Investigation of Mitochondrial Adaptations to Modulation of Carbohydrate Supply during Adipogenesis of 3T3-L1 Cells by Targeted 1H-NMR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation
2.2. Extracellular (EC) and Intracellular (IC) Metabolic Extracts Preparation and 1H-NMR Spectra Acquirement
2.3. Analytical Steps of the IC and EC Metabolic Contents through a Two-Step 1H-NMR-Based Metabonomic Study
3. Untargeted Exploratory Approach
3.1. Multivariate Date Analyses
3.2. Identification of Discriminant Variables (Metabolites)
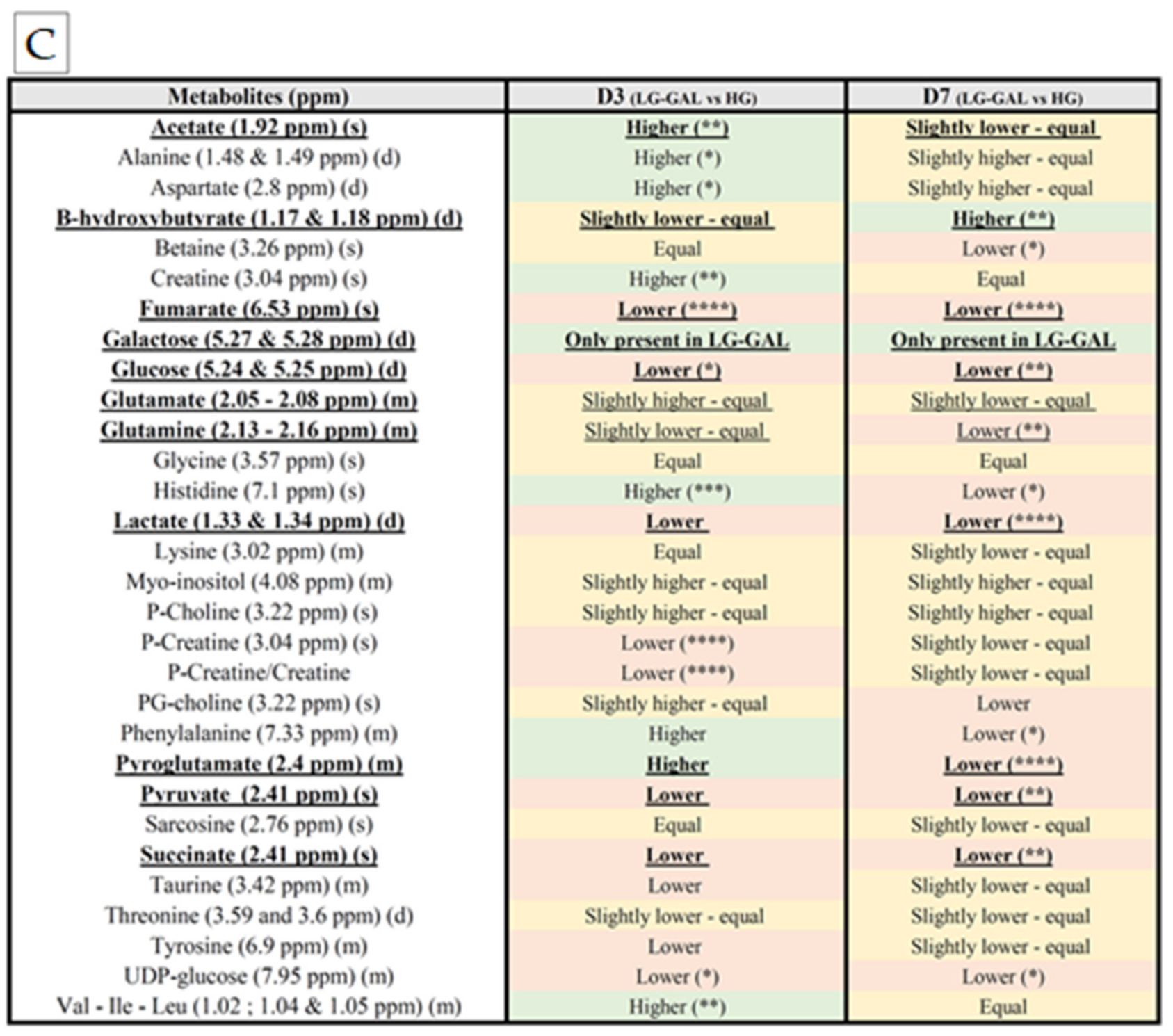
3.3. Relative Levels of the Mitochondrial Metabolic Markers’ Assessment
3.4. Selection of Mitochondrial Metabolic Markers
4. Targeted Approach
Statistical Analyses
5. Results
5.1. Selection of Mitochondrial Metabolic Markers
