Hydrocephalus Induced by Intraventricular Peroxiredoxin-2: The Role of Macrophages in the Choroid Plexus
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparation and Intracerebroventricular Injection
2.2. Experimental Groups
2.3. Immunohistochemistry and Immunofluorescence Double Staining
2.4. Magnetic Resonance Imaging and Ventricular Volume Measurement
2.5. Ventricular Wall Damage Measurements and Cell Counting
2.6. Statistical Analysis
3. Results
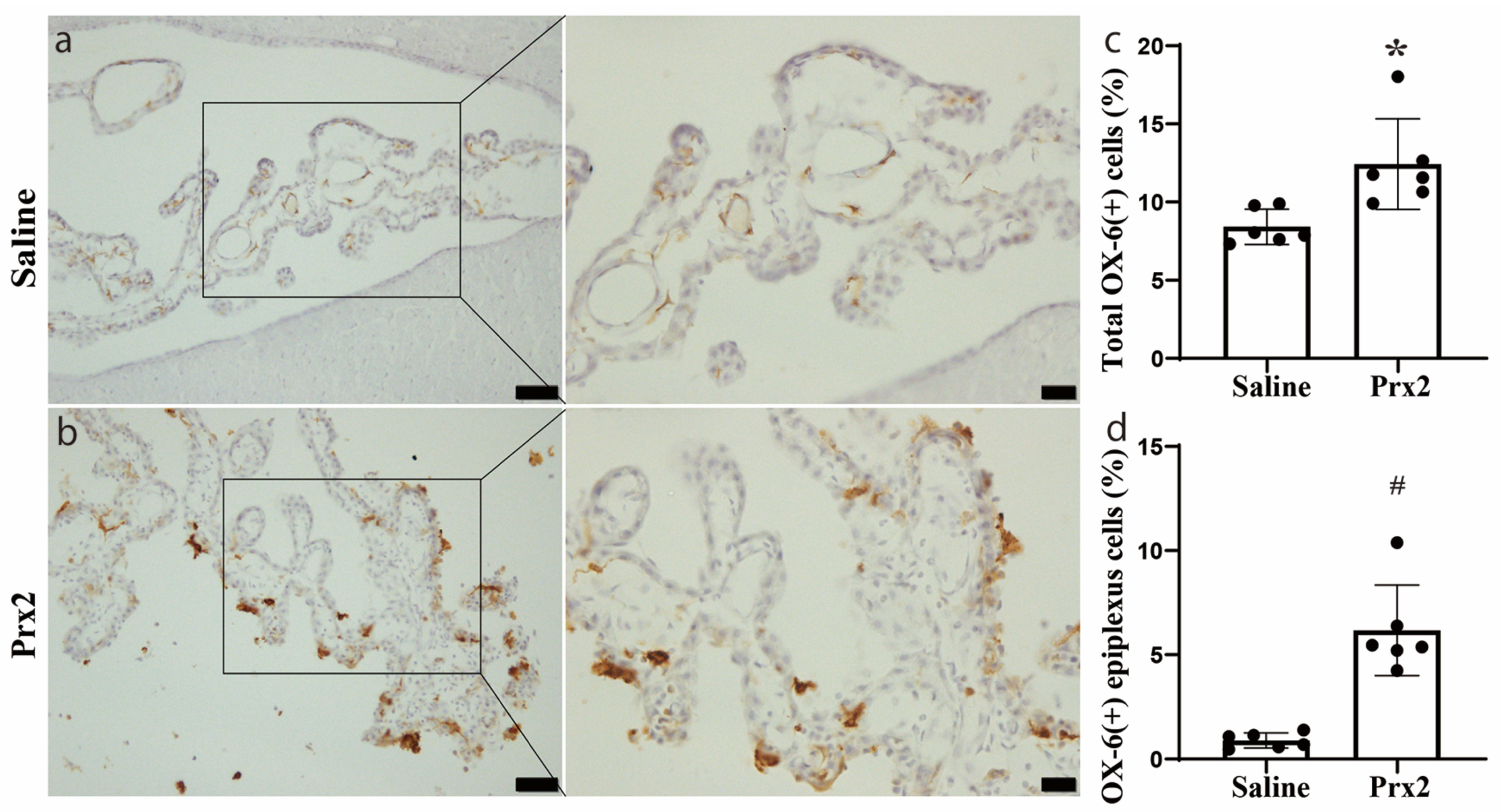
3.1. OX-6 Positive Cells Were Increased after Prx2 Injection
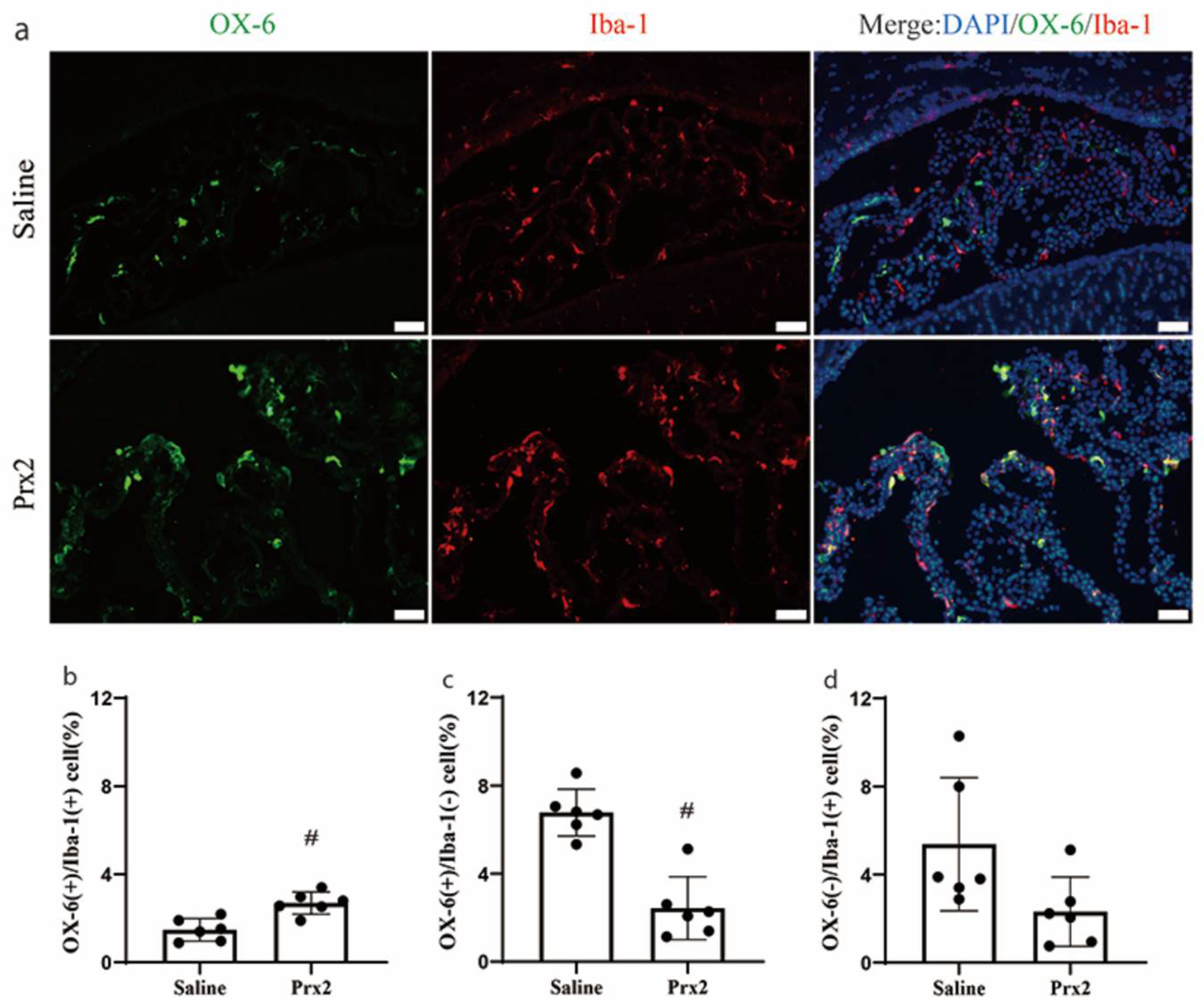
3.2. Immunophenotypic Changes of CP OX-6 Positive Cells after Prx2 Injection
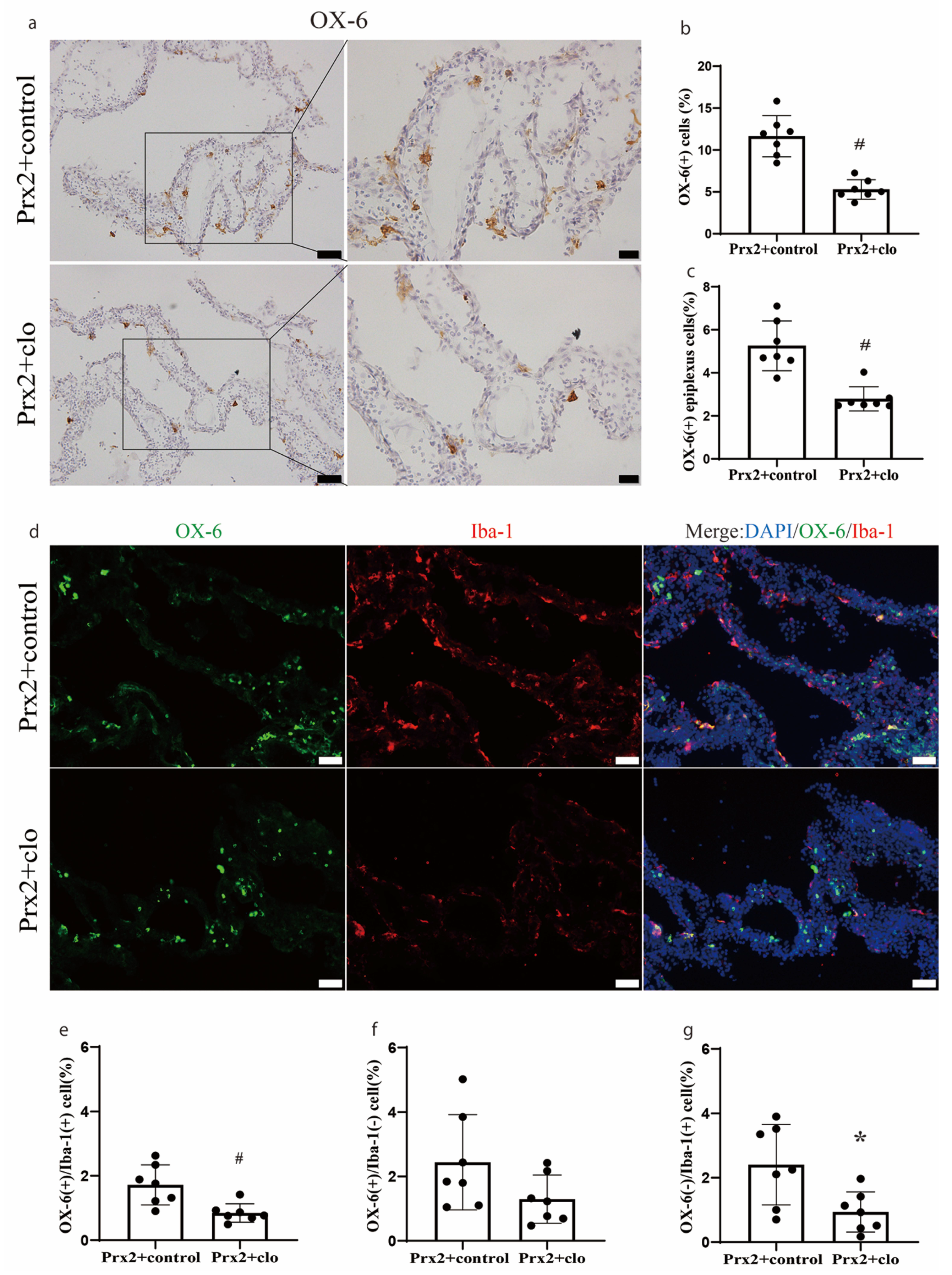
3.3. CP Macrophages Were Depleted by Intracerebroventricular Clodronate Liposome
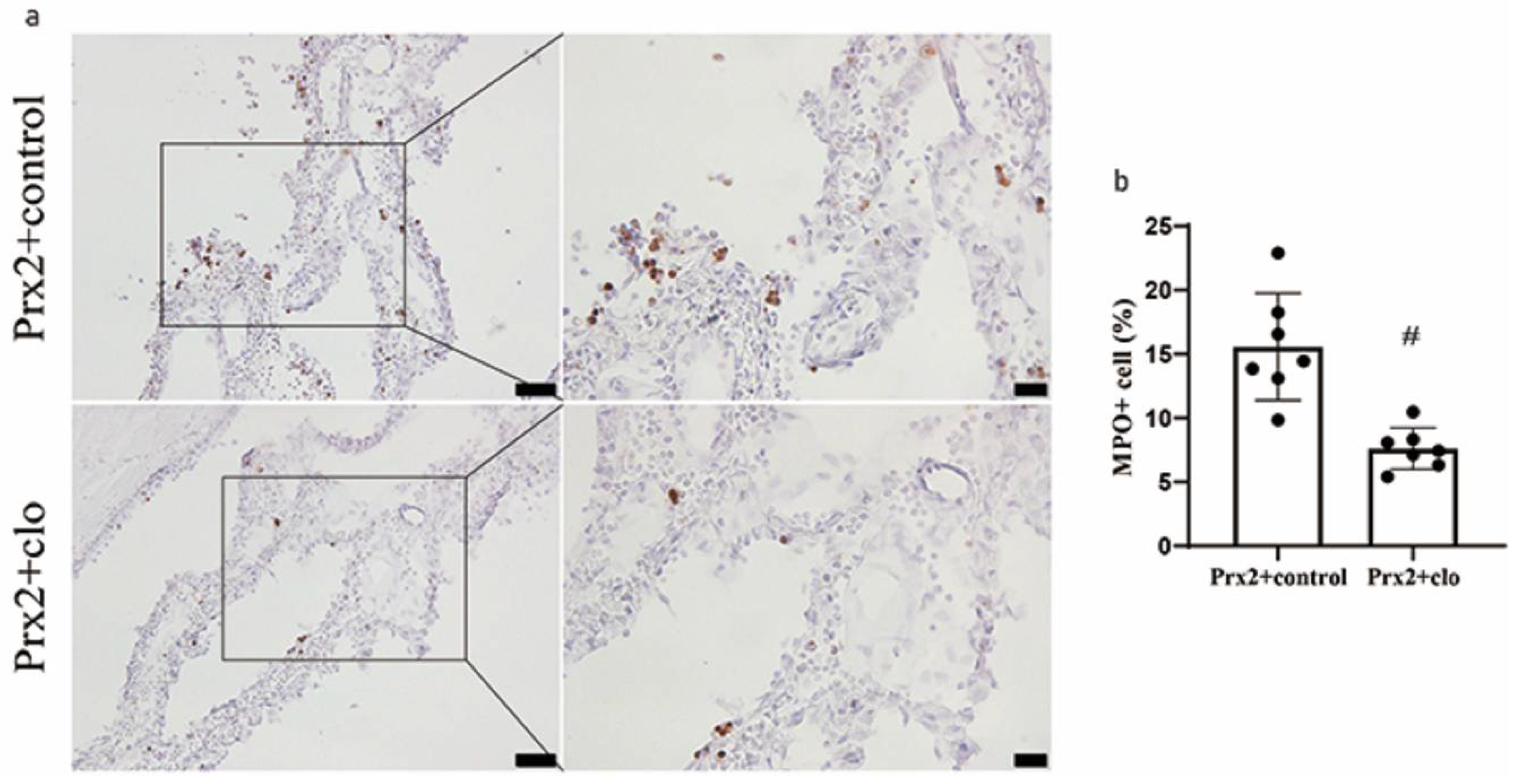
3.4. Macrophage Depletion Attenuated Neutrophil Infiltration in CP
3.5. Macrophage Depletion Attenuated Hydrocephalus and Ventricular Wall Damage
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| (+) | positive |
| (−) | negative |
| APCs | Antigen-presenting cells |
| CNS | Central nervous system |
| CPs | Choroid plexuses |
| DCs | Dendritic cells |
| Iba-1 | Ionized calcium-binding adaptor molecule 1 |
| Icv | Intracerebroventricular |
