Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium cruentum. (S.F.Gray) Nägeli
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Biological Material
2.3. Extraction of Polysaccharides
2.4. Total Carbon (C), Hydrogen (H), Nitrogen (N) and Sulfur (S)
2.5. Biochemical Composition of Biomass from P. cruentum
2.6. Fourier Transform Infrared Spectroscopy (FT-IR)
2.7. Gas Chromatography–Mass Spectrometry (GC-MS)
2.7.1. Chemicals and Reagents
2.7.2. Gas Chromatography/Mass Spectrometry (GC-MS) Analysis
2.8. Lipopolysaccharides (LPS) Contamination Assay
2.9. Antioxidant Capacity as ABTS Assay Scavenging of Free Radical in PcSPs and Biomass
2.10. Cell Cultures
2.11. Cytotoxic Effect Assay
2.12. Cell Cycle Analysis by Flow Cytometry
2.13. Macrophage Proliferation Assay (RAW 264.7)
2.14. Determination of Cytokines
2.15. In Vivo Study
2.15.1. Animals and Ethics Statement
2.15.2. Experimental Design and Treatment
2.15.3. White Blood Cell Differential
2.15.4. Peritoneal Macrophage Extraction and Counting
2.16. Statistical Analysis
3. Results
3.1. Chemical Characterization
3.1.1. Elemental Composition of PcSPs and Biomass and Chemical Composition of Biomass
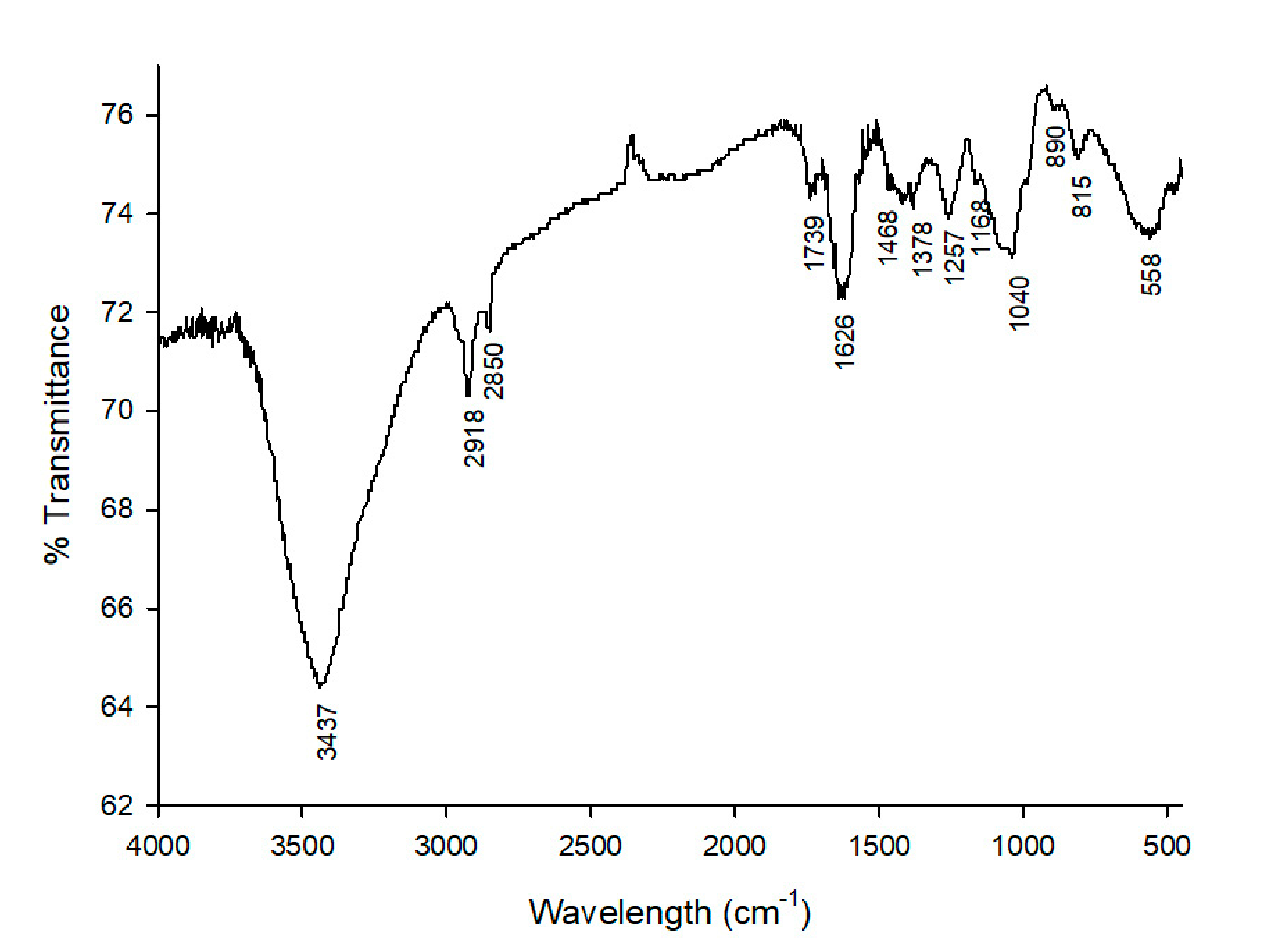
3.1.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.1.3. Gas Chromatography–Mass Spectrometry (GC-MS)
3.2. Biological Assessment
3.2.1. LPS Contamination
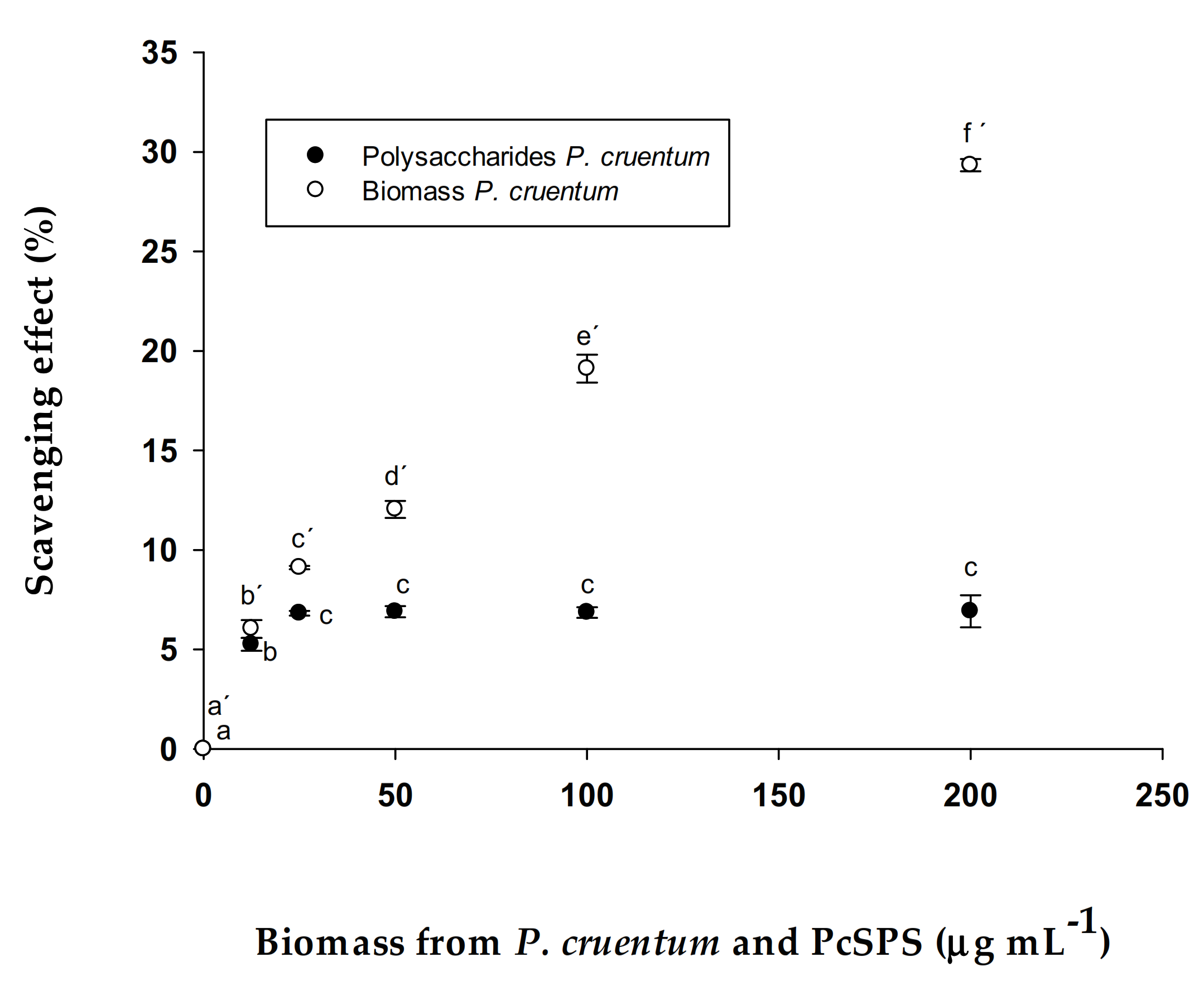
3.2.2. Antioxidant Activity (ABTS method) in PcSPs and Biomass
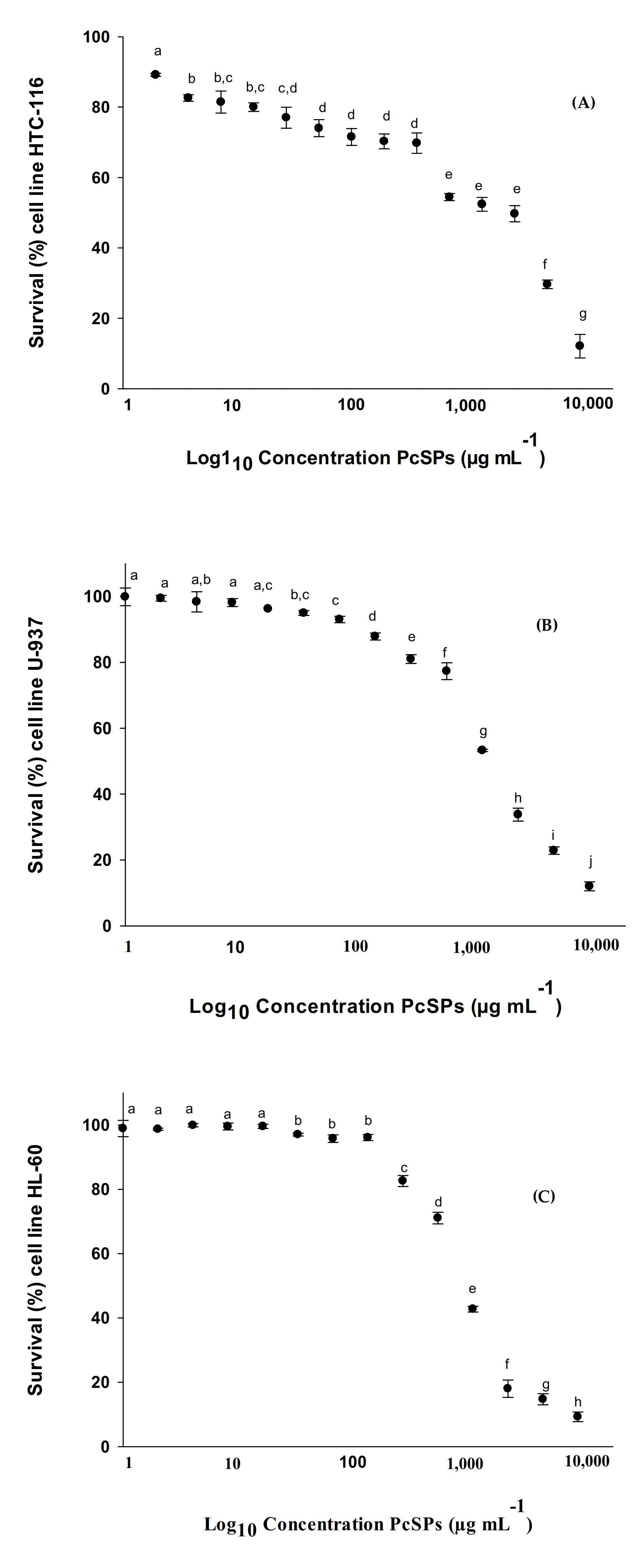
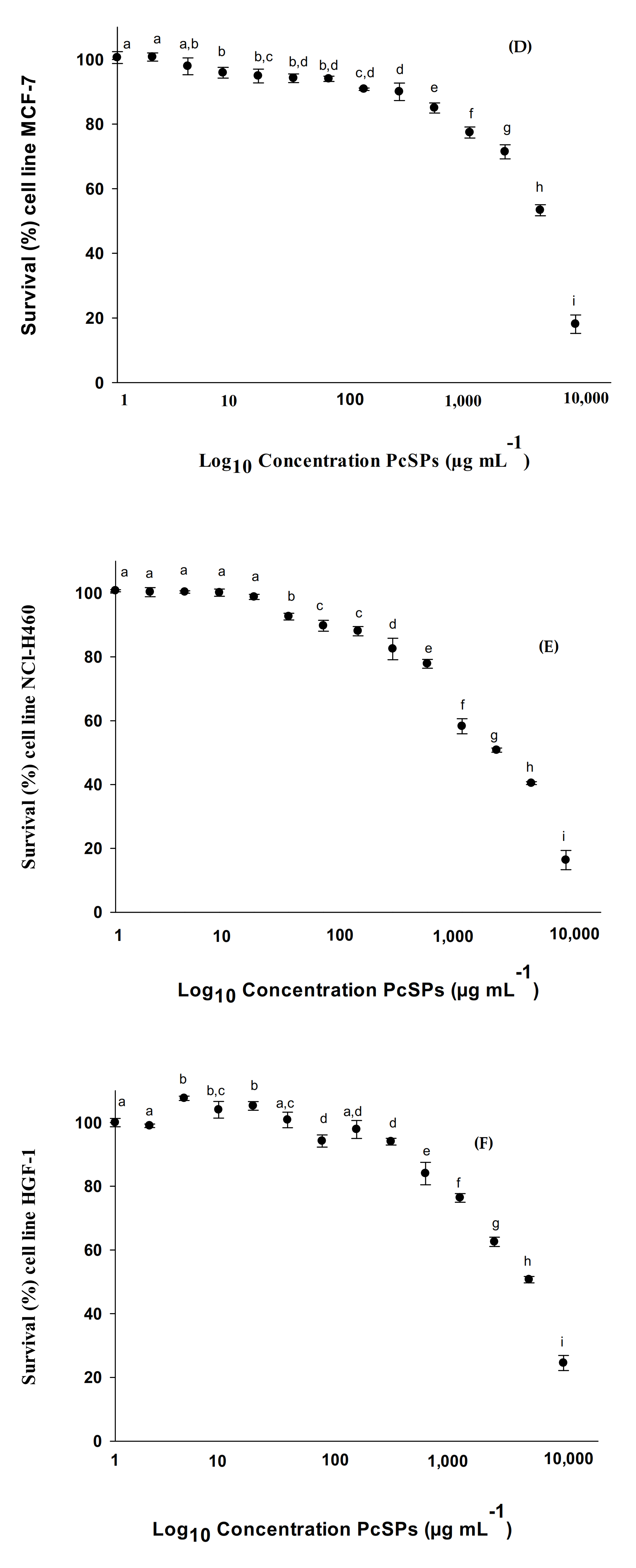
3.2.3. Cytotoxic Effect in HTC-116, U-937, HL-60, MCF-7, NCI-H460 and HGF-1 Cell Lines
3.2.4. Cell Cycle Analysis by Flow Cytometry in Cell Line HL-60
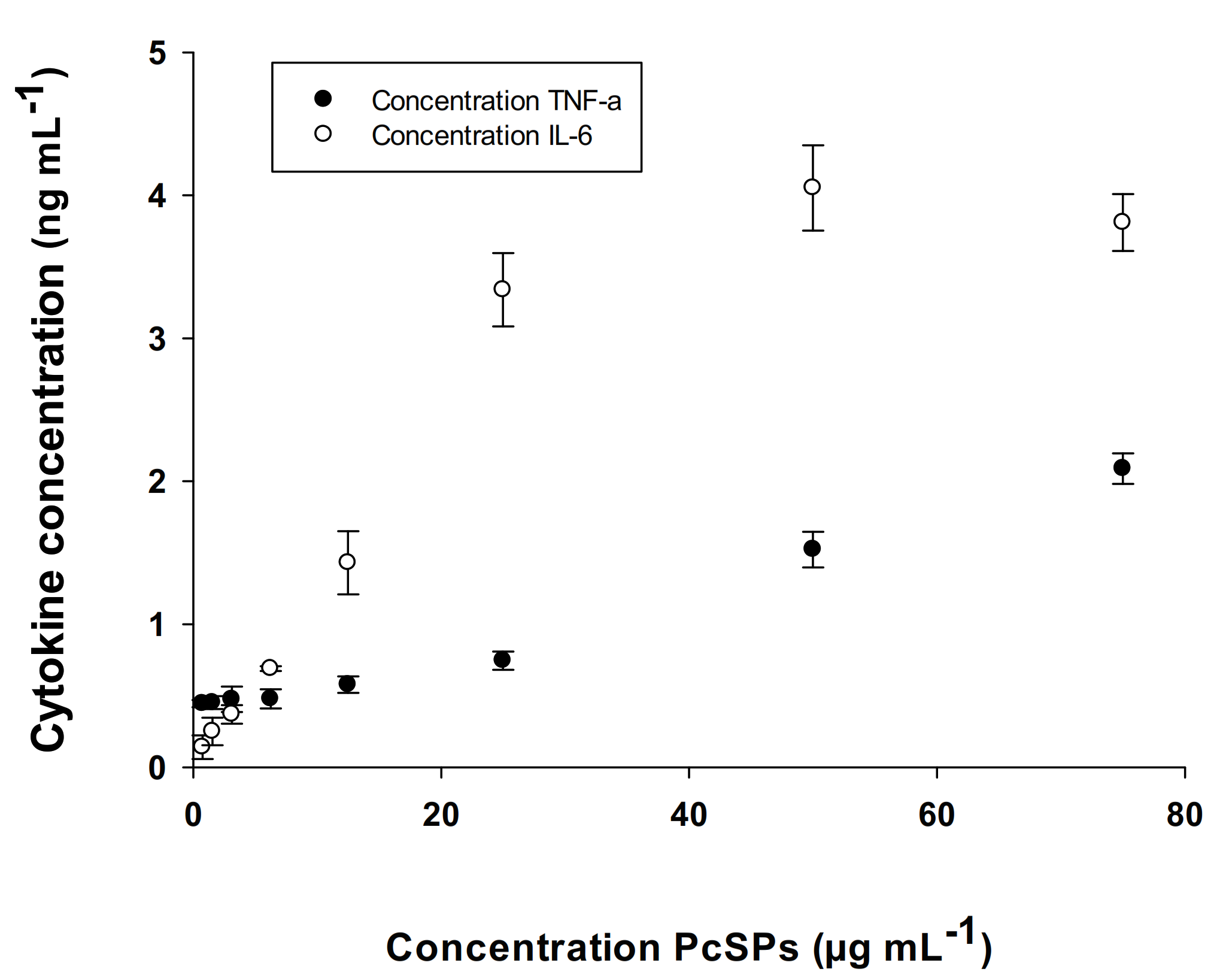
3.2.5. Determination of Cytokines (IL-6 and TNF-α)
3.3. In Vivo Study
3.3.1. Effects of Treatments on White Blood Cell Differential
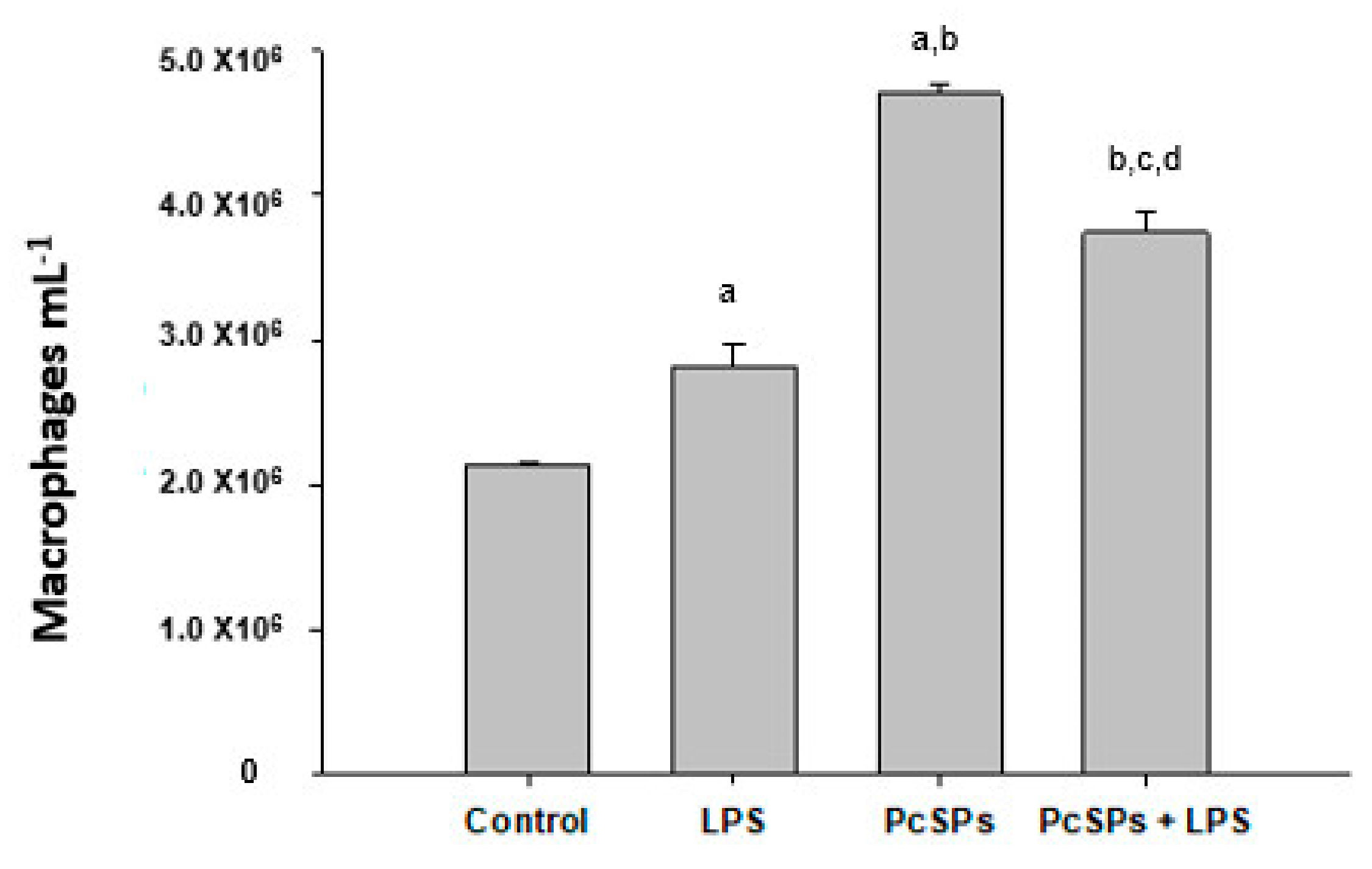
3.3.2. Effects of Treatments on Amount of Peritoneal Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falkowski, P.G. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature 1997, 387, 272–275. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2008, 25, 35–94. [Google Scholar] [CrossRef]
