Inherent Safety Assessment of Industrial-Scale Production of Chitosan Microbeads Modified with TiO2 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
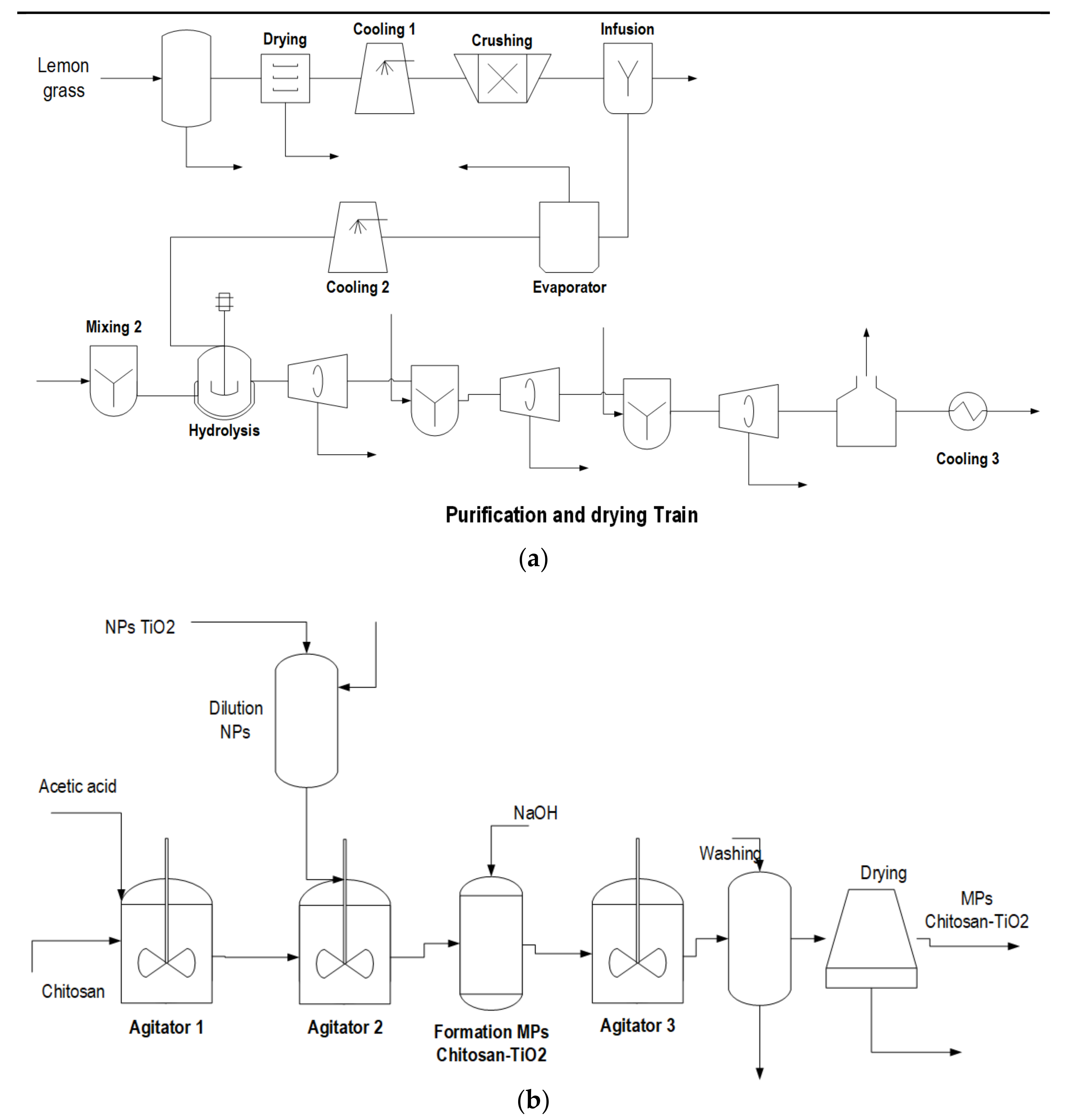
2.1. Process Description
2.2. Process Safety Assessment
3. Results
3.1. Material Characteristics
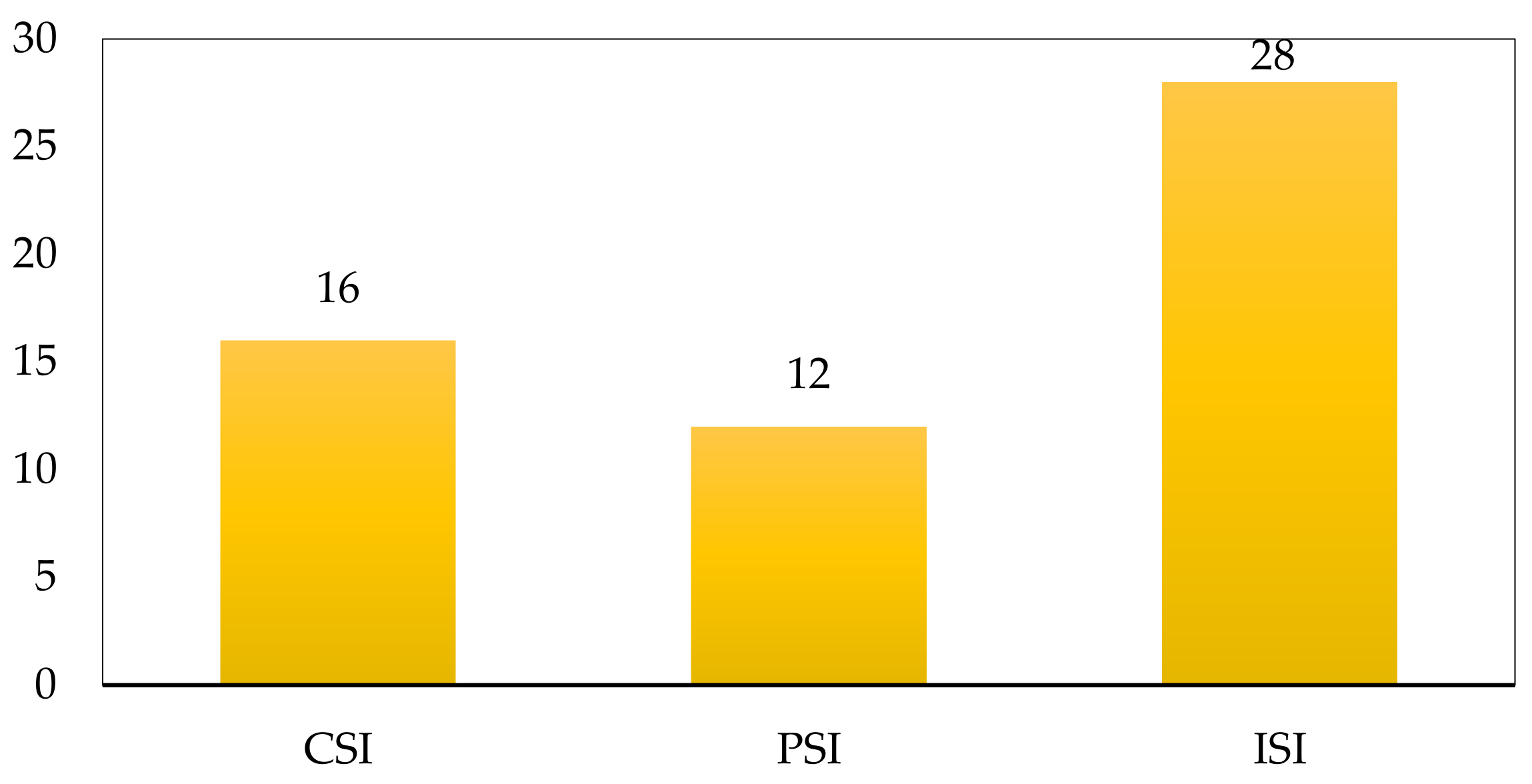
3.2. Safety Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meramo-Hurtado, S.; Ojeda-Delgado, K.; Sanchez-Tuiran, E. Environmental assessment of a biorefinery: Case study of a purification stage in biomass gasification. Contemp. Eng. Sci. 2018, 11, 113–120. Available online: http://www.m-hikari.com/ces/ces2018/ces1-4-2018/813.html (accessed on 15 January 2021). [CrossRef]
- Mangili, P.V.; Prata, D.M. Improvement of the butyl acetate process through heat integration: A sustainability-based assessment. Chem. Eng. Process. Process Intensif. 2019, 135, 93–107. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.; Patiño, D.; Cogollo-herrera, K.; Patino-Ruiz, D.; Cogollo-herrera, K.; Herrera, A.; Gonzalez-Delgado, A. Physico-Chemical Characterization of Superficial Water and Sediments from Cartagena Bay. Contemp. Eng. Sci. 2018, 11, 1571–1578. [Google Scholar] [CrossRef][Green Version]
- Choi, S.K.; Kim, J.H.; Park, Y.S.; Kim, Y.J.; Chang, H.I. An Efficient Method for the Extraction of Astaxanthin from the Red Yeast Xanthophyllomyces dendorhous. J. Microbiol. Biotechnol. 2007, 17, 847–852. [Google Scholar]
