Abstract
Protein homeostasis is an essential component of proper cellular function; however, sustaining protein health is a challenging task, especially during the aerobic lifestyle. Natural cellular oxidants may be involved in cell signaling and antibacterial defense; however, imbalanced levels can lead to protein misfolding, cell damage, and death. This merges together the processes of protein homeostasis and redox regulation. At the heart of this process are redox-regulated proteins or thiol-based switches, which carefully mediate various steps of protein homeostasis across folding, localization, quality control, and degradation pathways. In this review, we discuss the “redox code” of the proteostasis network, which shapes protein health during cell growth and aging. We describe the sources and types of thiol modifications and elaborate on diverse strategies of evolving antioxidant proteins in proteostasis networks during oxidative stress conditions. We also highlight the involvement of cysteines in protein degradation across varying levels, showcasing the importance of cysteine thiols in proteostasis at large. The individual examples and mechanisms raised open the door for extensive future research exploring the interplay between the redox and protein homeostasis systems. Understanding this interplay will enable us to re-write the redox code of cells and use it for biotechnological and therapeutic purposes.
1. Introduction
The stability of the cellular proteome is constantly challenged by conditions that cause proteotoxic stress, including errors during protein synthesis, undesirable protein modification (e.g., oxidation), inherited polymorphisms, and native changes in physiological conditions, such as aging [1,2,3]. It is, therefore, not surprising that protein homeostasis (or proteostasis) is among the most important mechanisms maintaining the proper balance between protein biogenesis and its cellular function. Thus, proteostasis can be seen as a pivotal player in maintaining the functional proteome specifically and cell survival more broadly.
The delicate balance in proteostasis is achieved by a carefully and timely regulated protein network (i.e., the proteostasis network), which is composed of a high number of proteins carrying out different functions (folding, protein editing, transfer, and degradation) across different cellular organelles, including the cytosol [4], nucleus [5], mitochondria [6,7], and others. This protein network is adaptive and comprises thousands of proteins in high eukaryotes [4,8], some of which are redundant in their function and some specific to either cellular condition [9,10] or client protein [11]. Despite the overall high energy cost in protein production within the cell, proteins mediating protein folding and degradation comprise a large portion of its proteome, emphasizing the significant role of this system in cell functionality. The composition and dynamics of the proteostasis network are shaped by various changes in cellular requirements and conditions, (e.g., the accumulation of oxidants, environmental temperatures such as heat shock, and pH), each of which may challenge cellular homeostasis and protein stability. Stress response mechanisms heavily rely on adaptation of the proteostasis system often in a near-instantaneous manner [12,13] in order to ensure temporal control of “health” and the functionality of thousands of cellular proteins. While some of these adaptive processes may involve relatively few proteins, others may lead to a significant rearrangement of the cellular proteome and formation of stress bodies, particularly in combination with other stress factors [14,15,16,17].
Proteostasis defines and carefully synchronizes every step from a protein’s initial formation by the ribosome through trafficking to subcellular compartments under appropriate conditions, assembly to sophisticated and dynamic macro-complexes, protein modification, functional regulation, signaling, and finally its “death” and turnover. A breakdown of this process can lead to the formation of misfolded proteins and potential accumulation of toxic protein aggregates, damaging cell growth and activity [14,15,18].
The heart of the protein proteostasis system is, thus, chaperones and co-chaperones, which are responsible for recognizing aggregation-prone proteins and assisting in their proper folding [4,19,20]. Chaperones range in function and behavior from the broadly conserved and highly prevalent (e.g., Hsp40/DnaJ, Hsp70/DnaK, etc.) [4] to the client- or condition-specific proteins (e.g., redox-regulated Hsp33 [21], pH-regulated HdeA [22] and HdeB [23], heat-induced small heat shock proteins (sHSPs) [24,25,26]). The chaperones differ not only in their mechanistic functions but also in localization or specificity, all of which may shape the mode of engagement with client proteins and subsequent chaperone activity. The canonical chaperones such as Hsp70, Hsp90, Hsp100, and others are ATP-fueled machines that assist folding of a wide range of cellular proteins and recognize differential conformational forms of the client proteins across their life span [4,27]. It was suggested that the expansion of proteomes from bacteria to mammalian cells led to an increase of more than six-fold in aggregation-prone proteins, requiring the evolution of chaperone and co-chaperone networks to facilitate a functional proteome in mammalian cells [28,29].
One of the cellular strategies for dealing with the expanded number of protein sequences and increased diversity in biochemical properties of evolved proteins is to increase the repertoire of chaperone-assisting proteins, i.e., co-chaperones. Co-chaperones are able to recognize misfolded proteins and ensure their interaction with the related chaperone via transfer to the substrate-binding domain of the ATP-dependent chaperone and enhancement of the chaperone’s ATPase activity [4,30,31]. Today, more than fifty different co-chaperones of human Hsp90 have been identified, varying in substrate specificity, catalytic activity, stress specificity, and even tissue specificity [32,33].
Another canonical chaperone, Hsp70, fulfills its function via assessment of a broad family of J-domain proteins (JDPs), including DnaJ in E. coli, Ydj1 [34] and Sis1 [35,36] in yeast, and DNAJB1/Hsp40 in mammalian cells [37,38,39]. It was shown that a crosstalk between different JDPs is essential for recognizing protein aggregates during proteotoxic stresses and aging [40]. One such J-protein is the mammalian ERdj5 (DNJ-27 in C. elegans), which comprises a typical JDP cysteine-rich domain fused with a thioredoxin-like domain [41]. ERdj5 is a functional reductase localized to the endoplasmic reticulum (ER), which is crucial for reducing undesirable disulfide bonds in misfolded proteins in the ER [42], tightening the redox and protein quality control functions together. Many of the known J-proteins harbor zinc (Zn)-binding domains that are crucial for their activity and might respond toward changes in cellular oxidation. In some of them, the redox status of the cysteines forming these Zn-binding regions defines anti-aggregation activity (e.g., ERdj3 in mammalian cells). Co-chaperones, including the J-proteins, have a crucial role in making the “life–death” decision and targeting the misfolded protein (instead of the folding one) to degradation by the proteasome [43,44].
To assist the ATP-dependent chaperones and their co-chaperones during stress conditions, other ATP-independent chaperones take on an essential role in maintaining a healthy proteome. This is particularly relevant under conditions associated with a drop in intracellular ATP reservoirs [45,46,47] (e.g., oxidative stress, mitochondrial dysfunction) or cellular locations depleted of ATP (e.g., bacterial periplasm). These are “holdases” or holding chaperones, which serve as the first line of defense in conditions leading to protein misfolding, for example Hsp33 [21], CnoX [48], and Get3 [49] during oxidative unfolding, HdeA/B [22,23] during acidic unfolding, and others. As chaperones may also target different aspects of protein quality control itself (e.g., protein folding, unfolding, assembly, disaggregation, etc.), they frequently work in tandem with different ATP-dependent chaperones to carry out their function. The ATP-independent activity and the ability to be a part of the cellular chaperone network serve as the foundation for the working cycles of redox-dependent chaperones, which maintain protein homeostasis during oxidative stress conditions.
Here, we will discuss the broad-scale relationship between cellular oxidation and proteostasis, focusing on the role of protein thiols as redox sensors and switches of the protein homeostasis network.
2. Cellular Oxidants: Origin, Targets and Benefits
The aerobic lifestyle has a proven advantage in efficient energy production. That said, it is also a major source of intracellular reactive oxygen and nitrogen species (ROS and RNS, respectively). From the quantitative aspect, oxygen-dependent ATP production in mitochondria is the largest contributor to the cellular ROS reservoir. As far back as the 1960s, Jensen and others showed that oxygen reduction in mitochondria leads to a flux of superoxide anions (O•−2) [50], which is further converted into hydrogen peroxide (H2O2) [51,52]. Formation of superoxide mainly occurs in complexes I and III in the respiration electron transfer chain (ETC) as a byproduct of oxygen reduction [53,54]. Since superoxide is a very reactive yet unstable radical, it is rapidly converted into hydrogen peroxide spontaneously or enzymatically through distant superoxide dismutase (SOD) enzymes [55,56,57].
In addition to mitochondria, cellular ROS is produced by a variety of enzymatic reactions in a regulatory way. This includes oxidative protein folding in the ER [58], lipid oxidation in peroxisomes [58], the inflammation process via activity of a diverse family of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) [59,60,61], oxidation of monoamine neurotransmitters using monoamino oxidases (MAO) [62], and many other processes [63,64].
Another group of chemically reactive endogenous molecules is that of reactive nitrogen species (RNS), which are mainly derived from nitric oxide (NO) interactions. One of the major sources of cellular nitric oxide molecules is the enzymatic activity of a family of nitric oxide synthetase (NOS) enzymes involved in cell signaling and immune defense [65,66]. Reactions between NO, ROS, and metals result in derivatives of other reactive molecules such as peroxynitrite (ONOO-), NO radicals (NO-), S-nitrosothiols (SNOs), and others [67,68]. The origins and crosstalk between RNS and ROS are well detailed in the following reviews [69,70].
Not surprisingly, organisms have found a way to use ROS and RNS reservoirs for cellular activity, maintaining a delicate balance between producing the needed oxidants at the right time and place, while detoxifying others. Impairment of this balance leads to oxidative and nitrosative stress, causing potential damage to macromolecules in cells.
One of the first pieces of evidence for the positive role of ROS was made by Babior, Kipnes, and Curnutte in 1973, by demonstrating that activated phagocytes (leukocytes and granulocytes) produce superoxide during phagocytosis [71]. This suggested that ROS can serve as a native antibacterial agent during the immune response. Later, this phenomenon led to the uncovering of a fundamental class of protein complexes (NOXs) that actively produce superoxide molecules to kill pathogens. Since then, other ROS-dependent pathways have been discovered and characterized, many of which utilize hydrogen peroxide as a molecular messenger or mediator of signaling pathways, including neurotransmission [72], cell proliferation and inflammation [57,73], and protein quality control, which will be discussed in further detail below.
Like ROS, RNS are important chemical messengers. In 1992, Daniel Koshland named nitric oxide as “the molecule of the year” [74] due to its important role in medicine (i.e., maintaining blood pressure), the immune response, and neuron function. Six years laterin 1998, Ferid Murad received the Nobel Prize for the discovery of the beneficial role of NO in cell transduction through activation of soluble guanylyl cyclase (sGC), which produces the signaling molecule 3′,5′-cyclic guanosine monophosphate (cGMP). Numerous modern studies have subsequently defined the molecular basis of NO and other RNS in cellular metabolism, apoptosis, and cellular proliferation through post-translational site-specific S-nitrosylation of signaling proteins. The detailed molecular basis of RNS and ROS-mediated cell signaling processes are well reviewed in [69,75,76,77].
Thus, cells have developed an elegant way to recycle ROS and RNS byproducts and convert them into biological readouts through site-specific oxidation of the target proteins, especially in cysteine thiols.
3. Cysteine Thiols: The Central Components of Redox-Regulation of Proteostasis
The cellular redox status sits at the junction between protein homeostasis and the global stress response, in large part through the coordinated role of molecular chaperones and the degradation machinery in maintaining protein homeostasis [4]. More specifically, oxidative stress has long been established as one of the primary sources of different forms of cellular damage, whether through direct modifications on individual cysteine residues or perturbations of existing protein complexes through disulfide-bridge formation, which all might result in protein misfolding and aggregation [78,79]. Numerous age-related diseases and disorders have been associated with changes in both redox and protein homeostasis, bridging these two defense systems together [80,81]. This includes neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases, which are associated with the buildup of cellular oxidants, mitochondrial dysfunction, and accumulation of “toxic” misfolded proteins (TAU and α/β amyloids in Alzheimer’s and α-synuclein in Parkinson’s diseases) into cellular inclusion bodies, followed by progressive death of specific neural cells [82,83,84,85,86,87].
This comes alongside the beneficial role of oxidants mediating “normal” signaling and biological responses triggered by cellular oxidation [88,89], which raise the question as to how cells maintain the balance between oxidation and proteostasis at large.
One of the major and most studied mechanisms for sensing and protecting cells against oxidation is utilizing the reactivity of cysteines, specifically sulfhydryl (thiol) groups, serving as oxidant sensors. Cysteines are highly reactive for oxidation, and this sensitivity makes them “sweet and sour” spots in cellular proteins. On the one hand, the accumulation of intracellular oxidants results in undesirable thiol (over)oxidation, leading to the addition of negatively charged modifications to the protein in the form of sulfenic (RSOH) or sulfinic acids (RSO2H). It may also introduce non-native disulfide bonds affecting protein structure and stability or even a covalent crosslink with other macromolecules. On the other hand, while the majority of proteins might lose function upon non-specific oxidation, other types of proteins use a site-specific thiol oxidation as an “on–off” switch for rapid function regulation. This mode of activation is similar to other post-translational modifications, which immediately change the chemical properties of a protein, leading to a loss or gain of function. In recent years, many such “redox switches” (or “thiol switches”) [90] have been identified as redox-regulated proteins, spanning from bacteria to mammals and fulfilling diverse biological functions [91,92,93].
Reactive thiols of redox-regulated proteins usually have distinctive chemical properties that define reactivity towards oxidants and reversibility. They can be modified in various ways: sulfenylation, nitrosylation, glutathionation, persulfidation, and disulfide formation, responding to different oxidants (Figure 1). Some modifications may emerge as either reversible (e.g., palmitoylation, sulfenic acid) or irreversible (e.g., prenylation, sulfinic, and sulfonic acid).
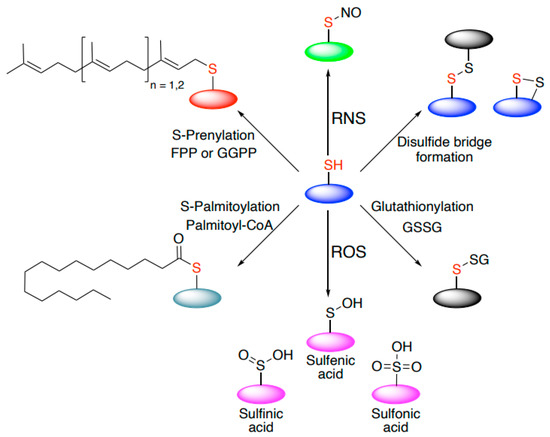
Figure 1.
Thiol-based cysteine modifications. Cysteines may undergo a wide range of thiol-based chemical modifications. These include both reversible (e.g., sulfenic acid, disulfide bridge formation, palmitoylation) and irreversible modifications (e.g., sulfinic acid, sulfonic acid, prenylation).
