Dehydration-Induced WRKY Transcriptional Factor MfWRKY70 of Myrothamnus flabellifolia Enhanced Drought and Salinity Tolerance in Arabidopsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Treatments
2.2. Cloning and Bioinformatic Analysis of MfWRKY70
2.3. Subcellular Localization of MfWRKY70
2.4. Vector Construction and Generation of Transgenic Lines
2.5. Water Loss Rate
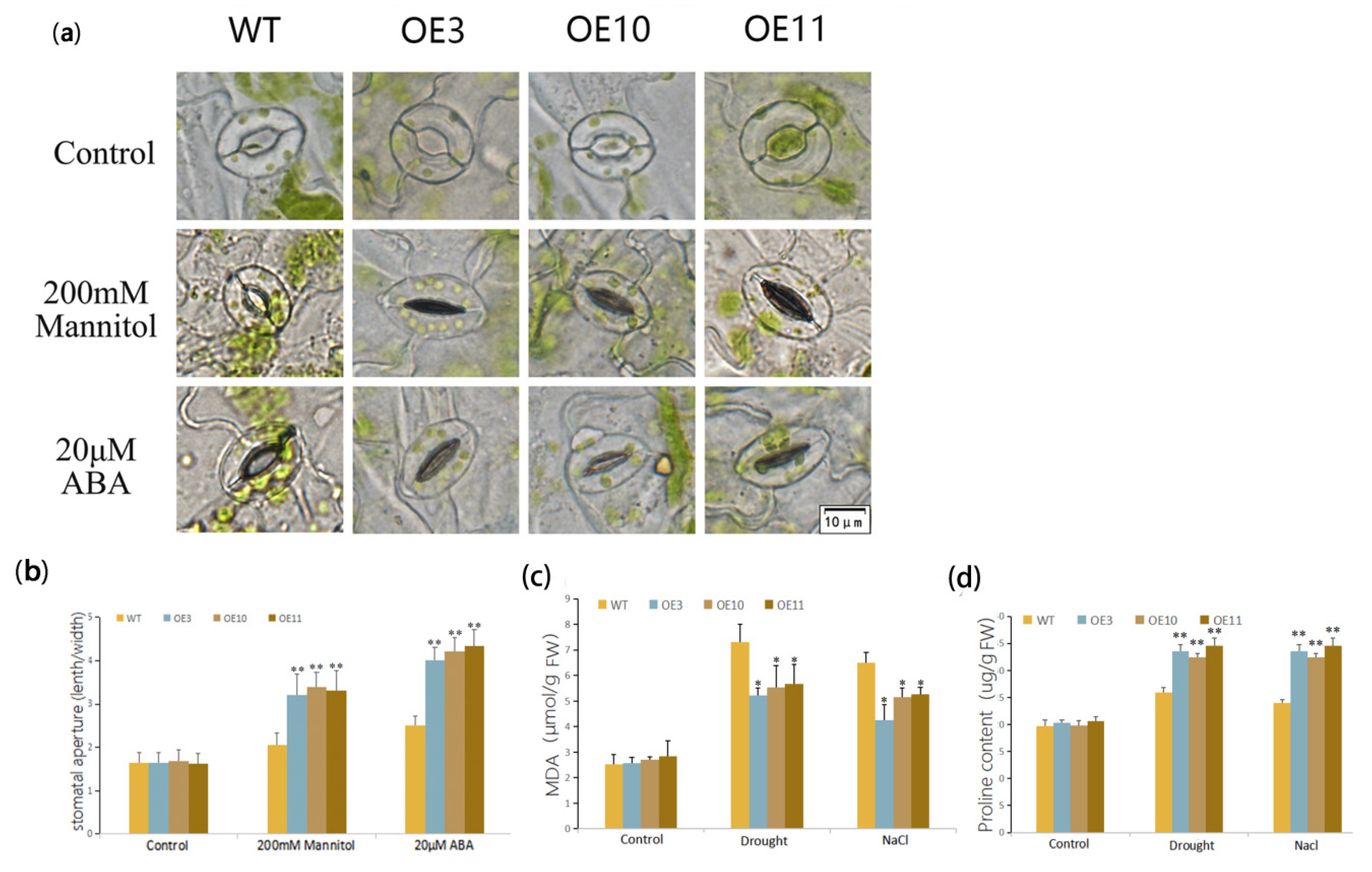
2.6. Stomatal Aperture Analysis
2.7. Physiological Measurements
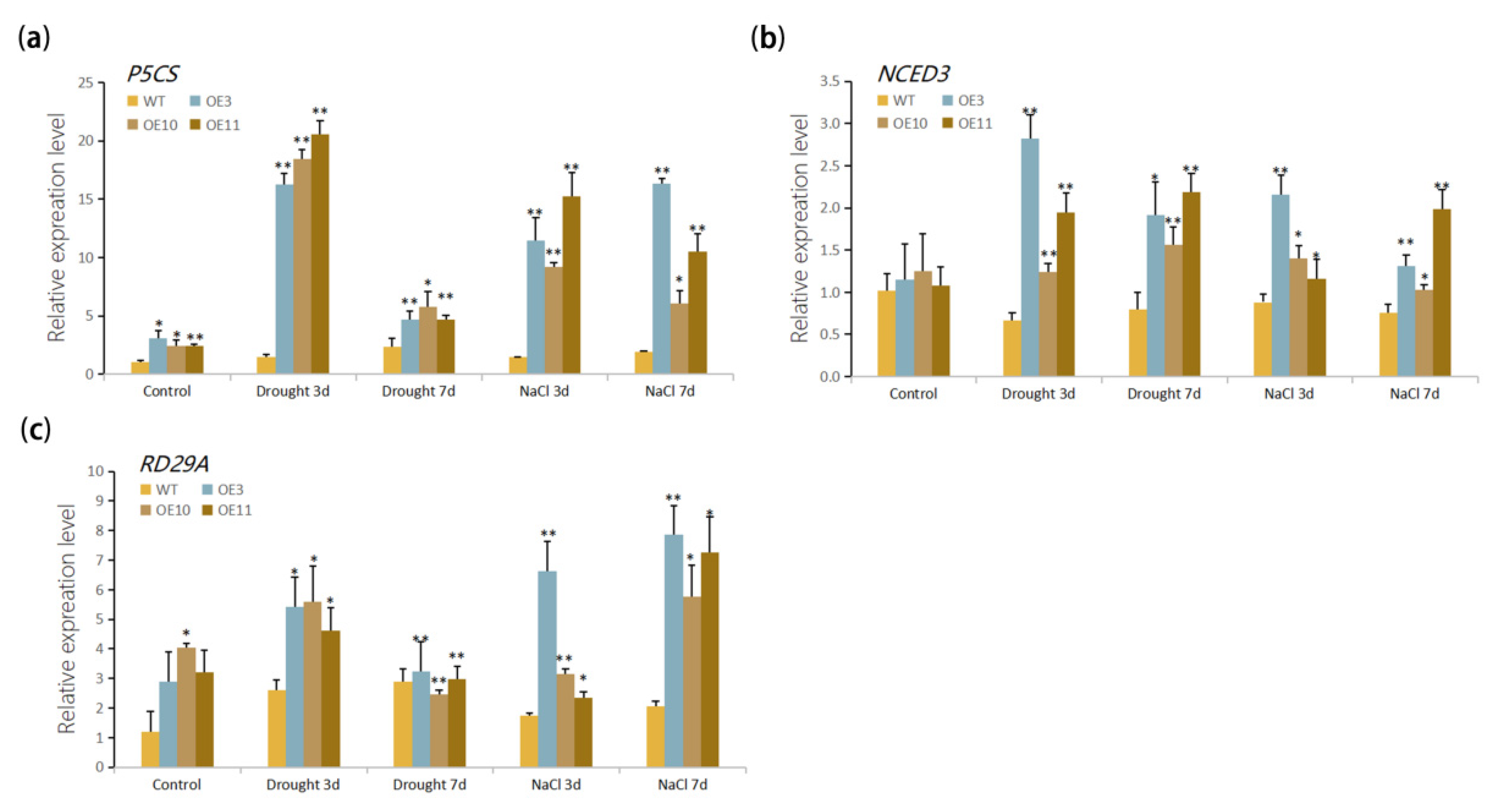
2.8. Reverse Transcription PCR (RT-PCR) and Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analyses
3. Results
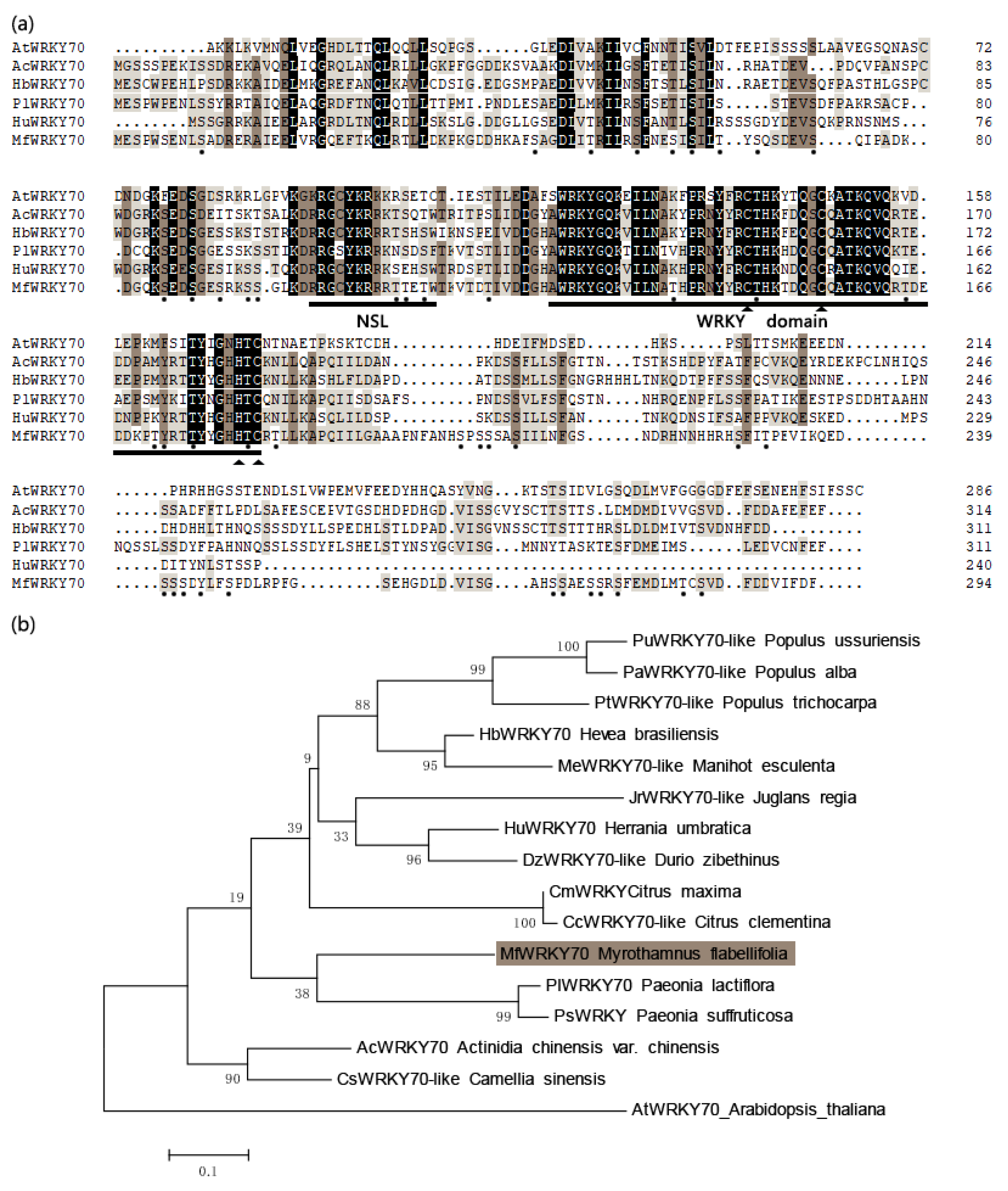
3.1. Isolation and Characterization of MfWRKY70
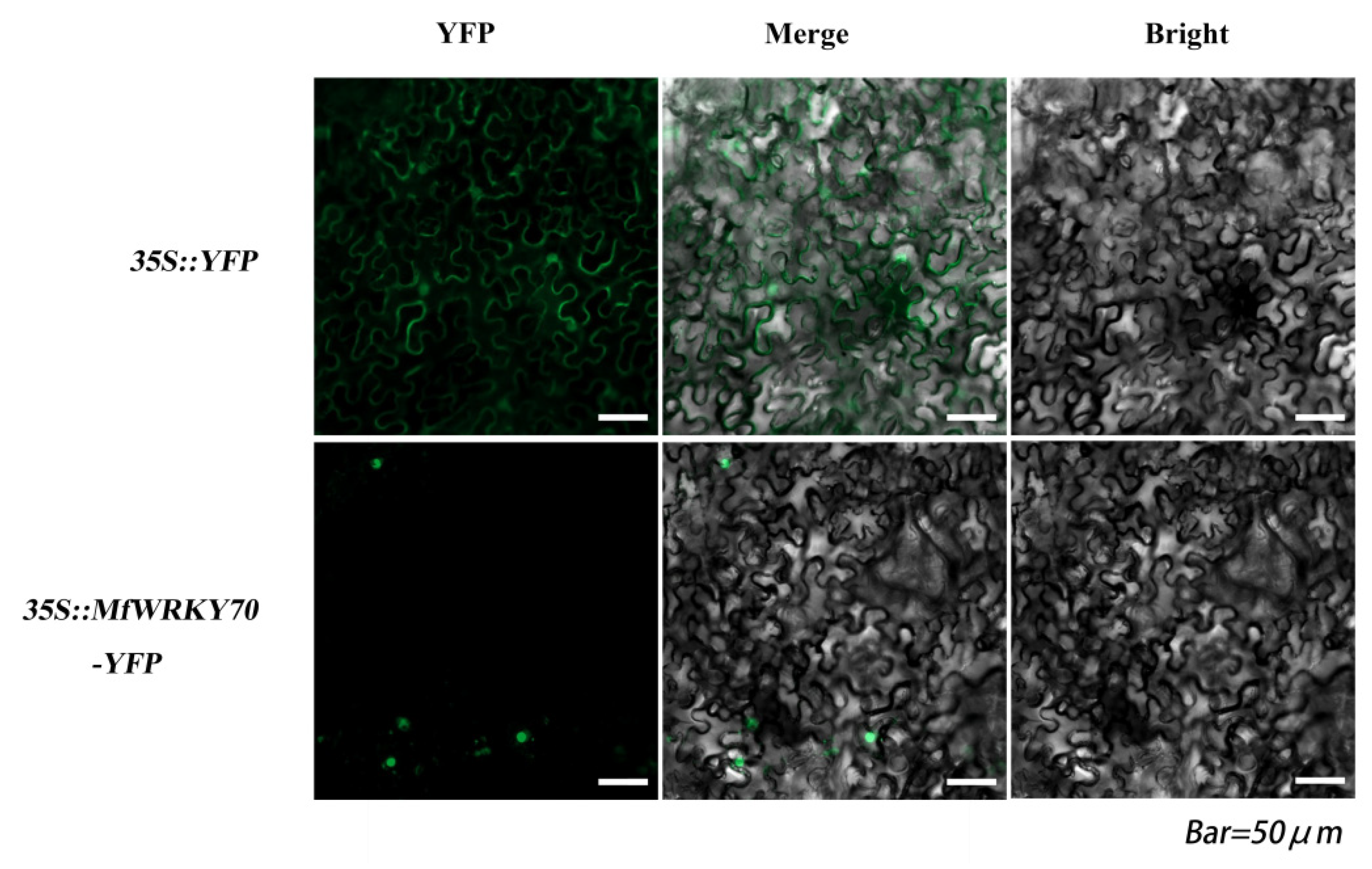
3.2. Subcellular Localization of MfWRKY70
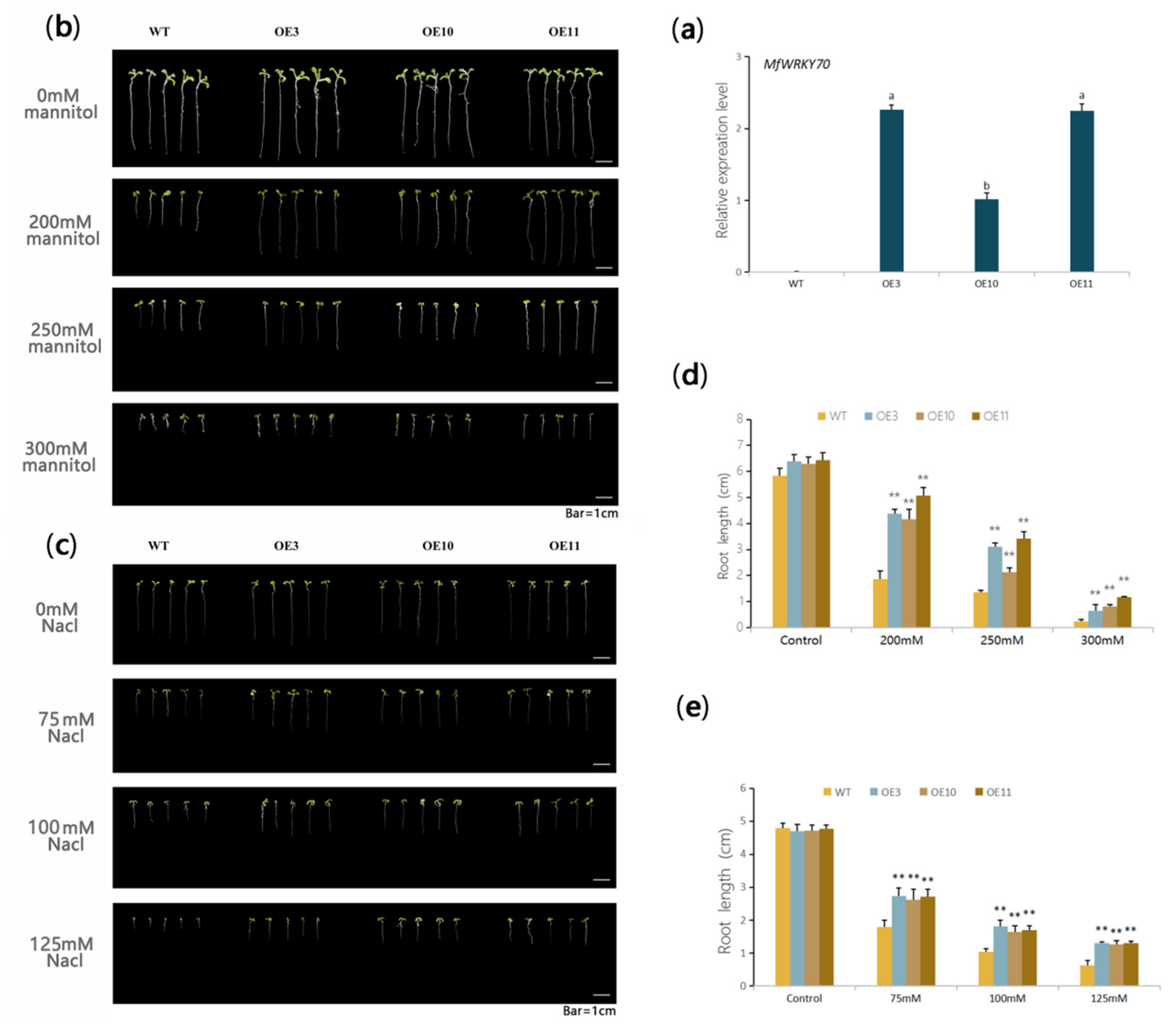
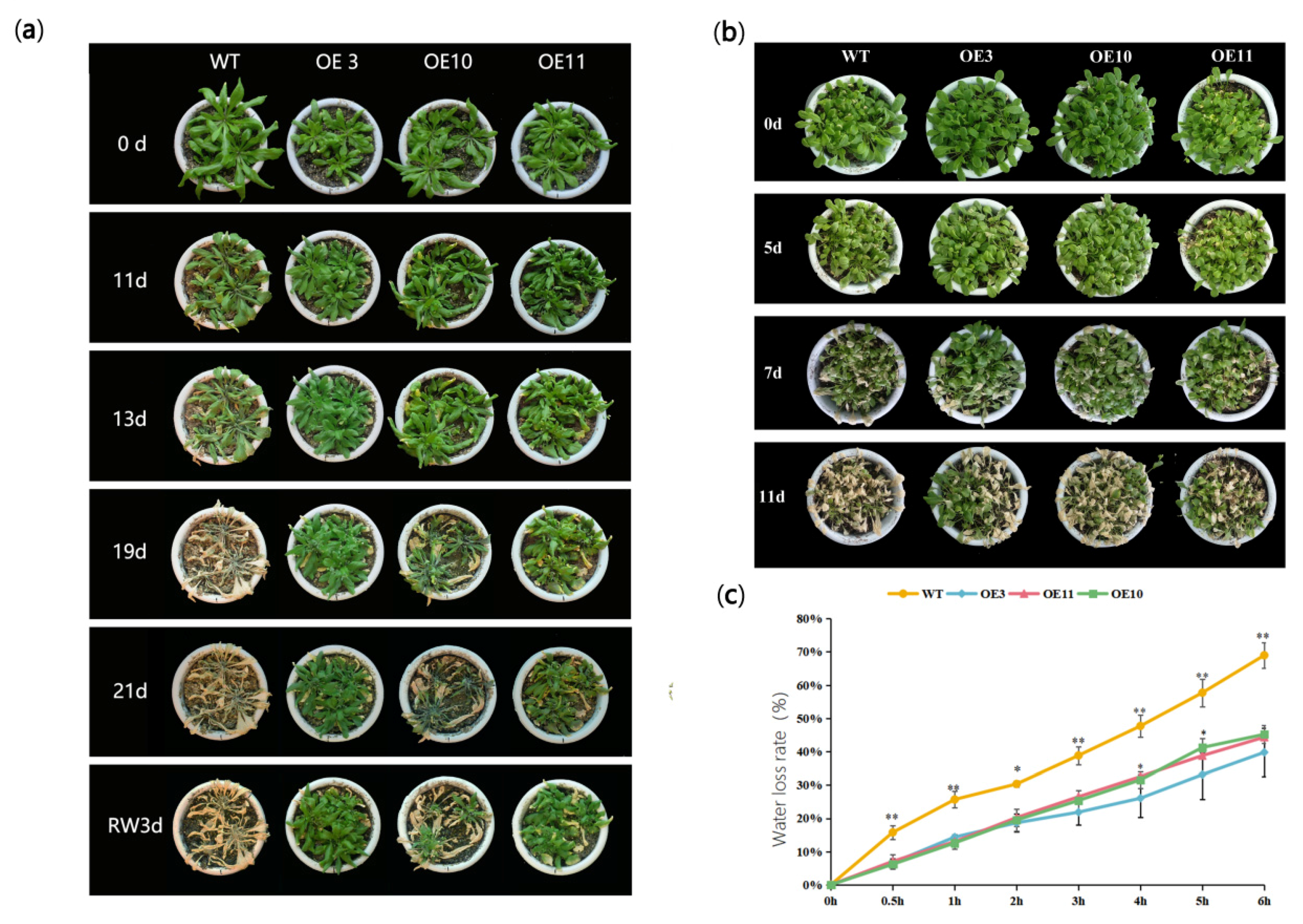
3.3. Heterologous Expression of MfWRKY70 Improved Salt and Osmotic Tolerance in Arabidopsis
3.4. Overexpression of MfWRKY70 Affected Antioxidant Metabolism Levels in Arabidopsis under Drought and Salinity Stress
3.5. Stress Response Genes Were Up-Regulated by Overexpression of MfWRKY70
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TFs | transcription factors |
| HDTs | homoiochlorophyllous desiccation-tolerant plants |
| NLS | nuclear localization signal |
| WT | wild type |
| P5CS | ∆-1-pyrroline-5-carboxylate synthetase |
| NCED | 9- cis-epoxycarotenoid dioxygenase |
| OE | Over-Expression |
| DAB | 3,3′-Diaminobenzidine |
| NBT | Nitrotetrazolium blue chloride |
| POD | peroxidase |
| SOD | superoxide dismutase |
| CAT | catalase |
| MDA | malondialdehyde |
References
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and Drought Stress Signal Transduction in Plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ling, H.; Wu, Q.; Xu, L.; Que, Y. The choice of reference genes for assessing gene expression in sugarcane under salinity and drought stresses. Sci. Rep. 2014, 4, 7042. [Google Scholar] [CrossRef]
