A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin
Abstract
1. Introduction
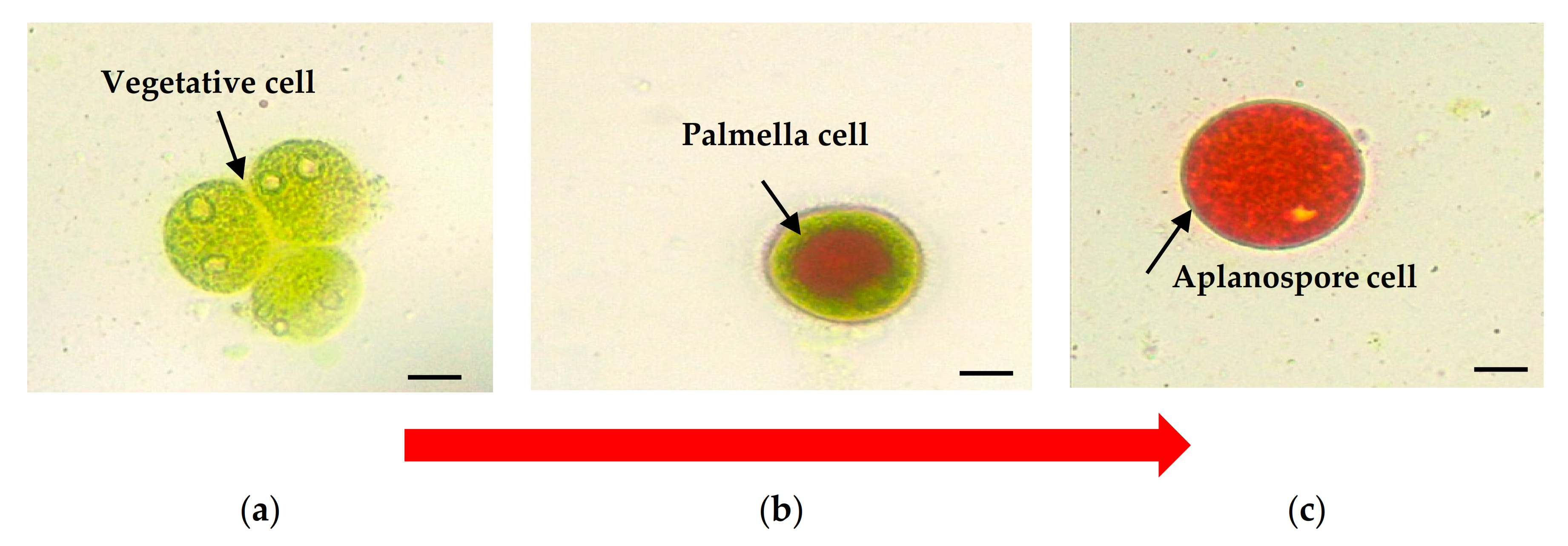
2. Cell Morphology of Haematococcus pluvialis
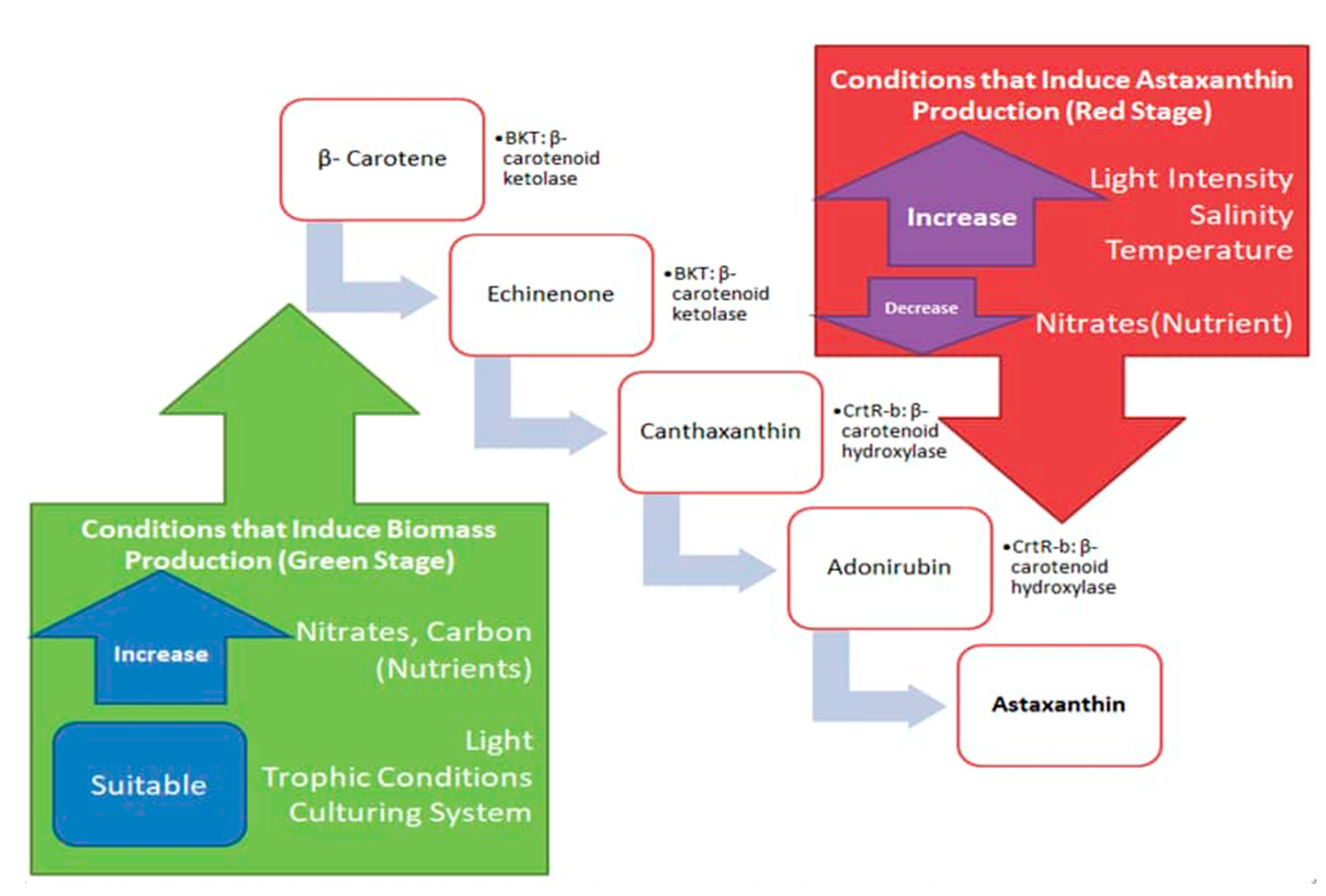
3. Current Production Strategy to Induce Biomass at the Green Stage
3.1. Effect of Nitrogen Sources
3.2. Effect of Carbon Sources
3.3. Effect of Illumination Intensity
3.4. Effect of Different Trophic Conditions
3.5. Effect of Culturing System
4. Current Strategies Inducing Astaxanthin in the Red Stage
4.1. Effect of Salinity
4.2. Nitrogen Depletion Strategy
4.3. Effect of Illumination Intensity
4.4. Effect of Temperature
4.5. Effect of Metal Concentration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Farahin, A.W.; Yusoff, F.M.; Basri, M.; Nagao, N.; Shariff, M. Use of microalgae: Tetraselmis tetrathele extract in formulation of nanoemulsions for cosmeceutical application. J. Appl. Phycol. 2018, 31, 1743–1752. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Muskhazli, M.; Nor Azwady, A.A.; Ahmad, S.A.; Adli, A.A. Three dimensional optimisation for the enhancement of astaxanthin recovery from shrimp shell wastes by Aeromonas hydrophila. Biocatal. Agric. Biotechnol. 2020, 27, 101649. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.; Aziz, N.A.A.; Rusea, G.; Azmi, N.U.; Ghafar, N.S.A.; Adli, A.A.; Mustafa, M. The Availability of Astaxanthin from Shrimp Shell Wastes through Microbial Fermentations, Aeromonas hydrophila and Cell Disruptions. Int. J. Agric. Biol. 2016, 16, 277–284. [Google Scholar]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other Nutrients from Haematococcus pluvialis—Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Yusoff, F.M.; Banerjee, S.; Nagao, N.; Imaizumi, Y.; Shariff, M.; Toda, T. Use of Microalgae Pigments in Aquaculture. In Pigments from Microalgae Handbook; Springer: Cham, Switherlands, 2020; pp. 471–513. ISBN 978-3-030-50970-5. [Google Scholar]
- Niizawa, I.; Espinaco, B.Y.; Leonardi, J.R.; Heinrich, J.M.; Sihufe, G.A. Enhancement of astaxanthin production from Haematococcus pluvialis under autotrophic growth conditions by a sequential stress strategy. Prep. Biochem. Biotechnol. 2018, 48, 528–534. [Google Scholar] [CrossRef]
- Wang, F.; Gao, B.; Wu, M.; Huang, L.; Zhang, C. A novel strategy for the hyper-production of astaxanthin from the newly isolated microalga Haematococcus pluvialis JNU35. Algal Res. 2019, 39, 101466. [Google Scholar] [CrossRef]
- Radzali, S.A.; Baharin, B.S.; Othman, R.; Markom, M.; Rahman, R.A. Co-solvent selection for supercritical fluid extraction of astaxanthin and other carotenoids from Penaeus monodon waste. J. Oleo Sci. 2014, 63, 769–777. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Dietary administration of astaxanthin improves feed utilization, growth performance and survival of Asian seabass, Lates calcarifer (Bloch, 1790). Aquac. Nutr. 2019, 25, 1410–1421. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S.; Nagao, N. Dietary supplementation of astaxanthin enhances hemato-biochemistry and innate immunity of Asian seabass, Lates calcarifer (Bloch, 1790). Aquaculture 2019, 512, 734339. [Google Scholar] [CrossRef]
