- Kienzle, A.; Servais, A.B.; Ysasi, A.B.; Gibney, B.C.; Valenzuela, C.D.; Wagner, W.L.; Ackermann, M.; Mentzer, S.J. Free-Floating Mesothelial Cells in Pleural Fluid After Lung Surgery. Front. Med. 2018, 5, 89–97. [Google Scholar] [CrossRef]
- Kawanishi, K. Diverse properties of the mesothelial cells in health and disease. Pleura Peritoneum 2016, 1, 79–89. [Google Scholar] [CrossRef]
- Servais, A.B.; Kienzle, A.; Valenzuela, C.D.; Ysasi, A.B.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural Heteropolysaccharide Adhesion to the Glycocalyx of Visceral Mesothelium. Tissue Eng. Part A 2018, 24, 199–206. [Google Scholar] [CrossRef]
- Markov, A.G.; Amasheh, S. Tight junction physiology of pleural mesothelium. Front. Physiol. 2014, 5, 221–228. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Prêle, C.M.; Pengelly, S.; Herrick, S.E. Mesothelial cells and peritoneal homeostasis. Fertil. Steril. 2016, 106, 1018–1024. [Google Scholar] [CrossRef]
- Shaw, T.J.; Zhang, X.Y.; Huo, Z.; Robertson, D.; Lovell, P.A.; Dalgleish, A.G.; Barton, D.P. Human Peritoneal Mesothelial Cells Display Phagocytic and Antigen-Presenting Functions to Contribute to Intraperitoneal Immunity. Int. J. Gynecol. Cancer 2016, 26, 833–838. [Google Scholar] [CrossRef]
- Haney, A.F. Identification of macrophages at the site of peritoneal injury: Evidence supporting a direct role for peritoneal macrophages in healing injured peritoneum. Fertil. Steril. 2000, 73, 988–995. [Google Scholar] [CrossRef]
- Wang, J.; Kubes, P. A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell 2016, 165, 668–678. [Google Scholar] [CrossRef]
- Yung, S.; Davies, M. Response of the human peritoneal mesothelial cell to injury: An in vitro model of peritoneal wound healing. Kidney Int. 1998, 54, 2160–2169. [Google Scholar] [CrossRef]
- Whitaker, D.; Papadimitriou, J. Mesothelial healing: Morphological and kinetic investigations. J. Pathol. 1985, 145, 159–175. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Whitaker, D.; Papadimitriou, J.M. Mesothelial regeneration is not dependent on subserosal cells. J. Pathol. 2000, 190, 86–92. [Google Scholar] [CrossRef]
- Foley-Comer, A.J.; Herrick, S.E.; Al-Mishlab, T.; Prêle, C.M.; Laurent, G.J.; Mutsaers, S.E. Evidence for incorporation of free-floating mesothelial cells as a mechanism of serosal healing. J. Cell Sci. 2002, 115, 1383–1389. [Google Scholar] [CrossRef]
- Shapiro, L.; Holste, J.L.; Muench, T.; diZerega, G. Rapid reperitonealization and wound healing in a preclinical model of abdominal trauma repair with a composite mesh. Int. J. Surg. 2015, 22, 86–91. [Google Scholar] [CrossRef]
- Bolen, J.W.; Hammar, S.P.; McNutt, M.A. Reactive and neoplastic serosal tissue. A light-microscopic, ultrastructural, and immunocytochemical study. Am. J. Surg. Pathol. 1986, 10, 34–47. [Google Scholar] [CrossRef]
- Amari, M.; Taguchi, K.; Iwahara, M.; Oharaseki, T.; Yokouchi, Y.; Naoe, S.; Takahashi, K. Interaction between mesothelial cells and macrophages in the initial process of pleural adhesion: Ultrastructural studies using adhesion molecules. Med. Mol. Morphol. 2006, 39, 187–192. [Google Scholar] [CrossRef]
- Carmona, R.; Cano, E.; Grueso, E.; Ruiz-Villalba, A.; Bera, T.K.; Gaztambide, J.; Segovia, J.C.; Muñoz-Chápuli, R. Peritoneal repairing cells: A type of bone marrow derived progenitor cells involved in mesothelial regeneration. J. Cell Mol. Med. 2011, 15, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, M.; Yanagihara, D.L.; Rodgers, K.E.; DiZerega, G.S. The mitogenic activity of peritoneal tissue repair cells: Control by growth factors. J. Surg. Res. 1989, 47, 45–51. [Google Scholar] [CrossRef]
- Abe, H.; Rodgers, K.E.; Campeau, J.D.; Girgis, W.; Ellefson, D.; DiZerega, G.S. The effect of intraperitoneal administration of sodium tolmetin-hyaluronic acid on the postsurgical cell infiltration in vivo. J. Surg. Res. 1990, 49, 322–327. [Google Scholar] [CrossRef]
