Abstract
Marine sponges (porifera) have proved to be a prolific source of unique bioactive secondary metabolites, among which the alkaloids occupy a special place in terms of unprecedented structures and outstanding biological activities. Identification of active cytotoxic alkaloids extracted from marine animals, particularly sponges, is an important strive, due to lack of knowledge on traditional experiential and ethnopharmacology investigations. In this report, a comprehensive survey of demospongian bioactive alkaloids in the range 1987–2020 had been performed with a special emphasis on the potent cytotoxic activity. Different resources and databases had been investigated, including Scifinder (database for the chemical literature) CAS (Chemical Abstract Service) search, web of science, Marin Lit (marine natural products research) database. More than 230 representatives of different classes of alkaloids had been reviewed and classified, different genera belonging to the phylum porifera had been shown to be a prolific source of alkaloidal molecules, including Agelas sp., Suberea sp., Mycale sp., Haliclona sp., Epipolasis sp., Monanchora sp., Crambe sp., Reniera sp., and Xestospongia sp., among others. The sufficient production of alkaloids derived from sponges is a prosperous approach that requires more attention in future studies to consider the constraints regarding the supply of drugs, attained from marine organisms.
1. Introduction
Marine alkaloids present unique chemical structures that have been widely distributed among marine organisms. Some of them represent derivative molecules of the commonly encountered terrestrial alkaloids, whereas others show unprecedented novel structures confined to the marine systems. Their purification, structure elucidation, stereochemistry, chemical modification, synthesis, and pharmaceutical activity have acquired outstanding interdisciplinary attention from various fields of research aside from chemistry, including physiology, and pharmacology, ecology, biotechnology [1]. Alkaloids represent one of the most important classes of natural products that are widely distributed among different biological sources, including plants, animals, fungi, cyanobacteria, actinomycetes, dinoflagellates, red algae, cnidarians, and bryozoans; however, their presence in marine invertebrates as major constituents is limited to specific phylum, including some sponge genera, ascidians, mollusks, red algae, and bryozoans. Although the exact physiological function of alkaloids remains unclear, many of them had been developed as defense chemical weapons against predation—this is of great importance, especially for vulnerable sessile organisms, like sponges, and consequently, they are expected to be very potent molecules demonstrating toxicity at low doses [2,3,4].
This class of compounds demonstrates potent biological activities that can be considered as lead compounds for the development of potent antibiotics, antifungal, antiviral, anti-inflammatory, antimalarial, immune-modulating, or neuro-suppressive [5,6,7,8,9,10,11,12]. They also demonstrated promising cytotoxic activity versus diverse types of cancer cells [13,14]. Sponges (porifera), phylogenetically the oldest metazoan, have been recognized as a precious origin of unique secondary metabolites. About 5000 sponge species had been identified and classified with major groups belonging to Demospongiae [15]. They demonstrated wide distribution from intertidal coastal regions to great depths up to 8000 m depth [16]. The outstanding secondary metabolites produced by sponges are assumed to be the result of a combination of various factors, including the sessile nature of the organism, the porous nature of the body, and the environmental and ecological factors surrounding the organism [17]. As a consequence, it is obvious that these indefensible immobile organisms were provided by potent allelopathic factors that lead to the biosynthesis of many new drugs.
In this review, a comprehensive survey of about 240 alkaloids isolated from marine sponges from 1987 to 2020 had been performed, to shed light on those alkaloids demonstrating potent in vitro cytotoxicity, different resources, and databases, including Scifinder (database for the chemical literature) CAS (Chemical Abstract Service) search, web of science, Marin Lit (marine natural products research) database, were used. Moreover, review articles reviewing secondary metabolites from sponges were included [1,18,19,20,21,22]. Throughout this review, the alkaloids were classified into 20 different chemical classes (acridine, β-carboline, bromotyrosine, brominated, dimeric aaptamine, guanidine, imidazole, indole, peptide, piperidine, pyrimidine, pyridine, pyrrole, pyrroloiminoquinone, quinoline and quinolizidine, tetrahydroisoqouinoline, steroidal, terpenoidal, manzamine, and sesquiterpene quinones/hydroquinones alkaloid), and representative examples of each class have been reviewed with about 240 molecules described. It is worth noting that an individual alkaloid may fall in more than one chemical class, and placing certain alkaloid in a specific class was based on the authors’ classification in the original articles.
2. Alkaloid Classification
2.1. Acridine Alkaloids
Acridine nucleus (C13H9N) is a polycyclic heteroarene in which one of the central CH groups is replaced by a nitrogen atom. Chemical investigation of the Dercitus sp., collected from the Bahamas at 160 depth, led to the isolation of a violet pigment, identified as dercitin (1); cytotoxic evaluation of this compound against a panel of cell lines revealed potent cytotoxic activity in nanomolar concentration [23]. Investigation of different sponge samples belonging to genus Xestospongia collected from Indonesia, and New Guinea had led to the isolation of neoamphimedine (2), 5-methoxyneoamphimedine (3), amphimedine (4), neoamphimedine Z (5), alpkinidine (6). Compounds 2–6 were identified as bisannulated acridines. Based on results, compounds 2,3, and 6 showed selective activity for solid tumors—among them, 2 was the most potent, and 3 showed the most selectivity for solid tumors [24]. Figure 1 illustrates the chemical structures of compounds 1–6; Table 1 summarizes the cytotoxic evaluation of compounds 1–6.
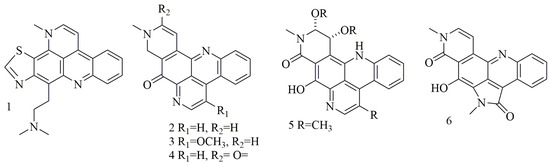
Figure 1.
Structures of acridine alkaloids isolated from sponges.

Table 1.
Cytotoxic activity of acridine alkaloids.
2.2. β-Carboline Alkaloid
Four β-carboline alkaloids 1,2,3,4-tetrahydronorharman-1-one (7) acanthomine A (8), annomontine (9), and ingenine E (10), were isolated from Acanthostrongylophora ingens. Compounds 7–10 showed the most potent cytotoxicity [25]. Figure 2 iIllustrates the chemical structures of compounds 7–10; Table 2 summarizes the cytotoxic evaluation of compounds 7–10.
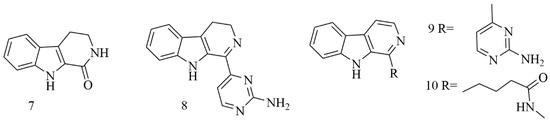
Figure 2.
Structures of β-carboline alkaloids isolated from sponges.

Table 2.
Cytotoxic activity of β-carboline alkaloids.
2.3. Bromotyrosine Alkaloids
Qun Göthel et al. reported three bromotyrosine alkaloids identified as 14-debromo-11-deoxyfistularin-3 (11), aplysinin A (12), and aplysinin B (13) from the sponge Aplysina lacunosa collected from Stirrup Cay in the Bahamas. Compounds 11–12 revealed a unique, five-membered oxazole ring with a spiro atom linked with the bromocyclohexa-diene ring [26]. Investigation of different sponge samples belonging to genus Hexadella sp., Jaspis sp. and Bubaris sp., collected from Indonesia by Tarazona, and co-workers led to the isolation and identification of new bromotyrosine alkaloids 14–15. Aplyzanzine B (14) was isolated from Jaspis sp., and Bubaris sp., whereas Anomoian B (15) was isolated from Hexadella sp. have shown potent cytotoxic activity against three human tumor cell lines A549, HT-29, and MDA-MB-231 (6.1, 1.6, and 7.8 μM, respectively) [27].
In another study, ma’edamines C (16) and ma’edamines D (17) were isolated by Japanese researchers from the Okinawan marine sponge Suberea sp. compounds 16–17 revealed cyclization of the side-chain nitrogen of tyrosine to add a quaternary pyridinium nucleus to the structure. Both compounds have shown selective cytotoxicity against murine leukemia L1210 cell line though, and they did not express cytotoxicity against KB cell line [28]. Investigation of the marine sponge Psammoclemma sp. collected from Bommie Bay, Queensland, Australia, led to the isolation of psammaplysene C (18) and psammaplysene D (19). Compounds 18–19 showed C6C3N moiety in one of their bromotyrosine uints instead of the conventional C6C2N arrangement [29]; both compounds showed potent cytotoxic activities. Another bromotyrosyn alkaloids were isolated from the Fijian sponge Druinella sp. These 10 bromotyrosine alkaloids 20–29, were identified as purealidin S (20), purpuramine J (21). PurealidinQ (22), aplysamine 2 (23), purpureamine I (24), aerophobin2 (25), aerophobin1 (26), purealidin J (27), araplysillin1 (28), and araplysillin 2 (29). Compounds 25–27 revealed replacing the other bromobenzene moiety commonly encountered in bromotyrosine alkaloids with an imidazole ring. Compounds 20–29 were evaluated for their cytotoxicity against two cell lines showing potent to moderate activities. Among them, compound 21 was the most potent cytotoxic compound [30]. Suberedamine A (30) and suberedamine B (31) were isolated from Suberea sp. These two new cytotoxic bromotyrosine alkaloids exhibited potent cytotoxic activity against L1210 and KB cell lines [31]. Figure 3 illustrates the chemical structures of compounds 11–31; Table 3 summarizes the cytotoxic evaluation of compounds 11–31.
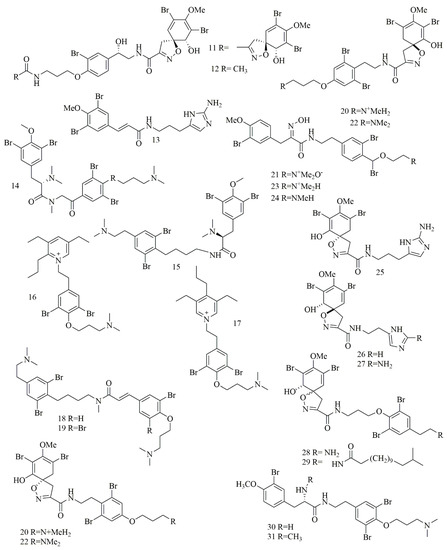
Figure 3.
Structures of bromotyrosine alkaloids isolated from sponges.

Table 3.
Cytotoxic effects of bromotyrosine alkaloids.
2.4. Dibrominated and Brominated Alkaloids
Tilvi et al. reported three new pyrrole-2-aminoimidazole alkaloids from the Agelas dendromorpha collected from New Caledonia. Cytotoxic investigation of the isolated compounds revealed that only one compound identified as Agelastatin E (32) that showed 100% activity at 3 and 30 µM against KB cell lines [32]. In Shaala et al. a dibrominated alkaloid, aerothionin (33) with potent cytotoxicity against HeLa cells was identified from the sponge, Suberea sp., collected from the Red Sea in Yanbu, Saudi Arabia [33]. Two brominated indolosulfonic acid derivatives were reported from the hydroalcoholic extract of the Psammoclemma sp., collected from New Caledonia. The compounds were identified as echinosulfonic acid D (34) and echinosulfonic acid B (35) based on extensive LC/MS/MS analysis besides conventional 1 and 2 D NMR analysis. Both compounds showed potent cytotoxicity against KB cells with equal IC50 of 2 µg/mL [34]. Figure 4 illustrates the chemical structures of compounds 32–35; Table 4 summarizes the cytotoxic evaluation of compounds 32–35.
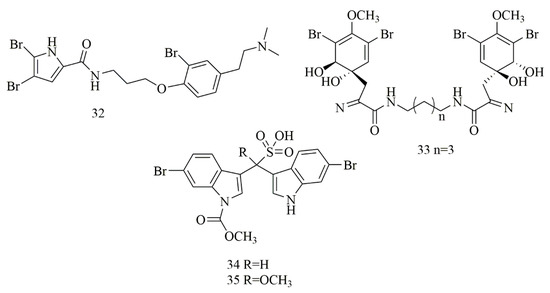
Figure 4.
Dibrominated and brominated alkaloids from marine sponges.

