Dopamine Receptors and the Kidney: An Overview of Health- and Pharmacological-Targeted Implications
Abstract
1. Introduction
2. Dopamine Receptors
3. Dopamine Receptors in the Kidney: Structure and Functions
4. Distribution of Renal Dopamine Receptors
5. Physiological Function of Renal Dopamine Receptors
6. Dopamine Receptors and Blood Pressure Regulation
7. Dopamine Renal System and Other Homeostasis Systems Interactions
8. Effects of Renal Dopamine Receptors in General Homeostasis
8.1. Dopamine Receptors and Oxidative Stress
8.2. Hypertension and Dopamine Receptors
8.3. Diabetes, Hyperinsulinemia, and Dopamine Receptors
9. Pharmacological Targets in the Renal Dopaminergic System
10. Summary
Funding
Conflicts of Interest
Abbreviations
| AADC | Aromatic Amino Acid Decarboxylase |
| ANP | Atrial Natriuretic Peptide |
| AT1R | Angiotensin II Receptor Type 1 |
| A1R | Adenosine Receptor 1 |
| A2R | Adenosine Receptor 2 |
| BP | Blood Pressure |
| cAMP | Cyclic AMP |
| CNS | Central Nervous System |
| Dahl-SR | Dahl Salt-Resistant Rats |
| Dahl-SS | Dahl Salt-Sensitive Rats |
| DRD1 | Dopamine Receptor D1 |
| DRD2 | Dopamine Receptor D2 |
| DRD3 | Dopamine Receptor D3 |
| DRD4 | Dopamine Receptor D4 |
| DRD5 | Dopamine Receptor D5 |
| ETAR | Endothelin A Receptor |
| ETBR | Endothelin B Receptor |
| GRK4 | G Protein Type 4 |
| HTN | Hypertension |
| L-DOPA | L-3,4-dihydroxiphenylalanine |
| NaCl | Sodium Chloride |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NHE3 | Sodium-Hydrogen Exchanger 3 |
| mRNA | Messenger RNA |
| mTAL | Medullary Thick Ascending Limb of Henle |
| PKA | Protein Kinase A |
| PKC | Protein Kinase C |
References
- Wang, Z.Q.; Siragy, H.M.; Felder, R.A.; Carey, R.M. Intrarenal dopamine production and distribution in the rat. Physiological control of sodium excretion. Hypertension 1997, 29, 228–234. [Google Scholar] [CrossRef]
- Jose, P.A.; Eisner, G.M.; Felder, R.A. Renal dopamine receptors in health and hypertension. Pharmacol. Ther. 1998, 80, 149–182. [Google Scholar] [CrossRef]
- Luippold, G.; Schneider, S.; Vallon, V.; Osswald, H.; Muhlbauer, B. Postglomerular vasoconstriction induced by dopamine D(3) receptor activation in anesthetized rats. Am. J. Physiol. Renal Physiol. 2000, 278, F570–F575. [Google Scholar] [CrossRef]
- Boyd, K.N.; Mailman, R.B. Dopamine receptor signaling and current and future antipsychotic drugs. Handb. Exp. Pharmacol. 2012, 212, 53–86. [Google Scholar]
- Niznik, H.B.; Grigoriadis, D.E.; Pri-Bar, I.; Buchman, O.; Seeman, P. Dopamine D2 receptors selectively labeled by a benzamide neuroleptic: [3H]-YM-09151–2. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1985, 329, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Gundlach, A.L.; Largent, B.L.; Snyder, S.H. 125I-Spiperone: A novel ligand for D2 dopamine receptors. Life Sci. 1984, 35, 1981–1989. [Google Scholar] [CrossRef]
- Weiss, S.; Sebben, M.; Garcia-Sainz, J.A.; Bockaert, J. D2-dopamine receptor-mediated inhibition of cyclic AMP formation in striatal neurons in primary culture. Mol. Pharmacol. 1985, 27, 595–599. [Google Scholar] [PubMed]
- Shonesy, B.C.; Stepheson, J.R.; Marks, C.R.; Colbran, R.J. Cyclic AMP-dependent protein kinase and D1 dopamine receptors regulate diacylglycerol lipase-α and synaptic 2-arachidonoyl glycerol signaling. J. Neurochem. 2020, 153, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Neve, K.A.; Seamans, J.K.; Trantham-Davidson, H. Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 2004, 24, 165–205. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, S.; Shukla, S. Physiological and Functional Basis of Dopamine Receptors and Their Role in Neurogenesis: Possible Implication for Parkinson’s disease. J. Exp. Neurosci. 2018, 12, 1179069518779829. [Google Scholar] [CrossRef]
- Sibley, D.R.; Monsma, F.J., Jr.; Shen, Y. Molecular neurobiology of dopaminergic receptors. Int. Rev. Neurobiol. 1993, 35, 391–415. [Google Scholar] [PubMed]
- Huff, R.M. Signal transduction pathways modulated by the D2 subfamily of dopamine receptors. Cell Signal. 1996, 8, 453–459. [Google Scholar] [CrossRef]
- Banday, A.A.; Lokhandwala, M.F. Dopamine receptors and hypertension. Curr. Hypertens. Rep. 2008, 10, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Hollon, T.R.; Bek, M.J.; Lachowicz Je Ariano, M.A.; Mezey, E.; Ramachandran, R.; Wersinger, S.R.; Soares-da-Silva, P.; Liu, Z.F.; Grinberg, A.; Drago, J.; et al. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J. Neurosci. 2002, 22, 10801–10810. [Google Scholar] [CrossRef] [PubMed]
- Konkalmatt, P.R.; Asico, L.D.; Zhang, Y.; Yang, Y.; Drachenberg, C.; Zheng, X.; Han, F.; Jose, P.A.; Armando, I. Renal rescue of dopamine D2 receptor function reverses renal injury and high blood pressure. JCI Insight 2016, 1, e85888. [Google Scholar] [CrossRef]
- Staudacher, T.; Pech, B.; Tappe, M.; Gross, G.; Mühlbauer, B.; Luippold, B. Arterial Blood Pressure and Renal Sodium Excretion in Dopamine D3 Receptor Knockout Mice. Hypertens. Res. 2007, 30, 93–101. [Google Scholar] [CrossRef]