- Campbell, J.H.; Efendy, J.L.; Campbell, G.R. Novel vascular graft grown within recipient’s own peritoneal cavity. Circ. Res. 1999, 85, 1173–1178. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Lara-Pezzi, E.; Selgas, R.; Ramírez-Huesca, M.; Domínguez-Jiménez, C.; Jiménez-Heffernan, J.A.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; Alvarez, V.; et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef]
- Yang, A.H.; Chen, J.Y.; Lin, J.K. Myofibroblastic conversion of mesothelial cells. Kidney Int. 2003, 63, 1530–1539. [Google Scholar] [CrossRef]
- López-Cabrera, M. Mesenchymal Conversion of Mesothelial Cells Is a Key Event in the Pathophysiology of the Peritoneum during Peritoneal Dialysis. Adv. Med. 2014, 2014, 473134–473152. [Google Scholar] [CrossRef]
- Strippoli, R.; Moreno-Vicente, R.; Battistelli, C.; Cicchini, C.; Noce, V.; Amicone, L.; Marchetti, A.; Del Pozo, M.A.; Tripodi, M. Molecular Mechanisms Underlying Peritoneal EMT and Fibrosis. Stem Cells Int. 2016, 2016, 3543678–3543690. [Google Scholar] [CrossRef]
- Strippoli, R.; Benedicto, I.; Perez Lozano, M.L.; Pellinen, T.; Sandoval, P.; Lopez-Cabrera, M.; del Pozo, M.A. Inhibition of transforming growth factor-activated kinase 1 (TAK1) blocks and reverses epithelial to mesenchymal transition of mesothelial cells. PLoS ONE 2012, 7, 1–13. [Google Scholar] [CrossRef]
- Wilson, R.B.; Archid, R.; Reymond, M.A. Reprogramming of Mesothelial-Mesenchymal Transition in Chronic Peritoneal Diseases by Estrogen Receptor Modulation and TGF-β1 Inhibition. Int. J. Mol. Sci. 2020, 21, 4158. [Google Scholar] [CrossRef]
- Margetts, P.J.; Bonniaud, P.; Liu, L.; Hoff, C.M.; Holmes, C.J.; West-Mays, J.A.; Kelly, M.M. Transient overexpression of TGF-{beta}1 induces epithelial mesenchymal transition in the rodent peritoneum. J. Am. Soc. Nephrol. 2005, 16, 425–436. [Google Scholar] [CrossRef]
- Patel, P.; Sekiguchi, Y.; Oh, K.H.; Patterson, S.E.; Kolb, M.R.; Margetts, P.J. Smad3-dependent and -independent pathways are involved in peritoneal membrane injury. Kidney Int. 2010, 77, 319–328. [Google Scholar] [CrossRef]
- Strippoli, R.; Benedicto, I.; Foronda, M.; Perez-Lozano, M.L.; Sánchez-Perales, S.; López-Cabrera, M.; Del Pozo, M. p38 maintains E-cadherin expression by modulating TAK1-NF-kappa B during epithelial-to-mesenchymal transition. J. Cell Sci. 2010, 123, 4321–4331. [Google Scholar] [CrossRef]
- Xie, J.Y.; Chen, N.; Ren, H.; Wang, W.M. Angiotensin II-mediated activation of fibrotic pathways through ERK1/2 in rat peritoneal mesothelial cells. Ren. Fail. 2010, 32, 871–879. [Google Scholar] [CrossRef]
- Yokoi, H.; Kasahara, M.; Mori, K.; Ogawa, Y.; Kuwabara, T.; Imamaki, H.; Kawanishi, T.; Koga, K.; Ishii, A.; Kato, Y.; et al. Pleiotrophin triggers inflammation and increased peritoneal permeability leading to peritoneal fibrosis. Kidney Int. 2012, 81, 160–169. [Google Scholar] [CrossRef]
- Morishita, Y.; Ookawara, S.; Hirahara, I.; Muto, S.; Nagata, D. HIF-1α mediates Hypoxia-induced epithelial-mesenchymal transition in peritoneal mesothelial cells. Ren. Fail. 2016, 38, 282–289. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chang, Y.T.; Pan, S.Y.; Chou, Y.H.; Chang, F.C.; Yeh, P.Y.; Liu, Y.H.; Chiang, W.C.; Chen, Y.M.; Wu, K.D.; et al. Lineage tracing reveals distinctive fates for mesothelial cells and submesothelial fibroblasts during peritoneal injury. J. Am. Soc. Nephrol. 2014, 25, 2847–2858. [Google Scholar] [CrossRef]
- Namvar, S.; Woolf, A.S.; Zeef, L.A.; Wilm, T.; Wilm, B.; Herrick, S.E. Functional molecules in mesothelial-to-mesenchymal transition revealed by transcriptome analyses. J. Pathol. 2018, 245, 491–501. [Google Scholar] [CrossRef]
