Monitoring and Modulating Inflammation-Associated Alterations in Synaptic Plasticity: Role of Brain Stimulation and the Blood–Brain Interface
Abstract
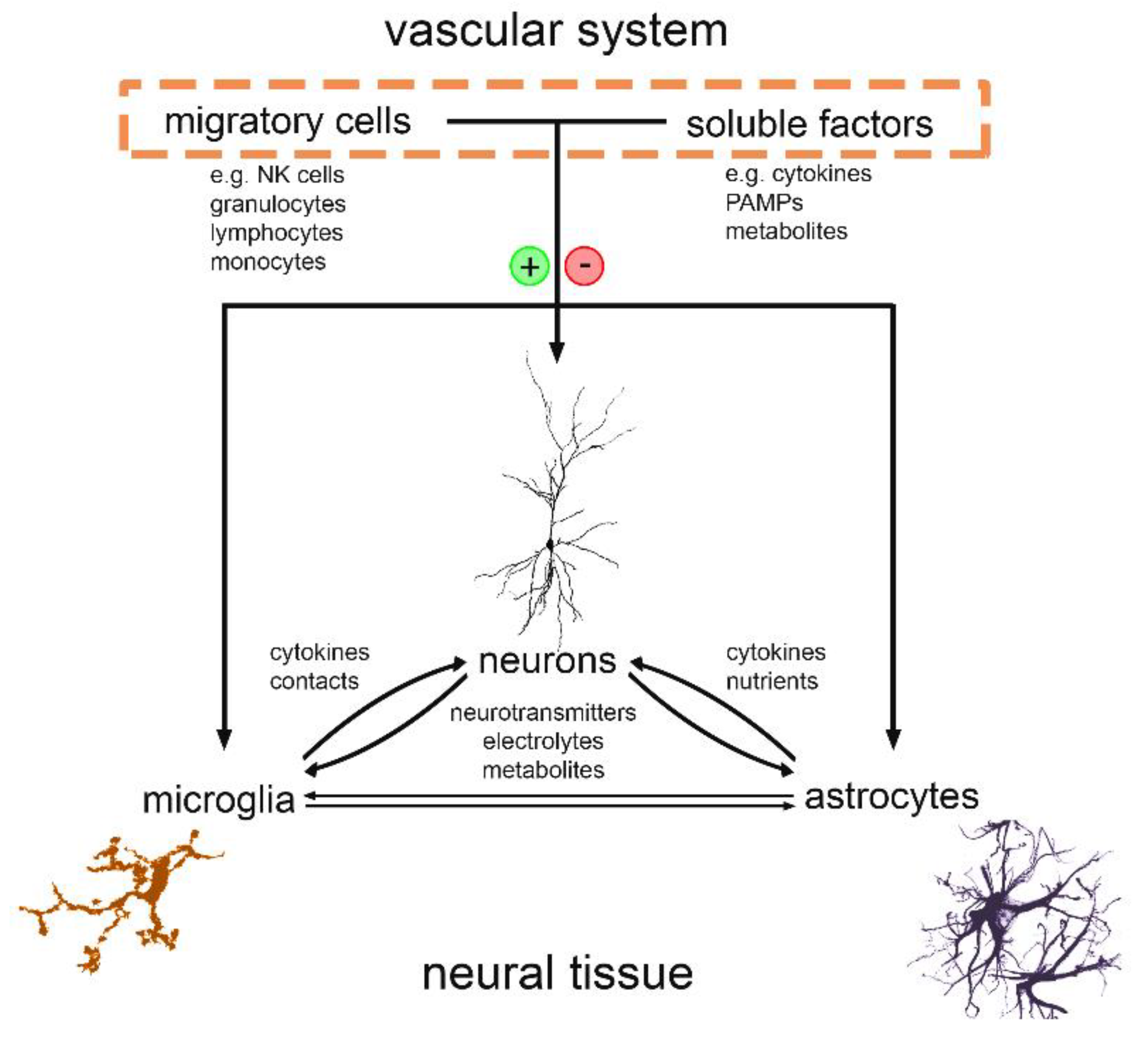
1. Introduction
2. Effects of Pro-Inflammatory Cytokines on Synaptic Plasticity in Health and Disease
3. Restraining Inflammation Restores Alterations in Synaptic Plasticity
4. Non-Invasive Brain Stimulation as a Tool to Monitor and Potentially Modulate Cytokine Expression
Funding
Conflicts of Interest
References
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef]
- Cho, H.; Proll, S.C.; Szretter, K.J.; Katze, M.G.; Gale, M., Jr.; Diamond, M.S. Differential innate immune response programs in neuronal subtypes determine susceptibility to infection in the brain by positive-stranded RNA viruses. Nat. Med. 2013, 19, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.M.; Kar, S.; Singh, R.; Chakraborty, D.; Vipat, V.; Raut, C.G.; Mishra, A.C.; Gore, M.M.; Ghosh, D. Immunomodulatory cytokines determine the outcome of Japanese encephalitis virus infection in mice. J. Med. Virol. 2010, 82, 304–310. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Collodel, A.; Generoso, J.S.; Scaini, G.; Wassather, R.; Selvaraj, S.; Hasbun, R.; Dal-Pizzol, F.; Petronilho, F.; Barichello, T. Neuroinflammation trajectories precede cognitive impairment after experimental meningitis-evidence from an in vivo PET study. J. Neuroinflammation 2020, 17, 5. [Google Scholar] [CrossRef]
- Soldan, S.S.; Alvarez Retuerto, A.I.; Sicotte, N.L.; Voskuhl, R.R. Dysregulation of IL-10 and IL-12p40 in secondary progressive multiple sclerosis. J. Neuroimmunol. 2004, 146, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Wedmore, C.V.; Williams, T.J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature 1981, 289, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, S.F.; Wade, M.S.; Acosta-Herrera, M.; Villar, J.; Pino-Yanes, M.; Zhou, T.; Liu, B.; Belvitch, P.; Moitra, J.; et al. Functional promoter variants in sphingosine 1-phosphate receptor 3 associate with susceptibility to sepsis-associated acute respiratory distress syndrome. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L467–L477. [Google Scholar] [CrossRef] [PubMed]
- Tiruppathi, C.; Minshall, R.D.; Paria, B.C.; Vogel, S.M.; Malik, A.B. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul. Pharmacol. 2002, 39, 173–185. [Google Scholar] [CrossRef]
- Vigl, B.; Aebischer, D.; Nitschke, M.; Iolyeva, M.; Rothlin, T.; Antsiferova, O.; Halin, C. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood 2011, 118, 205–215. [Google Scholar] [CrossRef]
- Heidland, A.; Klassen, A.; Rutkowski, P.; Bahner, U. The contribution of Rudolf Virchow to the concept of inflammation: What is still of importance? J. Nephrol. 2006, 19 (Suppl. 10), S102–S109. [Google Scholar]
- Liu, M.; Kalbasi, A.; Beatty, G.L. Functio Laesa: Cancer Inflammation and Therapeutic Resistance. J. Oncol. Pract. 2017, 13, 173–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sierra, A.; de Castro, F.; Del Rio-Hortega, J.; Rafael Iglesias-Rozas, J.; Garrosa, M.; Kettenmann, H. The “Big-Bang” for modern glial biology: Translation and comments on Pio del Rio-Hortega 1919 series of papers on microglia. Glia 2016, 64, 1801–1840. [Google Scholar] [CrossRef]
