Functional and Rheological Properties of Vicia faba L. Protein Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Protein Isolates
2.2. Hydrodynamic and Surface Properties
2.2.1. Electrophoretic Research
2.2.2. -Potential
2.2.3. Hydrophobicity of Isolate Proteins
2.2.4. Dynamic Light Scattering (DLS)
2.2.5. Measurement of Surface Tension and Determination of the Value of the Relaxation Time
2.3. Functional Properties Study
2.3.1. Solubility and Water Holding Capacity (WHC)
2.3.2. Preparation of Foams and Testing Their Basic Properties
2.3.3. Preparation of Model O/W Emulsions and Stability Testing
2.3.4. Rheological Studies of Emulsions
2.4. Statistical Analysis
3. Results
3.1. Hydrodynamic and Surface Properties
3.2. Functional Properties
3.2.1. Solubility and WHC
3.2.2. Surface Tension
3.2.3. Foaming Properties
3.2.4. Emulsifying Properties
3.3. Rheological Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DLS | dynamic light scattering |
| EAI | emulsifying activity index |
| ESI | emulsion stability |
| FC | foaming capacity |
| FO | foam overrun |
| IEP | isoelectric point |
| LD | liquid drainage |
| SDS-PAGE | sodium dodecyl sulphate–polyacrylamide gel electrophoresis |
| WHC | water holding capacity |
References
- Messina, M.J. Legumes and soybeans: Overview of their nutritional profiles and health effects. Am. J. Clin. Nutr. 1999, 70, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, K.D.; Rauschal, E.J.; Robowsky, K.D. Functional properties of plant proteins Part IV. Foaming properties of modified proteins from faba beans. Food/Nahrung 1983, 27, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Vioque, J.; Alaiz, M.; Girón-Calle, J. Nutritional and functional properties of Vicia faba protein isolates and related fractions. Food Chem. 2012, 132, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Nivala, O.; Mäkinen, O.E.; Kruus, K.; Nordlund, E.; Ercili-Cura, D. Structuring colloidal oat and faba bean protein particles via enzymatic modification. Food Chem. 2017, 231, 87–95. [Google Scholar] [CrossRef]
- Martínez-Velasco, A.; Lobato-Calleros, C.; Hernández-Rodríguez, B.E.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. High intensity ultrasound treatment of faba bean (Vicia faba L.) protein: Effect on surface properties, foaming ability and structural changes. Ultrason. Sonochem. 2018, 44, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Mession, J.L.; Chihi, M.L.; Sok, N.; Saurel, R. Effect of globular pea proteins fractionation on their heat-induced aggregation and acid cold-set gelation. Food Hydrocoll. 2015, 46, 233–243. [Google Scholar] [CrossRef]
