BanLec-eGFP Chimera as a Tool for Evaluation of Lectin Binding to High-Mannose Glycans on Microorganisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Plasmids
2.2. Design, Cloning of eGFP in pET-23b-BL
2.3. Expression of BanLec-eGFP
2.4. Purification of Recombinant BanLec-eGFP
2.5. Analysis of the BanLec-eGFP Structure by CD Spectroscopy, Fluorescent and Mass Spectrometry
2.6. Competitive FLLSA (Fluorescence-Linked Lectin Sorbent Assay)
2.7. Assessment of Fluorescence-Linked Lectin Binding to Bacterial Cells with Flow Cytometry
3. Results
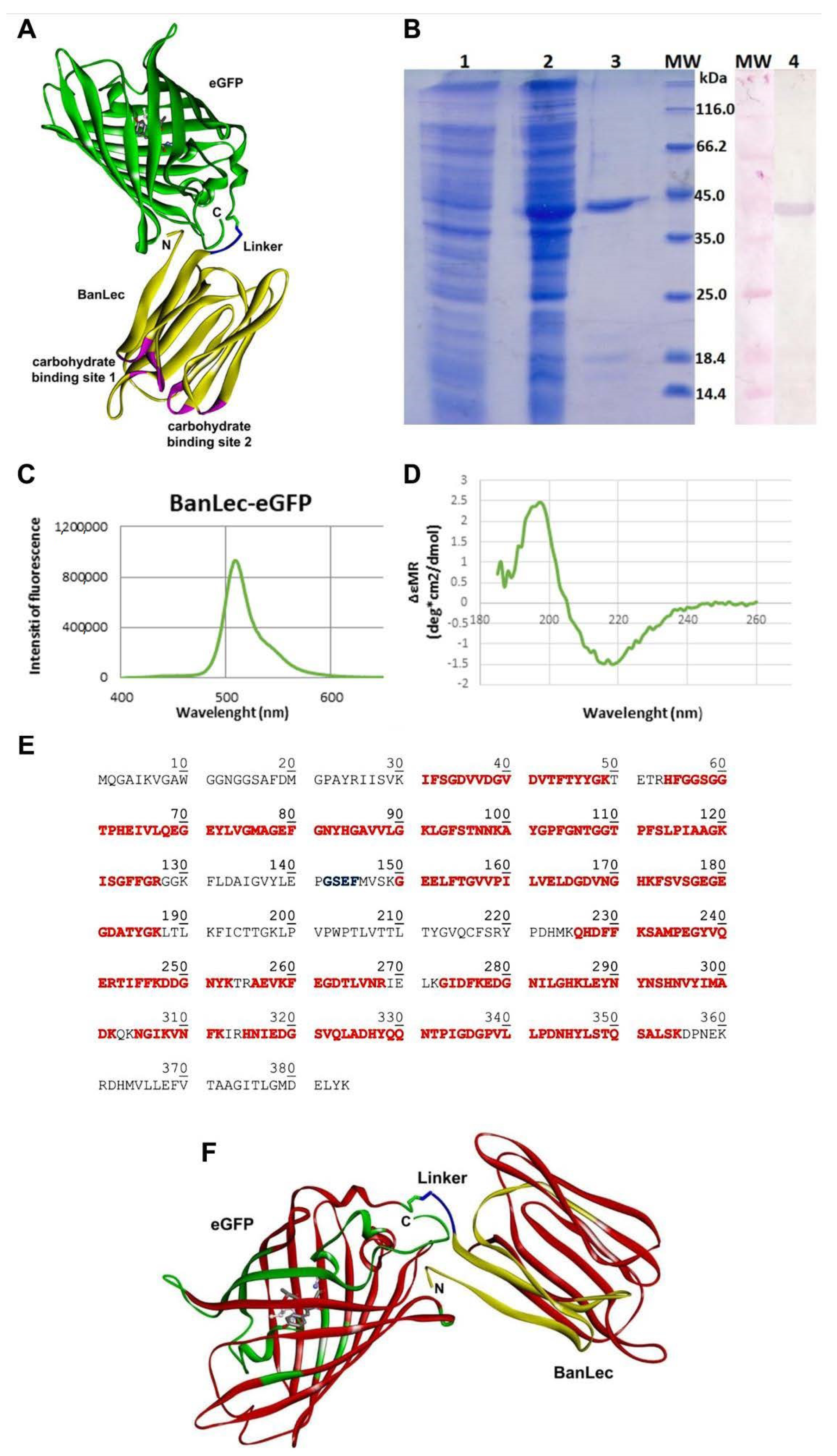
3.1. Design, Cloning, Expression, and Purification of Recombinant BanLec-eGFP
3.2. Physical-Chemical Characterization of BanLec-eGFP
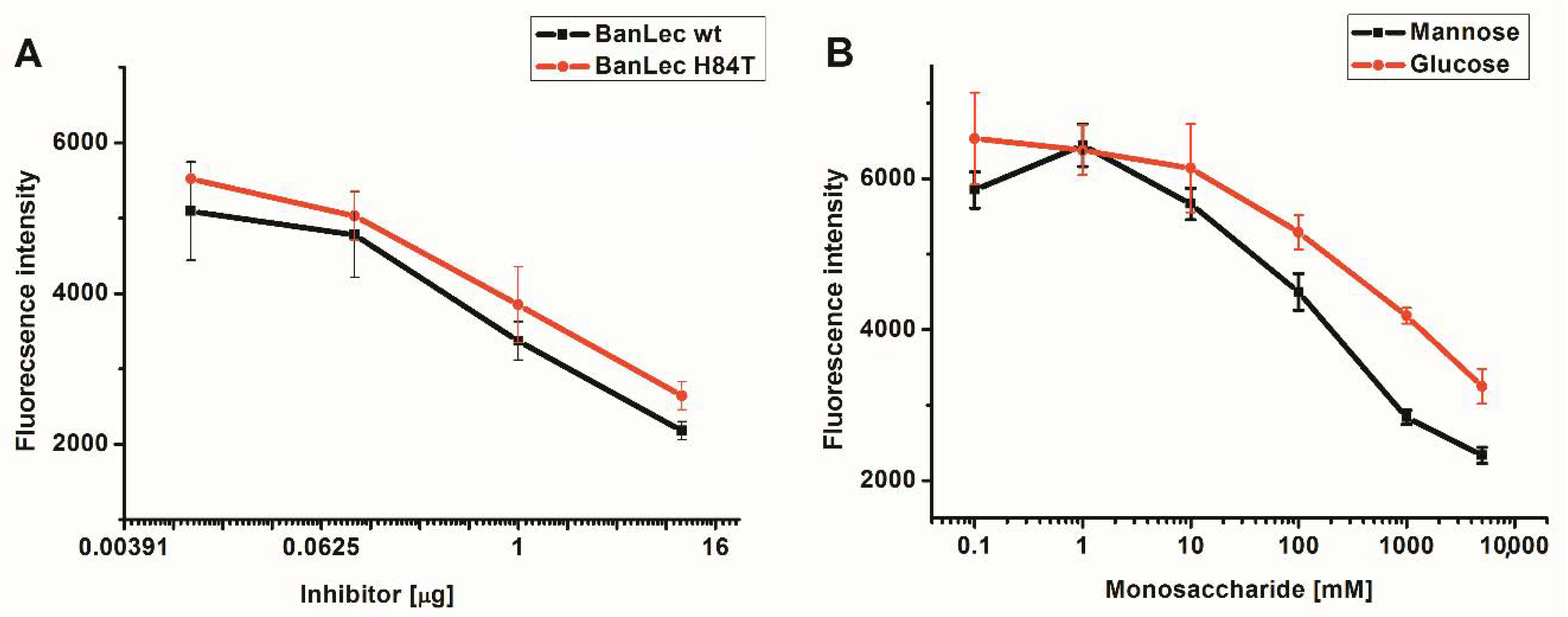
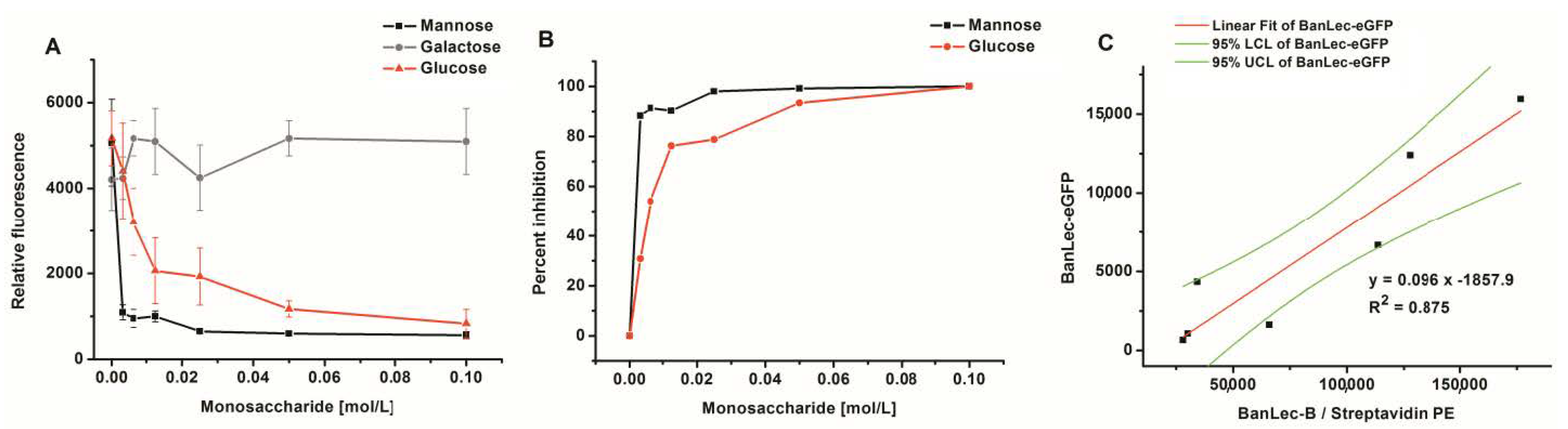
3.3. Competitive Inhibition of BanLec-eGFP Binding to Influenza Vaccine High-Mannose Glycans
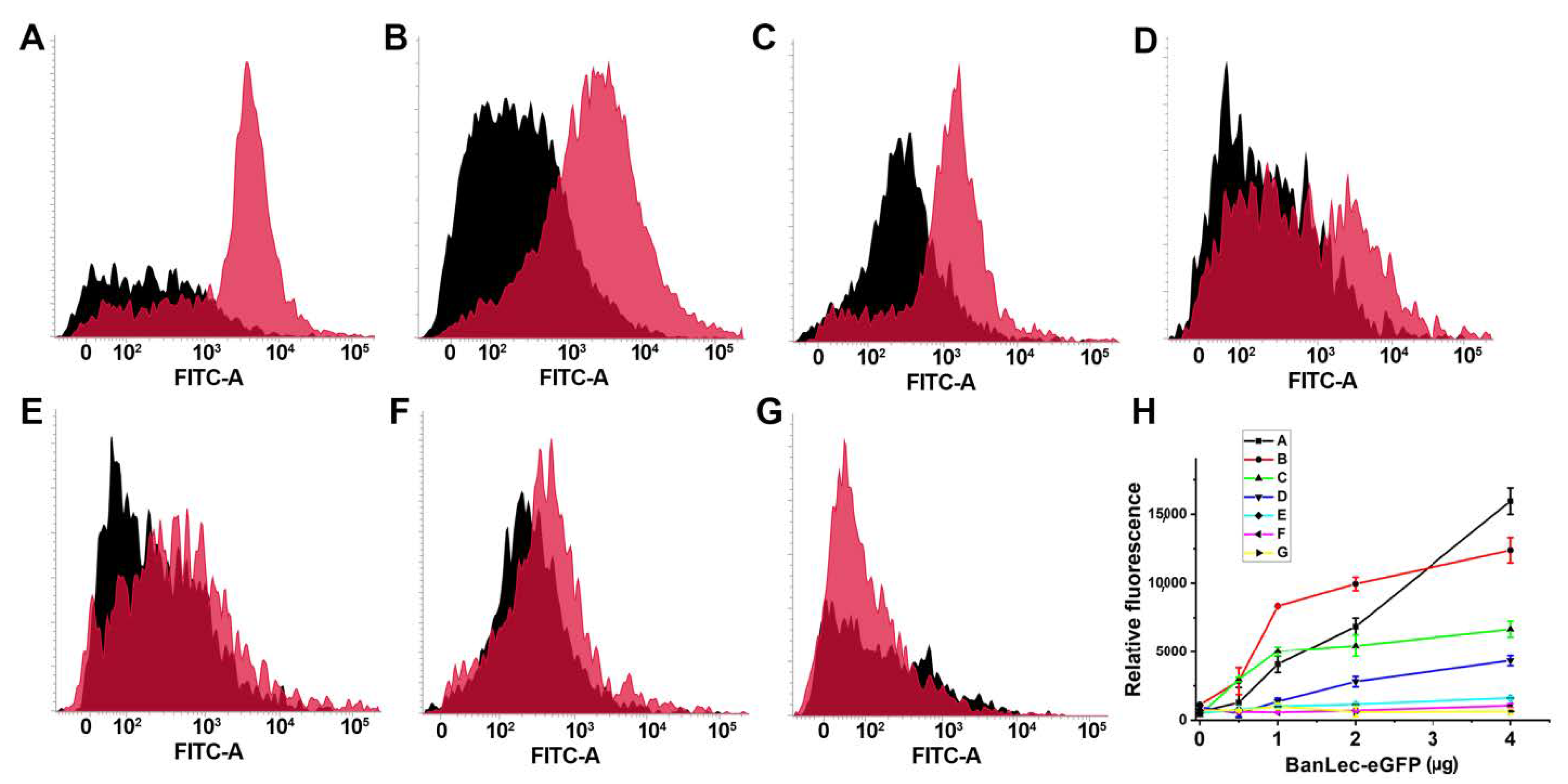
3.4. Flow Cytometric Detection of BanLec-eGFP binding to bacteria
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lam, S.-K.; Ng, T. Lectins: Production and practical applications. Appl. Microbiol. Biotechnol. 2010, 89, 45–55. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. Lectins as cell recognition molecules. Science 1989, 246, 227–234. [Google Scholar] [CrossRef]