| MRI | Magnetic resonance imaging |
| MHC II | Major histocompatibility complex II |
| MPO | Myeloperoxidase |
| Prx2 | Peroxiredoxin 2 |
| RBCs | Red blood cells |
References
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat. Rev. Neurosci. 2015, 16, 445–457. [Google Scholar] [CrossRef]
- Gu, C.; Hao, X.; Li, J.; Hua, Y.; Keep, R.F.; Xi, G. Effects of minocycline on epiplexus macrophage activation, choroid plexus injury and hydrocephalus development in spontaneous hypertensive rats. Br. J. Pharmacol. 2019, 39, 1936–1948. [Google Scholar] [CrossRef]
- Karimy, J.K.; Zhang, J.; Kurland, D.B.; Theriault, B.C.; Duran, D.; Stokum, J.A.; Furey, C.G.; Zhou, X.; Mansuri, M.S.; Montejo, J.; et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat. Med. 2017, 23, 997–1003. [Google Scholar] [CrossRef]
- Sarafian, T.A.; Verity, M.A.; Vinters, H.V.; Shih, C.C.; Shi, L.; Ji, X.D.; Dong, L.; Shau, H. Differential expression of peroxiredoxin subtypes in human brain cell types. J. Neurosci. Res. 1999, 56, 206–212. [Google Scholar] [CrossRef]
- Salzano, S.; Checconi, P.; Hanschmann, E.