- Waniska, R.D.; Kinsella, J.E. Foaming Properties of Proteins: Evaluation of a Column Aeration Apparatus Using Ovalbumin. J. Food Sci. 1979, 44, 1398–1402. [Google Scholar] [CrossRef]
- Johnson, E.A.; Brekke, C.J. Functional Properties of Acylated Pea Protein Isolates. J. Food Sci. 1983, 48, 722–725. [Google Scholar] [CrossRef]
- Dickinson, E. Stability and rheological implications of electrostatic milk protein–polysaccharide interactions. Trends Food Sci. Technol. 1998, 9, 347–354. [Google Scholar] [CrossRef]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Galazka, V.; Dickinson, E.; Ledward, D. Emulsifying behaviour of 11S globulin Vicia faba in mixtures with sulphated polysaccharides: Comparison of thermal and high-pressure treatments. Food Hydrocoll. 1999, 13, 425–435. [Google Scholar] [CrossRef]
- Gürbüz, G.; Liu, C.; Jiang, Z.Q.; Pulkkinen, M.; Piironen, V.; Sontag-Strohm, T.; Heinonen, M. Protein–lipid co-oxidation in emulsions stabilized by microwave-treated and conventional thermal-treated faba bean proteins. Food Sci. Nutr. 2018, 6, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Prahl, L.; Schwenke, K.D. Functional properties of plant proteins Part 7. Rheological properties of succinylated protein isolates from faba beans (Vicia faba L.). Food/Nahrung 1986, 30, 311–318. [Google Scholar] [CrossRef]
- Giménez, M.; Drago, S.; Greef, D.D.; Gonzalez, R.; Lobo, M.; Samman, N. Rheological, functional and nutritional properties of wheat/broad bean (Vicia faba) flour blends for pasta formulation. Food Chem. 2012, 134, 200–206. [Google Scholar] [CrossRef]
- Da Gulão, E.S.; de Souza, C.J.F.; da Costa, A.R.; da Rocha-Leão, M.H.M.; Garcia-Rojas, E.E. Stability and rheological behavior of coconut oil-in-water emulsions formed by biopolymers. Polímeros 2018, 28, 413–421. [Google Scholar] [CrossRef]
- Otegui, I.; Fernández-Quintela, A.; Diego, A.D.; Cid, C.; Macarulla, M.T.; Partearroyo, M.A. Properties of spray-dried and freeze-dried faba bean protein concentrates. Int. J. Food Sci. Technol. 1997, 32, 439–443. [Google Scholar] [CrossRef]
- Duthie, I.F.; Edwards, D.G.; Rogers, B.; Andrews, R.J.; Wright, J.A. Preliminary studies on suitability of field bean (Vicia faba L.) protein isolate for lambs and calves. Proc. Nutr. Soc. 1974, 33, 40A–41A. [Google Scholar] [PubMed]
- Duc, G. Faba bean (Vicia faba L.). Field Crop. Res. 1997, 53, 99–109. [Google Scholar] [CrossRef]