- Wei, W.; Cui, M.-Y.; Hu, Y.; Gao, K.; Xie, Y.-G.; Jiang, Y.; Feng, J.-Y. Ectopic expression of FvWRKY42, a WRKY transcription factor from the diploid woodland strawberry (Fragaria vesca), enhances resistance to powdery mildew, improves osmotic stress resistance, and increases abscisic acid sensitivity in Arabidopsis. Plant Sci. 2018, 275, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Li, Y.; Wang, F.; Cheng, Y.; Fan, B.; Yu, J.-Q.; Chen, Z. Arabidopsis Sigma Factor Binding Proteins Are Activators of the WRKY33 Transcription Factor in Plant Defense. Plant Cell 2011, 23, 3824–3841. [Google Scholar] [CrossRef]
- Robatzek, S. Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev. 2002, 16, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, L.; Su, H.; Guo, L.; Zhang, J.; Li, Y.; Xu, J.; Zhang, X.; Guo, Y.-D.; Zhang, N. Jasmonate and aluminum crosstalk in tomato: Identification and expression analysis of WRKYs and ALMTs during JA/Al-regulated root growth. Plant Physiol. Biochem. 2020, 154, 409–418. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Mingyu, Z. WRKY transcription factor superfamily: Structure, origin and functions. Afr. J. Biotechnol. 2012, 11. [Google Scholar] [CrossRef]
- Xu, H.; Shi, X.; Wang, Z.; Gao, C.; Wang, C.; Wang, Y. Transcription factor ThWRKY4 binds to a novel WLS motif and a RAV1A element in addition to the W-box to regulate gene expression. Plant Sci. 2017, 261, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, G.; Meng, X.; Liu, Y.; Ji, X.; Li, Y.; Nie, X.; Wang, Y. A WRKY gene from Tamarix hispida, ThWRKY4, mediates abiotic stress responses by modulating reactive oxygen species and expression of stress-responsive genes. Plant Mol. Biol. 2013, 82, 303–320. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Li, H.; Luo, W.; Zhou, C.; Zhang, L.; Lv, J. A wheat R2R3 MYB gene TaMpc1-D4 negatively regulates drought tolerance in transgenic Arabidopsis and wheat. Plant Sci. 2020, 299, 110613. [Google Scholar] [CrossRef] [PubMed]
