Exploration of Galectin Ligands Displayed on Gram-Negative Respiratory Bacterial Pathogens with Different Cell Surface Architectures
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Culture and Labelling and LPS/LOSs Isolation
2.2. Galectins
2.3. Microarray Binding Assays
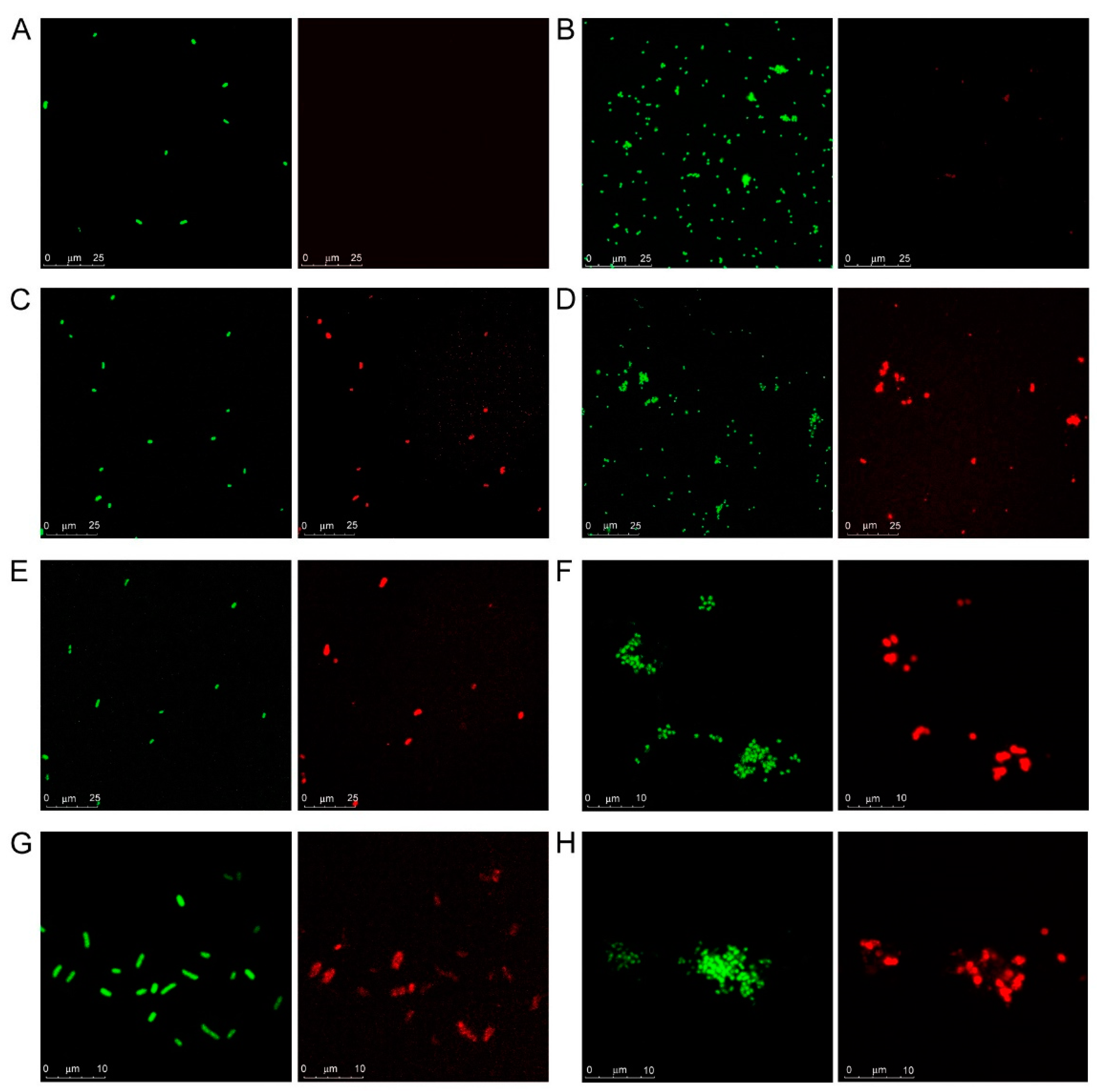
2.4. Confocal Microscopy
3. Results
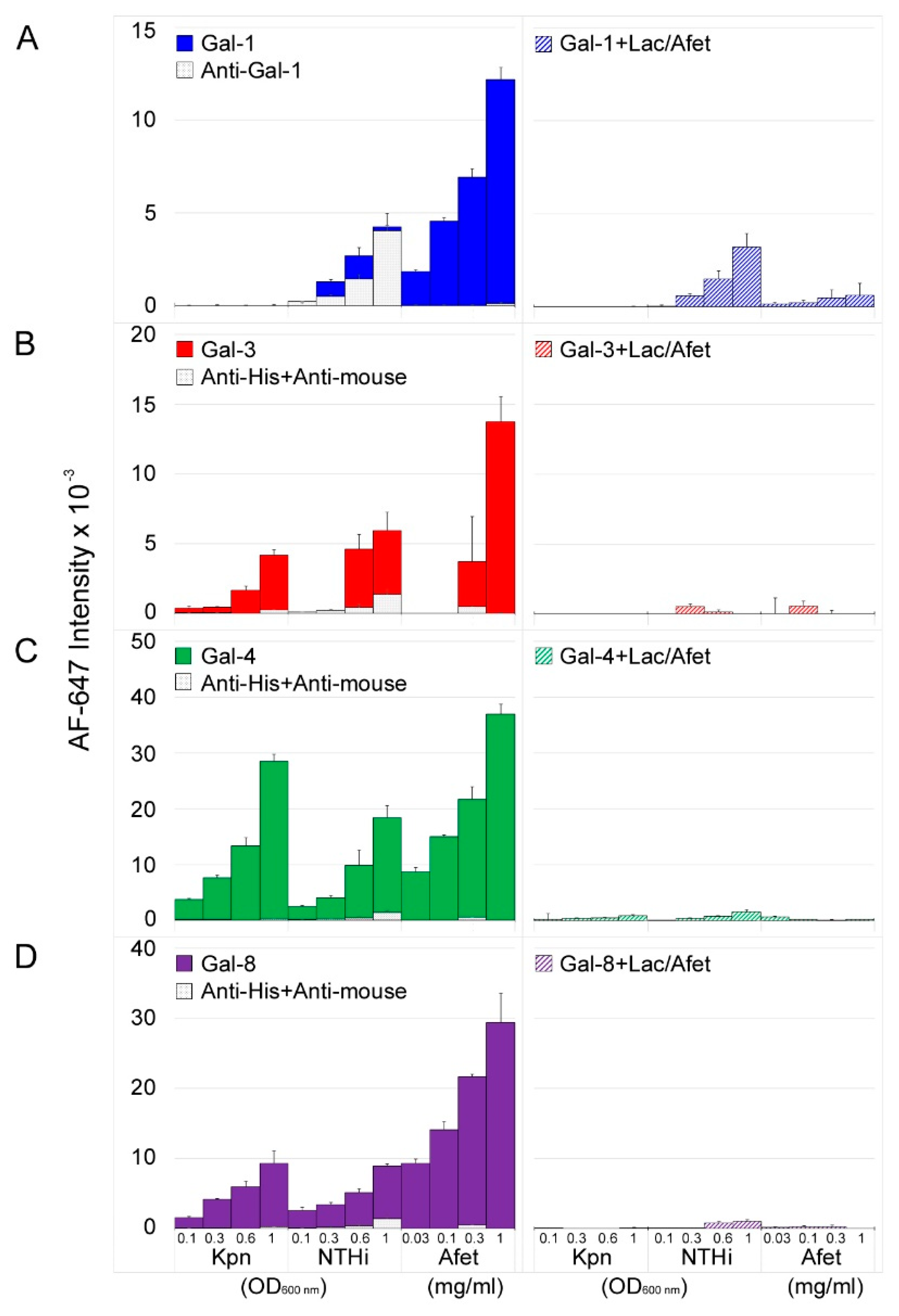
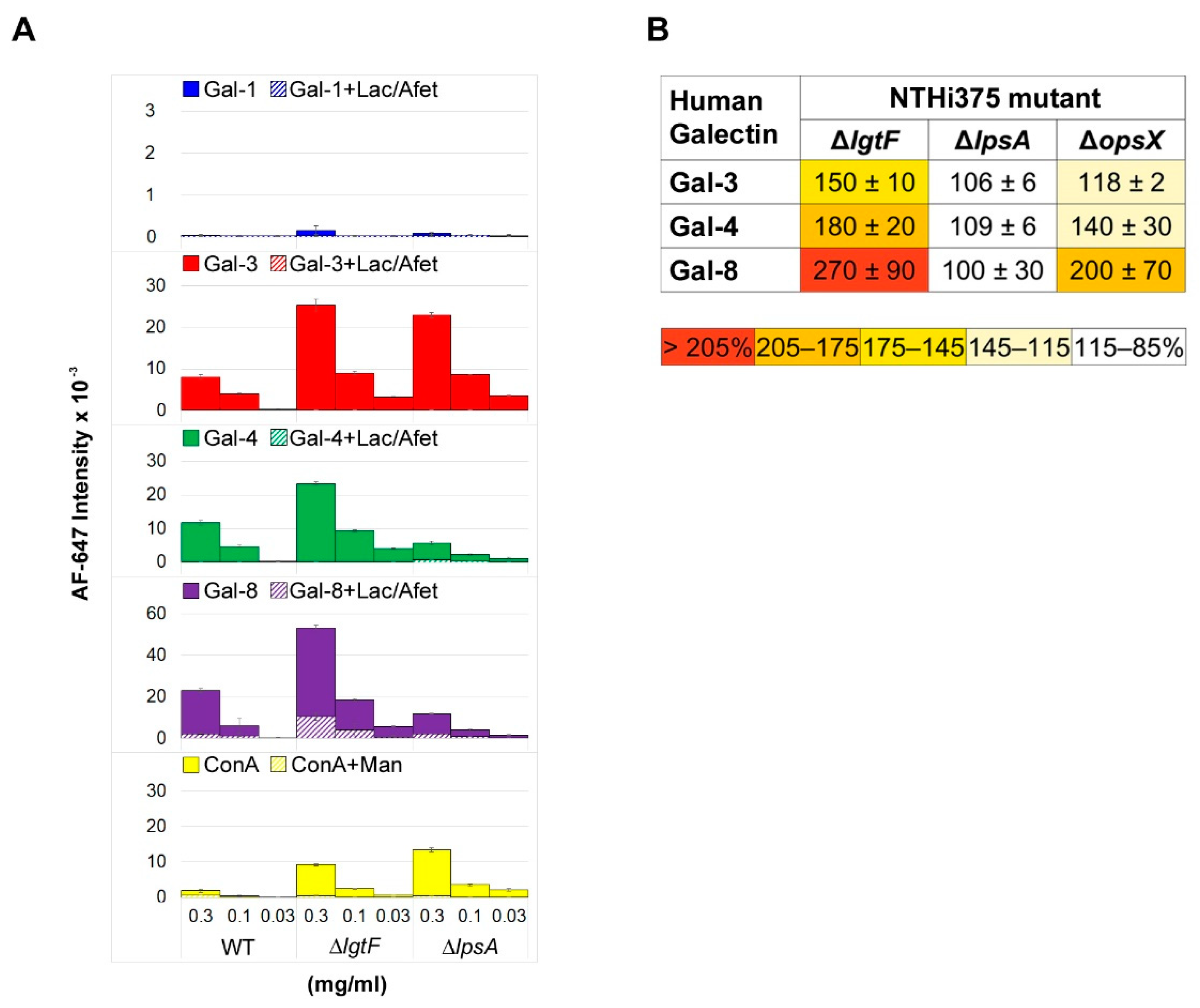
3.1. Microarray and Confocal Microscopy Analyses of Galectin Binding to Bacterial Cells
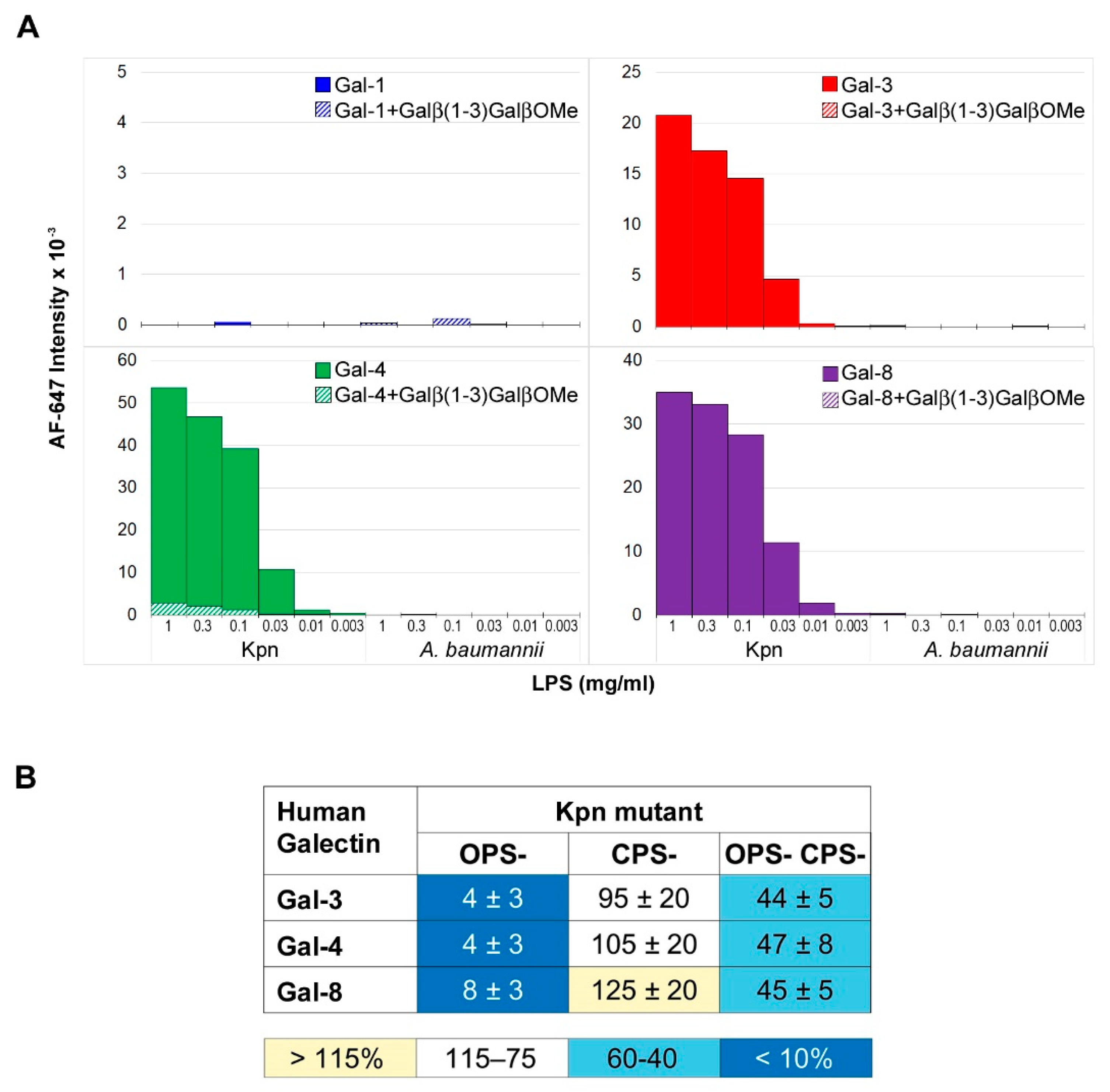
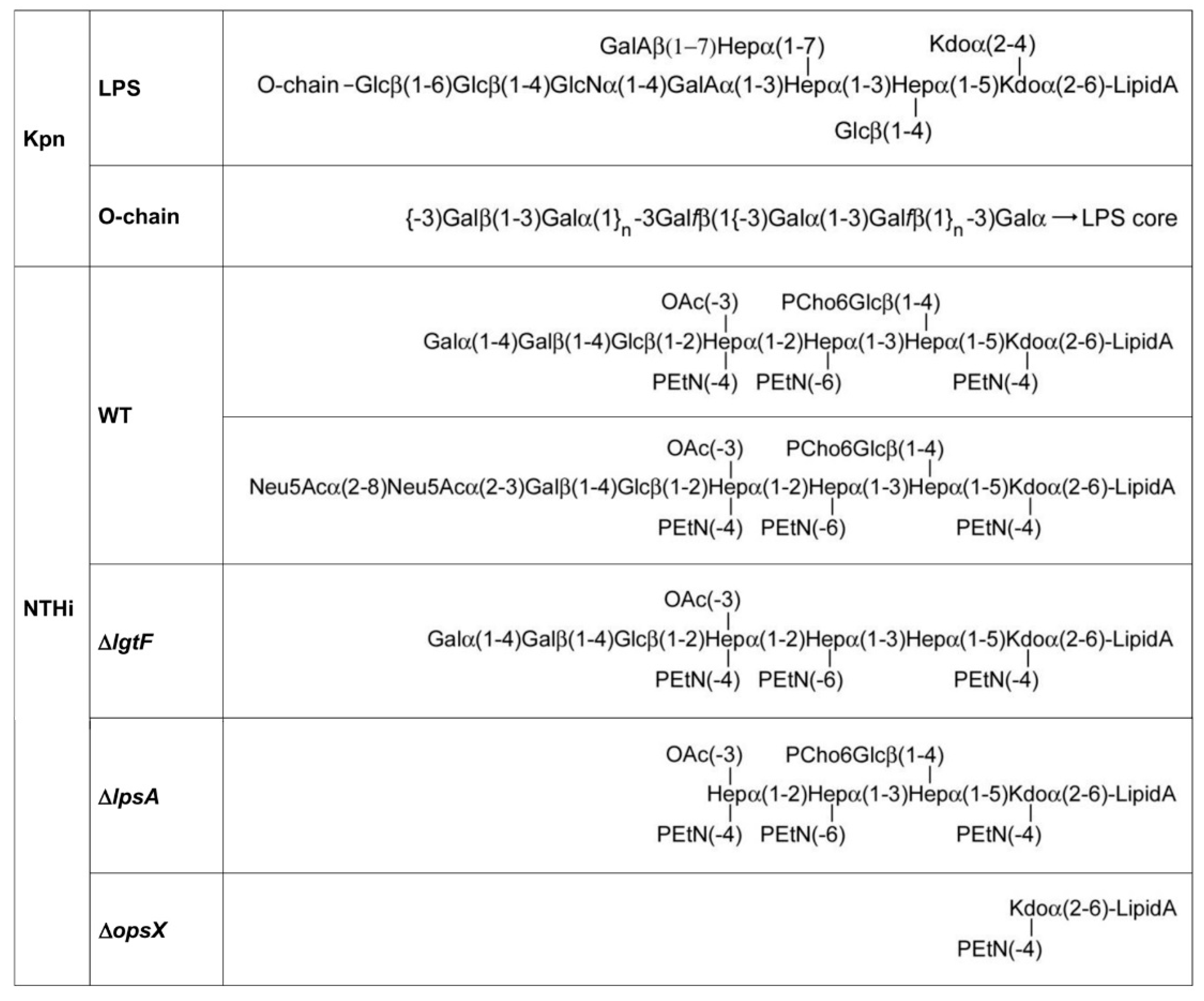
3.2. Involvement of Kpn LPS in Galectin Binding to Bacterial Cells
3.3. Involvement of NTHi LOS in Galectin Binding to Bacterial Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arthur, C.M.; Patel, S.R.; Mener, A.; Kamili, N.A.; Fasano, R.M.; Meyer, E.; Winkler, A.M.; Sola-Visner, M.; Josephson, C.D.; Stowell, S.R. Innate immunity against molecular mimicry: Examining galectin-mediated antimicrobial activity. Bioessays 2015, 37, 1327–1337. [Google Scholar] [CrossRef]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef]
- Liu, F.T.; Rabinovich, G.A. Galectins: Regulators of acute and chronic inflammation. Ann. N. Y. Acad. Sci. 2010, 1183, 158–182. [Google Scholar] [CrossRef] [PubMed]