There are also specific cysteine-containing motifs, which may be involved in redox switch activity, such as derivatives of the CxxC sequence motifs (where “x” represents any amino acid), metal-binding sites, or cysteines that are available for disulfide bridge formation. Among the first identified and very well-characterized redox switches are antioxidant proteins, which use rapid changes in the redox state of their catalytic cysteines to restore the redox status of cellular proteins (e.g., thioredoxins, glutaredoxins) or detoxify an excess of ROS and their byproducts (e.g., peroxiredoxins) [94,95]. These proteins use catalytic cysteine residues in highly specific and regulated manners, often through conserved motifs that determine cysteine thiol-based interactions. It was shown that sensitivity of thiol cysteines in these canonical antioxidant proteins is defined by their position in the protein sequence and protein environment, which can affect their pKa value and redox potential. Location near basic residues decreased the pKa of the redox-sensitive thiol, lowering the thiol pKa value from 9 to 5–7, turning it into an efficient nucleophile [96]. The canonical antioxidant proteins utilize reversible oxidation–reduction cycles of their highly conserved pair of redox-sensitive cysteines to restore the redox states of cellular proteins and mediate redox-dependent signaling events in cells. The mechanisms and roles of thiol modifications in cell survival and apoptosis were reviewed in detail in Benhar (2020) [97]. Recent studies have also shown how conformationally adjacent regions may impact the glutaredoxin activity, beyond the cysteine residues themselves [98]. Liedgens et al. determined that various additional residues (both motif-adjacent and conformationally available) are part of the glutathione-scaffold site, mutation of which alters either the reductive or oxidative half reaction, pointing to an expanded involvement of nearby regions in functional cysteine oxidation.
Interestingly, despite a large number of canonical antioxidant enzymes sharing similar functions and a wide range of thiol modification types, these enzymes are not redundant in their substrate selection, taking care of a defined set of client proteins. Moreover, proteomic profiles of thiol modifications show that they are site- and type-specific, with minimal crosstalk between them [99,100,101,102]. In contrast, the proteostasis network relies on a redundant function of its members, ensuring proper folding and degradation of similarly misfolded proteins [4,103].
The thiol-redox switch group may be present in proteins of varying roles, however there are notable recent advancements in identifying proteostasis-related redox switches, opening up a new era of understanding molecular mechanisms of redox regulation of the proteostasis function in cells [13,104] (Figure 2). Discovery of a dual function of peroxiredoxin proteins was one of the major breakthroughs in understanding the link between anti-aggregation and antioxidant activities. Peroxiredoxins are antioxidant enzymes that reduce cellular peroxide or peroxynitrite derivatives into harmless water molecules [105]. However, severe oxidative stress converts some of these enzymes (e.g., Tsa1 in yeast, Prx1, and Prx2 in humans) into powerful ATP-independent chaperones without the antioxidant activity [106,107]. This transition is triggered by overoxidation of one of the catalytic cysteines, which forms sulfinic acid coupled with substantial oligomerization changes. Along with conformational changes, thiol-specific oxidation might recruit other members of the proteostatic family in order to facilitate refolding of the misfolded proteins captured by peroxiredoxin. For example, hyperoxidation of its Cys48 in yeast peroxiredoxin Tsa1 recruits Hsp70 chaperones and the Hsp104 disaggregates to H2O2-induced aggregates [108]. However, the overoxidation of peroxiredoxins can be restored by sulfiredoxin enzymes, which reduce cysteine–sulfinic acid in an ATP-dependent manner [109]. This restores the antioxidant activity of peroxiredoxins during non-stress conditions [110,111]. It is important to note that the chaperone activity of peroxiredoxins is not limited to oxidation and can occur during other protein unfolding stresses, such as heat shock [112,113], metal deficiency [107], and acidic stress [114]. Additional recent advancements showcase the importance of persulfidation as a conserved cysteine modification that plays a role in protecting cysteine residues from irreversible overoxidation during oxidative stress [115]. This was specifically shown through modifications of mouse peroxiredoxins as mediated by thioredoxin-related proteins (TrxR1 and TRP14), with an additional protective effect on several redox-sensitive proteins with roles relating to protein quality control pathways, some of which will be discussed in further depth (e.g., PTP1B [116], HSP90 [4], and KEAP1 [117]) [118]. Taken together, these place peroxiredoxins at the center of both protective and potentially harmful roles (e.g., as treatment against ionizing radiation and upregulation in cancer cells, respectively) [119,120].
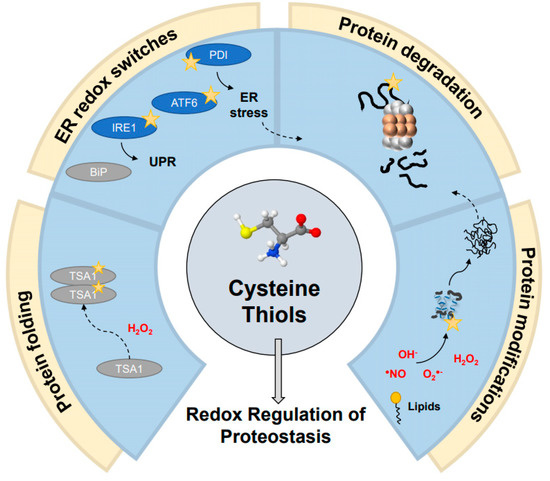
Figure 2.
Redox switches in proteostasis. Cysteine thiol switches are involved in regulating various aspects of proteostasis, such as protein folding, ER quality control and the unfolded protein response (UPR), protein degradation across various stages, and protein modifications and maturation. Numerous examples of redox-sensitive thiols have been found across each of these stages of protein quality control, such as redox regulation of the peroxiredoxin TSA1, the ER protein disulfide isomerase (PDI) and BiP chaperones, members of the UPR mechanism IRE1 and ATF6, and the proteasome itself. These emerge from oxidative modifications of varying sorts, including reactive oxygen species (ROS), reactive nitrogen species (RNS), and lipid modifications.
Meanwhile, there remain many other redox-sensitive proteins without explicit antioxidant behavior, which actively interact with the oxidative stress response in some form and play a direct role in proteostasis (Table 1). These include a wide range of chaperones that are necessary for assisting proteins that become damaged as a result of oxidative stress, whether through direct refolding (partial or complete), “holding” functions that may interact with the misfolded proteins along with other chaperones, or indeed relaying misfolded proteins to degradation itself. In this way, members of the protein quality control (PQC) system may themselves be recruited during oxidative stress conditions in order to maintain the necessary homeostasis. This regulation is critical, as a breakdown of the PQC system can lead to the formation of misfolded proteins as well as the potential toxic protein aggregates that may follow [5,121].

Table 1.
Table summarizing select chaperones involved in maintaining protein homeostasis through redox regulation.
Subsequent research of thiol-redox switches in a proteostasis context revealed an important property of some of the thiol-redox switches—a requirement to undergo substantial conformational rearrangements regulated by its redox status in order to gain anti-aggregation activity [104]. One such redox-regulated chaperone, which uses protein plasticity for its activation, is a highly conserved ATP-independent holdase chaperone, Hsp33. Hsp33 is a predominantly bacterial chaperone, also found in unicellular pathogens such as Trypanosoma and Leishmania [122], which protects microbes against a wide range of oxidants similar to those applied by the host immune system [21]. Exposure to oxidants or chlorine species (e.g., HOCl) triggers large conformational changes and exposure of hydrophobic regions involved in the anti-aggregation activity [91,123,126,127]. Upon return to reducing conditions, Hsp33 refolds due to tight and highly specific interactions between its domains [91], leading to a destabilization of the bound client protein and transfer to the foldase chaperone system, DnaK/J [126]. The reduction in Hsp33 is mediated by physiological antioxidant enzymes, such as thioredoxins and glutaredoxins. While constitutive (non-redox dependent) activation of Hsp33 can be easily achieved by either chemical denaturants, extreme heat, or single mutations [128], its inactivation mechanism is highly conserved and difficult to alter [91]. Thus, the evolutionary path of Hsp33 appears invested in providing unique structural features enabling reversibility of its activity, which prevents constitutive binding with misfolded proteins during normal, non-oxidative conditions.
Another example of a redox-sensitive chaperone is the recently identified CnoX bacterial chaperone, which protects cells against hypochlorous acid (HOCl) [48,129]. CnoX is reversibly activated by site specific chlorination, which leads to exposure of hydrophobic regions, most probably crucial for anti-aggregation activity. Similar to Hsp33, CnoX transfers its misfolded client proteins to the ATP-dependent system, GroEL and DnaK/J. It is still unknown if conformational changes on CnoX are coupled with this process. Interestingly, as with peroxiredoxins, CnoX from specific bacterial strains (including C. crescentus) acts as an oxidoreductase, utilizing its CxxC domain located in a thioredoxin fold. It was shown that C. crescentus CnoX is a constitutive chaperone with oxidoreductase activity, raising an intriguing evolutionary question as to the reason for this loss of function in specific bacterial strains such as E. coli.
Furthermore, the yeast Get3 (TRC40 or Asna1 in mammals) is an additional “moonlighting” redox switch with multiple functions [49]. Under reducing conditions, Get3 serves as a delivery protein together with other Get proteins, ensuring post-translational delivery of the thiol-anchoring (TA) proteins to the ER in an ATP-dependent manner. Under oxidative conditions, similar to Hsp33, the oxidation of Get3 leads to the formation of disulfide bonds and a structural rearrangement followed by oligomerization, converting the oxidized form of Get3 into a general, ATP-independent chaperone. These three examples demonstrate some of the diversity among redox-sensitive chaperones and the ways in which these proteins intersect with additional protein quality control pathways.
4. Integrative Approaches for Discovering New Redox Switches in PQC
Intrigued by the moonlighting function of redox-regulated proteins, which apply structural plasticity for their redox-sensitive function, Erdos et al. developed a computationally based structural prediction web server IUPred2A that points to cysteine-containing regions, which might undergo disorder-to-order transitions in response to changes in their redox status [130]. IUPred2A is a new generation of a very well-established prediction algorithm of intrinsically disordered proteins (IUPred), which was trained on outcomes of redox proteomic studies and sets of known redox-switch proteins to obtain valid predictions of potential redox switches. This study estimated that around 5% of proteomes harbor redox-sensitive thermobile regions, while viruses are among the most divergent proteomes regarding the predicted number of redox-regulating proteins. Moreover, this study suggests that metal binding regions are the most frequent among conditionally disordered regions including many experimentally validated redox-sensitive DnaJ co-chaperones such as Ydj1 and others [130].
To go beyond the individual cases and bioinformatic prediction, the advances in thiol labeling coupled with quantitative proteomics enable identification and accurate estimation of redox-sensitive thiols in diverse protein families. These mass spectrometry-based methods utilize differentially labeling thiol-specific alkylating reagents (e.g., isotope-coded affinity tags (ICAT), cysteine-reactive phosphate tags, tandem mass tags (TMT), and dimedone-based probes, as well as relatively simple N-ethylmaleimide (NEM) and iodoacetamide (IAM) reagents [131,132,133,134,135,136,137]), which are used to broadly map out cysteine oxidation in a quantitative manner. These wide-scale assessments may then be applied to specific cellular functions or conditions (e.g., different stresses), though these do not necessarily address proteostasis directly. However, these varying methods could easily be applied to study the redoxomes (i.e., redox proteomes) of individual systems within the PQC under diverse conditions. Undoubtedly, identification of the specific redox-sensitive thiols is only the first stage, to be followed by extensive biochemical analysis and validation in the context of physiologically relevant conditions.
Moreover, modern high-sensitivity mass spectrometry, genomic sequencing, and RNA-sequencing methods have enabled identification of proteins more generally associated with changes in global oxidation [13,138,139,140,141]. While this does not guarantee identification of individual thiol switches, it serves as a useful guidepost for defining proteins involved in maintaining redox homeostasis in some form. Indeed, a comparison of the proteomes of endogenously oxidized versus reduced cells revealed that reduced cells had relatively higher levels of proteins involved in folding pathways and the proteasome, as well as proteins involved in stress granule formation [140]. All in all, large-scale quantitative methodologies seem poised to fill in many of the gaps in our current understanding of redox switch regulation of protein homeostasis.
5. Cysteine-Mediated Modifications: An Efficient Mechanism to Regulate Signal Transduction and Protein Localization in Cells
Direct oxidation is not the only form of modification found on cysteine residues, nor is it the only form of cysteine-mediated regulation of proteostasis. Cysteines are primed to undergo an extraordinarily wide range of modifications, from disulfide bonds to S-nitrosylation through to reversible fatty acid modifications such as palmitoylation, many of which have been extensively studied through mass spectrometry-based proteomic techniques (Table 2) [142]. Due to the importance of post-translational modifications in proper protein folding, function, and secretion, cysteine modifications are crucial for different cellular regulatory processes and healthy cellular growth. Despite the difficulty in studying lipid modifications on a large scale, individual case studies have been able to identify specific cysteine residues involved in protein quality control on varying levels.

Table 2.
Table of select proteins that contain different cysteine modifications, related to Figure 3. Cysteine modification sites are noted where experimentally verified, alongside general associated processes in which the protein is involved or during which it undergoes the relevant modifications.
Among these different potential modifications, palmitoylation is a major cysteine-specific modification responsible for regulating signaling pathways within the cell. Here, either the presence or absence of palmitoylation may play a role in protein localization or in determining protein export from the ER [148]. This has been found in many different forms and utilizing different mechanisms. In some cases, palmitoylation specifically enables protein export from the ER (e.g., Wnt signaling protein LRP6) [149]. However, palmitoylation may also regulate protein aggregation within the ER prior to export, as seen for proteins such as yeast chitin synthase, Chs3 [145] (Figure 3), or alternatively, stabilize proteins for aggregation as in human adult-onset neuronal ceroid lipofuscinosis-causing CSPα mutants [150]. Whether playing a role in general protein export or through involvement in direct folding or aggregation-prevention, the presence of the cysteine-based palmitoylation is, thus, critical in determining proper protein function, localization, and behavior.
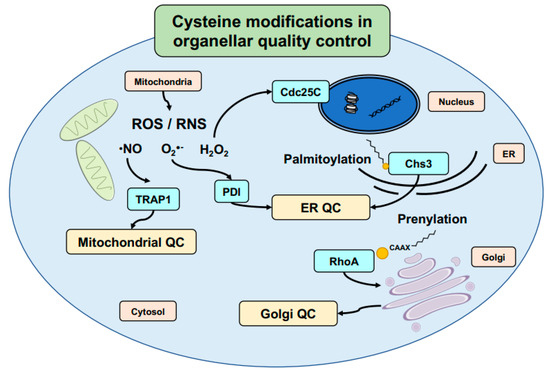
Figure 3.
Thiol-modifications define protein folding and translocation. Thiol modifications come in many forms, ranging from reactive oxygen/nitrogen species (ROS/RNS) to lipid modifications during protein maturation. Various individual proteins have been found to have redox-regulated functional changes affecting organellar quality control. Cysteine modifications on chaperones such as TRAP1 and PDI regulate quality control in the mitochondria and ER, respectively, while lipid modifications play additional roles in protein translocation within the cell. Palmitoylation of Chs3 regulates its proper exit from the ER and prevents its aggregation, while prenylation of RhoA alters its interactions with the SmgGDS chaperone. These and other cysteine modifications regulate various additional processes within the cell, such as the cell cycle in the case of hydrogen peroxide regulation of the checkpoint control protein Cdc25C.