- Jain, P.; Bhuiyan, M.H.; Hossain, K.R.; Bachar, S.C. Antibacterial and antioxidant activities of local seeded banana fruits. Afr. J. Pharm. Pharmacol. 2011, 5, 1398–1403. [Google Scholar] [CrossRef]
- Koshte, V.L.; Van Dijk, W.; Van Der Stelt, M.E.; Aalberse, R.C. Isolation and characterization of BanLec-I, a mannoside-binding lectin from Musa paradisiac (banana). Biochem. J. 1990, 272, 721–726. [Google Scholar] [CrossRef]
- Peumans, W.J.; Zhang, W.; Barre, A.; Astoul, C.H.; Balint-Kurti, P.J.; Rovira, P.; Rougé, P.; May, G.D.; Van Leuven, F.; Truffa-Bachi, P.; et al. Fruit-specific lectins from banana and plantain. Planta 2000, 211, 546–554. [Google Scholar] [CrossRef]
- Singh, D.D.; Saikrishnan, K.; Kumar, P.; Dauter, Z.; Sekar, K.; Surolia, A.; Vijayan, M. Purification, crystallization and preliminary X-ray structure analysis of the banana lectin fromMusa paradisiaca. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2104–2106. [Google Scholar] [CrossRef]
- Khan, J.M.; Qadeer, A.; Ahmad, E.; Ashraf, R.; Bhushan, B.; Chaturvedi, S.K.; Rabbani, G.; Khan, R.H. Monomeric Banana Lectin at Acidic pH Overrules Conformational Stability of Its Native Dimeric Form. PLoS ONE 2013, 8, 1–12. [Google Scholar] [CrossRef]
