Surface Modification of Basalt Fibres with ZnO Nanorods and Its Effect on Thermal and Mechanical Properties of PLA-Based Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of ZnO Nanorods on Basalt Fabrics
2.3. Morphological Characterization by Scanning Electron Microscopy (SEM)
2.4. X-ray Diffraction (XRD) Analysis
2.5. Static Contact Angle Measurements
2.6. Composite Manufacturing
2.7. Mechanical Characterization of Composite Laminates
2.8. Thermal Characterization
3. Results and Discussion
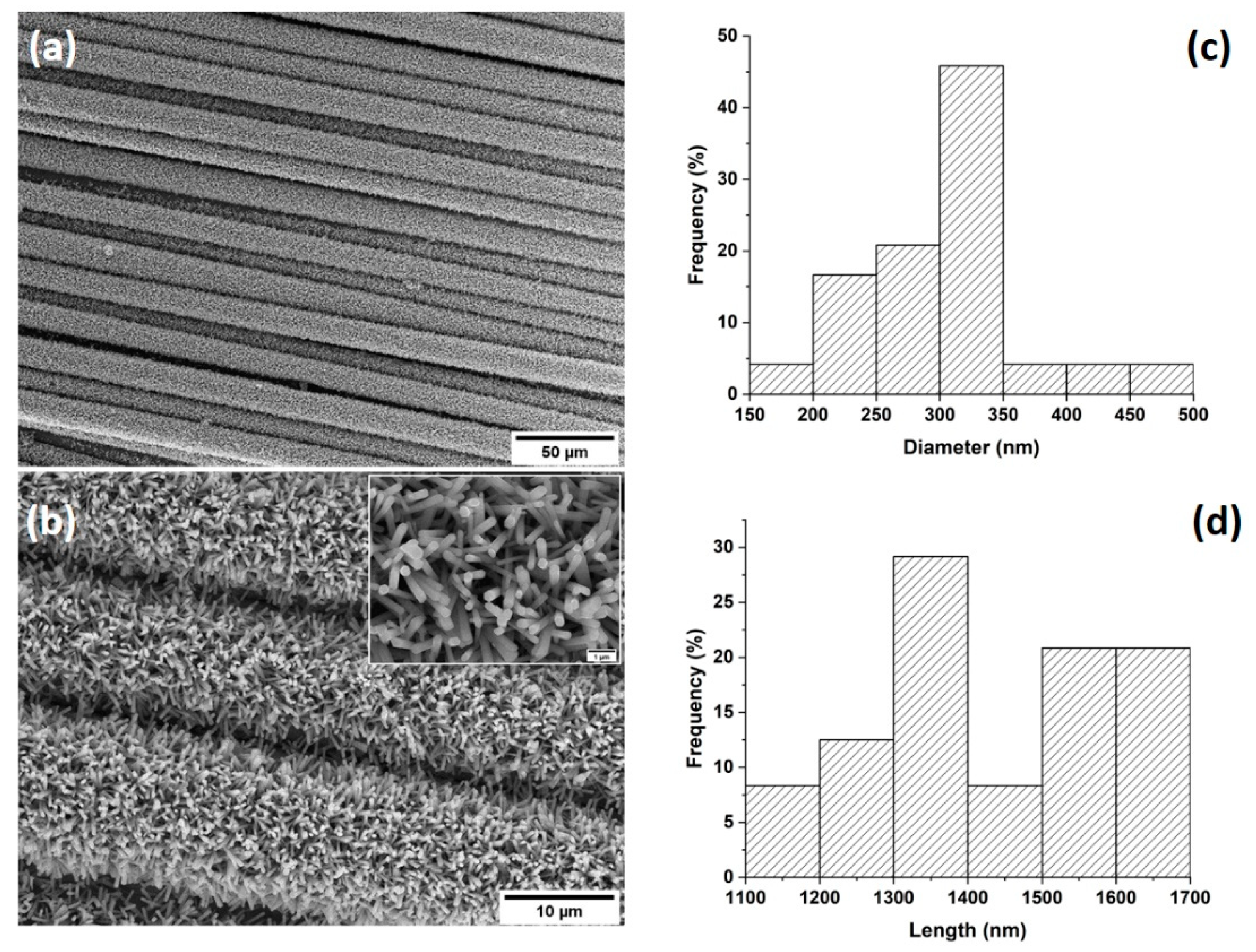
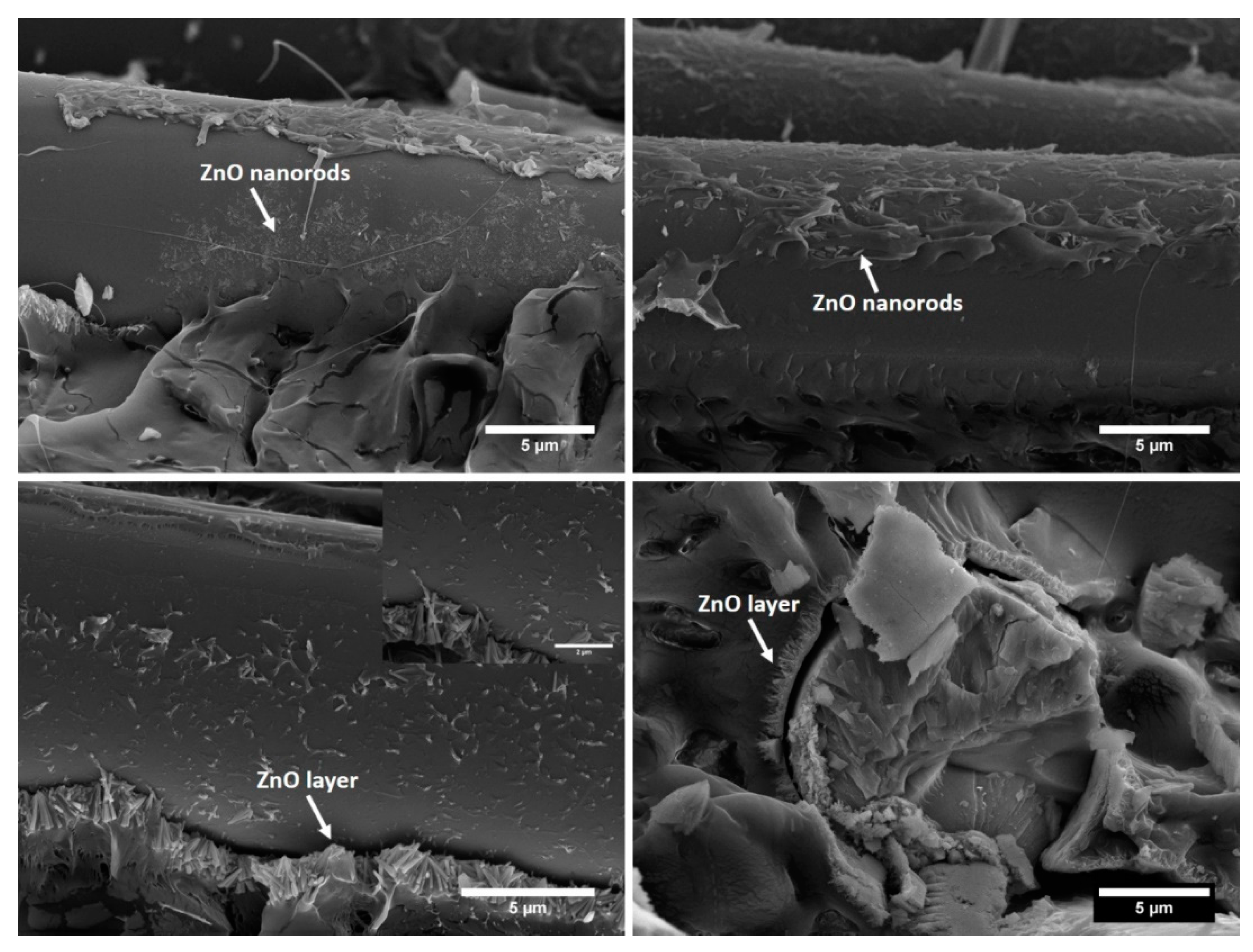
3.1. Characterization of ZnO-Decorated Basalt Fabrics
3.2. Thermal Characterization of PLA-Based Composites
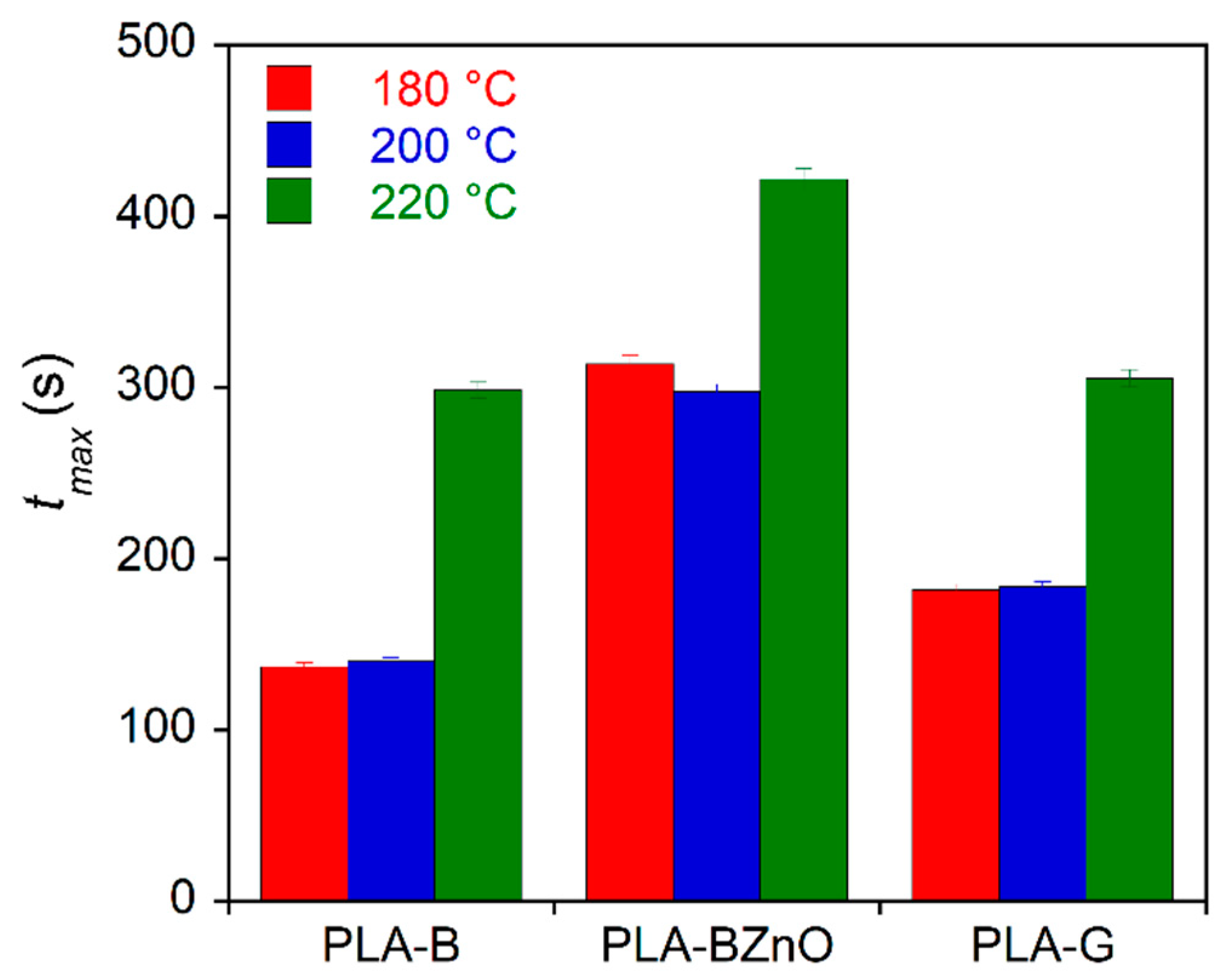
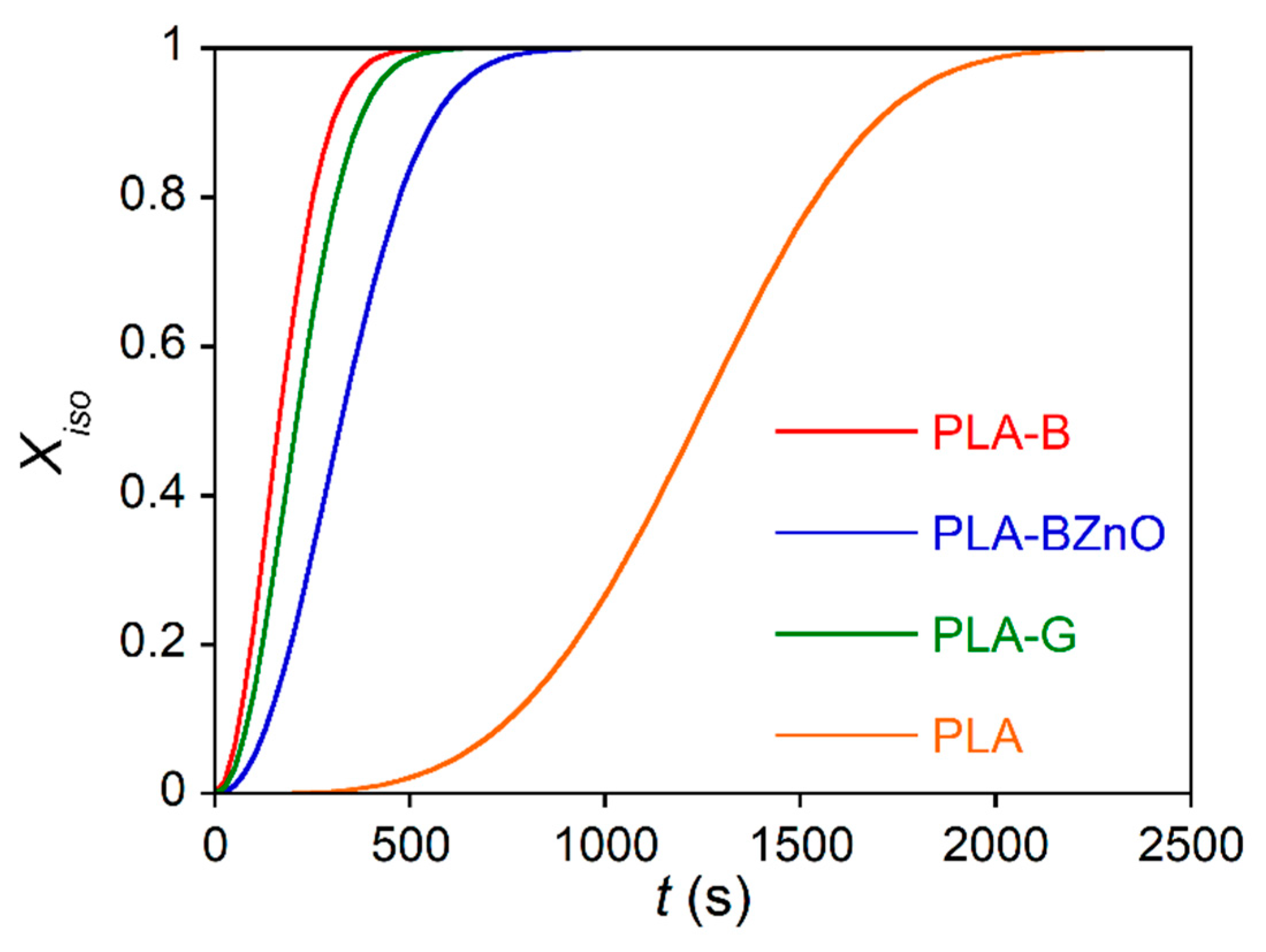
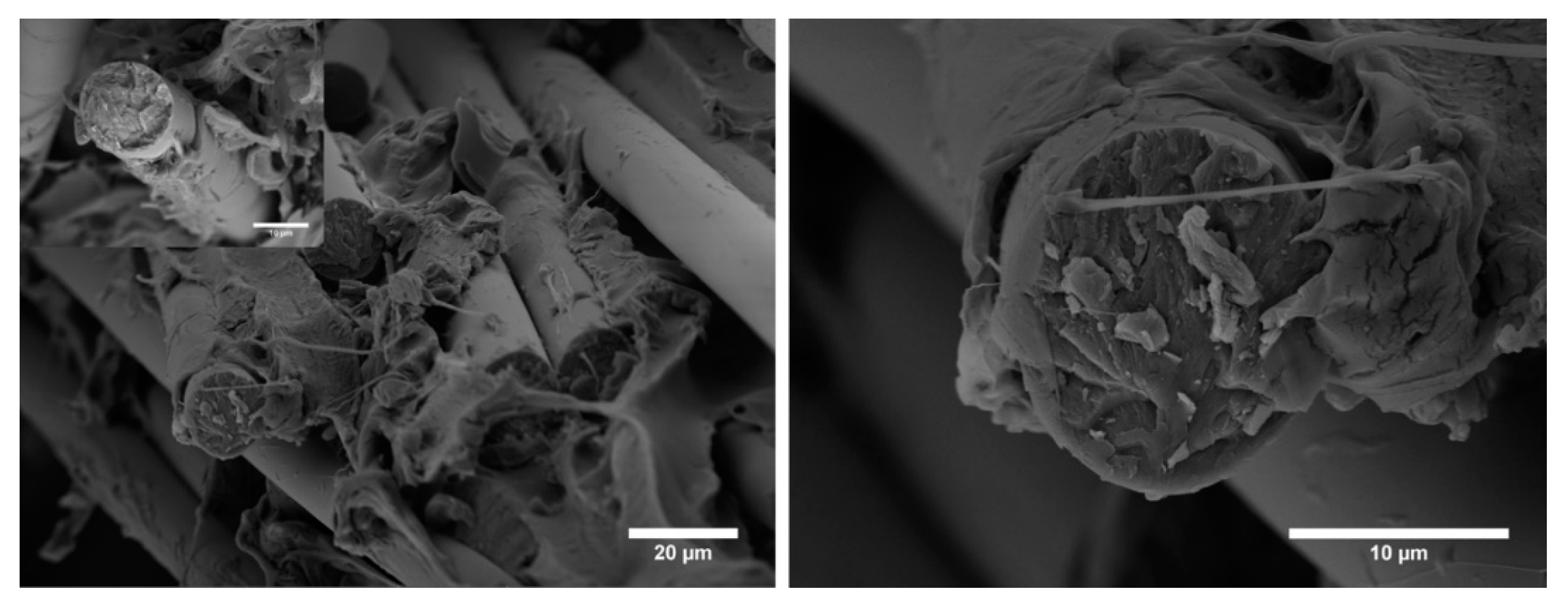
3.3. Mechanical Characterization of Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pickering, K.L.; Aruan Efendy, M.G.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Chatiras, N.; Georgiopoulos, P.; Christopoulos, A.; Kontou, E. Thermomechanical characterization of basalt fiber reinforced biodegradable polymers. Polym. Compos. 2019, 40, 4340–4350. [Google Scholar] [CrossRef]
- Eselini, N.; Tirkes, S.; Akar, A.O.; Tayfun, U. Production and characterization of poly (lactic acid)-based biocomposites filled with basalt fiber and flax fiber hybrid. J. Elastomers Plast. 2020, 52, 701–716. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Matykiewicz, D.; Kloziński, A.; Andrzejewski, J.; Piasecki, A. Synergistic effect of different basalt fillers and annealing on the structure and properties of polylactide composites. Polym. Test. 2020, 89, 106628. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Jaszkiewicz, A.; Scherzer, D. Mechanical properties of PLA composites with man-made cellulose and abaca fibres. Compos. Part A Appl. Sci. Manuf. 2009, 40, 404–412. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Piorkowska, E.; Kulpinski, P.; Pracella, M. Mechanical and thermal properties of PLA composites with cellulose nanofibers and standard size fibers. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1509–1514. [Google Scholar] [CrossRef]
