Erectile Function and Sexual Behavior: A Review of the Role of Nitric Oxide in the Central Nervous System
Abstract
1. Introduction
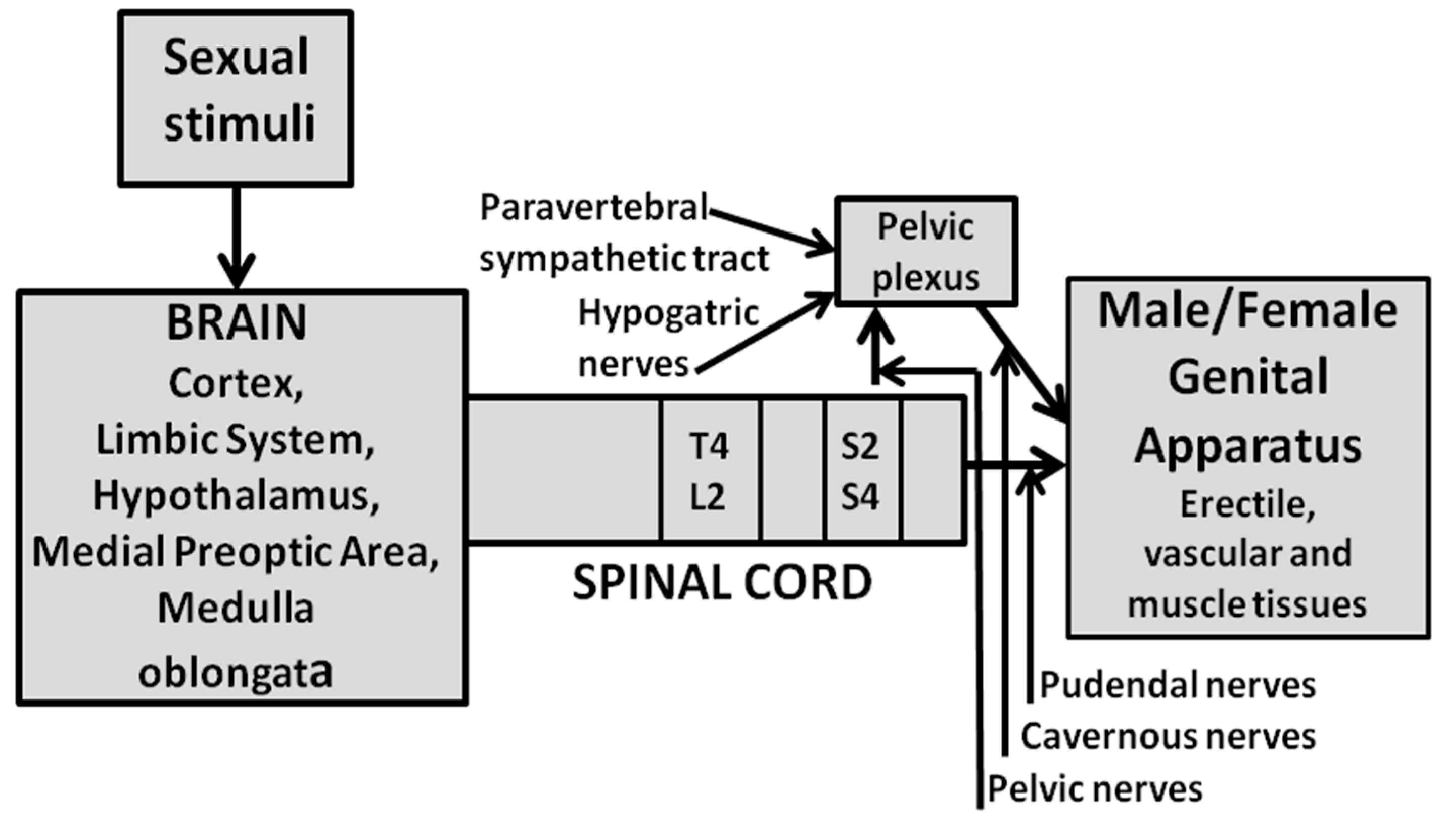
2. Central NO and Erectile Function
2.1. Central NO Facilitates Erectile Function in Male Rats: Effect of NO Synthase Inhibitors
2.2. Central NO, PVN and Erectile Function