- Koshte, V.; Aalbers, M.; Calkhoven, P.; Aalberse, R. The Potent lgG4-lnducing Antigen in Banana Is a Mannose-Binding Lectin, BanLec-I. Int. Arch. Allergy Immunol. 1992, 97, 17–24. [Google Scholar] [CrossRef]
- Swanson, M.D.; Winter, H.C.; Goldstein, I.J.; Markovitz, D.M. A Lectin Isolated from Bananas Is a Potent Inhibitor of HIV Replication. J. Biol. Chem. 2010, 285, 8646–8655. [Google Scholar] [CrossRef]
- Hopper, J.T.; Ambrose, S.; Grant, O.C.; Krumm, S.A.; Allison, T.M.; Degiacomi, M.T.; Tully, M.D.; Pritchard, L.K.; Ozorowski, G.; Ward, A.B.; et al. The Tetrameric Plant Lectin BanLec Neutralizes HIV through Bidentate Binding to Specific Viral Glycans. Structure 2017, 25, 773–782. [Google Scholar] [CrossRef]
- Gavrovic-Jankulovic, M.; Poulsen, K.; Brckalo, T.; Bobic, S.; Lindner, B.; Petersen, A. A novel recombinantly produced banana lectin isoform is a valuable tool for glycoproteomics and a potent modulator of the proliferation response in CD3+, CD4+, and CD8+ populations of human PBMCs. Int. J. Biochem. Cell Biol. 2008, 40, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, M.; Zivkovic, I.; Petrušić, V.; Kosec, D.J.; Dimitrijević, R.D.; Jankov, R.M.; Dimitrijević, L.A.; Gavrovic-Jankulovic, M. In vitro stimulation of Balb/c and C57 BL/6 splenocytes by a recombinantly produced banana lectin isoform results in both a proliferation of T cells and an increased secretion of interferon-gamma. Int. Immunopharmacol. 