Table 4.
Cytotoxic activity of dibrominated and brominated alkaloids from sponges.
2.5. Aaptamine Alkaloids
Bioassay-guided fractionation of the Indonesian sponge Aaptos suberitoides resulted in the isolation of three benzonaphthryidine derivatives identified as aaptamine (36), isoaaptamine (37), and demethylaaptamine (38). Biological evaluation of compounds 36–38 revealed potent cytotoxicity against HeLa cell lines with IC50 values of 15, 3.1, and 1.4 µg/mL, respectively. Moreover, the activity had been attributed to proteasome inhibitory action [35]. Investigation of a different sample from the same sponge A. suberitoides, collected from Xisha islands in the South China Sea (NaiHai), has led to the isolation of additional aaptamine derivatives with dimerization in the benzonathyridine moiety, the compounds were identified as suberitine A (39), suberitine B (40), suberitine C (41) and suberitine D (42). Cytotoxic evaluation of 39–42 against P388, HeLa, and K562 cell lines revealed selective, potent activity of compounds 40 and 42 with IC50 values of 1.8 and 3.5 μM, respectively, against P388 cell line with the absence of activity on other lines. Interestingly, compounds 39 and 41 did not show significant effects against any of the cell lines, revealing the possible need of exocyclic double bond in one of the monomer units either as ketone as in 42 or exocyclic terminal methylene as in 40 [36]. Figure 5 illustrates the chemical structures of compounds 36–42; Table 5 summarizes the cytotoxic evaluation of compounds 36–42.
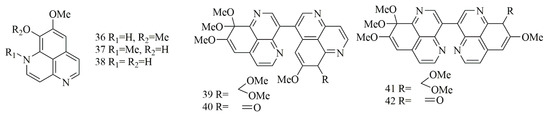
Figure 5.
Dimeric aaptamine alkaloids from marine sponges.

Table 5.
Cytotoxic activity of Dimeric aaptamine alkaloids.
2.6. Guanidine Alkaloids
Monanchora pulchra collected from the Pacific Ocean near Urup islands, was the source of guanidine alkaloid monanchocidin A (43). It is composed of a five-membered spiro-ring in the pentacyclic guanidine core along with an uncommon branched long alkyl chain and a heavily oxygenated morpholinone fragment [37]. Evaluation of 43 against monocytic anemia cell lines THP-1, Hela, and JB6-C141 cell lines revealed potent cytotoxicity with IC50 values against 5.1 µM, 11.8 µM, and 12.3 µM, respectively [38]. As this sponge is a great source of guanidine alkaloids, additional pentacyclic guanidine alkaloids, monanchocidin B-E (44–47) were reported by Makarieva at al. Compounds 44–47 revealed strong inhibitory activities with IC50 values of 540, 200, 110, 830, and 650 nM, respectively against HL-60 cells [39]. Investigation of different sample of the Monanchora sp. sponge, collected from a depth of 20 m from the coast of Hiva Oa Island (French Polynesia) led to the isolation of nine cytotoxic pentacyclic guanidine alkaloids identified as monanchoradin A (48), dehydrocrambescin A2 418(49), crambescidin 786(50), (−)-crambescidin 814 (51), monalidine A (52), (−)-crambescin 406 (53), crambescidin 800 (54), crambescidin 826 (55) and 20-norcrambescidic acid (56). Cytotoxic evaluation of compounds 48–56 had been performed against a panel of cell lines revealed interesting cytotoxic activity [40]. Crambescidin-816 (57) has been isolated from Crambe crambe (oyster sponge) belongs to the pentacyclic guanidine alkaloid. Biological evaluation of 57 revealed its ability to reduce cell viability of the tumor-derived cell line HepG2 at concentrations higher than 150 nM. Compound 57 also affected the human tumor-derived cell lines OVCAR (ovary carcinoma), HOP-92 (pulmonary carcinoma), MCF-7, PC3 (prostatic carcinoma), SK-MEL-28 (melanoma), UO-31 (kidney carcinoma), and HT-29 by reducing the cell viability at the three concentrations in all the cell lines except MCF-7 [41]. Detailed biological study of 57 attributed to its activity on calcium voltage-dependent channel blockage and decreasing neuronal viability in cortical neurons [42]. Extract of the Marine sponge Clathria bulbotoxa, collected from Samalona island, Indonesia, yielded five cytotoxic guanidine alkaloids 58–62, identified as crambescidins 345 (58), crambescidins 361 (59) crambescidins 373 (60), crambescidins 359 (61), and crambescidins 657 (62). All compounds demonstrated cytotoxic activity less than 10 μM against A431 cell line. Compounds 61 and 62 had the most powerful cytotoxicity with IC50 of 12 and 48 nM. Also 58–60 had modest cytotoxic with IC50 of 7.0, 2.5, 0.94 and 3.1 μM [43]. Continued investigation of the sponge Monanchora pulchra had has yielded three pentacyclic guanidine alkaloids normonanchocidin A (63), normonanchocidins B (64), and normonanchocidin D (65). Compounds 64 and 65 were not isolated in pure form and usually yielded a 1:1 mixture. The cytotoxic activity of compounds 63 and the mixture of 64 and 65 (1:1) against THP-1 showed IC50 values of 2.1 µM, 3.7 µM, 3.8 µM, and 6.8 µM, against HeLa cells, respectively [44]. Additionally, two pentacyclic guanidine alkaloid, namely, monanchomycalin C (66) and ptilomycalin A (67), were found in a different sample of Monanchora pulchra (Lambe, 1894, family Crambeidae) collected from Kunashir Island. The cytotoxic activity of the 66 and 67 against human breast cancer MDA-MB-231 cells displayed IC50 values of 8.2 µM and 4.3 µM, respectively [45]. Another different sample of Monanchora pulchra collected off the Chirpoi sland was a source of monanchoxymycalin C (68), a pentacyclic guanidine alkaloid. Compound 68 was able to inhibit the colony formation of HeLa cells, at a concentrations of ≥1.25 μM [46]. Tricyclic guanidine alkaloids have been reported from some sponges, including Biemna laboutei, collected from Salary Bay, Madagascar, seven Tricyclic guanidine alkaloids, were isolated from the methylene chloride: Methanol extract of this sponge, however only one compound, netamine M (69), revealed potent cytotoxicity against KB cell line with IC50 value of 1 μM [47]. Further investigation on B. laboutei, led to the isolation of additional seven netamine derivatives, among which, only netamine O (70), netamine Q (71), revealed cytotoxic activity against KB cells in the range of 10 µM [48]. Zanissine (72), a new bicyclic 4, 5-guanidino-pyridaine alkaloid was yielded from the CH2Cl2 extract of Anchinoe pauperta, collected from Zarzis, Tunisia. Compound 72 showed cytotoxic activity against P-388, KB, and NSCLC-N6 cell lines with IC50 of 12 μg/mL, 5 μg/mL, and 10 μg/mL [49]. The Marine sponge Monanchora sp., collected off the coast of Hiva Oa Island (French Polynesia) at 20 m depth, yielded three acyclic cytotoxic bis-guanidine alkaloids Unguiculin A (73), Unguiculin B (74), and Unguiculin C (75). Cytotoxic evaluation of 73–75 against KB cancer cell lines had revealed potent cytotoxicity with IC50 of 0.2, 0.08, and 0.03 μM, respectively [50]. Figure 6 illustrates the chemical structures of compounds 43–75; Table 6 summarizes the cytotoxic evaluation of compounds 43–75.
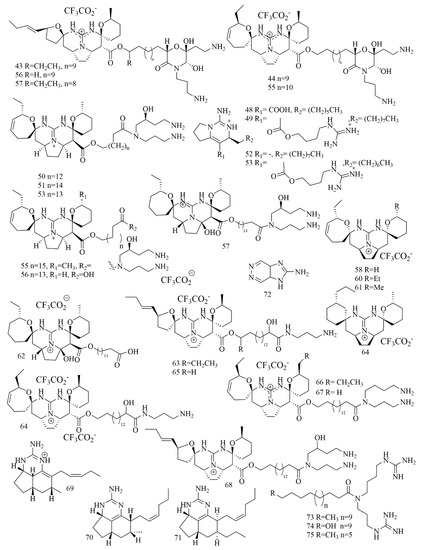
Figure 6.
Guanidine alkaloids from marine sponges.

Table 6.
Cytotoxic activity of Guanidine alkaloids from marine sponges.
2.7. Imidazole Alkaloid
Imidazole metabolites were reported from the Chinese sponge Leucetta chagosensis collected off the Yongxing islands in the South China Sea and yielded (−)-calcaridine (76) and (2E, 9E)-pyronaamidine-9-(N-methylimine) (77). Both compounds revealed selective cytotoxicity against MCF-7 cell lines with IC50 values of 25.3 and 24.2 μM, respectively, but no cytotoxic effect was detected on A549 and PC9 cell lines [51]. Two cytotoxic bi-imidazole alkaloids, naamidine J (78) and naamidine H (79) were isolated from Pericharax heteroraphis collected from the South Sea (Yongxing islands) at a depth of 12 m. Cytotoxic evaluation of the two alkaloids 78–79 revealed potent activity against cancer cell lines, where compound 78 inhibited the growth of K562 cell line with IC50 values of 11.3 µM, on the other hand, compound 79 showed an inhibitory effect on K562, HeLa, and A549 cell lines with IC50 values of 9.4, 21.4 and 22.4 µM, respectively [52]. Naamine J (80), a guanidine-based imidazole alkaloid was isolated from Leucandra sp., collected off Woody islands in the South China Sea. Evaluation of the cytotoxic effects of 80 on four MCF-7, A549, HeLa, and PC9 cell lines showed it possessed an inhibitory effect with IC50 values of 20.1, 23.7, 28.2, and 45.3 μM, respectively [53]. Naamidine I (81) along with 79 were isolated from Leucetta chagosensis (Calcarea sponge) collected from a depth of ten meters in North Sulawesi, Indonesia. Compounds 79 and 81 revealed cytotoxic effect on the HeLa cell line with IC50 values of 11.3 and 29.6 μM, respectively [54]. Isonaamine C (82), isonaamidine E (83), and two cytotoxic imidazole alkaloids isolated from Leucetta chagosensis from Bougainville Reef, the Great Barrier Reef, Australia. Cytotoxic effects of all compounds on HM02, HepG2, and Huh7 cell lines showed that compound 83 had growth inhibitory effect with GI50 values of 15.1, 15.1, and 2.8 µM, respectively. Whereas, compound 82 showed GI50 values of 15.0, 6.2, and 5.9 µM, respectively [55]. Another imidazole alkaloid, leucosolenamine B (84), isolated from Leucosolenia sp., collected off Milne Bay in Papua New Guinea, showed potent cytotoxic effect on the C-38 cell line with IC50 value of 19.6 µM [56]. Further investigation demonstrated Leucetta chagosensis collected from the South China Sea is a source of unique metal complexes, chagosendine A (85), chagosendine B (86), and chagosendine C (87) along with pyronaamidine (88) obtained by reacting chagosendine B with Na2S and 80 from reacted chagosendine C with Na2S. Cytotoxic evaluation of 86 and 87 showed potent inhibition against K562, HepG2, and HeLa cell lines, where 86 inhibited K562, HepG2, and Hella with IC50 of 0.62, 1.19, and 0.58 μM and 87 showed IC50 value of 0.62, 0.31 and 4.43 μM, respectively. Whereas, 85 displayed weak activity IC50 more than 10 μM against the cell lines. Compound 88 inhibited K562 and Hela with IC50 values of 6.87 and 5.62 μM [57]. Figure 7 illustrates the chemical structures of 76–88; Table 7 summarizes the cytotoxic evaluation of 76–88.
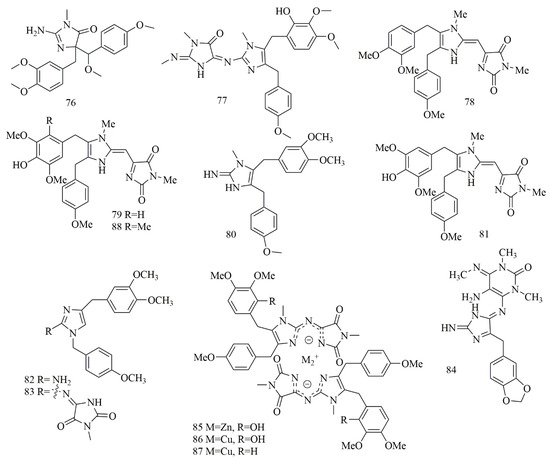
Figure 7.
Cytotoxic imidazole alkaloid structures in marine sponges.