- Ruiz-Carpio, V.; Sandoval, P.; Aguilera, A.; Albar-Vizcaíno, P.; Perez-Lozano, M.L.; González-Mateo, G.T.; Acuña-Ruiz, A.; García-Cantalejo, J.; Botías, P.; Bajo, M.A.; et al. Genomic reprograming analysis of the Mesothelial to Mesenchymal Transition identifies biomarkers in peritoneal dialysis patients. Sci. Rep. 2017, 7, 44941–44956. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Asahina, K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc. Natl. Acad. Sci. USA 2013, 110, 2324–2329. [Google Scholar] [CrossRef]
- Wong, A.S.; Auersperg, N. Normal ovarian surface epithelium. Cancer Treat Res. 2002, 107, 161–183. [Google Scholar] [CrossRef]
- De Giorgio-Miller, A.; Bottoms, S.; Laurent, G.; Carmeliet, P.; Herrick, S. Fibrin-induced skin fibrosis in mice deficient in tissue plasminogen activator. Am. J. Pathol. 2005, 167, 721–732. [Google Scholar] [CrossRef]
- Holmdahl, L. The role of fibrinolysis in adhesion formation. Eur. J. Surg. Suppl. 1997, 24–31. [Google Scholar]
- Xu, X.; Rivkind, A.; Pappo, O.; Pikarsky, A.; Levi-Schaffer, F. Role of mast cells and myofibroblasts in human peritoneal adhesion formation. Ann. Surg. 2002, 236, 593–601. [Google Scholar] [CrossRef] [PubMed]
- DiZerega, G.S.; Campeau, J.D. Peritoneal repair and post-surgical adhesion formation. Hum. Reprod. Update 2001, 7, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, H.; Dawson, L.; Laurent, G.J.; Bellingan, G.J.; Herrick, S.E. Role of plasminogen activators in peritoneal adhesion formation. Biochem. Soc. Trans. 2002, 30, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Hinoi, T.; Ikeda, S.; Adachi, T.; Kawaguchi, Y.; Tokunaga, M.; Sasada, T.; Egi, H.; Tanabe, K.; Okajima, M.; et al. Preservation of peritoneal fibrinolysis owing to decreased transcription of plasminogen activator inhibitor-1 in peritoneal mesothelial cells suppresses postoperative adhesion formation in laparoscopic surgery. Surgery 2013, 153, 344–356. [Google Scholar] [CrossRef]
- Honjo, K.; Munakata, S.; Tashiro, Y.; Salama, Y.; Shimazu, H.; Eiamboonsert, S.; Dhahri, D.; Ichimura, A.; Dan, T.; Miyata, T.; et al. Plasminogen activator inhibitor-1 regulates macrophage-dependent postoperative adhesion by enhancing EGF-HER1 signaling in mice. FASEB J. 2017, 31, 2625–2637. [Google Scholar] [CrossRef]
- Brokelman, W.; Holmdahl, L.; Falk, P.; Klinkenbijl, J.; Reijnen, M. The peritoneal fibrinolytic response to conventional and laparoscopic colonic surgery. J. Laparoendosc. Adv. Surg. Tech. A 2009, 19, 489–493. [Google Scholar] [CrossRef]
- Hellebrekers, B.W.; Trimbos-Kemper, T.C.; Boesten, L.; Jansen, F.W.; Kolkman, W.; Trimbos, J.B.; Press, R.R.; van Poelgeest, M.I.; Emeis, S.J.; Kooistra, T. Preoperative predictors of postsurgical adhesion formation and the Prevention of Adhesions with Plasminogen Activator (PAPA-study): Results of a clinical pilot study. Fertil. Steril. 2009, 91, 1204–1214. [Google Scholar] [CrossRef]
- Chung, D.R.; Chitnis, T.; Panzo, R.J.; Kasper, D.L.; Sayegh, M.H.; Tzianabos, A.O. CD4+ T cells regulate surgical and postinfectious adhesion formation. J. Exp. Med. 2002, 195, 1471–1478. [Google Scholar] [CrossRef]
- Holsti, M.A.; Chitnis, T.; Panzo, R.J.; Bronson, R.T.; Yagita, H.; Sayegh, M.H.; Tzianabos, A.O. Regulation of postsurgical fibrosis by the programmed death-1 inhibitory pathway. J. Immunol. 2004, 172, 5774–5781. [Google Scholar] [CrossRef]
- Tsai, J.M.; Shoham, M.; Fernhoff, N.B.; George, B.M.; Marjon, K.D.; McCracken, M.N.; Kao, K.S.; Sinha, R.; Volkmer, A.K.; Miyanishi, M.; et al. Neutrophil and monocyte kinetics play critical roles in mouse peritoneal adhesion formation. Blood Adv. 2019, 3, 2713–2721. [Google Scholar] [CrossRef]
- Krause, T.J.; Katz, D.; Wheeler, C.J.; Ebner, S.; McKinnon, R.D. Increased levels of surgical adhesions in TGFbeta1 heterozygous mice. J. Investig. Surg. 1999, 12, 31–38. [Google Scholar] [CrossRef]