- Bonfante-Alvarez, H.; De Avila-Montiel, G.; Herrera-Barros, A.; Torrenegra-Alarcón, M.; González-Delgado, Á.D. Evaluation of five chitosan production routes with astaxanthin recovery from shrimp exoskeletons. Chem. Eng. Trans. 2018, 70, 1969–1974. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85051413604&doi=10.3303%2FCET1870329&partnerID=40&md5=5c31cdd8fd183a644edbdebf5279eec3 (accessed on 16 January 2021). [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Mahata, S.; Mahato, S.S.; Nandi, M.M.; Mondal, B. Synthesis of TiO2 nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. AIP Conf. Proc. 2011, 1461, 225–228. [Google Scholar] [CrossRef]
- Hendershot, D.C. Green chemistry and process safety. J. Chem. Health Saf. 2015, 22, 39. [Google Scholar] [CrossRef]
- Valanciene, E.; Jonuskiene, I.; Syrpas, M.; Augustiniene, E.; Matulis, P.; Simonavicius, A.; Malys, N. Advances and prospects of phenolic acids production, biorefinery and analysis. Biomolecules 2020, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, V.; Romanazzi, F.; Gubitosa, J.; Fini, P.; Romita, R.; Agostiano, A.; Petrella, A.; Cosma, P. Chitosan film as eco-friendly and recyclable bio-adsorbent to remove/recover diclofenac, ketoprofen, and their mixture from wastewater. Biomolecules 2019, 9, 571. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Hashim, H.; Hassim, M.H. Inherent safety assessment technique for preliminary design stage. Chem. Eng. Trans. 2017, 56, 1345–1350. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.; Urbina-Suarez, N.; González-Delgado, Á. Computer-aided environmental and exergy analyses of a large-scale production of chitosan microbeads modified with TiO2 nanoparticles. J. Clean. Prod. 2019, 273. [Google Scholar] [CrossRef]