- Nassiopoulos, E.; Njuguna, J. Thermo-mechanical performance of poly(lactic acid)/flax fibre-reinforced biocomposites. Mater. Des. 2015, 66, 473–485. [Google Scholar] [CrossRef]
- Ferreira, R.T.L.; Amatte, I.C.; Dutra, T.A.; Bürger, D. Experimental characterization and micrography of 3D printed PLA and PLA reinforced with short carbon fibers. Compos. Part B Eng. 2017, 124, 88–100. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Wan, G.; Li, B.; Zhao, G. Glass fiber reinforced PLA composite with enhanced mechanical properties, thermal behavior, and foaming ability. Polymer (Guildf). 2019, 181, 121803. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Li, B.; Wan, G.; Zhao, G.; Zhang, A. Strong and thermal-resistance glass fiber-reinforced polylactic acid (PLA) composites enabled by heat treatment. Int. J. Biol. Macromol. 2019, 129, 448–459. [Google Scholar] [CrossRef]
- Aliotta, L.; Gigante, V.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A.; Seggiani, M. Thermo-Mechanical Properties of PLA/Short Flax Fiber Biocomposites. Appl. Sci. 2019, 9, 3797. [Google Scholar] [CrossRef]
- Chaitanya, S.; Singh, I.; Song, J. Il Recyclability analysis of PLA/Sisal fiber biocomposites. Compos. Part B Eng. 2019, 173, 106895. [Google Scholar] [CrossRef]
- Oksman, K.; Skrifvars, M.; Selin, J.-F. Natural fibres as reinforcement in polylactic acid (PLA) composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- Wei, B.; Cao, H.; Song, S. Tensile behavior contrast of basalt and glass fibers after chemical treatment. Mater. Des. 2010, 31, 4244–4250. [Google Scholar] [CrossRef]
- Liu, S.; Wu, G.; Yu, J.; Chen, X.; Guo, J.; Zhang, X.; Wang, P.; Yin, X. Surface modification of basalt fiber (BF) for improving compatibilities between BF and poly lactic acid (PLA) matrix. Compos. Interfaces 2019, 26, 275–290. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Gu, N. A novel basalt fiber-reinforced polylactic acid composite for hard tissue repair. Biomed. Mater. 2010, 5, 044104. [Google Scholar] [CrossRef]