- Abdala Díaz, R.T.; Chabrillón, M.; Cabello-Pasini, A.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Characterization of polysaccharides from Hypnea spinella (Gigartinales) and Halopithys incurva (Ceramiales) and their effect on RAW 264.7 macrophage activity. J. Appl. Phycol. 2010, 23, 523–528. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–233. [Google Scholar] [CrossRef] [PubMed]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, Y.; Monteiro, M.; Bandarra, N.; Gökpinar, Ş.; Işik, O. The effect of low temperature on fatty acid composition and tocopherols of the red microalga, Porphyridium cruentum. J. Appl. Phycol. 2007, 19, 223–227. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Navarro-Juárez, R.; López-Martínez, J.; Campra-Madrid, P.; Rebolloso-Fuentes, M.M. Functional properties of the biomass of three microalgal species. J. Food Eng. 2004, 65, 511–517. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Bergman, M.; Van-Moppes, D.; Grossman, S.; Arad, S. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Trommer, H.; Neubert, R.H.H. The examination of polysaccharides as potential antioxidative compounds for topical administration using a lipid model system. Int. J. Pharm. 2005, 298, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Parish, C.R.; Coombe, D.R.; Jakobsen, K.B.; Bennett, F.A.; Underwood, P.A. Evidence that sulphated polysaccharides inhibit tumour metastasis by blocking tumour-cell-derived heparanases. Int. J. Cancer 1987, 40, 511–518. [Google Scholar] [CrossRef]
- Ley, A.C.; Butler, W.L.; Bryant, D.A.; Glazer, A.N. Isolation and function of allophycocyanin B of Porphyridium cruentum. Plant Physiol. 1977, 59, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Shiran, D.; Khozin, I.; Heimer, Y.M.; Cohen, Z. Biosynthesis of eicosapentaenoic acid in the microalga Porphyridium cruentum. I: The use of externally supplied fatty acids. Lipids 1996, 31, 1277–1282. [Google Scholar] [CrossRef]
- Cohen, Z.; Shiran, D.; Khozin, I.; Heimer, Y.M. Fatty acid unsaturation in the red alga Porphyridium cruentum. Is the methylene interrupted nature of polyunsaturated fatty acids an intrinsic property of the desaturases? Biochim. Biophys. Acta-Lipids Lipid Metab. 1997, 1344, 59–64. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Yu, H.Z.; Adlerstein, D.; Didi-Cohen, S.; Heimer, Y.M.; Cohen, Z. Triacylglycerols of the red microalga Porphyridium cruentum can contribute to the biosynthesis of eukaryotic galactolipids. Lipids 2000, 35, 881–889. [Google Scholar] [CrossRef]