Such modification-based regulation of protein transport is not limited to the ER and has also been identified in the Golgi. Known palmitoylated cargo proteins were found to have a higher rate of transport within the Golgi as compared with un-palmitoylated mutant proteins [151]. Taken alongside an increasing awareness of Golgi quality control (GQC) as an independent and multipronged system for quality control within the secretory pathway [152], the potential for palmitoylation (or other cysteine-based modifications) as a PQC-regulating modification remains intriguing, albeit under-explored.
This similarly applies to other cysteine-specific modifications such as prenylation, which plays a central role in cell cycle and cancer regulation [153]. Once again, cysteine modifications have been found to play an important role in the quality control. For example, the cancer-associated chaperone SmgGDS has been found to associate differently with prenylated RhoA (Figure 3), and that different splice variants are in fact structurally designed to accommodate cysteine modification [147]. This suggests that prenylation (including farnesylation and geranylgeranylation specifically, as the lipid modifications) may be viewed as both structure- and function-modifying. In another example, altering a cysteine-based lipid modification in the Schizosaccharomyces pombe yeast Rho1 was recently shown to trigger the formation of protein aggregate centers (PACs) under mild heat stress [154]. By blocking access to the prenylation target, Rho1 was found to no longer localize to the plasma membrane at permissive temperatures and the aggregate-like PACs at 37 °C. These PACs, in turn, demonstrated a widespread rearrangement of PQC members within the cell, including the presence of Hsp70- and Hsp40-family chaperones, Hsp104, and more. This demonstrates, in part, the importance these modifications play in the PQC system specifically through regulation of proper protein folding and localization, as well as protein–protein interactions more generally. Given the widespread nature of the different lipid modifications in diverse eukaryotic proteomes, we may speculate that many other, yet undiscovered proteins similarly require cysteine-specific lipid modifications in order to fully mature and function under healthy conditions.
Other types of cysteine modifications may play a regulatory role in protein quality control as well. S-nitrosylation is well studied in the context of RNS, as previously described in detailed reviews on its implications for both cardiovascular and neurodegenerative diseases [155,156]. The latter is particularly interesting as a model for S-nitrosylation involvement in protein folding and aggregation. S-nitrosylation also occurs on chaperones such as protein disulfide isomerase (PDI) [157,158] and the mitochondrial TRAP1 [143] at the center of quality control mechanisms, emphasizing the varied relationship S-nitrosylation may have with protein quality control mechanisms (Figure 3). Interestingly, both in vitro and in vivo PDI retained fairly stable levels of S-nitrosylation, with only slow-reducing reversibility by glutathione, which may suggest a more direct involvement of S-nitrosylated PDI in neurodegenerative disorders [144,157].
In another example, studies in plants have also identified S-nitrosylation of several conserved cysteine residues in the Nicotiana tabacum Cdc48 [159]. Among these, Cys526 was found to be involved in ATPase activity and protein conformation (without affecting protein structure at large), alongside redox regulation of the conserved cysteine in the mammalian homolog VCP [160]. Interestingly, another identified S-nitrosylated conserved cysteine residue in plant Cdc48 was found to undergo palmitoylation in the human homolog [161]. Together, these point to the ways in which S-nitrosylation specifically and cysteine modifications more generally regulate proteostasis on individual protein levels. New methodologies combined with an increased proteomic sensitivity introduce the potential for large-scale screens to identify additional cysteine-specific modifications at varying stages of proteostasis.
6. Thiol Editing in the ER Is Mediated by Molecular Redox Switches
Cysteine-specific modifications that define protein activity or stability are not alone in bridging proteostasis and redox regulation. The ER itself plays a pivotal role in the PQC system, as this is frequently the site of protein folding and assembly within the cell [121,162]. Importantly, the ER is also where many proteins undergo redox modifications, which themselves often tie into PQC more broadly. Disulfide bond formation in the ER is largely mediated by Ero1 (ER oxidoreductin) and protein disulfide isomerase (PDI), as well as additional proteins belonging to the PDI family [163,164]. As previously mentioned, oxidative modification of cysteine residues in PDI itself has been linked to ER stress, for example in human neurodegenerative diseases [144,165].
This balance between redox homeostasis and the ER is further played out through the unfolded protein response (UPR) (Figure 2). The UPR follows the failure of the ER-associated degradation (ERAD) pathway to clear toxic or aberrant proteins [166], leading to a cascade of different signaling pathways, which may ultimately result in apoptosis [3]. Different proteins within UPR signaling have been found to contain redox-sensitive regulation; indeed, the UPR at large has been suggested to be broadly regulated by changes in oxidation [167] and during aging [168]. Among its major players, the ER stress-activated ATF6 transcription factor has been suggested to undergo a redox-dependent regulation in its response to an increase in misfolded proteins within the ER [169]. Ire1, another major UPR factor that is present in mammals, worms, and yeast, has also been found to contain an evolutionarily conserved cysteine (Cys663 in C. elegans) residue, which regulates its activity [170,171], while a different cysteine residue (Cys148) has been implicated in a potential cysteine oxidation-dependent interaction with a member of the PDI family in C. elegans [172]. The roles these prominent UPR factors play again demonstrate the intimate relationship between proteostasis quality control systems and individual cysteine residues.
7. Regulation of Protein Degradation during Oxidative Stress
The relationship between cysteine–thiol regulation within the ER and ER-related degradation pathways demonstrates the important role cysteines may play in mediating protein “preparation” or identification for degradation. Damage to proteins following oxidative stress draws a clear connection between protein oxidation and proteostasis, largely due to the importance of clearing these aberrant proteins from within the cell. This process may ultimately lead to degradation pathways, when no refolding alternative is available [13,17]. Various studies have identified redox-based regulation of different subunits or cofactors of the proteasome, including through specific cysteine modifications [173,174]. Moreover, the proteasome itself has demonstrated a degree of oxidative sensitivity, with differences between the 20S and 26S complexes in terms of maintaining activity during oxidative stress across yeast and mammalian models [175]. That the proteasome has been found to directly interact and degrade certain oxidized proteins, thus, only strengthens the regulatory relationship between the oxidative stress response and proteostasis at large [176].
Proteasome-mediated degradation of irreparably misfolded proteins is, therefore, one of the most important and well-regulated stages of the oxidative stress response [177]. The proteasome is responsible for the majority of ubiquitin-dependent and -independent degradation of oxidized proteins, through either the 26S or 20S proteasome. Under regular conditions, a vast portion of cellular proteins that are targeted for proteolysis are ubiquitylated and degraded by the 26S proteasome (Figure 4). The 26S proteasome—a 2.5 MDa complex—comprises a 20S core segment, with two 19S regulatory particle (RP) ATP-dependent segments wrapping the 20S from both sides, which can asymmetrically attach to the 20S core particle [178]. More regulatory particles are attached to the 20S proteasome, including the proteasome activator PA200 in mammals (Bml10 in yeast) and the PA28/11S particle [179].
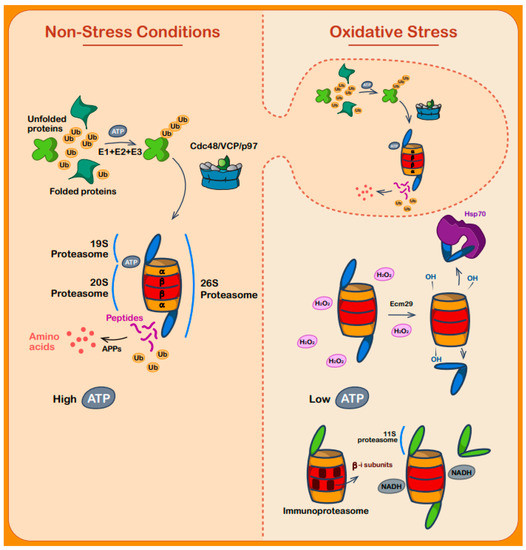
Figure 4.
Proteasome-mediated protein degradation during oxidative stress. To the left, under non-stress conditions and in a high ATP environment, unfolded and misfolded proteins undergo degradation by the ubiquitin system by tagging the targeted proteins with ubiquitin (as carried out by the E1, E2, and E3 enzymes), then reaching the 26S proteasome with the aid of different shuttle proteins. The client proteins are initially recognized by the 19S particle. Following binding and utilization of the energy stored in ATP molecules, the substrate unfolds to its primary structure and enters the hollow barrel-like structure of the 20S particle. The substrate is then degraded into peptides by the catalytic units of the beta-subunits, while aminopeptidases (APPs) break them down to amino acids after the peptides exit the proteasome. To the right, oxidative stress conditions alter the cell mechanism of dealing with misfolded and unfolded proteins. The previously described ubiquitin-based degradation system is minimized by the decrease in ATP molecules, and the 26S proteasome comes apart. The 19S and 20S particles split, mediated by the Ecm29 protein and Hsp70. While the 19S is held by Hsp70, the now-oxidized 20S particle begins to function by itself in an ATP-independent manner and degrades unfolded and misfolded proteins. Under these conditions, another kind of proteasome—the 20Si (immunoproteasome)—is upregulated. This proteasome is combined with 3 different beta-subunits (see text), enhancing the proteasome’s catalytic abilities. The 11S or PA28 subunits are upregulated, serving as an alternative regulatory unit for the 20S and 20Si proteasomes, while NADH stabilizes the proteasome structure in the absence of ATP.
Ubiquitylation of proteins by itself is insufficient for protein degradation by the proteasome, requiring the presence of unfolded and conformationally available domains in the substrates. Two highly important steps determine substrate fate: (1) a preliminary reversible step of ubiquitylation, which is dependent on ATP binding, and (2) recognition of unfolded regions in the client protein. The second is an engagement step for a cascade of substrate processing in the proteasome, leading to the final degradation of the protein [180].
The 19S particle is the recognition subunit of the 26S proteasome and is composed of several subunits itself, assembling the base and the lid (Figure 4). Rpt1-6 (regulatory particle triple-A protein) is responsible for ATPase activity, alongside non-ATPase subunits (Rpn) 1, 2, 10, and 13, forming the base. The lid, meanwhile, is composed of Rpn3, 5-9, 11, 12, and Sem1 [181]. Rpn10 and 13 both bind ubiquitin chains and ubiquitin-like (UBL) domains alike [182,183]. Although these two subunits are the main recognition particles of ubiquitinylated proteins or UBLs, the 19S particle has additional subunits capable of initially binding to substrates [183]. Shuttle proteins are crucial just prior to the initial binding to the proteasome, delivering targeted proteins to the proteasome. One such “shuttle” protein is the previously mentioned Cdc48/p97/VCP ATPase, which is a key factor in collecting and guiding targeted proteins to the proteasome [184]. Another important shuttle protein is UBQLN2, which has been found to have amyotrophic lateral sclerosis (ALS)-causing mutations [185,186].
Under normal conditions, the different components of the proteasome undergo several conformational changes. After its initial recognition and binding to unfolded regions, the 19S faces a widening of its ATPase pore and correspondingly of the 20S as well. Three consecutive ATP-dependent processes subsequently occur: substrate unfolding, gate opening in the 20S, and protein translocation [187]. The 20S core particle is itself made up of two inner β-rings (comprising seven β-subunits) that operate the proteolytic activity, while two outer α rings (comprising seven α-subunits) tightly control the gate opening for substrate entry, which prevent unwanted proteins from being degraded. Meanwhile, β1, β2, and β5 perform caspase-like, trypsin-like, and chymotrypsin-like activity, respectively [188].
Under oxidative stress, however, the two 19S caps dissociate from the main 20S core segment, leaving behind only the 20S core [189] (Figure 4). This process is mediated by the Ecm29 protein in yeast (encoded in mammalian cells by the KIAA0368 gene), as shown by crosslinking coupled with mass spectrometry. Specifically, mutants of Ecm29 displayed reduced dissociation of the 26S proteasome and subsequent sensitivity to H2O2 stress, emphasizing the importance of this proteasome dissociation within the 26S complex triggered by oxidative stress [189,190,191].
Moreover, the previously discussed canonical ATP-dependent chaperone Hsp70 is also involved in the 26S disassociation upon oxidative stress. Not only does it help in complex decoupling, it withholds the 19S cap and reconstitutes the complex after the return to non-stress conditions [192].
The subsequent degradation of proteins by the 20S is coordinated by NAD(P)H:quinone-oxidoreductase-1 (NQO1) and protein deglycase DJ-1, which are similar in structure, both sharing a Rossman fold and activity regulated by oxidative stress [173]. NQO1 and DJ-1 bind directly the 20S proteasome and enhance protein degradation specifically during oxidative stress conditions. The activity of these factors has feedback loop regulation: DJ-1 is involved in the Nrf2-dependent oxidative stress response that leads to upregulation of NQO1, an apo form of which is degraded by the 20S proteasome [193,194].
An additional recent study using a bioinformatic analysis utilized the structural and sequence similarity of DJ-1 and Nrf2 to extend the family of 20S regulators (named catalytic core regulators or CCRs), adding 17 other potential regulators into this club including some which have been experimentally verified [195]. Specifically, several of the identified CCRs were found to affect 20S proteasome activity in vitro, while their overexpression in vivo also demonstrated 20S proteasome inhibition.
Abi-Habib et al. showed that the dissociation of the 19S gives the green light for a direct interaction of oxidized and unfolded proteins with the 20S particle. This occurs through exposure of hydrophobic regions in an ATP-independent manner, which is especially central as oxidative stress leads to ATP depletion [196]. With that being said, depletion of ATP is itself directly connected to the disassembly of the 26S proteasome complex. The stability of the complex can be maintained by the NADH molecule as a compensating agent. Five subunits among those of the 19S complex are suspected of having an NADH binding motif, clarifying the important role NADH has under depletion of ATP conditions, as under oxidative stress [197].
Another subtype of proteasomes found in cells is the immunoproteasome (20S IP) (Figure 4). The 20S IP has a similar structure to the 20S proteasome, but instead of the β1,2 and 5 subunits, it comprises β1i,2i and 5i subunits, expression of which are induced by IFN-gamma in mammals [179]. The β5i plays a critical role in the degradation of oxidized proteins alongside intrinsically disordered proteins [196]. Additionally, an alternative ATP-independent 11S cap or PA28 multimer is expressed under oxidative stress. Overexpression of PA28 showed increased protection against oxidative stress-induced apoptosis and clearance of oxidized and misfolded proteins by the proteasome [198]. This was done using a combination of DNA fragmentation and degradation assays to assess degradation of GFPu, a known proteasome substrate [198].