- Pan-utai, W.; Parakulsuksatid, P.; Phomkaivon, N. Effect of inducing agents on growth and astaxanthin production in Haematococcus pluvialis: Organic and inorganic. Biocatal. Agric. Biotechnol. 2017, 12, 152–158. [Google Scholar] [CrossRef]
- Pereira, S.; Otero, A. Haematococcus pluvialis bioprocess optimization: Effect of light quality, temperature and irradiance on growth, pigment content and photosynthetic response. Algal Res. 2020, 51, 102027. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef]
- Begum, H.; Yusoff, F.M.D.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and utilization of pigments from microalgae. Crit. Rev. Food Sci. Nutr. 2015, 56, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cai, M.; Lin, M.; Huang, X.; Wang, J.; Ke, H.; Wang, C.; Zheng, X.; Chen, D.; Yang, S. Enhanced Biomass and Astaxanthin Production of Haematococcus pluvialis by a Cell Transformation Strategy with Optimized Initial Biomass Density. Mar. Drugs 2020, 18, 341. [Google Scholar] [CrossRef] [PubMed]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Poonkum, W.; Powtongsook, S.; Pavasant, P. Astaxanthin induction in microalga H. pluvialis with flat panel airlift photobioreactors under indoor and outdoor conditions. Prep. Biochem. Biotechnol. 2015, 45, 1–17. [Google Scholar] [CrossRef]
- Azizi, M.; Moteshafi, H.; Hashemi, M. Distinctive nutrient designs using statistical approach coupled with light feeding strategy to improve the Haematococcus pluvialis growth performance and astaxanthin accumulation. Bioresour. Technol. 2020, 300, 122594. [Google Scholar] [CrossRef]
- Wayama, M.; Ota, S.; Matsuura, H.; Nango, N.; Hirata, A.; Kawano, S. Three-Dimensional Ultrastructural Study of Oil and Astaxanthin Accumulation during Encystment in the Green Alga Haematococcus pluvialis. PLoS ONE 2013, 8, e53618. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Z.; Xu, X.; Cheng, J.; Chen, S.; Tian, J.; Yang, W.; Crocker, M. Simultaneous promotion of photosynthesis and astaxanthin accumulation during two stages of Haematococcus pluvialis with ammonium ferric citrate. Sci. Total Environ. 2020, 750, 141689. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.O.; McDougall, G.J.; Campbell, R.; Stanley, M.S.; Day, J.G. Media screening for obtaining Haematococcus pluvialis red motile macrozooids rich in astaxanthin and fatty acids. Biology 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Evens, T.J.; Niedz, R.P.; Kirkpatrick, G.J. Temperature and irradiance impacts on the growth, pigmentation and photosystem II quantum yields of Haematococcus pluvialis (Chlorophyceae). J. Appl. Phycol. 2008, 20, 411–422. [Google Scholar] [CrossRef]
- Hang Ho, Y. Maximization of Astaxanthin Production from Green Microalga Haematococcus pluvialis Using Internally-Illuminated Photobioreactor. Adv. Biosci. Bioeng. 2018, 6, 10. [Google Scholar] [CrossRef]
- Zhang, W.W.; Zhou, X.F.; Zhang, Y.L.; Cheng, P.F.; Ma, R.; Cheng, W.L.; Chu, H.Q. Enhancing astaxanthin accumulation in haematococcus pluvialis by coupled light intensity and nitrogen starvation in column photobioreactors. J. Microbiol. Biotechnol. 2018, 28, 2019–2028. [Google Scholar] [CrossRef]