2010, 10, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijević, R.; Stojanovic, M.; Micic, M.; Gavrovic-Jankulovic, M. Recombinant banana lectin as mucosal immunostimulator. J. Funct. Foods 2012, 4, 636–641. [Google Scholar] [CrossRef]
- Swanson, M.D.; Boudreaux, D.M.; Salmon, L.; Chugh, J.; Winter, H.C.; Meagher, J.L.; André, S.; Murphy, P.V.; Oscarson, S.; Roy, R.; et al. Engineering a Therapeutic Lectin by Uncoupling Mitogenicity from Antiviral Activity. Cell 2015, 163, 746–758. [Google Scholar] [CrossRef]
- Brawek, B.; del Moral, M.O.; Garaschuk, O. In Vivo Visualization of Microglia Using Tomato Lectin. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019. [Google Scholar]
- Liao, W.-Y.; Fugmann, S.D. Lectins identify distinct populations of coelomocytes in Strongylocentrotus purpuratus. PLoS ONE 2017, 12, 1–21. [Google Scholar] [CrossRef]
- Lohr, M.; Kaltner, H.; Schwartz-Albiez, R.; Sinowatz, F.; Gabius, H.-J. Towards Functional Glycomics by Lectin Histochemistry: Strategic Probe Selection to Monitor Core and Branch-end Substitutions and Detection of Cell-type and Regional Selectivity in Adult Mouse Testis and Epididymis. Anat. Histol. Embryol. 2010, 39, 481–493. [Google Scholar] [CrossRef]