- Cerliani, J.P.; Blidner, A.G.; Toscano, M.A.; Croci, D.O.; Rabinovich, G.A. Translating the ‘Sugar Code’ into immune and vascular signaling programs. Trends Biochem. Sci. 2017, 42, 255–273. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Gruppi, A. Galectins as immunoregulators during infectious processes: From microbial invasion to the resolution of the disease. Parasite Immunol. 2005, 27, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.S.; Arthur, C.M.; Evavold, B.; Roback, E.; Kamili, N.A.; Stowell, C.S.; Vallecillo-Zúniga, M.L.; Van Ry, P.M.; Dias-Baruffi, M.; Cummings, R.D.; et al. The sweet-side of leukocytes: Galectins as master regulators of neutrophil function. Front. Immunol. 2019, 10, 1762. [Google Scholar] [CrossRef] [PubMed]
- Casals, C.; Campanero-Rhodes, M.A.; García-Fojeda, B.; Solís, D. The role of collectins and galectins in lung innate immune defense. Front. Immunol. 2018, 9, 1998. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.; Wang, S.F.; Bernardes, E.S.; Liu, F.T. Galectins in host defense against microbial infections. Adv. Exp. Med. Biol. 2020, 1204, 141–167. [Google Scholar]
- Chen, H.Y.; Weng, I.C.; Hong, M.H.; Liu, F.T. Galectins as bacterial sensors in the host innate response. Curr. Opin. Microbiol. 2014, 17, 75–81. [Google Scholar] [CrossRef]
- Baum, L.G.; Garner, O.B.; Schaefer, K.; Lee, B. Microbe-host interactions are positively and negatively regulated by galectin-glycan interactions. Front. Immunol. 2014, 5, 284. [Google Scholar] [CrossRef][Green Version]
- Freudenberg, M.A.; Galanos, C. Bacterial lipopolysaccharides: Structure, metabolism and mechanisms of action. Int. Rev. Immunol. 1990, 6, 207–221. [Google Scholar] [CrossRef]