- Johnson, T.L.; Tulis, D.A.; Keeler, B.E.; Virag, J.A.; Lust, R.M.; Clemens, S. The dopamine D3 receptor knockout mouse mimics aging-related changes in autonomic function and cardiac fibrosis. PLoS ONE 2013, 8, e74116. [Google Scholar] [CrossRef]
- Sen, S.; Nesse, R.; Sheng, L.; Stoltenberg, S.F.; Gleiberman, L.; Burmeister, M.; Weder, A.B. Association between a dopamine-4 receptor polymorphism and blood pressure. Am. J. Hypertens. 2005, 18, 1206–1210. [Google Scholar] [CrossRef]
- Martin, Y.C. The Discovery of Novel Selective D1 Dopaminergic Agonists: A-68930, A-77636, A-86929, and ABT-413. Int. J. Med. Chem. 2011, 2011, 424535. [Google Scholar] [CrossRef]
- Bueschbell, B.; Barreto, C.A.V.; Preto, A.J.; Schiedel, A.C.; Moreira, I.S. A complete assessment of dopamine receptor-ligand interactions through computational methods. Molecules 2019, 24, 1196. [Google Scholar] [CrossRef]
- Moreland, R.B.; Patel, M.; Hsieh, G.C.; Wetter, J.M.; Marsh, K.; Brioni, J.D. A-412997 is a selective dopamine D4 receptor agonist in rats. Pharmacol. Biochem. Behav. 2005, 82, 140–147. [Google Scholar] [CrossRef]
- Bourne, J.A. SCH 23390: The first selective dopamine D1-like receptor antagonist. CNS Drug Rev. 2001, 7, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Clément, P.; Pozzato, C.; Heidbreder, C.; Alexandre, L.; Giuliano, F.; Melotto, S. Delay of ejaculation induced by SB-277011, a selective dopamine D3 receptor antagonist, in the rat. J. Sex. Med. 2009, 6, 980–988. [Google Scholar] [CrossRef]
- Hall, H.; Sedvall, G.; Magnusson, O.; Kopp, J.; Halldin, C.; Farde, L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology 1994, 11, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.L.; Jones-Tabah, J.; O’Dowd, B.F.; George, S.R. A physiological role for the dopamine D5 receptor as a regulator or BDNF and Akt signalling in rodent prefrontal cortex. Int. J. Neuropsychopharmacol. 2013, 16, 477–483. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Lu, X.; Yang, J.; Wang, H.; Chen, C.; Han, Y.; Ren, H.; Zheng, S.; He, D.; Zhou, L.; et al. Regulation of renalase expression by D5 dopamine receptors in rat renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 2014, 306, F588–F596. [Google Scholar] [CrossRef]
- Armando, I.; Villar, V.A.M.; Jose, P.A. Dopamine and renal function and blood pressure regulation. Compr. Physiol. 2011, 1, 1075–1117. [Google Scholar]
- Cuevas, S.; Villar, V.A.; Jose, P.A.; Armando, I. Renal dopamine receptors, oxidative stres and hypertension. Int. J. Mol. Sci. 2013, 14, 17553–17572. [Google Scholar] [CrossRef]
- Chen, Y.; Asico, L.D.; Zheng, S.; Villar, V.A.M.; He, D.; Zhou, D.; Zeng, C.; Jose, P.A. Gastrin and D1 dopamine receptor interact to induce natriuresis and diuresis. Hypertesion 2013, 62, 927–933. [Google Scholar] [CrossRef]
- Vieira-Coelho, M.A.; Gomes, P.; Serrao, M.P.; Soares-Da-Silva, P. D1-like dopamine receptor activation and natriuresis by nitrocatechol COMT inhibitors. Kidney Int. 2001, 59, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Schmauss, C.; Cuenca, A.; Ratcliffe, E.; Gershon, M.D. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: Analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J. Neurosci. 2006, 26, 2798–2807. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.-M.; Chen, X.; Luo, D.-Z.; Zhang, X.-H.; Xue, H.; Zheng, L.-F.; Yang, N.; Wang, X.-M.; Zhu, J.-X. Alteration of dopaminergic markers in gastrointestinal tract of different rodent models of Parkinson’s disease. Neuroscience 2008, 153, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, H.; Mogi, M.; Kan-No, H.; Tsukuda, K.; Ohshima, K.; Wang, X.L.; Chisaka, T.; Bai, H.-Y.; Shan, B.-S.; Kukida, M.; et al. Angiotensin II type 2 receptor signaling affects dopamine levels in the brain and prevents binge eating disorder. J. Renin Angiotensin. Aldosterone Syst. 2015, 16, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Bek, M.; Asico, L.D.; Yang, Z.; Grandy, D.K.; Goldstein, D.S.; Rubinstein, M.; Eisner, G.M.; Jose, P.A. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice. Hypertesion 2001, 38, 303–308. [Google Scholar] [CrossRef]
- Marin-Grez, M.; Angchanpen, P.; Gambaro, G.; Schnermann, J.; Schubert, G.; Briggs, J.P. Evidence for an involvement of dopamine receptors in the natriuretic response to atrial natriuretic peptide. Klin. Wochenschr. 1987, 65, 97–102. [Google Scholar]
- Fitzgerald, P.; Dinan, T.G. Prolactin and dopamine: What is the connection? A review article. J. Psychopharmacol. 2008, 22, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Lisbon, A. Low-dose dopamine in the intensive care unit. Semin. Dial. 2006, 19, 465–471. [Google Scholar] [CrossRef]
- Debaveye, Y.A.; Van den Berghe, G.H. Is there still a place for dopamine in the modern intensive care unit? Anesth. Analg. 2004, 98, 461–468. [Google Scholar] [CrossRef]
- Lei, S. Cross interaction of dopaminergic and adrenergic systems in neural modulation. Int. J. Physiol. Patholphysiol. Pharmacol. 2014, 6, 137–142. [Google Scholar]