- Tremblay, M.E.; Lecours, C.; Samson, L.; Sanchez-Zafra, V.; Sierra, A. From the Cajal alumni Achucarro and Rio-Hortega to the rediscovery of never-resting microglia. Front. Neuroanat. 2015, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Webster, H.; Astrom, K.E. Gliogenesis: Historical perspectives, 1839-1985. Adv. Anat. Embryol. Cell Biol. 2009, 202, 1–109. [Google Scholar]
- Penfield, W. Microglia and the Process of Phagocytosis in Gliomas. Am. J. Pathol. 1925, 1, 77–90. [Google Scholar]
- Sierra, A.; Paolicelli, R.C.; Kettenmann, H. Cien Anos de Microglia: Milestones in a Century of Microglial Research. Trends Neurosci. 2019, 42, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Suter, T.; Biollaz, G.; Gatto, D.; Bernasconi, L.; Herren, T.; Reith, W.; Fontana, A. The brain as an immune privileged site: Dendritic cells of the central nervous system inhibit T cell activation. Eur. J. Immunol. 2003, 33, 2998–3006. [Google Scholar] [CrossRef]
- Obermeier, B.; Verma, A.; Ransohoff, R.M. The blood-brain barrier. Handb. Clin. Neurol. 2016, 133, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.G.; et al. Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflammation 2015, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Halliday, M.R.; Rege, S.V.; Ma, Q.; Zhao, Z.; Miller, C.A.; Winkler, E.A.; Zlokovic, B.V. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2016, 36, 216–227. [Google Scholar] [CrossRef]
- Hammer, C.; Stepniak, B.; Schneider, A.; Papiol, S.; Tantra, M.; Begemann, M.; Siren, A.L.; Pardo, L.A.; Sperling, S.; Mohd Jofrry, S.; et al. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol. Psychiatry 2014, 19, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Herz, J.; Alme, M.N.; Salvador, A.F.; Dong, M.Q.; Viar, K.E.; Herod, S.G.; Knopp, J.; Setliff, J.C.; Lupi, A.L.; et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018, 21, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Pascual, O.; Ben Achour, S.; Rostaing, P.; Triller, A.; Bessis, A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. USA 2012, 109, E197–E205. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Wu, Y.; Dissing-Olesen, L.; MacVicar, B.A.; Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 2015, 36, 605–613. [Google Scholar] [CrossRef]
- Carpentier, P.A.; Palmer, T.D. Immune influence on adult neural stem cell regulation and function. Neuron 2009, 64, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Gardoni, F.; Boraso, M.; Zianni, E.; Corsini, E.; Galli, C.L.; Cattabeni, F.; Marinovich, M.; Di Luca, M.; Viviani, B. Distribution of interleukin-1 receptor complex at the synaptic membrane driven by interleukin-1beta and NMDA stimulation. J. Neuroinflam. 2011, 8, 14. [Google Scholar] [CrossRef]
- Becker, D.; Deller, T.; Vlachos, A. Tumor necrosis factor (TNF)-receptor 1 and 2 mediate homeostatic synaptic plasticity of denervated mouse dentate granule cells. Sci. Rep. 2015, 5, 12726. [Google Scholar] [CrossRef]
- Habbas, S.; Santello, M.; Becker, D.; Stubbe, H.; Zappia, G.; Liaudet, N.; Klaus, F.R.; Kollias, G.; Fontana, A.; Pryce, C.R.; et al. Neuroinflammatory TNFalpha Impairs Memory via Astrocyte Signaling. Cell 2015, 163, 1730–1741. [Google Scholar] [CrossRef]
- Heir, R.; Stellwagen, D. TNF-Mediated Homeostatic Synaptic Plasticity: From in vitro to in vivo Models. Front. Cell Neurosci. 2020, 14, 565841. [Google Scholar] [CrossRef]