- Kuki, Y.; Ohno, R.; Yoshida, K.; Takumi, S. Heterologous expression of wheat WRKY transcription factor genes transcriptionally activated in hybrid necrosis strains alters abiotic and biotic stress tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 150, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pnueli, L.; Hallak-Herr, E.; Rozenberg, M.; Cohen, M.; Goloubinoff, P.; Kaplan, A.; Mittler, R. Molecular and biochemical mechanisms associated with dormancy and drought tolerance in the desert legume Retama raetam. Plant J. 2002, 31, 319–330. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Mittler, R. The Combined Effect of Drought Stress and Heat Shock on Gene Expression in Tobacco. Plant Physiol. 2002, 130, 1143–1151. [Google Scholar] [CrossRef]
- He, G.-H.; Xu, J.-Y.; Wang, Y.-X.; Liu, J.-M.; Li, P.-S.; Chen, M.; Ma, Y.-Z.; Xu, Z.-S. Drought-responsive WRKY transcription factor genes TaWRKY1 and TaWRKY33 from wheat confer drought and/or heat resistance in Arabidopsis. BMC Plant Biol. 2016, 16, 1–16. [Google Scholar] [CrossRef]
- HuiYuan, Z.; YongWei, L.; JunFeng, Y.; ShuangXi, Z.; TaiFei, Y.; Jun, C.; Ming, C.; YongBin, Z.; Ma, Y.; ZhaoShi, X.; et al. Identification and Analysis of Salt Tolerance of Wheat Transcription Factor TaWRKY33 Protein. Sci. Agric. Sin. 2018, 51, 4591–4602. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, T.; Lin, Z.; Guo, B.; Xing, C.; Zhao, L.; Dong, H.; Gao, J.; Xie, Z.; Zhang, S.; et al. A WRKY transcription factor PbrWRKY53 from Pyrus betulaefolia is involved in drought tolerance and AsA accumulation. Plant Biotechnol. J. 2019, 17, 1770–1787. [Google Scholar] [CrossRef]
- Moore, J.P.; Lindsey, G.G.; Farrant, J.M.; Brandt, W.F. BOTANICAL BRIEFINGAn Overview of the Biology of the Desiccation-tolerant Resurrection Plant Myrothamnus flabellifolia. Ann. Bot. 2007, 99, 1241–1242. [Google Scholar] [CrossRef]
- Moore, J.P.; Nguema-Ona, E.; Chevalier, L.; Lindsey, G.G.; Brandt, W.F.; Lerouge, P.; Farrant, J.M.; Driouich, A. Response of the Leaf Cell Wall to Desiccation in the Resurrection Plant Myrothamnus flabellifolius. Plant Physiol. 2006, 141, 651–662. [Google Scholar] [CrossRef]