Table 7.
Cytotoxic effects of Imidazole alkaloid.
2.8. Indole, Bisindole, and Trisindole Alkaloids
The aqueous extract of the sponge Thorectandra sp., was a source of quaternary indole alkaloids identified as demethoxyfascaplysin (89), 1-deoxysecofascaplysin A (90), and fascaplysin (91). Compound 89 showed cytotoxic effects on breast cancer cell line (IC50 of 20.4 µM), 90 inhibited the growth of MCF-7, OVCAR-3, and A549 (IC50 of 4.9, 7.2 and 43.2 µM), whereas compound 91 showed cytotoxicity (IC50 0.11–1.40 µM) against MCF-7, OVCAR-3, MALME-3M and A549 [58]. Four bisindole alkaloids identified as dragmacidin G (92), dragmacidin H (93), topsentin B2 (bromotopsentin) (94), and topsentin B1 (topsentin) (95) were reported from Lipastrotethya sp., (Dictyonellidae family) collected off north of Hachijo Island, Japan. Compounds 92–95 exhibited cytotoxicity on HeLa cells with IC50 values of 4.2, 4.6, 1.7, and 4.4 μM, respectively [59]. LCMS analysis of a Hyrtios sp. extract, collected from Unten-Port, Okinawa, yielded 2,5-disubstituted pyrimidine bisindole alkaloid hyrtinadine A (96), with potent cytotoxicity on L1210 and KB cell lines with IC50 of 2.9 and 8.7 µM [60]. Hyrtios erectus collected from Hurghada, Egypt, was a source of indole alkaloids those were identified as hyrtioerectine A (97), hyrtioerectine B (98), and hyrtioerectine C (99). Compounds 97–99 demonstrated cytotoxic effects on HeLa cell line with IC50 (25.8, 20.3, and 20.4 μM) [61]. Two brominated tris-indole alkaloids were isolated from Callyspongia siphonella, collected from Hurghada, Egypt. The two alkaloids were identified as 5-bromotrisindoline (100) and 6-bromotrisindoline (101). Results from HT-29, OVCAR-3, and MM.1S cell line displayed that 100 and 101 proved to be toxic with IC50 values 8, 7, and 9 μM, for 100 and 12.5, 9, and 11 μM, for 101 [62]. Four simple bromoindole alkaloids, 5-bromo-l-tryptophan (102), 5-bromoabrine (103), 5,6-dibromoabrine (104), and 5-bromoindole-3-acetic acid (105), were isolated from Smenospongia sp., collected from Batanes, Philippines. Compounds 102–105 were evaluated against HCT-116 cell lines by means of an MTT assay, and they exhibited the least cytotoxicity in a set of isogenic HCT116 cell lines. Based upon the result the reduction in activity against the p53−/− cell line was evidenced [63]. Damirine A (106), a guanidine-based bis-indole alkaloid, was isolated and identified from the sponge Damiria sp., collected from Phuket Island, Thailand. Results from MALME-3M, Sw620, HCC-2998, MOLT-4, k562cell line revealed that 106 proved to be cytotoxic with GI50 of 1.9, 3.3, 2.3, 1.9, 2.2 μM [64]. 6″-Debromohamacanthin A (DBHA) (107), another bisindole alkaloid, was identified in a few sponges, including Spongosorites sp., and 107 did not show substantial cell viability up to 10 μM, but over 20 μM DBHA displayed a cytotoxic effect in mES cell line (IC50: 28.5 μM) [65]. Biossay-guided isolation of the methanol extract of Spongosorites sp., collected from the coast of Jeju Island, Korea, yielded the isolation of indole alkaloids (108–118), identified as I-6′′-debromohamacanthin A (108), (R)-6′-debromohamacanthin A (109), and (S)-6′′-debromohamacanthin B (110), categorized as hamacanthin class and dibromodeoxytopsentin (111) a bisindole alkaloid of the Topsentin class, along with trans-3,4-dihydrohamacanthin A (112), cis-3,4-dihydrohamacanthin B (113), topsentin(114), bromotopsentin (115), deoxytopsentin (116), bromodeoxytopsentin (117) and isobromodeoxytopsentin (118). Compounds 108–118 were evaluated for cytotoxicity against A549, SK-OV-3, SK-MEL-2, XF498, and HCT15 cell lines. Compounds 108, 112, 113, and 118 displayed modest to substantial cytotoxicity to all of the cancer cell lines established. Compounds 114 and 115 displayed cytotoxicity on P388 with IC50 values of 5.8 and 16.6 µM, respectively. Compounds 117 and 118 were described to exhibit cytotoxicity against the cell-line K-562 with IC50 values of 1.4 and 5.1 µM, respectively [66]. Figure 8 illustrates the chemical structures of compounds 89–118; Table 8 summarizes the cytotoxic evaluation of compounds 89–118.
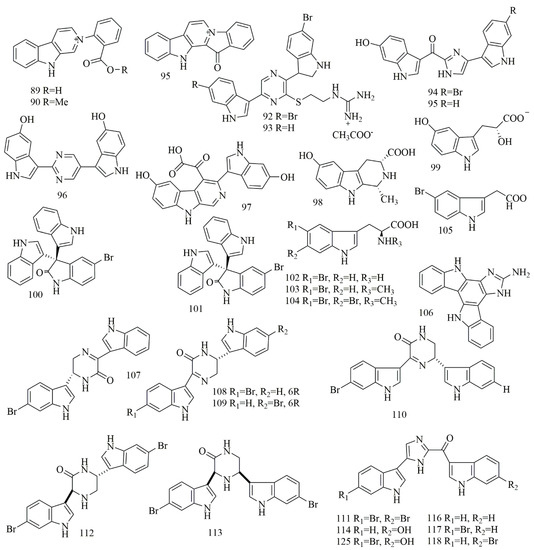
Figure 8.
Cytotoxic Indol, Bisindol, and Trisindol alkaloid structures in marine sponges.

Table 8.
Cytotoxic activity of Indol, Bisindol, and Trisindol alkaloids.
2.9. Peptide Alkaloid
Scleritodermin A (119), a potent sulfonated thiazol hexapeptide was isolated from the marine sponge Scleritoderma nodosum collected from the northwest side of Olango Island, Cebu, Philippines. The purified compound demonstrated low µM cytotoxicity to HCT116, A2780, and SKBR3 cell lines with IC50 of 1.9, 0.940, and 0.670 µM, respectively [67]. Figure 9 illustrates the chemical structures of compound 119; Table 9 summarizes the cytotoxic evaluation of compound 119.
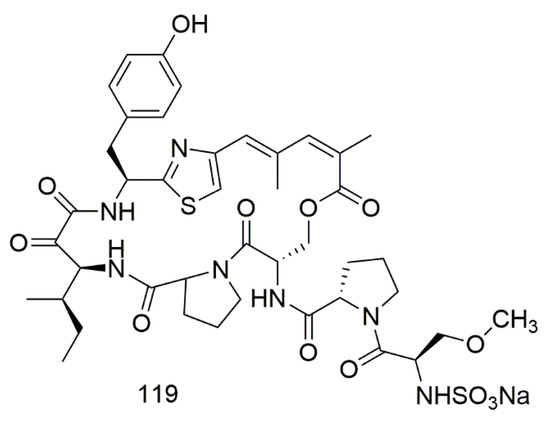
Figure 9.
Peptide alkaloids from sponge.

Table 9.
Cytotoxic activity of peptide alkaloid.
2.10. Piperidine Alkaloids
Arenosclera brasiliensis endemic to the southeastern Brazilian coastline collected from Joa˜o Fernandinho Beach, Bu’zios (Rio de Janeiro state) was the source of alkylpiperidine alkaloids identified as arenosclerin A (120), arenosclerin B (121), arenosclerin C (122) and haliclonacyclamine E (123). Results of cytotoxic activity displayed that 121–123 had cytotoxic effects in the range of 0.5–2.1 μg/mL to HL-60, B16, L929, and U138 cancer cell lines [68]. Madangamine F (124) haliclonacyclamine F (125), arenosclerins D (126), and arenosclerins E (127), were found in Pachychalina alcaloidifera as one of the richest national source of bis-pyridine alkaloid. Results proved the cytotoxic activity of 124–127 on SF295, MDA-MB435, HCT8, and HL60 cell lines. The most potent activity was against MDA-MB435 cell lines with IC50 values 16.2, 1, 1.2, and 3.1 μg/mL, respectively [69]. Neopetrosiamine A (128) was isolated from Neopetrosia proxima collected from Mona Island of Puerto Rico, which is belonging to the order Haplosclerida that is an abundant source of 3-alkylpiperidine alkaloids. Compound 128 displayed inhibitory effect on MALME-3M, CCRF-CEM, and MCF7 with IC50 values of 1.5, 2.0, and 3.5 μM, respectively [70]. Further, 1,5-diazacyclohenicosane (36) isolated from the marine sponge Mycale sp., collected from Kenya, was identified as a new macrocyclic diamine alkaloid. The compound showed interesting cytotoxic activity against the panel of cell lines [71]. Ingenamine G (129), isolated from Pachychalina sp., collected in Ilha do Pai (Father’s Island), NiteIo´I, Rio de Janeiro. Compound 129 was identified as macrocyclic dipipridine alkaloid. Cytotoxic evaluation of 129 against HCT-8, B16, and MCF-7 revealed potent cytotoxicity [72]. Papuamine (130) and haliclonadiamine (131) were identified as polycyclic alkaloids isolated from Neopetrosia cf exigua collected from Old Derawan Pier, Indonesia, at depth of 24 m. Compounds 130 and 131 were tested on human glioblastoma cell line (SF-295), and human renal cancer cell lines (UO-31 and A498)—and results proved their cytotoxic effects; in another study, 130 was reidentified from Haliclona sp, also collected from Indonesia, with remarkable cytotoxicity on MCF-7 cell [73,74]. Figure 10 illustrates the chemical structures of 120–131; Table 10 summarizes the cytotoxic evaluation of 120–131.
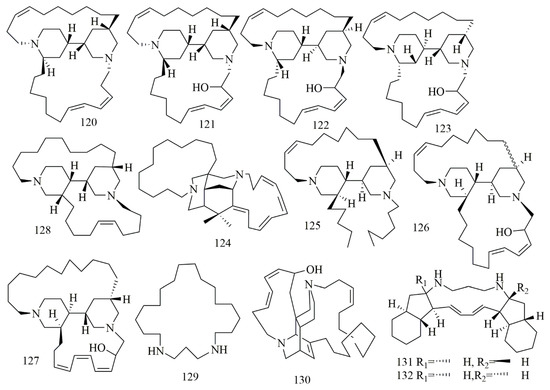
Figure 10.
Piperidine alkaloid structure in marine sponges.

Table 10.
Cytotoxic activity of piperidine alkaloid.
2.11. Pyrimidine Alkaloids
Lanesoic acid (133), was isolated as zwitterionic alkaloid from Theonella swinhoei a sponge of Theonella family collected from Lanes in Indo-Pacific, Indonesia. Compound 133 displayed an unusual 1, 4, 5, 6-tetrahydropyrimidine cation rarely exhibits in natural sources. Compound 133 was evaluated for cytotoxicity against a panel of cell lines, such as PSN1, HT-29, breast MD-MB-231, and A549, that revealed selective cytotoxicity against PSN1cells with an IC50 value of 28.2 μM and was inactive against NSCLC lung tumor, MD-MB-23, and HT-29 cells [75]. The alkaloid variolin B (134), heterocyclic constitute from the Antractic sponge Kirkpatrickia variolosa had been evaluated for cytotoxicity against a wide range of cancer cell lines, where it showed potent activity against most of the tested cell lines with the activity against androgen-sensitive prostate adenocarcinoma cell line with GI50 0.05 µM [76]. Figure 11 illustrates the chemical structures of 133–134; Table 11 summarizes the cytotoxic evaluation of 133–134.
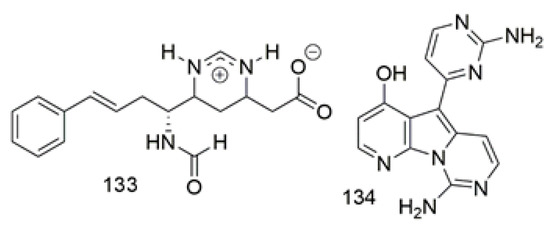
Figure 11.
Pyrimidine alkaloid structure isolated from sponges.