- Stellwagen, D.; Malenka, R.C. Synaptic scaling mediated by glial TNF-alpha. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef]
- Gonzalez, Y.; Herrera, M.T.; Soldevila, G.; Garcia-Garcia, L.; Fabian, G.; Perez-Armendariz, E.M.; Bobadilla, K.; Guzman-Beltran, S.; Sada, E.; Torres, M. High glucose concentrations induce TNF-alpha production through the down-regulation of CD33 in primary human monocytes. BMC Immunol. 2012, 13, 19. [Google Scholar] [CrossRef]
- Lenz, M.; Eichler, A.; Kruse, P.; Strehl, A.; Rodriguez-Rozada, S.; Goren, I.; Yogev, N.; Frank, S.; Waisman, A.; Deller, T.; et al. Interleukin 10 Restores Lipopolysaccharide-Induced Alterations in Synaptic Plasticity Probed by Repetitive Magnetic Stimulation. Front. Immunol. 2020, 11, 614509. [Google Scholar] [CrossRef]
- Sawada, M.; Kondo, N.; Suzumura, A.; Marunouchi, T. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res. 1989, 491, 394–397. [Google Scholar] [CrossRef]
- Maggio, N.; Vlachos, A. Tumor necrosis factor (TNF) modulates synaptic plasticity in a concentration-dependent manner through intracellular calcium stores. J. Mol. Med. 2018, 96, 1039–1047. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Merlini, M.; Rafalski, V.A.; Ma, K.; Kim, K.Y.; Bushong, E.A.; Rios Coronado, P.E.; Yan, Z.; Mendiola, A.S.; Sozmen, E.G.; Ryu, J.K.; et al. Microglial Gi-dependent dynamics regulate brain network hyperexcitability. Nat. Neurosci. 2021, 24, 19–23. [Google Scholar] [CrossRef]
- Shemer, A.; Scheyltjens, I.; Frumer, G.R.; Kim, J.S.; Grozovski, J.; Ayanaw, S.; Dassa, B.; Van Hove, H.; Chappell-Maor, L.; Boura-Halfon, S.; et al. Interleukin-10 Prevents Pathological Microglia Hyperactivation following Peripheral Endotoxin Challenge. Immunity 2020, 53, 1033–1049 e1037. [Google Scholar] [CrossRef] [PubMed]
- Santello, M.; Bezzi, P.; Volterra, A. TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 2011, 69, 988–1001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Kuo, T.T.; Chu, M.T.; Ma, H.I.; Chiang, Y.H.; Huang, E.Y. Postnatal systemic inflammation exacerbates impairment of hippocampal synaptic plasticity in an animal seizure model. Neuroimmunomodulation 2013, 20, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.; O’Neill, L.A.; O’Mara, S.M. The effects of the bacterial endotoxin lipopolysaccharide on synaptic transmission and plasticity in the CA1-subiculum pathway in vivo. Neuroscience 2001, 102, 273–280. [Google Scholar] [CrossRef]
- Maggio, N.; Shavit-Stein, E.; Dori, A.; Blatt, I.; Chapman, J. Prolonged systemic inflammation persistently modifies synaptic plasticity in the hippocampus: Modulation by the stress hormones. Front. Mol. Neurosci. 2013, 6, 46. [Google Scholar] [CrossRef]
- Strehl, A.; Lenz, M.; Itsekson-Hayosh, Z.; Becker, D.; Chapman, J.; Deller, T.; Maggio, N.; Vlachos, A. Systemic inflammation is associated with a reduction in Synaptopodin expression in the mouse hippocampus. Exp. Neurol. 2014, 261, 230–235. [Google Scholar] [CrossRef]
- Nistico, R.; Mori, F.; Feligioni, M.; Nicoletti, F.; Centonze, D. Synaptic plasticity in multiple sclerosis and in experimental autoimmune encephalomyelitis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130162. [Google Scholar] [CrossRef] [PubMed]