- Stowell, S.R.; Arthur, C.M.; Dias-Baruffi, M.; Rodrigues, L.C.; Gourdine, J.P.; Heimburg-Molinaro, J.; Ju, T.; Molinaro, R.J.; Rivera-Marrero, C.; Xia, B.; et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat. Med. 2010, 16, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Arthur, C.M.; McBride, R.; Berger, O.; Razi, N.; Heimburg-Molinaro, J.; Rodrigues, L.C.; Gourdine, J.P.; Noll, A.J.; von Gunten, S.; et al. Microbial glycan microarrays define key features of host-microbial interactions. Nat. Chem. Biol. 2014, 10, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Mey, A.; Leffler, H.; Hmama, Z.; Normier, G.; Revillard, J.P. The animal lectin galectin-3 interacts with bacterial lipopolysaccharides via two independent sites. J. Immunol. 1996, 156, 1572–1577. [Google Scholar] [PubMed]
- Gupta, S.K.; Masinick, S.; Garrett, M.; Hazlett, L.D. Pseudomonas aeruginosa lipopolysaccharide binds galectin-3 and other human corneal epithelial proteins. Infect. Immun. 1997, 65, 2747–2753. [Google Scholar] [CrossRef]
- Kalograiaki, I.; Euba, B.; Proverbio, D.; Campanero-Rhodes, M.A.; Aastrup, T.; Garmendia, J.; Solís, D. Combined bacteria microarray and quartz crystal microbalance approach for exploring glycosignatures of nontypeable Haemophilus influenzae and recognition by host lectins. Anal. Chem. 2016, 88, 5950–5957. [Google Scholar] [CrossRef] [PubMed]