Many different oxidative stress-induced post-translational modifications have been identified on the proteasome, including carbonylation, glycoxidation, lipoxidation, and glutathionylation, affecting the stability of the proteasome assembly [199]. S-glutathionylation of cysteines within the α5 subunit was also shown to be involved in redox regulation of the proteasome, increasing proteasomal activity by supporting ring opening of the α subunits [174].
These findings highlight the degree to which different aspects of proteasomal degradation rely on cysteine oxidation as a means of functional regulation. In addition to its role in degrading oxidation-damaged proteins, the proteasome itself undergoes significant rearrangement during oxidative conditions, which shape its interactions and mechanisms of behavior. This proteasomal redox sensitivity ultimately appears to be an integral part of the global redox homeostasis, and it seems likely that future research will clarify this role even further.
8. Protein Degradation by Redox Sensitive Proteins
While the relationship between protein degradation and the cellular redox state is frequently thought of through the lens of oxidation-associated damage, increasing studies point to direct redox regulation of ubiquitin and ubiquitin-like degradation. This begins with the relationship between oxidation and E1/E2 ubiquitin enzymes. Initial binding of the ubiquitin molecule to the E1 ubiquitin-activating enzyme is specifically triggered through formation of a thiol ester bond [200]. Subsequent transfer of the activated ubiquitin to an E2 ubiquitin-conjugating enzyme is also mediated by an active cysteine residue within the E2 [201].
Furthermore, numerous ubiquitin E3 ligases have been found to contain reactive cysteines that regulate ubiquitylation. Some E3 ligases or complex members undergo cysteine modifications, which in turn, affect ligase activity or interactions. A well-studied example of a directly redox-regulated E3 ligase adaptor is Keap1, which has multiple reactive cysteine residues determining interaction with the redox-associated transcription factor Nrf2, including in response to varying degrees of mild H2O2 treatment (100–400 μM) alongside other inducing conditions [117,202]. Thus, cysteine oxidation not only controls ubiquitylation and subsequent proteasomal degradation of a protein target, it can also serve as a regulation mechanism for ROS sensing [203].
A well-studied E3 ligase in the context of different redox regulation or cysteine modification is Parkin [204,205,206]. Parkin belongs to a family of RING-between-RING (RBR) ubiquitin ligase enzymes, further containing an in-between-RING (IBR) domain [207]. These domains are considered important for ubiquitylation of Parkin substrates and interaction with ubiquitin-conjugating enzymes [208,209,210]. RING0, RING1, RING2, and the IBR domains in the Parkin protein are cysteine-rich subdomains, which bind up to eight Zn2+ ions [211]. Moreover, Parkin is translocated to the mitochondria under oxidative stress in the absence of DJ-1 [212]. As previously discussed, DJ-1 is a known cellular regulator of ROS [213] and its deficiency leads to the appearance of fragmented mitochondria. This phenotype is rescued by Parkin or PTEN-induced kinase 1 (PINK1) expression [214].
However, ubiquitin enzymes or ligases are not the only redox-sensitive proteins when looking at ubiquitin-related pathways. Interestingly, similar redox-regulation has been identified for both SUMO-associated subunits as well as NEDD8/Rub1 [215]. For SUMOylation, oxidation of specific cysteines between the E1 and E2 enzymes Uba2 and Ubc9 have been directly implicated in the formation of a disulfide bridge between the two enzymes, which in turn, inactivates them [216]. This was later found to play a direct role in the cellular redox response, such that variants that directly affected the disulfide bond’s stability severely altered cell survival during oxidative stress [217].
Redox regulation also plays an important role in the interplay between SUMOylation and ubiquitylation itself. The mammalian SUMO protease SENP3 is constantly regulated by the ubiquitin-proteasome system, with a cysteine modification controlling its association with the chaperone Hsp90 and, thus, its degradation [218,219]. Additional reactive cysteines have been identified in other SUMO proteases, leaving open the possibility of a broader role for cysteine oxidation in SUMOylation across different organisms [220,221]. A similar redox-regulatory role also exists for another ubiquitin-like pathway, NEDDylation/rubylation, with changes in rubylation following oxidation of the yeast cullin Cul1 under both exogenous (4.4 mM H2O2) and endogenous oxidative conditions [222].
Taken together, these results place cysteine-mediated redox regulation of ubiquitin/ubiquitin-like degradation pathways at the center of varying mechanisms and across different model organisms.
9. Aging, Subcellular Localization, and Cysteine Oxidation
Aging is another process that raises particularly interesting questions as to the relationship between protein homeostasis and thiol oxidation. The link between proteostasis and aging has been well established [15,223], with much research into the relationship between proteostatic stress and different hallmarks or types of aging (e.g., replicative aging in yeast [224], diseases in various organisms [8,223]). This is of particular interest when studying neurodegenerative diseases, which are predominantly found in older patients and have been shown in correlation with a breakdown of the proteostasis network [14,223]. This further correlates with the well-studied changes in cellular oxidation during aging across different models, with correlations between replicative aging in yeast and changes in cellular oxidation as well as during chronological aging [140,225,226], though there are additional organisms, which suggest different mechanisms. For this reason, studying cysteine oxidation during aging may provide a relevant context for understanding the pathologies of highly prevalent aging-related disorders.
Several cysteine oxidation mapping methodologies (predominantly proteomic) have been applied in the context of cellular or system-wide aging. With the understanding that the PQC undergoes significant changes during aging, these studies may demonstrate the tight relationship between PQC and redox switches specifically, with many proteins and pathways identified as having aging-dependent changes in cysteine oxidation. Moreover, many of the observed changes are in proteins that are directly involved in regulating protein homeostasis at large, and as such suggest that these thiol switches serve functional redox-dependent roles within the cell, possibly in PQC itself [132,227].
Different studies focus on cysteine oxidation during aging from multiple perspectives. In yeast, for example, a varied group of proteins was identified based on cysteine residues, which underwent “early” oxidation, prior to the global redox collapse associated with chronological aging (under both normal and caloric restriction conditions) [227]. This experiment was conducted using redox proteomic methodologies to track individual thiol oxidation during extended stationary growth, as compared to “full” thiol oxidation under 500 μM H2O2 treatment [102]. Of the early oxidized cysteines, several were found in chaperones or PQC-related proteins (e.g., Cdc48 [228,229,230], Ydj1 [231,232], Ubc4 [233]), with others belonging to the ribosomal 40S and 60S complexes. This points toward potential functional roles for these cysteines in regulating proteostasis.
Intriguingly, the early oxidized cysteine residue in Cdc48 is the same conserved cysteine identified as undergoing different modifications in other organisms, as previously discussed. This comes alongside additional research, which has found that the highly prevalent, ERAD-associated AAA-ATPase has a functionally redox-sensitive cysteine in one of its ATPase domains, with ATPase activity specifically regulated by oxidative stress [160]. Furthermore, Ydj1 and additional ribosomal 40S and 60S subunits are predicted to have conditionally unfolded regions [130], which may further point to functional changes following potential cysteine oxidation. Ydj1′s cysteines have more broadly been implicated in H2O2-induced oxidation and subsequent inactivation [102,108], which leaves open intriguing questions regarding a potential role in regulating proteostasis at large. While these do not directly address aging, they remain curious avenues for future research and raise questions as to the roles individual cysteine residues may play in regulating the redox response under different growth conditions.
However, further additional studies into the relationship between cysteine oxidation and aging suggest the involvement of several other pathways and individual proteins in maintaining protein homeostasis. A wide-scale proteomic screen of reversible tissue-specific cysteine oxidation in mice (establishing the Oximouse technique) demonstrated the presence of cysteine oxidation networks with stark changes in thiol oxidation during aging, with notable differences mapped to human disease-associated proteins [132]. Many of the changes were tissue-specific and were interestingly not limited to oxidation alone, with cases of thiol reduction during aging as well. On a mechanistic level, tissue-specific cysteine oxidation has been found to play a functional role in the stabilization of the human lipid biosynthesis regulator Insig-2 [234]. These pathways resemble those regulated by other proteins with potential redox switches, such as the Cdc48 ergosterol degradation pathway [235] and Txnip-regulated lipid homeostasis [236]. Other identified redox networks also included tRNA multi-synthetase complex members, which suggested oxidation-based regulation of translation. Meanwhile, the presence of thiol reduction during aging (alternatively viewed as the “loss” of a highly oxidized site in older mice) raises several new questions in terms of oxidative changes during aging on the whole, which will likely need to be studied to a greater degree in the future.
Oxidation of cysteine residues across unique pathways is not limited to changes during aging. Studies have been able to identify large-scale thiol oxidation in yeast, finding that proteins with highly oxidized cysteine residues were preferentially localized to organelles such as the mitochondria, ER, and vacuole [92]. Furthermore, oxidative treatment with 1 mM H2O2 in the same study revealed specific thiol oxidation in proteins linked to translation, ribosome biogenesis, and subsequently, ribosomal degradation mechanisms [92]. Taken alongside recent research, which has identified ribosomes as frequent targets for ubiquitin-mediated degradation during oxidative stress [237], this further points to the precise balance between proteostasis, ribosomes, and the oxidative stress response [238]. Similar changes in cysteine oxidation among chaperones were found in C. elegans during treatment with H2O2, alongside cysteine oxidation in multiple ribosomal proteins [239]. Despite not addressing aging conditions specifically, there are several parallels between these pathways and those identified in the context of different forms of aging (e.g., translation initiation as also seen in Xiao et al. [132]).
Additional studies have found diverse links between specific thiol oxidation and protein homeostasis across numerous other organisms. In plants, for example, many different redox switches have been identified as playing a role in ROS homeostasis, whether in chloroplasts or stroma [240]. Interestingly, studies have shown that cysteine oxidation in Drosophila melanogaster does not follow aging, rather that cysteines undergo clear oxidation following fasting [241]. This comes alongside drosophila oocytes, which undergo changes in cysteine oxidation during embryonic development [242], leaving open the possibility of another as-of-yet unknown mechanism of aging- or development-associated oxidation, as well as casting an interesting light on existing aging-associated oxidation changes in mammals, yeast, and plants.
It is important to note that the vastly different experimental setups across various studies may complicate our understanding of the relationship between aging, protein localization, and cysteine oxidation. Varying H2O2 concentrations are of particular relevance, as these may follow differences in reagent stability and sensitivity. More focused, rigorous studies for each individual protein or pathway discussed above would be required to reconcile the effects measured at different concentrations of H2O2, different mediums, and other factors. Nonetheless, the range of cysteine modifications identified during aging, caloric restriction, and mild oxidative stress in different organisms provide extensive insights into redox-sensitive cysteines, which may yet emerge as thiol-based switches or functional sensors.
10. Cell Cycle and Redox Status Are Highly Connected
In the same way that aging is tightly linked to proteostasis at large, so too is the cell cycle. Another of the most important and well-regulated cellular processes, the cell cycle encompasses a wide range of genetic and proteomic changes during different cellular stages. The link between redox, proteostasis, and the cell cycle is most notable when examining disease models such as cancer, where changes in the cell cycle can lead to catastrophic organism-level damage.
Numerous recent links have been identified between the cell cycle and different forms of redox regulation, whether in the form of individual cysteine redox regulation of checkpoint proteins, such as the human Cdc25C [146] (Figure 3) or the role redox-regulating proteins such as glutaredoxin (i.e., Grx1) may play in activating DNA damage repair pathways [243]. Cell proliferation in particular has been well reviewed as regulated through oxidation [244,245], with accumulation of ROS within the cell ultimately leading to oxidative modifications of numerous cysteines in different cell cycle regulators. Oxidation for the most part leads to inhibition of the individual pathways, though it may on occasion activate proliferation. More recent studies have also shown that this regulation extends to the embryonic level, with ROS levels fluctuating throughout the cell cycle and suggesting cyclic cysteine oxidation during embryonic development [246]. These ROS fluctuations have also been identified at different stages of the cell cycle in human cell lines, with an increase in oxidative damage during mitosis in particular [247]. Taken together, these studies demonstrate the ways in which oxidation—and indeed specific cysteine oxidation—regulate and are regulated by the cell cycle. This has particularly interesting implications that relate to aging and aging-associated disorders, especially in light of cases where single cysteines may regulate folding and function of tumor suppressors (e.g., p16INK4A) [248]. Furthermore, numerous studies have identified links between cell division, replicative aging, and oxidation [140,249], finding that increased cell division events correlate with higher ROS levels and global cellular oxidation. Many questions remain as to the mechanisms behind these relationships and present a particularly interesting avenue for future research.
11. Conclusions and Perspectives
In this review, we have discussed the intersections between cysteine thiol switches, redox regulation, and protein homeostasis. Thiol switches can be found across a range of proteostasis-associated pathways, with molecular mechanisms that vary widely. These include cysteine modifications on both chaperones and aggregation-prone substrates, as well as redox sensitive cysteines at the heart of the degradation machinery itself. The diversity in modifications and mechanisms—some protective, others harmful—point to robustness in the role cysteine thiols may play within the cell, particularly in these regulatory or sensing roles. Rather than viewing cysteines (and subsequent modifications) as homogenous in either reactivity, chemical mechanism, or “damaging” effect, we find that cysteine modifications appear in many configurations and form a rich tapestry of different molecular mechanisms.
Meanwhile, advancements in redox proteomics and genomic screens as well as in assessing the global redox status have opened the door for further study of the role individual cysteine thiols play in regulating proteostasis. Thus, various studies over the past decade have identified a remarkable range of proteins (many of them explicit chaperones or co-chaperones), which may be additional candidates for redox regulation of proteostasis at large. Individual mechanisms for many such cysteines remain to be studied; however, as we have discussed above, an increasing volume of research into protein folding, localization, and degradation hinges on specific cysteine thiols across the proteostasis network. This may point to a greater number of yet undiscovered dual-functionality within the members of the protein homeostasis system mediated by rapid changes in the redox status of specific redox-sensitive cysteines upon oxidative stress conditions.
Moreover, the pathways that currently demonstrate some form of redox regulation of proteostasis are of particular interest in understanding different human diseases. The links between cellular aging and oxidation have been well studied in correlation with neurodegenerative diseases in particular but may also contribute to a broader understanding of “inevitable” cellular dysfunction and its prevention. Similarly, links between replication and changes in redox regulation have been studied concerning embryonic development and cancer [250], yet may provide insights into the changing landscape of the proteostasis network itself. Together, these are crucial for a deeper understanding of the homeostasis mechanisms themselves and potential treatment or inhibition of harmful processes [251].
The findings presented in this review are but the tip of the iceberg. Future advances in redox proteomics and redox biology will likely reveal an extensive network of oxidation dependence in proteostasis and will uncover novel mechanisms for maintaining a “healthy” proteome during an aerobic lifestyle. To best understand and address when and how the proteome function begins to break down, it is of crucial importance to combine large-scale studies alongside investigating specific cysteine modifications in proteostasis. It is, therefore, also tempting to speculate that this fascinating journey into the redox biology of proteostasis will provide us with detailed knowledge of the cellular redox code and will allow us to use it for biotechnological and therapeutic purposes.