- Scibilia, L.; Girolomoni, L.; Berteotti, S.; Alboresi, A.; Ballottari, M. Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal Res. 2015, 12, 170–181. [Google Scholar] [CrossRef]
- Minyuk, G.; Sidorov, R.; Solovchenko, A. Effect of nitrogen source on the growth, lipid, and valuable carotenoid production in the green microalga Chromochloris zofingiensis. J. Appl. Phycol. 2020, 32, 923–935. [Google Scholar] [CrossRef]
- Dhup, S. Understanding Urea Assimilation and its Effect on Lipid Production and Fatty Acid Composition of Scenedesmus sp. SOJ Biochem. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Minyuk, G.S.; Dantsyuk, N.V.; Chelebieva, E.S.; Chubchikova, I.N.; Drobetskaya, I.V.; Solovchenko, A.E. The effect of diverse nitrogen sources in the nutrient medium on the growth of the green microalgae Chromochloris zofingiensis in the batch culture. Mar. Biol. J. 2019, 4, 41–52. [Google Scholar] [CrossRef]
- Buchheim, M.A.; Sutherland, D.M.; Buchheim, J.A.; Wolf, M. The blood alga: Phylogeny of Haematococcus (Chlorophyceae) inferred from ribosomal RNA gene sequence data. Eur. J. Phycol. 2013, 48, 318–329. [Google Scholar] [CrossRef]
- Sarada, R.; Tripathi, U.; Ravishankar, G. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem. 2002, 37, 623–627. [Google Scholar] [CrossRef]
- Yoshimura, S.; Ranjbar, R.; Inoue, R.; Katsuda, T.; Katoh, S. Effective utilization of transmitted light for astaxanthin production by Haematococcus pluvialis. J. Biosci. Bioeng. 2006, 102, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Wijanarko, A. Effect of the Presence Of Substituted Urea and Also Ammonia as Nitrogen Source in Cultivied Medium on Chlorella Lipid Content. In Progress in Biomass and Bioenergy Production; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Cui, J.; Yu, C.; Zhong, D.B.; Zhao, Y.; Yu, X. Melatonin and calcium act synergistically to enhance the coproduction of astaxanthin and lipids in Haematococcus pluvialis under nitrogen deficiency and high light conditions. Bioresour. Technol. 2020, 305, 123069. [Google Scholar] [CrossRef]
- Jeon, Y.C.; Cho, C.W.; Yun, Y.S. Combined effects of light intensity and acetate concentration on the growth of unicellular microalga Haematococcus pluvialis. Enzyme Microb. Technol. 2006, 39, 490–495. [Google Scholar] [CrossRef]
- Göksan, T.; Ak, I.; Gökpinar, Ş. An alternative approach to the traditional mixotrophic cultures of Haematococcus pluvialis flotow (Chlorophyceae). J. Microbiol. Biotechnol. 2010, 20, 1276–1282. [Google Scholar] [CrossRef]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Christian, D.; Zhang, J.; Sawdon, A.J.; Peng, C.A. Enhanced astaxanthin accumulation in Haematococcus pluvialis using high carbon dioxide concentration and light illumination. Bioresour. Technol. 2018, 256, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, K.; Yang, Z.; Zhou, J.; Cen, K. Enhancing the growth rate and astaxanthin yield of Haematococcus pluvialis by nuclear irradiation and high concentration of carbon dioxide stress. Bioresour. Technol. 2016, 204, 49–54. [Google Scholar] [CrossRef]