- Ginzberg, A.; Cohen, M.; Sod-Moriah, U.A.; Shany, S.; Rosenshtrauch, A.; Arad, S. Chickens fed with biomass of the red microalga Porphyridium sp. have reduced blood cholesterol level and modified fatty acid composition in egg yolk. J. Appl. Phycol. 2000, 12, 325–330. [Google Scholar] [CrossRef]
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, G.; Gislerød, H.R. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Gloaguen, V.; Ruiz, G.; Morvan, H.; Mouradi-Givernaud, A.; Maes, E.; Krausz, P.; Strecker, G. The extracellular polysaccharide of Porphyridium sp.: An NMR study of lithium-resistant oligosaccharidic fragments. Carbohydr. Res. 2004, 339, 97–103. [Google Scholar] [CrossRef]
- Geresh, S.; Arad, S. The extracellular polysaccharides of the red microalgae: Chemistry and rheology. Bioresour. Technol. 1991, 38, 195–201. [Google Scholar] [CrossRef]
- Geresh, S.; Arad, S.; Levy-Ontman, O.; Zhang, W.; Tekoah, Y.; Glaser, R. Isolation and characterization of poly- and oligosaccharides from the red microalga Porphyridium sp. Carbohydr. Res. 2009, 344, 343–349. [Google Scholar] [CrossRef]
- Fabregas, J.; García, D.; Fernandez-Alonso, M.; Rocha, A.I.; Gómez-Puertas, P.; Escribano, J.M.; Otero, A.; Coll, J.M. In vitro inhibition of the replication of haemorrhagic septicaemia virus (VHSV) and African swine fever virus (ASFV) by extracts from marine microalgae. Antivir. Res. 1999, 44, 67–73. [Google Scholar] [CrossRef]
- Huheihel, M.; Ishanu, V.; Tal, J.; Arad, S.M. Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods 2002, 50, 189–200. [Google Scholar] [CrossRef]
- Talyshinsky, M.M.; Souprun, Y.Y.; Huleihel, M.M. Anti-viral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int. 2002, 2, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.H.; Jolles, P. Immunostimulating agents: What next? A review of their present and potential medical applications. Eur. J. Biochem. 1996, 242, 1–19. [Google Scholar] [CrossRef]
- Morris Quevedo, H.J.; Martínez Manrique, C.E.; Abdala Díaz, R.T.; Cobas Pupo, G. Evidencias preliminares de la actividad inmunomoduladora de la fracción polisacárida de origen marino Pc-1. Rev. Cuba. Oncol. 2000, 16, 171–177. [Google Scholar]
- Vonshak, A. Porphyridium; Borowitzka, M.A., Borowitzka, J.L., Eds.; Cambridge Univ. Press: Cambridge, UK, 1988. [Google Scholar]