2.2.1. NO Synthase Inhibitors Injected into the PVN Abolish/Reduce Drug- and Peptide-Induced Penile Erection in Male Rats
2.2.2. NO Donors Injected i.c.v. or Directly into the PVN Induce Penile Erection in Male Rats
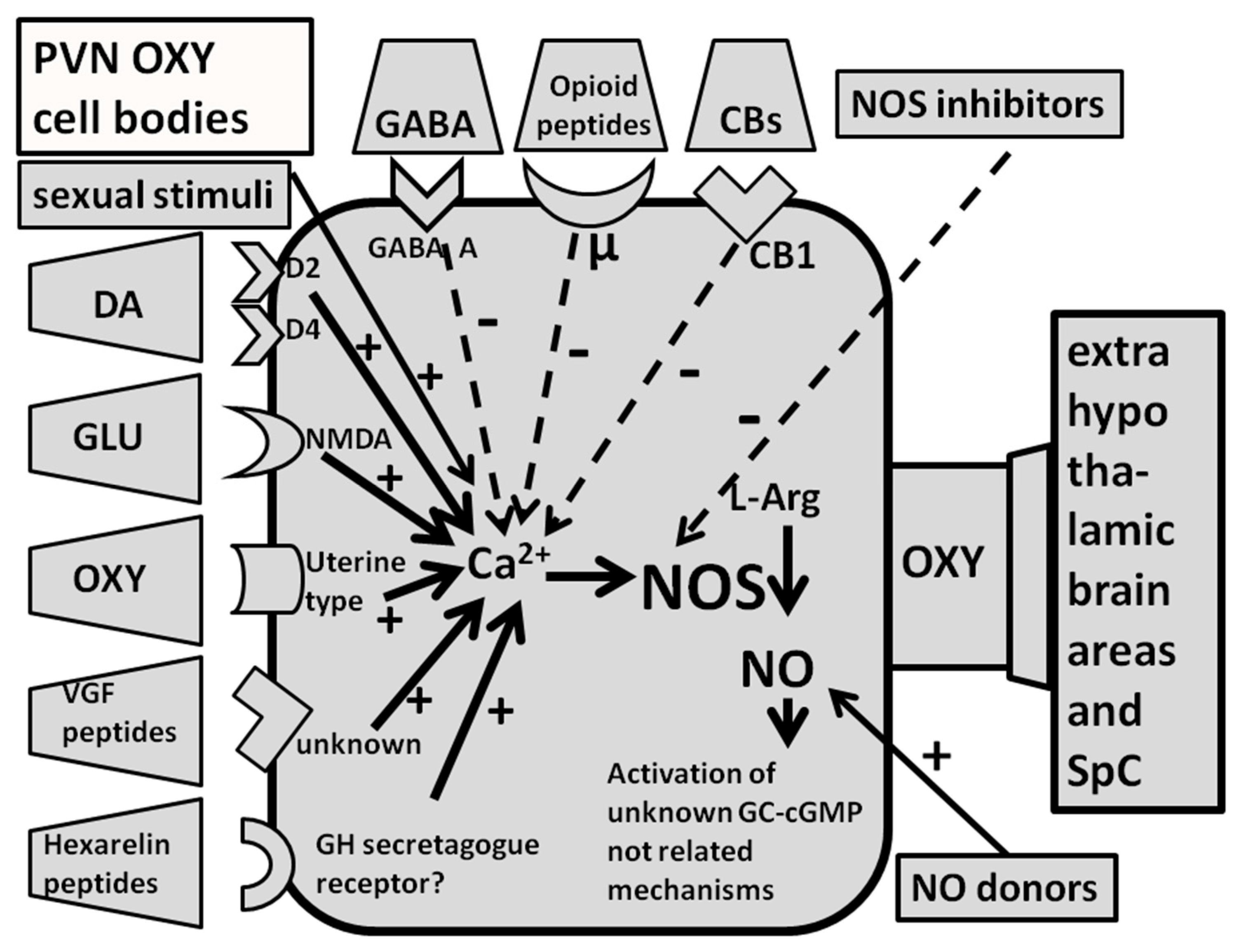
2.2.3. Dopamine Agonists, Oxytocin, NMDA, Hexarelin