-M.; Lillig, C.H.; Bowler, L.D.; Chan, P.; Vaudry, D.; Mengozzi, M.; Coppo, L.; Sacre, S.; et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. USA 2014, 111, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Hua, Y.; Garton, H.J.L.; Novakovic, N.; Keep, R.F.; Xi, G. Activation of epiplexus macrophages in hydrocephalus caused by subarachnoid hemorrhage and thrombin. CNS Neurosci. Ther. 2019, 25, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Chen, J.; Keep, R.F.; Xi, G.; Hua, Y. Prx2 (Peroxiredoxin 2) as a Cause of Hydrocephalus After Intraventricular Hemorrhage. Stroke 2020, 51, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.G. Depleting Macrophages In Vivo with Clodronate-Liposomes. Methods Mol. Biol. 2018, 1784, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Bian, L.; Wang, M.; Keep, R.F.; Xi, G.; Hua, Y. Enhancement of Hematoma Clearance with CD47 Blocking Antibody in Experimental Intracerebral Hemorrhage. Stroke 2019, 50, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Faraco, G.; Sugiyama, Y.; Lane, D.; Garcia-Bonilla, L.; Chang, H.; Santisteban, M.M.; Racchumi, G.; Murphy, M.; Van Rooijen, N.; Anrather, J.; et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J. Clin. Investig. 2016, 126, 4674–4689. [Google Scholar] [CrossRef] [PubMed]