Funding
This work was funded by the Israel Science Foundation (individual research grants 1765/13 and 1537/18 for D. R and 1072/14 and 783/18 for N.M.), Legacy Heritage Biomedical Science Partnership (1649/16) and the NSFC-ISF program (2629/16). The doctoral fellowship of M.R was funded by the Azrieli Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Yael Sidkiyahu for her assistance with figure preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brandman, O.; Stewart-Ornstein, J.; Wong, D.; Larson, A.; Williams, C.C.; Li, G.-W.; Zhou, S.; King, D.; Shen, P.S.; Weibezahn, J.; et al. A Ribosome-Bound Quality Control Complex Triggers Degradation of Nascent Peptides and Signals Translation Stress. Cell 2012, 151, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Brandman, O.; Hegde, R.S. Ribosome-associated protein quality control. Nat. Struct. Mol. Biol. 2016, 23, 7–15. [Google Scholar] [CrossRef]
- Santiago, A.M.; Gonçalves, D.L.; Morano, K.A. Mechanisms of sensing and response to proteotoxic stress. Exp. Cell Res. 2020, 395, 112240. [Google Scholar] [CrossRef]
- Kim, Y.E.; Hipp, M.S.; Bracher, A.; Hayer-Hartl, M.; Ulrich Hartl, F. Molecular Chaperone Functions in Protein Folding and Proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. [Google Scholar] [CrossRef]
- Enam, C.; Geffen, Y.; Ravid, T.; Gardner, R.G. Protein Quality Control Degradation in the Nucleus. Annu. Rev. Biochem. 2018, 87, 725–749. [Google Scholar] [CrossRef]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Moehle, E.A.; Shen, K.; Dillin, A. Mitochondrial proteostasis in the context of cellular and organismal health and aging. J. Biol. Chem. 2019, 294, 5396–5407. [Google Scholar] [CrossRef] [PubMed]
- Klaips, C.L.; Jayaraj, G.G.; Hartl, F.U. Pathways of cellular proteostasis in aging and disease. J. Cell Biol. 2018, 217, 51–63. [Google Scholar] [CrossRef]
- Bardwell, J.C.A.; Jakob, U. Conditional disorder in chaperone action. Trends Biochem. Sci. 2012, 37, 517–525. [Google Scholar] [CrossRef]
- Suss, O.; Reichmann, D. Protein plasticity underlines activation and function of ATP-independent chaperones. Front. Mol. Biosci. 2015, 2. [Google Scholar] [CrossRef]
- Mayer, M.P.; Gierasch, L.M. Recent advances in the structural and mechanistic aspects of Hsp70 molecular chaperones. J. Biol. Chem. 2019, 294, 2085–2097. [Google Scholar] [CrossRef]
- Laskowska, E.; Kuczyńska-Wiśnik, D.; Lipińska, B. Proteomic analysis of protein homeostasis and aggregation. J. Proteom. 2018, 198, 98–112. [Google Scholar] [CrossRef]
- Reichmann, D.; Voth, W.; Jakob, U. Maintaining a Healthy Proteome during Oxidative Stress. Mol. Cell 2018, 69, 203–213. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Labbadia, J.; Morimoto, R.I. The Biology of Proteostasis in Aging and Disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef]
- Saarikangas, J.; Barral, Y. Protein aggregation as a mechanism of adaptive cellular responses. Curr. Genet. 2016, 62, 711–724. [Google Scholar] [CrossRef]
- Korovila, I.; Hugo, M.; Castro, J.P.; Weber, D.; Höhn, A.; Grune, T.; Jung, T. Proteostasis, oxidative stress and aging. Redox Biol. 2017, 13, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Sitia, R.; Braakman, I. Quality control in the endoplasmic reticulum protein factory. Nature 2003, 426, 891–894. [Google Scholar] [CrossRef]
- Tsai, F.T.F.; Jeng, W.; Lee, S.; Sung, N.; Lee, J. Molecular chaperones: Guardians of the proteome in normal and disease states. F1000Research 2015, 4, 1448. [Google Scholar]
- Brandvold, K.R.; Morimoto, R.I. The Chemical Biology of Molecular Chaperones—Implications for Modulation of Proteostasis. J. Mol. Biol. 2015, 427, 2931–2947. [Google Scholar] [CrossRef]
- Jakob, U.; Muse, W.; Eser, M.; Bardwell, J.C.A. Chaperone activity with a redox switch. Cell 1999, 96, 341–352. [Google Scholar] [CrossRef]
- Tapley, T.L.; Körnera, J.L.; Barge, M.T.; Hupfeld, J.; Schauerte, J.A.; Gafni, A.; Jakob, U.; Bardwell, J.C.A. Structural plasticity of an acid-activated chaperone allows promiscuous substrate binding. Proc. Natl. Acad. Sci. USA 2009, 106, 5557–5562. [Google Scholar] [CrossRef]
- Dahl, J.U.; Koldewey, P.; Salmon, L.; Horowitz, S.; Bardwell, J.C.A.; Jakob, U. HdeB functions as an acid-protective chaperone in bacteria. J. Biol. Chem. 2015, 290, 65–75. [Google Scholar] [CrossRef]
- Haslbeck, M.; Weinkauf, S.; Buchner, J. Small heat shock proteins: Simplicity meets complexity. J. Biol. Chem. 2019, 294, 2121–2132. [Google Scholar] [CrossRef]
- Alderson, T.R.; Ying, J.; Bax, A.; Benesch, J.L.P.; Baldwin, A.J. Conditional Disorder in Small Heat-shock Proteins. J. Mol. Biol. 2020, 432, 3033–3049. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Mogk, A.; Kummer, E.; Bukau, B. Cooperation of Hsp70 and Hsp100 chaperone machines in protein disaggregation. Front. Mol. Biosci. 2015, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Rebeaud, M.; Mallik, S.; Goloubinoff, P.; Tawfik, D. On the evolution of chaperones and co-chaperones and the expansion of proteomes across the Tree of Life. bioRxiv 2020. [Google Scholar] [CrossRef]
- Powers, E.T.; Balch, W.E. Diversity in the origins of proteostasis networks-a driver for protein function in evolution. Nat. Rev. Mol. Cell Biol. 2013, 14, 237–248. [Google Scholar] [CrossRef]
- Russell, R.; Karzai, A.W.; Mehl, A.F.; McMacken, R. DnaJ dramatically stimulates ATP hydrolysis by DnaK: Insight into targeting of Hsp70 proteins to polypeptide substrates. Biochemistry 1999, 38, 4165–4176. [Google Scholar] [CrossRef]
- Xu, H. Cochaperones enable Hsp70 to use ATP energy to stabilize native proteins out of the folding equilibrium. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef]
- Dean, M.E.; Johnson, J.L. Human Hsp90 cochaperones: Perspectives on tissue-specific expression and identification of cochaperones with similar in vivo functions. Cell Stress Chaperones 2020, 26. [Google Scholar] [CrossRef]
- Cyr, D.M. Cooperation of the molecular chaperone Ydj1 with specific Hsp70 homologs to suppress protein aggregation. FEBS Lett. 1995, 359, 129–132. [Google Scholar] [CrossRef]
- Luke, M.M.; Sutton, A.; Arndt, K.T. Characterization of SIS1, a Saccharomyces cerevisiae homologue of bacterial dnaJ proteins. J. Cell Biol. 1991, 114, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kukushkin, Y.; Gupta, R.; Chen, T.; Konagai, A.; Hipp, M.S.; Hayer-Hartl, M.; Hartl, F.U. PolyQ proteins interfere with nuclear degradation of cytosolic proteins by sequestering the Sis1p chaperone. Cell 2013, 154, 134–145. [Google Scholar] [CrossRef]
- Faust, O.; Abayev-Avraham, M.; Wentink, A.S.; Maurer, M.; Nillegoda, N.B.; London, N.; Bukau, B.; Rosenzweig, R. HSP40 proteins use class-specific regulation to drive HSP70 functional diversity. Nature 2020, 587, 489–494. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Andreasson, C.; Barducci, A.; Cheetham, M.E.; Cyr, D.; Emanuelsson, C.; Genevaux, P.; Gestwicki, J.E.; Goloubinoff, P.; Huerta-Cepas, J.; et al. Function, evolution, and structure of J-domain proteins. Cell Stress Chaperones 2019, 24, 7–15. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Kirstein, J.; Arnsburg, K.; Scior, A.; Szlachcic, A.; Guilbride, D.L.; Morimoto, R.I.; Bukau, B.; Nillegoda, N.B. In vivo properties of the disaggregase function of J-proteins and Hsc70 in Caenorhabditis elegans stress and aging. Aging Cell 2017, 16, 1414–1424. [Google Scholar] [CrossRef]
- Feleciano, D.R.; Arnsburg, K.; Kirstein, J. Interplay between redox and protein homeostasis. Worm 2016, 5, e1170273. [Google Scholar] [CrossRef][Green Version]
- Ushioda, R.; Hoseki, J.; Araki, K.; Jansen, G.; Thomas, D.Y.; Nagata, K. ERdj5 is required as a disulfide reductase for degradation of misfolded proteins in the ER. Science 2008, 321, 569–572. [Google Scholar] [CrossRef]
- Abildgaard, A.B.; Gersing, S.K.; Larsen-Ledet, S.; Nielsen, S.V.; Stein, A.; Lindorff-Larsen, K.; Hartmann-Petersen, R. Co-chaperones in targeting and delivery of misfolded proteins to the 26s proteasome. Biomolecules 2020, 10, 1141. [Google Scholar] [CrossRef]
- Shiber, A.; Ravid, T. Chaperoning proteins for destruction: Diverse roles of Hsp70 chaperones and their co-chaperones in targeting misfolded proteins to the proteasome. Biomolecules 2014, 4, 704–724. [Google Scholar] [CrossRef]
- Tiwari, B.S.; Belenghi, B.; Levine, A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 2002, 128, 1271–1281. [Google Scholar] [CrossRef]
- Colussi, C.; Albertini, M.C.; Coppola, S.; Rovidati, S.; Galli, F.; Ghibelli, L. H2O2-induced block of glycolysis as an active ADP-ribosylation reaction protecting cells from apoptosis. FASEB J. 2000, 14, 2266–2276. [Google Scholar] [CrossRef]
- Schraufstatter, I.U.; Hyslop, P.A.; Hinshaw, D.B.; Spragg, R.G.; Sklar, L.A.; Cochrane, C.G. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc. Natl. Acad. Sci. USA 1986, 83, 4908–4912. [Google Scholar] [CrossRef]
- Goemans, C.V.; Vertommen, D.; Agrebi, R.; Collet, J.F. CnoX Is a Chaperedoxin: A Holdase that Protects Its Substrates from Irreversible Oxidation. Mol. Cell 2018, 70, 614–627.e7. [Google Scholar] [CrossRef]
- Voth, W.; Schick, M.; Gates, S.; Li, S.; Vilardi, F.; Gostimskaya, I.; Southworth, D.R.; Schwappach, B.; Jakob, U. The protein targeting factor Get3 functions as ATP-Independent chaperone under oxidative stress conditions. Mol. Cell 2014, 56, 116–127. [Google Scholar] [CrossRef]
- Jensen, P.K. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles I. pH dependency and hydrogen peroxide formation. BBA Enzymol. Biol. Oxid. 1966, 122, 157–166. [Google Scholar] [CrossRef]
- Loschen, G.; Azzi, A.; Richter, C.; Flohé, L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974, 42, 68–72. [Google Scholar] [CrossRef]
- Loschen, G.; Azzi, A.; Flohé, L. Mitochondrial H2O2 formation: Relationship with energy conservation. FEBS Lett. 1973, 33, 84–88. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide dismutases. Annu. Rev. Biochem. 1975, 44, 147–159. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Yoboue, E.D.; Sitia, R.; Simmen, T. Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis. 2018, 9, 331. [Google Scholar] [CrossRef]
- Suh, Y.-A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401, 79–82. [Google Scholar] [CrossRef]
- Takeya, R.; Sumimoto, H. Regulation of novel superoxide-producing NAD(P)H oxidases. Antioxid. Redox Signal. 2006, 8, 1523–1532. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Maker, H.S.; Weiss, C.; Silides, D.J.; Cohen, G. Coupling of Dopamine Oxidation (Monoamine Oxidase Activity) to Glutathione Oxidation Via the Generation of Hydrogen Peroxide in Rat Brain Homogenates. J. Neurochem. 1981, 36, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Shao, L.; Spitz, D.R. Reactive oxygen species in normal and tumor stem cells. In Advances in Cancer Research; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 122, pp. 1–67. [Google Scholar]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- Fourquet, S.; Guerois, R.; Biard, D.; Toledano, M.B. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J. Biol. Chem. 2010, 285, 8463–8471. [Google Scholar] [CrossRef]
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric oxide in macrophage immunometabolism: Hiding in plain sight. Metabolites 2020, 10, 429. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Snapshot: Reactive oxygen intermediates (ROI). Cell 2010, 140, 951–951.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Loake, G.J.; Chu, C. Cross-talk of nitric oxide and reactive oxygen species in plant programed cell death. Front. Plant Sci. 2013, 4, 314. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Feldman, N.B.; Lutsenko, S.V. ROS and RNS signalling: Adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic. Res. 2018, 52, 507–543. [Google Scholar] [CrossRef]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar] [CrossRef]
- Rice, M.E. H2O2: A dynamic neuromodulator. Neuroscientist 2011, 17, 389–406. [Google Scholar] [CrossRef]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003, 15, 247–254. [Google Scholar] [CrossRef]
- Koshland, D.E. The molecule of the year. Science 1992, 258, 1861. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Del Valle, N.R.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef]
- Benhar, M. Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radic. Biol. Med. 2018, 127, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Fröhlich, E.; Ligr, M.; Grey, M.; Sigrist, S.J.; Wolf, D.H.; Fröhlich, K.U. Oxygen stress: A regulator of apoptosis in yeast. J. Cell Biol. 1999, 145, 757–767. [Google Scholar] [CrossRef]