- Abdala Díaz, R.T.; Chabrillón, M.; Cabello Pasini, A.; López Soler, B.; Figueroa, F.L. Effect of Porphyridium cruentum polysaccharides on the activity of murine macrophage cell line RAW 264.7. Cienc. Mar. 2010, 36, 345–353. [Google Scholar] [CrossRef]
- Abdala Díaz, R.T.; Casas Arrojo, V.; Arrojo Agudo, M.A.; Cárdenas, C.; Dobretsov, S.; Figueroa, F.L. Immunomodulatory and antioxidant activities of sulfated polysaccharides from Laminaria ochroleuca, Porphyra umbilicalis and Gelidium corneum. Mar. Biotechnol. 2019, 21, 577–587. [Google Scholar] [CrossRef]
- Folch, J.; Less, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Lavín, P.L.; Lanfer Marquez, U.M.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Biochem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ismail, B.P. Ash Content Determination. In Food Analysis Laboratory Manual; Food Science Text Series; Springer International: St Paul, MN, USA, 2017; pp. 117–119. ISBN 978-3-319-44125-2. [Google Scholar]
- Ojeda, J.J.; Dittrich, M. Fourier transform infrared spectroscopy for molecular analysis of microbial cells. Microb. Syst. Biol. 2012, 881, 187–211. [Google Scholar] [CrossRef]
- Kim, J.S.; Laskowich, E.R.; Arumugham, R.G.; Kaiser, R.E.; Macmichael, G.J. Determination of saccharide content in pneumococcal polysaccharides and conjugate vaccines by GC-MSD. Anal. Biochem. 2005, 347, 262–274. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Vijayabaskar, P.; Vaseela, N. In vitro antioxidant properties of sulfated polysaccharide from brown marine algae Sargassum tenerrimum. Asian Pac. J. Trop. Dis. 2012, 2, S890–S896. [Google Scholar] [CrossRef]
- Martınez, C.; Delgado, M.; Pozo, D.; Leceta, J.; Calvo, J.R.; Ganea, D.; Gomariz, R.P. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide modulate endotoxin-induced IL-6 production by murine peritoneal macrophages. J. Leukoc. Biol. 1998, 63, 591–601. [Google Scholar] [CrossRef]
- Lundberg, P.; Skoda, R. Hematology testing in mice. Curr. Protoc. Mouse Biol. 2011, 1, 323–346. [Google Scholar] [CrossRef] [PubMed]
- Houwen, B. Blood film preparation and staining procedures. Lab. Hematol. 2002, 22, 1–14. [Google Scholar] [CrossRef]
- Ray, A.; Dittel, B.N. Isolation of mouse peritoneal cavity cells. J. Vis. Exp. 2010, 10–12. [Google Scholar] [CrossRef]