- Wolters-Eisfeld, G.; Schumacher, U. Lectin Histochemistry for Metastasizing and Non-metastasizing Cancer Cells. In Histochemestry of Single Molecules; Humana Press: New York, NY, USA, 2017; Volume 1560, pp. 121–132. [Google Scholar] [CrossRef]
- Neves, A.A.; Di Pietro, M.; O’Donovan, M.; Waterhouse, D.J.; Bohndiek, S.E.; Brindle, K.M.; Fitzgerald, R.C. Detection of early neoplasia in Barrett’s esophagus using lectin-based near-infrared imaging: An ex vivo study on human tissue. Endoscopy 2018, 50, 618–625. [Google Scholar] [CrossRef]
- Carvalho, M.E.T.; De Oliveira, W.F.; Cunha, C.R.; Coelho, L.C.; Silva, M.V.; Junior, L.B.C.; Santos, B.S.; Filho, P.E.C.; Fontes, A.; Correia, M.T. Evaluating the glycophenotype on breast cancer tissues with quantum dots-Cramoll lectin conjugates. Int. J. Biol. Macromol. 2019, 138, 302–308. [Google Scholar] [CrossRef]
- Stevens, J.; Blixt, O.; Paulson, J.C.; Wilson, I.A. Glycan microarray technologies: Tools to survey host specificity of influenza viruses. Nat. Rev. Genet. 2006, 4, 857–864. [Google Scholar] [CrossRef]
- Fei, Y.; Sun, Y.-S.; Li, Y.; Lau, K.; Yu, H.; Chokhawala, H.A.; Huang, S.; Landry, J.P.; Chen, X.; Zhu, X. Fluorescent labeling agents change binding profiles of glycan-binding proteins. Mol. BioSyst. 2011, 7, 3343–3352. [Google Scholar] [CrossRef]