- Cornil, C.A.; Ball, G.F. Interplay Among Catecholamine Systems: Dopamine Binds to a2-adrenergic Receptors in Birds and Mammals. J. Comp. Neurol. 2008, 511, 610–627. [Google Scholar] [CrossRef]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine Hydroxylase and Regulation of Dopamine Synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- Wassenberg, T.; Monnens, L.A.; Geurtz, B.; Wevers, R.A.; Verbeek, M.M.; Willemsen, M. The Paradox of Hyperdopaminuria in Aromatic l-Amino Acid Deficiency Explained. JIMD Rep. 2012, 4, 39–45. [Google Scholar] [PubMed]
- Zhang, M.Z.; Yao, B.; Wang, S.; Fan, X.; Wu, G.; Yang, H.; Yin, H.; Yang, S.; Harris, R.C. Intrarenal dopamine defiency leads to hypertension and decreased longevity in mice. J. Clin. Investig. 2011, 121, 2845–2854. [Google Scholar] [CrossRef]
- Goldberg, L.I. L-dopa effect on renal function. N. Engl. J. Med. 1977, 297, 112–113. [Google Scholar] [PubMed]
- Grupp, C.; Begher, M.; Cohen, D.; Raghunath, M.; Franz, H.E.; Müller, G.A. Isolation and characterization of the lower portion of the thin limb of Henle in primary culture. Am. J. Physiol. Renal Physiol. 1998, 274, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Katayama, E.; Ogura, T.; Ota, Z. Characteristics of rat kidney dopamine receptors and the effects of renal denervation and dopamine infusion on these receptors. Nephron 1989, 53, 358–363. [Google Scholar] [CrossRef]
- Ohbu, K.; Felder, R.A. DA1 dopamine receptors in renal cortical collecting duct. Am. J. Physiol. 1991, 261, 890–895. [Google Scholar] [CrossRef]
- Han, F.; Konkalmatt, P.; Chen, J.; Gildea, J.; Felder, R.A.; Jose, P.A.; Armando, I. MiR-217 mediates the protective effects of the dopamine D2 receptor on fibrosis in human renal proximal tubule cells. Hypertens. 2015, 65, 1118–1125. [Google Scholar] [CrossRef]
- Gao, D.Q.; Canessa, L.M.; Mouradian, M.M.; Jose, P.A. Expression of the D2 subfamily of dopamine receptor genes in kidney. Am. J. Physiol. Renal Physiol. 1994, 266, 646–650. [Google Scholar] [CrossRef]
- Nürnberger, A.; Räbiger, M.; Mack, A.; Diaz, J.; Sokoloff, P.; Mühlbauer, B.; Luippold, G. Subapical Localization of the Dopamine D3Receptor in Proximal Tubules of the Rat Kidney. J. Histochem. Cytochem. 2004, 52, 1647–1655. [Google Scholar] [CrossRef]
- Ricci, A.; Marchal-Victorion, S.; Bronzetti, E.; Parini, A.; Amenta, F.; Tayebati, S.K. Dopamine D4 receptor expression in rat kidney: Evidence for pre- and postjunctional localization. J. Histochem. Cytochem. 2002, 50, 1091–1096. [Google Scholar] [CrossRef]
- Shin, Y.; Kumar, U.; Patel, Y.; Patel, S.C.; Sidhu, A. Differential expression of D2-like dopamine receptors in the kidney of the spontaneously hypertensive rat. J. Hypertens. 2003, 21, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Schafer, J.A.; Li, L.; Sun, D. The collecting duct, dopamine and vasopressin-dependent hypertension. Acta Physiol. Scand. 2000, 168, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Armando, I.; Yu, P.; Escano, C.; Mueller, S.C.; Asico, L.; Pascua, A.; Lu, Q.; Wang, X.; Villar, V.A.M.; et al. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J. Clin. Investig. 2008, 118, 2180–2189. [Google Scholar] [CrossRef]
- Goldberg, L.I. Cardiovascular and renal actions of dopamine: Potential clinical applications. Pharmacol. Rev. 1972, 24, 1–29. [Google Scholar]
- McCormick, J.A.; Ellison, D.H. Distal convoluted tubule. Compr. Physiol. 2015, 5, 45–98. [Google Scholar]
- Kiryluz, K. Renal function and genetic variation in dopamine D1 receptor: Is the case strong enough? Kidney Int. 2009, 86, 1019–1022. [Google Scholar] [CrossRef][Green Version]
- Gildea, J.J.; Shah, I.T.; Van Sciver, R.E.; Israel, J.A.; Enzensperger, C.; McGrath, H.E.; Jose, P.A.; Felder, R.A. The cooperative roles of the dopamine receptors, D1R and D5R, on the regulation of renal sodium transport. Kidney Int. 2014, 86, 118–126. [Google Scholar] [CrossRef]
- Zhang, Y.; Cuevas, S.; Asico, L.D.; Escano, C.; Yang, Y.; Pascua, A.M.; Wang, X.; Jones, J.E.; Grandy, D.; Eisner, G.; et al. Deficient Dopamine D2 Receptor Function Causes Renal Inflammation Independently of High Blood Pressure. PLoS ONE 2012, 7, e38745. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.-L.P.; Koch, A.; Mühlbauer, B.; Adamczak, M.; Ziebart, H.; Drescher, K.; Gross, G.; Berger, I.; Amann, K.U.; Ritz, E. Renoprotective effect of a dopamine D3 receptor antagonist in experimental type II diabetes. Lab. Investig. 2006, 86, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Deng, K.; Wang, X.; Wang, Z.; Zheng, S.; Ren, H.; He, D.; Han, Y.; Asico, L.D.; Jose, P.A.; et al. Activation of D 4 Dopamine Receptor Decreases Angiotensin II Type 1 Receptor Expression in Rat Renal Proximal Tubule Cells. Hypertens. 2015, 65, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Olsen, N.V.; Olsen, M.H.; Bonde, J.; Kanstrup, I.-L.; Plum, I.; Strandgaard, S.; Leyssac, P.P. Dopamine natriuresis in salt-repleted, water-loaded humans: A dose-response study. Br. J. Clin. Pharmacol. 1997, 43, 509–520. [Google Scholar] [CrossRef]
- Yatsu, T.; Arai, Y.; Takizawa, K.; Kasai-Nakagawa, C.; Takanashi, M.; Uchida, W.; Inagaki, O.; Tanaka, A.; Asano, M.; Honda, K.; et al. Renal effect of YM435, a new dopamine D1 receptor agonist, in anesthetized dogs. Eur. J. Pharmacol. 1997, 322, 45–53. [Google Scholar] [CrossRef]