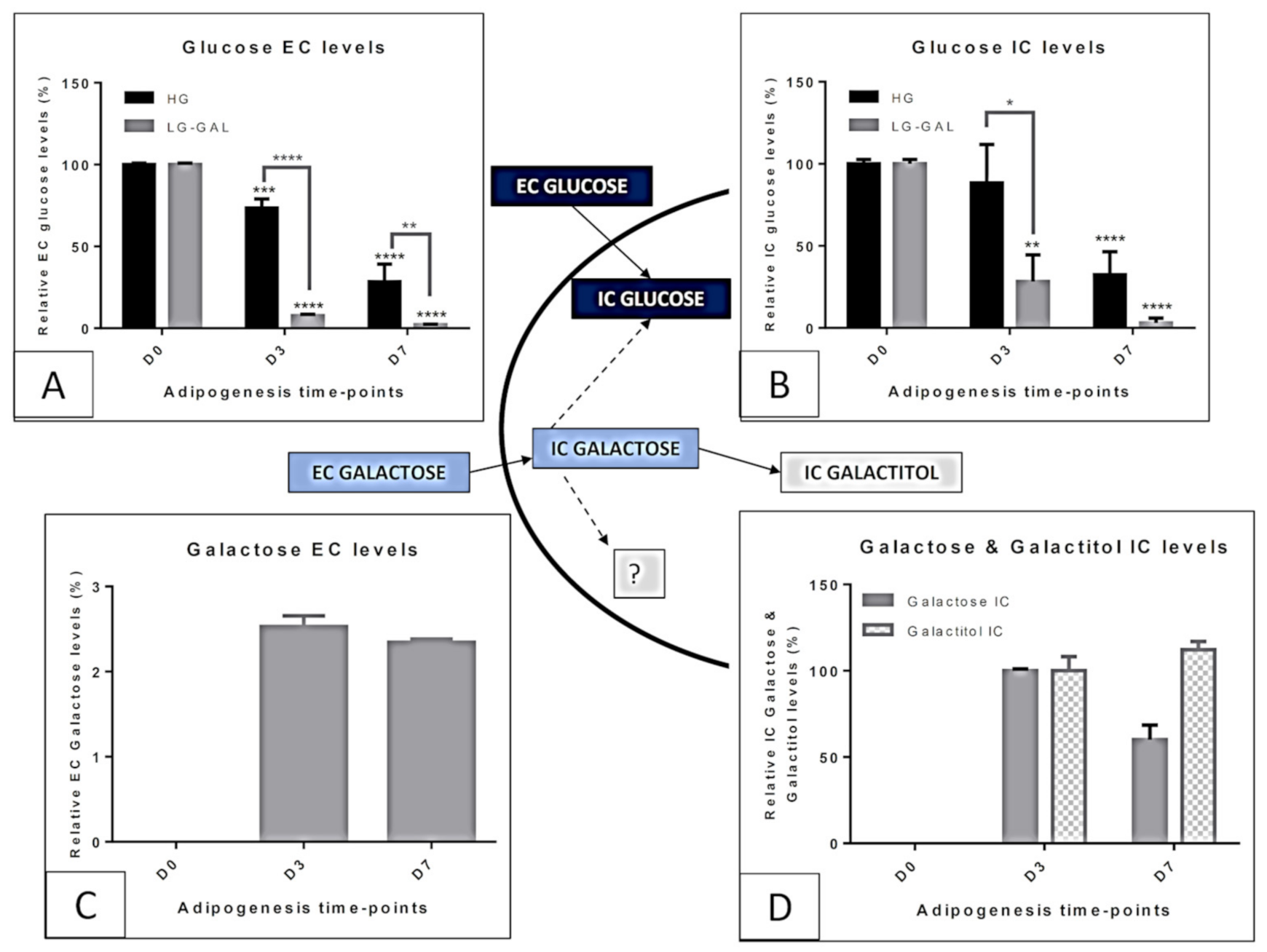
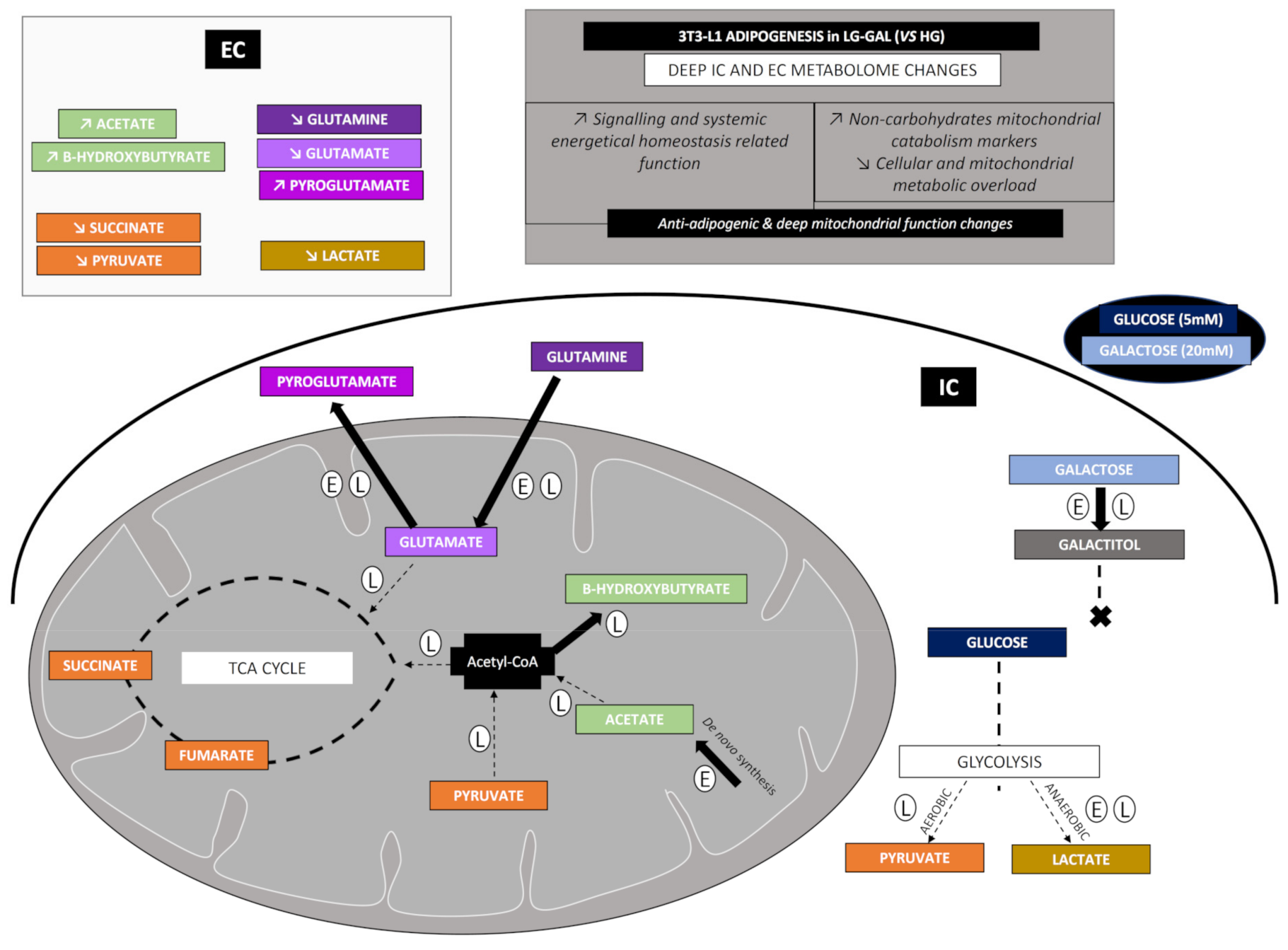
Carbohydrates Supply Intake and Consumption
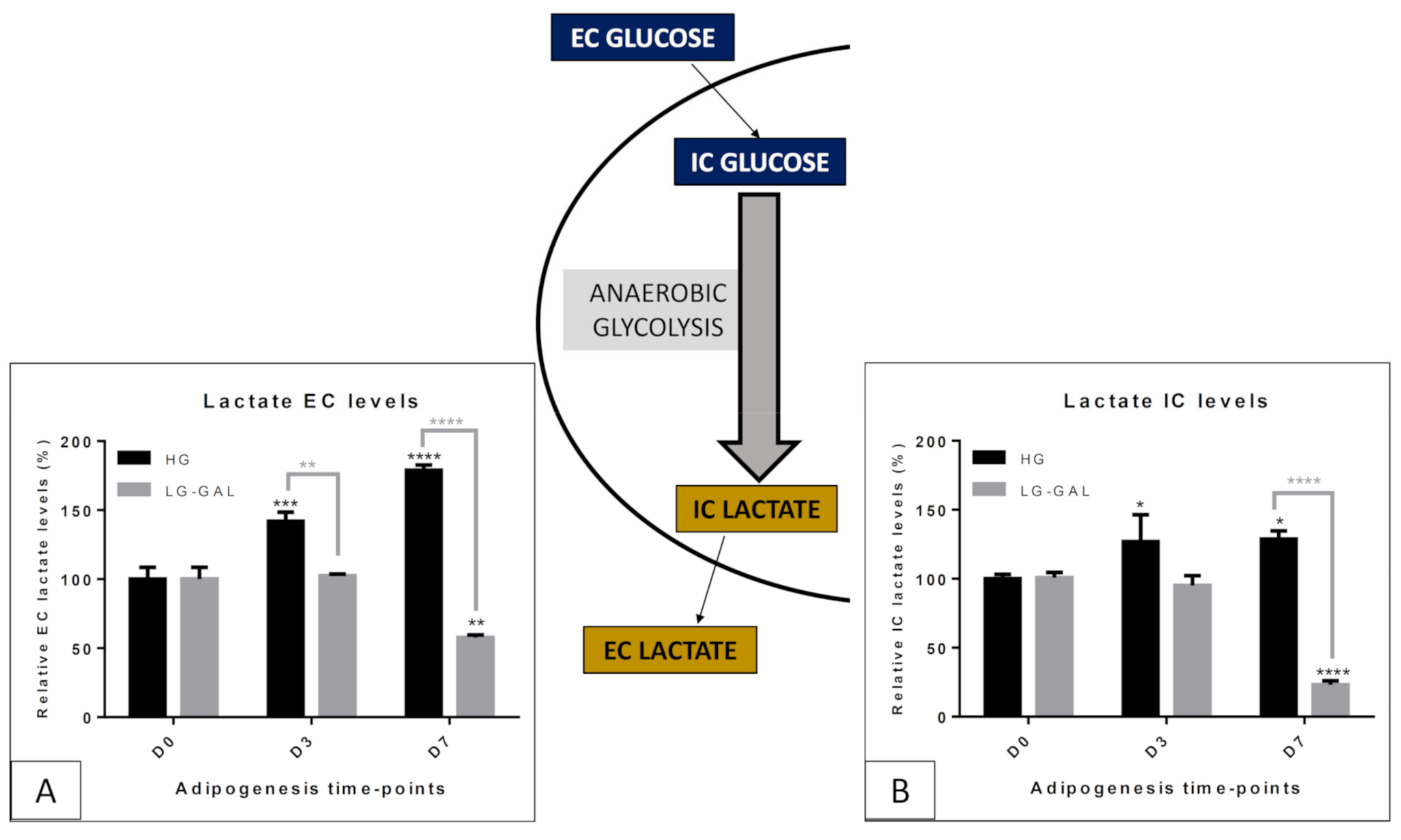
5.2. Carbohydrate Metabolism-Anaerobic Glycolysis
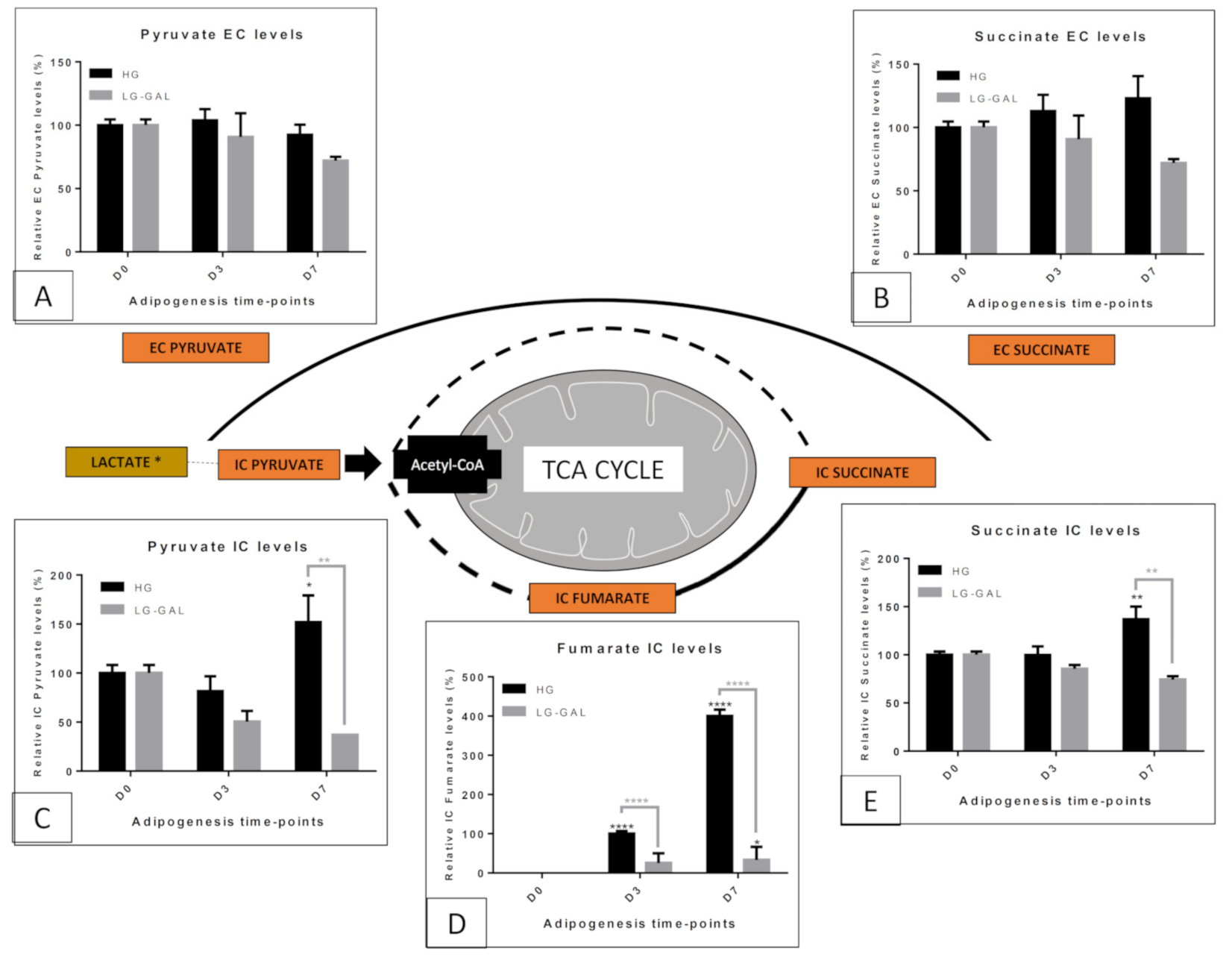
5.3. Carbohydrate and Related Mitochondrial Catabolism—TCA (TriCarboxylic Acid) Cycle
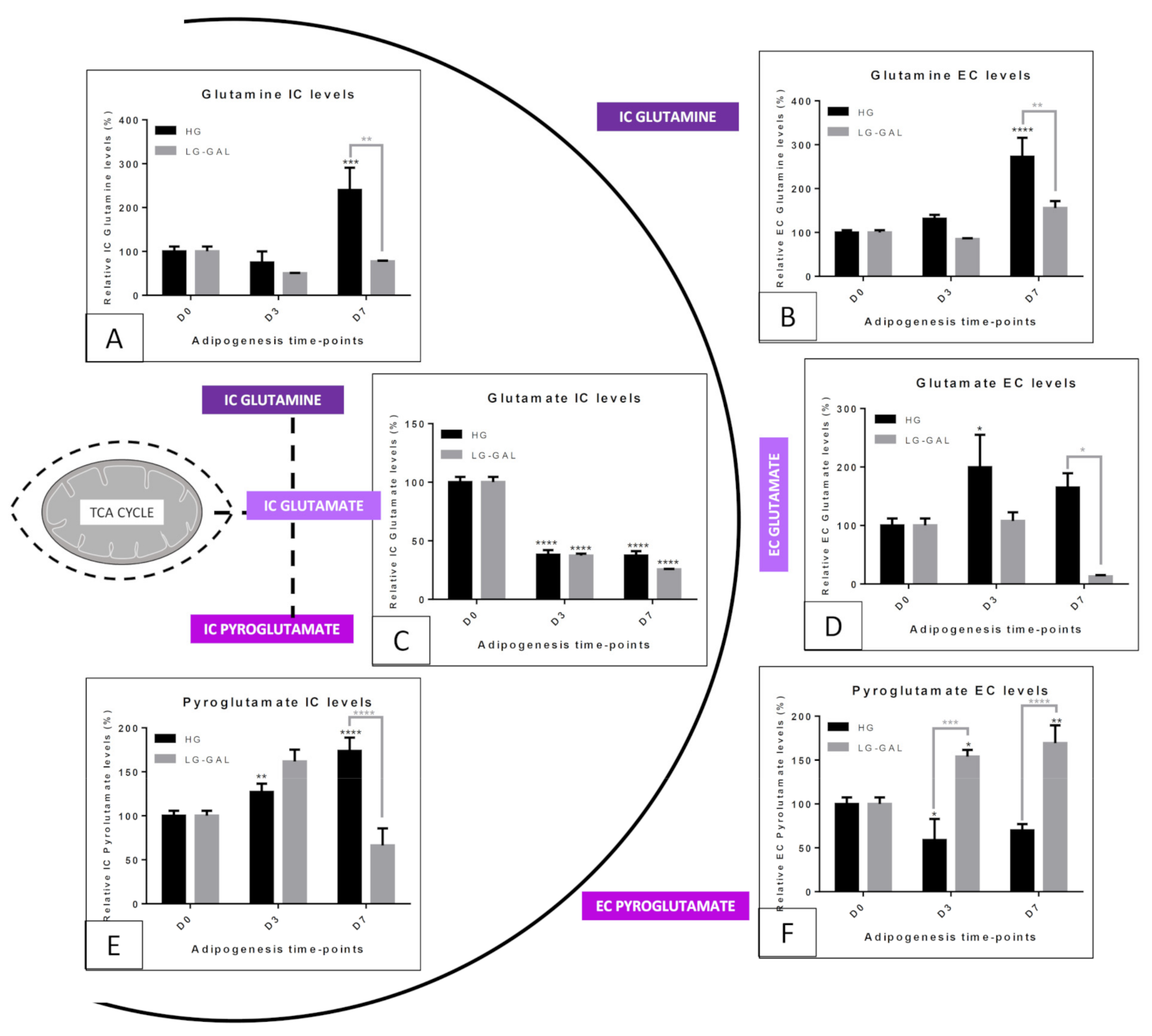
5.4. Mitochondrial Metabolism—Non-Glycolytic “Glutamine–Glutamate–Pyroglutamate” Group
5.5. Mitochondrial Metabolism Markers—Acetate and β-hydroxybutyrate
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef]
- Vishvanath, L.; Gupta, R.K. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Investig. 2019, 129, 4022–4031. [Google Scholar] [CrossRef]
