Abstract
Parkinson’s disease (PD) usually presents in older adults and typically has both motor and non-motor dysfunctions. PD is a progressive neurodegenerative disorder resulting from dopaminergic neuronal cell loss in the mid-brain substantia nigra pars compacta region. Outlined here is an integrative medicine and health strategy that highlights five treatment options for people with Parkinson’s (PwP): rehabilitate, therapy, restorative, maintenance, and surgery. Rehabilitating begins following the diagnosis and throughout any additional treatment processes, especially vis-à-vis consulting with physical, occupational, and/or speech pathology therapist(s). Therapy uses daily administration of either the dopamine precursor levodopa (with carbidopa) or a dopamine agonist, compounds that preserve residual dopamine, and other specific motor/non-motor-related compounds. Restorative uses strenuous aerobic exercise programs that can be neuroprotective. Maintenance uses complementary and alternative medicine substances that potentially support and protect the brain microenvironment. Finally, surgery, including deep brain stimulation, is pursued when PwP fail to respond positively to other treatment options. There is currently no cure for PD. In conclusion, the best strategy for treating PD is to hope to slow disorder progression and strive to achieve stability with neuroprotection. The ultimate goal of any management program is to improve the quality-of-life for a person with Parkinson’s disease.
1. Introduction
It is estimated that one million people in the United States are living with Parkinson’s disease (PD), with approximately 60,000 new cases diagnosed nationally each year [1,2,3,4,5]. The global prevalence of PD is believed to be up to 10 million people. PD symptoms occur due to the progressive loss of dopamine-producing neurons in the substantia nigra pars compacta region of the brain. Symptoms typically occur gradually over several years, making diagnosis challenging [5]. PD is traditionally characterized as a motor system disorder with four cardinal symptoms: bradykinesia (slowness of movement); rigidity (stiffness of the limbs and trunk); postural instability (impaired balance and coordination); and tremor (trembling in hands, arms, legs, and face) [6,7,8,9]. Though not as visible as these motor symptoms, non-motor symptoms are also experienced by many PwP as a part of their disease. The most common non-motor symptoms of PD include constipation, urinary dysfunction, depression, psychosis, apathy, and sleep disorders [9,10,11,12].
PD occurs most commonly in people aged over 60 years old [5]. In this group, most cases of PD occur sporadically and due to etiologies including neuroinflammation and oxidative stress, dysfunction of the innate and/or adaptive immune systems, mitochondrial activity disruption, genetic mutation, intracellular protein denaturation and aggregation, and environmental factors [1,2,3,4,5]. Interestingly, cases of PD in younger people are usually linked to particular genotypes [13]. At present, PD remains an incurable disease. As such, treatment goals in PD management center on slowing or halting disease progression [1,14]. The complexity of the factors that contribute to the development of the major sporadic form of PD demands a multi-pronged therapeutic intervention and plan to halt or slow PD progression. Accordingly, this review aims to describe a comprehensive and integrative treatment protocol for PD.
2. Treatment Plan for PD
The traditional approach for treating PD typically begins with a pharmacologic dopamine replacement strategy [1,5,14,15]. The first line for such therapy is either daily oral carbidopa/levodopa or a dopamine agonist. Some drugs prolong the lifetime of endogenous dopamine. Either along with or alternative to dopamine replacement, complementary and alternative medicine (CAM) and integrative medicine approaches are used by many to improve brain and overall health in PwP [16,17,18,19,20,21]. Lifestyle modifications can provide therapeutic benefits, as different forms of strenuous aerobic exercise are neuroprotective, on top of the general quality-of-life (QoL) benefits offered by regular exercise [10,12,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. PD is a complicated disorder such that two PwP might have different symptoms with varied rates of progression and likely follow different treatment strategies, despite diagnosis with the same disease. Non-motor symptoms are prevalent in PD. Thus, PwP must communicate clearly with their healthcare team to address these issues and treat PD’s motor and non-motor symptoms.
The treatment plan described here incorporates these aforementioned traditional and non-traditional approaches that could help keep PD from progressing. The treatment strategy complements these options with an initial rehabilitation program to carefully assess and address PD before beginning other treatment options and with surgery, in cases where PD has resisted management by the other treatment options. Each of the five steps of this treatment plan for PD offers a comprehensive healthcare strategy and therapy that has been studied or found to be effective in either human or rodent animal studies. Described here is an integrative medicine and health strategy for PD that features five treatment options: rehabilitate, therapy, restorative, maintenance, and surgery (Figure 1).
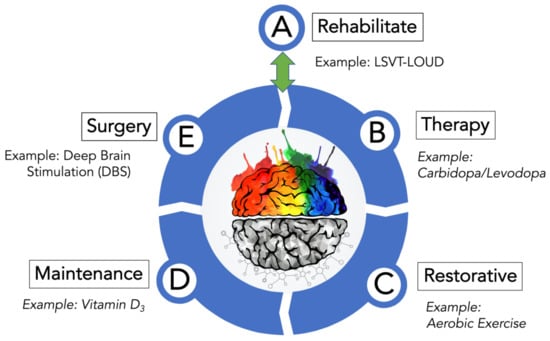
Figure 1.
Treatment options for PD. As depicted, this PD-directed integrative and health strategy features five areas where intervention can be used to manage the numerous symptoms of PD. A treatment example from each category is given. Although drawn as a stepwise progression from A–E, this is not suggesting that PwP engage all five treatment options at all or in order of their presentation (Figure 1). Treatment option A (Rehabilitate) is considered a reversible entry and exit point to the treatment wheel described by options B (Therapy), C (Restorative), D (Maintenance), and E (Surgery). Please note that the various treatment options are not isolated silos that can only be accessed after fulfilling the prior treatment option. It is more likely that PwP, with guidance and advice from movement disorder neurologists, would likely use several components found in treatment options A–D that may change as their disorder progresses before possibly moving finally to option E (Surgery). Post-surgery-PD patients, assuming a successful response to surgery and under continual guidance from their medical team, would re-engage the A–D treatment options. Furthermore, aspects of the Therapy, Restorative, and Maintenance treatment options overlap and complement one another to develop an effective PD treatment plan.
2.1. Rehabilitate Options for Treating PD
Before PwP begin pharmacological therapy for PD, movement disorder specialists are likely to recommend PwP visit a physical therapist, occupational therapist, or a speech pathology therapist. The goal of such consultation would be to begin management of some of the altered motor symptoms. The reduction of dopamine in PD typically softens the voice and limits the body movements of PwP. Two programs called LSVT-LOUD [43,44,45] and LSVT-BIG [31,46,47,48] are directly targeted to helping the PwP speak louder and make larger movements, respectively.
The majority of PwP have speech/voice dysfunction negatively impact communication. LSVT (Lee Silverman Voice Treatment)-LOUD enhances the voice, increases vocal loudness (by improving articulation, vocal quality, and intonation), and positively alters PwP functional skills communication [43,44,45]. PwP also typically have a movement that is slow (bradykinesia) and hesitant (akinesia) with smaller amplitude (hypokinesia). LSVT-BIG uses intensive exercises of large-amplitude movements to overcome bradykinesia and hypokinesia in PwP [31,46,47,48]. Furthermore, LSVT-BIG yields movement focused on amplitude, resulting in bigger, faster, and increased movement precision.
LSVT-LOUD and LSVT-BIG require a neurologist trained to administer the programs, as are the physical therapist (LSVT-BIG) and speech pathology therapist (LSVT-LOUD) certified to oversee them. Both programs are for one hour per day, four days/week, for a total of four weeks. Afterwards, PwP can use these exercises from each program to continue on their own. While there are other programs that provide similar assistance, LSVT-LOUD [43,44,45] and LSVT-BIG [31,46,47,48] pioneered these programs specifically to help rehabilitate PwP [49].
An essential feature of rehabilitation is regular exercise. As mentioned above, many of the motor defects associated with PD cause stiffness, impaired balance, and slow movement. Under the ongoing guidance of their movement disorder neurologists, PwP should develop a regular exercise routine that incorporates stretching, movement, strength training, and aerobic exercise. There are many well-trained physical therapists with expertise in exercise-specific routines for PD. There are also numerous exercise modalities that PwP use to improve their QoL, including PWR!Moves, Rock Steady Boxing, and Dance for PD programs, power walking with poles, stationary biking, tai chi, and yoga [8,16,22,24,50,51,52]. Thus, rehabilitation therapy should be utilized throughout all stages of the disorder.