- Schweda, E.K.; Richards, J.C.; Hood, D.W.; Moxon, E.R. Expression and structural diversity of the lipopolysaccharide of Haemophilus influenzae: Implication in virulence. Int. J. Med. Microbiol. 2007, 297, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Kalograiaki, I.; Euba, B.; Fernández-Alonso, M.D.C.; Proverbio, D.; St Geme, J.W.; Aastrup, T.; Garmendia, J.; Cañada, F.J.; Solís, D. Differential recognition of Haemophilus influenzae whole bacterial cells and isolated lipooligosaccharides by galactose-specific lectins. Sci. Rep. 2018, 8, 16292. [Google Scholar] [CrossRef]
- Campanero-Rhodes, M.A.; Llobet, E.; Bengoechea, J.A.; Solís, D. Bacteria microarrays as sensitive tools for exploring pathogen surface epitopes and recognition by host receptors. RSC Adv. 2015, 5, 7173–7181. [Google Scholar] [CrossRef]
- Yi, E.C.; Hackett, M. Rapid isolation method for lipopolysaccharide and lipid A from gram-negative bacteria. Analyst 2000, 125, 651–656. [Google Scholar] [CrossRef]
- Bertuzzi, S.; Gimeno, A.; Núñez-Franco, R.; Bernardo-Seisdedos, G.; Delgado, S.; Jiménez-Osés, G.; Millet, O.; Jiménez-Barbero, J.; Ardá, A. Unravelling the time scale of conformational plasticity and allostery in glycan recognition by human galectin-1. Chemistry 2020, 26, 15643–15653. [Google Scholar] [CrossRef]
- Kalograiaki, I.; Campanero-Rhodes, M.A.; Proverbio, D.; Euba, B.; Garmendia, J.; Aastrup, T.; Solís, D. Bacterial surface glycans: Microarray and QCM strategies for glycophenotyping and exploration of recognition by host receptors. Methods Enzymol. 2018, 598, 37–70. [Google Scholar]