- Fève, B. Adipogenesis: Cellular and molecular aspects. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 483–499. [Google Scholar] [CrossRef]
- Lowe, C.E.; O’Rahilly, S.; Rochford, J.J. Adipogenesis at a glance. J. Cell Sci. 2011, 124, 2681–2686. [Google Scholar] [CrossRef] [Green Version]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3428766/ (accessed on 18 November 2020). [CrossRef] [Green Version]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Morrison, S.; McGee, S.L. 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte 2015, 4, 295–302. [Google Scholar] [CrossRef] [Green Version]
- De Pauw, A.; Tejerina, S.; Raes, M.; Keijer, J.; Arnould, T. Mitochondrial (Dys)function in Adipocyte (De)differentiation and Systemic Metabolic Alterations. Am. J. Pathol. 2009, 175, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Gao, Z.; Chen, Y.; Guan, M.-X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell 2017, 8, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, A.J.; Jackson, T.D.; Stroud, D.A.; Stojanovski, D. Mitochondria—Hubs for regulating cellular biochemistry: Emerging concepts and networks. Open Biol. 2019, 9, 190126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinelli, J.B.; Haigis, M.C. The Multifaceted Contributions of Mitochondria to Cellular Metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.G.; Green, D.R. Mitochondria and cell signalling. J. Cell Sci. 2012, 125, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandel, N.S. Evolution of Mitochondria as Signaling Organelles. Cell Metab. 2015, 22, 204–206. [Google Scholar] [CrossRef] [Green Version]