Table 11.
Cytotoxic activity of pyrimidine alkaloids from sponges.
2.12. Pyridine Alkaloids
The sample of Haliclona sp., collected from Indonesia, contained pyridine alkaloid cyano -3-dodecyl pyridine (135). Compound 135 showed moderate cytotoxity against A549, MCF-7, and Hela cell lines with IC50 values of 41.8, 48.4, and 33.2 μM, respectively [77]. Four pyridine glycosides had been isolated from Amphimedon sp., collected from Hachijo Island, Japan. The glycosidal alkaloids were identified as amphimedoside A (136) as a β-glucopyranose, amphimedoside C (137), a positional isomer of amphimedoside B (138) in the acetylenic bond with same molecular formula C26H42N2O6, amphimedoside D (139) and amphimedoside E (140). The results proved the cytotoxicity of 136–140 against P388 with IC50 values 90.0, 10.4, 23.0, 0.9, and 4.5 µM, respectively. These β-glucopyranosyl 3-alkylpyridine alkaloids exhibited comparable cytotoxic activities with aglycones [78]. Interestingly, from two different sponges Xestospongia sp. and Amphimedon sp., collected from Hachijo island, Japan l seven related 3-alkylpyridine alkaloids were reported: Hachijodine A (141) hachijodine B(142), hachijodine C(143), and hachijodine D(144) from Xestospongia sp., and hachijodine E (145), hachijodine F (146), and hachijodine G (147) from Amphimedon sp. [79]. N-methylniphatyne A (148), a 3-alkylpyridine alkaloid, was isolated from marine sponge of Xestospongia sp., collected off Java Island, Indonesia. Compound 148 was identified as N-methyl derivative of niphatyne A (149), which was reported from Fijian sponge of Niphates sp. Compound 148 revealed potent cytotoxicity against PANC-1 cells with IC50 value of 16 µM, whereas 149 showed cytotoxic effect of 2 µM on P388 cells [80]. Pyrinodemin A-D (150–153) were isolated from Okinawan sponge Amphimedon sp., collected off Nakijin, Okinawa. Compounds 151–153 showed strong cytotoxicity on L1210 with IC50 value (0.12, 0.10 and 0.14 µM, respectively) and KB with IC50 value (0.89, 0.89, 0.91 µM, respectively) [81]. Pyrinadines B-G (154–159), bis-3-alkylpyridine alkaloids with an azoxy moiety, were reported from the Okinawan sponge Cribrochalina sp., collected from Unten Port, Okinawa. Compounds 154–159 showed potent cytotoxic effects on L1210 cell line with IC50 values (23.0, 18.1, 17.3, 16.0, 11.9 and 11.9 µM, respectively). However, they did not this effect on KB cell line (IC50 > 20 µg/mL) [82]. Figure 12 illustrates the chemical structures of 135–159; Table 12 summarizes the cytotoxic evaluation of 135–159.
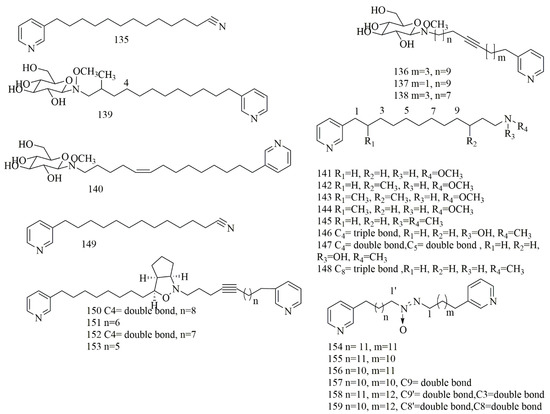
Figure 12.
Pyridine alkaloids from sponges.

Table 12.
Cytotoxic activity of pyridine alkaloids.
2.13. Pyrrole and Bromopyrrole Alkaloids
Stylissa carteri collected from the Red Sea, Hurghada, Egypt, was the source of (−) clathramide C (160) (+)-dibromophakelline (161), (Z)-spongiacidin D (162), (Z)-hymenialdisine (163), (Z)-3-bromohymenialdisine (164) and 3,4-dibromo-1H-pyrrole-2-carbamide (165). Compounds 160–164 were evaluated for their cytotoxic activity against L5178Y and HCT116 cell lines. Regarding L5178Y cell line, 161 and 164 displayed cytotoxic effects with inhibition of growth 57.0% and 60.5%, respectively (10 μg/mL), while the cytotoxicity of 160, 162, 163 and 165 were not potent (growth inhibition of 25.3%, 36.7%, 37.0% and 38.4%, respectively) [83]. Oroidin (166), a pyrrole alkaloid from Agelas oroides., showed the cytotoxic activity with the GI50 of 42 µM on MCF-7, 24 µM on A2780 cells and more than 50 µM on HT29, SW480, H460, A431, Du145, BE2-C, SJ-G2, MIA, SMA, and U87 cell lines [84]. Figure 13 illustrates the chemical structures of 160–166; Table 13 summarizes the cytotoxic evaluation of 160–166.
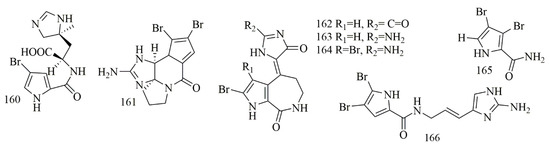
Figure 13.
Pyrrole and bromopyrrole alkaloid structures from sponges.

Table 13.
Cytotoxic effects of pyrrole and bromopyrrole alkaloids.
2.14. Pyrroloiminoquinone Alkaloids
Four different species of marine sponges collected off Algoa bay of South Africa, namely, Tsitsikamma pedunculata, Tsitsikamma favus, Latrunculia bellae, Strongylodesma algoaensis, had been investigated for cytotoxic alkaloids yielding twenty one alkaloidal pigments (167–187) belong to pyrroloiminoquinone class and could be describesd as pyrroloacridine alkaloids, the compounds were identified as 14-bromodiscorhabdin C (167), 14-bromo-3-dihydrodiscorhabdin C (168), 3-dihydrodiscorhabdin C (169), 3-dihydro-7,8-dehydrodiscorhabdin C (170), 14-bromo-3-dihydro-7,8-dehydrodiscorhabdin C (171), discorhabdin V (172), and 14-bromo-1-hydroxydiscorhabdin V (173), from T. pedunculata. Tsitsikammamine A (174), tsitsikammamine B (175), tsitsikammamine A N-18 oxime (176), and tsitsikammamine B N-18 oxime (177), from T.favus. 1-Methoxydiscorhabdin D (178), 1-aminodiscorhabdin D (179), damirone B (180), makaluvic acid A (181), makaluvamine C (182), discorhabdin G (183), and discorhabdin N (184), from L. bellae. Discorhabdin A (185), discorhabdin D (186), discorhabdin H (187), from S. algoaensis. All the purified compounds 167–187 revealed potent cytotoxic activity against HCT-116 cell lines. Among them, 185 was the most potent with IC50 value of 0.007 µM, the tsitsikammamines intercalate DNA and cleave DNA via inhibition of topoisomerase I [85]. Further discorhabdins were reported from the Patagonian sponge Latrunculia brevis, collected from Patagonia, Argentina. These compounds identified by spectroscopic analysis as discorhabdin L (188), and discorhabdin I (189), showed potent cytotoxic effects on the HT-29 cell line with GI50 values of 0.12 and 0.35 μM, respectively [86]. Chemical investigation on Higginsia sp., collected from Deal island led to the purification of 185 and 186. In addition, (+)-dihydrodiscorhabdin A (190), (+)-debromodiscorhabdin A (191), was obtained from Spongosorites sp., collected from Port Campbell along with (+)-discorhabdin X (192), makaluvamine J (193), damirone A (194), and (+)-dihydrodiscorhabdin L (195). Compounds 185, 190, 193–195 were subjected to cytotoxic assay against a panel of cell lines. Compounds 193–194 showed the least activity (IC50 5–10 μg/mL). Compounds 190 and 195 showed more potent activity with IC50 0.1–0.5 μg/mL, and finally, 185 showed the most potent cytotoxicity with IC50 0.05–0.1 μg/mL [87]. Deniz Tasdemir et al. investigated the Philippine sponge Smenospongia sp. This study ended with the isolation of makaluvamine O (196). This compound demonstrated activity with IC50 values of 71, 79, 94, and 8.6 µM against p53+/+, p53−/−, p21+/+, and p21−/− cell lines [63]. Makaluvamine P (197), was reported from Zyzzya cf. fuliginosa, collected from the coasts of Vanuatu Islands. Compound 197 demonstrated an inhibitory effect on the KB cell line with IC50 values of 1.4 µM [88]. Investigation of the Caribbean sponge Batzella sp., yielded eight cytotoxic prroloiminoquinone alkaloids that identified as batzelline A (198), batzelline B (199), isobatzelline A (200), isobatzelline C (201), isobatzelline D (202), isobatzelline E (203), secobatzelline A (204), and secobatzelline B (205). The results proved the cytotoxic activity of 198–205 against four diverse cell lines: Panc-1, AsPC-1, BxPC-3, and MIA-PaCa2. Compounds 200–203 displayed cytotoxicity in all pancreatic cell lines with IC50 of less than 10 μM. Also, their cytotoxic activities on AsPC-1, BxPC3, and MIA PaCa2 had greater activity in comparison with 5-fluorouracil, a current treatment for pancreatic cancer. Interestingly, the batzellines showed less cytotoxicity in the Vero cells, which is a preferential cytotoxic ability towards tumor cell lines [89]. Figure 14 illustrates the chemical structures of 167–205; Table 14 summarizes the cytotoxic evaluation of 167–205.
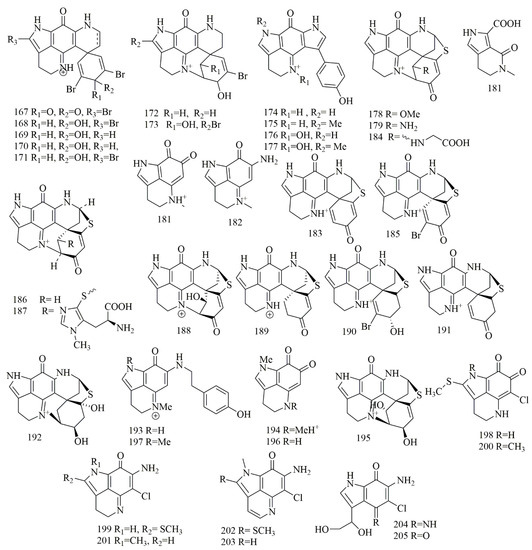
Figure 14.
Pyrroloiminoquinone alkaloids structures isolated from sponges.