- Nistico, R.; Mango, D.; Mandolesi, G.; Piccinin, S.; Berretta, N.; Pignatelli, M.; Feligioni, M.; Musella, A.; Gentile, A.; Mori, F.; et al. Inflammation subverts hippocampal synaptic plasticity in experimental multiple sclerosis. PLoS ONE 2013, 8, e54666. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Vlachos, A. The neuroimmunological synapse: From synaptic homeostasis to brain disease. Neuroforum 2019, 25, 163–172. [Google Scholar] [CrossRef]
- Charrad, R.; Berraies, A.; Hamdi, B.; Ammar, J.; Hamzaoui, K.; Hamzaoui, A. Anti-inflammatory activity of IL-37 in asthmatic children: Correlation with inflammatory cytokines TNF-alpha, IL-beta, IL-6 and IL-17A. Immunobiology 2016, 221, 182–187. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Su, D.L.; Lu, Z.M.; Shen, M.N.; Li, X.; Sun, L.Y. Roles of pro- and anti-inflammatory cytokines in the pathogenesis of SLE. J. Biomed. Biotechnol. 2012, 2012, 347141. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Aste-Amezaga, M.; Valiante, N.M.; Ma, X.; Kubin, M.; Trinchieri, G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993, 178, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Gruber, M.F.; Williams, C.C.; Gerrard, T.L. Macrophage-colony-stimulating factor expression by anti-CD45 stimulated human monocytes is transcriptionally up-regulated by IL-1 beta and inhibited by IL-4 and IL-10. J. Immunol. 1994, 152, 1354–1361. [Google Scholar]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the immune response in the brain: IL-10 and its regulation. J. Neuroinflammation 2016, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.J.; Walters, T.D.; Guo, C.H.; Kugathasan, S.; Klein, C.; Turner, D.; Wolters, V.M.; Bandsma, R.H.; Mouzaki, M.; Zachos, M.; et al. IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflamm. Bowel. Dis. 2013, 19, 115–123. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Nenov, M.N.; Konakov, M.V.; Teplov, I.Y.; Levin, S.G. Interleukin-10 Facilitates Glutamatergic Synaptic Transmission and Homeostatic Plasticity in Cultured Hippocampal Neurons. Int. J. Mol. Sci. 2019, 20, 3375. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayanan, A.; Carter, J.M.; Landin, J.D.; Morrow, A.L.; Werner, D.F.; Spigelman, I. Role of interleukin-10 (IL-10) in regulation of GABAergic transmission and acute response to ethanol. Neuropharmacology 2016, 107, 181–188. [Google Scholar] [CrossRef]
- Woiciechowsky, C.; Asadullah, K.; Nestler, D.; Eberhardt, B.; Platzer, C.; Schoning, B.; Glockner, F.; Lanksch, W.R.; Volk, H.D.; Docke, W.D. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat. Med. 1998, 4, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Ledeboer, A.; Breve, J.J.; Wierinckx, A.; van der Jagt, S.; Bristow, A.F.; Leysen, J.E.; Tilders, F.J.; Van Dam, A.M. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur. J. Neurosci. 2002, 16, 1175–1185. [Google Scholar] [CrossRef]
- Williams, K.; Dooley, N.; Ulvestad, E.; Becher, B.; Antel, J.P. IL-10 production by adult human derived microglial cells. Neurochem. Int. 1996, 29, 55–64. [Google Scholar] [CrossRef]
- Bohlen, C.J.; Bennett, F.C.; Tucker, A.F.; Collins, H.Y.; Mulinyawe, S.B.; Barres, B.A. Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 2017, 94, 759–773.e758. [Google Scholar] [CrossRef]