- Campanero-Rhodes, M.; Childs, R.; Kiso, M.; Komba, S.; Le Narvor, C.; Warren, J.; Otto, D.; Crocker, P.; Feizi, T. Carbohydrate microarrays reveal sulphation as a modulator of siglec binding. Biochem. Biophys. Res. Commun. 2006, 344, 1141–1146. [Google Scholar] [CrossRef]
- Dam, T.; Gabius, H.; Andre, S.; Kaltner, H.; Lensch, M.; Brewer, C. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry 2005, 44, 12564–12571. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, E.; Frirdich, E.; MacLean, L.L.; Perry, M.B.; Petersen, B.O.; Duus, J.; Whitfield, C. Structures of lipopolysaccharides from Klebsiella pneumoniae. Eluicidation of the structure of the linkage region between core and polysaccharide O chain and identification of the residues at the non-reducing termini of the O chains. J. Biol. Chem. 2002, 277, 25070–25081. [Google Scholar] [CrossRef]
- Tomás, J.M.; Camprubi, S.; Merino, S.; Davey, M.R.; Williams, P. Surface exposure of O1 serotype lipopolysaccharide in Klebsiella pneumoniae strains expressing different K antigens. Infect. Immun. 1991, 59, 2006–2011. [Google Scholar] [CrossRef]
- Kohatsu, L.; Hsu, D.K.; Jegalian, A.G.; Liu, F.T.; Baum, L.G. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J. Immunol. 2006, 177, 4718–4726. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Ippel, H.; Suylen, D.; Klyosov, A.A.; Traber, P.G.; Hackeng, T.; Mayo, K.H. Binding of polysaccharides to human galectin-3 at a noncanonical site in its carbohydrate recognition domain. Glycobiology 2016, 26, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Garner, O.B.; Yun, T.; Pernet, O.; Aguilar, H.C.; Park, A.; Bowden, T.A.; Freiberg, A.N.; Lee, B.; Baum, L.G. Timing of galectin-1 exposure differentially modulates Nipah virus entry and syncytium formation in endothelial cells. J. Virol. 2015, 89, 2520–2529. [Google Scholar] [CrossRef]
- St-Pierre, C.; Manya, H.; Ouellet, M.; Clark, G.F.; Endo, T.; Tremblay, M.J.; Sato, S. Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J. Virol. 2011, 85, 11742–11751. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.L.; Chen, Y.H.; Wang, S.W.; Huang, Y.J.; Leu, C.H.; Yeh, N.C.; Chu, C.Y.; Lin, C.C.; Shieh, G.S.; Chen, Y.L.; et al. Galectin-1 binds to influenza virus and ameliorates influenza virus pathogenesis. J. Virol. 2011, 85, 10010–10020. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Pelletier, I.; Ouellet, M.; Vargas, A.; Tremblay, M.J.; Sato, S.; Barbeau, B. Induction of galectin-1 expression by HTLV-I Tax and its impact on HTLV-I infectivity. Retrovirology 2008, 5, 105. [Google Scholar] [CrossRef]
- Nita-Lazar, M.; Banerjee, A.; Feng, C.; Amin, M.N.; Frieman, M.B.; Chen, W.H.; Cross, A.S.; Wang, L.X.; Vasta, G.R. Desialylation of airway epithelial cells during influenza virus infection enhances pneumococcal adhesion via galectin binding. Mol. Immunol. 2015, 65, 1–16. [Google Scholar] [CrossRef]