Table 14.
Cytotoxic activity of pyrroloiminoquinone alkaloids in sponges.
2.15. Quinoline and Quinolizinde Alkaloids
Renierol (206), a cytotoxic quinoline alkaloid, was isolated from the blue hard sponge Xestospongia collected at Sand Island, Suva Harbor, Fiji. Compound 206 had displayed potent cytotoxic activity against L1210 cell line with IC50 of 9.5 µM [90]. Lihouidine (207), is a spiro nonacyclic polyaromatic cytotoxic alkaloid isolated from Suberea sponge, obtained from Lihou reef in the Coral Sea, showed moderate cytotoxicity against P388D with IC50 3 µg/mL (5 µM) [91]. A specimen of Reniera sarai collected from the Naples gulf was the source of six quinolizidine alkaloids identified as saraine A (208), saraine B (209), saraine C (210), saraine 1(211), saraine 2 (212), and saraine 3 (213). Compounds 208–214 displayed preliminary cytotoxicity in the brine shrimp cytotoxic bioassay, revealing that compounds 211–213 were more powerfully cytotoxic (2.5 < LD50 > 6.4 μg/mL) than 208–210 (4.5 < LD50 > 46.7 μg/mL) [92]. Compounds 208–213 could also be classified as piperidine alkaloids. Eight cytotoxic macrocyclic bis-quinolizidine alkaloids 214–221 were identified from Xestospongia muta, collected off the coral reef of Vinh Moc, Quang Tri, Vietnam. The other isolated compounds were identified as meso-araguspongine C (214) araguspongines A, C, E, L, N−P (215–221). The result of cytotoxic assay proved 214–221 their cytotoxic activities against human cancer cell lines HepG-2, HL-60, LU-1, MCF-7, and SK-Mel-2. Stereochemistry of 216 and its meso-isomer 214 were established by NMRspectroscopy and conformational analyses. Results showed outstanding cytotoxic activities with IC50 values of the range of 0.43 ± 0.03 to 1.02 ± 0.11 μM, compared to positive control ellipticine (IC50 value of 1.18 ± 0.12 to 1.91 ± 0.28 μM) [93]. Figure 15 illustrates the chemical structures of 206–221; Table 15 summarizes the cytotoxic evaluation of 206–221.
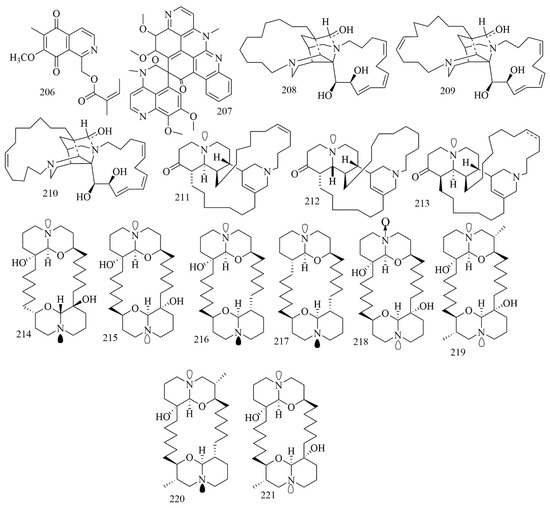
Figure 15.
Quinoline and quinolizidine alkaloid structures in sponges.

Table 15.
Cytotoxic activity of quinoline and quinolizidine alkaloids.
2.16. Tetrahydroisoqouinoline Alkaloids
Based on the study by Sirimangkalakitti et al., bistetrahydroisoquinoline alkaloid, including renieramycin M (222), are mainly identified from sponges of the genera Reniera, Haliclona, Xestospongia, Neopetrosia, and Cribrochalina. Other renieramycin-type alkaloids, such as jorunnamycin A (223), were purified from the sponge-eating nudibranch Jorunnaf unebris, 222–223 were also reported from the Thai blue sponge Xestospongia sp., collected off Sichang island, the Gulf of Thailand. Compounds 222–223 revealed outstanding cytotoxic activity versus H292 cell line with IC50 (23 ± 4 and 220 ± 20 nM, respectively) and H460 cell line with IC50 8.3 ± 0.6 and 160 ± 10 nM, respectively [94]. Renieramycin J (224), which has been reported from Neopetrosia sp., collected off Kuchinoerabu-jima island also showed outstanding cytotoxicity on 3Y1, HeLa, and P388 cells with IC50 values 5.3, 12.3, and 0.53 nM, respectively [95]. Figure 16 illustrates the chemical structures of 222–224; Table 16 summarizes the cytotoxic evaluation of 222–224.
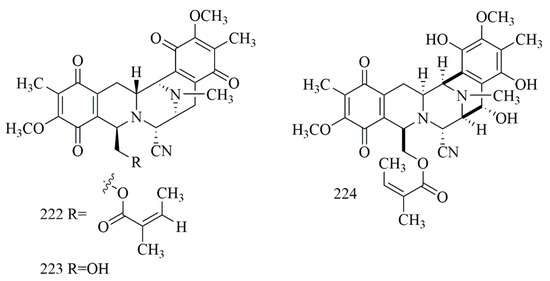
Figure 16.
Tetrahydroisoqouinoline alkaloid structures isolated from sponges.

Table 16.
Cytotoxic activity of tetrahydroisoqouinoline alkaloids.
2.17. Steroidal Alkaloid
Four steroidal alkaloids have been reported from the Philippine sponge Corticium niger, collected off Boracay island. The compounds were elucidated by interpretation of spectroscopic data as plakinamine I-K (225–227) and dihydroplakinamine K (228). Compounds 225–228 showed potent cytotoxicity against HCT-116 cell lines, while 227 and 228 exhibited 1.4 µM as an IC50 value, 225 and 226 showed IC50 10.6 and 6.1 µM, respectively [96]. Sunassee et al. investigated the same sponge, which yielded more steroidal alkaloids plakinamine N (229) and plakinamine O (230), along with 225 and 226. Compounds 229, 230, and 226 were tested for antiproliferative activity in the NCI-60 panel. The test showed enhanced inhibitory effects versus all of the colon cell lines with mean GI50 values of 11.5, 2.4, and 1.4 μM, respectively [97]. Figure 17 illustrates the chemical structures of 225–230; Table 17 summarizes the cytotoxic evaluation of 225–230.
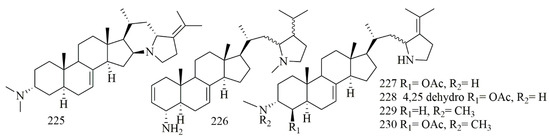
Figure 17.
Steroid alkaloid structures isolated from marine sponges.

Table 17.
Cytotoxic activity of Steroidal alkaloids.
2.18. Manzamine Alkaloids
Samoylenko et al. have reported two tertiary bases, (+)-8-hydroxymanzamine A (231) and (+)-manzamine A (232). In addition to their hydrochloride salts 231a and 232a, from Acanthostrongylophora ingens known for the biosynthesis of broad range of manzamine alkaloids as natural hydrochloride salts. Cytotoxic activity of 231, 232 and their salts versus a panel of cell lines were tested. The hydrochloride salt 231a was more toxic to HepG2, in comparison with 232 with IC50 1.55 vs. 4.4 μg/mL, as well as being 3-fold more toxic than 232 towards kidney epithelial (non-cancer) cells (IC50 0.69 vs. 2.15 μg/mL) [98]. Figure 18 illustrates the chemical structures of 231–232; Table 18 summarizes the cytotoxic evaluation of 231–232.
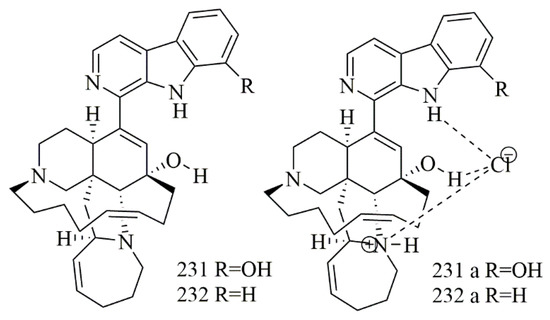
Figure 18.
Manzamine alkaloid structures isolated from sponges.

Table 18.
Cytotoxic activity of manzamine alkaloids.
2.19. Diterpene Alkaloid
Paige Stout et al. isolated nine diterpene alkaloids from the Caribbean sponge Agelas citrina, among which only agelasine E (233) showed potent cytotoxicity against CLL cell line with IC50 = 10 μM [99]. Guangmin Yao et al. isolated cytotoxic sulfated sesterterpene alkaloid 19-oxofasciospongine A (234) from Fasciospongia sp., and 234 demonstrated cytotoxic effect versus LNCaP, LU-1, and MCF-7 cell lines with IC50 21.8, 5.0 and 13.4 µM, respectively [100]. Chu and coworkers reported three cytotoxic diterpene alkaloids iso-agelasine C (235) agelasine J (236), and nemoechine G (237) from Agelas nakamurai collected off the South China Sea. The results confirmed the cytotoxicity of 235 versus HCT-116, K562, and HL-60, cell lines with IC50 values of 19.8, 16.0, and 12.4µM, respectively [101]. Figure 19 illustrates the chemical structures of 233–237; Table 19 summarizes the cytotoxic evaluation of 233–237.
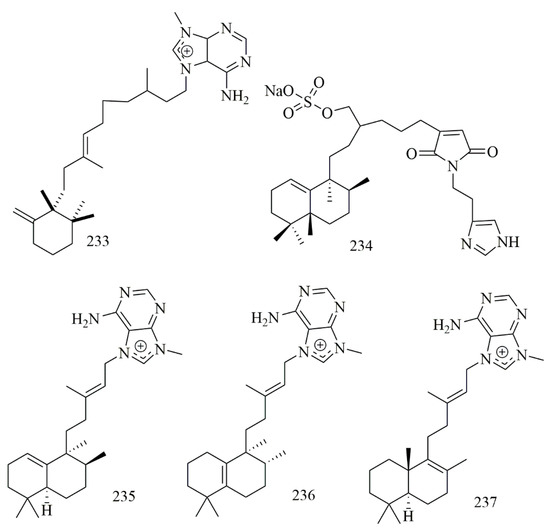
Figure 19.
Diterpen alkaloid structures isolated from sponges.

Table 19.
Cytotoxic activity of diterpen alkaloids.
2.20. Sesquiterpene Quinones/Hydroquinones Alkaloid
The sponge Dysidea avara is a great source of sesquiterpene quinones/hydroquinone alkaloid, which the isolated compounds showed antileishmanial, antiplasmodial, antischistosomal, and cytotoxic activity [102]. The sesquiterpene(−)-4’-methylaminoavarone (238). (−)-N-methylmelemeleone-A (239), and (−)-3’-methylaminoavarone (240) isolated from Dysidea avara collected from the Mediterranean Sea in Fethiye, Turkey. The results revealed that compound 240 proved to be highly toxic, while 239 displayed very weak cytotoxic activity L5178Y cell line. The results from HCT116 cells revealed weak toxicity for 238–240 were IC50-values of 9,> 50, 45 µM, respectively. Results from H4IIE cells exhibited similar results—239 demonstrated weak cytotoxic activity with IC50- value more than 50 µM, while 238 and 240 showed IC50-values of 40 and25 µM, respectively [103]. Figure 20 illustrates the chemical structures of 238–240; Table 20 summarizes the cytotoxic evaluation of 238–240.
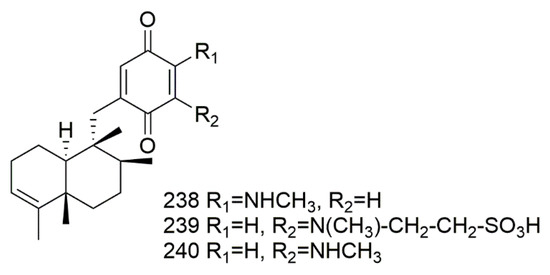
Figure 20.
Sesquiterpene quinones/hydroquinones alkaloid structures isolated from sponges.