- Liu, S.Q.; Yu, J.J.; Wu, G.H.; Wang, P.; Liu, M.F.; Zhang, Y.; Zhang, J.; Yin, X.L.; Li, F.; Zhang, M. Effect of silane KH550 on interface of basalt fibers (BFs)/poly (lactic acid) (PLA) composites. Ind. Textila 2019, 70, 408–412. [Google Scholar] [CrossRef]
- Ying, Z.; Wu, D.; Zhang, M.; Qiu, Y. Polylactide/basalt fiber composites with tailorable mechanical properties: Effect of surface treatment of fibers and annealing. Compos. Struct. 2017, 176, 1020–1027. [Google Scholar] [CrossRef]
- Kurniawan, D.; Kim, B.S.; Lee, H.Y.; Lim, J.Y. Atmospheric pressure glow discharge plasma polymerization for surface treatment on sized basalt fiber/polylactic acid composites. Compos. Part B Eng. 2012, 43, 1010–1014. [Google Scholar] [CrossRef]
- He, H.; Yang, P.; Duan, Z.; Wang, Z.; Liu, Y. Reinforcing effect of hybrid nano-coating on mechanical properties of basalt fiber/poly(lactic acid) environmental composites. Compos. Sci. Technol. 2020, 199, 108372. [Google Scholar] [CrossRef]
- Förster, T.; Hao, B.; Mäder, E.; Simon, F.; Wölfel, E.; Ma, P.-C.; Förster, T.; Hao, B.; Mäder, E.; Simon, F.; et al. CVD-Grown CNTs on Basalt Fiber Surfaces for Multifunctional Composite Interphases. Fibers 2016, 4, 28. [Google Scholar] [CrossRef]
- Ehlert, G.J.; Galan, U.; Sodano, H.A. Role of Surface Chemistry in Adhesion between ZnO Nanowires and Carbon Fibers in Hybrid Composites. ACS Appl. Mater. Interfaces 2013, 5, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.; Eslami-Farsani, R.; Khosravi, H. Evaluating the Mechanical Behavior of Basalt Fibers/Epoxy Composites Containing Surface-modified CaCO3 Nanoparticles. Fibers Polym. 2018, 19, 635–640. [Google Scholar] [CrossRef]
- Kuzmin, K.L.; Timoshkin, I.A.; Gutnikov, S.I.; Zhukovskaya, E.S.; Lipatov, Y.V.; Lazoryak, B.I. Effect of silane/nano-silica on the mechanical properties of basalt fiber reinforced epoxy composites. Compos. Interfaces 2017, 24, 13–34. [Google Scholar] [CrossRef]
- Nasser, J.; Steinke, K.; Sodano, H. ZnO Nanostructured Interphase for Multifunctional and Lightweight Glass Fiber Reinforced Composite Materials under Various Loading Conditions. ACS Appl. Nano Mater. 2020, 3, 1363–1372. [Google Scholar] [CrossRef]