- Aon, M.A.; Camara, A.K.S. Mitochondria: Hubs of cellular signaling, energetics and redox balance. A rich, vibrant, and diverse landscape of mitochondrial research. Front. Physiol. 2015, 6, 94. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4374463/ (accessed on 26 October 2020). [CrossRef] [PubMed] [Green Version]
- Wang, B.; Yang, Q.; Harris, C.L.; Nelson, M.L.; Busboom, J.R.; Zhu, M.-J.; Du, M. Nutrigenomic regulation of adipose tissue development—Role of retinoic acid: A review. Meat Sci. 2016, 120, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Khalilpourfarshbafi, M.; Gholami, K.; Murugan, D.D.; Abdul Sattar, M.Z.; Abdullah, N.A. Differential effects of dietary flavonoids on adipogenesis. Eur. J. Nutr. 2019, 58, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Bouwman, L.M.S.; Fernández-Calleja, J.M.S.; van der Stelt, I.; Oosting, A.; Keijer, J.; van Schothorst, E.M. Replacing Part of Glucose with Galactose in the Postweaning Diet Protects Female but Not Male Mice from High-Fat Diet-Induced Adiposity in Later Life. J. Nutr. 2019, 149, 1140–1148. [Google Scholar] [CrossRef]
- Delcourt, M.; Tagliatti, V.; Delsinne, V.; Colet, J.-M.; Declèves, A.-E. Influence of Nutritional Intake of Carbohydrates on Mitochondrial Structure, Dynamics, and Functions during Adipogenesis. Nutrients 2020, 12, 2984. [Google Scholar] [CrossRef]
- Cowan, K.J. The Mitochondria: Powerhouse of the Cell. In Functional Metabolism: Regulation and Adaptation; Storey, K.B., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 211–241. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/047167558X.ch8 (accessed on 26 October 2020).