- Chekanov, K.; Schastnaya, E.; Solovchenko, A.; Lobakova, E. Effects of CO2 enrichment on primary photochemistry, growth and astaxanthin accumulation in the chlorophyte Haematococcus pluvialis. J. Photochem. Photobiol. B Biol. 2017, 171, 58–66. [Google Scholar] [CrossRef]
- Pang, N.; Gu, X.; Fu, X.; Chen, S. Effects of gluconate on biomass improvement and light stress tolerance of Haematococcus pluvialis in mixotrophic culture. Algal Res. 2019, 43, 101647. [Google Scholar] [CrossRef]
- Lu, Z.; Zheng, L.; Liu, J.; Dai, J.; Song, L. A novel fed-batch strategy to boost cyst cells production based on the understanding of intracellular carbon and nitrogen metabolism in Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121744. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Perspectives on microalgal CO2-emission mitigation systems—A review. Biotechnol. Adv. 2011, 29, 189–198. [Google Scholar] [CrossRef]
- Imamoglu, E.; Sukan, F.V.; Dalay, M.C. Effect of Different Culture Media and Light Intensities on Growth of Haematococcus Pluvialis. Int. J. Nat. Eng. Sci. 2007, 1, 5–9. [Google Scholar]
- Kiperstok, A.C.; Sebestyén, P.; Podola, B.; Melkonian, M. Biofilm cultivation of Haematococcus pluvialis enables a highly productive one-phase process for astaxanthin production using high light intensities. Algal Res. 2017, 21, 213–222. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Z.; Ding, Y.; Geng, Y.; Li, Y. Enhancing the production of astaxanthin by mixotrophic cultivation of Haematococcus pluvialis in open raceway ponds. Aquac. Int. 2020, 28, 625–638. [Google Scholar] [CrossRef]
- Hong, M.E.; Hwang, S.K.; Chang, W.S.; Kim, B.W.; Lee, J.; Sim, S.J. Enhanced autotrophic astaxanthin production from Haematococcus pluvialis under high temperature via heat stress-driven Haber–Weiss reaction. Appl. Microbiol. Biotechnol. 2015, 99, 5203–5215. [Google Scholar] [CrossRef]
- Kwan, P.P.; Banerjee, S.; Shariff, M.; Yusoff, F. Influence of light on biomass and lipid production in microalgae cultivation. Aquac. Res. 2020. [Google Scholar] [CrossRef]
- Kamalanathan, M.; Chaisutyakorn, P.; Gleadow, R.; Beardall, J. A comparison of photoautotrophic, heterotrophic, and mixotrophic growth for biomass production by the green alga Scenedesmus sp. (Chlorophyceae). Phycologia 2018, 57, 309–317. [Google Scholar] [CrossRef]
- Aslanbay Guler, B.; Deniz, I.; Demirel, Z.; Imamoglu, E. Computational fluid dynamics simulation in scaling-up of airlift photobioreactor for astaxanthin production. J. Biosci. Bioeng. 2020, 129, 86–92. [Google Scholar] [CrossRef]
- Do, T.T.; Ong, B.N.; Tran, M.L.N.; Nguyen, D.; Melkonian, M.; Tran, H.D. Biomass and astaxanthin productivities of Haematococcus pluvialis in an angled twin-layer porous substrate photobioreactor: Effect of inoculum density and storage time. Biology 2019, 8, 68. [Google Scholar] [CrossRef]
- Yusoff, F.M.; Nagao, N.; Imaizumi, Y.; Toda, T. Bioreactor for Microalgal Cultivation Systems: Strategy and Development. In Prospects of Renewable Bioprocessing in Future Energy Systems. Biofuel and Biorefinery Technologies; Springer: Cham, Switherlands, 2019; Volume 10, pp. 117–159. [Google Scholar]