- Wei, B.; Cao, H.; Song, S. Surface modification and characterization of basalt fibers with hybrid sizings. Compos. Part A Appl. Sci. Manuf. 2011, 42, 22–29. [Google Scholar] [CrossRef]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc Oxide for Functional Textile Coatings: Recent Advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef]
- Frade, T.; Melo Jorge, M.E.; Gomes, A. One-dimensional ZnO nanostructured films: Effect of oxide nanoparticles. Mater. Lett. 2012, 82, 13–15. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.G.; Kim, Y.S.; Seo, H.K.; Shin, H.S. Room temperature synthesis of needle-shaped ZnO nanorods via sonochemical method. Appl. Surf. Sci. 2007, 253, 7622–7626. [Google Scholar] [CrossRef]
- Cui, J. Zinc oxide nanowires. Mater. Charact. 2012, 64, 43–52. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Chang, P.C.; Fan, Z.; Wang, D.; Tseng, W.Y.; Chiou, W.A.; Hong, J.; Lu, J.G. ZnO nanowires synthesized by vapor trapping CVD method. Chem. Mater. 2004, 16, 5133–5137. [Google Scholar] [CrossRef]
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S.; Ali, E.S. Synthesis of ZnO Nanostructures Using Sol-Gel Method. Procedia Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 18. [Google Scholar] [CrossRef]
- Pal, U.; Santiago, P. Controlling the morphology of ZnO nanostructures in a low-temperature hydrothermal process. J. Phys. Chem. B 2005, 109, 15317–15321. [Google Scholar] [CrossRef]
- Nasser, J.; Steinke, K.; Hwang, H.; Sodano, H. Nanostructured ZnO Interphase for Carbon Fiber Reinforced Composites with Strain Rate Tailored Interfacial Strength. Adv. Mater. Interfaces 2020, 7, 1901544. [Google Scholar] [CrossRef]
- Lilli, M.; Sbardella, F.; Bavasso, I.; Bracciale, M.P.; Scheffler, C.; Rivilla, I.; Tirillo’, J.; Xin, W.; De Rosa, I.M.; Sarasini, F. Tailoring the interfacial strength of basalt fibres/epoxy composite with ZnO-nanorods. Compos. Interfaces 2020, 1–23. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Jordá-Beneyto, M.; Rosa, E.; Lara-Lledo, M.; Lorente, I. ZnO-PLA nanocomposite coated paper for antimicrobial packaging application. LWT-Food Sci. Technol. 2017, 78, 250–257. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Pantani, R.; Turng, L.-S. Manufacturing of advanced biodegradable polymeric components. J. Appl. Polym. Sci. 2015, 132, 1–3. [Google Scholar] [CrossRef]