Analogue Peptides, VGF-Related Peptides Induce Penile Erection by Increasing NO Production in the PVN of Male Rats
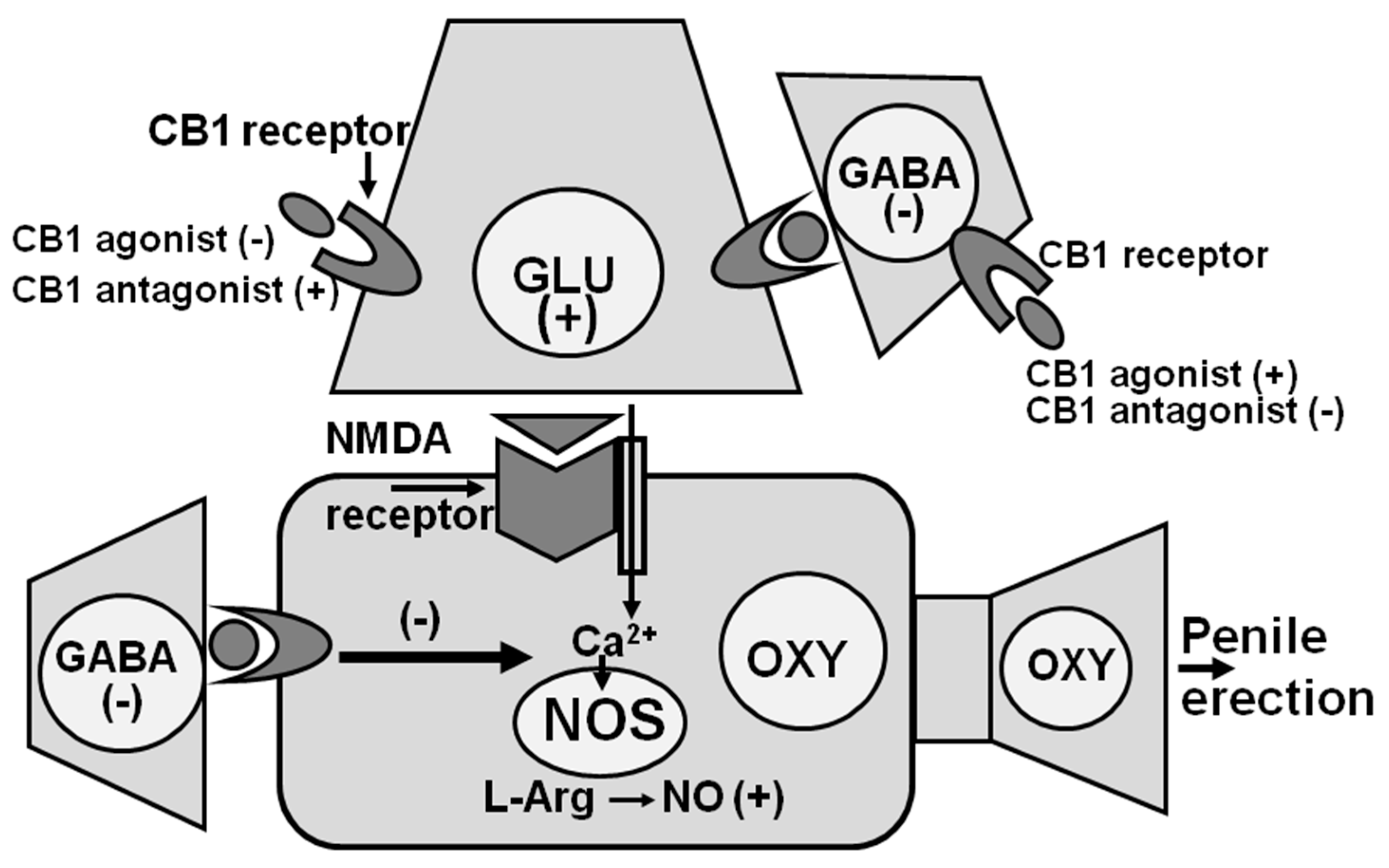
2.2.4. GABA Agonists, Opioid Peptides/Opiates and Cannabinoids Reduce Spontaneous or Drug- and Peptide-Induced Penile Erection by Inhibiting NO Synthase in the PVN of Male Rats