- Giepmans, B.N.; Adams, S.R.; Ellisman, M.H.; Tsien, R.Y. The fluorescent toolbox for assessing protein location and function. Science 2006, 312, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Baader, S.L.; Sixt, M.; Kappler, J.; Rauch, U. Neurocan–GFP Fusion Protein. J. Histochem. Cytochem. 2004, 52, 915–922. [Google Scholar] [CrossRef]
- Lannoo, N.; Peumans, W.J.; Van Pamel, E.; Alvarez, R.; Xiong, T.-C.; Hause, G.; Mazars, C.; Van Damme, E.J.M. Localization and in vitro binding studies suggest that the cytoplasmic/nuclear tobacco lectin can interact in situ with high-mannose and complexN-glycans. FEBS Lett. 2006, 580, 6329–6337. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.-Y.; Lee, K.J.; Kim, Y.J.; Kwon, O.; Lee, S.-G.; Park, W.S.; Heo, W.D.; Oh, D.-B. Development of fluorescent probes for the detection of fucosylated N-glycans using an Aspergillus oryzae lectin. Appl. Microbiol. Biotechnol. 2012, 93, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Arpino, J.A.J.; Rizkallah, P.J.; Jones, D.D. Crystal Structure of Enhanced Green Fluorescent Protein to 1.35 Å Resolution Reveals Alternative Conformations for Glu222. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Discovery Studio Modeling Environment, Release 4.5; BIOVIA, Dassault Systèmes: San Diego, CA, USA, 2015.