- Gashi, B.; Babani, F.; Kongjika, E. Chlorophyll fluorescence imaging of photosynthetic activity and pigment contents of the resurrection plants Ramonda serbica and Ramonda nathaliae during dehydration and rehydration. Physiol. Mol. Biol. Plants 2013, 19, 333–341. [Google Scholar] [CrossRef]
- Ülker, B.; Mukhtar, M.S.; Somssich, I.E. The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta 2007, 226, 125–137. [Google Scholar] [CrossRef]
- Knoth, C.; Ringler, J.; Dangl, J.L.; Eulgem, T. Arabidopsis WRKY70 Is Required for Full RPP4-Mediated Disease Resistance and Basal Defense against Hyaloperonospora parasitica. Mol. Plant Microbe Interact. 2007, 20, 120–128. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Huang, Z.-Y.; Xie, P.; Gu, C.; Li, K.; Wang, D.-C.; Yu, Y.-Y.; Fan, Z.-H.; Wang, C.-J.; Wang, Y.-P.; et al. Transcription factors WRKY70 and WRKY11 served as regulators in rhizobacterium Bacillus cereus AR156-induced systemic resistance to Pseudomonas syringae pv. tomatoDC3000 in Arabidopsis. J. Exp. Bot. 2015, 67, 157–174. [Google Scholar] [CrossRef]
- Abbasi, S.; Sadeghi, A.; Safaie, N. Streptomyces alleviate drought stress in tomato plants and modulate the expression of transcription factors ERF1 and WRKY70 genes. Sci. Hortic. 2020, 265, 109206. [Google Scholar] [CrossRef]
- Li, J.; Besseau, S.; Törönen, P.; Sipari, N.; Kollist, H.; Holm, L.; Palva, E.T. Defense-related transcription factors WRKY 70 and WRKY 54 modulate osmotic stress tolerance by regulating stomatal aperture in Arabidopsis. New Phytol. 2013, 200, 457–472. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, J.; Li, K.; Liu, G. Populus simonii × Populus nigra WRKY70 is involved in salt stress and leaf blight disease responses. Tree Physiol. 2017, 37, 827–844. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Macnish, A.J.; Estrada-Melo, A.C.; Lin, J.; Chang, Y.; Reid, M.S.; Jiang, C.-Z. Transcriptomic analysis reveals numerous diverse protein kinases and transcription factors involved in desiccation tolerance in the resurrection plant Myrothamnus flabellifolia. Hortic. Res. 2015, 2, 15034. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Yadav, S.; Gill, S.S.; Passricha, N.; Gill, R.; Badhwar, P.; Anjum, N.A.; Francisco, J.