2.2. Therapy Options for Treating Motor Symptoms of PD
Dopamine has the chemical structure of 3,4-dihydroxyphenethylamine and is a member of the catecholamine and phenethylamine molecular families [53]. Like other neurotransmitters, dopamine delivers messages throughout the central nervous system (CNS) [54]. Dopamine is a derivative of the amino acid tyrosine (Tyr), where the enzyme tyrosine hydroxylase converts Tyr to levodopa (DOPA) [53,54]. From there, DOPA decarboxylase removes carbon dioxide from DOPA to produce dopamine [53,54]. The structures of dopamine and its precursors and some dopamine-regulating therapeutics are shown in Figure 2.
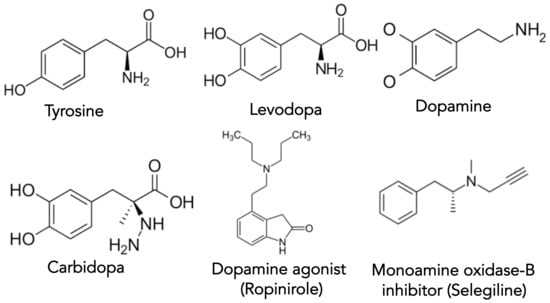
Figure 2.
Structures of some of the key molecules involved in dopamine synthesis, drug therapy, and PD.
Table 1 gives a list of the majority of drugs approved by the FDA that are available to treat the motor symptoms of PD [55]. As PD is first and foremost a disorder of dopamine deficiency, dopamine replacement remains the standard therapeutic aim [56]. The combination of levodopa with carbidopa, an aromatic l-amino acid decarboxylase inhibitor, provides the most significant amount of symptomatic relief with the least adverse side-effects in treating PD [57,58]. The addition of carbidopa prevents the conversion of levodopa (i.e., DOPA) to dopamine in peripheral tissues, allowing for a successful transport of levodopa to the CNS [14]. Interestingly, the blood-brain barrier allows levodopa access into the CNS but denies entry to both dopamine and carbidopa (compare molecular structural differences in Figure 2). There are multiple formulations for carbidopa/levodopa tablets (Table 1). Alternatively, Duodopa is a continuously infused intrajejunal gel of carbidopa/levodopa [59]. Additionally, subcutaneous infusion of carbidopa/levodopa is under evaluation [60,61]. The major side-effects of carbidopa/levodopa are the development over time of dyskinesia and fluctuating ‘off-on’ periods of effectiveness [5]. The potential neurotoxicity of carbidopa/levodopa has been suggested [62]. However, Ahlskog recently reviewed and refuted the evidence that carbidopa/levodopa is neurotoxic [63].

Table 1.
Therapeutic Options for Treating the Motor Symptoms of Parkinson’s Disease.
Dopamine agonists mimic dopamine by binding to dopamine receptors in the CNS. They are used as a monotherapy for many PwP early in the treatment of their disease [64]. The original drug used to treat PD is apomorphine injection, a dopamine agonist, which is comparable in effect to levodopa but it has a shorter duration time [65]. Dopamine agonists are also frequently paired in combination with carbidopa/levodopa, especially as a “bridge” to stabilize the on–off periods PwP may experience on long-term carbidopa/levodopa therapy [1,14]. There are multiple dopamine agonists with both immediate- and extended-release forms (Table 1). There are several troubling side-effects that a minority of PwP encounter with dopamine agonists, mostly centered around impulse control disorders (e.g., pathologic gambling, shopping, internet use, and hypersexuality) [1,14].
Monoamine oxidase B (MAO-B) inhibitors are substances that inactivate the enzyme responsible for the inactivation of dopamine [66]. The MAO-B inhibitors Selegiline and Safinamide are used adjunctively with carbidopa/levodopa while Rasagiline is used either as monotherapy or in concert with carbidopa/levodopa (Table 1) [5]. MAO-B inhibitors may provide relief from symptoms as they help regulate the degradation of dopamine in peripheral tissue, which leads to increased half-life and availability of levodopa in the CNS. The SELEDO (from Selegiline plus Levodopa) study was a 5-year trial to assess the potential advantage of combining Selegiline and Levodopa in PD [67]. In treating early-stage PD, the combination of Selegiline and Levodopa was better than Levodopa alone.
Inhibitors of cathecol-O-methyl transferase (COMT) enzymes prevent the processing of levodopa to 3-O-methyldopa [68,69]. COMT inhibitors increase the half-life of levodopa, allowing more levodopa remain in the patient’s CNS for a longer period of time. Similar in concept to the MAO-B inhibitors but different in mechanism, COMT inhibitors preserve levodopa in PwP experiencing motor fluctuations with carbidopa/levodopa therapy. Similar to MAO-B inhibitors, COMT inhibitors can be used adjunctively with carbidopa/levodopa [1,5].
The history behind the use of Amantadine in PD is fascinating [70,71]. Amantadine was made and used initially as an anti-influenza medication. It turns out that PwP taking Amantadine to prevent the flu showed better control over their tremor. Amantadine provides help with most PD motor symptoms and it might be useful in PwP who have a prominent tremor or levodopa-induced dyskinesia.
2.3. Therapy Options for Treating Non-Motor Symptoms of PD
PD can also be considered a neuropsychiatric disorder [72]. Several neuropsychiatric symptoms are related to emotional and cognitive problems [73]. The neuropsychiatric symptoms are a significant disruption that contributes to disability in PwP [74,75]. There are symptoms related to the disease itself, including apathy, depression, and anxiety [76,77]. These non-motor symptoms are frequently present in the earliest PD stages, even preceding the origination of the motor symptoms. This suggests that both non-motor and motor-related symptoms of PD are associated with reduced dopaminergic production.
The second type of PD neuropsychiatric symptoms exists as a side effect of dopaminergic replacement therapy [73,78]. The impact of the medication can result in addiction, hypomania, nocturnal hyperactivity, and punding [73,78]. Managing the non-motor symptoms of PD presents a challenge to the physician because they must differentiate the contribution from medication, disorder progression, and the PD patient’s emotional state.
Table 2 lists most of the drugs approved by the FDA that are available to treat the non-motor symptoms of PD related to depression and anxiety, excessive drooling, and gastrointestinal problems [79,80,81,82,83]. Depression occurs in up to 50% of PwP at some point during the disorder [84]. PwP with depression are typically treated with a standard antidepressant from the class of SSRIs, SNRIs, and other similar neurotransmitter reuptake inhibitors (Table 2). Anxiety in PD takes many forms but is generally described as feelings of worry, panic, unease, and jitteriness [84]. Besides psychotherapy, medication options include SSRIs, and Buspirone appears to deal with generalized anxiety effectively. The benzodiazepine compounds are also effective at reducing symptoms of panic and worry (Table 2).

Table 2.
Therapeutic Options for Treating Non-Motor Symptoms of Parkinson’s Disease: Depression and Anxiety, Drooling, and Gastrointestinal Problems.
Sialorrhea, or excessive drooling, occurs not from making too much saliva but from the slowing of the swallowing reflect the action that routinely happens [85]. Several forms of treatment range from atropine drops, Botulinum toxin, and glycopyrrolate (an oral anticholinergic) (Table 2). Constipation is a common gastrointestinal problem in PD [86]. PwP should follow a good diet and preventative maintenance (e.g., drink plenty of fluids, use dietary fiber products). Several medications can be used for treating constipation (Table 2), but Reglan, Compazine, and Phenergan should be avoided since they are dopamine-blocking compounds.
Table 3 gives a list of the majority of drugs approved by the FDA that are available to treat the non-motor symptoms of PD related to dementia and psychosis, sleep disorders, cognition, orthostatic hypotension, and urinary incontinence [79,80,81,82,83]. Mild cognitive impairment that progresses to dementia is a major concern to PwP [87]. The group of acetylcholinesterase inhibitors (Donepezil, Galantamine, and Rivastigmine) are frequently used in treating PwP for cognitive impairment and dementia. If PwP begin experiencing visual hallucinations or delusions, in addition to other symptoms of psychosis [88], besides a detailed assessment by the healthcare team, Pimavanserin, Clozapine, and Quetiapine have been used in PD. An interesting side effect of Clozapine is an anti-tremor effect in PD [89,90].

Table 3.
Therapeutic Options for Treating Non-Motor Symptoms of Parkinson’s Disease: Dementia and Psychosis, Sleep Disorders, Cognition, Orthostatic Hypotension, and Urinary Incontinence.
There are many different forms of sleep disorders in PD [91]. Sleeping disorders can range from poor tremor control and reduced bed mobility, restless leg syndrome, insomnia and rapid-eye-movement (REM)-sleep behavior (RBD). A related and frequent coexisting problem in PD is obstructive sleep apnea, which is evaluated by an overnight evaluation in a sleep laboratory. Combined with sleeping disorders is a widespread occurrence of excessive daytime sleepiness. The healthcare team will carefully assess treatment strategies to describe the sleeping history of the PD patient.