Table 20.
Cytotoxic activity of sesquiterpene quinones/hydroquinones alkaloids.
3. Conclusions
Isolation and identification of new cytotoxic alkaloids extracted from marine animals, particularly sponges, is a multidisciplinary and intricate endeavor. This is because of the lack of knowledge on traditional experiential and ethnopharmacology investigations that have endorsed this zoochemical scrutiny. Hence, not only is it fundamental to assemble a database to address the shortcomings in the scholarly publication, but it is also bound to be on natural products identification. The representative survey depicted the study on marine cytotoxic alkaloid originated from sponge accomplished between 1987 and 2020 in different resources and databases had been investigated, including Scifinder (database for the chemical literature) CAS (Chemical Abstract Service) search, web of science, Marin Lit (marine natural products research) database. This investigation ended in 80 closely-correlated articles, certifying, as an example, the considerable chemodiversity of sponges as original of secondary cytotoxic metabolite. During these 33 years database search, natural products originated, including novel, new, and known compounds, were reported as cytotoxic chemical structures. Several of these structures have demonstrated authenticated in vitro cytotoxic bioactivities versus a panel of cancer/tumor cell lines. In addition, several of them are attracting the interest for future in vivo assay. The main chemical classes of these alkaloid structures were confined, particularly to the Pyrroloiminoquinone alkaloids (16.4%), Guanidine alkaloids (13.9%), Indole alkaloids (12.6%), Pyridine alkaloids (10.6%), and Bromotyrosine alkaloids (8.9%)—demonstrating the five major chemical classes of alkaloid structures observed in sponges during this time, which along with other chemical classes as Quinolizidine and Brominated alkaloids, and others (47.6%), exhibited a various range of cytotoxicity activity. Verongida, Poecilosclerida, Agelasida, Haplosclerida, Halichondrida, Dictyonellidae, and Homosclerophorida were the sponge’s main orders in this review, and were also discovered to be the most prosperous genera, due to their potential to produce promising bioactive alkaloid compounds. The examination of the contribution from an individual species revealed that regardless of the order, each species contributed, on average, 2–5 new compounds. In addition, alkaloids with cytotoxic activities have been isolated from 49 genus of sponges. Genus Agelas was discovered to be the most lucrative producer of potent alkaloid compounds, then Biemna, Suberea, and Pachychalina were established. The geniuses Mycale, kirkpatrickia, Scleritoderma, Zyzzya, and Dercitus only had one cytotoxic alkaloid. Among the reported alkaloid in this review, Dercitine (acridin alkaloid), Crambescidin 814 (guanidine alkaloid), Crambescidin 816 (guanidine alkaloid), Unguiculin A-C (guanidine alkaloid), Dihydrodiscorhabdin A and L (Pyrroloiminoquinone alkaloid), Discorhabdin A (Pyrroloiminoquinone alkaloid), Renierol (quinolone alkaloid), Renieramycin(quinolone alkaloid), Renieramycin J (quinolone alkaloid) and Jurunnamycin A (quinolone alkaloid) showed the best cytotoxic activity in micro molar to nano molar ranges.
Evaluation of cytotoxicity is requisite to approach the cytotoxic level of alkaloid structures. In this survey, the evaluation of cytotoxicity of the alkaloid compounds, extracted from marine sponges, was determined through the most usual panels of cytotoxic cell lines—comprised of either in human [breast cancer (MCF-7 and MDA-MB-231), colorectal carcinoma HCT-116, HCT15, HCT8, Sw620, and HCC-2998), alveolar adenocarcinomic (A549), lung Adenocarcinoma (PC9), lung fibroblast (MALME3M), fibroblast (FS4-LTM and MRC-5), leukemia (THP-1, HL-60, K562, MOLT-4, and CCRF-CEM), cervical cells (Hela), ovarian cell line (A2780, OVCAR-3 and SK-OV-3), melanoma (SK-MEL-2), renal (A498 and UO-31), melanoma (SK-MEL-2 and MM.1S), Glioblastoma (U138 and SF-295), liver (HepG2 and Huh7), PSN1 (pancreatic), and CNS solid tumors (XF498)], or murine [lymphocytic leukemia (L1210and P388D1); melanoma (B16 and B16F10)] cancerous cell-lines.
Nevertheless, in infrequent studies were wide-range and have consisted of the NCI-60 panel of cell lines for screening anticancer characteristics of the drug. These kinds of screening system are great implements for clarifying the relationship between cytotoxicity and anticancer drugs, for the exploration of molecular-targeted antineoplastic drugs, and improve the compounds, selection as candidates for antineoplastic drug. Even though it was not the object of this survey to consider preclinical and clinical perspective of the cytotoxic isolated alkaloids, it is obvious that the present gap with regard to the natural approachability of these cytotoxic alkaloids to carry out studies, since they need higher amounts of compounds. As far as the amounts of substances are concerned, preparing synthetic compounds influences the preclinical phase for drug candidates. Synthesis of natural products and their chemical derivatives display the potential to assert or modify isolated alkaloids, as well as to prepare effective analogs. Sponge aquatic environment and microorganisms engineering are attracted interesting to support these kinds of compounds. Sufficient production of alkaloids derived from sponges is a prosperous approach that requires more attention in future to consider the constraints regarding the supply of drugs, attained from marine organism. In conclusion, this review is able to point out the importance of cytotoxic alkaloids to explore new, effective anticancer drugs.
Author Contributions
Conceptualization, P.M.P.; methodology, M.L.A. and P.M.P.; investigation, N.M.; writing-original draft preparation, E.S.D., A.M.E., and N.M.; writing-review and editing, M.L.A., A.M.E., and P.M.P.; supervision, M.L.A. and P.M.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gul, W.; Hamann, M.T. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005, 78, 442–453. [Google Scholar] [CrossRef]
- Fattorusso, E.; Taglialatela-Scafati, O. Modern Alkaloids: Structure, Isolation, Synthesis, And Biology; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine Indole Alkaloids: Potential New Drug Leads for the Control of Depression and Anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Casertano, M.; Menna, M.; Imperatore, C. The Ascidian-Derived Metabolites with Antimicrobial Properties. Antibiotics 2020, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Yamashita, T.; Tsukamoto, S.; Fusetani, N. Three New Antibacterial Alkaloids from a Marine SpongeStellettaSpecies. J. Nat. Prod. 1999, 62, 1202–1204. [Google Scholar] [CrossRef]
- Baker, B.J.; Scheuer, P.J.; Shoolery, J.N. Papuamine, an antifungal pentacyclic alkaloid from a marine sponge, Haliclona sp. J. Am. Chem. Soc. 1988, 110, 965–966. [Google Scholar] [CrossRef]
- Perry, N.B.; Ettouati, L.; Litaudon, M.; Blunt, J.W.; Munro, M.H.; Parkin, S.; Hope, H. Alkaloids from the antarctic sponge Kirkpatrickia varialosa.: Part 1: Variolin b, a new antitumour and antiviral compound. Tetrahedron 1994, 50, 3987–3992. [Google Scholar] [CrossRef]
- Johnson, T.A.; Sohn, J.; Vaske, Y.M.; White, K.N.; Cohen, T.L.; Vervoort, H.C.; Tenney, K.; Valeriote, F.A.; Bjeldanes, L.F.; Crews, P. Myxobacteria versus sponge-derived alkaloids: The bengamide family identified as potent immune modulating agents by scrutiny of LC–MS/ELSD libraries. Bioorganic Med. Chem. 2012, 20, 4348–4355. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Saraswati, S.; Mathur, R.; Pandey, M. Brucine, a plant derived alkaloid inhibits inflammatory angiogenesis in a murine sponge model. Biomed. Prev. Nutr. 2011, 1, 180–185. [Google Scholar] [CrossRef]
- Xu, M.; Andrews, K.T.; Birrell, G.W.; Tran, T.L.; Camp, D.; Davis, R.A.; Quinn, R.J. Psammaplysin H, a new antimalarial bromotyrosine alkaloid from a marine sponge of the genus Pseudoceratina. Bioorganic Med. Chem. Lett. 2011, 21, 846–848. [Google Scholar] [CrossRef]
- Ravichandran, S.; Kathiresan, K.; Balaram, H. Anti-malarials from marine sponges. Biotechnol. Mol. Biol. Rev. 2007, 2, 33–38. [Google Scholar]
- Munro, M.H.G.; Blunt, J.W.; Dumdei, E.J.; Hickford, S.J.; Lill, R.E.; Li, S.; Battershill, C.N.; Duckworth, A.R. The discovery and development of marine compounds with pharmaceutical potential. J. Biotechnol. 1999, 70, 15–25. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T.; Srebnik, M. Lipid compounds of freshwater sponges: Family Spongillidae, class Demospongiae. Chem. Phys. Lipids 2003, 123, 117–155. [Google Scholar] [CrossRef]
- Vacelet, J. Diversity and evolution of deep-sea carnivorous sponges. Porifera research: Biodiversity, innovation and sustainability. Série Livros 2007, 28, 107–115. [Google Scholar]
- Mioso, R.; Marante, F.J.T.; Bezerra, R.S.; Borges, F.V.P.; Santos, B.V.D.O.; De Laguna, I.H.B. Cytotoxic Compounds Derived from Marine Sponges. A Review (2010–2012). Molecules 2017, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Peng, J.; Hamann, M.T.; Hu, J.-F. Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms. Chem. Rev. 2007, 108, 264–287. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Sujatha, S. Pharmacologically important natural products from marine sponges. J. Nat. Prod. 2011, 4, 5–12. [Google Scholar]
- Kim, S.K.; Kalimuthu, S. Introduction to anticancer drugs from marine origin. In Handbook of Anticancer Drugs from Marine Origin; Springer: Cham, Switzerland, 2015; pp. 1–13. [Google Scholar]
- Han, B.N.; Hong, L.L.; Gu, B.B.; Sun, Y.T.; Wang, J.; Liu, J.T.; Lin, H.W. Natural Products from Sponges. In Symbiotic Microbiomes of Coral Reefs Sponges and Corals; Springer: Dordrecht, The Netherlands, 2019; pp. 329–463. [Google Scholar]
- Hanan, M.A.; Musarat, A. Marine sponge alkaloids: A source of novel anticancer agents. In Phytochemistry: Volume 3: Marine Sources, Industrial Applications, and Recent Advances; Egbuna, C., Chinenye Ifemeje, J., Kumar, S., Sharif, N., Eds.; Apple Academic Press Inc.: Palm Bay, FL, USA, 2018; pp. 35–64. [Google Scholar]
- Burres, N.S.; Sazesh, S.; Gunawardana, G.P.; Clement, J.J. Antitumor activity and nucleic acid binding properties of dercitin, a new acridine alkaloid isolated from a marine Dercitus species sponge. Cancer Res. 1989, 49, 5267–5274. [Google Scholar]