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- MacKerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, R.; Jadranin, M.; Burazer, L.; Ostojic, S.; Gavrovic-Jankulovic, M. Evaluation of the thermal stability and digestibility of heterologously produced banana lectin. Food Chem. 2010, 120, 1113–1118. [Google Scholar] [CrossRef]
- Meagher, J.L.; Winter, H.C.; Ezell, P.; Goldstein, I.J.; Stuckey, J.A. Crystal structure of banana lectin reveals a novel second sugar binding site. Glycobiology 2005, 15, 1033–1042. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Ramessar, K.; O’Keefe, B.R. Antiviral lectins: Selective inhibitors of viral entry. Antivir. Res. 2017, 142, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Resende, R.; Wennekes, T.; Chen, H.-M.; Bance, N.; Buchini, S.; Watts, A.G.; Pilling, P.; Streltsov, V.A.; Petric, M.; et al. Mechanism-Based Covalent Neuraminidase Inhibitors with Broad-Spectrum Influenza Antiviral Activity. Science 2013, 340, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wang, X.; Liu, H.; Guo, L.; Su, Q.; Wang, H.; Vasiliadis, T.; Ho, W.; Li, J. GalNAc-Specific Soybean Lectin Inhibits HIV Infection of Macrophages through Induction of Antiviral Factors. J. Virol. 2017, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Koharudin, L.M.; Gronenborn, A.M. Antiviral lectins as potential HIV microbicides. Curr. Opin. Virol. 2014, 7, 95–100. [Google Scholar] [CrossRef]
- Covés-Datson, E.M.; King, S.R.; Legendre, M.; Gupta, A.; Chan, S.M.; Gitlin, E.; Kulkarni, V.V.; García, J.P.; Smee, D.F.; Lipka, E.; et al. A molecularly engineered antiviral banana lectin inhibits fusion and is efficacious against influenza virus infection in vivo. Proc. Natl. Acad. Sci. USA 2020, 117, 2122–2132. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Smee, D.F.; Turpin, J.A.; Saucedo, C.J.; Gustafson, K.R.; Mori, T.; Blakeslee, D.; Buckheit, R.; Boyd, M.R. Potent Anti-Influenza Activity of Cyanovirin-N and Interactions with Viral Hemagglutinin. Antimicrob. Agents Chemother. 2003, 47, 2518–2525. [Google Scholar] [CrossRef]
- Gordts, S.C.; Renders, M.; Férir, G.; Huskens, D.; Van Damme, E.J.M.; Peumans, W.; Balzarini, J.; Schols, D. NICTABA and UDA, two GlcNAc-binding lectins with unique antiviral activity profiles. J. Antimicrob. Chemother. 2015, 70, 1674–1685. [Google Scholar] [CrossRef]
- Haugh, M.; Gresset-Bourgeois, V.; Macabeo, B.; Woods, A.; Samson, S.I. A trivalent, inactivated influenza vaccine (Vaxigrip®): Summary of almost 50 years of experience and more than 1.8 billion doses distributed in over 120 countries. Expert Rev. Vaccines 2017, 16, 545–564. [Google Scholar] [CrossRef]
- Dragacevic, L.; Djordjevic, B.; Gavrovic-Jankulovic, M.; Ilic, V.; Kanazir, D.; Minić, R. ELLSA based profiling of surface glycosylation in microorganisms reveals that ß-glucan rich yeasts’ surfaces are selectively recognized with recombinant banana lectin. Glycoconj. J. 2019, 37, 95–105. [Google Scholar] [CrossRef]
- Goldstein, I.J.; Winter, H.C.; Mo, H.; Misaki, A.; Van Damme, E.J.M.; Peumans, W.J. Carbohydrate binding properties of banana (Musa acuminata ) lectin. JBIC J. Biol. Inorg. Chem. 2001, 268, 2616–2619. [Google Scholar] [CrossRef]