- Baginsky, C.; Peña-Neira, Á.; Cáceres, A.; Hernández, T.; Estrella, I.; Morales, H.; Pertuzé, R. Phenolic compound composition in immature seeds of fava bean (Vicia faba L.) varieties cultivated in Chile. J. Food Compos. Anal. 2013, 31, 1–6. [Google Scholar] [CrossRef]
- Crépon, K.; Marget, P.; Peyronnet, C.; Carrouée, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crop. Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Köpke, U.; Nemecek, T. Ecological services of faba bean. Field Crop. Res. 2010, 115, 217–233. [Google Scholar] [CrossRef]
- Ofuya, Z.M.; Akhidue, V. The role of pulses in human nutrition: A review. J. Apply Sci. Environ. Manag. 2005, 9, 99–104. [Google Scholar] [CrossRef]
- Frühbeck, G.; Monreal, I.; Santidrián, S. Hormonal implications of the hypocholesterolemic effect of intake of field beans (Vicia faba L.) by young men with hypercholesterolemia. Am. J. Clin. Nutr. 1997, 66, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Macarulla, M.T.; Medina, C.; Diego, M.A.D.; Chávarri, M.; Ángeles Zulet, M.; Martínez, J.A.; Nöel-Suberville, C.; Higueret, P.; Portillo, M.P. Effects of the whole seed and a protein isolate of faba bean (Vicia faba) on the cholesterol metabolism of hypercholesterolaemic rats. Br. J. Nutr. 2001, 85, 607–614. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Hou, W.; Li, P.; Sha, W.; Tian, Y. Structure and function of seed storage proteins in faba bean (Vicia faba L.). 3 Biotech 2017, 7, 74. [Google Scholar] [CrossRef]
- Warsame, A.O.; O’Sullivan, D.M.; Tosi, P. Seed Storage Proteins of Faba Bean (Vicia faba L): Current Status and Prospects for Genetic Improvement. J. Agric. Food Chem. 2018, 66, 12617–12626. [Google Scholar] [CrossRef]
- Yanping, W.; Shuangxi, L.; Ahmed, Z.; Qingming, S. Extraction of broad bean protein and effects of NaCl concentration and pH value on its solubility and emulsibility. Trans. Chin. Soc. Agric. Eng. 2010, 26, 380–384. [Google Scholar] [CrossRef]
- Arogundade, L.A.; Tshay, M.; Shumey, D.; Manazie, S. Effect of ionic strength and/or pH on Extractability and physico-functional characterization of broad bean (Vicia faba L.) Protein concentrate. Food Hydrocoll. 2006, 20, 1124–1134. [Google Scholar] [CrossRef]
- Rahma, E.H. Functional and electrophoretic characteristics of faba bean (Vicia faba) flour proteins as affected by germination. Food/Nahrung 1988, 32, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Husband, F.; Wilde, P.; Clark, D.; Rawel, H.; Muschiolik, G. Foaming properties of modified faba bean protein isolates. Food Hydrocoll. 1994, 8, 455–468. [Google Scholar] [CrossRef]