- Rebolloso Fuentes, M.M.; Acién Femández, F.G.; Sánchez Pérez, J.A.; Gil García, M.D.; Guil Guerrero, J.L. Note. Nutrient composition of the biomass of the microalga Porphyridium cruentum. Food Sci. Technol. Int. 2000, 6, 129–135. [Google Scholar] [CrossRef]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; de Oliveira, J.V.; Junior, A.F.; Derner, R.B.; Sant’Anna, E.S. Chemical characterization of six microalgae with potential utility for food application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Rebolloso Fuentes, M.M.; Acién Fernández, F.G.; Sánchez Pérez, J.A.; Guil Guerrero, J.L. Biomass nutrient profiles of the microalga Porphyridium cruentum. Food Chem. 2000, 70, 345–353. [Google Scholar] [CrossRef]
- Shanura Fernando, I.P.; Asanka Sanjeewa, K.K.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kim, E.A.; Gunasekara, U.K.; Abeytunga, D.T.U.; Nanayakkara, C.; De Silva, E.D.; et al. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. Algae 2017, 32, 75–86. [Google Scholar] [CrossRef]
- Trabelsi, L.; Chaieb, O.; Mnari, A.; Abid-Essafi, S.; Aleya, L. Partial characterization and antioxidant and antiproliferative activities of the aqueous extracellular polysaccharides from the thermophilic microalgae Graesiella sp. BMC Complement. Altern. Med. 2016, 16, 210. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Liu, H.; Zhou, A.; Cao, Y.; Liu, X. Preliminary characterization of the structure and immunostimulatory and anti-aging properties of the polysaccharide fraction of: Haematococcus pluvialis. RSC Adv. 2018, 8, 9243–9252. [Google Scholar] [CrossRef]
- Liao, M.L.; Chiovitti, A.; Munro, S.L.A.; Craik, J.D.; Kraft, G.T.; Bacic, A. Sulfated galactans from Australian specimens of the red alga Phacelocarpus peperocarpos (Gigartinales, Rhodophyta). Carbohydr. Res. 1996, 296, 237–247. [Google Scholar] [CrossRef]
- Percival, E.; Foyle, R.A.J. The extracellular polysaccharides of Porphyridium cruentum and Porphyridium aerugineum. Carbohydr. Res. 1979, 72, 165–176. [Google Scholar] [CrossRef]
- Huang, J.; Chen, B.; You, W. Studies on separation of extracellular polysaccharide from Porphyridium cruentum and its anti-HBV activity in vitro. Chin. J. Mar. Drugs 2005, 24, 18–21. [Google Scholar]
- De Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Radonić, A.; Thulke, S.; Achenbach, J.; Kurth, A.; Vreemann, A.; König, T.; Walter, C.; Possinger, K.; Nitsche, A. Anionic polysaccharides from phototrophic microorganisms exhibit antiviral activities to vaccinia virus. J. Antivir. Antiretrovir. 2010, 2, 051–055. [Google Scholar] [CrossRef]