- Javadov, S.; Kuznetsov, A.V. Mitochondria: The cell powerhouse and nexus of stress. Front. Physiol. 2013, 4, 207. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3735979/ (accessed on 26 October 2020). [CrossRef] [Green Version]
- Yang, C.; Ko, B.; Hensley, C.T.; Jiang, L.; Wasti, A.T.; Kim, J.; Sudderth, J.; Calvaruso, M.A.; Lumata, L.; Mitsche, M.; et al. Glutamine Oxidation Maintains the TCA Cycle and Cell Survival during Impaired Mitochondrial Pyruvate Transport. Mol. Cell 2014, 56, 414–424. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.D.; Goetze, A.M.; Bass, R.B.; Flynn, G.C. N-terminal Glutamate to Pyroglutamate Conversion In Vivo for Human IgG2 Antibodies. J. Biol. Chem. 2011, 286, 11211–11217. [Google Scholar] [CrossRef] [Green Version]
- Weber, G.; Banerjee, G.; Ashmore, J. Activities of enzymes involved in glycolysis, glucogenesis and hexosemonophosphate shunt in rat adipose tissue. Biochem. Biophys. Res. Commun. 1960, 3, 182–186. [Google Scholar] [CrossRef]
- Atsumi, T.; Nishio, T.; Niwa, H.; Takeuchi, J.; Bando, H.; Shimizu, C.; Yoshioka, N.; Bucala, R.; Koike, T. Expression of Inducible 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase/PFKFB3 Isoforms in Adipocytes and Their Potential Role in Glycolytic Regulation. Diabetes 2005, 54, 3349–3357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acheson, K.J.; Schutz, Y.; Bessard, T.; Anantharaman, K.; Flatt, J.P.; Jéquier, E. Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. Am. J. Clin. Nutr. 1988, 48, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansson, P.-A.; Smith, U.; Lönnroth, P. Evidence for lactate production by human adipose tissue in vivo. Diabetologia 1990, 33, 253–256. [Google Scholar] [CrossRef] [Green Version]
- DiGirolamo, M.; Newby, F.D.; Lovejoy, J. Lactate production in adipose tissue: A regulated function with extra-adipose implications. FASEB J. 1992, 6, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- King, J.L.; DiGirolamo, M. Lactate production from glucose and response to insulin in perifused adipocytes from mesenteric and epididymal regions of lean and obese rats. Obes. Res. 1998, 6, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.F.; Guichardant, M.; Cozzone, D.; Bernoud-Hubac, N.; Bouzaïdi-Tiali, N.; Lagarde, M.; Géloën, A. Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radic. Biol. Med. 2005, 38, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Hirano, I.; Inui, H.; Yamaji, R. Stereoselective effects of lactate enantiomers on the enhancement of 3T3-L1 adipocyte differentiation. Biochem. Biophys. Res. Commun. 2018, 498, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Sabater, D.; Arriarán, S.; del Mar Romero, M.; Agnelli, S.; Remesar, X.; Fernández-López, J.A.; Alemany, M. Cultured 3T3L1 adipocytes dispose of excess medium glucose as lactate under abundant oxygen availability. Sci. Rep. 2014, 4, 3663. [Google Scholar] [CrossRef] [Green Version]
- Krycer, J.R.; Quek, L.-E.; Francis, D.; Fazakerley, D.J.; Elkington, S.D.; Diaz-Vegas, A.; Cooke, K.C.; Weiss, F.C.; Duan, X.; Kurdyukov, S.; et al. Lactate production is a prioritized feature of adipocyte metabolism. J. Biol. Chem. 2020, 295, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Philp, A.; Macdonald, A.L.; Watt, P.W. Lactate—A signal coordinating cell and systemic function. J. Exp. Biol. 2005, 208, 4561–4575. [Google Scholar] [CrossRef] [Green Version]
- Carrière, A.; Lagarde, D.; Jeanson, Y.; Portais, J.-C.; Galinier, A.; Ader, I.; Casteilla, L. The emerging roles of lactate as a redox substrate and signaling molecule in adipose tissues. J. Physiol. Biochem. 2020, 76, 241–250. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef]
- Baltazar, F.; Afonso, J.; Costa, M.; Granja, S. Lactate Beyond a Waste Metabolite: Metabolic Affairs and Signaling in Malignancy. Front. Oncol. 2020, 10, 231. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2020.00231/full (accessed on 30 September 2020). [CrossRef] [Green Version]
- Petersen, C.; Nielsen, M.D.; Andersen, E.S.; Basse, A.L.; Isidor, M.S.; Markussen, L.K.; Viuff, B.M.; Lambert, I.H.; Hansen, J.B.; Pendersen, S.F. MCT1 and MCT4 Expression and Lactate Flux Activity Increase during White and Brown Adipogenesis and Impact Adipocyte Metabolism. Sci. Rep. 2017, 7, 13101. [Google Scholar] [CrossRef]