- Makri, E.A.; Doxastakis, G.I. Study of emulsions stabilized with Phaseolus vulgaris or Phaseolus coccineus with the addition of Arabic gum, locust bean gum and xanthan gum. Food Hydrocoll. 2006, 20, 1141–1152. [Google Scholar] [CrossRef]
- Felix, M.; Romero, A.; Carrera-Sanchez, C.; Guerrero, A. Assessment of interfacial viscoelastic properties of Faba bean (Vicia faba) protein-adsorbed O/W layers as a function of pH. Food Hydrocoll. 2019, 90, 353–359. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Hayakawa, S.; Nakai, S. Relationships of Hydrophobicity and Net Charge to the Solubility of Milk and Soy Proteins. J. Food Sci. 1985, 50, 486–491. [Google Scholar] [CrossRef]
- Mccoy, J.L.; Muthukumar, M. Dynamic light scattering studies of ionic and nonionic polymer gels with continuous and discontinuous volume transitions. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 2193–2206. [Google Scholar] [CrossRef]
- Raoufi, N.; Kadkhodaee, R.; Fang, Y.; Phillips, G.O. pH-Induced structural transitions in whey protein isolate and ultrasonically solubilized Persian gum mixture. Ultrason. Sonochem. 2020, 68, 105190. [Google Scholar] [CrossRef]
- Zhmud, B.V.; Tiberg, F.; Kizling, J. Dynamic Surface Tension in Concentrated Solutions of CnEm Surfactants: A Comparison between the Theory and Experiment. Langmuir 2000, 16, 2557–2565. [Google Scholar] [CrossRef]
- Rodriguez-Abreu, C.; Kunieda, H. Equilibrium and Dynamic Surface Tension Properties of Aqueous Solutions of Sulfonated Cationic-Nonionic Fluorocarbon Surfactants. J. Dispers. Sci. Technol. 2005, 26, 435–440. [Google Scholar] [CrossRef]
- Liao, Y.C.; Basaran, O.A.; Franses, E.I. Micellar dissolution and diffusion effects on adsorption dynamics of surfactants. AIChE J. 2003, 49, 3229–3240. [Google Scholar] [CrossRef]
- ISO 1871:2009. Food and Feed Products–General Guidelines for the Determination of Nitrogen by the Kjeldahl Method; Standard, International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Sorgentini, D.A.; Wagner, J.R.; Anon, M.C. Effects of Thermal Treatment of Soy Protein Isolate on the Characteristics and Structure-Function Relationship of Soluble and Insoluble Fractions. J. Agric. Food Chem. 1995, 43, 2471–2479. [Google Scholar] [CrossRef]