- Lucas, P.A.; Warejcka, D.J.; Young, H.E.; Lee, B.Y. Formation of abdominal adhesions is inhibited by antibodies to transforming growth factor-beta1. J. Surg. Res. 1996, 65, 135–138. [Google Scholar] [CrossRef]
- Gorvy, D.A.; Herrick, S.E.; Shah, M.; Ferguson, M.W. Experimental manipulation of transforming growth factor-beta isoforms significantly affects adhesion formation in a murine surgical model. Am. J. Pathol. 2005, 167, 1005–1019. [Google Scholar] [CrossRef]
- Holmdahl, L.; Kotseos, K.; Bergström, M.; Falk, P.; Ivarsson, M.L.; Chegini, N. Overproduction of transforming growth factor-beta1 (TGF-beta1) is associated with adhesion formation and peritoneal fibrinolytic impairment. Surgery 2001, 129, 626–632. [Google Scholar] [CrossRef]
- Torres, K.; Pietrzyk, Ł.; Plewa, Z.; Załuska-Patel, K.; Majewski, M.; Radzikowska, E.; Torres, A. TGF-β and inflammatory blood markers in prediction of intraperitoneal adhesions. Adv. Med. Sci. 2018, 63, 220–223. [Google Scholar] [CrossRef]
- Gómez-Gil, V.; Pascual, G.; Pérez-Köhler, B.; Cifuentes, A.; Buján, J.; Bellón, J.M. Involvement of transforming growth factor-β3 and betaglycan in the cytoarchitecture of postoperative omental adhesions. J. Surg. Res. 2014, 187, 699–711. [Google Scholar] [CrossRef]
- Chegini, N. TGF-beta system: The principal profibrotic mediator of peritoneal adhesion formation. Semin. Reprod. Med. 2008, 26, 298–312. [Google Scholar] [CrossRef]
- Kawanishi, K.; Nitta, K. Cell sheet-based tissue engineering for mesothelial cell injury. Contrib. Nephrol. 2015, 185, 66–75. [Google Scholar] [CrossRef]
- Bresson, L.; Leblanc, E.; Lemaire, A.S.; Okitsu, T.; Chai, F. Autologous peritoneal grafts permit rapid reperitonealization and prevent postoperative abdominal adhesions in an experimental rat study. Surgery 2017, 162, 863–870. [Google Scholar] [CrossRef]
- Rout, U.K.; Saed, G.M.; Diamond, M.P. Expression pattern and regulation of genes differ between fibroblasts of adhesion and normal human peritoneum. Reprod. Biol. Endocrinol. 2005, 3, 1–13. [Google Scholar] [CrossRef]
- Macarak, E.J.; Lotto, C.E.; Koganti, D.; Jin, X.; Wermuth, P.J.; Olsson, A.K.; Montgomery, M.; Rosenbloom, J. Trametinib prevents mesothelial-mesenchymal transition and ameliorates abdominal adhesion formation. J. Surg. Res. 2018, 227, 198–210. [Google Scholar] [CrossRef]
- Foster, D.S.; Marshall, C.D.; Gulati, G.S.; Chinta, M.S.; Nguyen, A.; Salhotra, A.; Jones, R.E.; Burcham, A.; Lerbs, T.; Cui, L.; et al. Elucidating the fundamental fibrotic processes driving abdominal adhesion formation. Nat. Commun. 2020, 11, 4061–4079. [Google Scholar] [CrossRef]
- Burnett, S.H.; Beus, B.J.; Avdiushko, R.; Qualls, J.; Kaplan, A.M.; Cohen, D.A. Development of peritoneal adhesions in macrophage depleted mice. J. Surg. Res. 2006, 131, 296–301. [Google Scholar] [CrossRef]
- Katz, S.; Zsiros, V.; Kiss, A.L. Under inflammatory stimuli mesenteric mesothelial cells transdifferentiate into macrophages and produce pro-inflammatory cytokine IL-6. Inflamm. Res. 2019, 68, 525–528. [Google Scholar] [CrossRef]
- Hoshino, A.; Kawamura, Y.I.; Yasuhara, M.; Toyama-Sorimachi, N.; Yamamoto, K.; Matsukawa, A.; Lira, S.A.; Dohi, T. Inhibition of CCL1-CCR8 interaction prevents aggregation of macrophages and development of peritoneal adhesions. J. Immunol. 2007, 178, 5296–5304. [Google Scholar] [CrossRef]
- Zindel, J.; Peiseler, M.; Hossain, M.; Deppermann, C.; Lee, W.Y.; Haenni, B.; Zuber, B.; Deniset, J.F.; Surewaard, B.G.J.; Candinas, D.; et al. Primordial GATA6 macrophages function as extravascular platelets in sterile injury. Science 2021, 371. [Google Scholar] [CrossRef]
- Sandoval, P.; Jiménez-Heffernan, J.A.; Guerra-Azcona, G.; Pérez-Lozano, M.L.; Rynne-Vidal, Á.; Albar-Vizcaíno, P.; Gil-Vera, F.; Martín, P.; Coronado, M.J.; Barcena, C.; et al. Mesothelial-to-mesenchymal transition in the pathogenesis of post-surgical peritoneal adhesions. J. Pathol. 2016, 239, 48–59. [Google Scholar] [CrossRef]