- Newton, B.W.; Cologna, S.M.; Moya, C.; Russell, D.H.; Russell, W.K.; Jayaraman, A. Proteomic analysis of 3T3-L1 adipocyte mitochondria during differentiation and enlargement. J. Proteome Res. 2011, 10, 4692–4702. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Sokolov, A.P.; Kondrashova, M.N. Nonezymatic formation of succinate in mitochondria under oxidative stress. Free Radic. Biol. Med. 2006, 41, 56–64. [Google Scholar] [CrossRef]
- Nagai, R.; Brock, J.W.; Blatnik, M.; Baatz, J.E.; Bethard, J.; Walla, M.D.; Thorpe, S.R.; Baynes, J.W.; Frizzell, N. Succination of protein thiols during adipocyte maturation: A biomarker of mitochondrial stress. J. Biol. Chem. 2007, 282, 34219–34228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frizzell, N.; Rajesh, M.; Jepson, M.J.; Nagai, R.; Carson, J.A.; Thorpe, S.R.; Baynes, J.W. Succination of thiol groups in adipose tissue proteins in diabetes: Succination inhibits polymerization and secretion of adiponectin. J. Biol. Chem. 2009, 284, 25772–25781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frizzell, N.; Thomas, S.A.; Carson, J.A.; Baynes, J.W. Mitochondrial stress causes increased succination of proteins in adipocytes in response to glucotoxicity. Biochem. J. 2012, 445, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Kirk, K.; Shurubor, Y.I.; Zhao, D.; Arreguin, A.J.; Shahi, I.; Valsecchi, F.; Primiano, G.; Calder, E.L.; Carelli, V.; et al. Rewiring of glutamine metabolism is a bioenergetic adaptation of human cells with mitochondrial DNA mutations. Cell Metab. 2018, 27, 1007–1025. [Google Scholar] [CrossRef] [Green Version]
- Petrus, P.; Lecoutre, S.; Dollet, L.; Wiel, C.; Sulen, A.; Gao, H.; Tavira, B.; Laurencikiene, J.; Rooyackers, O.; Checa, A.; et al. Glutamine Links Obesity to Inflammation in Human White Adipose Tissue. Cell Metab. 2020, 31, 375–390. [Google Scholar] [CrossRef]
- Brennan, A.M.; Tchernof, A.; Gerszten, R.E.; Cowan, T.E.; Ross, R. Depot-Specific Adipose Tissue Metabolite Profiles and Corresponding Changes Following Aerobic Exercise. Front. Endocrinol. 2018, 9, 759. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6297272/ (accessed on 20 November 2020). [CrossRef] [PubMed]
- Shi, L.; Tu, B.P. Acetyl-CoA and the Regulation of Metabolism: Mechanisms and Consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl Coenzyme A: A Central Metabolite and Second Messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Cooper, D.E.; Cluntun, A.A.; Warmoes, M.O.; Zhao, S.; Reid, M.A.; Liu, J.; Lund, P.J.; Lopes, M.; Garcia, B.A.; et al. Acetate Production from Glucose and Coupling to Mitochondrial Metabolism in Mammals. Cell 2018, 175, 502–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.; Johnston, D.G.; Gill, A.; Barnes, A.J.; Orskov, H. Hormonal regulation of ketone-body metabolism in man. Biochem. Soc. Symp. 1978, 43, 163–182. [Google Scholar]
- Wang, W.; Ishibashi, J.; Trefely, S.; Shao, M.; Cowan, A.J.; Sakers, A.; Lim, H.-W.; O’Connor, S.; Doan, M.T.; Cohen, P.; et al. A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab. 2019, 30, 174–189. [Google Scholar] [CrossRef]


| Total AUC of Doublet at 5.25 ppm | % (Relative to the Mean of the Glucose AUC in HG Media) | |
|---|---|---|
| Glucose EC levels in HG media | 4.87 | 100% |
| Glucose EC levels LG media | 0.97 | 19.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delcourt, M.; Delsinne, V.; Colet, J.-M.; Declèves, A.-E.; Tagliatti, V. Investigation of Mitochondrial Adaptations to Modulation of Carbohydrate Supply during Adipogenesis of 3T3-L1 Cells by Targeted 1H-NMR Spectroscopy. Biomolecules 2021, 11, 662. https://doi.org/10.3390/biom11050662
Delcourt M, Delsinne V, Colet J-M, Declèves A-E, Tagliatti V. Investigation of Mitochondrial Adaptations to Modulation of Carbohydrate Supply during Adipogenesis of 3T3-L1 Cells by Targeted 1H-NMR Spectroscopy. Biomolecules. 2021; 11(5):662. https://doi.org/10.3390/biom11050662
Chicago/Turabian StyleDelcourt, Manon, Virginie Delsinne, Jean-Marie Colet, Anne-Emilie Declèves, and Vanessa Tagliatti. 2021. "Investigation of Mitochondrial Adaptations to Modulation of Carbohydrate Supply during Adipogenesis of 3T3-L1 Cells by Targeted 1H-NMR Spectroscopy" Biomolecules 11, no. 5: 662. https://doi.org/10.3390/biom11050662
APA StyleDelcourt, M., Delsinne, V., Colet, J.-M., Declèves, A.-E., & Tagliatti, V. (2021). Investigation of Mitochondrial Adaptations to Modulation of Carbohydrate Supply during Adipogenesis of 3T3-L1 Cells by Targeted 1H-NMR Spectroscopy. Biomolecules, 11(5), 662. https://doi.org/10.3390/biom11050662