- Squier, T.C. Oxidative stress and protein aggregation during biological aging. Exp. Gerontol. 2001, 36, 1539–1550. [Google Scholar] [CrossRef]
- Fernando, R.; Drescher, C.; Nowotny, K.; Grune, T.; Castro, J.P. Impaired proteostasis during skeletal muscle aging. Free Radic. Biol. Med. 2019, 132, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Soares, T.R.; Reis, S.D.; Pinho, B.R.; Duchen, M.R.; Oliveira, J.M.A. Targeting the proteostasis network in Huntington’s disease. Ageing Res. Rev. 2019, 49, 92–103. [Google Scholar] [CrossRef]
- Mesika, R.; Reichmann, D. When safeguarding goes wrong: Impact of oxidative stress on protein homeostasis in health and neurodegenerative disorders. In Advances in Protein Chemistry and Structural Biology; Academic Press Inc.: Cambridge, MA, USA, 2019; Volume 114, pp. 221–264. ISBN 9780128155578. [Google Scholar]
- Butterfield, D.A. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: Implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic. Res. 2002, 36, 1307–1313. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S38. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.V.; Hora, S.; Pal, A.; Jha, S.; Taneja, R. Stressing the (Epi)Genome: Dealing with Reactive Oxygen Species in Cancer. Antioxid. Redox Signal. 2017, 29, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The Free Radical Theory of Aging. Antioxid. Redox Signal. 2003, 5, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-W.; Chen, Y.-C.; Hsieh, W.-L.; Chiou, S.-H.; Kao, C.-L. Ageing and neurodegenerative diseases. Ageing Res. Rev. 2010, 9, S36–S46. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.; Chang, C. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef]
- Paiva, C.N.; Bozza, M.T. Are Reactive Oxygen Species Always Detrimental to Pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef]
- Brandes, N.; Schmitt, S.; Jakob, U. Thiol-Based Redox Switches in Eukaryotic Proteins. Antioxid. Redox Signal. 2009, 11, 997–1014. [Google Scholar] [CrossRef]
- Rimon, O.; Suss, O.; Goldenberg, M.; Fassler, R.; Yogev, O.; Amartely, H.; Propper, G.; Friedler, A.; Reichmann, D. A role of metastable regions and their connectivity in the inactivation of a redox-regulated chaperone and its inter-chaperone crosstalk. Antioxid. Redox Signal. 2017, 27, 1252–1267. [Google Scholar] [CrossRef]
- Topf, U.; Suppanz, I.; Samluk, L.; Wrobel, L.; Böser, A.; Sakowska, P.; Knapp, B.; Pietrzyk, M.K.; Chacinska, A.; Warscheid, B. Quantitative proteomics identifies redox switches for global translation modulation by mitochondrially produced reactive oxygen species. Nat. Commun. 2018, 9, 324. [Google Scholar] [CrossRef]
- Knuesting, J.; Scheibe, R. Small Molecules Govern Thiol Redox Switches. Trends Plant Sci. 2018, 23, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A. Antioxidant Function of Thioredoxin and Glutaredoxin Systems. Antioxid. Redox Signal. 2000, 2, 811–820. [Google Scholar] [CrossRef]
- Rhee, S.G.; Kil, I.S. Multiple Functions and Regulation of Mammalian Peroxiredoxins. Annu. Rev. Biochem. 2017, 86, 749–775. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef]
- Benhar, M. Oxidants, Antioxidants and Thiol Redox Switches in the Control of Regulated Cell Death Pathways. Antioxidants 2020, 9, 309. [Google Scholar] [CrossRef]
- Liedgens, L.; Zimmermann, J.; Wäschenbach, L.; Geissel, F.; Laporte, H.; Gohlke, H.; Morgan, B.; Deponte, M. Quantitative assessment of the determinant structural differences between redox-active and inactive glutaredoxins. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Roede, J.R.; Walker, D.I.; Duong, D.M.; Seyfried, N.T.; Orr, M.; Liang, Y.; Pennell, K.D.; Jones, D.P. Selective targeting of the cysteine proteome by thioredoxin and glutathione redox systems. Mol. Cell. Proteom. 2013, 12, 3285–3296. [Google Scholar] [CrossRef]
- Gould, N.S.; Evans, P.; Martínez-Acedo, P.; Marino, S.M.; Gladyshev, V.N.; Carroll, K.S.; Ischiropoulos, H. Site-Specific Proteomic Mapping Identifies Selectively Modified Regulatory Cysteine Residues in Functionally Distinct Protein Networks. Chem. Biol. 2015, 22, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, D.E.; Marino, S.M.; Gladyshev, V.N. Functional diversity of cysteine residues in proteins and unique features of catalytic redox-active cysteines in thiol oxidoreductases. Mol. Cells 2008, 26, 228–235. [Google Scholar]
- Brandes, N.; Reichmann, D.; Tienson, H.; Leichert, L.I.; Jakob, U. Using quantitative redox proteomics to dissect the yeast redoxome. J. Biol. Chem. 2011, 286, 41893–41903. [Google Scholar] [CrossRef] [PubMed]
- Genest, O.; Wickner, S.; Doyle, S.M. Hsp90 and Hsp70 chaperones: Collaborators in protein remodeling. J. Biol. Chem. 2019, 294, 2109–2120. [Google Scholar] [CrossRef]
- Ulrich, K.; Schwappach, B.; Jakob, U. Thiol-based switching mechanisms of stress-sensing chaperones. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Wood, Z.A.; Schröder, E.; Harris, J.R.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef]
- Jang, H.H.; Lee, K.O.; Chi, Y.H.; Jung, B.G.; Park, S.K.; Park, J.H.; Lee, J.R.; Lee, S.S.; Moon, J.C.; Yun, J.W.; et al. Two enzymes in one: Two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 2004, 117, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Macdiarmid, C.W.; Taggart, J.; Kerdsomboon, K.; Kubisiak, M.; Panascharoen, S.; Schelble, K.; Eide, D.J. Peroxiredoxin chaperone activity is critical for protein homeostasis in zinc-deficient yeast. J. Biol. Chem. 2013, 288, 31313–31327. [Google Scholar] [CrossRef] [PubMed]
- Hanzén, S.; Vielfort, K.; Yang, J.; Roger, F.; Andersson, V.; Zamarbide-Forés, S.; Andersson, R.; Malm, L.; Palais, G.; Biteau, B.; et al. Lifespan Control by Redox-Dependent Recruitment of Chaperones to Misfolded Proteins. Cell 2016, 166, 140–151. [Google Scholar] [CrossRef]
- Biteau, B.; Labarre, J.; Toledano, M.B. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 2003, 425, 980–984. [Google Scholar] [CrossRef]
- Sunico, C.R.; Sultan, A.; Nakamura, T.; Dolatabadi, N.; Parker, J.; Shan, B.; Han, X.; Yates, J.R.; Masliah, E.; Ambasudhan, R.; et al. Role of sulfiredoxin as a peroxiredoxin-2 denitrosylase in human iPSC-derived dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2016, 113, E7564–E7571. [Google Scholar] [CrossRef]
- Detienne, G.; De Haes, W.; Mergan, L.; Edwards, S.L.; Temmerman, L.; Van Bael, S. Beyond ROS clearance: Peroxiredoxins in stress signaling and aging. Ageing Res. Rev. 2018, 44, 33–48. [Google Scholar] [CrossRef]
- Kamariah, N.; Eisenhaber, B.; Eisenhaber, F.; Grüber, G. Molecular mechanism of the Escherichia coli AhpC in the function of a chaperone under heat-shock conditions. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.; Castro, H.; Cruz, T.; Tse, E.; Koldewey, P.; Southworth, D.R.; Tomás, A.M.; Jakob, U. Mitochondrial peroxiredoxin functions as crucial chaperone reservoir in Leishmania infantum. Proc. Natl. Acad. Sci. USA 2015, 112, E616–E624. [Google Scholar] [CrossRef] [PubMed]
- Saccoccia, F.; Di Micco, P.; Boumis, G.; Brunori, M.; Koutris, I.; Miele, A.E.; Morea, V.; Sriratana, P.; Williams, D.L.; Bellelli, A.; et al. Moonlighting by different stressors: Crystal structure of the chaperone species of a 2-Cys peroxiredoxin. Structure 2012, 20, 429–439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zivanovic, J.; Kouroussis, E.; Kohl, J.B.; Adhikari, B.; Bursac, B.; Schott-Roux, S.; Petrovic, D.; Miljkovic, J.L.; Thomas-Lopez, D.; Jung, Y.; et al. Selective Persulfide Detection Reveals Evolutionarily Conserved Antiaging Effects of S-Sulfhydration. Cell Metab. 2019, 30, 1152–1170.e13. [Google Scholar] [CrossRef]
- Gu, F.; Duc, T.N.; Stuible, M.; Dubé, N.; Tremblay, M.L.; Chevet, E. Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. J. Biol. Chem. 2004, 279, 49689–49693. [Google Scholar] [CrossRef]
- Suzuki, T.; Muramatsu, A.; Saito, R.; Iso, T.; Shibata, T.; Kuwata, K.; Kawaguchi, S.; Iwawaki, T.; Adachi, S.; Suda, H.; et al. Molecular Mechanism of Cellular Oxidative Stress Sensing by Keap1. Cell Rep. 2019, 28, 746–758.e4. [Google Scholar] [CrossRef]
- Dóka, É.; Ida, T.; Dagnell, M.; Abiko, Y.; Luong, N.C.; Balog, N.; Takata, T.; Espinosa, B.; Nishimura, A.; Cheng, Q.; et al. Control of protein function through oxidation and reduction of persulfidated states. Sci. Adv. 2020, 6, eaax8358. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Novoselov, V.I.; Penkov, N.V.; Fesenko, E.E.; Vedunova, M.V.; Bruskov, V.I.; Gudkov, S.V. Protective and adaptogenic role of peroxiredoxin 2 (Prx2) in neutralization of oxidative stress induced by ionizing radiation. Free Radic. Biol. Med. 2019, 134, 76–86. [Google Scholar] [CrossRef]
- Ding, C.; Fan, X.; Wu, G. Peroxiredoxin 1—An antioxidant enzyme in cancer. J. Cell. Mol. Med. 2017, 21, 193–202. [Google Scholar] [CrossRef]
- McCaffrey, K.; Braakman, I. Protein quality control at the endoplasmic reticulum. Essays Biochem. 2016, 60, 227–235. [Google Scholar] [CrossRef]
- Aramin, S.; Fassler, R.; Chikne, V.; Goldenberg, M.; Arian, T.; Kolet Eliaz, L.; Rimon, O.; Ram, O.; Michaeli, S.; Reichmann, D. TrypOx, a Novel Eukaryotic Homolog of the Redox-Regulated Chaperone Hsp33 in Trypanosoma brucei. Front. Microbiol. 2020, 11, 1844. [Google Scholar] [CrossRef]
- Ilbert, M.; Horst, J.; Ahrens, S.; Winter, J.; Graf, P.C.F.; Lilie, H.; Jakob, U. The redox-switch domain of Hsp33 functions as dual stress sensor. Nat. Struct. Mol. Biol. 2007, 14, 556–563. [Google Scholar] [CrossRef]
- Winter, J.; Ilbert, M.; Graf, P.C.F.; Özcelik, D.; Jakob, U. Bleach Activates a Redox-Regulated Chaperone by Oxidative Protein Unfolding. Cell 2008, 135, 691–701. [Google Scholar] [CrossRef]
- Xie, J.L.; Bohovych, I.; Wong, E.O.Y.; Lambert, J.P.; Gingras, A.C.; Khalimonchuk, O.; Cowen, L.E.; Leach, M.D. Ydj1 governs fungal morphogenesis and stress response, and facilitates mitochondrial protein import via Mas1 and Mas2. Microb. Cell 2017, 4, 342–361. [Google Scholar] [CrossRef]
- Reichmann, D.; Xu, Y.; Cremers, C.M.; Ilbert, M.; Mittelman, R.; Fitzgerald, M.C.; Jakob, U. Order out of disorder: Working cycle of an intrinsically unfolded chaperone. Cell 2012, 148, 947–957. [Google Scholar] [CrossRef]
- Groitl, B.; Horowitz, S.; Makepeace, K.A.T.; Petrotchenko, E.V.; Borchers, C.H.; Reichmann, D.; Bardwell, J.C.A.; Jakob, U. Protein unfolding as a switch from self-recognition to high-affinity client binding. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Cremers, C.M.; Reichmann, D.; Hausmann, J.; Ilbert, M.; Jakob, U. Unfolding of metastable linker region is at the core of Hsp33 activation as a redox-regulated chaperone. J. Biol. Chem. 2010, 285, 11243–11251. [Google Scholar] [CrossRef]
- Goemans, C.V.; Beaufay, F.; Arts, I.S.; Agrebi, R.; Vertommen, D.; Collet, J.F. The Chaperone and Redox Properties of CnoX Chaperedoxins Are Tailored to the Proteostatic Needs of Bacterial Species. MBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Erdős, G.; Mészáros, B.; Reichmann, D.; Dosztányi, Z. Large-Scale Analysis of Redox-Sensitive Conditionally Disordered Protein Regions Reveals Their Widespread Nature and Key Roles in High-Level Eukaryotic Processes. Proteomics 2019, 19, 1800070. [Google Scholar] [CrossRef]
- Leichert, L.I.; Gehrke, F.; Gudiseva, H.V.; Blackwell, T.; Ilbert, M.; Walker, A.K.; Strahler, J.R.; Andrews, P.C.; Jakob, U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 8197–8202. [Google Scholar] [CrossRef]
- Xiao, H.; Jedrychowski, M.P.; Schweppe, D.K.; Huttlin, E.L.; Yu, Q.; Heppner, D.E.; Li, J.; Long, J.; Mills, E.L.; Szpyt, J.; et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell 2020, 180, 968–983.e24. [Google Scholar] [CrossRef]
- McDonagh, B.; Sakellariou, G.K.; Smith, N.T.; Brownridge, P.; Jackson, M.J. Differential cysteine labeling and global label-free proteomics reveals an altered metabolic state in skeletal muscle aging. J. Proteome Res. 2014, 13, 5008–5021. [Google Scholar] [CrossRef]
- Rosenwasser, S.; Van Creveld, S.G.; Schatz, D.; Malitsky, S.; Tzfadia, O.; Aharoni, A.; Levin, Y.; Gabashvili, A.; Feldmesser, E.; Vardi, A. Mapping the diatom redox-sensitive proteome provides insight into response to nitrogen stress in the marine environment. Proc. Natl. Acad. Sci. USA 2014, 111, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Carroll, K.S.; Liebler, D.C. The expanding landscape of the thiol redox proteome. Mol. Cell. Proteom. 2016, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Carroll, K.S. Activity-Based Sensing for Site-Specific Proteomic Analysis of Cysteine Oxidation. Acc. Chem. Res. 2020, 53, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Li, L.; Parisien, M.; Wu, J.; Bederman, I.; Gao, Z.; Krokowski, D.; Chirieleison, S.M.; Abbott, D.; Wang, B.; et al. Discovery of a redox thiol switch: Implications for cellular energy metabolism. Mol. Cell. Proteom. 2020, 19, 852–870. [Google Scholar] [CrossRef] [PubMed]