Orthostatic hypotension is a significant decrease in blood pressure when someone rises from a sitting or lying position to a standing position [92]. Orthostatic hypotension can be enhanced by dopamine agonists and carbidopa/levodopa [93]. As mentioned above for sleep disorders, a careful history by the healthcare team may decide to lower the motor-symptom medication, change in lifestyle (e.g., drink more fluids, wear support stockings) before moving on to medications that increase blood pressure. The loss of bladder control (urinary incontinence) and urinary urgency/frequency are relatively common issues in PD [94,95]. There are several types of medications that help regain control of the bladder. However, the healthcare team needs to fully assess this non-motor PD problem before using medication to relax the bladder (e.g., exclude urinary tract infection and enlarged prostate in men).
2.4. Restorative Options for Treating PD
One key goal for any PD treatment strategy is to achieve some form of neuroprotection. Of the therapeutic compounds featured in Table 1, there is evidence of neuroprotection from only Selegiline (Eldepryl, Zelapar) and Rasagiline (Azilect) in cell culture and rodent models of PD [96]. In the DATATOP (Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism) study, researchers showed a significant but small symptomatic benefit for Selegine; however, it was not classified as truly neuroprotective [97,98]. Azilect was studied in large follow-up clinical studies, named ADAGIO (Attenuation of Disease progression with Azilect Given Once-daily) [99] and TEMPO (Rasagiline in Early Monotherapy for Parkinson’s Disease Outpatients) [100]. Ultimately, the FDA did not give these studies approval to label Rasagiline as neuroprotective. Thus, the long-term neuroprotective effect for Selegiline and Rasagiline remains an open question.
The potential for exercise to be neuroprotective in PD has been widely studied [10,12,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. There is emerging evidence that neuroinflammation contributes to the progression of PD [101,102]. Exercise is believed to help mitigate this pathogenic effect and so has been studied as a potential therapy. Exercise studies of a mouse model of PD found that exercise preserved remaining dopaminergic neurons and was associated with both an increase in brain-derived neurotropic factors and a reduction of pro-inflammatory markers [32,103,104,105]. Collectively, the animal results have shown that strenuous aerobic exercise is neuroprotective in PD through the inhibition of alpha-synuclein accumulation. Furthermore, the animal studies have suggested strenuous aerobic exercise promotes both anti-oxidation and anti-inflammatory properties not only in the brain but systemically. Human studies have shown that moderate exercise improves QoL in PwP and that strenuous aerobic exercise likely has a neuroprotective effect in PD [22,25,46,106,107].
Stress and mindfulness studies are beginning to demonstrate that being mindful can substantially benefit PwP [108,109]. Exercise studies have also been performed to address non-motor symptoms in PD [10,11,12,35,50]. Aerobic exercise continues to be a key treatment in potentially being neuroprotective in PD [110,111]; however, as mentioned before, there are several exercise routines that improve QoL in PD [8,16,22,24,50,51,52].
There is a growing interest in trying to understand the interplay between muscle and bone factors synthesized in response to exercise [112,113,114]. Exercise has been found to promote substances that have been termed ‘exerkines’, shown to influence homeostasis [115,116]. One recently described exerkine is interleukin-13 (IL-13), which is produced in mouse skeletal tissue and increases with exercise [117,118]. The impact of exercise led to the increased synthesis of IL-13 that promoted endurance in the animal. This and other related findings suggest these circulating bioactive substances may cross the blood-brain barrier and possibly offer protection from PD and other neurodegenerative disorders.
2.5. Maintenance Options for Treating PD
With the exception of strenuous aerobic exercise, there is no known therapy or drug treatment regimen found to slow down PD progression. In this absence of many neuroprotective options, numerous PwP have turned to a CAM and integrative medicine strategy [16,17,18,19,20,21]. Such strategies aim to preserve remaining dopaminergic neurons with the added potential to reduce neuroinflammation, maintaining PD from progressing further. Discussed below are the potential benefit of various compounds in PD maintenance.
There is renewed interest in understanding how vitamin D3 impacts the progression of PD [119,120,121,122]. PwP with deficiencies in vitamin D3 have impaired motor function and increased disease severity [120,122]. PwP with higher levels of vitamin D3 had better cognitive performance with improved verbal fluency and verbal memory [121]. Magnesium l-threonate has been shown to cross the blood-brain barrier and may assist dopaminergic cell survival [123,124,125]. Vitamin B1 has been found to generally support cognition and is vital for healthy nerves [126,127,128]. Taurine has been shown to down-regulate pro-inflammatory microglial cells in a mouse model of PD [129,130,131].
The ultimate goal of CAM therapy is to help maintain the health of remaining dopaminergic cells healthy in PwP. Curcumin is a well-known polyphenol with antioxidant properties [132,133,134]. Curcumin both inhibits NFkB and MAPK and prevents free radical damage. Alpha lipoic acid and acetyl-l-carnitine have been shown to reverse and partially restore mitochondrial function and reduce oxidative vulnerability in experiments using aging mice [135,136,137,138]. This suggests that these compounds may offer benefit to the aging population. N-acetyl-cysteine (NAC), one of the building blocks of the important antioxidant glutathione, crosses the blood-brain barrier and reduces oxidative stress. However, NAC also contributes to the reducing potential of the brain, as NAC is thought to be the rate-limiting factor in the production of glutathione [139,140]. Trans-resveratrol is a potent antioxidant capable of reducing free radicals in the environment of the brain [141,142]. Finally, melatonin regulates the sleep-wake cycle and also has been shown to protect mitochondria [143]. Collectively, these compounds may help preserve remaining dopaminergic neurons in the PD brain. Their molecular structures are depicted in Figure 3.
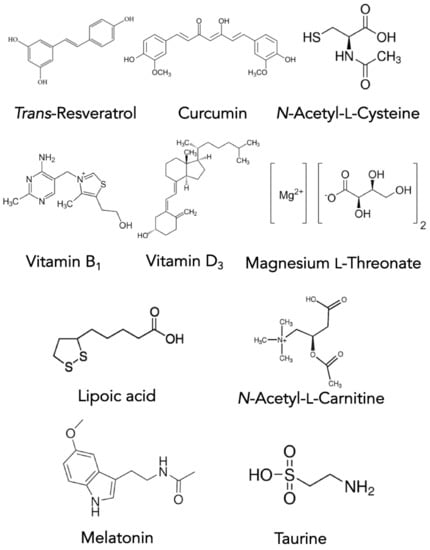
Figure 3.
Structures of the CAM maintenance compounds used for PD treatment.
2.6. Surgery Options for Treating PD
Deep brain stimulation (DBS) is the surgery used for PD treatment typically in PwP suffering long-term complications from carbidopa/levodopa therapy. Currently, DBS surgery for PD is considered reversible, as brain tissue is not destroyed, stimulation can be adjusted as the disease progresses, and DBS can be performed bilaterally without significant increase in adverse events [144,145,146,147,148].
Refinement of the DBS technique has led to increased understanding of the connection between basal ganglia and PD pathophysiology [149,150,151]. Typically, DBS targets three structures in the brain, specifically the thalamus, globus pallidus, and subthalamic nucleus [144,145,146,147,148]. PwP are recommended for DBS surgery because of motor complications that do not respond further to medical therapy [5]. It should be noted that the PD patient still has a good response to carbidopa/levodopa, but this is complicated by excessive dyskinesia.
3. PD Clinical Trials in Progress and Novel (and Emerging) PD Therapies
MacFarthing et al. recently reviewed the current number of clinical trial pipelines with a goal of either stopping, slowing, or reversing PD [152]. They found 145 registered and ongoing clinical trials targeting PD spread out among Phase 1, Phase 2, and Phase 3. Interestingly, 57 clinical trials are aimed at long-term disease modifying therapies while 88 clinical trials are centered on symptomatic relief. Furthermore, 50 of these clinical trials are testing repurposed therapies [152]. One recent approved repurposed therapy is a new inhaled version of levodopa (Inbrija), approved by the FDA in December 2018. Two such compounds considered disease modifying therapy include Exenatide and glial cell-derived neurotrophic factor (GDNF). Exenatide (currently there are five clinical trials) is a glucagon-like peptide-1 receptor agonist that reduces glucose in type 2 diabetes; however, early results suggest that Exenatide is neuroprotective in PD [152]. Based on neurorestorative and neuroprotective effects in animal models of PD, there are currently three clinical trial evaluating the influence of GDNF in PD [152]. This detailed review of on-going clinical trials by MacFarthing et al. offers some encouraging results, describing a wide range of therapeutic approaches in multiple phases of clinical testing and evaluation in PD [152].