- Yatsu, T.; Takizawa, K.; Kasai-Nakagawa, C.; Uchida, W.; Tanaka, A.; Asano, M.; Honda, K.; Takenaka, T. Hemodynamic Characterization of YM435, a Novel Dopamine DA1 Receptor Agonist, in Anesthetized Dogs. J. Cardiovasc. Pharmacol. 1997, 29, 382–388. [Google Scholar] [CrossRef]
- Lang, W.J.; Woodman, O.L. Comparison of the vasodilator action of dopamine and dopamine agonists in the renal and coronary beds of the dog. Br. J. Pharmacol. 1982, 77, 23–28. [Google Scholar] [CrossRef]
- Leigh, F.S.M.; Young, J.B. Evidence that circulating 3,4 dihydroyphenylalanine (dopa) is not of neuronal origin. Clin. Res. 1990, 38, 342A. [Google Scholar]
- Alkadhi, K.A.; Sabouni, M.H.; Ansari, A.F.; Lokhandwala, M.F. Activation of DA1 receptors by dopamine or fenoldopam increases cyclic AMP levels in the renal artery but not in the superior cervical ganglion of the rat. J. Pharmacol. Exp. Ther. 1988, 238, 547–553. [Google Scholar]
- Han, G.; Kryman, J.P.; McMillin, P.J.; White, R.E.; Carrier, G.O. A novel transduction mechanism mediating dopamine-induced vascular relaxation: Opening of BKCa channels by cyclic AMP-induced stimulation of the cyclic GMP-dependent protein kinase. J. Cardiovasc. Pharmacol. 1999, 34, 619–627. [Google Scholar] [CrossRef] [PubMed]
- White, R.E.; Kryman, J.P.; El-Mowafy, A.M.; Han, G.; Carrier, G.O. cAMP dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BK(Ca) channel activity in coronary artery smooth muscle cells. Circ. Res. 2000, 86, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, U.; Chen, C.; Lokhandwala, M.F. The role of intrarenal nitric oxide in the natriuretic response to dopamine-receptor activation. Clin. Exp. Hypertens. 2000, 22, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Weinbaum, S.; Weinstein, A.M. Regulation of glomerulotubular balance: Flow-activated proximal tubule function. Pflugers Arch. 2017, 469, 643–654. [Google Scholar] [CrossRef]
- Rashed, S.M.; Songu-Mize, E. Regulation of Na(+)-pump activity by dopamine in rat tail arteries. Eur. J. Pharmacol. 1995, 284, 289–297. [Google Scholar] [CrossRef]
- Amenta, F. Light microscope autoradiography of peripheral dopamine receptor subtypes. Clin. Exp. Hypertens. 1997, 19, 27–41. [Google Scholar] [CrossRef]
- Cavallotti, C.; Nuti, F.; Bruzzone, P.; Mancone, M. Age-related changes in dopamine D2 receptors in rat heart and coronary vessels. Clin. Exp. Pharmacol. Physiol. 2002, 29, 412–418. [Google Scholar] [CrossRef]
- Sanada, H.; Yao, L.; Jose, P.A.; Carey, R.M.; Felder, R.A. Dopamine D3 receptors in rat juxtaglomerular cells. Clin. Exp. Hypertens. 1997, 19, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Lokhandwala, M.F.; Steenberg, M.L. Selective activation by LY141865 and apomorphine of presynaptic dopamine receptors in the rat kidney and influence of stimulation parameters in the action of dopamine. J. Pharmacol. Exp. Ther. 1984, 228, 161–167. [Google Scholar]
- Dupont, A.G.; Vanderniepen, P.; Lefebvre, R.A.; Bogaert, M.G. Pharmacological characterization of neuronal dopamine receptors in the rat hindquarters, renal and superior mesenteric vascular beds. J. Auton. Pharmacol. 1986, 6, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, L.I.; Kohli, J.D.; Glock, D. Conclusive evidence for two subtypes of peripheral dopamine receptors. In Dopaminergic Systems and Their Regulation; The MacMillan Press Ltd.: London, UK, 1986; pp. 195–212. [Google Scholar]
- Bughi, S.; Jost-Vu, E.; Antonipillai, I.; Nadler, J.; Horton, R. Effect of dopamine 2 blockade on renal function under varied sodium intake. J. Clin. Endocrinol. Metab. 1994, 78, 1079–1084. [Google Scholar] [PubMed]
- Neve, K.A.; Kozlowski, M.R.; Rosser, M.P. Dopamine D2 receptor stimulation of Na+/H +exchange assessed by quantification of extracellular acidification. J. Biol. Chem 1992, 267, 25748–25753. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, D.; Asico, L.D.; Welch, W.J.; Wilcox, C.S.; Hopfer, U.; Eisner, G.M.; Felder, R.A.; Jose, P.A. Aberrant D 1 and D 3 Dopamine Receptor Transregulation in Hypertension. Hypertens. 2004, 43, 654–660. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, D.; Yang, Z.; Wang, Z.; Asico, L.D.; Wilcox, C.S.; Eisner, G.M.; Welch, W.J.; Felder, R.A.; Jose, P.A. Dopamine D 1 Receptor Augmentation of D 3 Receptor Action in Rat Aortic or Mesenteric Vascular Smooth Muscles. Hypertens. 2004, 43, 673–679. [Google Scholar] [CrossRef]
- Jose, P.A.; Eisner, G.M.; Drago, J.; Carey, R.M.; Felder, R.A. Dopamine receptor signaling defects in spontaneous hypertension. Am. J. Hypertens. 1996, 9, 400–405. [Google Scholar] [CrossRef]