- Lee, K.Y.; Lee, S.H.; Lee, J.E.; Lee, S.Y. Biosorption of radioactive cesium from contaminated water by microalgae Haematococcus pluvialis and Chlorella vulgaris. J. Environ. Manag. 2019, 233, 83–88. [Google Scholar] [CrossRef]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A Review on the Use of Microalgae for Sustainable Aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Jin, E.; Lee, C.G.; Polle, J.E.W. Secondary carotenoid accumulation in Haematococcus (chlorophyceae): Biosynthesis, regulation, and biotechnology. J. Microbiol. Biotechnol. 2006, 16, 821–831. [Google Scholar]
- Wang, Y.; Chen, T. The biosynthetic pathway of carotenoids in the astaxanthin-producing green alga Chlorella zofingiensis. World J. Microbiol. Biotechnol. 2008, 24, 2927–2932. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in microalgae: Pathways, functions and biotechnological implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Huang, J.C.; Chen, F.; Sandmann, G. Stress-related differential expression of multiple β-carotene ketolase genes in the unicellular green alga Haematococcus pluvialis. J. Biotechnol. 2006, 122, 176–185. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front. Microbiol. 2016, 7, 546. [Google Scholar] [CrossRef]
- Ji, X.; Cheng, J.; Gong, D.; Zhao, X.; Qi, Y.; Su, Y.; Ma, W. The effect of NaCl stress on photosynthetic efficiency and lipid production in freshwater microalga—Scenedesmus obliquus XJ002. Sci. Total Environ. 2018, 633, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Shanmugam, J.; Nallamuthu, T. Salt stress enhancing the production of Phytochemicals in Chlorella vulgaris and Chlamydomonas reinhardtii. J. Algal Biomass Utln. 2015, 7, 37–44. [Google Scholar]
- Guilian, M.; Xing, X.; Zhaozhen, X. Advances in physiological and biochemical research of salt tolerance in plant. Chin. J. Eco Agric. 2004, 12, 43–46. [Google Scholar]
- Gao, Z.; Meng, C.; Zhang, X.; Xu, D.; Miao, X.; Wang, Y.; Yang, L.; Lv, H.; Chen, L.; Ye, N. Induction of salicylic acid (SA) on transcriptional expression of eight carotenoid genes and astaxanthin accumulation in Haematococcus pluvialis. Enzym. Microb. Technol. 2012, 51, 225–230. [Google Scholar] [CrossRef]
- Gao, Z.; Meng, C.; Chen, Y.C.; Ahmed, F.; Mangott, A.; Schenk, P.M.; Li, Y. Comparison of astaxanthin accumulation and biosynthesis gene expression of three Haematococcus pluvialis strains upon salinity stress. J. Appl. Phycol. 2015, 27, 1853–1860. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A. Astaxanthin Accumulation in the Green Alga Haematococcus pluvialis1. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Tam, L.T.; Hoang, D.D.; Ngoc Mai, D.T.; Hoai Thu, N.T.; Lan Anh, H.T.; Hong, D.D. Study on the effect of salt concentration on growth and Astaxanthin accumulation of microalgae Haematococcus pluvialis as the initial basis for two phase culture of astaxanthin production. J. Biol. 2012, 34, 213–223. [Google Scholar] [CrossRef]