The transplantation of neuronal cells (fetal midbrain tissue) into PD patients’ brains has led to various stem cell therapy forms [153,154]. There are currently two clinical trials using embryonic stem cells (ESC) therapy for PD (in Australia and China). A clinical trial was recently started in Japan using induced pluripotent stem cell (iPSC)-derived dopaminergic neurons [155]. Cell-based therapy offers a chance to renew and replace dopaminergic neurons in PD; therefore, there is much interest in these necessary clinical trials’ safety and outcome.
The red-light-helmet for treating PD is under investigation [156,157,158], which uses a helmet lined with light-emitting diodes (LEDs) of wavelengths across the red to near-infrared range (i.e., 670, 810, and 850 nm) with or without an intranasal LED device (660 nm). Preliminary results are promising regarding improved symptoms of the tested PwP [156,157,158].
4. Conclusions
PD is a complicated and chronic disorder featuring the development and progression of both motor and non-motor defects. This review lays out a five-part management strategy for PD. One of the confounding aspects of PD is the wide heterogeneity of patient presentations; one PwP may have had the disorder for many years with minimal symptoms while another PwP may be newly diagnosed and experiencing significant disease progression. Different disease presentations demand different management strategies. Hopefully, this review may aid PwP and their care teams in developing comprehensive and personalized management plans. In particular, it is hoped the overview provided here might help inform how not only to manage PD symptoms but also to improve QoL for PwP.
Author Contributions
F.C.C.: Conceptualization, writing—original draft preparation, writing—review and editing, visualization, project, and administration. The author has read and agreed to the published version of the manuscript.
Funding
F.C.C. received no external funding for this research.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
F.C.C. gratefully acknowledges Russell R. Broaddus, the Joe W. and Evelyn M. Grisham, and Department Chair in the Department of Pathology and Laboratory Medicine at UNC School of Medicine, for their continued support of his Parkinson’s disease research/scholarship.
Conflicts of Interest
The author declares no conflict of interest.
References
- Armstrong, M.J.; Okun, M.S. Diagnosis and treatment of parkinson disease: A review. J. Am. Med. Assoc. 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Werner, P.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar]
- Ahlskog, J.E. The New Parkinson’s Disease Treatment Book: Partnering with Your Doctor to Get the Most from Your Medications; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Santens, P.; Boon, P.; Van Roost, D.; Caemaert, J. The pathophysiology of motor symptoms in Parkinson’s disease. Acta Neurol. Belg. 2003, 103, 129–134. [Google Scholar]
- Jankovic, J. Motor fluctuations and dyskinesias in Parkinson’s disease: Clinical manifestations. Mov. Disord. 2005, 20, S11–S16. [Google Scholar] [CrossRef]
- Ferrazzoli, D.; Ortelli, P.; Cucca, A.; Bakdounes, L.; Canesi, M.; Volpe, D. Motor-cognitive approach and aerobic training: A synergism for rehabilitative intervention in Parkinson’s disease. Neurodegener. Dis. Manag. 2020, 10, 41–55. [Google Scholar] [CrossRef]
- Berganzo, K.; Tijero, B.; González-Eizaguirre, A.; Somme, J.; Lezcano, E.; Gabilondo, I.; Fernandez, M.; Zarranz, J.; Gómez-Esteban, J. Motor and non-motor symptoms of Parkinson’s disease and their impact on quality of life and on different clinical subgroups. Neurología 2016, 31, 585–591. [Google Scholar] [CrossRef]
- Crowley, E.K.; Nolan, Y.M.; Sullivan, A.M. Exercise as a therapeutic intervention for motor and non-motor symptoms in Parkinson’s disease: Evidence from rodent models. Prog. Neurobiol. 2019, 172, 2–22. [Google Scholar] [CrossRef]
- Carapellotti, A.M.; Stevenson, R.; Doumas, M. The efficacy of dance for improving motor impairments, non-motor symptoms, and quality of life in Parkinson’s disease: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0236820. [Google Scholar] [CrossRef]
- Amara, A.W.; Memon, A.A. Effects of exercise on non-motor symptoms in Parkinson’s disease. Clin. Ther. 2018, 40, 8–15. [Google Scholar] [CrossRef]
- Bandres-Ciga, S.; Diez-Fairen, M.; Kim, J.J.; Singleton, A.B. Genetics of Parkinson’s disease: An introspection of its journey towards precision medicine. Neurobiol. Dis. 2020, 137, 104782. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. J. Am. Med. Assoc. 2014, 311, 1670–1683. [Google Scholar] [CrossRef]
- Ahlskog, J.E. Cheaper, simpler, and better: Tips for treating seniors with Parkinson disease. Mayo Clin. Proc. 2011, 86, 1211–1216. [Google Scholar] [CrossRef]
- Hall, M.-F.E.; Church, F.C. Integrative medicine and health therapy for Parkinson disease. Top. Geriatr. Rehabil. 2020, 36, 176–186. [Google Scholar] [CrossRef]
- HP-200 in Parkinson’s Disease Study Group. An alternative medicine treatment for Parkinson’s disease: Results of a multicenter clinical trial. J. Altern. Complement. Med. 1995, 1, 249–255. [Google Scholar] [CrossRef]
- Bishop, F.L.; Yardley, L.; Lewith, G.T. Systematic review of beliefs involved in the use of complementary and alternative medicine. J. Health Psychol. 2007, 12, 851–867. [Google Scholar] [CrossRef]
- Bega, D.; Gonzalez-Latapi, P.; Zadikoff, C.; Simuni, S. A review of the clinical evidence for complementary and alternative therapies in Parkinson’s disease. Curr. Treat. Options Neurol. 2014, 16, 314. [Google Scholar] [CrossRef]
- Seung-Nam, K.; Wang, X.; Park, H.-J. Integrative approach to Parkinson’s disease. Front. Aging Neurosci. 2019, 11, 339. [Google Scholar]
- Pickut, B.A.; Mischley, L.K.; Reversa, R.J. Integrative medicine and Parkinson’s disease. Integr. Neurol. 2020, 7, 141–183. [Google Scholar]
- Ahlskog, J.E. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 2011, 77, 288–294. [Google Scholar] [CrossRef]
- Crowley, E.K.; Nolan, Y.M.; Sullivan, A.M. Exercise as therapy for Parkinson’s? Aging 2018, 10, 1536. [Google Scholar] [CrossRef]
- David, F.J.; Rafferty, M.R.; Robichaud, J.A.; Prodoehl, J.; Kohrt, W.M.; Vaillancourt, D.E.; Corcos, D.M. Progressive resistance exercise and Parkinson’s disease: A review of potential mechanisms. Parkinson’s Dis. 2012, 124527. [Google Scholar] [CrossRef]
- De Carvalho, A.O.; Filho, A.S.S.; Murillo-Rodriguez, E.; Rocha, N.B.; Carta, M.G.; Machado, S. Physical exercise for Parkinson’s disease: Clinical and experimental evidence. Clin. Pract. Epidemiol. Ment. Health 2018, 14, 89. [Google Scholar]
- Ebersbach, G.; Grust, U.; Ebersbach, A.; Wegner, B.; Gandor, F.; Kühn, A.A. Amplitude-oriented exercise in Parkinson’s disease: A randomized study comparing LSVT-BIG and a short training protocol. J. Neural Transm. 2015, 122, 253–256. [Google Scholar] [CrossRef]
- El-Sayes, J.; Harasym, D.; Turco, C.V.; Locke, M.B.; Nelson, A.J. Exercise-induced neuroplasticity: A mechanistic model and prospects for promoting plasticity. Neuroscientist 2019, 25, 65–85. [Google Scholar] [CrossRef]