- Gerashchenko, M.V.; Lobanov, A.V.; Gladyshev, V.N. Genome-wide ribosome profiling reveals complex translational regulation in response to oxidative stress. Proc. Natl. Acad. Sci. USA 2012, 109, 17394–17399. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, H.G.; Son, C.G. Tissue-specific profiling of oxidative stress-associated transcriptome in a healthy mouse model. Int. J. Mol. Sci. 2018, 19, 3174. [Google Scholar] [CrossRef]
- Radzinski, M.; Fassler, R.; Yogev, O.; Breuer, W.; Shai, N.; Gutin, J.; Ilyas, S.; Geffen, Y.; Tsytkin-Kirschenzweig, S.; Nahmias, Y.; et al. Temporal profiling of redox-dependent heterogeneity in single cells. Elife 2018, 7, e37623. [Google Scholar] [CrossRef]
- Ayer, A.; Fellermeier, S.; Fife, C.; Li, S.S.; Smits, G.; Meyer, A.J.; Dawes, I.W.; Perrone, G.G. A Genome-Wide Screen in Yeast Identifies Specific Oxidative Stress Genes Required for the Maintenance of Sub-Cellular Redox Homeostasis. PLoS ONE 2012, 7, e44278. [Google Scholar] [CrossRef]
- Kim, H.H.-J.; Ha, S.; Lee, H.Y.; Lee, K.-J.K. ROSics: Chemistry and proteomics of cysteine modifications in redox biology. Mass Spectrom. Rev. 2015, 34, 184–208. [Google Scholar] [CrossRef]
- Rizza, S.; Montagna, C.; Cardaci, S.; Maiani, E.; Di Giacomo, G.; Sanchez-Quiles, V.; Blagoev, B.; Rasola, A.; De Zio, D.; Stamler, J.S.; et al. S-nitrosylation of the mitochondrial chaperone TRAP1 sensitizes hepatocellular carcinoma cells to inhibitors of succinate dehydrogenase. Cancer Res. 2016, 76, 4170–4182. [Google Scholar] [CrossRef]
- Uehara, T.; Nakamura, T.; Yao, D.; Shi, Z.Q.; Gu, Z.; Ma, Y.; Masliah, E.; Nomura, Y.; Lipton, S.A. S-Nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 2006, 441, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.K.Y.; Davey, M.; Sun, B.; Roth, A.F.; Davis, N.G.; Conibear, E. Palmitoylation by the DHHC protein Pfa4 regulates the ER exit of Chs3. J. Cell Biol. 2006, 174, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Savitsky, P.A.; Finkel, T. Redox regulation of Cdc25C. J. Biol. Chem. 2002, 277, 20535–20540. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Toma-Fukai, S.; Kontani, K.; Katada, T.; Shimizu, T.; Goody, R.S. GEF mechanism revealed by the structure of SmgGDS-558 and farnesylated RhoA complex and its implication for a chaperone mechanism. Proc. Natl. Acad. Sci. USA 2018, 115, 9563–9568. [Google Scholar] [CrossRef]
- Resh, M.D. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat. Chem. Biol. 2006, 2, 584–590. [Google Scholar] [CrossRef]
- Abrami, L.; Atrice Kunz, B.; Iacovache, I.; Gisou Van Der Goot, F. Palmitoylation and Ubiquitination Regulate Exit of the Wnt Signaling Protein LRP6 from the Endoplasmic Reticulum. Proc. Natl. Acad. Sci. USA 2008, 105, 5384–5389. [Google Scholar] [CrossRef] [PubMed]
- Diez-Ardanuy, C.; Greaves, J.; Munro, K.R.; Tomkinson, N.C.O.; Chamberlain, L.H. A cluster of palmitoylated cysteines are essential for aggregation of cysteine-string protein mutants that cause neuronal ceroid lipofuscinosis. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Ernst, A.M.; Syed, S.A.; Zaki, O.; Bottanelli, F.; Zheng, H.; Hacke, M.; Xi, Z.; Rivera-Molina, F.; Graham, M.; Rebane, A.A.; et al. S-Palmitoylation Sorts Membrane Cargo for Anterograde Transport in the Golgi. Dev. Cell 2018, 47, 479–493.e7. [Google Scholar] [CrossRef]
- Sun, Z.; Brodsky, J.L. Protein quality control in the secretory pathway. J. Cell Biol. 2019, 218, 3171–3187. [Google Scholar] [CrossRef]
- Berndt, N.; Hamilton, A.D.; Sebti, S.M. Targeting protein prenylation for cancer therapy. Nat. Rev. Cancer 2011, 11, 775–791. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Boronat, S.; Marte, L.; Vega, M.; Pérez, P.; Ayté, J.; Hidalgo, E. Chaperone-Facilitated Aggregation of Thermo-Sensitive Proteins Shields Them from Degradation during Heat Stress. Cell Rep. 2020, 30, 2430–2443.e4. [Google Scholar] [CrossRef]
- Nakamura, T.; Lipton, S.A. Nitric oxide-dependent protein post-translational modifications impair mitochondrial function and metabolism to contribute to neurodegenerative diseases. Antioxid. Redox Signal. 2020, 32, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, A.; Lamas, S. S-nitrosylation: A potential new paradigm in signal transduction. Cardiovasc. Res. 2004, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ogura, J.; Ruddock, L.W.; Mano, N. Cysteine 343 in the substrate binding domain is the primary S-Nitrosylated site in protein disulfide isomerase. Free Radic. Biol. Med. 2020, 160, 103–110. [Google Scholar] [CrossRef]
- Wang, K.; Liu, J.Q.; Zhong, T.; Liu, X.L.; Zeng, Y.; Qiao, X.; Xie, T.; Chen, Y.; Gao, Y.Y.; Tang, B.; et al. Phase Separation and Cytotoxicity of Tau are Modulated by Protein Disulfide Isomerase and S-nitrosylation of this Molecular Chaperone. J. Mol. Biol. 2020, 432, 2141–2163. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Besson-Bard, A.; Lamotte, O.; Bertoldo, J.; Bourque, S.; Terenzi, H.; Wendehenne, D. Nitric oxide inhibits the ATPase activity of the chaperone-like AAA + ATPase CDC48, a target for S-nitrosylation in cryptogein signalling in tobacco cells. Biochem. J. 2012, 447, 249–260. [Google Scholar] [CrossRef]
- Noguchi, M.; Takata, T.; Kimura, Y.; Manno, A.; Murakami, K.; Koike, M.; Ohizumi, H.; Hori, S.; Kakizuka, A. ATPase activity of p97/valosin-containing protein is regulated by oxidative modification of the evolutionally conserved cysteine 522 residue in walker a motif. J. Biol. Chem. 2005. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, X.; Zhang, L.; Gao, X.; Yang, P.; Lu, H. Identification of Palmitoylated Transitional Endoplasmic Reticulum ATPase by Proteomic Technique and Pan Antipalmitoylation Antibody. J. Proteome Res. 2016, 15, 956–962. [Google Scholar] [CrossRef]
- Kleizen, B.; Braakman, I. Protein folding and quality control in the endoplasmic reticulum. Curr. Opin. Cell Biol. 2004, 16, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Sevier, C.S.; Kaiser, C.A. Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 549–556. [Google Scholar] [CrossRef]
- Ellgaard, L.; Ruddock, L.W. The human protein disulphide isomerase family: Substrate interactions and functional properties. EMBO Rep. 2005, 6, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Farg, M.A.; Bye, C.R.; McLean, C.A.; Horne, M.K.; Atkin, J.D. Protein disulphide isomerase protects against protein aggregation and is S-nitrosylated in amyotrophic lateral sclerosis. Brain 2010, 133, 105–116. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Eletto, D.; Chevet, E.; Argon, Y.; Appenzeller-Herzog, C. Redox controls UPR to control redox. J. Cell Sci. 2014, 127, 3649–3658. [Google Scholar] [CrossRef]
- Chadwick, S.R.; Fazio, E.N.; Etedali-Zadeh, P.; Genereaux, J.; Duennwald, M.L.; Lajoie, P. A functional unfolded protein response is required for chronological aging in Saccharomyces cerevisiae. Curr. Genet. 2019, 66, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Teuber, V.; Albert-Gasco, H.; Auyeung, V.C.; Papa, F.R.; Mallucci, G.R.; Hetz, C. Small Molecules to Improve ER Proteostasis in Disease. Trends Pharmacol. Sci. 2019, 40, 684–695. [Google Scholar] [CrossRef]
- Hourihan, J.M.; Moronetti Mazzeo, L.E.; Fernández-Cárdenas, L.P.; Blackwell, T.K. Cysteine Sulfenylation Directs IRE-1 to Activate the SKN-1/Nrf2 Antioxidant Response. Mol. Cell 2016, 63, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Moreno, A.; Ang, J.; Welsch, H.; Jochem, M.; Hanna, J. Regulation of the unfolded protein response in yeast by oxidative stress. FEBS Lett. 2019, 593, 1080–1088. [Google Scholar] [CrossRef]
- Eletto, D.; Eletto, D.; Dersh, D.; Gidalevitz, T.; Argon, Y. Protein Disulfide Isomerase A6 Controls the Decay of IRE1α Signaling via Disulfide-Dependent Association. Mol. Cell 2014, 53, 562–576. [Google Scholar] [CrossRef]
- Deshmukh, F.K.; Yaffe, D.; Olshina, M.A.; Ben-Nissan, G.; Sharon, M. The contribution of the 20s proteasome to proteostasis. Biomolecules 2019, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.M.; Netto, L.E.S.; Simões, V.; Santos, L.F.A.; Gozzo, F.C.; Demasi, M.A.A.; Oliveira, C.L.P.; Bicev, R.N.; Klitzke, C.F.; Sogayar, M.C.; et al. Redox control of 20S proteasome gating. Antioxid. Redox Signal. 2012, 16, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.T.; Kaake, R.M.; Wang, X.; Huang, L. Oxidative Stress-Mediated Regulation of Proteasome Complexes. Mol. Cell. Proteom. 2011. [Google Scholar] [CrossRef]
- Shringarpure, R.; Grune, T.; Mehlhase, J.; Davies, K.J.A. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J. Biol. Chem. 2003, 278, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.A. Degradation of oxidized proteins by the 20S proteasome. In Proceedings of the Biochimie; Elsevier Masson SAS: Amsterdam, The Netherlands, 2001; Volume 83, pp. 301–310. [Google Scholar]
- Yu, Z.; Yu, Y.; Wang, F.; Myasnikov, A.G.; Coffino, P.; Cheng, Y. Allosteric coupling between α-rings of the 20S proteasome. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.; Bertolotti, A. Regulation of proteasome assembly and activity in health and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Peth, A.; Uchiki, T.; Goldberg, A.L. ATP-Dependent steps in the binding of ubiquitin conjugates to the 26s proteasome that commit to degradation. Mol. Cell 2010, 40, 671–681. [Google Scholar] [CrossRef]
- Aufderheide, A.; Beck, F.; Stengel, F.; Hartwig, M.; Schweitzer, A.; Pfeifer, G.; Goldberg, A.L.; Sakata, E.; Baumeister, W.; Förster, F. Structural characterization of the interaction of Ubp6 with the 26S proteasome. Proc. Natl. Acad. Sci. USA 2015, 112, 8626–8631. [Google Scholar] [CrossRef]
- Finley, D.; Chen, X.; Walters, K.J. Gates, Channels, and Switches: Elements of the Proteasome Machine. Trends Biochem. Sci. 2016, 41, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Husnjak, K.; Elsasser, S.; Zhang, N.; Chen, X.; Randles, L.; Shi, Y.; Hofmann, K.; Walters, K.J.; Finley, D.; Dikic, I. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature 2008, 453, 481–488. [Google Scholar] [CrossRef]
- Richly, H.; Rape, M.; Braun, S.; Rumpf, S.; Hoege, C.; Jentsch, S. A Series of Ubiquitin Binding Factors Connects CDC48/p97 to Substrate Multiubiquitylation and Proteasomal Targeting. Cell 2005, 120, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.A.; Goldberg, A.L. The Logic of the 26S Proteasome. Cell 2017, 169, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.X.; Chen, W.; Hong, S.T.; Boycott, K.M.; Gorrie, G.H.; Siddique, N.; Yang, Y.; Fecto, F.; Shi, Y.; Zhai, H.; et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 2011, 477, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Benaroudj, N.; Zwickl, P.; Seemüller, E.; Baumeister, W.; Goldberg, A.L. ATP hydrolysis by the proteasome regulatory complex PAN serves multiple functions in protein degradation. Mol. Cell 2003, 11, 69–78. [Google Scholar] [CrossRef]
- Groll, M.; Ditzel, L.; Löwe, J.; Stock, D.; Bochtler, M.; Bartunik, H.D.; Huber, R. Structure of 20S proteasome from yeast at 2.4 Å resolution. Nature 1997, 386, 463–471. [Google Scholar] [CrossRef]
- Wang, X.; Yen, J.; Kaiser, P.; Huang, L. Regulation of the 26S proteasome complex during oxidative stress. Sci. Signal. 2010, 3, ra88. [Google Scholar] [CrossRef]
- Haratake, K.; Sato, A.; Tsuruta, F.; Chiba, T. KIAA0368-deficiency affects disassembly of 26S proteasome under oxidative stress condition. J. Biochem. 2016, 159, 609–618. [Google Scholar] [CrossRef]
- Wang, X.; Chemmama, I.E.; Yu, C.; Huszagh, A.; Xu, Y.; Viner, R.; Block, S.A.; Cimermancic, P.; Rychnovsky, S.D.; Ye, Y.; et al. The proteasome-interacting Ecm29 protein disassembles the 26S proteasome in response to oxidative stress. J. Biol. Chem. 2017, 292, 16310–16320. [Google Scholar] [CrossRef]
- Grune, T.; Catalgol, B.; Licht, A.; Ermak, G.; Pickering, A.M.; Ngo, J.K.; Davies, K.J.A. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic. Biol. Med. 2011, 51, 1355–1364. [Google Scholar] [CrossRef]
- Moscovitz, O.; Ben-Nissan, G.; Fainer, I.; Pollack, D.; Mizrachi, L.; Sharon, M. The Parkinson’s-associated protein DJ-1 regulates the 20S proteasome. Nat. Commun. 2015, 6, 6609. [Google Scholar] [CrossRef]
- Moscovitz, O.; Tsvetkov, P.; Hazan, N.; Michaelevski, I.; Keisar, H.; Ben-Nissan, G.; Shaul, Y.; Sharon, M. A Mutually Inhibitory Feedback Loop between the 20S Proteasome and Its Regulator, NQO1. Mol. Cell 2012, 47, 76–86. [Google Scholar] [CrossRef]