-B.J.; Tuteja, N. Genome-wide analysis and transcriptional expression pattern-assessment of superoxide dismutase (SOD) in rice and Arabidopsis under abiotic stresses. Plant Gene 2019, 17, 100165. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Zheng, X.; Tian, S.; Meng, X.; Li, B. Physiological and biochemical responses in peach fruit to oxalic acid treatment during storage at room temperature. Food Chem. 2007, 104, 156–162. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lorens, G.F.; Bennett, J.M.; Loggale, L.B. Differences in Drought Resistance between Two Corn Hybrids. I. Water Relations and Root Length Density 1. Agron. J. 1987, 79, 802–807. [Google Scholar] [CrossRef]
- Ekanayake, I.J.; O’Toole, J.C.; Garrity, D.P.; Masajo, T.M. Inheritance of Root Characters and their Relations to Drought Resistance in Rice 1. Crop. Sci. 1985, 25, 927–933. [Google Scholar] [CrossRef]
- O’Toole, J.C. Soemartono Evaluation of a simple technique for characterizing rice root systems in relation to drought resistance. Euphytica 1981, 30, 283–290. [Google Scholar] [CrossRef]
- Reicosky, D.C.; Deaton, D.E. Soybean Water Extraction, Leaf Water Potential, and Evapotranspiration during Drought 1. Agron. J. 1979, 71, 45–50. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, S.; Chen, S.; Jiang, J.; Liu, G. Phylogenetic and stress-responsive expression analysis of 20 WRKY genes in Populus simonii × Populus nigra. Gene 2015, 565, 130–139. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, D. Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environ. Exp. Bot. 2009, 65, 35–47. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, Y.; Jiang, Q.; Yang, J.; Zhao, W.; A Taylor, I.; Peng, Y.-L.; Wang, D.; Liu, J. Structural basis of dimerization and dual W-box DNA recognition by rice WRKY domain. Nucleic Acids Res. 2019, 47, 4308–4318. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Yoshida, R.; Kishi-Kaboshi, M.; Matsushita, A.; Jiang, C.-J.; Goto, S.; Takahashi, A.; Hirochika, H.; Takatsuji, H. Abiotic Stresses Antagonize the Rice Defence Pathway through the Tyrosine-Dephosphorylation of OsMPK6. PLoS Pathog. 2015, 11, e1005231. [Google Scholar] [CrossRef] [PubMed]