- Ellis, T.; Rochester, L. Mobilizing Parkinson’s disease: The future of exercise. J. Parkinson’s Dis. 2018, 8, S95–S100. [Google Scholar] [CrossRef]
- Fisher, B.E.; Petzinger, G.M.; Nixon, K.; Hogg, E.; Bremmer, S.; Meshul, C.K.; Jakowec, M.W. Exercise-induced behavioral recovery and neuroplasticity in the 1-Methyl-4-Phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned mouse basal ganglia. J. Neurosci. Res. 2004, 77, 378–390. [Google Scholar] [CrossRef]
- Fisher, B.E.; Wu, A.D.; Salem, G.J.; Song, J.E.; Lin, C.-H.; Yip, J.; Cen, S.; Gordon, J.; Jakowec, M.; Petzinger, G. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch. Phys. Med. Rehabil. 2008, 89, 1221–1229. [Google Scholar] [CrossRef]
- Hirsch, M.A.; Farley, B.G. Exercise and neuroplasticity in persons living with Parkinson’s disease. Eur. J. Phys. Rehab. Med. 2009, 45, 215–229. [Google Scholar]
- Jang, Y.; Koo, J.-H.; Kwon, I.; Kang, E.-B.; Um, H.-S.; Soya, H.; Lee, Y.; Cho, J.-Y. Neuroprotective effects of endurance exercise against neuroinflammation in MPTP-induced Parkinson’s disease mice. Brain Res. 2017, 1655, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Freidle, M.; Ekman, U.; Schalling, E.; Leavy, B.; Svenningsson, P.; Hagströmer, M.; Franzén, E. Feasibility aspects of exploring exercise-induced neuroplasticity in Parkinson’s disease: A pilot randomized controlled trial. Parkinson’s Dis. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Paillard, T.; Rolland, Y.; Barreto, P.D.S. Protective effects of physical exercise in Alzheimer’s disease and Parkinson’s disease: A narrative review. J. Clin. Neurol. 2015, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Niewiadomski, W.; Gasiorowska, A.; Wysocka, A.; Stepniewska, A.; Niewiadomska, G. Exercise-induced neuroprotection and recovery of motor function in animal models of Parkinson’s disease. Front. Neurol. 2019, 10, 1143. [Google Scholar] [CrossRef]
- Palmer, S.S.; A Mortimer, J.; Webster, D.D.; Bistevins, R.; Dickinson, G.L. Exercise therapy for Parkinson’s disease. Arch. Phys. Med. Rehabil. 1986, 67, 741–745. [Google Scholar] [CrossRef]
- Petzinger, G.M.; Fisher, B.E.; McEwen, S.; Beeler, J.A.; Walsh, J.P.; Jakowec, M.W. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013, 12, 716–726. [Google Scholar] [CrossRef]
- Petzinger, G.M.; Fisher, B.E.; Van Leeuwen, J.-E.; Vukovic, M.; Akopian, G.; Meshul, C.K.; Holschneider, D.P.; Nacca, A.; Walsh, J.P.; Jakowec, M.W. Enhancing neuroplasticity in the basal ganglia: The role of exercise in Parkinson’s disease. Mov. Disord. 2010, 25, S141–S145. [Google Scholar] [CrossRef]
- Rafferty, M.R.; Prodoehl, J.; Robichaud, J.A.; David, F.J.; Poon, C.; Goelz, L.C.; Vaillancourt, D.E.; Kohrt, W.M.; Comella, C.L.; Corcos, D.M. Effects of two years of exercise on gait impairment in people with Parkinson’s Disease: The PRET-PD randomized trial. J. Neurol. Phys. 2017, 41, 21. [Google Scholar]
- Rafferty, M.R.; Schmidt, P.N.; Luo, S.T.; Li, K.; Marras, C.; Davis, T.L.; Guttman, M.; Cubillos, F.; Simuni, T.; on behalf of all NPF-QII Investigators. Regular exercise, quality of life, and mobility in Parkinson’s disease: A longitudinal analysis of national Parkinson foundation quality improvement initiative data. J. Parkinson’s Dis. 2017, 7, 193–202. [Google Scholar] [CrossRef]
- Schenkman, M.; Moore, C.G.; Kohrt, W.M.; Hall, D.A.; Delitto, A.; Comella, C.L.; Josbeno, D.A.; Christiansen, C.L.; Berman, B.D.; Kluger, B.M. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. J. Am. Med. Assoc. Neurol. 2018, 75, 219–226. [Google Scholar] [CrossRef]
- Silveira, C.R.; Roy, E.A.; Intzandt, B.N.; Almeida, Q.J. Aerobic exercise is more effective than goal-based exercise for the treatment of cognition in Parkinson’s disease. Brain Cogn. 2018, 122, 1–8. [Google Scholar] [CrossRef]
- Bryans, L.A.; Palmer, A.D.; Anderson, S.; Schindler, J.; Graville, D.J. The impact of Lee Silverman Voice Treatment (Lsvt Loud®) on voice, communication, and participation: Findings from a prospective, longitudinal study. J. Commun. Disord. 2020, 89, 106031. [Google Scholar] [CrossRef]
- Foltynie, T.; Grover, T. Not only loud but also intelligible. EClinicalMedicine 2020, 24, 100456. [Google Scholar] [CrossRef]
- Yuan, F.; Guo, X.; Wei, X.; Xie, F.; Zheng, J.; Huang, Y.; Huang, Z.; Chang, Z.; Li, H.; Guo, Y.; et al. Lee Silverman Voice Treatment for dysarthria in patients with Parkinson’s disease: A systematic review and meta-analysis. Eur. J. Neurol. 2020, 27, 1957–1970. [Google Scholar] [CrossRef]
- Farley, B.G.; Fox, C.M.; Ramig, L.O.; McFarland, D.H. Intensive amplitude-specific therapeutic approaches for Parkinson’s disease: Toward a neuroplasticity-principled rehabilitation model. Top. Geriatr. Rehabil. 2008, 24, 99–114. [Google Scholar] [CrossRef]
- Farley, B.G.; Koshland, G.F. Training BIG to move faster: The application of the speed–amplitude relation as a rehabilitation strategy for people with Parkinson’s disease. Exp. Brain Res. 2005, 167, 462–467. [Google Scholar] [CrossRef]
- Ebersbach, G.; Ebersbach, A.; Edler, D.; Kaufhold, O.; Kusch, M.; Kupsch, A.; Wissel, J. Comparing exercise in Parkinson’s disease—The Berlin LSVT®BIG study. Mov. Disord. 2010, 25, 1902–1908. [Google Scholar] [CrossRef]
- Fox, C.; Ebersbach, G.; Ramig, L.; Sapir, S. LSVT LOUD and LSVT BIG: Behavioral treatment programs for speech and body movement in Parkinson disease. Parkinson’s Dis. 2012, 2012. [Google Scholar] [CrossRef]
- LaMotte, G.; Rafferty, M.R.; Prodoehl, J.; Kohrt, W.M.; Comella, C.L.; Simuni, T.; Corcos, D.M. Effects of endurance exercise training on the motor and non-motor features of Parkinson’s disease: A review. J. Parkinson’s Dis. 2015, 5, 21–41. [Google Scholar] [CrossRef]
- van der Kolk, N.M.; de Vries, N.M.; Kessels, R.P.C.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol. 2019, 18, 998–1008. [Google Scholar] [CrossRef]
- Ridgel, A.L.; Vitek, J.L.; Alberts, J.L. Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabilit. Neural Repair 2009, 23, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, J.D.; Roth, R.H. Dopamine synthesis, uptake, metabolism, and receptors: Relevance to gene therapy of Parkinson’s disease. Exp. Neurol. 1997, 144, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.R. The biochemistry of Parkinson’s disease. Annu. Rev. Biochem. 2005, 74, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.H.; Katzenschlager, R.; Lim, S.-Y.; Barton, B.; De Bie, R.M.A.; Seppi, K.; Coelho, M.; Sampaio, C.; on behalf of the Movement Disorder Society Evidence-Based Medicine Committee. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2018, 33, 1248–1266. [Google Scholar] [CrossRef]
- Abbott, A. Levodopa: The story so far. Nat. Cell Biol. 2010, 466, S6–S7. [Google Scholar] [CrossRef]
- Cotzias, G.C.; Papavasiliou, P.S.; Gellene, R. Modification of Parkinsonism—Chronic treatment with L-Dopa. N. Engl. J. Med. 1969, 280, 337–345. [Google Scholar] [CrossRef]
- Lees, A.J.; Tolosa, E.; Olanow, C.W. Four pioneers of L-dopa treatment: Arvid Carlsson, Oleh Hornykiewicz, George Cotzias, and Melvin Yahr. Mov. Disord. 2015, 30, 19–36. [Google Scholar] [CrossRef]