- Siragy, H.M.; Felder, R.A.; Howell, N.L.; Chevalier, R.L.; Peach, M.J.; Carey, R.M. Evidence that intrarenal dopamine acts as a paracrine substance at the renal tubule. Am. J. Physiol. 1989, 257, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.; Ward, A.S.; Foltin, R.W.; Fischman, M.W. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology 2001, 155, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Asico, L.D.; Yu, P.; Wang, Z.; Jones, J.E.; Escano, C.S.; Wang, X.; Quinn, M.T.; Sibley, D.R.; Romero, G.G.; et al. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am. J. Physiol. Integr. Comp. Physiol. 2006, 290, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Yang, Z.; Asico, L.D.; Jose, P.A. Regulation of blood pressure by D5 dopamine receptors. Cardiovasc. Hematol. Agents Med. Chem. 2007, 5, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guan, W.; Han, Y.; Ren, H.; Tang, X.; Zhang, H.; Liu, Y.; Fu, J.; He, D.; Asico, L.D.; et al. Stimulation of Dopamine D3 Receptor Attenuates Renal Ischemia-Reperfusion Injury via Increased Linkage With Gα12. Transplant. 2015, 99, 2274–2284. [Google Scholar] [CrossRef]
- Choi, M.R.; Kouyoumdzian, N.M.; Rukavina Mikusic, N.L.; Kravetz, M.C.; Rosón, M.I.; Rodríguez Fermepin, M.; Enrique Fernández, B. Renal dopaminergic system: Pathophysiological implications and clinical perspectives. World J. Nephrol. 2015, 4, 196–212. [Google Scholar] [CrossRef]
- Zeng, C.; Asico, L.D.; Yu, C.; Villar, V.A.M.; Shi, W.; Luo, Y.; Wang, Z.; He, D.; Liu, Y.; Huang, L.; et al. Renal D3 dopamine receptor stimulation induces natriuresis by endothelin B receptor interactions. Kidney Int. 2008, 74, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, C.; Asico, L.D.; Villar, V.A.M.; Ren, H.; He, D.; Wang, Z.; Yang, J.; Jose, P.A.; Zeng, C. Role of Gα12- and Gα13-protein subunit linkage of D3 dopamine receptors in the natriuretic effect of D3 dopamine receptor in kidney. Hypertens. Res. 2011, 34, 1011–1016. [Google Scholar] [CrossRef]
- Bacic, D.; Kaissling, B.; McLeroy, P.; Zou, L.; Baum, M.; Moe, O.W. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int. 2003, 64, 2133–2141. [Google Scholar] [CrossRef]
- Gomes, P.; Soares-Da-Silva, P. D2-like receptor-mediated inhibition of Na+-K+-ATPase activity is dependent on the opening of K+ channels. Am. J. Physiol. Renal Physiol. 2002, 283, 114–123. [Google Scholar] [CrossRef]
- Pedrosa, R.; Jose, P.A.; Soares-da-Silva, P. Defective D1-like receptor-mediated inhibition of the Cl-/HCO3-exchanger in immortalized SHR proximal tubular epithelial cells. Am. J. Physiol. Renal Physiol. 2004, 286, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Carranza, A.; Nowicki, S.; Barontini, M.; Armando, I. L-Dopa uptake and dopamine production in proximal tubular cells are regulated by b2- adrenergic receptors. Am. J. Physiol. Renal Physiol. 2000, 279, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, W.C.; Jin, L.; Xie, K.Q.; Zhu, X.Z. Activation of adenosine A1 receptor modulates dopamine D1 receptor activity in stably cotransfected human embryonic kidney 293 cells. Eur. J. Pharmacol. 2006, 548, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Ferre, S.; Agnati, L.; Torvinen, M.; Gines, S.; Hillion, J.; Casado, V.; Lledo, P.-M.; Zoli, M.; Lluis, C.; et al. Evidence for Adenosine/Dopamine Receptor Interactions: Indications for Heteromerization. Neuropsychopharmacology 2000, 23, S50–S59. [Google Scholar] [CrossRef]
- Häberle, D.A.; Königbauer, B.; Kawabata, M.; Ushiogi, Y. Renal blood flow control by tubuloglomerular feedback (TGF) in normal and spontaneously hypertensive rats—A role for dopamine and adenosine. Klin. Wochenschr. 1991, 69, 587–596. [Google Scholar] [CrossRef]
- Asico, L.D.; Ladines, C.; Fuchs, S.; Accili, D.; Carey, R.M.; Semeraro, C.; Pocchiari, F.; Felder, R.A.; Eisner, G.M.; Jose, P.A. Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J. Clin. Investig. 1998, 102, 493–498. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Yao, L.; Sanada, H.; Ozono, R.; Mouradian, M.M.; Carey, R.M.; Jose, P.A.; Felder, R.A. Characterization of dopamine D1A receptors in rat juxtaglomerular cells. Hypertension 1997, 29, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Bek, M.J.; Wang, X.; Asico, L.D.; Jones, J.E.; Zheng, S.; Li, X.; Eisner, G.M.; Grandy, D.K.; Carey, R.M.; Soares-Da-Silva, P.; et al. Angiotensin-II Type 1 Receptor–Mediated Hypertension in D 4 Dopamine Receptor–Deficient Mice. Hypertens. 2006, 47, 288–295. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Chen, W.; Jiang, X.; Zhang, Y.; Wang, Z.; Yang, J.; Jones, J.E.; Jose, P.A.; Yang, Z. Regulation of blood pressure, oxidative stress and AT1R by high salt diet in mutant human dopamine D5 receptor transgenic mice. Hypertens. Res. 2015, 38, 394–399. [Google Scholar] [CrossRef]
- Chugh, G.; Pokkunuri, I.; Asghar, M. Renal dopamine and angiotensin II receptor signaling in age-related hypertension. Am. J. Physiol. Renal Physiol. 2013, 304, F1–F7. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.R.; Eisner, G.M.; Armando, I.; Browning, S.; Pezzullo, J.C.; Rhee, L.; Dajani, M.; Carey, R.M.; Jose, P.A. The Renin-Angiotensin and Renal Dopaminergic Systems Interact in Normotensive Humans. J. Am. Soc. Nephrol. 2015, 27, 265–279. [Google Scholar] [CrossRef][Green Version]