- Gosselin, D.; Link, V.M.; Romanowski, C.E.; Fonseca, G.J.; Eichenfield, D.Z.; Spann, N.J.; Stender, J.D.; Chun, H.B.; Garner, H.; Geissmann, F.; et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 2014, 159, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Delbridge, A.R.D.; Huh, D.; Brickelmaier, M.; Burns, J.C.; Roberts, C.; Challa, R.; Raymond, N.; Cullen, P.; Carlile, T.M.; Ennis, K.A.; et al. Organotypic Brain Slice Culture Microglia Exhibit Molecular Similarity to Acutely-Isolated Adult Microglia and Provide a Platform to Study Neuroinflammation. Front. Cell. Neurosci. 2020, 592005. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipovic, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Profice, P.; Pilato, F.; Dileone, M.; Oliviero, A.; Ziemann, U. The effects of motor cortex rTMS on corticospinal descending activity. Clin. Neurophysiol. 2010, 121, 464–473. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U. Thirty years of transcranial magnetic stimulation: Where do we stand? Exp. Brain Res. 2017, 235, 973–984. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef]
- Gersner, R.; Kravetz, E.; Feil, J.; Pell, G.; Zangen, A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: Differential outcomes in anesthetized and awake animals. J. Neurosci. 2011, 31, 7521–7526. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Platschek, S.; Priesemann, V.; Becker, D.; Willems, L.M.; Ziemann, U.; Deller, T.; Muller-Dahlhaus, F.; Jedlicka, P.; Vlachos, A. Repetitive magnetic stimulation induces plasticity of excitatory postsynapses on proximal dendrites of cultured mouse CA1 pyramidal neurons. Brain Struct. Funct. 2015, 220, 3323–3337. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, A.; Muller-Dahlhaus, F.; Rosskopp, J.; Lenz, M.; Ziemann, U.; Deller, T. Repetitive magnetic stimulation induces functional and structural plasticity of excitatory postsynapses in mouse organotypic hippocampal slice cultures. J. Neurosci. 2012, 32, 17514–17523. [Google Scholar] [CrossRef] [PubMed]
- Maeda, F.; Keenan, J.P.; Tormos, J.M.; Topka, H.; Pascual-Leone, A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin. Neurophysiol. 2000, 111, 800–805. [Google Scholar] [CrossRef]
- Lenz, M.; Vlachos, A. Releasing the Cortical Brake by Non-Invasive Electromagnetic Stimulation? rTMS Induces LTD of GABAergic Neurotransmission. Front. Neural Circuits 2016, 10, 96. [Google Scholar] [CrossRef]
- Clarke, D.; Penrose, M.A.; Penstone, T.; Fuller-Carter, P.I.; Hool, L.C.; Harvey, A.R.; Rodger, J.; Bates, K.A. Frequency-specific effects of repetitive magnetic stimulation on primary astrocyte cultures. Restor. Neurol. Neurosci. 2017, 35, 557–569. [Google Scholar] [CrossRef]
- Badimon, A.; Strasburger, H.J.; Ayata, P.; Chen, X.; Nair, A.; Ikegami, A.; Hwang, P.; Chan, A.T.; Graves, S.M.; Uweru, J.O.; et al. Negative feedback control of neuronal activity by microglia. Nature 2020, 586, 417–423. [Google Scholar] [CrossRef]
- Becker, D.; Zahn, N.; Deller, T.; Vlachos, A. Tumor necrosis factor alpha maintains denervation-induced homeostatic synaptic plasticity of mouse dentate granule cells. Front. Cell Neurosci. 2013, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, C.C.; Turrigiano, G.G. Tumor necrosis factor-alpha signaling maintains the ability of cortical synapses to express synaptic scaling. J. Neurosci. 2010, 30, 14685–14690. [Google Scholar] [CrossRef]
