Oligosaccharides Derived from Tramesan: Their Structure and Activity on Mycotoxin Inhibition in Aspergillus flavus and Aspergillus carbonarius
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Growth Conditions
2.2. Purification of Polysaccharides Produced by T. versicolor
2.3. Tramesan© Oligosaccharide Production and Characterization of Tramesan© Oligosaccharides
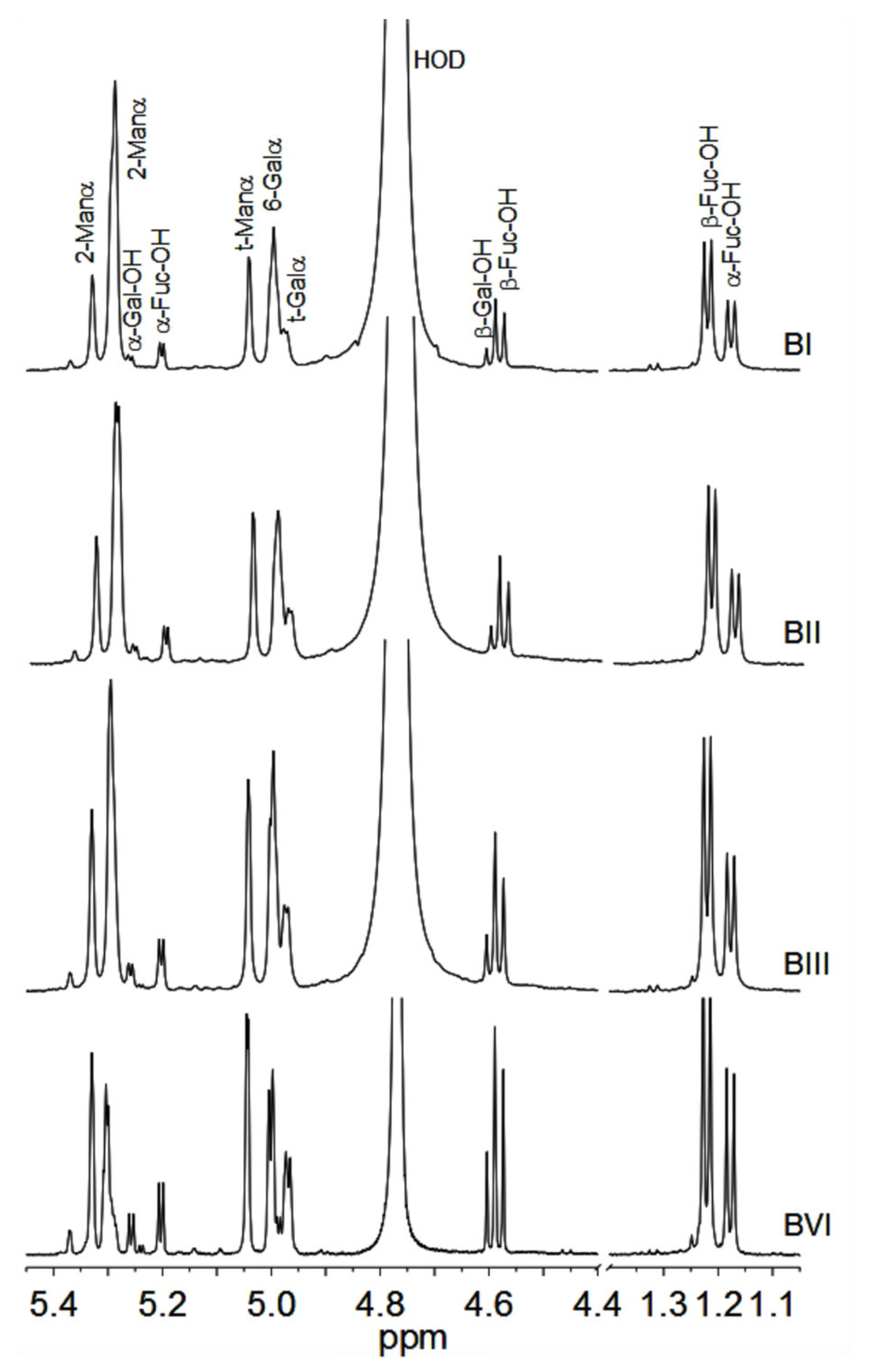
2.4. NMR Spectroscopy
2.5. ESI Mass Spectrometry
2.6. Inhibition of Aflatoxin B1 and OTA Biosynthesis in A. flavus 3357 and A. carbonarius by Tramesan© Oligosaccharide Fractions
3. Results
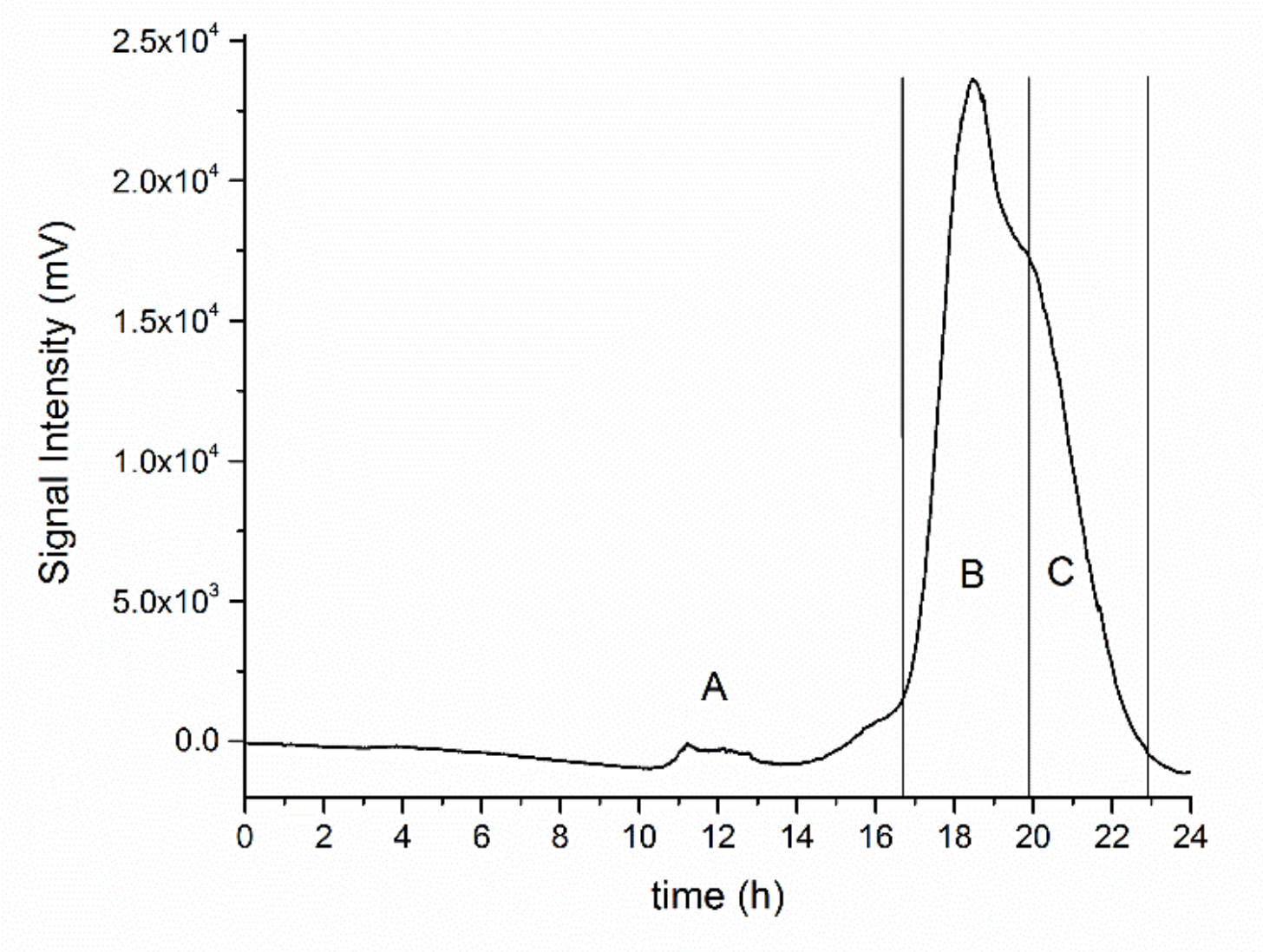
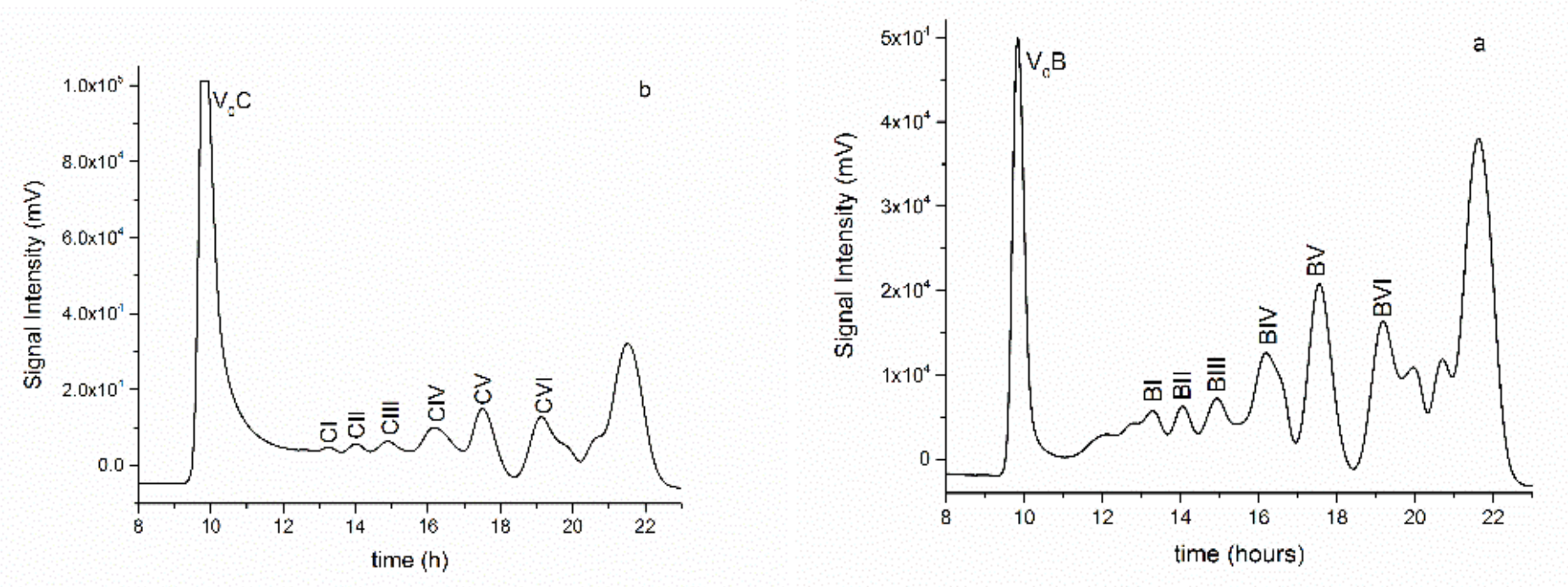
3.1. Production and Purification of Oligosaccharides from Tramesan©