- Imamoglu, E.; Dalay, M.C.; Sukan, F.V. Influences of different stress media and high light intensities on accumulation of astaxanthin in the green alga Haematococcus pluvialis. N. Biotechnol. 2009, 26, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Nahidian, B.; Ghanati, F.; Shahbazi, M.; Soltani, N. Effect of nutrients on the growth and physiological features of newly isolated Haematococcus pluvialis TMU. Bioresour. Technol. 2018, 255, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Xia, F.; Liu, M.; Cui, X.; Wahid, F.; Jia, S. Metabolomic profiling of the astaxanthin accumulation process induced by high light in Haematococcus pluvialis. Algal Res. 2016, 20, 35–43. [Google Scholar] [CrossRef]
- Hu, C.; Cui, D.; Sun, X.; Shi, J.; Xu, N. Primary metabolism is associated with the astaxanthin biosynthesis in the green algae Haematococcus pluvialis under light stress. Algal Res. 2020, 46, 101768. [Google Scholar] [CrossRef]
- Giannelli, L.; Yamada, H.; Katsuda, T.; Yamaji, H. Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J. Biosci. Bioeng. 2015, 119, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Djearamane, S.; Lim, Y.M.; Wong, L.S.; Lee, P.F. Cellular accumulation and cytotoxic effects of zinc oxide nanoparticles in microalga Haematococcus pluvialis. PeerJ 2019, 7, e7582. [Google Scholar] [CrossRef]
- Mehrgan, M.S.; Rastar, M.; Shekarabi, S.P.H.; Sabzi, S. Effects of iron and zinc concentrations on growth performance and biochemical composition of Haematococcus pluvialis: A comparison between nanoparticles and their corresponding metals bulks. J. Algal Biomass Utln 2018, 9, 59–67. [Google Scholar]
- He, M.; Yan, Y.; Pei, F.; Wu, M.; Gebreluel, T.; Zou, S.; Wang, C. Improvement on lipid production by Scenedesmus obliquus triggered by low dose exposure to nanoparticles. Sci. Rep. 2017, 7, 15526. [Google Scholar] [CrossRef]
- Kadar, E.; Rooks, P.; Lakey, C.; White, D.A. The effect of engineered iron nanoparticles on growth and metabolic status of marine microalgae cultures. Sci. Total Environ. 2012, 439, 8–17. [Google Scholar] [CrossRef]
- Sibi, G.; Kumar, D.A.; Gopal, T.; Harinath, K.; Banupriya, C. Metal Nanoparticle Triggered Growth and Lipid Production in Chlorella Vulgaris. Int. J. Sci. Res. Env. Sci. Toxicol. 2017, 2, 1–8. [Google Scholar]
- Wan, M.; Zhang, J.; Hou, D.; Fan, J.; Li, Y.; Huang, J.; Wang, J. The effect of temperature on cell growth and astaxanthin accumulation of Haematococcus pluvialis during a light-dark cyclic cultivation. Bioresour. Technol. 2014, 167, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Baroni, É.G.; Yap, K.Y.; Webley, P.A.; Scales, P.J.; Martin, G.J.O. The effect of nitrogen depletion on the cell size, shape, density and gravitational settling of Nannochloropsis salina, Chlorella sp. (marine) and Haematococcus pluvialis. Algal Res. 2019, 39, 101454. [Google Scholar] [CrossRef]
- Sarada, R.; Bhattacharya, S.; Ravishankar, G.A. Optimization of culture conditions for growth of the green alga Haematococcus pluvialis. World J. Microbiol. Biotechnol. 2002, 18, 517–521. [Google Scholar] [CrossRef]
| Optimal Haematococcus pluvialis Growth Conditions(Green Stage) | Optimal Stress Condition for Inducing Astaxanthin Production by Haematococcus pluvialis (Red Stage) | References | ||||||