- Okumura, C.Y.; Baum, L.G.; Johnson, P.J. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell Microbiol. 2008, 10, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Barboni, E.; Coade, S.; Fiori, A. The binding of mycolic acids to galectin-3: A novel interaction between a host soluble lectin and trafficking mycobacterial lipids? FEBS Lett. 2005, 579, 6749–6755. [Google Scholar] [CrossRef] [PubMed]
- Bogoeva, V.; Rangelov, M.; Todorova, N.; Lambert, A.; Bridot, C.; Yordanova, A.; Roos, G.; Grandjean, C.; Bouckaert, J. Binding of Gold(III) Porphyrin by the pro-metastatic regulatory protein human Galectin-3. Molecules 2019, 24, 4561. [Google Scholar] [CrossRef] [PubMed]
- Grass, S.; Lichti, C.F.; Townsend, R.R.; Gross, J.; St Geme, J.W. The Haemophilus influenzae HMW1C protein is a glycosyltransferase that transfers hexose residues to asparagine sites in the HMW1 adhesin. PLoS Pathog. 2010, 6, e1000919. [Google Scholar] [CrossRef]
- Lujan, A.L.; Croci, D.O.; Gambarte Tudela, J.A.; Losinno, A.D.; Cagnoni, A.J.; Mariño, K.V.; Damiani, M.T.; Rabinovich, G.A. Glycosylation-dependent galectin-receptor interactions promote Chlamydia trachomatis infection. Proc. Natl. Acad. Sci. USA 2018, 115, E6000–E6009. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.; Wang, J. Galectin-mediated immune recognition: Opsonic roles with contrasting outcomes in selected shrimp and bivalve mollusk species. Dev. Comp. Immunol. 