- Kidam, K.; Hassim, M.H.; Hurme, M. Enhancement of Inherent Safety in Chemical Industry. Chem. Eng. Trans. 2008, 13, 287. [Google Scholar]
- Heikkila, A.-M. Inherent Safety in Process Plant Design; VTT Technical Research Centre of Finland: Espoo, Finland, 1999; pp. 1–132. [Google Scholar]
- Meramo-Hurtado, S.I.; González-Delgado, Á.D.; Rehmann, L.; Quiñones-Bolaños, E.; Mehrvar, M. Comparison of Biobutanol Production Pathways via Acetone–Butanol–Ethanol Fermentation Using a Sustainability Exergy-Based Metric. ACS Omega 2020, 5, 18710–18730. [Google Scholar] [CrossRef] [PubMed]
- Koller, G.; Fischer, U.; Hungerbühler, K. Assessing Safety, Health, and Environmental Impact Early during Process Development. Ind. Eng. Chem. Res. 2000, 39, 960–972. [Google Scholar] [CrossRef]
- Gonzalez-Delgado, A. Computer-assisted Hira Assessment of Hazardous in the Production of Crude Palm Oil. Palmas 2019, 40, 56–66. [Google Scholar]
- Khan, F.I.; Amyotte, P.R. I2SI: A comprehensive quantitative tool for inherent safety and cost evaluation. J. Loss Prev. Process Ind. 2005, 18, 310–326. [Google Scholar] [CrossRef]
- Rathnayaka, S.; Khan, F.; Amyotte, P. Risk-based process plant design considering inherent safety. Saf. Sci. 2014, 70, 438–464. [Google Scholar] [CrossRef]
- Zuorro, A.; Cassiani-Cassiani, D.; Meza-González, D.A.; Moreno-Sader, K.A.; González-Delgado, Á.D. Evaluation of shrimp waste valorization combining computer-aided simulation and numerical descriptive inherent safety technique (NuDIST). Appl. Sci. 2020, 10, 5339. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.; Moreno-Sader, K.; González-Delgado, Á. Simulation and exergy analysis of TiO2 nanoparticles production via green chemistry. PeerJ 2019, e8113. [Google Scholar] [CrossRef] [PubMed]