- Jezek, M.; Blatt, M.R. The Membrane Transport System of the Guard Cell and Its Integration for Stomatal Dynamics. Plant Physiol. 2017, 174, 487–519. [Google Scholar] [CrossRef] [PubMed]
- Zoulias, N.; Harrison, E.L.; Casson, S.A.; Gray, J.E. Molecular control of stomatal development. Biochem. J. 2018, 475, 441–454. [Google Scholar] [CrossRef]
- Molinari, H.B.C.; Marur, C.J.; Daros, E.; De Campos, M.K.F.; De Carvalho, J.F.R.P.; Filho, J.C.B.; Pereira, L.F.P.; Vieira, L.G.E. Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): Osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiol. Plant. 2007, 130, 218–229. [Google Scholar] [CrossRef]
- Sánchez, F.J.; Manzanares, M.; De Andres, E.F.; Tenorio, J.L.; Ayerbe, L. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crop. Res. 1998, 59, 225–235. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Papadakis, A.K.; Stephanou, E.G.; Roubelakis-Angelakis, K.A. Abiotic Stress Generates ROS That Signal Expression of Anionic Glutamate Dehydrogenases to Form Glutamate for Proline Synthesis in Tobacco and Grapevine. Plant Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Silva, V.M.; Boleta, E.H.M.; Lanza, M.G.D.B.; Lavres, J.; Martins, J.T.; Santos, E.F.; Dos Santos, F.L.M.; Putti, F.F.; Junior, E.F.; White, P.J.; et al. Physiological, biochemical, and ultrastructural characterization of selenium toxicity in cowpea plants. Environ. Exp. Bot. 2018, 150, 172–182. [Google Scholar] [CrossRef]
- Taulavuori, E.; Hellström, E.; Taulavuori, K.; Laine, K. Comparison of two methods used to analyse lipid peroxidation from Vaccinium myrtillus (L.) during snow removal, reacclimation and cold acclimation. J. Exp. Bot. 2001, 52, 2375–2380. [Google Scholar] [CrossRef]
- Ampofo, J.O.; Ngadi, M. Stimulation of the phenylpropanoid pathway and antioxidant capacities by biotic and abiotic elicitation strategies in common bean (Phaseolus vulgaris) sprouts. Process. Biochem. 2021. [Google Scholar] [CrossRef]