- Cheftel, J.C.; Cuq, J.L.; Lorient, D. Amino Acids, Peptides and Proteins; Marcel Dekker Inc.: New York, NY, USA, 1985; pp. 245–369. [Google Scholar]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Qi, M.; Hettarachchy, N.; Kalapathy, U. Solubility and Emulsifying Properties of Soy Protein Isolates Modified by Pancreatin. J. Food Sci. 1997, 62, 1110–1115. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Derbyshire, E.; Wright, D.; Boulter, D. Legumin and vicilin, storage proteins of legume seeds. Phytochemistry 1976, 15, 3–24. [Google Scholar] [CrossRef]
- Mori, T.; Utsumi, S. Purification and Properties of Storage Proteins of Broad Bean. Agric. Biol. Chem. 1979, 43, 577–583. [Google Scholar] [CrossRef]
- Wright, D.J.; Boulter, D. Purification and subunit structure of legumin of Vicia faba L. (broad bean). Biochem. J. 1974, 141, 413–418. [Google Scholar] [CrossRef]
- Wüstneck, R.; Krägel, J.; Miller, R.; Fainerman, V.; Wilde, P.; Sarker, D.; Clark, D. Dynamic surface tension and adsorption properties of beta-casein and beta-lactoglobulin. Food Hydrocoll. 1996, 10, 395–405. [Google Scholar] [CrossRef]
- Ward, A.F.H.; Tordai, L. Time-Dependence of Boundary Tensions of Solutions I. The Role of Diffusion in Time-Effects. J. Chem. Phys. 1946, 14, 453–461. [Google Scholar] [CrossRef]
- Chove, B.E.; Grandison, A.S.; Lewis, M.J. Emulsifying properties of soy protein isolates obtained by microfiltration. J. Sci. Food Agric. 2002, 82, 267–272. [Google Scholar] [CrossRef][Green Version]
- Fernández-Quintela, A.; Macarulla, M.T.; del Barrio, A.S.; Martínez, J.A. Composition and functional properties of protein isolates obtained from commercial legumes grown in northern Spain. Plant Foods Hum. Nutr. 1997, 51, 331–341. [Google Scholar] [CrossRef] [PubMed]
- López de Ogara, M.C.; Delgado de Layño, M.; Pilosof, A.M.; Macchi, R.A. Functional properties of soy protein isolates as affected by heat treatment during isoelectric precipitation. J. Am. Oil Chem. Soc. 1992, 69, 184–187. [Google Scholar] [CrossRef]
- Liang, H.N.; Tang, C.H. pH-dependent emulsifying properties of pea [Pisum sativum (L.)] proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- Jarpa-Parra, M.; Bamdad, F.; Tian, Z.; Zeng, H.; Temelli, F.; Chen, L. Impact of pH on molecular structure and surface properties of lentil legumin-like protein and its application as foam stabilizer. Colloids Surf. B Biointerfaces 2015, 132, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Dagorn-Scaviner, C.; Gueguen, J.; Lefebvre, J. Emulsifying Properties of Pea Globulins as Related to Their Adsorption Behaviors. J. Food Sci. 1987, 52, 335–341. [Google Scholar] [CrossRef]