- Murariu, M.; Doumbia, A.; Bonnaud, L.; Dechief, A.L.; Paint, Y.; Ferreira, M.; Campagne, C.; Devaux, E.; Dubois, P. High-performance polylactide/ZnO nanocomposites designed for films and fibers with special end-use properties. Biomacromolecules 2011, 12, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, Y.; Liu, H.; Belfiore, L.A. Effects of organic nucleating agents and zinc oxide nanoparticles on isotactic polypropylene crystallization. Polymer (Guildf). 2004, 45, 2081–2091. [Google Scholar] [CrossRef]
- Wang, B.; Wen, T.; Zhang, X.; Tercjak, A.; Dong, X.; Müller, A.J.; Wang, D.; Cavallo, D. Nucleation of Poly(lactide) on the Surface of Different Fibers. Macromolecules 2019, 52, 6274–6284. [Google Scholar] [CrossRef]
- Pan, H.; Kong, J.; Chen, Y.; Zhang, H.; Dong, L. Improved heat resistance properties of poly(L-lactide)/basalt fiber biocomposites with high crystallinity under forming hybrid-crystalline morphology. Int. J. Biol. Macromol. 2019, 122, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Cao, Z.; Chen, Y.; Wang, X.; Jia, S.; Yang, H.; Zhang, H.; Dong, L. Effect of molecular stereoregularity on the transcrystallinization properties of poly(L-lactide)/basalt fiber composites. Int. J. Biol. Macromol. 2019, 137, 238–246. [Google Scholar] [CrossRef]
- Turner, J.F.; Riga, A.; O’Connor, A.; Zhang, J.; Collis, J. Characterization of drawn and undrawn poly-L-lactide films by differential scanning calorimetry. J. Therm. Anal. Calorim. 2004, 75, 257–268. [Google Scholar] [CrossRef]
- Li, J.Y.; Chen, X.L.; Li, H.; He, M.; Qiao, Z.Y. Fabrication of zinc oxide nanorods. J. Cryst. Growth 2001, 233, 5–7. [Google Scholar] [CrossRef]
- Wang, X.J.; Huang, Z.; Wei, M.Y.; Lu, T.; Nong, D.D.; Zhao, J.X.; Gao, X.Y.; Teng, L. jun Catalytic effect of nanosized ZnO and TiO2 on thermal degradation of poly(lactic acid) and isoconversional kinetic analysis. Thermochim. Acta 2019, 672, 14–24. [Google Scholar] [CrossRef]