- Lefevre-Borg, F.; Lorrain, J.; Lechaire, J.; Thiry, C.; Hicks, P.E.; Cavero, I. Studies on the mechanisms of the development of tolerance to the hypotensive effects of fenoldopam in rats. J. Cardiovasc. Pharmacol. 1988, 11, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Yang, Z.; Wang, Z.; Jones, J.; Wang, X.; Altea, J.; Mangrum, A.J.; Hopfer, U.; Sibley, D.R.; Eisner, G.M.; et al. Interaction of AT1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension 2005, 45, 804–810. [Google Scholar] [CrossRef]
- Hirata, Y.; Fukui, K.; Hayakawa, H.; Suzuki, E.; Sugimoto, T.; Kimura, K.; Matsuoka, H.; Sugimoto, E.S.T. Renal Effects of Atrial Natriuretic Peptide During Dopamine Infusion. Am. J. Hypertens. 1990, 3, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Hansell, P.; Fasching, A.; Sjöquist, M.; Andén, N.E.; Ulfendahl, H.R. The dopamine receptor antagonist haloperidol blocks natriuretic but not hypotensive effects of the atrial natriuretic factor. Acta Physiol. Scand. 1987, 130, 401–407. [Google Scholar] [CrossRef]
- Lucarini, A.R.; Arrighi, P.; Favilla, S.; Simonini, N.; Salvetti, A. The influence of dopamine-1 receptor blockade on the humoral and renal effects of low-dose atrial natriuretic factor in human hypertensives. J. Hypertens. 1989, 7, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yang, Z.; Ren, H.; Zhang, Y.; Han, Y.; He, D.; Lu, Q.; Wang, X.; Wang, X.; Yang, C.; et al. D3 dopamine receptor regulation of ETB receptors in renal proximal tubule cells from WKY and SHRs. Am. J. Hypertens. 2009, 22, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Armando, I.; Asico, L.D.; Wang, X.; Jones, J.E.; Serrão, M.P.; Cuevas, S.; Grandy, D.K.; Soares-Da-Silva, P.; Jose, P.A. Antihypertensive effect of etamicastat in dopamine D2 receptor-deficient mice. Hypertens. Res. 2018, 41, 489–498. [Google Scholar] [CrossRef]
- Lokhandwala, M.F.; Hussain, T. Defective renal dopamine D1-like receptor signal transduction in obese hypertensive rats. Acta Physiol. Scand. 2000, 168, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, K.; Hosoya, H.; Takano, S.; Ohta, M.; Sekime, A.; Kanai, S.; Matsui, T.; Funakoshi, A. DIFFERENCES IN ETHANOL INGESTION BETWEEN CHOLECYSTOKININ-A RECEPTOR DEFICIENT AND -B RECEPTOR DEFICIENT MICE. Alcohol Alcohol. 2005, 40, 176–180. [Google Scholar] [CrossRef]
- Li, H.; Han, W.; Villar, V.A.M.; Keever, L.B.; Lu, Q.; Hopfer, U.; Quinn, M.T.; Felder, R.A.; Jose, P.A.; Yu, P. D1-Like Receptors Regulate NADPH Oxidase Activity and Subunit Expression in Lipid Raft Microdomains of Renal Proximal Tubule Cells. Hypertens. 2009, 53, 1054–1061. [Google Scholar] [CrossRef]
- Han, W.; Li, H.; Villar, V.A.M.; Pascua, A.M.; Dajani, M.I.; Wang, X.; Natarajan, A.; Quinn, M.T.; Felder, R.A.; Jose, P.A.; et al. Lipid Rafts Keep NADPH Oxidase in the Inactive State in Human Renal Proximal Tubule Cells. Hypertens. 2008, 51, 481–487. [Google Scholar] [CrossRef]
- Yu, P.; Han, W.X.; Sun, M.; Villar, V.A.M.; Jose, P.A. Protein kinase C inhibits NADPH oxidase activity via cross-talk with protein kinase A in HEK-293 heterologously expressing D1 receptor cells. J. Am. Soc. Nephrol. 2009, 20, 533A. [Google Scholar]
- Lu, Q.; Yang, Y.; Villar, V.A.; Asico, L.D.; Jones, J.E.; Yu, P.; Li, H.; Weinman, E.J.; Eisner, G.M.; Jose, P.A. D5 dopamine receptor decreases NADPH oxidase, reactive oxygen species and blood pressure via heme oxygenase-1. Hypertens. Res. 2013, 36, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Bellner, L.; Martinelli, L.; Halilovic, A.; Patil, K.; Puri, N.; Dunn, M.W.; Regan, R.F.; Schwartzman, M.L. Heme Oxygenase-2 Deletion Causes Endothelial Cell Activation Marked by Oxidative Stress, Inflammation, and Angiogenesis. J. Pharmacol. Exp. Ther. 2009, 331, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, S.; Zhang, Y.; Yang, Y.; Escano, C.; Asico, L.; Jones, J.E.; Armando, I.; Jose, P.A. Role of Renal DJ-1 in the Pathogenesis of Hypertension Associated With Increased Reactive Oxygen Species Production. Hypertens. 2012, 59, 446–452. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Cuevas, S.; Villar, V.A.; Escano, C.S.; Asico, L.D.; Yu, P.; Grandy, D.K.; Felder, R.A.; Armando, I.; et al. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free. Radic. Biol. Med. 2012, 53, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Armando, I.; Wang, X.; Villar, V.A.M.; Jones, J.E.; Asico, L.D.; Escano, C.; Jose, P.A. Reactive Oxygen Species–Dependent Hypertension in Dopamine D 2 Receptor–Deficient Mice. Hypertens. 2007, 49, 672–678. [Google Scholar] [CrossRef]
- Yang, S.; Yang, Y.; Yu, P.; Yang, J.; Jiang, X.; Villar, V.A.M.; Sibley, D.R.; Jose, P.A.; Zeng, C. Dopamine D1 and D5 receptors differentially regulate oxidative stress through paraoxonase 2 in kidney cells. Free. Radic. Res. 2015, 49, 397–410. [Google Scholar] [CrossRef]
- Niewiarowska-Sendo, A.; Kozik, A.; Guevara-Lora, I. Influence of bradykinin B2 receptor and dopamine D2 receptor on the oxidative stress, inflammatory response, and apoptotic process in human endothelial cells. PLoS ONE 2018, 13, e0206443. [Google Scholar] [CrossRef]
- Zeng, C.; Jose, P.A. Dopamine receptors: Important antihypertensive counterbalance against hypertensive factors. Hypertension 2011, 57, 11–17. [Google Scholar] [CrossRef]