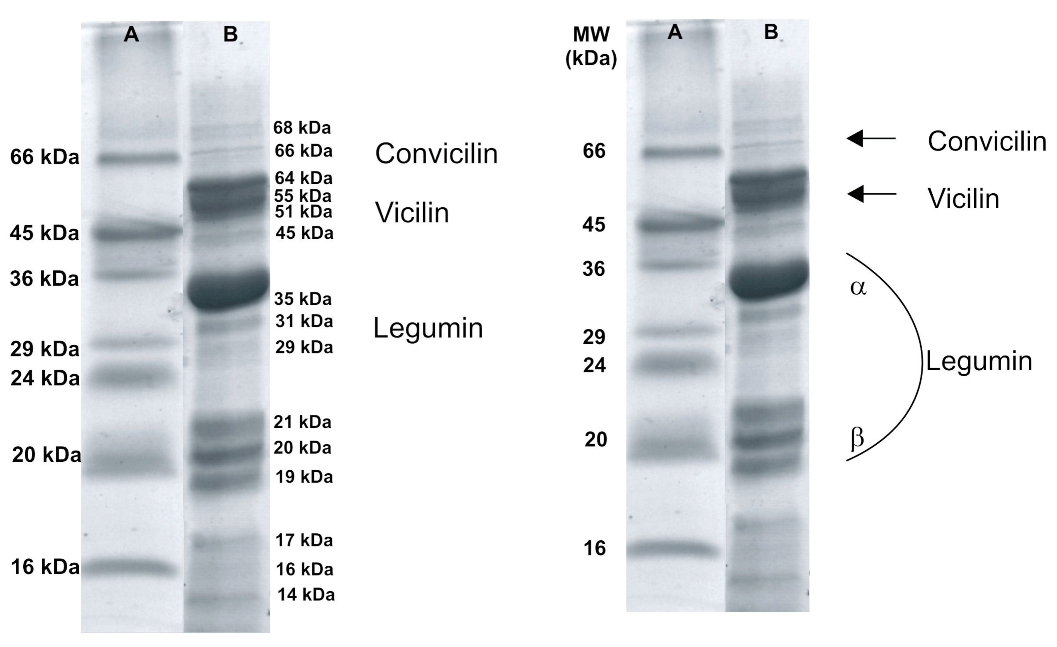
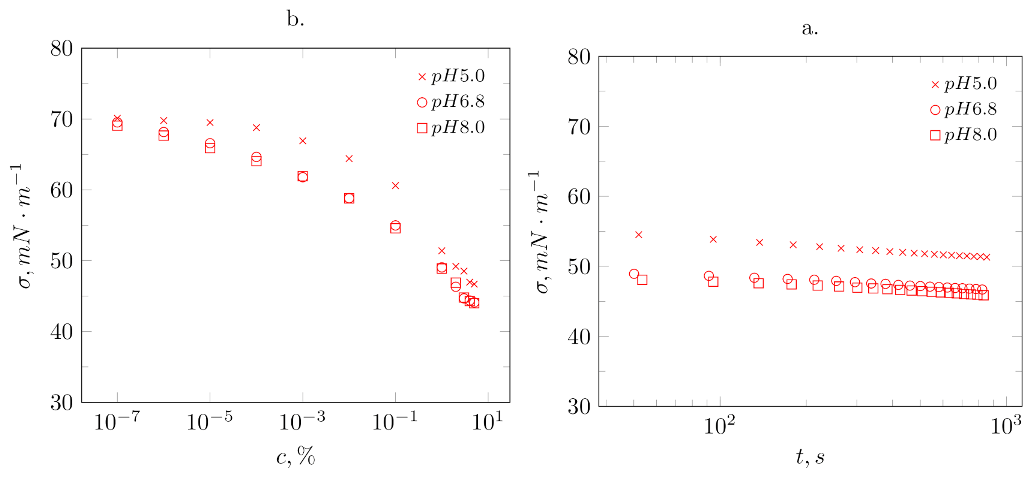

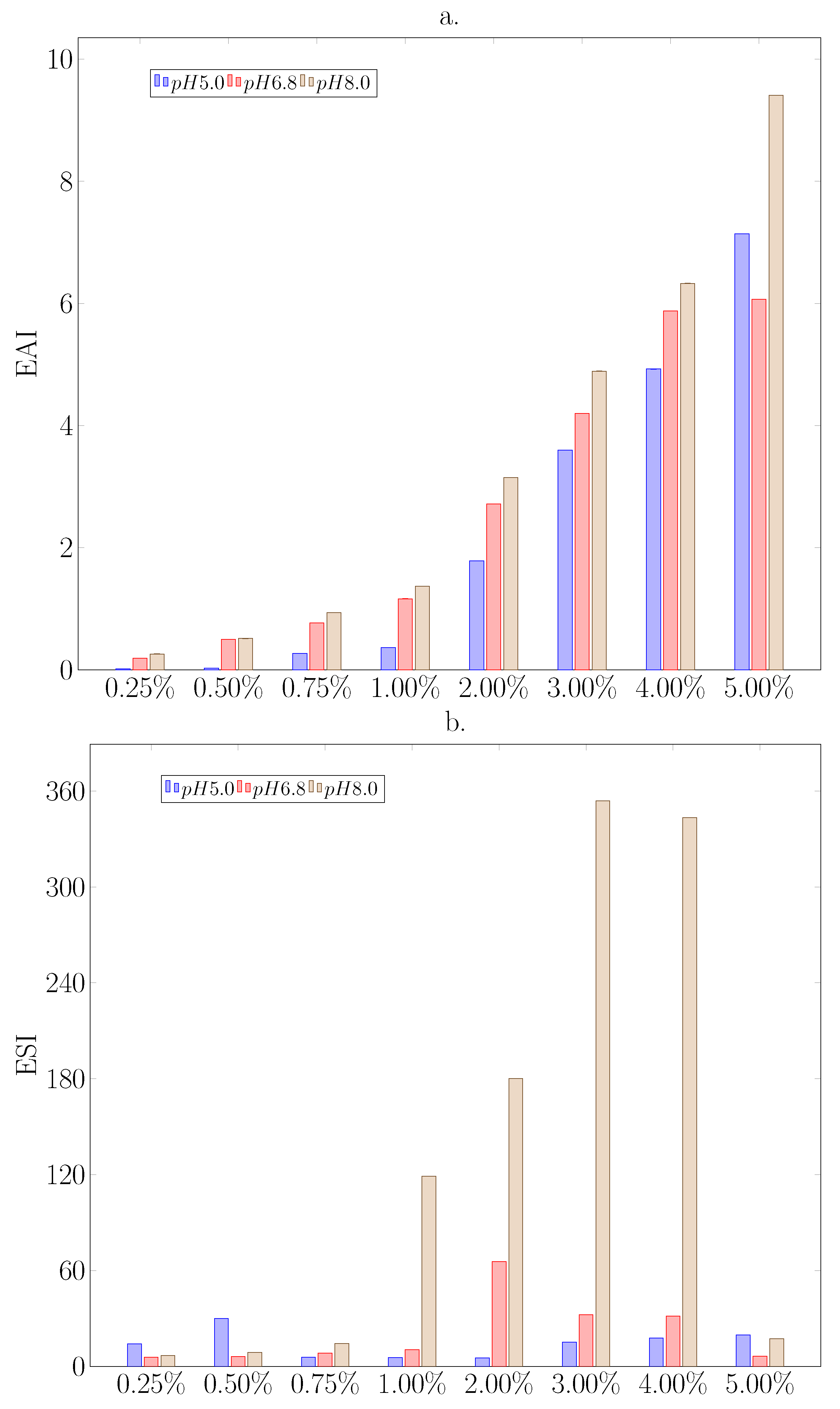

| Surface Properties | Hydrodynamic Properties | |||||||
|---|---|---|---|---|---|---|---|---|
| pH | c,% | , mN·m | , s | , mms | , nm | , mV | H | |
| 5.0 | 0.001 | 72 | 186,000 | 0.01 | - | - | - | - |
| 0.01 | 67 | 18,800 | 0.35 | - | - | - | - | |
| 0.1 | 64 | 14,800 | 1.25 | - | - | - | - | |
| 1.0 | 54 | 15,700 | 2.32 | 3 | −20.86 | 1.64 | ||
| 6.8 | 0.001 | 72 | 72,400 | 0.17 | - | - | - | - |
| 0.01 | 66 | 18,000 | 0.61 | - | - | - | - | |
| 0.1 | 57 | 6800 | 1.19 | - | - | - | - | |
| 1.0 | 49 | 17,600 | 0.57 | 30 | −28.73 | 7.49 | ||
| 8.0 | 0.001 | 60 | 16700 | 0.75 | - | - | - | - |
| 0.01 | 56 | 8200 | 1.17 | - | - | - | - | |
| 0.1 | 55 | 5700 | 2.45 | - | - | - | - | |
| 1.0 | 48 | 17,900 | 0.31 | 10 | −34.03 | 7.40 | ||
| pH | |||||
|---|---|---|---|---|---|
| 4.0 | 5.0 | 6.0 | 6.8 | 8.0 | |
| Solubility | 0.025 ± 0.003 | 0.040 ± 0.003 | 0.049 ± 0.003 | 0.155 ± 0.004 | 0.223 ± 0.001 |
| WHC | 2.255 ± 0.002 | 1.991 ± 0.001 | 2.585 ± 0.002 | 3.469 ± 0.002 | 3.494 ± 0.002 |
| pH | c,% | K, s | m, - | , Pa·s | , Pa·s | |
|---|---|---|---|---|---|---|
| 5.0 | 1 | 0.0003 | 2.85 | 1.384 | 0.014 | 0.09048 |
| 3 | 0.0002 | 2.85 | 1.049 | 0.005 | 0.02977 | |
| 5 | 0.0051 | 1.68 | 0.124 | 0.005 | 0.00052 | |
| 6.8 | 1 | 0.0107 | 1.45 | 0.020 | 0.003 | 0.00002 |
| 3 | 0.0053 | 1.72 | 0.045 | 0.004 | 0.00007 | |
| 5 | 0.0032 | 1.94 | 0.052 | 0.005 | 0.00010 | |
| 8.0 | 1 | 0.0022 | 1.73 | 0.014 | 0.003 | 0.00001 |
| 3 | 0.0064 | 1.38 | 0.020 | 0.004 | 0.00001 | |
| 5 | 0.0075 | 1.47 | 0.030 | 0.006 | 0.00002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żmudziński, D.; Goik, U.; Ptaszek, P. Functional and Rheological Properties of Vicia faba L. Protein Isolates. Biomolecules 2021, 11, 178. https://doi.org/10.3390/biom11020178
Żmudziński D, Goik U, Ptaszek P. Functional and Rheological Properties of Vicia faba L. Protein Isolates. Biomolecules. 2021; 11(2):178. https://doi.org/10.3390/biom11020178
Chicago/Turabian StyleŻmudziński, Daniel, Urszula Goik, and Paweł Ptaszek. 2021. "Functional and Rheological Properties of Vicia faba L. Protein Isolates" Biomolecules 11, no. 2: 178. https://doi.org/10.3390/biom11020178
APA StyleŻmudziński, D., Goik, U., & Ptaszek, P. (2021). Functional and Rheological Properties of Vicia faba L. Protein Isolates. Biomolecules, 11(2), 178. https://doi.org/10.3390/biom11020178