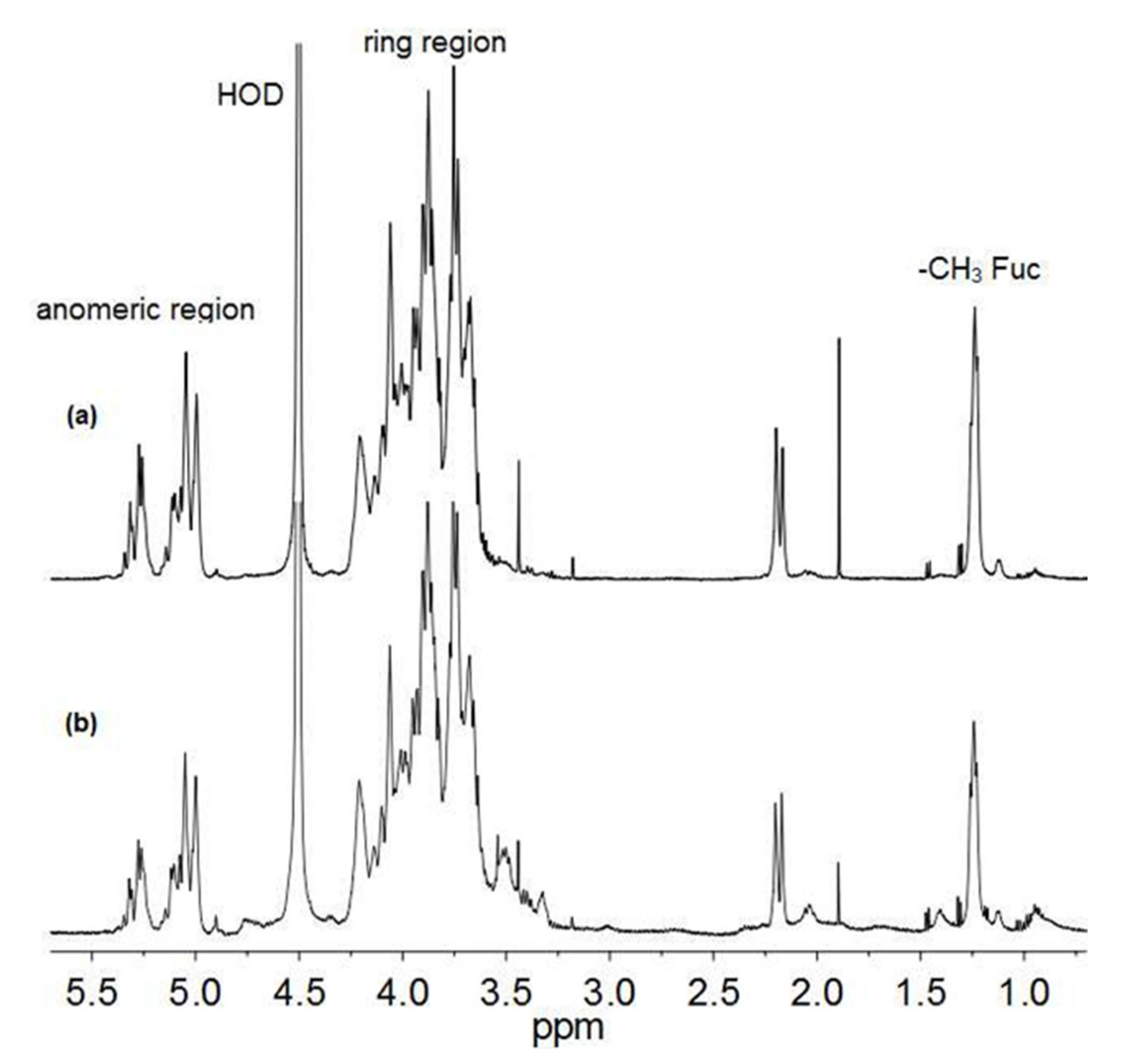
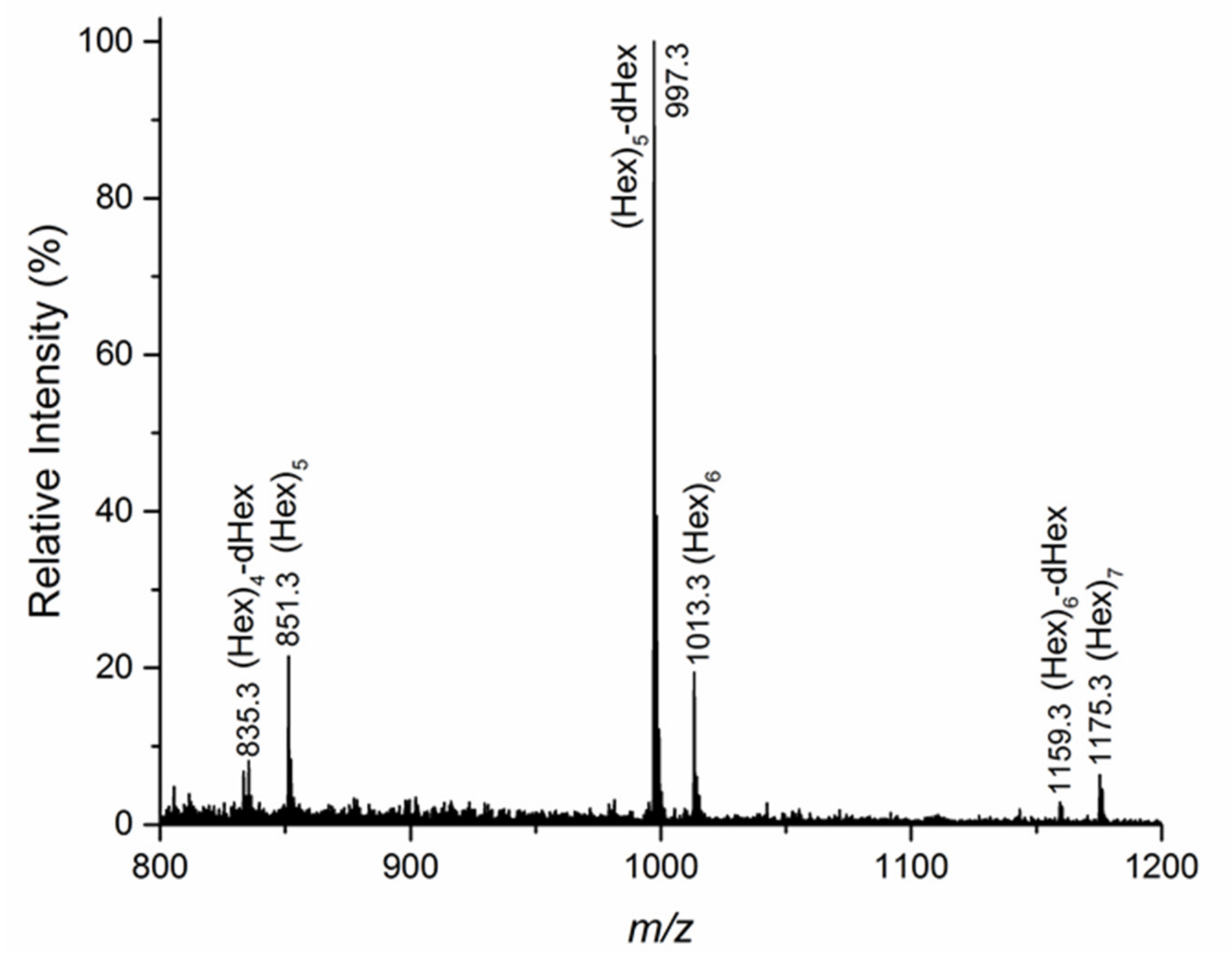
3.2. Structural Characterization of the Oligosaccharides Obtained after Mild Acid Hydrolysis of Tramesan©
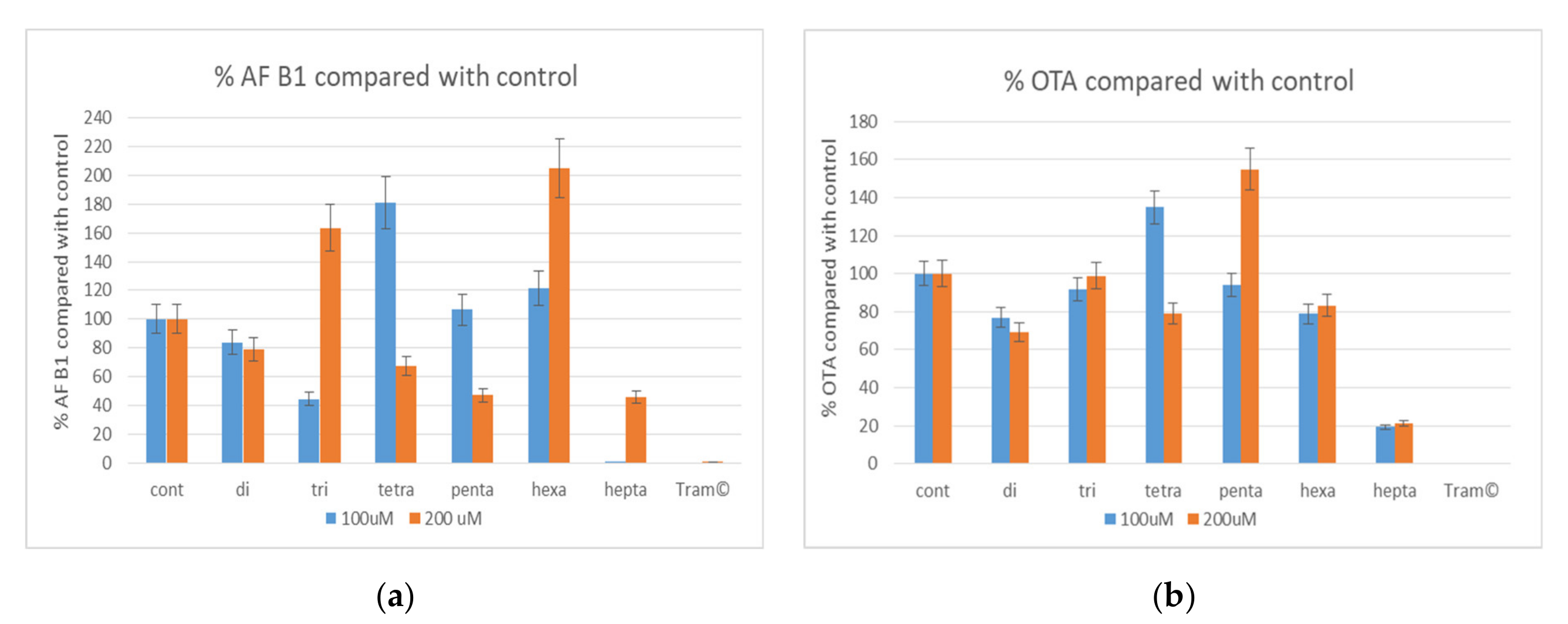
3.3. AFB1 and OTA Inhibition Assay for the Oligosaccharides of Tramesan©
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef]
- Dellafiora, L.; Dall’Asta, C. Forthcoming challenges in mycotoxins toxicology research for safer food-a need for multi-omics approach. Toxins 2017, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Medina, A.; Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre-and postharvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EU) No 105/2010 of 5 February 2010 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Ochratoxin AEU no 105/2010. Available online: http://www.efsa.europa.eu/en/scdocs/doc/contam_op_ej365_ (accessed on 30 December 2020).
- COMMISSION REGULATION (EU) No 165/2010 of 26 February 2010 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxins” EN Official Journal of the European Union L 50/8. Available online: http://www.efsa (accessed on 30 December 2020).