2020, 110. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanero-Rhodes, M.A.; Kalograiaki, I.; Euba, B.; Llobet, E.; Ardá, A.; Jiménez-Barbero, J.; Garmendia, J.; Solís, D. Exploration of Galectin Ligands Displayed on Gram-Negative Respiratory Bacterial Pathogens with Different Cell Surface Architectures. Biomolecules 2021, 11, 595. https://doi.org/10.3390/biom11040595
Campanero-Rhodes MA, Kalograiaki I, Euba B, Llobet E, Ardá A, Jiménez-Barbero J, Garmendia J, Solís D. Exploration of Galectin Ligands Displayed on Gram-Negative Respiratory Bacterial Pathogens with Different Cell Surface Architectures. Biomolecules. 2021; 11(4):595. https://doi.org/10.3390/biom11040595
Chicago/Turabian StyleCampanero-Rhodes, María A., Ioanna Kalograiaki, Begoña Euba, Enrique Llobet, Ana Ardá, Jesús Jiménez-Barbero, Junkal Garmendia, and Dolores Solís. 2021. "Exploration of Galectin Ligands Displayed on Gram-Negative Respiratory Bacterial Pathogens with Different Cell Surface Architectures" Biomolecules 11, no. 4: 595. https://doi.org/10.3390/biom11040595
APA StyleCampanero-Rhodes, M. A., Kalograiaki, I., Euba, B., Llobet, E., Ardá, A., Jiménez-Barbero, J., Garmendia, J., & Solís, D. (2021). Exploration of Galectin Ligands Displayed on Gram-Negative Respiratory Bacterial Pathogens with Different Cell Surface Architectures. Biomolecules, 11(4), 595. https://doi.org/10.3390/biom11040595