- Carrasco, F.; Pags, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Kinetics of the thermal decomposition of processed poly(lactic acid). Polym. Degrad. Stab. 2010, 95, 2508–2514. [Google Scholar] [CrossRef]
- Mazzanti, V.; Salzano de Luna, M.; Pariante, R.; Mollica, F.; Filippone, G. Natural fiber-induced degradation in PLA-hemp biocomposites in the molten state. Compos. Part A Appl. Sci. Manuf. 2020, 137, 105990. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.; Jiang, L.; Liaw, J.; Hartman, K. Insight on the influence of nano zinc oxide on the thermal, dynamic mechanical, and flow characteristics of Poly(lactic acid)– zinc oxide composites. Polym. Eng. Sci. 2019, 59, 1242–1249. [Google Scholar] [CrossRef]
- Lizundia, E.; Ruiz-Rubio, L.; Vilas, J.L.; León, L.M. Poly(l-lactide)/zno nanocomposites as efficient UV-shielding coatings for packaging applications. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Rashidi, H.; Najaf Oshani, B.; Hejazi, I.; Seyfi, J. Tuning crystallization and hydrolytic degradation behaviors of poly(lactic acid) by using silver phosphate, zinc oxide and their nano-hybrids. Polym. Technol. Mater. 2020, 59, 72–82. [Google Scholar] [CrossRef]
- Jayaramudu, J.; Das, K.; Sonakshi, M.; Siva Mohan Reddy, G.; Aderibigbe, B.; Sadiku, R.; Sinha Ray, S. Structure and properties of highly toughened biodegradable polylactide/ZnO biocomposite films. Int. J. Biol. Macromol. 2014, 64, 428–434. [Google Scholar] [CrossRef]
- Sun, X.; Xue, B.; Yang, S.; Huo, K.; Liao, X.; Li, X.; Xie, L.; Qin, S.; Zheng, Q. Structural conversion of PLLA/ZnO composites facilitated by interfacial crystallization to potential application in oil-water separation. Appl. Surf. Sci. 2020, 517, 146135. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, H.; Liu, W.; Zhang, M.; Du, Z.; Wang, X. The synergistic effect of zinc oxide and phenylphosphonic acid zinc salt on the crystallization behavior of poly (lactic acid). Polym. Degrad. Stab. 2015, 122, 25–35. [Google Scholar] [CrossRef]