- Du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol. 2020, 40, 1769–1777. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, C.; Hua, Y.; Keep, R.F.; Muraszko, K.; Xi, G. Role of Iron in Brain Injury After Intraventricular Hemorrhage. Stroke 2011, 42, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Okubo, S.; Strahle, J.; Keep, R.F.; Hua, Y.; Xi, G. Subarachnoid Hemorrhage-Induced Hydrocephalus in Rats. Stroke 2013, 44, 547–550. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, P.G. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J. Comp. Neurol. 1999, 405, 553–562. [Google Scholar] [CrossRef]
- Matyszak, M.; Lawson, L.; Perry, V.; Gordon, S. Stromal macrophages of the choroid plexus situated at an interface between the brain and peripheral immune system constitutively express major histocompatibility class II antigens. J. Neuroimmunol. 1992, 40, 173–181. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, S.; Konings, J.; Van Der Pol, S.; Kamermans, A.; Amor, S.; Van Horssen, J.; Witte, M.E.; Kooij, G.; De Vries, H.E. Inflammation of the choroid plexus in progressive multiple sclerosis: Accumulation of granulocytes and T cells. Acta Neuropathol. Commun. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012, 12, 623–635. [Google Scholar] [CrossRef]
- Worbs, T.; Hammerschmidt, S.I.; Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2016, 17, 30–48. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Broeren, C.P.; Wauben, M.H.; Lucassen, M.A.; Van Meurs, M.; Van Kooten, P.J.; Boog, C.J.; Claassen, E.; Van Eden, W. Activated rat T cells synthesize and express functional major histocompatibility class II antigens. Immunology 1995, 84, 193–201. [Google Scholar]
- Llovera, G.; Benakis, C.; Enzmann, G.; Cai, R.; Arzberger, T.; Ghasemigharagoz, A.; Mao, X.; Malik, R.; Lazarevic, I.; Liebscher, S.; et al. The choroid plexus is a key cerebral invasion route for T cells after stroke. Acta Neuropathol. 2017, 134, 851–868. [Google Scholar] [CrossRef]
- Strominger, I.; Elyahu, Y.; Berner, O.; Reckhow, J.; Mittal, K.; Nemirovsky, A.; Monsonego, A. The Choroid Plexus Functions as a Niche for T-Cell Stimulation within the Central Nervous System. Front. Immunol. 2018, 9, 1066. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.M.; Rothwell, N.J.; Le Feuvre, R.A. CNS injury: The role of the cytokine IL-1. Veter J. 2004, 168, 230–237. [Google Scholar] [CrossRef]
- Pedragosa, J.; Salas-Perdomo, A.; Gallizioli, M.; Cugota, R.; Miró-Mur, F.; Briansó, F.; Justicia, C.; Pérez-Asensio, F.; Marquez-Kisinousky, L.; Urra, X.; et al. CNS-border associated macrophages respond to acute ischemic stroke attracting granulocytes and promoting vascular leakage. Acta Neuropathol. Commun. 