- Tsai, J.M.; Sinha, R.; Seita, J.; Fernhoff, N.; Christ, S.; Koopmans, T.; Krampitz, G.W.; McKenna, K.M.; Xing, L.; Sandholzer, M.; et al. Surgical adhesions in mice are derived from mesothelial cells and can be targeted by antibodies against mesothelial markers. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, R.; Sandoval, P.; Moreno-Vicente, R.; Rossi, L.; Battistelli, C.; Terri, M.; Pascual-Antón, L.; Loureiro, M.; Matteini, F.; Calvo, E.; et al. Caveolin1 and YAP drive mechanically induced mesothelial to mesenchymal transition and fibrosis. Cell Death Dis. 2020, 11, 647–668. [Google Scholar] [CrossRef]
- Fang, C.C.; Huang, J.W.; Shyu, R.S.; Yen, C.J.; Shiao, C.H.; Chiang, C.K.; Hu, R.H.; Tsai, T.J. Fibrin-Induced epithelial-to-mesenchymal transition of peritoneal mesothelial cells as a mechanism of peritoneal fibrosis: Effects of pentoxifylline. PLoS ONE 2012, 7, 1–11. [Google Scholar] [CrossRef]
- Funayama, N.; Sato, Y.; Matsumoto, K.; Ogura, T.; Takahashi, Y. Coelom formation: Binary decision of the lateral plate mesoderm is controlled by the ectoderm. Development 1999, 126, 4129–4138. [Google Scholar] [CrossRef] [PubMed]
- Winters, N.I.; Thomason, R.T.; Bader, D.M. Identification of a novel developmental mechanism in the generation of mesothelia. Development 2012, 139, 2926–2934. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.F.; Pritchard-Jones, K.; Bickmore, W.A.; Hastie, N.D.; Bard, J.B. The expression of the Wilms’ tumour gene, WT1, in the developing mammalian embryo. Mech. Dev. 1993, 40, 85–97. [Google Scholar] [CrossRef]
- Wilm, B.; Ipenberg, A.; Hastie, N.D.; Burch, J.B.; Bader, D.M. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 2005, 132, 5317–5328. [Google Scholar] [CrossRef]
- Hastie, N.D. Wilms’ tumour 1 (WT1) in development, homeostasis and disease. Development 2017, 144, 2862–2872. [Google Scholar] [CrossRef]
- Wilm, B.; Muñoz-Chapuli, R. The Role of WT1 in Embryonic Development and Normal Organ Homeostasis. Methods Mol. Biol. 2016, 1467, 23–39. [Google Scholar] [CrossRef]
- Que, J.; Wilm, B.; Hasegawa, H.; Wang, F.; Bader, D.; Hogan, B.L. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc. Natl. Acad. Sci. USA 2008, 105, 16626–16630. [Google Scholar] [CrossRef]
- Lüdtke, T.H.; Rudat, C.; Kurz, J.; Häfner, R.; Greulich, F.; Wojahn, I.; Aydoğdu, N.; Mamo, T.M.; Kleppa, M.J.; Trowe, M.O.; et al. Mesothelial mobilization in the developing lung and heart differs in timing, quantity, and pathway dependency. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, 767–783. [Google Scholar] [CrossRef]
- Von Gise, A.; Stevens, S.M.; Honor, L.B.; Oh, J.H.; Gao, C.; Zhou, B.; Pu, W.T. Contribution of Fetal, but Not Adult, Pulmonary Mesothelium to Mesenchymal Lineages in Lung Homeostasis and Fibrosis. Am. J. Respir. Cell Mol. Biol. 2016, 54, 222–230. [Google Scholar] [CrossRef]
- Zhou, B.; Pu, W.T. Epicardial epithelial-to-mesenchymal transition in injured heart. J. Cell Mol. Med. 2011, 15, 2781–2783. [Google Scholar] [CrossRef]
- Rudat, C.; Kispert, A. Wt1 and epicardial fate mapping. Circ. Res. 2012, 111, 165–169. [Google Scholar] [CrossRef]
- Asahina, K.; Zhou, B.; Pu, W.T.; Tsukamoto, H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 2011, 53, 983–995. [Google Scholar] [CrossRef]
- Kendall, T.J.; Duff, C.M.; Boulter, L.; Wilson, D.H.; Freyer, E.; Aitken, S.; Forbes, S.J.; Iredale, J.P.; Hastie, N.D. Embryonic mesothelial-derived hepatic lineage of quiescent and heterogenous scar-orchestrating cells defined but suppressed by WT1. Nat. Commun. 2019, 10, 4688–4706. [Google Scholar] [CrossRef]