- Meramo, S.I.; Bonfante, H.; De Avila-Montiel, G.; Herrera-Barros, A.; Gonzalez-Delgado, A. Environmental assessment of a large-scale production of TiO2 nanoparticles via green chemistry. Chem. Eng. Trans. 2018, 70, 1063–1068. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85051387159&doi=10.3303%2FCET1870178&partnerID=40&md5=b42cc3cbf5f56bd22e3e25ce9794661d (accessed on 7 January 2021). [CrossRef]
- Meramo-Hurtado, S.; Herrera-Barros, A.; Gonzalez-Delgado, A. Evaluation of large-scale production of chitosan microbeads modified with nanoparticles based on exergy analysis. Energies 2019, 12, 1200. [Google Scholar] [CrossRef]
- Park, S.; Xu, S.; Rogers, W.; Pasman, H.; El-Halwagi, M.M. Incorporating inherent safety during the conceptual process design stage: A literature review. J. Loss Prev. Process Ind. 2020, 63, 104040. [Google Scholar] [CrossRef]
- Zheng, K.; Lou, H.H.; Gangadharan, P.; Kanchi, K. Incorporating sustainability into the conceptual design of chemical process-reaction routes selection. Ind. Eng. Chem. Res. 2012, 51, 9300–9309. [Google Scholar] [CrossRef]
- Lawrence, D. Quantifying Inherent Safety of Chemical Process Routes. Ph.D. Thesis, Loughborough University, Loughborough, UK, 1996. [Google Scholar]
- Vargas, M.A.; Rodríguez-Páez, J.E. Amorphous TiO2 nanoparticles: Synthesis and antibacterial capacity. J. Non. Cryst. Solids 2017, 459, 192–205. [Google Scholar] [CrossRef]