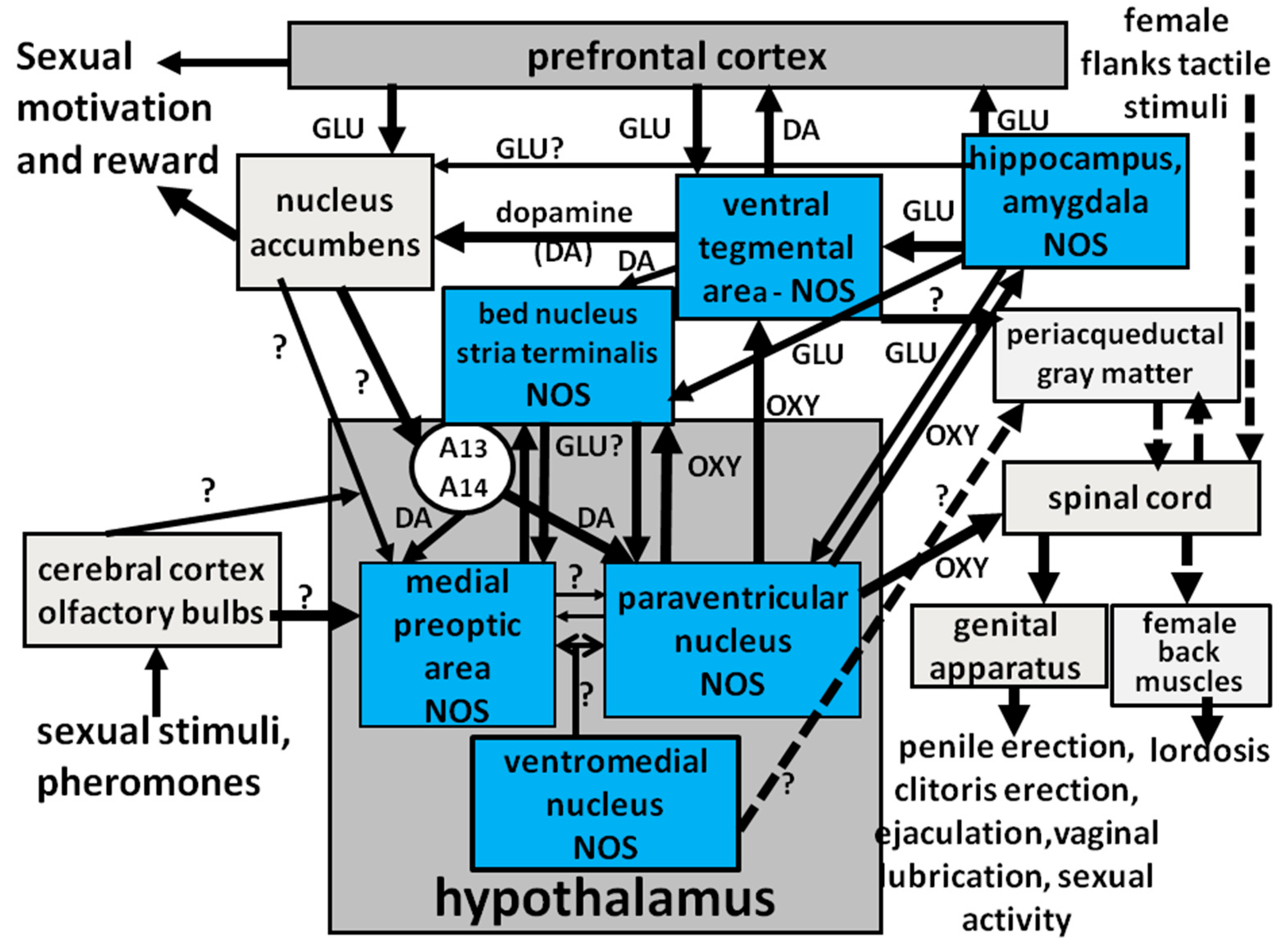
2.3. NO, Erectile Function and Extrahypothalamic Brain Areas
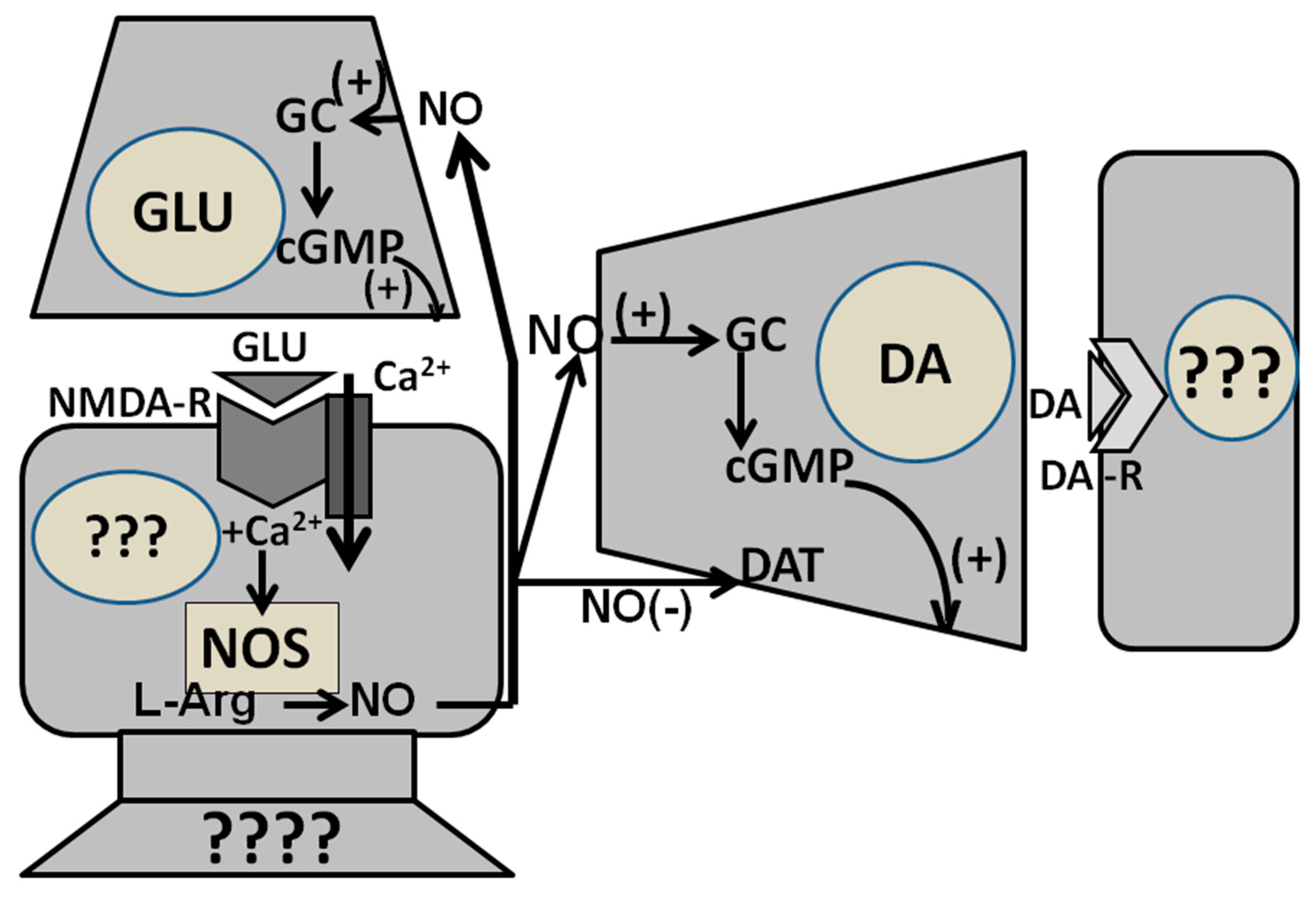
2.4. Facilitation of Erectile Function by Central NO: Mechanism of Action
2.4.1. NO Facilitates Erectile Function in Male Rats by Activating Central Oxytocinergic Neurotransmission
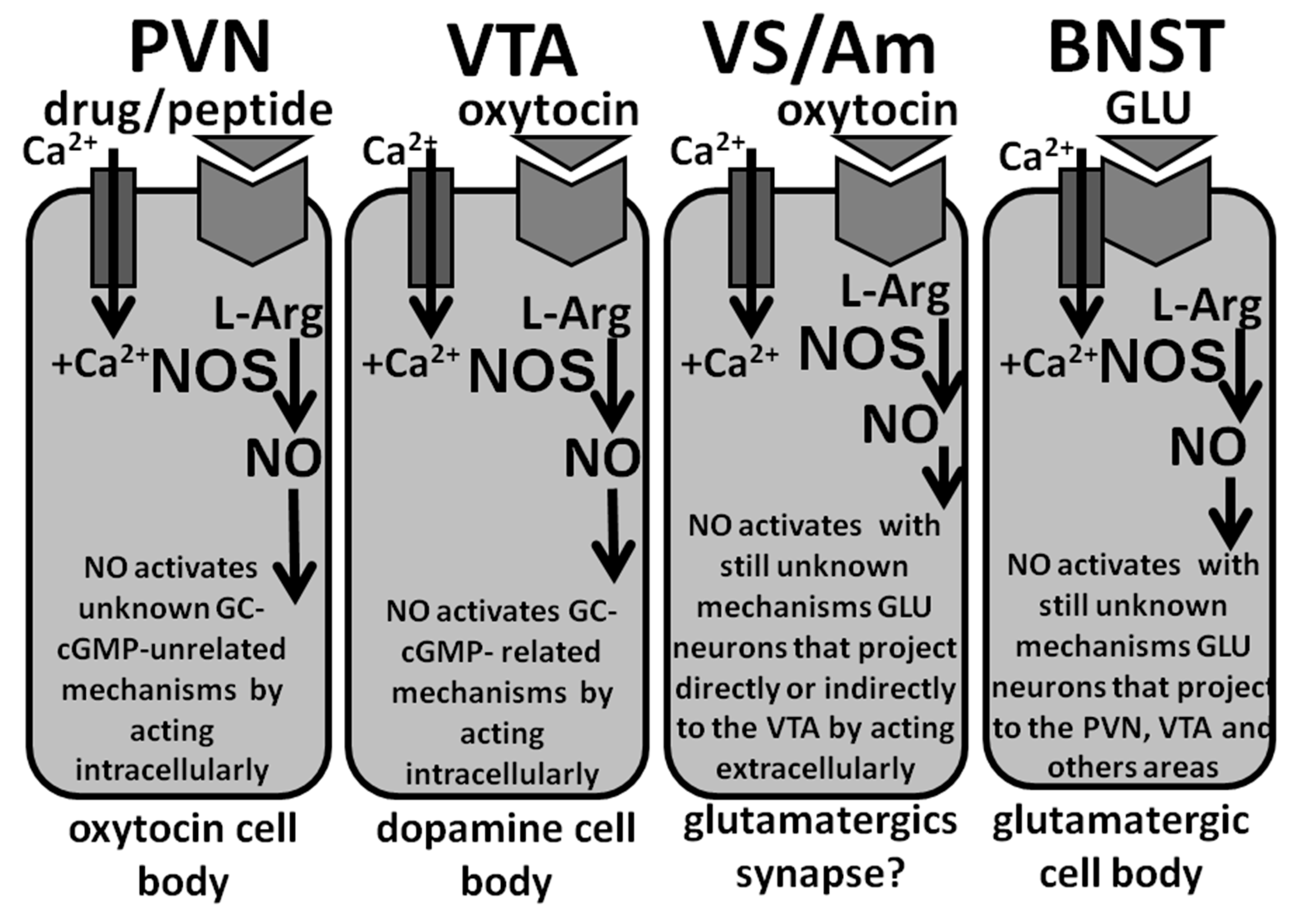
2.4.2. Is Guanylate Cyclase the NO Target in the PVN?
2.4.3. Guanylate Cyclase Is the NO Target in the Ventral Tegmental Area of Male Rats
2.4.4. NO Facilitates Erectile Function in Male Rats by Acting Intracellularly in the PVN and in the Ventral Tegmental Area and Intercellularly in the Ventral Subiculum
3. Central NO and Male Sexual Behavior
3.1. Medial Preoptic Area
3.1.1. NO Facilitates Erectile Function and Sexual Behavior by Acting Intercellularly as a Retrograde Messenger in the Medial Preoptic Area of Male Rats
3.2. PVN