- Albrecht, F.E.; Drago, J.; Felder, R.A.; Printz, M.P.; Eisner, G.M.; Robillard, J.E.; Sibley, D.R.; Westphal, H.J.; Jose, P.A. Role of the D1A dopamine receptor in the pathogenesis of genetic hypertension. J. Clin. Investig. 1996, 97, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Ladines, C.A.; Zeng, C.; Asico, L.D.; Sun, X.; Pocchiari, F.; Semeraro, C.; Pisegna, J.; Wank, S.; Yamaguchi, I.; Eisner, G.M.; et al. Impaired renal D(1)-like and D(2)-like dopamine receptor interaction in the spontaneously hypertensive rat. Am. J. Physiol. Integr. Comp. Physiol. 2001, 281, R1071–R1078. [Google Scholar] [CrossRef]
- O’Connell, D.P.; Ragsdale, N.V.; Boyd, D.G.; Felder, R.A.; Carey, R.M. Differential human renal tubular responses to dopamine type 1 receptor stimulation are determined by blood pressure status. Hypertension 1997, 29, 115–122. [Google Scholar] [CrossRef]
- Felder, R.A.; Sanada, H.; Xu, J.; Yu, P.-Y.; Wang, Z.; Watanabe, H.; Asico, L.D.; Wang, W.; Zheng, S.; Yamaguchi, I.; et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc. Natl. Acad. Sci. USA 2002, 99, 3872–3877. [Google Scholar] [CrossRef]
- Vandell, A.G.; Lobmeyer, M.T.; Gawronski, B.E.; Langaee, T.Y.; Gong, Y.; Gums, J.G.; Beitelshees, A.L.; Turner, S.T.; Chapman, A.B.; Cooper-DeHoff, R.M.; et al. G protein receptor kinase 4 polymorphisms: β-blocker pharmacogenetics and treatment-related outcomes in hypertension. Hypertension 2012, 60, 957–964. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Liu, S.; Yang, L. Association between GRK4 and DRD1 gene polymorphisms and hypertension: A meta-analysis. Clin. Interv. Aging 2015, 11, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Sodium sensitivity of blood pressure in Chinese populations. Curr. Hypertens. Rep. 2010, 12, 127–134. [Google Scholar] [CrossRef]
- Du, B.; Jia, X.; Tian, W.; Yan, X.; Wang, N.; Cai, D.; Li, X.; Zhang, H.; Jin, M.; Wu, N.; et al. Associations of SUCNR1, GRK4, CAMK1D gene polymorphisms and the susceptibility of type 2 diabetes mellitus and essential hypertension in a northern Chinese Han population. J. Diabetes its Complicat. 2020, 35, 107752. [Google Scholar] [CrossRef]
- Luippold, G.; Zimmermann, C.; Mai, M.; Kloor, D.; Starck, D.; Gross, G.; Mühlbauer, B. Dopamine D(3) receptors and salt-dependent hypertension. J. Am. Soc. Nephrol. 2001, 12, 2272–2279. [Google Scholar]
- Carey, R.M. Renal dopamine system. Paracrine regulator of sodium homeostasis and blood pressure. Hypertension 2001, 38, 297–302. [Google Scholar] [CrossRef]
- US Renal Data System. USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2008. [Google Scholar]
- Vallon, V.; Richter, K.; Blantz, R.C.; Thomson, S.; Osswald, H. Glomerular hyperfiltration in experimental diabetes mellitus: Potential role of tubular reabsorption. J. Am. Soc. Nephrol. 1999, 10, 2569–2576. [Google Scholar]
- Thomson, S.C.; Vallon, V.; Blantz, R.C. Kidney function in early diabetes: The tubular hypothesis of glomerular filtration. Am. J. Physiol. Renal Physiol. 2004, 286, 8–15. [Google Scholar] [CrossRef]
- Hussain, T.; Beheray, S.A.; Lokhandwala, M.F. Defective dopamine receptor function in proximal tubules of obese zucker rats. Hypertension 1999, 34, 1091–1096. [Google Scholar] [CrossRef]
- Kuzhikandathil, E.V.; Clark, L.; Li, Y. The extracellular cAMP adenosine pathway regulates expression of renal D1 dopamine receptors in diabetic rats. J. Biol. Chem. 2011, 286, 32454–32463. [Google Scholar] [CrossRef] [PubMed]
- Barthelmebs, M.; Mayer, P.; Thomas, A.; Grima, M.; Imbs, J.L. Pathophysiological role of dopamine in the kidney: Effects in diabetes mellitus and after contralateral nephrectomy. Hypertens. Res. 1995, 18, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Luippold, G.; Beilharz, M.; Mühlbauer, B. Reduction of glomerular hyperfiltration by dopamine D(2)-like receptor blockade in experimental diabetes mellitus. Nephrol. Dial. Transplant. 2001, 16, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Marinosci, G.Z.; De Robertis, E.; De Benedictis, G.; Piazza, O. Dopamine Use in Intensive Care: Are We Ready to Turn it Down? Transl. Med. UniSa 2012, 4, 90–94. [Google Scholar]
- Chamorro, C.; Romera, M.A.; Martinez-Melgar, J.L.; Pardo, C.; Silva, J.A. Dopamine dose and renal damage. Lancet 2001, 357, 1707–1708. [Google Scholar] [CrossRef]
- Lee, M.R. Dopamine and the kidney: Ten years on. Clin. Sci. 1993, 84, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Beheray, S.; Kansra, V.; Hussain, T.; Lokhandwala, M.F. Diminished natriuretic response to dopamine in old rats is due to an impaired D1-like receptor-signaling pathway. Kidney Int. 2000, 58, 712–720. [Google Scholar] [CrossRef] [PubMed]

| RECEPTOR | D1-Like | D2-Like | |||
|---|---|---|---|---|---|
| D1 | D5 | D2 | D3 | D4 | |
| Gene | DRD1 | DRD5 | DRD2 | DRD3 | DRD4 |
| Length (amino acids) | 446 | 477 | 443 | 400 | 419 |
| Structural information | Intronless | Intronless | 7 exons | 7 exons | 4 exons |
| Chromosomal localization | 5q 34.2 | 4p16.1 | 11q23.2 | 3q13.31 | 11p15.5 |