- Olshina, M.A.; Arkind, G.; Kumar Deshmukh, F.; Fainer, I.; Taranavsky, M.; Hayat, D.; Ben-Dor, S.; Ben-Nissan, G.; Sharon, M. Regulation of the 20S Proteasome by a Novel Family of Inhibitory Proteins. Antioxid. Redox Signal. 2020, 32, 636–655. [Google Scholar] [CrossRef]
- Abi Habib, J.; De Plaen, E.; Stroobant, V.; Zivkovic, D.; Bousquet, M.P.; Guillaume, B.; Wahni, K.; Messens, J.; Busse, A.; Vigneron, N.; et al. Efficiency of the four proteasome subtypes to degrade ubiquitinated or oxidized proteins. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Myers, N.; Eliav, R.; Adamovich, Y.; Hagai, T.; Adler, J.; Navon, A.; Shaul, Y. NADH Binds and stabilizes the 26S proteasomes independent of ATP. J. Biol. Chem. 2014, 289, 11272–11281. [Google Scholar] [CrossRef]
- Li, J.; Powell, S.R.; Wang, X. Enhancement of proteasome function by PA28α overexpression protects against oxidative stress. FASEB J. 2011, 25, 883–893. [Google Scholar] [CrossRef]
- Jung, T.; Höhn, A.; Grune, T. The proteasome and the degradation of oxidized proteins: Part III-Redox regulation of the proteasomal system. Redox Biol. 2014, 2, 388–394. [Google Scholar] [CrossRef]
- Haas, A.L.; Rose, I.A. The Mechanism of Ubiquitin Activating Enzyme. J. Biol. Chem. 1982, 257, 10329–10337. [Google Scholar] [CrossRef]
- Shang, F.; Taylor, A. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic. Biol. Med. 2011, 51, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Hannink, M. Distinct Cysteine Residues in Keap1 Are Required for Keap1-Dependent Ubiquitination of Nrf2 and for Stabilization of Nrf2 by Chemopreventive Agents and Oxidative Stress. Mol. Cell. Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef] [PubMed]
- Stankovic-Valentin, N.; Melchior, F. Control of SUMO and Ubiquitin by ROS: Signaling and disease implications. Mol. Asp. Med. 2018, 63, 3–17. [Google Scholar] [CrossRef]
- Yao, D.; Gu, Z.; Nakamura, T.; Shi, Z.-Q.; Ma, Y.; Gaston, B.; Palmer, L.A.; Rockenstein, E.M.; Zhang, Z.; Uehara, T.; et al. Nitrosative Stress Linked to Sporadic Parkinson’s Disease: S-Nitrosylation of Parkin Regulates Its E3 Ubiquitin Ligase Activity. Proc. Natl. Acad. Sci. USA 2004, 101, 10810–10814. [Google Scholar] [CrossRef]
- Ozawa, K.; Komatsubara, A.T.; Nishimura, Y.; Sawada, T.; Kawafune, H.; Tsumoto, H.; Tsuji, Y.; Zhao, J.; Kyotani, Y.; Tanaka, T.; et al. S-nitrosylation regulates mitochondrial quality control via activation of parkin. Sci. Rep. 2013, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yao, D.; Shi, Y.; Kabakoff, J.; Wu, W.; Reicher, J.; Ma, Y.; Moosmann, B.; Masliah, E.; Lipton, S.A.; et al. Oxidation of the cysteine-rich regions of parkin perturbs its E3 ligase activity and contributes to protein aggregation. Mol. Neurodegener. 2011, 6, 1–15. [Google Scholar] [CrossRef]
- Marín, I.; Ferrúst, A. Comparative genomics of the RBR family, including the Parkinson’s disease-related gene parkin and the genes of the ariadne subfamily. Mol. Biol. Evol. 2002, 19, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, J.; Chung, K.K.K.; Huang, H.; Dawson, V.L.; Dawson, T.M. Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc. Natl. Acad. Sci. USA 2000, 97, 13354–13359. [Google Scholar] [CrossRef]
- Imai, Y.; Soda, M.; Takahashi, R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J. Biol. Chem. 2000, 275, 35661–35664. [Google Scholar] [CrossRef] [PubMed]
- Shimura, H.; Hattori, N.; Kubo, S.I.; Mizuno, Y.; Asakawa, S.; Minoshima, S.; Shimizu, N.; Iwai, K.; Chiba, T.; Tanaka, K.; et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 2000, 25, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Hristova, V.A.; Beasley, S.A.; Rylett, R.J.; Shaw, G.S. Identification of a novel Zn2+-binding domain in the autosomal recessive juvenile Parkinson-related E3 ligase parkin. J. Biol. Chem. 2009, 284, 14978–14986. [Google Scholar] [CrossRef]
- Joselin, A.P.; Hewitt, S.J.; Callaghan, S.M.; Kim, R.H.; Chung, Y.H.; Mak, T.W.; Shen, J.; Slack, R.S.; Park, D.S. ROS-dependent regulation of parkin and DJ-1 localization during oxidative stress in neurons. Hum. Mol. Genet. 2012, 21, 4888–4903. [Google Scholar] [CrossRef]
- Canet-Avilés, R.M.; Wilson, M.A.; Miller, D.W.; Ahmad, R.; McLendon, C.; Bandyopadhyay, S.; Baptista, M.J.; Ringe, D.; Petsko, G.A.; Cookson, M.R. The Parkinson’s disease DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA 2004, 101, 9103–9108. [Google Scholar] [CrossRef]
- Irrcher, I.; Aleyasin, H.; Seifert, E.L.; Hewitt, S.J.; Chhabra, S.; Phillips, M.; Lutz, A.K.; Rousseaux, M.W.C.; Bevilacqua, L.; Jahani-Asl, A.; et al. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 2010, 19, 3734–3746. [Google Scholar] [CrossRef]
- Pick, E. The necessity of NEDD8/Rub1 for vitality and its association with mitochondria-derived oxidative stress. Redox Biol. 2020, 37, 101765. [Google Scholar] [CrossRef] [PubMed]
- Bossis, G.; Melchior, F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol. Cell 2006, 21, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Stankovic-Valentin, N.; Drzewicka, K.; König, C.; Schiebel, E.; Melchior, F. Redox regulation of SUMO enzymes is required for ATM activity and survival in oxidative stress. EMBO J. 2016, 35, 1312–1329. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Sun, X.; Xiang, B.; Cang, H.; Kang, X.; Chen, Y.; Li, H.; Shi, G.; Yeh, E.T.H.; Wang, B.; et al. Redox regulation of the stability of the SUMO protease SENP3 via interactions with CHIP and Hsp90. EMBO J. 2010, 29, 3773–3786. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Han, Y.; Wang, Y.; Sun, X.; Yan, S.; Yeh, E.T.H.; Chen, Y.; Cang, H.; Li, H.; Shi, G.; et al. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J. 2009, 28, 2748–2762. [Google Scholar] [CrossRef]
- Xu, Z.; Lam, L.S.M.; Lam, L.H.; Chau, S.F.; Ng, T.B.; Au, S.W.N. Molecular basis of the redox regulation of SUMO proteases: A protective mechanism of intermolecular disulfide linkage against irreversible sulfhydryl oxidation. FASEB J. 2008, 22, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Hickey, C.M.; Wilson, N.R.; Hochstrasser, M. Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol. 2012, 13, 755–766. [Google Scholar] [CrossRef]
- Bramasole, L.; Sinha, A.; Gurevich, S.; Radzinski, M.; Klein, Y.; Panat, N.; Gefen, E.; Rinaldi, T.; Jimenez-Morales, D.; Johnson, J.; et al. Proteasome lid bridges mitochondrial stress with Cdc53/Cullin1 NEDDylation status. Redox Biol. 2019, 20, 533–543. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Proteostasis and aging. Nat. Med. 2015, 21, 1406–1415. [Google Scholar] [CrossRef]
- Moreno, D.F.; Aldea, M. Proteostatic Stress as a Nodal Hallmark of Replicative Aging. Exp. Cell Res. 2020, 394, 112163. [Google Scholar] [CrossRef] [PubMed]
- Leupold, S.; Hubmann, G.; Litsios, A.; Meinema, A.C.; Takhaveev, V.; Papagiannakis, A.; Niebel, B.; Janssens, G.; Siegel, D.; Heinemann, M. Saccharomyces cerevisiae goes through distinct metabolic phases during its replicative lifespan. Elife 2019, 8, e41046. [Google Scholar] [CrossRef]
- He, X.; Memczak, S.; Qu, J.; Belmonte, J.C.I.; Liu, G.H. Single-cell omics in ageing: A young and growing field. Nat. Metab. 2020, 2, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Brandes, N.; Tienson, H.; Lindemann, A.; Vitvitsky, V.; Reichmann, D.; Banerjee, R.; Jakob, U. Time line of redox events in aging postmitotic cells. Elife 2013, 2013, e306. [Google Scholar] [CrossRef]
- Ghosh, D.K.; Roy, A.; Ranjan, A. The ATPase VCP/p97 functions as a disaggregase against toxic Huntingtin-exon1 aggregates. FEBS Lett. 2018, 592, 2680–2692. [Google Scholar] [CrossRef]
- Baek, G.H.; Cheng, H.; Choe, V.; Bao, X.; Shao, J.; Luo, S.; Rao, H. Cdc48: A Swiss Army Knife of Cell Biology. J. Amino Acids 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Tran, J.R.; Brodsky, J.L. The Cdc48–Vms1 complex maintains 26S proteasome architecture. Biochem. J. 2014, 458, 459–467. [Google Scholar] [CrossRef]
- Saarikangas, J.; Caudron, F.; Prasad, R.; Moreno, D.F.; Bolognesi, A.; Aldea, M.M.; Barral, Y. Compartmentalization of ER-Bound Chaperone Confines Protein Deposit Formation to the Aging Yeast Cell. Curr. Biol. 2017, 27, 773–783. [Google Scholar] [CrossRef]
- Caplan, A.J.; Douglas, M.G. Characterization of YDJ1: A yeast homologue of the bacterial dnaJ protein. J. Cell Biol. 1991, 114, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.K.; Lima, C.D. Structure of a Ubiquitin E1-E2 Complex: Insights to E1-E2 Thioester Transfer. Mol. Cell 2013, 49, 884–896. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Li, M.X.; Liu, J.; Jiao, H.; Xia, J.M.; Shi, X.J.; Zhao, H.; Chu, L.; Liu, J.; Qi, W.; et al. Competitive oxidation and ubiquitylation on the evolutionarily conserved cysteine confer tissue-specific stabilization of Insig-2. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Kolawa, N.; Sweredoski, M.J.; Graham, R.L.J.; Oania, R.; Hess, S.; Deshaies, R.J. Perturbations to the ubiquitin conjugate proteome in yeast δubx mutants identify Ubx2 as a regulator of membrane lipid composition. Mol. Cell. Proteom. 2013, 12, 2791–2803. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, E.; Masaki, S.; Matsuo, Y.; Chen, Z.; Tian, H.; Yodoi, J. Thioredoxin/Txnip: Redoxisome, as a redox switch for the pathogenesis of diseases. Front. Immunol. 2013, 4, 514. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Jacob, S.; Wang, J.; Wiechecki, K.A.; Koh, H.W.L.; Simões, V.; Choi, H.; Vogel, C.; Silva, G.M. Polyubiquitin Chains Linked by Lysine Residue 48 (K48) Selectively Target Oxidized Proteins in Vivo. Antioxid. Redox Signal. 2019, 31, 1133–1149. [Google Scholar] [CrossRef]
- Shcherbik, N.; Pestov, D.G. The Impact of Oxidative Stress on Ribosomes: From Injury to Regulation. Cells 2019, 8, 1379. [Google Scholar] [CrossRef]
- Kumsta, C.; Thamsen, M.; Jakob, U. Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of Caenorhabditis elegans. Antioxid. Redox Signal. 2011, 14, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, Y.; Qin, Z.; Guo, S.; Li, Y.; Miao, Y.; Song, C.; Chen, S.; Dai, S. Plant Chloroplast Stress Response: Insights from Thiol Redox Proteomics. Antioxid. Redox Signal. 2020, 33, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Menger, K.E.; James, A.M.; Cochemé, H.M.; Harbour, M.E.; Chouchani, E.T.; Ding, S.; Fearnley, I.M.; Partridge, L.; Murphy, M.P. Fasting, but Not Aging, Dramatically Alters the Redox Status of Cysteine Residues on Proteins in Drosophila melanogaster. Cell Rep. 2015, 11, 1856–1865. [Google Scholar] [CrossRef]
- Petrova, B.; Liu, K.; Tian, C.; Kitaoka, M.; Freinkman, E.; Yang, J.; Orr-Weaver, T.L. Dynamic redox balance directs the oocyte-to-embryo transition via developmentally controlled reactive cysteine changes. Proc. Natl. Acad. Sci. USA 2018, 115, E7978–E7986. [Google Scholar] [CrossRef]
- Yang, F.; Yi, M.; Liu, Y.; Wang, Q.; Hu, Y.; Deng, H. Glutaredoxin-1 Silencing Induces Cell Senescence via p53/p21/p16 Signaling Axis. J. Proteome Res. 2018, 17, 1091–1100. [Google Scholar] [CrossRef]
- Chiu, J.; Dawes, I.W. Redox control of cell proliferation. Trends Cell Biol. 2012, 22, 592–601. [Google Scholar] [CrossRef]
- Foyer, C.H.; Wilson, M.H.; Wright, M.H. Redox regulation of cell proliferation: Bioinformatics and redox proteomics approaches to identify redox-sensitive cell cycle regulators. Free Radic. Biol. Med. 2018, 122, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ishibashi, S.; Iglesias-Gonzalez, J.; Chen, Y.; Love, N.R.; Amaya, E. Ca2+-Induced Mitochondrial ROS Regulate the Early Embryonic Cell Cycle. Cell Rep. 2018, 22, 218–231. [Google Scholar] [CrossRef]
- Patterson, J.C.; Joughin, B.A.; van de Kooij, B.; Lim, D.C.; Lauffenburger, D.A.; Yaffe, M.B. ROS and Oxidative Stress Are Elevated in Mitosis during Asynchronous Cell Cycle Progression and Are Exacerbated by Mitotic Arrest. Cell Syst. 2019, 8, 163–167.e2. [Google Scholar] [CrossRef] [PubMed]
- Göbl, C.; Morris, V.K.; van Dam, L.; Visscher, M.; Polderman, P.E.; Hartlmüller, C.; de Ruiter, H.; Hora, M.; Liesinger, L.; Birner-Gruenberger, R.; et al. Cysteine oxidation triggers amyloid fibril formation of the tumor suppressor p16INK4A. Redox Biol. 2020, 28, 101316. [Google Scholar] [CrossRef] [PubMed]
- Laun, P.; Pichova, A.; Madeo, F.; Fuchs, J.; Ellinger, A.; Kohlwein, S.; Dawes, I.; Fröhlich, K.-U.; Breitenbach, M. Aged mother cells of Saccharomyces cerevisiae show markers of oxidative stress and apoptosis. Mol. Microbiol. 2004, 39, 1166–1173. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; De Ciucis, C.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox homeostasis and cellular antioxidant systems: Crucial players in cancer growth and therapy. Oxid. Med. Cell. Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).