- Heikkilä, A.M.; Hurme, M.; Järveläinen, M. Safety considerations in process synthesis. Comput. Chem. Eng. 1996, 20. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.I.; Sanchez-Tuiran, E.; Ponce-Ortega, J.M.; El-Halwagi, M.M.; Ojeda-Delgado, K.A. Synthesis and Sustainability Evaluation of a Lignocellulosic Multifeedstock Biorefinery Considering Technical Performance Indicators. ACS Omega 2020, 5, 9259–9275. [Google Scholar] [CrossRef] [PubMed]
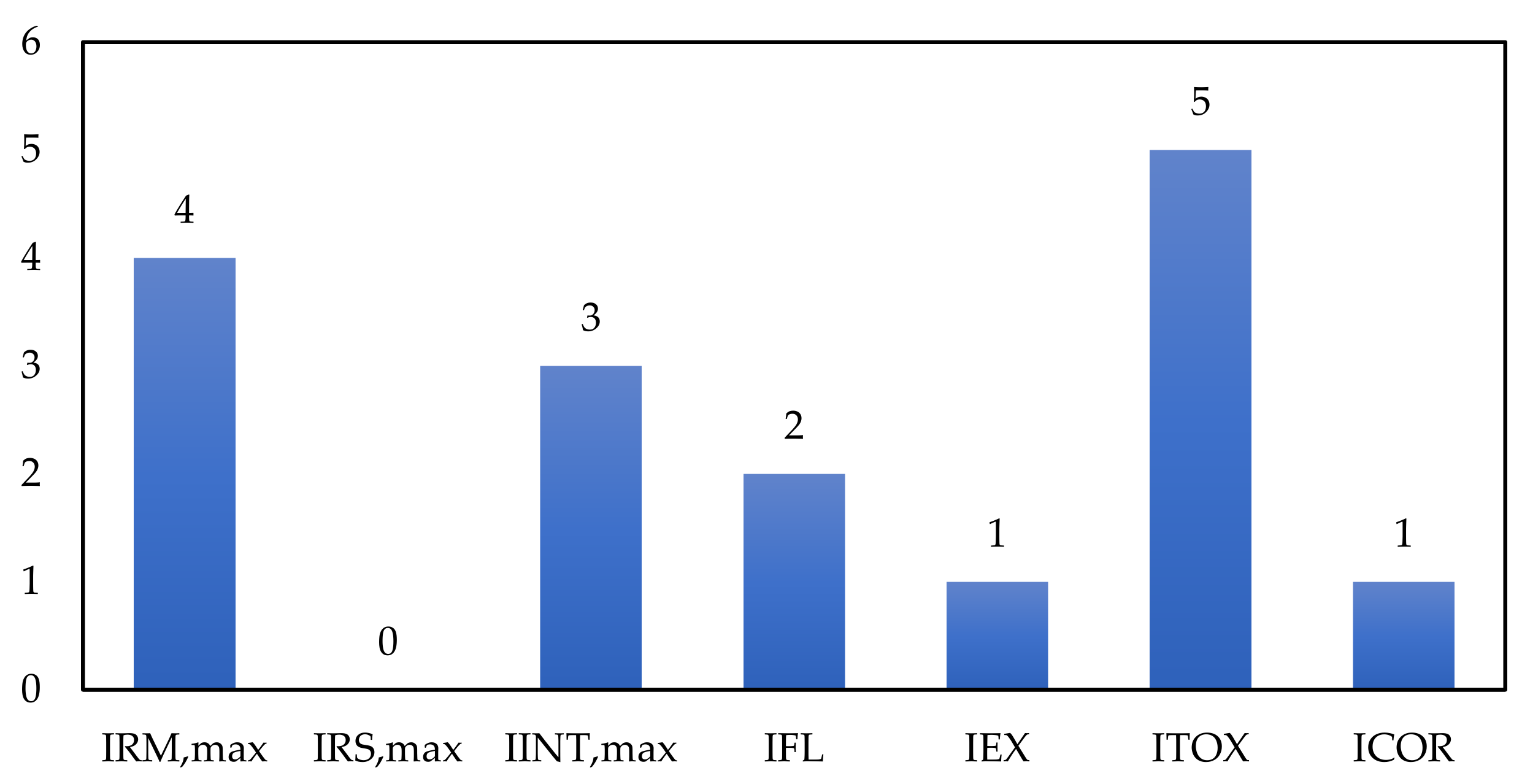

| Dangerous Chemical Reactions Sub-Index | Reference Ranges | |
|---|---|---|
| Heat of reaction (main) | IRM, MAX | 0 to 4 |
| Heat of reaction (sides) | IRS, MAX | 0 to 4 |
| Chemical interactions | IINT, MAX | 0 to 4 |
| Dangerous Substances Sub-Indexes | ||
| Inflammability | IFL | 0 to 4 |
| Explosivity | IEX | 0 to 4 |
| Toxicity | ITOX | 0 to 6 |
| Corrosivity | ICOR | 0 to 2 |
| Process Conditions Sub-Indexes | Reference Ranges | |
|---|---|---|
| Process inventory | II | 0 to 6 |
| Process temperature | IT, MAX | 0 to 4 |
| Process pressure | IP, MAX | 0 to 4 |
| Process System Sub-Indexes | ||
| Equipment | IEQ | 0 to 4 |
| Process structure | IST,MAX | 0 to 4 |
| Indicator for Main Reaction | |
|---|---|
| Main reaction | Hydrolysis |
| Products | |
| Substance | (J/g) |
| TiO2 | −12,491.5 |
| Propanol | −5292.8 |
| Reagents | |
| Water | −1587.8 |
| TTIP | −5526.5 |
| Total heat of reaction | −24,960.92 |
| IRM | 4 |
| Substance | TTIP | EtOH | PrOH | AA | NaOH |
|---|---|---|---|---|---|
| Flash point (°C) | 47.00 | 13.90 | 15.00 | 39.00 | − |
| IFLA | 2 | 3 | 3 | 2 | 0 |
| UEL-LEL | − | 19–3,3 | 2,2 | − | − |
| IEXP | 1 | 1 | 1 | 0 | 0 |