- Ciurleo, R.; Corallo, F.; Bonanno, L.; Buono, V.L.; Di Lorenzo, G.; Versaci, R.; Allone, C.; Palmeri, R.; Bramanti, P.; Marino, S. Assessment of Duodopa® effects on quality of life of patients with advanced Parkinson’s disease and their caregivers. J. Neurol. 2018, 265, 2005–2014. [Google Scholar] [CrossRef]
- Warren Olanow, C.; Espay, A.J.; Stocchi, F.; Ellenbogen, A.L.; Leinonen, M.; Adar, L.; Case, R.J.; Fuchs Orenbach, S.; Yardeni, T.; Oren, S.; et al. Continuous subcutaneous levodopa delivery for Parkinson’s disease: A randomized study. J. Parkinson’s Dis. 2020, 11, 177–186. [Google Scholar] [CrossRef]
- Ellenbogen, A.; Stocchi, F.; Espay, A.; Poewe, W.; Oren, S.; Case, R.; Olanow, C.W. Impact of Subcutaneous Levodopa Infusion with ND0612 on Patient Reported Outcomes (4506). Neurology 2021, 94, 4506. [Google Scholar]
- Braksick, S.A.; Nasr, D.M. Neurological emergencies from prescription drugs. In Neurological Emergencies in Clinical Practice; Springer: Cham, Switzerland, 2019; pp. 301–318. [Google Scholar]
- Ahlskog, J.E. Common myths and misconceptions that sidetrack Parkinson disease treatment, to the detriment of patients. Mayo Clin. Proc. 2020, 95, 2225–2234. [Google Scholar] [CrossRef]
- Stowe, R.; Ives, N.; Clarke, C.E.; Van Hilten, J.; Ferreira, J.; Hawker, R.J.; Shah, L.; Wheatley, K.; Gray, R. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst. Rev. 2008, CD006564. [Google Scholar] [CrossRef]
- Carbone, F.; Djamshidian, A.; Seppi, K.; Poewe, W. Apomorphine for Parkinson’s disease: Efficacy and safety of current and new formulations. CNS Drugs 2019, 33, 905–918. [Google Scholar] [CrossRef]
- Foley, P.; Gerlach, M.; Youdim, M.; Riederer, P. MAO-B inhibitors: Multiple roles in the therapy of neurodegenerative disorders? Parkinsonism Relat. Disord. 2000, 6, 25–47. [Google Scholar] [CrossRef]
- Przuntek, H.; Conrad, B.; Dichgans, J.; Kraus, P.; Krauseneck, P.; Pergande, G.; Rinne, U.; Schimrigk, K.; Schnitker, J.; Vogel, H. SELEDO: A 5-year long-term trial on the effect of selegiline in early parkinsonian patients treated with levodopa. Eur. J. Neurol. 1999, 6, 141–150. [Google Scholar] [CrossRef]
- Männistö, P.T.; Kaakkola, S. Catechol-O-methyltransferase (COMT): Biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol. Rev. 1999, 51, 593–628. [Google Scholar]
- Männistö, P.T.; Ulmanen, I.; Lundström, K.; Taskinen, J.; Tenhunen, J.; Tilgmann, C.; Kaakkola, S. Characteristics of catechol O-methyltransferase (COMT) and properties of selective COMT inhibitors. In Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progrès des Recherches Pharmaceutiques; Springer: Berlin/Heidelberg, Germany, 1992; Volume 39, pp. 291–350. [Google Scholar]
- Schwab, R.S.; England, A.C.; Poskanzer, D.C.; Young, R.R. Amantadine in the treatment of Parkinson’s disease. J. Am. Med. Assoc. 1969, 208, 1168–1170. [Google Scholar] [CrossRef]
- Santiago, P.L.; Rascol, R. Efficacy and safety of Amantadine for the treatment of L-Dopa-induced Dyskinesia. J. Neural Transm. 2018, 125, 1237–1250. [Google Scholar]
- Agid, Y.; Arnulf, I.; Bejjani, P.; Bloch, F.; Bonnet, A.M.; Damier, P.; Dubois, B.; François, C.; Houeto, J.L.; Iacono, D.; et al. Parkinson’s disease is a neuropsychiatric disorder. Adv. Neurol. 2003, 91, 365–370. [Google Scholar]
- Castrioto, A.; Thobois, S.; Carnicella, S.; Maillet, A.; Krack, P. Emotional manifestations of PD: Neurobiological basis. Mov. Disord. 2016, 31, 1103–1113. [Google Scholar] [CrossRef]
- Rieu, I.; Martinez-Martin, P.; Pereira, B.; De Chazeron, I.; Metman, L.V.; Jahanshahi, M.; Ardouin, C.; Chéreau, I.; Brefel-Courbon, C.; Ory-Magne, F.; et al. International validation of a behavioral scale in Parkinson’s disease without dementia. Mov. Disord. 2015, 30, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Rieu, I.; Houeto, J.L.; Pereira, B.; De Chazeron, I.; Bichon, A.; Chéreau, I.; Ulla, M.; Brefel-Courbon, C.; Ory-Magne, F.; Dujardin, K.; et al. Impact of Mood and Behavioral Disorders on Quality of Life in Parkinson’s disease. J. Parkinson’s Dis. 2016, 6, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; David, A.S.; Evans, A.H.; Grant, J.E.; Stacy, M. Clinical spectrum of impulse control disorders in Parkinson’s disease. Mov. Disord. 2015, 30, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.; Carnicella, S.; Strafella, A.P.; Bichon, A.; Lhommée, E.; Castrioto, A.; Chabardes, S.; Thobois, S.; Krack, P. Apathy and Impulse Control Disorders: Yin & Yang of Dopamine Dependent Behaviors. J. Parkinson’s Dis. 2015, 5, 625–636. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Wang, X.-X.; Feng, Y.; Fekete, R.; Jankovic, J.; Wu, Y.-C. Impulse Control Disorders in Parkinson’s disease: Epidemiology, Pathogenesis and Therapeutic Strategies. Front. Psychiatry 2021, 12, 97. [Google Scholar] [CrossRef]
- Seppi, K.; Ray Chaudhuri, K.; Coelho, M.; Fox, S.H.; Katzenschlager, R.; Perez Lloret, S.; Weintraub, D.; Sampaio, C.; Collaborators of the Parkinson’s disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease—An evidence-based medicine review. Mov. Disord. 2019, 34, 180–198. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Healy, D.G.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006, 5, 235–245. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Schapira, A.H.V. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Vuletić, V. Non-motor symptoms in Parkinson’s disease. In Mind and Brain; Springer International Publishing: Cham, Switzerland, 2020; pp. 109–118. [Google Scholar] [CrossRef]
- Pontone, G.M.; Mills, K.A. Optimal treatment of depression and anxiety in Parkinson’s disease. Am. J. Geriatr. Psychiatry 2021, in press. [Google Scholar] [CrossRef]
- Martimbianco, A.L.C.; Prosdocimi, F.C.; Anauate-Netto, C.; dos Santos, E.M.; Mendes, G.D.; Fragoso, Y.D. Evidence-based recommendations for the oral health of patients with Parkinson’s disease. Neurology 2021, 1–10. [Google Scholar] [CrossRef]
- Skjærbæk, C.; Knudsen, K.; Horsager, J.; Borghammer, P. Gastrointestinal dysfunction in Parkinson’s disease. J. Clin. Med. 2021, 10, 493. [Google Scholar] [CrossRef]
- Rashid, N.; Shim, A.; Andes, S.; Quale, S.; Abler, V. Treatment patterns with antipsychotics in long-term care patients with Parkinson’s disease psychosis. J. Appl. Gerontol. 2021, 0733464820987032. [Google Scholar] [CrossRef]
- Naasan, G.; Shdo, S.M.; Rodriguez, E.M.; Spina, S.; Grinberg, L.; Lopez, L.; Karydas, A.; Seeley, W.W.; Miller, B.L.; Rankin, K.P. Psychosis in neurodegenerative disease: Differential patterns of hallucination and delusion symptoms. Brain 2021, 144, 999–1012. [Google Scholar] [CrossRef]
- Pakkenberg, H.; Pakkenberg, B. Clozapine in the treatment of tremor. Acta Neurol. Scand. 1986, 73, 295–297. [Google Scholar] [CrossRef]
- Friedman, J.H.; Lannon, M.C. Clozapine-responsive tremor in Parkinson’s disease. Mov. Disord. 1990, 5, 225–229. [Google Scholar] [CrossRef]
- Keir, L.H.M.; Breen, D.P. New awakenings: Current understanding of sleep dysfunction and its treatment in Parkinson’s disease. J. Neurol. 2020, 267, 288–294. [Google Scholar] [CrossRef]
- Bhalke, R.D.; Giri, M.A.; Anil, R.Y.; Balasaheb, N.M.; Nanasaheb, P.A.; Pande, V.V. Hypotension: A comprehensive review. J. Pharmacogn. Phytochem. 2021, 10, 1945–1947. [Google Scholar]