- Krause, T.; Kalinka, G.; Auer, C.; Hinrichsen, G. Computer simulation of crystallization kinetics in fiber-reinforced composites. J. Appl. Polym. Sci. 1994, 51, 399–406. [Google Scholar] [CrossRef]
- Liu, T.; Yu, X.; Yu, F.; Zhao, X.; Lu, A.; Wang, J.; Wang, X.; Liu, T. Isothermal Crystallization Kinetics of Fiber/Polylactic Acid Composites and Morphology. Polym. Plast. Technol. Eng. 2012, 51, 597–604. [Google Scholar] [CrossRef]
- Yu, W.; Lan, C.H.; Wang, S.J.; Fang, P.F.; Sun, Y.M. Influence of zinc oxide nanoparticles on the crystallization behavior of electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanofibers. Polymer (Guildf). 2010, 51, 2403–2409. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. ZnO-reinforced poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bionanocomposites with antimicrobial function for food packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.; Díez-Vicente, A. Poly(3-hydroxybutyrate)/ZnO Bionanocomposites with Improved Mechanical, Barrier and Antibacterial Properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef] [PubMed]


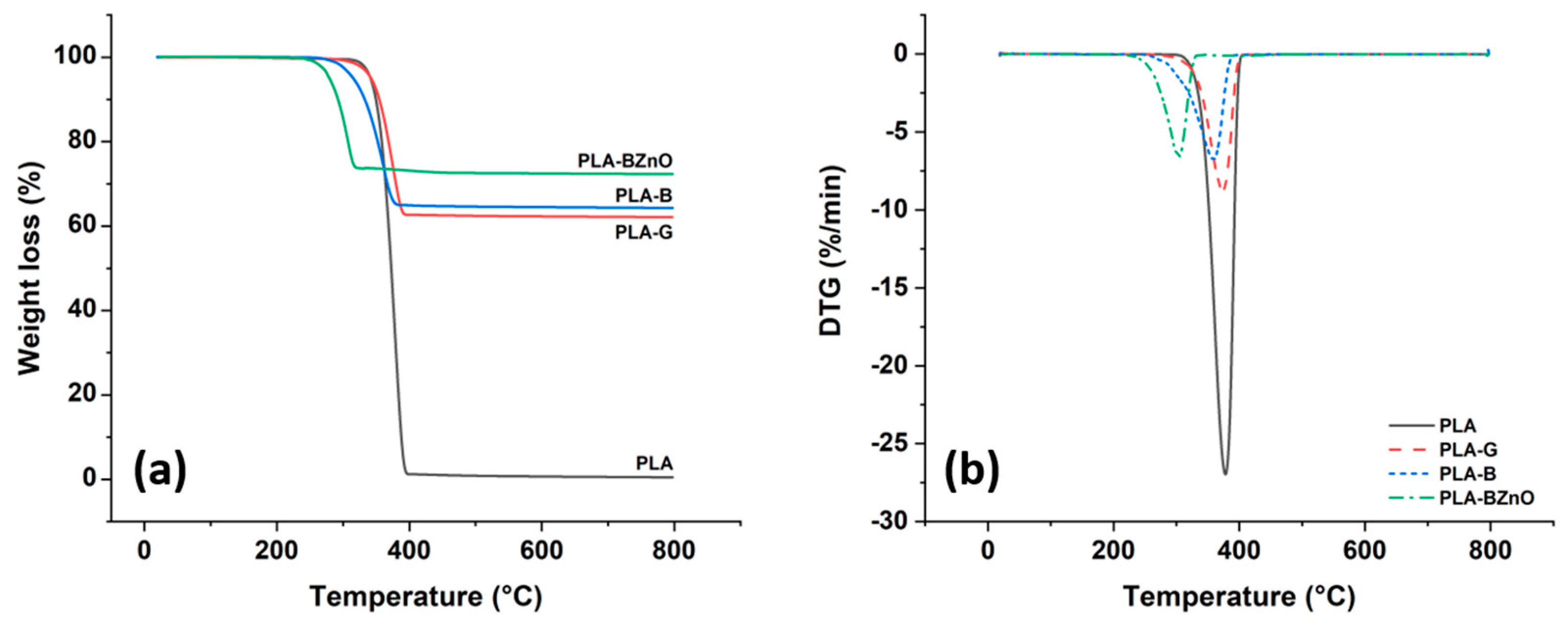





| Sample | Td5% (°C) | Td10% (°C) | Tdmax (°C) | WPLA (wt.%) |
|---|---|---|---|---|
| PLA | 341.4 ± 0.5 | 355.2 ± 0.4 | 372.1 ± 0.8 | 100 |
| PLA-B | 314.6 ± 1.2 | 332.1 ± 1.0 | 357.8 ± 1.4 | 34.3 ± 0.6 |
| PLA-BZnO | 278.7 ± 1.1 | 291.4 ± 1.3 | 302.2 ± 1.8 | 25.4 ± 1.3 |
| PLA-G | 342.5 ± 0.2 | 354.4 ± 0.3 | 372.9 ± 1.2 | 36.6 ± 0.4 |
| Sample | Tgos (°C) | Tccos (°C) | Tcc (°C) | Tm (°C) | ΔHcc (J g−1) | ΔHm (J g−1) | χc (%) |
|---|---|---|---|---|---|---|---|
| PLA second heating | 57 ± 1 | 109 ± 1 | 134 ± 2 | 166 ± 1 | 6.5 ± 0.2 | 9.3 ± 0.4 | 3 ± 1 |
| PLA-B first heating | 57 ± 3 | 77 ± 3 | 101 ± 3 | 164 ± 2 | 26 ± 5 | 37 ± 3 | 12 ± 8 |
| PLA-BZnO first heating | 56 ± 3 | 76 ± 2 | 101 ± 3 | 165 ± 1 | 30 ± 6 | 50 ± 5 | 21 ± 11 |
| PLA-G first heating | 56 ± 3 | 81 ± 1 | 108 ± 1 | 168 ± 1 | 21 ± 5 | 42 ± 6 | 22 ± 13 |
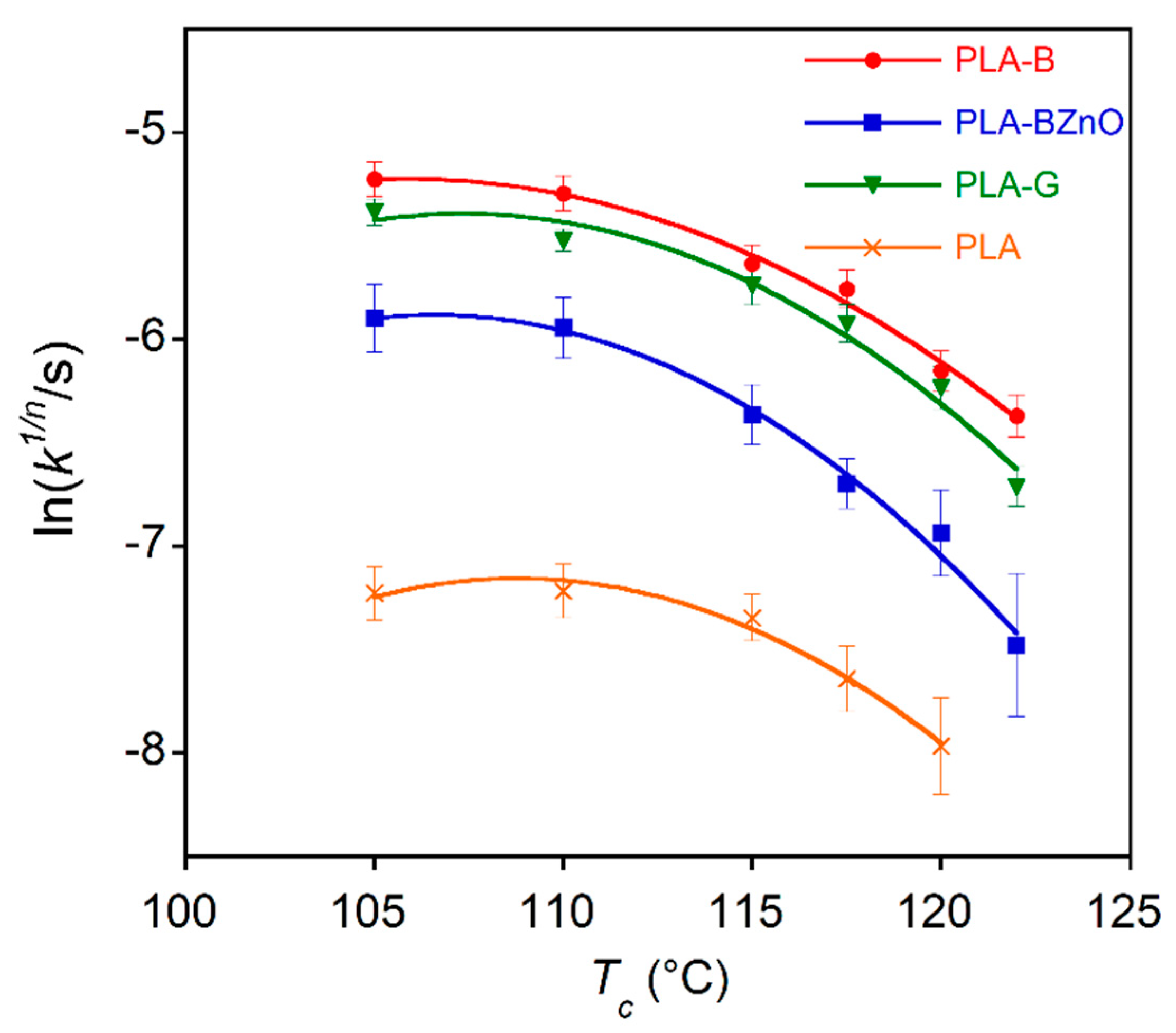
| Crystallization Temperature | PLA-B | PLA-BZnO | PLA-G | PLA | ||||
|---|---|---|---|---|---|---|---|---|
| Tc (°C) | k (s−n) | n | k (s−n) | n | k (s−n) | n | k (s−n) | n |