- Zhong, M.; Song, R.; Wang, Y.; Shu, S.; Sun, J.; Guo, S. TGase regulates salt stress tolerance through enhancing bound polyamines-mediated antioxidant enzymes activity in tomato. Environ. Exp. Bot. 2020, 179, 104191. [Google Scholar] [CrossRef]
- Tran, L.P.; Nakashima, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Chapter Six—Plant Gene Net-works in Osmotic Stress Response: From Genes to Regulatory Networks. In Methods in Enzymology; Häussinger, D., Sies, H., Eds.; Osmosensing and Osmosignaling; Academic Press: London, UK, 2007; Volume 428, pp. 109–128. [Google Scholar]
- Mattioli, R.; Marchese, D.; D’Angeli, S.; Altamura, M.M.; Costantino, P.; Trovato, M. Modulation of intracellular proline levels affects flowering time and inflorescence architecture in Arabidopsis. Plant Mol. Biol. 2008, 66, 277–288. [Google Scholar] [CrossRef]
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Furihata, T.; Abe, H.; Narusaka, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003, 34, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, M.; Liu, Q.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1999, 17, 287–291. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Jha, B. Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Biol. Plant. 2010, 54, 201–212. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, G.; Yu, D. Activated Expression of WRKY57 Confers Drought Tolerance in Arabidopsis. Mol. Plant 2012, 5, 1375–1388. [Google Scholar] [CrossRef]
- Anoop, N.; Gupta, A.K. Transgenic indica Rice cv IR-50 Over-expressing Vigna aconitifolia Δ1-Pyrroline -5- Carboxylate Synthetase cDNA Shows Tolerance to High Salt. J. Plant Biochem. Biotechnol. 2003, 12, 109–116. [Google Scholar] [CrossRef]
- Hmida-Sayari, A.; Gargouri-Bouzid, R.; Bidani, A.; Jaoua, L.; Savouré, A.; Jaoua, S. Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers salt tolerance in transgenic potato plants. Plant Sci. 2005, 169, 746–752. [Google Scholar] [CrossRef]
- Su, J.; Wu, R. Stress-inducible synthesis of proline in transgenic rice confers faster growth under stress conditions than that with constitutive synthesis. Plant Sci. 2004, 166, 941–948. [Google Scholar] [CrossRef]