- Garcia, D.; Morales, E.; Dominguez, A.; Fábregas, J. Productividad Mixotrófica del Exopolisacárido Sulfatado con la Microalga Marina Porphyridium cruentum. In Proceedings of the In Communicaciones del III Congreso Ibérico de Biotecnología—Biotec’96, Valladolid, Spain, 10–13 September 1996; Universidad de Valladolid: Valladolid, Spain, 1996; pp. 591–592. [Google Scholar]
- Nikolova, B.; Semkova, S.; Tsoneva, I.; Antov, G.; Ivanova, J.; Vasileva, I.; Kardaleva, P.; Stoineva, I.; Christova, N.; Nacheva, L.; et al. Characterization and potential antitumor effect of a heteropolysaccharide produced by the red alga Porphyridium sordidum. Eng. Life Sci. 2019, 19, 978–985. [Google Scholar] [CrossRef]
- Gardeva, E.; Toshkova, R.; Minkova, K.; Gigova, L. Cancer protective action of polysaccharide, derived from red microalga Porphyridium cruentum—A biological background. Biotechnol. Biotechnol. Equip. 2009, 23 (Suppl. S1), 783–787. [Google Scholar] [CrossRef]
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and fractionation of exopolysaccharides from Porphyridium cruentum. Bioresour. Technol. 2013, 145, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Bernaerts, T.M.M.; Kyomugasho, C.; Van Looveren, N.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Molecular and rheological characterization of different cell wall fractions of Porphyridium cruentum. Carbohydr. Polym. 2018, 195, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Guo, G.; Pu, Y.; Yan, B. The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef]
- Rudic, V.; Cepoi, L.; Rudic, L.; Chiriac, T.; Nicorici, A.; Todosiciuc, A.; Gutsul, T. Synthesis of CdSe Nanoparticles and their Effect on the Antioxidant Activity of Spirulina platensis and Porphyridium cruentum Cells. In Proceedings of the International Conference Nanotechnologies and Biomedical Engineering, Chisinau, Moldova, 6–9 July 2011; pp. 354–356. [Google Scholar]
- Guedes, A.C.; Amaro, H.M.; Gião, M.S.; Malcata, F.X. Optimization of ABTS radical cation assay specifically for determination of antioxidant capacity of intracellular extracts of microalgae and cyanobacteria. Food Chem. 2013, 138, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.C.; Walter, C.; Lange, H.A.; Buchholz, R. Microalgae as natural sources for antioxidative compounds. J. Appl. Phycol. 2012, 24, 1133–1139. [Google Scholar] [CrossRef]