- Ariza, L.; Cañete, A.; Rojas, A.; Muñoz-Chápuli, R.; Carmona, R. Role of the Wilms’ tumor suppressor gene Wt1 in pancreatic development. Dev. Dyn. 2018, 247, 924–933. [Google Scholar] [CrossRef]
- Ariza, L.; Rojas, A.; Muñoz-Chápuli, R.; Carmona, R. The Wilms’ tumor suppressor gene regulates pancreas homeostasis and repair. PLoS Genet. 2019, 15, 1–19. [Google Scholar] [CrossRef]
- Carmona, R.; Cano, E.; Mattiotti, A.; Gaztambide, J.; Muñoz-Chápuli, R. Cells derived from the coelomic epithelium contribute to multiple gastrointestinal tissues in mouse embryos. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Chau, Y.Y.; Bandiera, R.; Serrels, A.; Martínez-Estrada, O.M.; Qing, W.; Lee, M.; Slight, J.; Thornburn, A.; Berry, R.; McHaffie, S.; et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 2014, 16, 367–375. [Google Scholar] [CrossRef]
- Martínez-Estrada, O.M.; Lettice, L.A.; Essafi, A.; Guadix, J.A.; Slight, J.; Velecela, V.; Hall, E.; Reichmann, J.; Devenney, P.S.; Hohenstein, P.; et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat. Genet. 2010, 42, 89–93. [Google Scholar] [CrossRef]
- Von Gise, A.; Zhou, B.; Honor, L.B.; Ma, Q.; Petryk, A.; Pu, W.T. WT1 regulates epicardial epithelial to mesenchymal transition through β-catenin and retinoic acid signaling pathways. Dev. Biol. 2011, 356, 421–431. [Google Scholar] [CrossRef]
- Buechler, M.B.; Kim, K.W.; Onufer, E.J.; Williams, J.W.; Little, C.C.; Dominguez, C.X.; Li, Q.; Sandoval, W.; Cooper, J.E.; Harris, C.A.; et al. A Stromal Niche Defined by Expression of the Transcription Factor WT1 Mediates Programming and Homeostasis of Cavity-Resident Macrophages. Immunity 2019, 51, 119–130. [Google Scholar] [CrossRef]
- Smart, N.; Bollini, S.; Dubé, K.N.; Vieira, J.M.; Zhou, B.; Davidson, S.; Yellon, D.; Riegler, J.; Price, A.N.; Lythgoe, M.F.; et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011, 474, 640–644. [Google Scholar] [CrossRef]
- Kreidberg, J.A.; Sariola, H.; Loring, J.M.; Maeda, M.; Pelletier, J.; Housman, D.; Jaenisch, R. WT-1 is required for early kidney development. Cell 1993, 74, 679–691. [Google Scholar] [CrossRef]
- Cano, E.; Carmona, R.; Ruiz-Villalba, A.; Rojas, A.; Chau, Y.Y.; Wagner, K.D.; Wagner, N.; Hastie, N.D.; Muñoz-Chápuli, R.; Pérez-Pomares, J.M. Extracardiac septum transversum/proepicardial endothelial cells pattern embryonic coronary arterio-venous connections. Proc. Natl. Acad. Sci. USA 2016, 113, 656–661. [Google Scholar] [CrossRef]
- Ijpenberg, A.; Pérez-Pomares, J.M.; Guadix, J.A.; Carmona, R.; Portillo-Sánchez, V.; Macías, D.; Hohenstein, P.; Miles, C.M.; Hastie, N.D.; Muñoz-Chápuli, R. Wt1 and retinoic acid signaling are essential for stellate cell development and liver morphogenesis. Dev. Biol. 2007, 312, 157–170. [Google Scholar] [CrossRef]
- Diez-Roux, G.; Banfi, S.; Sultan, M.; Geffers, L.; Anand, S.; Rozado, D.; Magen, A.; Canidio, E.; Pagani, M.; Peluso, I.; et al. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011, 9, 1–14. [Google Scholar] [CrossRef]
- Lua, I.; Li, Y.; Zagory, J.A.; Wang, K.S.; French, S.W.; Sévigny, J.; Asahina, K. Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J. Hepatol. 2016, 64, 1137–1146. [Google Scholar] [CrossRef]
- Bera, T.K.; Pastan, I. Mesothelin is not required for normal mouse development or reproduction. Mol. Cell Biol. 2000, 20, 2902–2906. [Google Scholar] [CrossRef]
- Astarita, J.L.; Acton, S.E.; Turley, S.J. Podoplanin: Emerging functions in development, the immune system, and cancer. Front. Immunol. 2012, 3, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Tamura, S.; Suzuki-Inoue, K. New horizon in platelet function: With special reference to a recently-found molecule, CLEC-2. Thromb J. 2016, 14, 27–37. [Google Scholar] [CrossRef]