- Thale, Z.; Johnson, T.; Tenney, K.; Wenzel, P.J.; Lobkovsky, E.; Clardy, J.; Media, J.; Pietraszkiewicz, H.; Valeriote, F.A.; Crews, P. Structures and Cytotoxic Properties of Sponge-Derived Bisannulated Acridines. J. Org. Chem. 2002, 67, 9384–9391. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A. Ingenine E, a new cytotoxic β-carboline alkaloid from the Indonesian sponge Acanthostrongylophora ingens. J. Asian Nat. Prod. Res. 2017, 19, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Göthel, Q.; Sirirak, T.; Köck, M. Bromotyrosine-derived alkaloids from the Caribbean sponge Aplysina lacunosa. Beilstein J. Org. Chem. 2015, 11, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, G.; Santamaría, G.; Cruz, P.G.; Fernández, R.; Pérez, M.; Martínez-Leal, J.F.; Rodríguez, J.; Jiménez, C.; Cuevas, C. Cytotoxic Anomoian B and Aplyzanzine B, New Bromotyrosine Alkaloids from Indonesian Sponges. ACS Omega 2017, 2, 3494–3501. [Google Scholar] [CrossRef]
- Kurimoto, S.I.; Seino, S.; Fromont, J.; Kobayashi, J.I.; Kubota, T. Ma’edamines C and D, New Bromotyrosine Alkaloids Pos-sessing a Unique Tetrasubstituted Pyridinium Moiety from an Okinawan Marine Sponge Suberea sp. Org. Lett. 2019, 21, 8824–8826. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Addepalli, R.; Avery, V.M.; Hooper, J.N.; Quinn, R.J. Psammaplysenes C and D, cytotoxic alkaloids from Psammoclemma sp. J. Nat. Prod. 2007, 70, 1827–1829. [Google Scholar] [CrossRef] [PubMed]
- Tabudravu, J.N.; Jaspars, M. Purealidin S and purpuramine J, bromotyrosine alkaloids from the Fijian marine sponge Druinella sp. J. Nat. Prod. 2002, 65, 1798–1801. [Google Scholar] [CrossRef]
- Tsuda, M.; Sakuma, Y.; Kobayashi, J. Suberedamines A and B, New Bromotyrosine Alkaloids from a SpongeSubereaSpecies. J. Nat. Prod. 2001, 64, 980–982. [Google Scholar] [CrossRef]
- Tilvi, S.; Moriou, C.; Martin, M.T.; Gallard, J.F.; Sorres, J.; Patel, K.; Petek, S.; Debitus, C.; Ermolenko, L.; Al-Mourabit, A. Agelastatin E, agelastatin F, and benzosceptrin C from the marine sponge Agelas dendromorpha. J. Nat. Prod. 2010, 73, 720–723. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Sulaiman, M.; Khedr, A. Bioactive Secondary Metabolites from the Red Sea Marine Verongid Sponge Suberea Species. Mar. Drugs 2015, 13, 1621–1631. [Google Scholar] [CrossRef]
- Rubnov, S.; Chevallier, C.; Thoison, O.; Debitus, C.; Laprévote, O.; Guénard, D.; Sévenet, T. Echinosulfonic acid D: An ESI MS n evaluation of a new cytotoxic alkaloid from the New-Caledonian sponge Psammoclemma sp. Nat. Prod. Res. 2005, 19, 75–79. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Yamanokuchi, R.; Yoshitomi, M.; Sato, K.; Ikeda, T.; Rotinsulu, H.; Mangindaan, R.E.P.; De Voogd, N.J.; Van Soest, R.W.M.; Yokosawa, H. Aaptamine, an alkaloid from the sponge Aaptos suberitoides, functions as a proteasome inhibitor. Bioorganic Med. Chem. Lett. 2010, 20, 3341–3343. [Google Scholar] [CrossRef]
- Liu, C.; Tang, X.; Li, P.-L.; Li, G.-Q. Suberitine A–D, Four New Cytotoxic Dimeric Aaptamine Alkaloids from the Marine Sponge Aaptos suberitoides. Org. Lett. 2012, 14, 1994–1997. [Google Scholar] [CrossRef]
- Shubina, L.K.; Makarieva, T.N.; Guzii, A.G.; Denisenko, V.A.; Popov, R.S.; Dmitrenok, P.S.; Stonik, V.A. Absolute configuration of the cytotoxic marine alkaloid monanchocidin A. J. Nat. Prod. 2018, 81, 1113–1115. [Google Scholar] [CrossRef]
- Guzii, A.G.; Makarieva, T.N.; Denisenko, V.A.; Dmitrenok, P.S.; Kuzmich, A.S.; Dyshlovoy, S.A.; Krasokhin, V.B.; Stonik, V.A. Monanchocidin: A New Apoptosis-Inducing Polycyclic Guanidine Alkaloid from the Marine Sponge Monanchora pulchra. Org. Lett. 2010, 12, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Tabakmaher, K.M.; Guzii, A.G.; Denisenko, V.A.; Dmitrenok, P.S.; Shubina, L.K.; Kuzmich, A.S.; Lee, H.S.; Stonik, V.A. Monanchocidins B–E: Polycyclic guanidine alkaloids with potent antileukemic activities from the sponge Monanchora pulchra. J. Nat. Prod. 2011, 74, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A.; Moriou, C.; Martin, M.-T.; Rodrigues-Stien, A.D.S.; Petek, S.; Demoy-Schneider, M.; Hall, K.; Hooper, J.N.A.; Debitus, C.; Al-Mourabit, A. Cytotoxic Guanidine Alkaloids from a French Polynesian Monanchora n. sp. Sponge. J. Nat. Prod. 2016, 79, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, J.A.; López-Alonso, H.; Roel, M.; Vieytes, M.R.; Thomas, O.; Ternon, E.; Vega, F.V.; Botana, L.M. Mechanism of cytotoxic action of crambescidin-816 on human liver-derived tumour cells. Br. J. Pharmacol. 2014, 171, 1655–1667. [Google Scholar] [CrossRef]
- Mendez, A.G.; Juncal, A.B.; Silva, S.B.L.; Thomas, O.P.; Vázquez, V.M.; Alfonso, A.; Vieytes, M.R.; Vale, C.; Botana, L.M. The Marine Guanidine Alkaloid Crambescidin 816 Induces Calcium Influx and Cytotoxicity in Primary Cultures of Cortical Neurons through Glutamate Receptors. ACS Chem. Neurosci. 2017, 8, 1609–1617. [Google Scholar] [CrossRef]
- Kasmiati, K.; Yoshioka, Y.; Okamoto, T.; Ojika, M. New Crambescidin-Type Alkaloids from the Indonesian Marine Sponge Clathria bulbotoxa. Mar. Drugs 2018, 16, 84. [Google Scholar] [CrossRef]
- Tabakmakher, K.M.; Makarieva, T.N.; Denisenko, V.A.; Guzii, A.G.; Dmitrenok, P.S.; Kuzmich, A.S.; Stonik, V.A. Normonanchocidins A, B and D, New Pentacyclic Guanidine Alkaloids from the Far-Eastern Marine Sponge Monanchora pulchra. Nat. Prod. Commun. 2015, 10, 913–916. [Google Scholar] [CrossRef]
- Tabakmakher, K.M.; Denisenko, V.A.; Guzii, A.G.; Dmitrenok, P.S.; Dyshlovoy, S.A.; Lee, H.-S.; Makarieva, T.N. Monanchomycalin C, a New Pentacyclic Guanidine Alkaloid from the Far-Eastern Marine Sponge Monanchora Pulchra. Nat. Prod. Commun. 2013, 8, 1399–1402. [Google Scholar] [CrossRef]
- Shubina, L.K.; Makarieva, T.N.; Von Amsberg, G.; Denisenko, V.A.; Popov, R.S.; Dyshlovoy, S.A. Monanchoxymycalin C with anticancer properties, new analogue of crambescidin 800 from the marine sponge Monanchora pulchra. Nat. Prod. Res. 2019, 33, 1415–1422. [Google Scholar] [CrossRef]
- Gros, E.; Al-Mourabit, A.; Martin, M.T.; Sorres, J.; Vacelet, J.; Frederich, M.; Aknin, M.; Kashman, Y.; Gauvin-Bialecki, A. Netamines H–N, Tricyclic Alkaloids from the Marine Sponge Biemna laboutei and Their Antimalarial Activity. J. Nat. Prod. 2014, 77, 818–823. [Google Scholar] [CrossRef]
- Gros, E.; Martin, M.-T.; Sorres, J.; Moriou, C.; Vacelet, J.; Frederich, M.; Aknin, M.; Kashman, Y.; Gauvin-Bialecki, A.; Al-Mourabit, A. Netamines O-S, Five New Tricyclic Guanidine Alkaloids from the Madagascar Sponge Biemna laboutei, and Their Antimalarial Activities. Chem. Biodivers. 2015, 12, 1725–1733. [Google Scholar] [CrossRef]
- Bouaicha, N.; Amade, P.; Puel, D.; Roussakis, C. Zarzissine, a New Cytotoxic Guanidine Alkaloid from the Mediterranean Sponge Anchinoe paupertas. J. Nat. Prod. 1994, 57, 1455–1457. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Moriou, C.; Martin, M.-T.; Petek, S.; Debitus, C.; Al-Mourabit, A. Unguiculins A-C: Cytotoxic bis-guanidine alkaloids from the French Polynesian sponge, Monanchora n. sp. Nat. Prod. Res. 2018, 32, 1512–1517. [Google Scholar] [CrossRef]
- Tang, W.Z.; Yang, Z.Z.; Sun, F.; Wang, S.P.; Yang, F.; Jiao, W.H.; Lin, H.W. (−)-Calcaridine B, a new chiral aminoimidazole-containing alkaloid from the marine sponge Leucetta chagosensis. J. Asian Nat. Prod. Res. 2019, 21, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hu, X.; Yu, R.; Wan, S.-B.; Jiang, T. Efficient Total Synthesis of Lissodendrin B, 2-Aminoimidazole Marine Alkaloids Isolated from Lissodendoryx (Acanthodoryx) fibrosa. Mar. Drugs 2020, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-Z.; Yang, Z.-Z.; Sun, F.; Wang, S.-P.; Yang, F.; Lin, H.W. Leucanone A and naamine J, glycerol ether lipid and imidazole alkaloid from the marine sponge Leucandra sp. J. Asian Nat. Prod. Res. 2016, 19, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kawabata, T.; Kato, H.; Ohta, T.; Rotinsulu, H.; Mangindaan, R.E.P.; Van Soest, R.W.M.; Ukai, K.; Kobayashi, H.; Namikoshi, M. Naamidines H and I, Cytotoxic Imidazole Alkaloids from the Indonesian Marine Sponge Leucetta chagosensis. J. Nat. Prod. 2007, 70, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; Kehraus, S.; König, G.M.; Wörheide, G.; Wright, A.D. New and Biologically Active Imidazole Alkaloids from Two Sponges of the Genus Leucetta. J. Nat. Prod. 2002, 65, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Ralifo, P.; Tenney, K.; Valeriote, F.A.; Crews, P. A Distinctive Structural Twist in the Aminoimidazole Alkaloids from a Calcareous Marine Sponge: Isolation and Characterization of Leucosolenamines A and B. J. Nat. Prod. 2007, 70, 33–38. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Yin, F.; De Voogd, N.J.; Chen, X.; Cheng, W.; Lin, W. Chagosendines A—C, New Metal Complexes of Imidazole Alkaloids from the Calcareous Sponge Leucetta chagosensis. Chem. Biodivers. 2018, 15, e1700481. [Google Scholar] [CrossRef] [PubMed]
- Charan, R.D.; Mckee, T.C.; Boyd, M.R. Cytotoxic alkaloids from the marine sponge Thorectandra sp. Nat. Prod. Res. 2004, 18, 225–229. [Google Scholar] [CrossRef]
- Hitora, Y.; Takada, K.; Ise, Y.; Okada, S.; Matsunaga, S. Dragmacidins G and H, Bisindole Alkaloids Tethered by a Guanidino Ethylthiopyrazine Moiety, from a Lipastrotethya sp. Marine Sponge. J. Nat. Prod. 2016, 79, 2973–2976. [Google Scholar] [CrossRef]
- Endo, T.; Tsuda, M.; Fromont, J.; Kobayashi, J. Hyrtinadine A, a Bis-indole Alkaloid from a Marine Sponge. J. Nat. Prod. 2007, 70, 423–424. [Google Scholar] [CrossRef]
- Youssef, D.T. Hyrtioerectines A−C, Cytotoxic Alkaloids from the Red Sea Sponge Hyrtios erectus. J. Nat. Prod. 2005, 68, 1416–1419. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Rateb, M.E.; Amin, E.; Mohammed, T.A.; El-Mesery, M.; Bin Muhsinah, A.; Alsayari, A.; et al. Bioactive brominated oxindole alkaloids from the Red Sea sponge Callyspongia siphonella. Mar. Drugs 2019, 17, 465. [Google Scholar] [CrossRef]
- Tasdemir, D.; Bugni, T.S.; Mangalindan, G.C.; Concepción, G.P.; Harper, M.K.; Ireland, C.M. Cytotoxic bromoindole derivatives and terpenes from the Philippine marine sponge Smenospongia sp. Z. Nat. C J. Biosci. 2002, 57, 914–922. [Google Scholar] [CrossRef]