- Idiaquez, J.F.; Casar, J.C.; Biaggioni, I. Neurogenic orthostatic hypotension. Lessons from synucleinopathies. Am. J. Hypertens. 2021, 34, 125–133. [Google Scholar] [CrossRef]
- Gupta, A.; LaFaver, K.; Duque, K.R.; Lingaiah, A.; Meriwether, K.V.; Gaskins, J.; Gomes, J.; Espay, A.J.; Mahajan, A. Pelvic floor health in women with Parkinson’s disease. J. Parkinson’s Dis. 2021, 11, 857–864. [Google Scholar] [CrossRef]
- Osaki, Y.; Morita, Y.; Miyamoto, Y.; Furushima, T.; Furuta, K.; Furuya, H. Disease progression and phenotypes of non-motor symptoms in Parkinson’s disease. Neurol. Clin. Neurosci. 2021, 9, 83–90. [Google Scholar] [CrossRef]
- Oldfield, V.; Keating, G.M.; Perry, C.M. Rasagiline. Drugs 2007, 67, 1725–1747. [Google Scholar] [CrossRef] [PubMed]
- Parkinson Study Group. DATATOP: A multicenter controlled clinical trial in early Parkinson’s disease. Arch. Neurol. 1989, 46, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Shoulson, I.; Parkinson Study Group. DATATOP: A decade of neuroprotective inquiry. Ann. Neurol. 1998, 44, S160–S166. [Google Scholar] [CrossRef]
- Rascol, O.; Fitzer-Attas, C.J.; Hauser, R.; Jankovic, J.; Lang, A.; Langston, J.W.; Melamed, E.; Poewe, W.; Stocchi, F.; Tolosa, E.; et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease (the ADAGIO study): Prespecified and post-hoc analyses of the need for additional therapies, changes in UPDRS scores, and non-motor outcomes. Lancet Neurol. 2011, 10, 415–423. [Google Scholar] [CrossRef]
- Siderowf, A.; Stern, M.; Shoulson, I.; Kieburtz, K.; Oakes, D.; Day, D.; Shinaman, A.; Plumb, S.; Fahn, S.; Blindauer, K. A controlled trial of rasagiline in early Parkinson disease: The tempo study. Arch. Neurol. 2002, 59, 1937–1943. [Google Scholar]
- Reish, A.; Heather, E.; Standaert, D. Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. J. Parkinson’s Dis. 2005, 5, 1–19. [Google Scholar]
- Kannarkat, G.T.; Boss, J.M.; Tansey, M.G. The role of innate and adaptive immunity in Parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 493–514. [Google Scholar] [CrossRef]
- Salim, S.; Sarraj, N.; Taneja, M.; Saha, K.; Tejada-Simon, M.V.; Chugh, G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav. Brain Res. 2010, 208, 545–552. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.J.; Shang, N.N.; Liu, J.; Li, J.; Tang, D.H.; Li, Q. Moderate intensity treadmill exercise alters food preference via dopaminergic plasticity of ventral tegmental area-nucleus accumbens in obese mice. Neurosci. Lett. 2017, 641, 56–61. [Google Scholar] [CrossRef]
- Zhou, W.; Barkow, J.C.; Freed, C.R. Running wheel exercise reduces α-synuclein aggregation and improves motor and cognitive function in a transgenic mouse model of Parkinson’s disease. PLoS ONE 2017, 12, e0190160. [Google Scholar] [CrossRef]
- Sacheli, M.A.; Murray, D.K.; Vafai, N.; Cherkasova, M.V.; Dinelle, K.; Shahinfard, E.; Neilson, N.; McKenzie, J.; Schulzer, M.; Appel-Cresswell, S. Habitual exercisers versus sedentary subjects with Parkinson’s disease: Multimodal Pet and fMRI study. Mov. Disord. 2018, 33, 1945–1950. [Google Scholar] [CrossRef]
- Hall, M.-F.E.; Church, F.C. Exercise for older adults improves the quality of life in Parkinson’s disease and potentially enhances the immune response to COVID-19. Brain Sci. 2020, 10, 612. [Google Scholar] [CrossRef]
- Van der Heide, A.; Speckens, A.E.; Meinders, M.J.; Rosenthal, L.S.; Bloem, B.R.; Helmich, R.C. Stress and mindfulness in Parkinson’s disease—A survey in 5000 patients. NPJ Parkinson’s Dis. 2021, 7, 1–10. [Google Scholar]
- Van der Heide, A.; Meinders, M.J.; Speckens, A.E.M.; Peerbolte, T.F.; Bloem, B.R.; Helmich, R.C. Stress and mindfulness in Parkinson’s disease: Clinical effects and potential underlying mechanisms. Mov. Disord. 2021, 36, 64–70. [Google Scholar] [CrossRef]
- Rodríguez, M.Á.; Albillos-Almaraz, L.; López-Aguado, I.; Crespo, I.; Del Valle, M.; Olmedillas, H. Vigorous aerobic exercise in the management of Parkinson disease: A systematic review. PM&R 2020. [Google Scholar] [CrossRef]
- Gronek, P.; Haas, A.N.; Czarny, W.; Podstawski, R.; Delabary, M.D.S.; Clark, C.C.; Boraczyński, M.; Tarnas, M.; Wycichowska, P.; Pawlaczyk, M.; et al. The mechanism of physical activity-induced amelioration of Parkinson’s disease: A narrative review. Aging Dis. 2021, 12, 192–202. [Google Scholar] [CrossRef]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef]
- Taylor, J.M. Editorial overview: Muscle and bone are highly effective communicators. Curr. Opin. Pharmacol. 2017, 34. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Bente Klarlund, P. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar]
- Tari, A.R.; Norevik, C.S.; Scrimgeour, N.R.; Kobro-Flatmoen, A.; Storm-Mathisen, J.; Bergersen, L.H.; Wrann, D.C.; Selbæk, G.; Kivipelto, M.; Moreira, J.B.N. Are the neuroprotective effects of exercise training systemically mediated? Prog. Cardiovasc. Dis. 2019, 62, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.H.; Stanya, K.J.; Hyde, A.L.; Chalom, M.M.; Alexander, R.K.; Liou, Y.-H.; Starost, K.A.; Gangl, M.R.; Jacobi, D.; Liu, S.; et al. Interleukin-13 drives metabolic conditioning of muscle to endurance exercise. Science 2020, 368. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.C.; Ruas, J.L. Exercised cytokines promote endurance. Science 2020, 368, 470–471. [Google Scholar] [CrossRef]
- Hribar, C.A.; Cobbold, P.H.; Church, F.C. Potential role of vitamin D in the elderly to resist COVID-19 and to slow progression of Parkinson’s disease. Brain Sci. 2020, 10, 284. [Google Scholar] [CrossRef]
- Ding, H.; Dhima, K.; Lockhart, K.C.; Locascio, J.J.; Hoesing, A.N.; Duong, K.; Trisini-Lipsanopoulos, A.; Hayes, M.T.; Sohur, U.S.; Wills, A.-M.; et al. Unrecognized vitamin D3 deficiency is common in Parkinson disease: Harvard Biomarker Study. Neurology 2013, 81, 1531–1537. [Google Scholar] [CrossRef]
- Peterson, A.L.; Murchison, C.; Zabetian, C.; Leverenz, J.B.; Watson, G.S.; Montine, T.; Carney, N.; Bowman, G.L.; Edwards, K.; Quinn, J.F. Memory, mood, and vitamin D in persons with Parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 547–555. [Google Scholar] [CrossRef]
- Sleeman, I.; Aspray, T.; Lawson, R.; Coleman, S.; Duncan, G.; Khoo, T.K.; Schoenmakers, I.; Rochester, L.; Burn, D.; Yarnall, A. The role of vitamin D in disease progression in early Parkinson’s disease. J. Parkinson’s Dis. 2017, 7, 669–675. [Google Scholar] [CrossRef]
- Ebel, H.; Günther, T. Magnesium metabolism: A review. Clin. Chem. Lab. Med. 1980, 18, 257–270. [Google Scholar] [CrossRef]
- Vink, R. Magnesium in the CNS: Recent advances and developments. Magnes. Res. 2016, 29, 95–101. [Google Scholar]
- Shen, Y.; Dai, L.; Tian, H.; Xu, R.; Li, F.; Li, Z.; Zhou, J.; Wang, L.; Dong, J.; Sun, L. Treatment of magnesium-L-threonate elevates the magnesium level in the cerebrospinal fluid and attenuates motor deficits and dopamine neuron loss in a mouse model of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2019, 15, 3143–3153. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Tangalakis, K.; Bosevski, M.; Apostolopoulos, V. Cognitive decline: A vitamin B perspective. Maturitas 2016, 93, 108–113. [Google Scholar] [CrossRef]