- Mahtab, E.A.; Wijffels, M.C.; Van Den Akker, N.M.; Hahurij, N.D.; Lie-Venema, H.; Wisse, L.J.; Deruiter, M.C.; Uhrin, P.; Zaujec, J.; Binder, B.R.; et al. Cardiac malformations and myocardial abnormalities in podoplanin knockout mouse embryos: Correlation with abnormal epicardial development. Dev. Dyn. 2008, 237, 847–857. [Google Scholar] [CrossRef]
- Gittenberger-de Groot, A.C.; Mahtab, E.A.; Hahurij, N.D.; Wisse, L.J.; Deruiter, M.C.; Wijffels, M.C.; Poelmann, R.E. Nkx2.5-negative myocardium of the posterior heart field and its correlation with podoplanin expression in cells from the developing cardiac pacemaking and conduction system. Anat. Rec. 2007, 290, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Velecela, V.; Torres-Cano, A.; García-Melero, A.; Ramiro-Pareta, M.; Müller-Sánchez, C.; Segarra-Mondejar, M.; Chau, Y.Y.; Campos-Bonilla, B.; Reina, M.; Soriano, F.X.; et al. Epicardial cell shape and maturation are regulated by Wt1 via transcriptional control of. Development 2019, 146, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, C.C.; Schmaier, A.A.; Mericko, P.; Hess, P.R.; Zou, Z.; Chen, M.; Chen, C.Y.; Xu, B.; Lu, M.M.; Zhou, D.; et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 2010, 116, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Gil, H.J.; Ma, W.; Oliver, G. A novel podoplanin-GFPCre mouse strain for gene deletion in lymphatic endothelial cells. Genesis 2018, 56, 1–8. [Google Scholar] [CrossRef]
- Honda, A.; Ito, Y.; Takahashi-Niki, K.; Matsushita, N.; Nozumi, M.; Tabata, H.; Takeuchi, K.; Igarashi, M. Extracellular Signals Induce Glycoprotein M6a Clustering of Lipid Rafts and Associated Signaling Molecules. J. Neurosci. 2017, 37, 4046–4064. [Google Scholar] [CrossRef]
- Sato, Y.; Mita, S.; Fukushima, N.; Fujisawa, H.; Saga, Y.; Hirata, T. Induction of axon growth arrest without growth cone collapse through the N-terminal region of four-transmembrane glycoprotein M6a. Dev. Neurobiol. 2011, 71, 733–746. [Google Scholar] [CrossRef]
- Rudat, C.; Grieskamp, T.; Röhr, C.; Airik, R.; Wrede, C.; Hegermann, J.; Herrmann, B.G.; Schuster-Gossler, K.; Kispert, A. Upk3b is dispensable for development and integrity of urothelium and mesothelium. PLoS ONE 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Kuriyama, S.; Tamiya, Y.; Tanaka, M. Spatiotemporal expression of UPK3B and its promoter activity during embryogenesis and spermatogenesis. Histochem. Cell Biol. 2017, 147, 17–26. [Google Scholar] [CrossRef]
- Asahina, K.; Tsai, S.Y.; Li, P.; Ishii, M.; Maxson, R.E.; Sucov, H.M.; Tsukamoto, H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology 2009, 49, 998–1011. [Google Scholar] [CrossRef]
- Bochmann, L.; Sarathchandra, P.; Mori, F.; Lara-Pezzi, E.; Lazzaro, D.; Rosenthal, N. Revealing new mouse epicardial cell markers through transcriptomics. PLoS ONE 2010, 5, 1–13. [Google Scholar] [CrossRef]
- Onitsuka, I.; Tanaka, M.; Miyajima, A. Characterization and functional analyses of hepatic mesothelial cells in mouse liver development. Gastroenterology 2010, 138, 1525–1535. [Google Scholar] [CrossRef]
- Si, M.; Wang, Q.; Li, Y.; Lin, H.; Luo, D.; Zhao, W.; Dou, X.; Liu, J.; Zhang, H.; Huang, Y.; et al. Inhibition of hyperglycolysis in mesothelial cells prevents peritoneal fibrosis. Sci. Transl. Med. 2019, 11, 1–16. [Google Scholar] [CrossRef]
- Lansley, S.M.; Searles, R.G.; Hoi, A.; Thomas, C.; Moneta, H.; Herrick, S.E.; Thompson, P.J.; Newman, M.; Sterrett, G.F.; Prêle, C.M.; et al. Mesothelial cell differentiation into osteoblast- and adipocyte-like cells. J. Cell Mol. Med. 2011, 15, 2095–2105. [Google Scholar] [CrossRef]
- Do Amaral, R.J.; Benac, P.; Andrade, L.R.; Farina, M.; Bernardazzi, C.; Arcanjo, K.D.; Palumbo, A.; Cordeiro, I.R.; Brito, J.M.; El-Cheikh, M.C.; et al. Peritoneal Submesothelial Stromal Cells Support Hematopoiesis and Differentiate into Osteogenic and Adipogenic Cell Lineages. Cells Tissues Organs 2014, 200, 118–131. [Google Scholar] [CrossRef]