- Dellafiora, L.; Dall’Asta, C. Masked mycotoxins: An emerging issue that makes renegotiable what is ordinary. Food Chem. 2016, 213, 534–535. [Google Scholar] [CrossRef] [PubMed]
- Newberne, P.M. Mycotoxins: Toxicity, carcinogenicity, and the influence of various nutritional conditions. Environ. Health Perspect. 1974, 9, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Stark, A. Microbiol of Mycotoxins: DNA binding as a possible mode of action structure-activity relationship in aflatoxins and related compounds. Annu. Rev. Microbiol. 1980, 34, 235–262. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Grosse, Y.; Roubal, T.; Skarkova, J.; Ruprich, J. Monitoring the mycotoxins in food and their biomarkers in the Czech Republic. Mol. Nutr. Food Res. 2006, 50, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: A largely ignored global health issue. J. Carcinog. 2009, 31, 71–82. [Google Scholar] [CrossRef]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-year Odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011, 120, 28–48. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Abrar, M.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Randhawa, M.A.; Saeed, F.; Waqas, K. Aflatoxins: Biosynthesis, occurrence, toxicity, and remedies. Crit. Rev. Food Sci. Nutr. 2013, 53, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Scarpari, M.; Parroni, A.; Zaccaria, M.; Fattorini, L.; Bello, C.; Fabbri, A.A.; Bianchi, G.; Scala, V.; Zjalic, S.; Fanelli, C. Trametes versicolor bioactive compounds stimulate Aspergillus flavus antioxidant system and inhibit aflatoxin synthesis. Plant Biosyst. 2016, 150, 653–659. [Google Scholar] [CrossRef]
- Duarte, S.C.; Pena, A.; Lino, C.M. Human ochratoxin a biomarkers-from exposure to effect. Crit. Rev. Toxicol. 2011, 41, 187–212. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, A.E.; Atoui, A. Ochratoxin A: General overview and actual molecular status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef] [PubMed]
- Barug, D.; Van Egmond, H.; Van Osenbruggen, T.; Visconti, A. Meeting the Mycotoxin; Wageningen Academic Publishers: Wageningen, The Netherlands, 2004; pp. 295–305. ISBN 978-90-76998-28-2. [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Naturally Occurring Substances: Food Items and Con- Stituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993; pp. 1–599. [Google Scholar]
- Directive 2009/128/EC on the Sustainable Use of Pesticides European Implementation Assessment; Milan Remáč, European Parliamentary Research Service: Brussels, Belgium, 2018; ISBN 978-92-846-3330-2.
- Lakshmeesha, T.R.; Kalagatur, N.K.; Mudili, V.; Mohan, C.D.; Rangappa, S.; Prasad, B.D.; Ashwini, B.S.; Hashem, A.; Alqarawi, A.A.; Malik, J.A.; et al. Biofabrication of zinc oxide nanoparticles with Syzygium aromaticum flower buds extract and finding its novel application in controlling the growth and mycotoxins of Fusarium graminearum. Front. Microbiol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Degola, F.; Marzouk, B.; Gori, A.; Brunetti, C.; Dramis, L.; Gelati, S.; Buschini, A.; Restivo, F.M. Aspergillus flavus as a model system to test the biological activity of botanicals: An example on Citrullus colocynthis L. Schrad. organic extracts. Toxins 2019, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.V.; Tarazona, A.; Mateo-Castro, R.; Gimeno-Adelantado, J.V.; Jiménez, M.; Mateo, E.M. Selected plant essential oils and their main active components, a promising approach to inhibit aflatoxigenic fungi and aflatoxin production in food. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 1581–1595. [Google Scholar] [CrossRef] [PubMed]
- Elhouiti, F.; Tahri, D.; Takhi, D.; Ouinten, M.; Barreau, C.; Verdal-Bonnin, M.N.; Bombarda, I.; Yousfi, M. Variability of composition and effects of essential oils from Rhanterium adpressum Coss. & Durieu against mycotoxinogenic Fusarium strains. Arch. Microbiol. 2017, 199, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Parroni, A.; Bellabarba, A.; Beccaccioli, M.; Scarpari, M.; Reverberi, M.; Infantino, A. Use of the Secreted Proteome of Trametes versicolor for Controlling the Cereal Pathogen Fusarium langsethiae. Int. J. Mol. Sci. 2019, 20, 4167. [Google Scholar] [CrossRef]