|---|---|---|---|---|---|---|---|---|
| Basal Medium | Inoculum (Days) | Temp (°C) | pH | Light Intensity | Time Course (Days) | Stress Condition | Effect | |
| Strain JNU35 Modified BBM (mBBM) Modified BG-11 (mBG-11) | 7 days Initial biomass 0.4–0.5 g L−1 | 25 ± 1 | 6–7 | 150 μmol photons m−2s−1 | 15 | Different Initial Nitrogen: Sodium nitrate (NaNO3) Ammonium bicarbonate (NH4HCO3) Or urea ((NH2)2CO) | Best N source: Urea Max biomass (10.2 g L−1) Max Astaxanthin (5.4 mg L−1) | [9] |
| Strain ZY-18 NIES-C | Initial biomass 0.4 g L−1 | 25 | 7.5–8 | 250 μmol photons m−2s−1 12 h:12 h Light: Dark cycle | until achieved 10 g L−1 of biomass | Temperature: Daytime temperature range (8 °C to 33 °C) Night temperature (maintained at 28 °C) Night temperature range(8 °C to 33 °C) Daytime temperature(maintained at 28 °C) | Daytime temperature (23 to 28 °C) is best for photoinduction, and the night temperature should be kept below 28 °C. The net biomass and astaxanthin productivities under the controlled temperature (2.34 g m−2 d−1) (60 mg m−2 d−1) were 5-fold and 2.9-fold, while of those under the natural temperature (biomass: 0.47 g m−2 d−1; astaxanthin: 21 mg m−2 d−1), respectively. | [79] |
| Strain Flotow RM | Initial cell 6 × 104cell mL−1 | 25 | NR | 1.5 klux density 12 h:12 h Light: Dark cycle | 15 | Different salinity 0.8%, 1.5% and 2.5% NaCl | Astaxanthin increased 4.8 folds from 10 pg⋅cell−1 to 48 pg⋅cell−1 at 2.5% NaCl under high temperature. | [67] |
| Strain K-0084 BG-11 | 5 × 105 cell mL−1 | 22 ± 1 | NR | 16 h:8 h Light: Dark cycle | 10 | Nitrogen starvation and high light intensity | High light (400 μmol photons m−2s−1) combined with nitrogen starvation is the most effective condition to induce astaxanthin production | [27] |
| Strain com-mercial MLA medium | 5% (v/v) inoculum with 4.07 × 104 cell mL−1 | 20 ± 1.5 | NR | Photon flux density 65–75 μE m−2 s −1 14 h:10 h Light: Dark cycle | 17 | Nitrogen depletion Culture were grown autotrophically and undergo natural exhaustion of nitrate. | The cell size increased within the cell population, which the cell diameter average ≈30% and the cell density decreased during senescence. | [80] |
| Strain NIES-144; UTEX-2505 BG-11 | Initial density1 × 104 cell mL−1 | NR | 7–7.5 | 50 μmol photons m−2s−1(White LED) 12 h:12 h Light: Dark cycle | 9 | Different nutrient and light-feeding strategy Nutrients: MgSO4·7H2O, H3BO3 Na2CO3 | Biomass 0.15 g L−1d−1 Astaxanthin 13.33 mg L−1d−1 Utilizing RSM technique of under constant light intensity. | [20] |
| Strain NIES-144 Kobayashi basal medium | The initial cell biomass ~1.0 g L−1 | 25 ± 1 | 7.5 | 4 ± 1 μmol photons m−2s−1, provided by cool white fluorescent tubes | 12 | Different ratio of carbon to nitrogen (C/N) | Biomass 9.18 g L−1 (100% immotile cyst cells) Astaxanthin productivity 15.45 mg L−1d−1 | [43] |
| Strain (Isolate, Iran) BBM | Initial cell number 2 × 105 cell mL−1 | 25 ± 1 | NR | 20 μmol photons m−2s−1 16 h:8 h Light: Dark cycle | 15 | Different macro/micronutrients Nitrate and phosphate (macronutrients) Iron and boron(trace elements) | The modified BBM with 3-fold higher phosphate led to the highest cell density and up to 86% increase in the growth rate. | [69] |