- Lo, T.C.T.; Chang, C.A.; Chiu, K.H.; Tsay, P.K.; Jen, J.F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Geresh, S.; Mamontov, A.; Weinstein, J. Sulfation of extracellular polysaccharides of red microalgae: Preparation, characterization and properties. J. Biochem. Biophys. Methods 2002, 50, 179–187. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and antitumor activities of different-molecular-weight polysaccharides from Porphyridium cruentum. Carbohydr. Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- Gardeva, E.; Toshkova, R.; Yossifova, L.; Minkova, K.; Gigova, L. Cytotoxic and apoptogenic potential of red microalgal polysaccharides. Biotechnol. Biotechnol. Equip. 2012, 26, 3167–3172. [Google Scholar] [CrossRef]
- Na, Y.S.; Kim, W.J.; Kim, S.M.; Park, J.K.; Lee, S.M.; Kim, S.O.; Synytsya, A.; Park, Y.I. Purification, characterization and immunostimulating activity of water-soluble polysaccharide isolated from Capsosiphon fulvescens. Int. Immunopharmacol. 2010, 10, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A. The mononuclear phagocyte system. Curr. Opin. Immunol. 2006, 18, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Fragoso-Lona, J.M.; Ramírez-Bello, J.; Cruz-Robles, D.; Pérez-Méndez, O.; De La Peña, A.; Vargas-Alarcón, G. Marcadores pro y antiinflamatorios en la enfermedad arterial coronaria y el síndrome isquémico coronario agudo. Arch. Cardiol. Mex. 2009, 79, 54–62. [Google Scholar]
- Geresh, S.; Lupescu, N.; Arad, S.M. Fractionation and partial characterization of the sulphated polysaccharide of Porphyridium. Phytochemistry 1992, 31, 4181–4186. [Google Scholar] [CrossRef]
- Lavin, Y.; Winter, D.; Blecher-Gonen, R.; David, E.; Keren-Shaul, H.; Merad, M.; Jung, S.; Amit, I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 2014, 159, 1312–1326. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Rosas, M.; Smith, P.J.; Fraser, J.D.; Jones, S.A.; Taylor, P.R. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur. J. Immunol. 2011, 41, 2155–2164. [Google Scholar] [CrossRef]
- Celada, A.; Nathan, C. Macrophage activation revisited. Immunol. Today 1994, 15, 100–102. [Google Scholar] [CrossRef]

| Percentages | |
|---|---|
| Proteins | 15.96 ± 0.09% |
| Carbohydrates | 48.53 ± 0.25% |
| Lipids | 6.85 ± 0.12% |
| Inorganic compounds | 20.14 ± 0.96% |
| Moisture | 8.52 ± 0.19% |