- Reverberi, M.; Zjalic, S.; Ricelli, A.; Punelli, F.; Camera, E.; Fabbri, C.; Picardo, M.; Fanelli, C.; Fabbri, A.A. Modulation of antioxidant defense in Aspergillus parasiticus is involved in aflatoxin biosynthesis: A role for the Ap yapA gene. Eukaryot. Cell 2008, 7, 988–1000. [Google Scholar] [CrossRef]
- Christensen, S.A.; Kolomiets, M.V. The lipid language of plant-fungal interactions. Fungal Genet. Biol. 2011, 48, 4–14. [Google Scholar] [CrossRef]
- Islam, T.; Ganesan, K.; Xu, B. New insight into mycochemical profiles and antioxidant potential of edible and medicinal mushrooms: A review. Int. J. Med. Mushrooms 2019, 21, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Matsunaga, K.; Fujii, M. PSK as a chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 1993, 2, 271–276. [Google Scholar] [PubMed]
- Sun, C.; Wang, J.W.; Fang, L.; Gao, X.D.; Tan, R.X. Free radical scavenging and antioxidant activities of EPS2, an exopolysaccharide produced by a marine filamentous fungus Keissleriella sp. YS 4108. Life Sci. 2004, 75, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Zjalic, S.; Reverberi, M.; Ricelli, A.; Granito, M.V.; Fanelli, C.; Fabbri, A.A. Trametes versicolor: A possible tool for aflatoxin control. Int. J. Food Microbiol. 2006, 107, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Li, Y.; Zhou, T.; Xu, D.P.; Zhang, P.; Li, S.; Li, H.B. Bioactivities and health benefits of mushrooms mainly from China. Molecules 2016, 21, 938. [Google Scholar] [CrossRef]
- Scarpari, M.; Reverberi, M.; Parroni, A.; Scala, V.; Fanelli, C.; Pietricola, C.; Zjalic, S.; Maresca, V.; Tafuri, A.; Ricciardi, M.R.; et al. Tramesan, a novel polysaccharide from Trametes versicolor. Structural characterization and biological effects. PLoS ONE 2017, e0171412. [Google Scholar] [CrossRef] [PubMed]
- Ricelli, A.; Fabbri, A.A.; Trionfetti-Nisini, P.; Reverberi, M.; Zjalic, S.; Fanelli, C. Inhibiting effect of different edible and medicinal mushrooms on the growth of two ochratoxigenic microfungi. Int. J. Med. Mushrooms 2002, 4, 8. [Google Scholar] [CrossRef]
- Reverberi, M.; Fabbri, A.A.; Zjalic, S.; Ricelli, A.; Punelli, F.; Fanelli, C. Antioxidant enzymes stimulation in Aspergillus parasiticus by Lentinula edodes inhibits aflatoxin production. Appl. Microbiol. Biotechnol. 2005, 69, 207–215. [Google Scholar] [CrossRef]
- Reverberi, M.; Zjalic, S.; Ricelli, A.; Di Meo, C.; Scarpari, M.; Fanelli, C.; Fabbri, A.A. Mushrooms versus fungi: Natural compounds from Lentinula edodes inhibit aflatoxin biosynthesis by Aspergillus parasiticus. World Mycotoxin J. 2011, 4, 217–224. [Google Scholar] [CrossRef]
- Scarpari, M.; Parroni, A.; Zaccaria, M.; Fattorini, L.; Bello, C.; Fabbri, A.A.; Bianchi, G.; Scala, V.; Zjalic, S.; Fanelli, C. Aflatoxin control in maize by Trametes versicolor. Toxins 2014, 6, 3426–3437. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Zjalic, S.; Punelli, F.; Ricelli, A.; Fabbri, A.A.; Fanelli, C. Apyap1 affects aflatoxin biosynthesis during Aspergillus parasiticus growth in maize seeds. Food Addit. Contam. 2007, 24, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Punelli, M.; Scala, V.; Scarpari, M.; Uva, P.; Mentzen, W.I.; Dolezal, A.L.; Woloshuk, C.; Pinzari, F.; Fabbri, A.A.; et al. Genotypic and phenotypic versatility of Aspergillus flavus during maize exploitation. PLoS ONE 2013, 8, e68735. [Google Scholar] [CrossRef]
- Bock, K.; Lundt, I.; Pedersen, C. Assignment of anomeric structure to carbohydrates through geminal13C-H coupling constants. Tetrahedron Lett. 1973, 14, 1037–1040. [Google Scholar] [CrossRef]
- Fanelli, C.; Fabbri, A.A.; Passi, S. Aflatoxin production by Aspergillus flavus during incubation with lipid sources in culture media. Trans. Br. Mycol. Soc. 1981, 77, 416–419. [Google Scholar] [CrossRef]
- Giorni, P.; Magan, N.; Pietri, A.; Battilani, P. Growth and aflatoxin production of an Italian strain of Aspergillus flavus: Influence of ecological factors and nutritional substrates. World Mycotoxin J. 2011, 4, 425–432. [Google Scholar] [CrossRef]