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Moxon-Emre, I.; Schlichter, L.C. Neutrophil Depletion Reduces Blood-Brain Barrier Breakdown, Axon Injury, and Inflammation After Intracerebral Hemorrhage. J. Neuropathol. Exp. Neurol. 2011, 70, 218–235. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Tan, X.; Xia, F.; Hua, Y.; Keep, R.F.; Xi, G. Hydrocephalus Induced by Intraventricular Peroxiredoxin-2: The Role of Macrophages in the Choroid Plexus. Biomolecules 2021, 11, 654. https://doi.org/10.3390/biom11050654
Chen T, Tan X, Xia F, Hua Y, Keep RF, Xi G. Hydrocephalus Induced by Intraventricular Peroxiredoxin-2: The Role of Macrophages in the Choroid Plexus. Biomolecules. 2021; 11(5):654. https://doi.org/10.3390/biom11050654
Chicago/Turabian StyleChen, Ting, Xiaoxiao Tan, Fan Xia, Ya Hua, Richard F. Keep, and Guohua Xi. 2021. "Hydrocephalus Induced by Intraventricular Peroxiredoxin-2: The Role of Macrophages in the Choroid Plexus" Biomolecules 11, no. 5: 654. https://doi.org/10.3390/biom11050654
APA StyleChen, T., Tan, X., Xia, F., Hua, Y., Keep, R. F., & Xi, G. (2021). Hydrocephalus Induced by Intraventricular Peroxiredoxin-2: The Role of Macrophages in the Choroid Plexus. Biomolecules, 11(5), 654. https://doi.org/10.3390/biom11050654