| Locations | CNS and kidneys | CNS, kidneys, heart, blood vessels, adrenal glands, gastrointestinal tract, sympathetic ganglia | CNS, kidneys, cortex, heart, blood vessels, adrenal glands, gastrointestinal tract, sympathetic ganglia. | CNS, kidneys, gastrointestinal tract, mast cells. | CNS, kidneys, heart, blood vessels, adrenal glands, gastrointestinal tract, sympathetic ganglia |
| Type (G protein coupling) | Gs-coupled | Gs-coupled | Gi-coupled | Gi-coupled | Gi-coupled |
| Function | Actions dependent on CNS and control of HTN [2,13] | Actions dependent on CNS, control of HTN and endocrine functions [14] | Actions dependent on CNS, renal functions (control of HTN), gastrointestinal motility [15] | Actions dependent on CNS, control of HTN and endocrine functions [16,17] | Actions dependent on CNS, regulations of renal functions (control of HTN) and gastrointestinal motility [18] |
| Mechanism | cAMP (+) | cAMP (+) | cAMP (-) | cAMP (-) | cAMP (-) |
| Synaptic location | Postsynaptic | Both pre- and postsynaptic | |||
| Selective agonist | A-86929 [19] A-68930Doxanthrine | Same as D1 | Apomorphine [20] Ropinirole (DRD2>DRD3) | 7- oH-DPAT (DRD3>DRD2) ML417 | A-412997 [21] ABT-670 PD-168077 |
| Selective antagonist | SCH-23390 [22] SCH-39166 SKF-83566 | Same as D1 | Haloperidol Raclopride Sulpiride Spiperone Risperidone | Nafadotride GR-103691 GR-218231 SB-277011-A [23] NGB-2904 PG-01037 ABT-127 | A-381393 FAUC213 L-745870 L-750667 |
| RECEPTOR | D1-Like | D2-Like | |||
|---|---|---|---|---|---|
| D1 | D5 | D2 | D3 | D4 | |
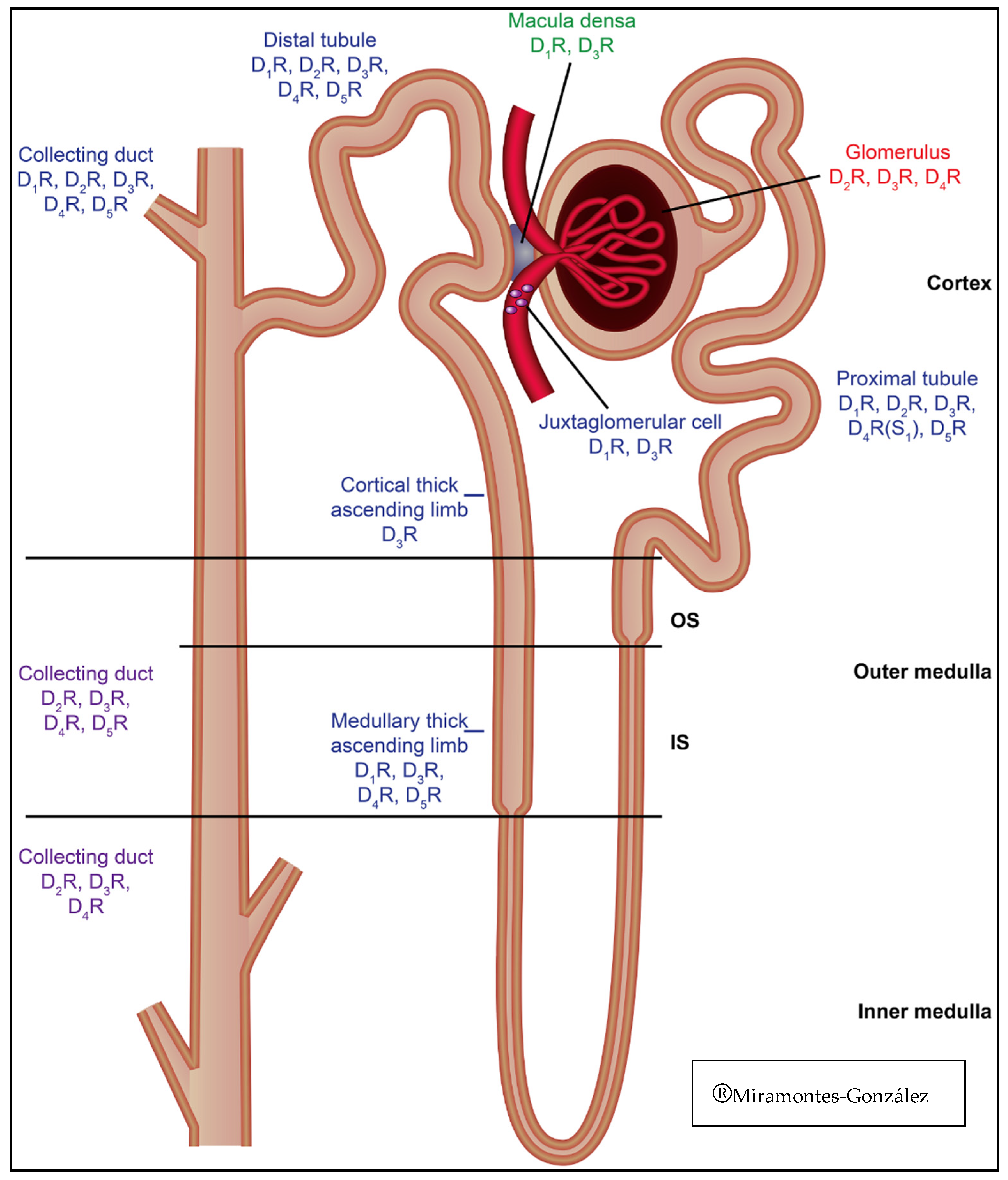
| Nephron distribution | Collecting duct. Distal tubule (including medullary thick ascending limb) [56]. Macula densa. Juxtaglomerular cell. Proximal tubule | Collecting duct. Distal tubule (including medullary thick ascending limb) [56]. Proximal tubule | Collecting Duct. Distal tubule. Proximal tubule. Glomerulus | Collecting duct. Distal tubule (including medullary thick ascending limb) [56]. Cortical thick ascending limb. Macula densa. Juxtaglomerular cell. Proximal tubule. Glomerulus | Collecting duct. Distal tubule (including medullary thick ascending limb) [56]. Proximal tubule. Glomerulus |
| Physiological responses | Inhibition of sodium transport in kidneys [57,58] and gastrointestinal tract. Vasodilation. Inhibition of AT1 receptor expression | Inhibition of sodium transport in kidneys and AT1 receptor expression [58] | Inhibition of sodium transport in kidneys.Antagonizes angiotensin II [59] | Inhibition of sodium transport in kidney [60].Inhibition of AT1 receptor expression and renin excretion. Vasodilation | Antagonize vasopressin- and aldosterone-dependent water and sodium reabsorption in the cortical collecting duct [61]. Inhibition of AT1 receptor expression |
| Characteristics of gene knockout mice | Hypertension. Sodium retention | Hypertension. Increased sympathetic activity. Sodium retention | Hypertension. Sodium retention | Hypertension. Sodium retention. Increased activities of α-adrenergic and ETB receptors | Hypertension with increased renal AT1 receptor expression |
| Dopamine Receptor Subfamily | Dopamine Receptor Subtype | Pro-Oxidant Enzymes (Inhibition) | Anti-Oxidant Enzymes (Stimulation) |
|---|---|---|---|
| D1-like | D1 receptor | NADPH oxidase, via PKA/PKC cross talk [122] | SOD, gluthatione peroxidase, glutamyl cysteine transferase, and HO-1 [122] |
| D5 receptor | NADPH oxidase, via PLD2 [122] | SOD, gluthatione peroxidase, glutamyl cysteine transferase, and HO-1 [122] | |
| D2-like | D2 receptor | NADPH oxidase [123] | DJ-1, PON2, HO-2 glutathione, catalase, and SOD [123] |
| D3 receptor | Unknown function | Unknown function | |
| D4 receptor | Unknown function | Unknown function |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares-Hernández, A.; Figuero-Pérez, L.; Cruz-Hernandez, J.J.; González Sarmiento, R.; Usategui-Martin, R.; Miramontes-González, J.P. Dopamine Receptors and the Kidney: An Overview of Health- and Pharmacological-Targeted Implications. Biomolecules 2021, 11, 254. https://doi.org/10.3390/biom11020254
Olivares-Hernández A, Figuero-Pérez L, Cruz-Hernandez JJ, González Sarmiento R, Usategui-Martin R, Miramontes-González JP. Dopamine Receptors and the Kidney: An Overview of Health- and Pharmacological-Targeted Implications. Biomolecules. 2021; 11(2):254. https://doi.org/10.3390/biom11020254
Chicago/Turabian StyleOlivares-Hernández, Alejandro, Luis Figuero-Pérez, Juan Jesus Cruz-Hernandez, Rogelio González Sarmiento, Ricardo Usategui-Martin, and José Pablo Miramontes-González. 2021. "Dopamine Receptors and the Kidney: An Overview of Health- and Pharmacological-Targeted Implications" Biomolecules 11, no. 2: 254. https://doi.org/10.3390/biom11020254
APA StyleOlivares-Hernández, A., Figuero-Pérez, L., Cruz-Hernandez, J. J., González Sarmiento, R., Usategui-Martin, R., & Miramontes-González, J. P. (2021). Dopamine Receptors and the Kidney: An Overview of Health- and Pharmacological-Targeted Implications. Biomolecules, 11(2), 254. https://doi.org/10.3390/biom11020254