- Vendruscolo, E.C.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinari, H.B.C.; Marur, C.J.; Vieira, L.G.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, X.; Pei, S.; Shengqiang, P.; Wang, X.; Tang, Q.; Jia, C.; Lu, Y.; Chunlin, J.; Zhou, G. The Miscanthus NAC transcription factor MlNAC9 enhances abiotic stress tolerance in transgenic Arabidopsis. Gene 2016, 586, 158–169. [Google Scholar] [CrossRef]
- Yang, R.; Wang, T.; Shi, W.; Li, S.; Liu, Z.; Wang, J.; Yang, Y. E3 ubiquitin ligase ATL61 acts as a positive regulator in abscisic acid mediated drought response in Arabidopsis. Biochem. Biophys. Res. Commun. 2020, 528, 292–298. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, X.-Y.; Chen, J.; Xu, W.-X.; Qiu, J.-R.; Song, L.; Wang, J.-T.; Tang, R.; Chen, D.; Jiang, C.-Z.; Huang, Z. Dehydration-Induced WRKY Transcriptional Factor MfWRKY70 of Myrothamnus flabellifolia Enhanced Drought and Salinity Tolerance in Arabidopsis. Biomolecules 2021, 11, 327. https://doi.org/10.3390/biom11020327
Xiang X-Y, Chen J, Xu W-X, Qiu J-R, Song L, Wang J-T, Tang R, Chen D, Jiang C-Z, Huang Z. Dehydration-Induced WRKY Transcriptional Factor MfWRKY70 of Myrothamnus flabellifolia Enhanced Drought and Salinity Tolerance in Arabidopsis. Biomolecules. 2021; 11(2):327. https://doi.org/10.3390/biom11020327
Chicago/Turabian StyleXiang, Xiang-Ying, Jia Chen, Wen-Xin Xu, Jia-Rui Qiu, Li Song, Jia-Tong Wang, Rong Tang, Duoer Chen, Cai-Zhong Jiang, and Zhuo Huang. 2021. "Dehydration-Induced WRKY Transcriptional Factor MfWRKY70 of Myrothamnus flabellifolia Enhanced Drought and Salinity Tolerance in Arabidopsis" Biomolecules 11, no. 2: 327. https://doi.org/10.3390/biom11020327
APA StyleXiang, X.-Y., Chen, J., Xu, W.-X., Qiu, J.-R., Song, L., Wang, J.-T., Tang, R., Chen, D., Jiang, C.-Z., & Huang, Z. (2021). Dehydration-Induced WRKY Transcriptional Factor MfWRKY70 of Myrothamnus flabellifolia Enhanced Drought and Salinity Tolerance in Arabidopsis. Biomolecules, 11(2), 327. https://doi.org/10.3390/biom11020327