| Monosaccharide | Retention Time (min) | Peak Area | % | |
|---|---|---|---|---|
| 1 | Ribose (Rib) | 18.42 | 101,886 | 0.4 |
| 2 | Fucose (Fuc) | 19.44 | 1,807,537 | 7 |
| 3 | Xylose (Xyl) | 20.97 | 178,746 | 0.7 |
| 4 | Arabinose (Arb) | 25.46 | 12,405,336 | 48.3 |
| 5 | Mannose (Mann) | 26.09 | 2,650,521 | 10.3 |
| 6–7 | α and β-Galactose (Gal) | 27.41–27.52 | 5,994,240 | 23.3 |
| 8 | Glucose (Glc) | 28.96 | 1,914,772 | 7.4 |
| 9 | Glucuronic acid (Glc-A) | 29.83 | 650,111 | 2.5 |
| WBCs | Basal | Day 7 | Day 14 | Day 21 | |||
|---|---|---|---|---|---|---|---|
| VEH | PcSPs | VEH | PcSPs | VEH | PcSPs | ||
| Neutrophils | 22.06 ± 5.33 | 17.95 ± 6.42 | 19.29 ± 4.15 | 22.63 ± 2.88 | 15.60 ± 4.97 Aa | 12.47 ± 4.09 c | 11.50 ± 4.31 c |
| Eosinophils | 0.78 ± 0.88 | 4.40 ± 2.56c | 1.57 ± 1.40 A | 1.50 ± 1.07 | 0.60 ± 0.52 | 4.16 ± 0.74 a | 5.50 ± 1.78 c |
| Basophils | 0.53 ± 0.61 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.13 ± 0.35 | 0.10 ± 0.32 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Lymphocytes | 69.89 ± 5.32 | 76.65 ± 7.34 a | 70.57 ± 5.50 | 63.38 ± 2.00 | 77.70 ± 5.58 Bb | 81.22 ± 4.29 c | 82.00 ± 6.86 c |
| Monocytes | 6.56 ± 3.22 | 1.71 ± 2.21 b | 9.83 ± 2.91 C | 6.38 ± 1.69 | 6.00 ± 3.09 | 2.40 ± 1.52 a | 2.83 ± 1.57 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Arrojo, V.; Decara, J.; de los Ángeles Arrojo-Agudo, M.; Pérez-Manríquez, C.; Abdala-Díaz, R.T. Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium cruentum. (S.F.Gray) Nägeli. Biomolecules 2021, 11, 488. https://doi.org/10.3390/biom11040488
Casas-Arrojo V, Decara J, de los Ángeles Arrojo-Agudo M, Pérez-Manríquez C, Abdala-Díaz RT. Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium cruentum. (S.F.Gray) Nägeli. Biomolecules. 2021; 11(4):488. https://doi.org/10.3390/biom11040488
Chicago/Turabian StyleCasas-Arrojo, Virginia, Juan Decara, María de los Ángeles Arrojo-Agudo, Claudia Pérez-Manríquez, and Roberto T. Abdala-Díaz. 2021. "Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium cruentum. (S.F.Gray) Nägeli" Biomolecules 11, no. 4: 488. https://doi.org/10.3390/biom11040488
APA StyleCasas-Arrojo, V., Decara, J., de los Ángeles Arrojo-Agudo, M., Pérez-Manríquez, C., & Abdala-Díaz, R. T. (2021). Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium cruentum. (S.F.Gray) Nägeli. Biomolecules, 11(4), 488. https://doi.org/10.3390/biom11040488