- Groisman, E.A.; Sturmoski, M.A.; Solomon, F.R.; Lin, R.; Ochman, H. Molecular, functional, and evolutionary analysis of sequences specific to Salmonella. Proc. Natl. Acad. Sci. USA 1993, 90, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Coburn, B.; Grassl, G.A.; Finlay, B.B. Salmonella, the host and disease: A brief review. Immunol. Cell Biol. 2007, 85, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, M.; Joiner, K.; Ezekowitz, R.A. The human mannose-binding protein functions as an opsonin. J. Exp. Med. 1989, 169, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Devyatyarova-Johnson, M.; Rees, I.H.; Robertson, B.D.; Turner, M.W.; Klein, N.J.; Jack, D.L. The Lipopolysaccharide Structures of Salmonella enterica Serovar Typhimurium and Neisseria gonorrhoeaeDetermine the Attachment of Human Mannose-Binding Lectin to Intact Organisms. Infect. Immun. 2000, 68, 3894–3899. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Nakamura, S.; Islam, S.; Guo, Y.; Ihara, K.; Tomioka, R.; Masuda, M.; Yoneyama, H.; Isogai, E. Mannose-Binding Lectin Inhibits the Motility of Pathogenic Salmonella by Affecting the Driving Forces of Motility and the Chemotactic Response. PLoS ONE 2016, 11, 1–14. [Google Scholar] [CrossRef]
| [M] Determined | [M] Theoretical | Peptide Sequence | Peptide Length (-AA) |
|---|---|---|---|
| 4474.01 | 4473.84 | HNIEDGSVQLADHYQQNTPIGDGPVLLPDNHYLSTQSALSK | 41 |
| 3914.708 | 3915.35 | HFGGSGGTPHEIVLQEGEYLVGMAGEFGNYHGAVVLGK | 38 |
| 2438.294 | 2437.73 | GEELFTGVVPILVELDGDVNGHK | 23 |
| 2082.038 | 2082.29 | IFSGDVVDGVDVTFTYYGK | 19 |
| 2023.074 | 2023.28 | AYGPFGNTGGTPFSLPIAAGK | 21 |
| 1973.927 | 1974.18 | LEYNYNSHNVYIMADK | 16 |
| 1542.819 | 1542.77 | GIDFKEDGNILGHK | 14 |
| 1503.769 | 1503.54 | FSVSGEGEGDATYGK | 15 |
| 1477.842 | 1477.75 | AEVKFEGDTLVNR | 13 |
| 1347.748 | 1347.64 | TIFFKDDGNYK | 11 |
| 1266.604 | 1266.39 | SAMPEGYVQER | 11 |
| 1050.576 | 1050.14 | FEGDTLVNR | 9 |
| 880.475 | 879.97 | LGFSTNNK | 8 |
| 821.416 | 820.90 | QHDFFK | 6 |
| 783.39 | 782.90 | ISGFFGR | 7 |
| 655.336 | 654.81 | TIFFK | 5 |
| 507.184 | 506.60 | VNFK | 4 |
| 430.225 | 430.50 | NGIK | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopandić, Z.; Dragačević, L.; Popović, D.; Andjelković, U.; Minić, R.; Gavrović-Jankulović, M. BanLec-eGFP Chimera as a Tool for Evaluation of Lectin Binding to High-Mannose Glycans on Microorganisms. Biomolecules 2021, 11, 180. https://doi.org/10.3390/biom11020180
Lopandić Z, Dragačević L, Popović D, Andjelković U, Minić R, Gavrović-Jankulović M. BanLec-eGFP Chimera as a Tool for Evaluation of Lectin Binding to High-Mannose Glycans on Microorganisms. Biomolecules. 2021; 11(2):180. https://doi.org/10.3390/biom11020180
Chicago/Turabian StyleLopandić, Zorana, Luka Dragačević, Dragan Popović, Uros Andjelković, Rajna Minić, and Marija Gavrović-Jankulović. 2021. "BanLec-eGFP Chimera as a Tool for Evaluation of Lectin Binding to High-Mannose Glycans on Microorganisms" Biomolecules 11, no. 2: 180. https://doi.org/10.3390/biom11020180
APA StyleLopandić, Z., Dragačević, L., Popović, D., Andjelković, U., Minić, R., & Gavrović-Jankulović, M. (2021). BanLec-eGFP Chimera as a Tool for Evaluation of Lectin Binding to High-Mannose Glycans on Microorganisms. Biomolecules, 11(2), 180. https://doi.org/10.3390/biom11020180