- Dauleh, S.; Santeramo, I.; Fielding, C.; Ward, K.; Herrmann, A.; Murray, P.; Wilm, B. Characterisation of Cultured Mesothelial Cells Derived from the Murine Adult Omentum. PLoS ONE 2016, 11, 1–22. [Google Scholar] [CrossRef]
- Shelton, E.L.; Galindo, C.L.; Williams, C.H.; Pfaltzgraff, E.; Hong, C.C.; Bader, D.M. Autotaxin Signaling Governs Phenotypic Heterogeneity in Visceral and Parietal Mesothelia. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef][Green Version]
- Auersperg, N.; Wong, A.S.; Choi, K.C.; Kang, S.K.; Leung, P.C. Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr. Rev. 2001, 22, 255–288. [Google Scholar] [CrossRef]
- Okabe, Y.; Medzhitov, R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 2014, 157, 832–844. [Google Scholar] [CrossRef]
- Karabulut, B.; Sönmez, K.; Türkyilmaz, Z.; Demiroğullari, B.; Karabulut, R.; Sezer, C.; Sultan, N.; Başaklar, A.C.; Kale, N. Omentum prevents intestinal adhesions to mesh graft in abdominal infections and serosal defects. Surg. Endosc. 2006, 20, 978–982. [Google Scholar] [CrossRef]
- Gómez-Gil, V.; Pascual, G.; García-Honduvilla, N.; Rodríguez, M.; Buján, J.; Bellón, J.M. Characterizing omental adhesions by culturing cells isolated from a novel in vivo adhesion model. Wound Repair Regen. 2009, 17, 51–61. [Google Scholar] [CrossRef]
- Liu, M.; Silva-Sanchez, A.; Randall, T.D.; Meza-Perez, S. Specialized immune responses in the peritoneal cavity and omentum. J. Leukoc. Biol. 2021, 109, 717–729. [Google Scholar] [CrossRef]
- Suzuki, T.; Kono, T.; Bochimoto, H.; Hira, Y.; Watanabe, T.; Furukawa, H. An injured tissue affects the opposite intact peritoneum during postoperative adhesion formation. Sci. Rep. 2015, 5, 7668–7679. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Koopmans, T.; Ramesh, P.; Christ, S.; Strunz, M.; Wannemacher, J.; Aichler, M.; Feuchtinger, A.; Walch, A.; Ansari, M.; et al. Post-surgical adhesions are triggered by calcium-dependent membrane bridges between mesothelial surfaces. Nat. Commun. 2020, 11, 3068–3083. [Google Scholar] [CrossRef]
| Gene Name, Molecule Type | Expression in Peritoneum | Role of Protein | |||
|---|---|---|---|---|---|
| Parietal | Intestine | Liver | Pancreas | Knockout | |
| GMP6A (Glycoprotein m6a) | Adult [32] | Embryonic, adult [67] | Increase in body fat / body weight [136] | ||
| PDPN (podoplanin) | Embryonic [131] | E12.5, adult [32,112,126] | Lymphatic and endothelial phenotype [133,134] | ||
| WT1 (Wilms’ tumour protein 1) | Embryonic, adult [103,104,116] | Embryonic, adult [103,104,116] | Embryonic, adult mesothelial and submesothelial [112,113,139] | Embryonic, adult [114,115] | Multi-systemic: kidneys, heart, visceral fat, bone, haematopoiesis, pancreas [114,115,117,122] |
| MSLN (Mesothelin) | Embryonic, adult [31,125] | Embryonic, adult [31,125] | Embryonic, adult [31,125,126] | No phenotype [127] | |
| UPK3B (Uroplakin 3b) | Embryonic, adult [137] | Embryonic, adult [137] | Embryonic, adult [137] | No phenotype [137] | |
| ALCAM (activated leukocyte cell adhesion molecule) | Embryonic, mesothelial and submesothelial [67,112,139] | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrick, S.E.; Wilm, B. Post-Surgical Peritoneal Scarring and Key Molecular Mechanisms. Biomolecules 2021, 11, 692. https://doi.org/10.3390/biom11050692
Herrick SE, Wilm B. Post-Surgical Peritoneal Scarring and Key Molecular Mechanisms. Biomolecules. 2021; 11(5):692. https://doi.org/10.3390/biom11050692
Chicago/Turabian StyleHerrick, Sarah E., and Bettina Wilm. 2021. "Post-Surgical Peritoneal Scarring and Key Molecular Mechanisms" Biomolecules 11, no. 5: 692. https://doi.org/10.3390/biom11050692
APA StyleHerrick, S. E., & Wilm, B. (2021). Post-Surgical Peritoneal Scarring and Key Molecular Mechanisms. Biomolecules, 11(5), 692. https://doi.org/10.3390/biom11050692