| TLV (ppm) | 0,86 | 530,71 | 81,36 | 10 | 1,22 |
| ITOX | 5 | 2 | 3 | 4 | 4 |
| IFLA + IEXP + ITOX | 8 | 6 | 7 | 6 | 4 |
| Temperature Indicator | Process Indicator | ||
|---|---|---|---|
| Maximum Temperature (°C) | 550 | Maximum pressure (atm) | 1 |
| IT, MAX | 3 | IP,MAX | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meramo-Hurtado, S.; Ceballos-Arrieta, N.; Cortes-Caballero, J.; Leon-Pulido, J.; Gonzalez-Quiroga, A.; Gonzalez-Delgado, Á.D. Inherent Safety Assessment of Industrial-Scale Production of Chitosan Microbeads Modified with TiO2 Nanoparticles. Biomolecules 2021, 11, 568. https://doi.org/10.3390/biom11040568
Meramo-Hurtado S, Ceballos-Arrieta N, Cortes-Caballero J, Leon-Pulido J, Gonzalez-Quiroga A, Gonzalez-Delgado ÁD. Inherent Safety Assessment of Industrial-Scale Production of Chitosan Microbeads Modified with TiO2 Nanoparticles. Biomolecules. 2021; 11(4):568. https://doi.org/10.3390/biom11040568
Chicago/Turabian StyleMeramo-Hurtado, Samir, Nicolas Ceballos-Arrieta, Jose Cortes-Caballero, Jeffrey Leon-Pulido, Arturo Gonzalez-Quiroga, and Ángel Dario Gonzalez-Delgado. 2021. "Inherent Safety Assessment of Industrial-Scale Production of Chitosan Microbeads Modified with TiO2 Nanoparticles" Biomolecules 11, no. 4: 568. https://doi.org/10.3390/biom11040568
APA StyleMeramo-Hurtado, S., Ceballos-Arrieta, N., Cortes-Caballero, J., Leon-Pulido, J., Gonzalez-Quiroga, A., & Gonzalez-Delgado, Á. D. (2021). Inherent Safety Assessment of Industrial-Scale Production of Chitosan Microbeads Modified with TiO2 Nanoparticles. Biomolecules, 11(4), 568. https://doi.org/10.3390/biom11040568