- Passi, S.; Nazzaro-Porro, M.; Fanelli, C.; Fabbri, A.A.; Fasella, P. Role of lipoperoxidation in aflatoxin production. Appl. Microbiol. Biotechnol. 1984, 19, 186–190. [Google Scholar] [CrossRef]
- Fabbri, A.A.; Fanelli, C.; Panfili, G. Lipoperoxidation and aflatoxin biosynthesis by Aspergillus parasiticus and A. flavus. J. Gen. Microbiol. 1983, 129, 3447–3452. [Google Scholar] [CrossRef]
- Luchese, R.H.; Harrigan, W.F. Biosynthesis of aflatoxin. J. Appl. Microbiol. 1993, 74, 5–14. [Google Scholar]
- Liu, J.; Sun, L.; Zhang, N.; Zhang, J.; Guo, J.; Li, C.; Rajput, S.A.; Qi, D. Effects of nutrients in substrates of different grains on aflatoxin B1 production by Aspergillus flavus. Biomed. Res. Int. 2016. [Google Scholar] [CrossRef]
- Davis, N.D.; Diener, U.L.; Agnihotri, V.P. Production of aflatoxins B1 and G1 in chemically defined medium. Mycopathol. Mycol. Appl. 1967, 31, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, Z.; Song, C.; Hu, Y.; Han, Z.; She, J.; Fan, F.; Wang, J.; Jin, C.; Chang, J.; et al. Chitin-induced dimerization activates a plant immune receptor. Science 2012, 336, 1160–1164. [Google Scholar] [CrossRef]
- Hayafunea, M.; Berisiob, R.; Marchettic, R.; Silipoc, A.; Kayamaa, M.; Desakia, Y.; Arimaa, S.; Squegliab, F.; Ruggierob, A.; Tokuyasud, K.; et al. Chitin-induced activation of immune signaling by the rice receptor CEBiP relies on a unique sandwich-type dimerization. Proc. Natl. Acad. Sci. USA 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Scala, V.; Pietricola, C.; Farina, V.; Beccaccioli, M.; Zjalic, S.; Quaranta, F.; Fornara, M.; Zaccaria, M.; Momeni, B.; Reverberi, M.; et al. Tramesan elicits durum wheat defense against the Septoria disease complex. Biomolecules 2020, 10, 608. [Google Scholar] [CrossRef] [PubMed]
| Tramesan Fractions | Size | Composition of Oligosaccharides |
|---|---|---|
| BVI and CVI | disaccharides | Hex-dHex and (Hex)2 |
| BV and CV | trisaccharides | (Hex)2-dHex and (Hex)3 |
| BIV and CIV | tetrasccharides | (Hex)3-dHex and (Hex)4 |
| BIII and CIII | pentasaccharides | (Hex)4-dHex and (Hex)5 |
| BII and CII | hexasaccharides | (Hex)5-dHex and (Hex)6 |
| BI and CI | heptasaccharides | (Hex)6-dHex and (Hex)7 |
| Tramesan Fractions | Size | Composition of Oligosaccharides |
|---|---|---|
| BVI and CVI | disaccharides | αMan-Fuc-OH and αGal-Gal-OH |
| BV and CV | trisaccharides | (αMan)2-Fuc-OH and (αGal)2-Gal-OH |
| BIV and CIV | tetrasccharides | (αMan)3-Fuc-OH and (αGal)3-Gal-OH |
| BIII and CIII | pentasaccharides | (αMan)4-Fuc-OH and (αGal)4-Gal-OH |
| BII and CII | hexasaccharides | (αMan)5-Fuc-OH and (αGal)5-Gal-OH |
| BI and CI | heptasaccharides | (αMan)6-Fuc-OH and (αGal)6-Gal-OH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loncar, J.; Bellich, B.; Parroni, A.; Reverberi, M.; Rizzo, R.; Zjalić, S.; Cescutti, P. Oligosaccharides Derived from Tramesan: Their Structure and Activity on Mycotoxin Inhibition in Aspergillus flavus and Aspergillus carbonarius. Biomolecules 2021, 11, 243. https://doi.org/10.3390/biom11020243
Loncar J, Bellich B, Parroni A, Reverberi M, Rizzo R, Zjalić S, Cescutti P. Oligosaccharides Derived from Tramesan: Their Structure and Activity on Mycotoxin Inhibition in Aspergillus flavus and Aspergillus carbonarius. Biomolecules. 2021; 11(2):243. https://doi.org/10.3390/biom11020243
Chicago/Turabian StyleLoncar, Jelena, Barbara Bellich, Alessia Parroni, Massimo Reverberi, Roberto Rizzo, Slaven Zjalić, and Paola Cescutti. 2021. "Oligosaccharides Derived from Tramesan: Their Structure and Activity on Mycotoxin Inhibition in Aspergillus flavus and Aspergillus carbonarius" Biomolecules 11, no. 2: 243. https://doi.org/10.3390/biom11020243
APA StyleLoncar, J., Bellich, B., Parroni, A., Reverberi, M., Rizzo, R., Zjalić, S., & Cescutti, P. (2021). Oligosaccharides Derived from Tramesan: Their Structure and Activity on Mycotoxin Inhibition in Aspergillus flavus and Aspergillus carbonarius. Biomolecules, 11(2), 243. https://doi.org/10.3390/biom11020243