| Strain Flotow EGE MACC-35 BG-11 | Seven-day-old culture of green cells about 0.26 mg mL−1 | 25 ± 1 | <8.0 | 100 µmol photons m−2s−1 | 14 | Different stress media with different light intensity Rudic’s medium (RM) Nitrogen-free RM medium (N-free) Phosphate-free RM medium (P-free) Nitrogen and phosphate-free RM medium (NP- free) and Distilled water with the sparging of CO2 Light Intensity 445 and 546 μmol photons m−2s−1 | Astaxanthin concentrations: Distilled water with CO2 (29.62 mg g−1) N-free RM medium (30.07 mg g−1) at 546 μmol photons m−2s−1 | [68] |
| Strain SAG 19-a BBM | Initial cell 4 × 105cell mL−1 | 25 ± 1 | NR | Under fluorescent light | 15 | Effect of the four variables Carbon dioxide 1.54% Sodium nitrate 1.06 g L−1 Inoculum volume 24.97% Light intensity 2.42 klux | Positive effect on cell growth leading to maximum yield of dried biomass at 0.51 g L−1 | [81] |
| Strain SAG 19-a Basal medium | 4-day old culture Inoculum 4.95 × 105 cell mL−1 | 25 ± 1 | 7 | Under a continuous light intensity of 1.5 klux | 12–16 | Effect of salinity with added sodium acetate (2.2 mM) Range 0.25, 0.5, 1.0, and 2.0% w/v Effect of nitrogen source with 0.25%NaCl and sodium acetate (4.4 mM) Calcium nitrate; potassium nitrate; ammonium nitrate; sodium nitrate Effect of pH with added sodium acetate (4.4 mM) pH 5–9 | Astaxanthin content was higher in acetate supplemented medium, in which an increment was obtained at 0.25 and 0.5% salinity. The maximum cell concentration was obtained in potassium nitrate (6.2 × 105 cell mL−1) and the lowest was obtained in ammonium nitrate (1.65 × 105 cell mL−1) grown cultures. There was a significant increase in astaxanthin productivity in media at pH 6–8.Older cells accumulated 8.3–10.69 mg L−1 astaxanthin compared to 0.95–8.1 mg L−1 in 4–8-day-old cultures, respectively. | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oslan, S.N.H.; Shoparwe, N.F.; Yusoff, A.H.; Rahim, A.A.; Chang, C.S.; Tan, J.S.; Oslan, S.N.; Arumugam, K.; Ariff, A.B.; Sulaiman, A.Z.; et al. A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules 2021, 11, 256. https://doi.org/10.3390/biom11020256
Oslan SNH, Shoparwe NF, Yusoff AH, Rahim AA, Chang CS, Tan JS, Oslan SN, Arumugam K, Ariff AB, Sulaiman AZ, et al. A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules. 2021; 11(2):256. https://doi.org/10.3390/biom11020256
Chicago/Turabian StyleOslan, Siti Nur Hazwani, Noor Fazliani Shoparwe, Abdul Hafidz Yusoff, Ainihayati Abdul Rahim, Chang Shen Chang, Joo Shun Tan, Siti Nurbaya Oslan, Kavithraashree Arumugam, Arbakariya Bin Ariff, Ahmad Ziad Sulaiman, and et al. 2021. "A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin" Biomolecules 11, no. 2: 256. https://doi.org/10.3390/biom11020256
APA StyleOslan, S. N. H., Shoparwe, N. F., Yusoff, A. H., Rahim, A. A., Chang, C. S., Tan, J. S., Oslan, S. N., Arumugam, K., Ariff, A. B., Sulaiman, A. Z., & Mohamed, M. S. (2021). A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules, 11(2), 256. https://doi.org/10.3390/biom11020256