- Tran, T.D.; Cartner, L.K.; Bokesch, H.R.; Henrich, C.J.; Wang, X.W.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P.; O’Keefe, B.R.; Gustafson, K.R. NMR characterization of rearranged staurosporine aglycone analogues from the marine sponge Damiria sp. Magn. Reson. Chem. 2019. [Google Scholar] [CrossRef]
- Kim, G.D.; Cheong, O.J.; Bae, S.Y.; Shin, J.; Lee, S.K. 6″-Debromohamacanthin A, a Bis (Indole) Alkaloid, Inhibits Angiogenesis by Targeting the VEGFR2-Mediated PI3K/AKT/mTOR Signaling Pathways. Mar. Drugs 2013, 11, 1087–1103. [Google Scholar] [CrossRef]
- Bao, B.; Sun, Q.; Yao, X.; Hong, J.; Lee, C.O.; Sim, C.J.; Im, K.S.; Jung, J.H. Cytotoxic bisindole alkaloids from a marine sponge Spongosorites sp. J. Nat. Prod. 2005, 68, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.W.; Raventos-Suarez, C.; Bifano, M.; Menendez, A.T.; Fairchild, C.R.; Faulkner, D.J. Scleritodermin A, a Cytotoxic Cyclic Peptide from the Lithistid Sponge Scleritoderma nodosum. J. Nat. Prod. 2004, 67, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Torres, Y.R.; Berlinck, R.G.; Magalhaes, A.; Schefer, A.B.; Ferreira, A.G.; Hajdu, E.; Muricy, G. Arenosclerins A−C and Haliclonacyclamine E, New Tetracyclic Alkaloids from a Brazilian Endemic Haplosclerid Sponge Arenosclera brasiliensis. J. Nat. Prod. 2000, 63, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.H.; Nascimento, A.M.; Kossuga, M.H.; Cavalcanti, B.C.; Pessoa, C.O.; Moraes, M.O.; Macedo, M.L.; Ferreira, A.G.; Hajdu, E.; Pinheiro, U.S.; et al. Cytotoxic alkylpiperidine alkaloids from the Brazilian marine sponge Pachychalina alcaloidifera. J. Nat. Prod. 2007, 70, 538–543. [Google Scholar] [CrossRef]
- Wei, X.; Nieves, K.; Rodriguez, A.D. Neopetrosiamine A, biologically active bis-piperidine alkaloid from the Caribbean sea sponge Neopetrosia proxima. Bioorganic Med. Chem. Lett. 2010, 20, 5905–5908. [Google Scholar] [CrossRef]
- Coello, L.; Martín, M.J.; Reyes, F. 1,5-Diazacyclohenicosane, a New Cytotoxic Metabolite from the Marine Sponge Mycale sp. Mar. Drugs 2009, 7, 445–450. [Google Scholar] [CrossRef]
- De Oliveira, J.H.; Grube, A.; Köck, M.; Berlinck, R.G.; Macedo, M.L.; Ferreira, A.G.; Hajdu, E. Ingenamine G and Cyclostellettamines G−I, K, and L from the New Brazilian Species of Marine Sponge Pachychalina sp. J. Nat. Prod. 2004, 67, 1685–1689. [Google Scholar] [CrossRef]
- Liang, Z.; Sulzmaier, F.J.; Yoshida, W.Y.; Kelly, M.; Ramos, J.W.; Williams, P.G. Neopetrocyclamines A and B, polycyclic diamine alkaloids from the sponge Neopetrosia cf exigua. J. Nat. Prod. 2015, 78, 543–547. [Google Scholar] [CrossRef]
- Kanno, S.I.; Yomogida, S.; Tomizawa, A.; Yamazaki, H.; Ukai, K.; Mangindaan, R.E.; Namikoshi, M.; Ishikawa, M. Papuamine causes autophagy following the reduction of cell survival through mitochondrial damage and JNK activation in MCF-7 human breast cancer cells. Int. J. Oncol. 2013, 43, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Jiménez, C.; Blanco, M.; Tarazona, G.; Fernández, R.; Cuevas, C. Lanesoic Acid: A Cytotoxic Zwitterion from Theonella sp. Org. Lett. 2016, 18, 5832–5835. [Google Scholar] [CrossRef]
- Fresneda, P.M.; Delgado, S.; Francesch, A.; Manzanares, I.; Cuevas, C.; Molina, P. Synthesis and Cytotoxic Evaluation of New Derivatives of the Marine Alkaloid Variolin B. J. Med. Chem. 2006, 49, 1217–1221. [Google Scholar] [CrossRef]
- Zhang, H.; Loveridge, S.T.; Tenney, K.; Crews, P. A new 3-alkylpyridine alkaloid from the marine sponge Haliclona sp. and its cytotoxic activity. Nat. Prod. Res. 2016, 30, 1262–1265. [Google Scholar] [CrossRef]
- Takekawa, Y.; Matsunaga, S.; van Soest, R.W.; Fusetani, N. Amphimedosides, 3-alkylpyridine glycosides from a marine sponge Amphimedon sp. J. Nat. Prod. 2006, 69, 1503–1505. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Takahashi, M.; Matsunaga, S.; Fusetani, N.; Van Soest, R.W. Hachijodines A−G: Seven new cytotoxic 3-alkylpyridine alkaloids from two marine sponges of the Genera Xestospongia and Amphimedon. J. Nat. Prod. 2000, 63, 682–684. [Google Scholar] [CrossRef]
- Arai, M.; Kamiya, K.; Shin, D.; Matsumoto, H.; Hisa, T.; Setiawan, A.; Kotoku, N.; Kobayashi, M. N-Methylniphatyne A, a New 3-Alkylpyridine Alkaloid as an Inhibitor of the Cancer Cells Adapted to Nutrient Starvation, from an Indonesian Marine Sponge of Xestospongia sp. Chem. Pharm. Bull. 2016, 64, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Kubota, T.; Tsuda, M.; Mikami, Y.; Kobayashi, J. Pyrinodemins B-D, Potent Cytotoxic bis-Pyridine Alkaloids from Marine Sponge Amphimedon sp. Chem. Pharm. Bull. 2000, 48, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Kubota, T.; Fromont, J.; Kobayashi, J. Pyrinadines B–G, new bis-pyridine alkaloids with an azoxy moiety from sponge Cribrochalina sp. Bioorganic Med. Chem. 2006, 14, 8415–8419. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.N.E.; Schmitz, R.; Bergermann, A.; Totzke, F.; Kubbutat, M.; Müller, W.E.; Youssef, D.T.; Bishr, M.M.; Kamel, M.; Edrada-Ebel, R.; et al. Bioactive pyrrole alkaloids isolated from the Red Sea: Marine sponge Stylissa carteri. Z. Nat. C 2018, 73, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Dyson, L.; Wright, A.D.; Young, K.A.; Sakoff, J.A.; McCluskey, A. Synthesis and anticancer activity of focused compound libraries from the natural product lead, oroidin. Bioorganic Med. Chem. 2014, 22, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Antunes, E.M.; Beukes, D.R.; Kelly, M.; Samaai, T.; Barrows, L.R.; Marshall, K.M.; Sincich, C.; Davies-Coleman, M.T. Cytotoxic Pyrroloiminoquinones from Four New Species of South African Latrunculid Sponges. J. Nat. Prod. 2004, 67, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Reyes, F.; Martín, R.; Rueda, A.; Fernández, R.; Montalvo, D.; Gómez, C.; Sánchez-Puelles, J.M. Discorhabdins I and L, Cytotoxic Alkaloids from the Sponge Latrunculia b revis. J. Nat. Prod. 2004, 67, 463–465. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.; Capon, R.J. Discorhabdins Revisited: Cytotoxic Alkaloids from Southern Australian Marine Sponges of the Genera Higginsia and Spongosorites. J. Nat. Prod. 2009, 72, 1368. [Google Scholar] [CrossRef]
- Casapullo, A.; Cutignano, A.; Bruno, I.; Bifulco, G.; Debitus, C.; Gomez-Paloma, L.; Riccio, R. Makaluvamine P, a new cytotoxic pyrroloiminoquinone from Zyzzya cf. fuliginosa. J. Nat. Prod. 2001, 64, 1354–1356. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.A.; Johnson, J.D.; Carrier, M.K.; Meyer, C.I.; Pitts, T.P.; Gunasekera, S.P.; Wright, A.E. Selective cytotoxic activity of the marine-derived batzelline compounds against pancreatic cancer cell lines. Anti-Cancer Drugs 2009, 20, 149–155. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.C.; Ireland, C.M. Cytotoxic and Antimicrobial Alkaloids from the Fijian Sponge Xestospongia caycedoi. J. Nat. Prod. 1987, 50, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.F.; McCool, B.J.; Willis, R.H. Lihouidine, a novel spiro polycyclic aromatic alkaloid from the marine sponge Suberea sp. (Aplysinellidae, Verongida). J. Org. Chem 2004, 69, 7791–7793. [Google Scholar] [CrossRef] [PubMed]
- Caprioll, V.; Cimino, G.; De Giulio, A.; Madaio, A.; Scognamiglio, G.; Trivellone, E. Selected biological activities of saraines. Comp. Biochem. Physiol. Part B Comp. Biochem. 1992, 103, 293–296. [Google Scholar] [CrossRef]
- Dung, D.T.; Hang, D.T.T.; Yen, P.H.; Quang, T.H.; Nhiem, N.X.; Tai, B.H.; Minh, C.V.; Kim, Y.C.; Kim, D.C.; Oh, H.; et al. Macrocyclic bis-quinolizidine alkaloids from Xestospongia muta. Nat. Prod. Res. 2019, 33, 400–406. [Google Scholar] [CrossRef]
- Sirimangkalakitti, N.; Chamni, S.; Charupant, K.; Chanvorachote, P.; Mori, N.; Saito, N.; Suwanborirux, K. Chemistry of Renieramycins. 15. Synthesis of 22-O-Ester Derivatives of Jorunnamycin A and Their Cytotoxicity against Non-Small-Cell Lung Cancer Cells. J. Nat. Prod. 2016, 79, 2089–2093. [Google Scholar] [CrossRef]
- Oku, N.; Matsunaga, S.; van Soest, R.W.; Fusetani, N. Renieramycin J, a highly cytotoxic tetrahydroisoquinoline alkaloid, from a marine sponge Neopetrosia sp. J. Nat. Prod. 2003, 66, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Ridley, C.P.; Faulkner, D.J. New Cytotoxic Steroidal Alkaloids from the Philippine Sponge Corticium niger. J. Nat. Prod. 2003, 66, 1536–1539. [Google Scholar] [CrossRef]
- Sunassee, S.N.; Ransom, T.; Henrich, C.J.; Beutler, J.A.; Covell, D.G.; McMahon, J.B.; Gustafson, K.R. Steroidal Alkaloids from the Marine Sponge Corticium niger That Inhibit Growth of Human Colon Carcinoma Cells. J. Nat. Prod. 2014, 77, 2475–2480. [Google Scholar] [CrossRef] [PubMed]
- Samoylenko, V.; Khan, S.I.; Jacob, M.R.; Tekwani, B.L.; Walker, L.A.; Hufford, C.D.; Muhammad, I. Bioactive (+)-Manzamine A and (+)-8-Hydroxymanzamine A Tertiary Bases and Salts from Acanthostrongylophora Ingens and Their Preparations. Nat. Prod. Commun. 2009, 4, 185–192. [Google Scholar] [CrossRef]
- Stout, E.P.; Yu, L.C.; Molinski, T.F. Antifungal diterpene alkaloids from the Caribbean sponge Agelas citrina: Unified configurational assignments of agelasidines and agelasines. Eur. J. Org. Chem. 2012, 2012, 5131–5135. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Kondratyuk, T.P.; Tan, G.T.; Pezzuto, J.M.; Chang, L.C. Bioactive sulfated sesterterpene alkaloids and sesterterpene sulfates from the marine sponge Fasciospongia sp. J. Nat. Prod. 2009, 72, 319–323. [Google Scholar] [CrossRef]
- Chu, M.-J.; Tang, X.-L.; Qin, G.-F.; Sun, Y.-T.; Li, L.; De Voogd, N.J.; Li, P.-L.; Li, G.-Q. Pyrrole Derivatives and Diterpene Alkaloids from the South China Sea Sponge Agelas nakamurai. Chem. Biodivers. 2017, 14, e1600446. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, C.; Gimmelli, R.; Persico, M.; Casertano, M.; Guidi, A.; Saccoccia, F.; Ruberti, G.; Luciano, P.; Aiello, A.; Parapini, S.; et al. Investigating the Antiparasitic Potential of the Marine Sesquiterpene Avarone, Its Reduced Form Avarol, and the Novel Semisynthetic Thiazinoquinone Analogue Thiazoavarone. Mar. Drugs 2020, 18, 112. [Google Scholar] [CrossRef]
- Hamed, A.N.E.-S.; Wätjen, W.; Schmitz, R.; Chovolou, Y.; Edrada-Ebel, R.; Youssef, D.T.A.; Kamel, M.S.; Proksch, P. A New Bioactive Sesquiterpenoid Quinone from the Mediterranean Sea Marine Sponge Dysidea avara. Nat. Prod. Commun. 2013, 8, 289–292. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).