- Vazana, U.; Schori, L.; Monsonego, U.; Swissa, E.; Pell, G.S.; Roth, Y.; Brodt, P.; Friedman, A.; Prager, O. TMS-Induced Controlled BBB Opening: Preclinical Characterization and Implications for Treatment of Brain Cancer. Pharmaceutics 2020, 12, 946. [Google Scholar] [CrossRef]
- Li, X.; Nahas, Z.; Lomarev, M.; Denslow, S.; Shastri, A.; Bohning, D.E.; George, M.S. Prefrontal cortex transcranial magnetic stimulation does not change local diffusion: A magnetic resonance imaging study in patients with depression. Cogn. Behav. Neurol. 2003, 16, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Ravnborg, M.; Knudsen, G.M.; Blinkenberg, M. No effect of pulsed magnetic stimulation on the blood-brain barrier in rats. Neuroscience 1990, 38, 277–280. [Google Scholar] [CrossRef]
- Ruber, T.; David, B.; Luchters, G.; Nass, R.D.; Friedman, A.; Surges, R.; Stocker, T.; Weber, B.; Deichmann, R.; Schlaug, G.; et al. Evidence for peri-ictal blood-brain barrier dysfunction in patients with epilepsy. Brain 2018, 141, 2952–2965. [Google Scholar] [CrossRef]
- Vazana, U.; Veksler, R.; Pell, G.S.; Prager, O.; Fassler, M.; Chassidim, Y.; Roth, Y.; Shahar, H.; Zangen, A.; Raccah, R.; et al. Glutamate-Mediated Blood-Brain Barrier Opening: Implications for Neuroprotection and Drug Delivery. J. Neurosci. 2016, 36, 7727–7739. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Stevens, B. The "quad-partite" synapse: Microglia-synapse interactions in the developing and mature CNS. Glia 2013, 61, 24–36. [Google Scholar] [CrossRef]
- Del Turco, D.; Deller, T. Organotypic entorhino-hippocampal slice cultures—A tool to study the molecular and cellular regulation of axonal regeneration and collateral sprouting in vitro. Methods Mol. Biol. 2007, 399, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Humpel, C. Organotypic Brain Slices of ADULT Transgenic Mice: A Tool to Study Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.M.; Joseph, K.; Wurm, J.; Behringer, S.; Garrelfs, N.; d’Errico, P.; Naseri, Y.; Franco, P.; Meyer-Luehmann, M.; Sankowski, R.; et al. Human organotypic brain slice culture: A novel framework for environmental research in neuro-oncology. Life Sci. Alliance 2019, 2. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenz, M.; Eichler, A.; Vlachos, A. Monitoring and Modulating Inflammation-Associated Alterations in Synaptic Plasticity: Role of Brain Stimulation and the Blood–Brain Interface. Biomolecules 2021, 11, 359. https://doi.org/10.3390/biom11030359
Lenz M, Eichler A, Vlachos A. Monitoring and Modulating Inflammation-Associated Alterations in Synaptic Plasticity: Role of Brain Stimulation and the Blood–Brain Interface. Biomolecules. 2021; 11(3):359. https://doi.org/10.3390/biom11030359
Chicago/Turabian StyleLenz, Maximilian, Amelie Eichler, and Andreas Vlachos. 2021. "Monitoring and Modulating Inflammation-Associated Alterations in Synaptic Plasticity: Role of Brain Stimulation and the Blood–Brain Interface" Biomolecules 11, no. 3: 359. https://doi.org/10.3390/biom11030359
APA StyleLenz, M., Eichler, A., & Vlachos, A. (2021). Monitoring and Modulating Inflammation-Associated Alterations in Synaptic Plasticity: Role of Brain Stimulation and the Blood–Brain Interface. Biomolecules, 11(3), 359. https://doi.org/10.3390/biom11030359