| 105 | (2.1 ± 0.01) 10−5 | 2.16 ± 0.02 | (2.18 ± 0.05) 10−5 | 1.82 ± 0.02 | (2.76 ± 0.1) 10−5 | 1.95 ± 0.03 | (2.1 ± 0.05) 10−11 | 3.4 ± 0.6 |
| 110 | (2.7 ± 0.08) 10−5 | 2.05 ± 0.01 | (1.86 ± 0.05) 10−6 | 2.2 ± 0.1 | (8.71 ± 0.03) 10−6 | 2.10 ± 0.02 | (1.0 ± 0.06) 10−13 | 3.8 ± 0.1 |
| 115 | (3 ± 0.1) 10−6 | 2.23 ± 0.02 | (3.16 ± 0.06) 10−6 | 2.0 ± 0.1 | (1.0 ± 0.5) 10−5 | 2.04 ± 0.05 | (1.3 ± 0.04) 10−11 | 3.4 ± 0.2 |
| 117.5 | (7 ± 0.01) 10−6 | 2.02 ± 0.01 | (2.28 ± 0.02) 10−6 | 1.92 ± 0.01 | (9.6 ± 0.1) 10−6 | 1.95 ± 0.03 | (1.9 ± 0.3) 10−11 | 3.2 ± 0.2 |
| 120 | (6 ± 0.02) 10−6 | 1.95 ± 0.02 | (1.89 ± 0.04) 10−6 | 1.96 ± 0.02 | (1.2 ± 0.1) 10−6 | 2.19 ± 0.05 | (1.4 ± 0.5) 10−14 | 4.0 ± 0.1 |
| 122 | (8 ± 0.2) 10−7 | 2.21 ± 0.01 | (7.2 ± 0.1) 10−8 | 2.2 ± 0.1 | (9 ± 1) 10−8 | 2.41 ± 0.05 | – | – |
| Mean | 2.1 ± 0.1 | 2.0 ± 0.2 | 2.1 ± 0.2 | 3.6 ± 0.3 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbardella, F.; Martinelli, A.; Di Lisio, V.; Bavasso, I.; Russo, P.; Tirillò, J.; Sarasini, F. Surface Modification of Basalt Fibres with ZnO Nanorods and Its Effect on Thermal and Mechanical Properties of PLA-Based Composites. Biomolecules 2021, 11, 200. https://doi.org/10.3390/biom11020200
Sbardella F, Martinelli A, Di Lisio V, Bavasso I, Russo P, Tirillò J, Sarasini F. Surface Modification of Basalt Fibres with ZnO Nanorods and Its Effect on Thermal and Mechanical Properties of PLA-Based Composites. Biomolecules. 2021; 11(2):200. https://doi.org/10.3390/biom11020200
Chicago/Turabian StyleSbardella, Francesca, Andrea Martinelli, Valerio Di Lisio, Irene Bavasso, Pietro Russo, Jacopo Tirillò, and Fabrizio Sarasini. 2021. "Surface Modification of Basalt Fibres with ZnO Nanorods and Its Effect on Thermal and Mechanical Properties of PLA-Based Composites" Biomolecules 11, no. 2: 200. https://doi.org/10.3390/biom11020200
APA StyleSbardella, F., Martinelli, A., Di Lisio, V., Bavasso, I., Russo, P., Tirillò, J., & Sarasini, F. (2021). Surface Modification of Basalt Fibres with ZnO Nanorods and Its Effect on Thermal and Mechanical Properties of PLA-Based Composites. Biomolecules, 11(2), 200. https://doi.org/10.3390/biom11020200