- Lu’O’ng, K.V.Q.; Nguyê∼n, L.T.H. Thiamine and Parkinson’s disease. J. Neurol. Sci. 2012, 316, 1–8. [Google Scholar] [CrossRef]
- Parkhomenko, Y.M.; Pavlova, A.S.; Mezhenskaya, O.A. Mechanisms responsible for the high sensitivity of neural cells to vitamin B1 deficiency. Neurophysiology 2016, 48, 429–448. [Google Scholar] [CrossRef]
- Wright, C.E.; Tallan, H.H.; Lin, Y.Y.; Gaull, G.E. Taurine: Biological update. Annu. Rev. Biochem. 1986, 55, 427–453. [Google Scholar] [CrossRef]
- Che, Y.; Hou, L.; Sun, F.; Zhang, C.; Liu, X.; Piao, F.; Zhang, D.; Li, H.; Wang, Q. Taurine protects dopaminergic neurons in a mouse Parkinson’s disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018, 9, 435. [Google Scholar] [CrossRef]
- Hou, L.; Che, Y.; Sun, F.; Wang, Q. Taurine protects noradrenergic locus coeruleus neurons in a mouse Parkinson’s disease model by inhibiting microglial M1 polarization. Amino Acids 2018, 50, 547–556. [Google Scholar] [CrossRef]
- Hu, S.; Maiti, P.; Ma, Q.; Zuo, X.; Jones, M.R.; Cole, G.M.; Frautschy, S.A. Clinical development of curcumin in neuro-degenerative disease. Expert Rev. Neurother. 2015, 15, 629–637. [Google Scholar] [CrossRef]
- Jiang, T.-F.; Zhang, Y.-J.; Zhou, H.-Y.; Wang, H.-M.; Tian, L.-P.; Liu, J.; Ding, J.-Q.; Chen, S.-D. Curcumin ameliorates the neurodegenerative pathology in A53T α-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J. Neuroimmune Pharmacol. 2013, 8, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-S.; Zhang, Z.-R.; Zhang, M.-M.; Sun, M.-X.; Wang, W.-W.; Xie, C.-L. Neuroprotective properties of curcumin in toxin-base animal models of Parkinson’s disease: A systematic experiment literatures review. BMC Complement. Altern. Med. 2017, 17, 412. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, O.T. Management of the aging risk factor for Parkinson’s disease. Neurobiol. Aging 2014, 35, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, O.T. Inhibition of aging in Parkinson’s disease: A case study. J. Altern. Complement. Med. 2013, 19, 851. [Google Scholar] [CrossRef] [PubMed]
- Hagen, T.M.; Ingersoll, R.T.; Wehr, C.M.; Lykkesfeldt, J.; Vinarsky, V.; Bartholomew, J.C.; Song, M.-H.; Ames, B.N. Acetyl-l-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc. Natl. Acad. Sci. USA 1998, 95, 9562–9566. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Atamna, H.; Kuratsune, H.; Ames, B.N. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann. N. Y. Acad. Sci. 2002, 959, 133–166. [Google Scholar] [CrossRef]
- Katz, M.; Won, S.J.; Park, Y.; Orr, A.; Jones, D.P.; Swanson, R.A.; Glass, G.A. Cerebrospinal fluid concentrations of N-Acetylcysteine after oral administration in Parkinson’s disease. Parkinsonism Relat. Disord. 2015, 21, 500–503. [Google Scholar] [CrossRef]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.W.; Wintering, N.A.; Cai, J.; Wei, X.; Bazzan, A.J.; Zhong, L.; Bowen, B.; et al. N-Acetyl cysteine may support dopamine neurons in Parkinson’s disease: Preliminary clinical and cell line data. PLoS ONE 2016, 11, e0157602. [Google Scholar] [CrossRef]
- Ferretta, A.; Gaballo, A.; Tanzarella, P.; Piccoli, C.; Capitanio, N.; Nico, B.; Annese, T.; Di Paola, M.; Dell’Aquila, C.; De Mari, M.; et al. Effect of resveratrol on mitochondrial function: Implications in parkin-associated familiar Parkinson’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 902–915. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1114–1123. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; Tan, D.X.; Antolín, I.; Rodríguez, C.; Reiter, R.J. Melatonin and Parkinson’s disease. Endocrine 2005, 27, 169–178. [Google Scholar] [CrossRef]
- Cernera, S.; Eisinger, R.S.; Wong, J.K.; Ho, K.W.D.; Lopes, J.L.; To, K.; Carbunaru, S.; Ramirez-Zamora, A.; Almeida, L.; Foote, K.D.; et al. Long-term Parkinson’s disease quality of life after staged DBS: STN vs GPi and first vs. second lead. NPJ Parkinson’s Dis. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Sharma, V.D.; Patel, M.; Miocinovic, S. Surgical treatment of Parkinson’s disease: Devices and lesion approaches. Neurotherapeutics 2020, 17, 1525–1538. [Google Scholar] [CrossRef]
- Artusi, C.A.; Lopiano, L.; Morgante, F. Deep brain stimulation selection criteria for Parkinson’s disease: Time to go beyond CAPSIT-PD. J. Clin. Med. 2020, 9, 3931. [Google Scholar] [CrossRef]
- Lozano, C.S.; Tam, J.; Lozano, A.M. The changing landscape of surgery for Parkinson’s disease. Mov. Disord. 2018, 33, 36–47. [Google Scholar] [CrossRef]
- Hariz, M. My 25 Stimulating years with DBS in Parkinson’s disease. J. Parkinson’s Dis. 2017, 7, S33–S41. [Google Scholar] [CrossRef]
- Dallapiazza, R.F.; De Vloo, P.; Fomenko, A.; Lee, D.J.; Hamani, C.; Munhoz, R.P.; Hodaie, M.; Lozano, A.M.; Fasano, A.; Kalia, S.K. Considerations for patient and target selection in deep brain stimulation surgery for Parkinson’s disease. Exon Publ. 2018, 145–160. [Google Scholar]
- Hitti, F.L.; Ramayya, A.G.; McShane, B.J.; Yang, A.I.; Vaughan, K.A.; Baltuch, G.H. Long-term outcomes following deep brain stimulation for Parkinson’s disease. J. Neurosurg. 2020, 132, 205–210. [Google Scholar] [CrossRef]
- Kurtis, M.M.; Rajah, T.; Delgado, L.F.; Dafsari, H.S. The effect of deep brain stimulation on the non-motor symptoms of Parkinson’s disease: A critical review of the current evidence. NPJ Parkinson’s Dis. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- McFarthing, K.; Buff, S.; Rafaloff, G.; Dominey, T.; Wyse, R.K.; Stott, S.R. Parkinson’s disease drug therapies in the clinical trial pipeline: 2020. J. Parkinson’s Dis. 2020, 10, 757–774. [Google Scholar] [CrossRef]
- Kordower, J.H.; Freeman, T.B.; Snow, B.J.; Vingerhoets, F.J.; Mufson, E.J.; Sanberg, P.R.; Hauser, R.A.; Smith, D.A.; Nauert, G.M.; Perl, D.P.; et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N. Engl. J. Med. 1995, 332, 1118–1124. [Google Scholar] [CrossRef]
- Freed, C.R.; Greene, P.E.; Breeze, R.E.; Tsai, W.-Y.; DuMouchel, W.; Kao, R.; Dillon, S.; Winfield, H.; Culver, S.; Trojanowski, J.Q.; et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 2001, 344, 710–719. [Google Scholar] [CrossRef]
- Doi, D.; Magotani, H.; Kikuchi, T.; Ikeda, M.; Hiramatsu, S.; Yoshida, K.; Amano, N.; Nomura, M.; Umekage, M.; Morizane, A. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Stone, J.; Johnstone, D.; Mitrofanis, J. The helmet experiment in Parkinson’s disease: An observation of the mechanism of neuroprotection by near infra-red light. In Proceedings of the 9th WALT Congress, Gold Coast, QLD, Australia, 28–30 September 2012. [Google Scholar]
- Hamilton, C.L.; El Khoury, H.; Hamilton, D.; Nicklason, F.; Mitrofanis, J. Buckets: Early observations on the use of red and infrared light helmets in Parkinson’s disease patients. Photobiomodul. Photomed. Laser Surg. 2019, 37, 615–622. [Google Scholar] [CrossRef]
- Johnstone, D.M.; Emoro, C.; Estone, J.; Benabid, A.-L.; Emitrofanis, J. Turning on lights to stop neurodegeneration: The potential of near infrared light therapy in Alzheimer’s and Parkinson’s disease. Front. Neurosci. 2016, 9, 500. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).