3.3. Ventral Tegmental Area, Ventral Subiculum, Amygdala and Bed Nucleus of the Stria Terminalis
4. Central NO and Female Sexual Behavior
5. Can Central NO Have a Role in Strategies Aimed to Improve Erectile Function and Sexual Behavior in Humans?
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Rees, D.D.; Palmer, R.M.; Schulz, R.; Hodson, H.F.; Moncada, S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990, 101, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Chachlaki, K.; Prevot, V. Nitric oxide signalling in the brain and its control of bodily functions. Br. J. Pharmacol. 2020, 177, 5437–5458. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Biosynthesis of nitric oxide from L-arginine. Biochem. Pharmacol. 1989, 38, 1709–1715. [Google Scholar] [CrossRef]
- Garthwaite, J.; Charles, S.L.; Chess-Williams, R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 1988, 336, 385–388. [Google Scholar] [CrossRef]
- Knowles, R.G.; Palacios, M.; Palmer, R.M.; Moncada, S. Formation of nitric oxide from L-arginine in the central nervous system: A transduction mechanism for stimulation of the soluble guanylate cyclase. Proc. Natl. Acad. Sci. USA 1989, 86, 5159–5163. [Google Scholar] [CrossRef]
- Bredt, D.S.; Snyder, S.H. Isolation of nitric oxide synthase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. USA 1990, 82, 682–685. [Google Scholar] [CrossRef]
- Bredt, D.S.; Hwang, P.M.; Snyder, S.H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 1990, 347, 768–770. [Google Scholar] [CrossRef]
- Ignarro, L.J. Biosynthesis and metabolism of endothelium derived nitric oxide. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 535–560. [Google Scholar] [CrossRef]
- Snyder, S.H. Nitric oxide: First in a new class of neurotransmitters? Science 1992, 257, 494–496. [Google Scholar] [CrossRef]
- Schuman, E.M.; Madison, D.V. Nitric oxide and synaptic function. Annu. Rev. Neurosci. 1994, 17, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Azadzoi, K.M.; Goldstein, I.; Saenz De Tejada, I. A nitric oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J. Clin. Investig. 1991, 88, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Rajfer, J.; Aronson, W.J.; Bush, P.A.; Dorey, F.J.; Ignarro, L.J. Nitric oxide as a mediator of relaxation of the corpus cavemosum in response to nonadrenergic, noncholinergic neurotransmission. N. Engl. J. Med. 1992, 326, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Sáenz de Tejada, I.; Angulo, J.; Cellek, S.; González-Cadavid, N.; Heaton, J.; Pickard, R.; Simonsen, U. Physiology of erectile function. J. Sex. Med. 2004, 1, 254–265. [Google Scholar] [CrossRef]
- Argiolas, A. Male erectile dysfunction: The chemical pharmacology of penile erection. Drugs Discov. Today Ther. Strateg. 2005, 2, 31–36. [Google Scholar] [CrossRef]
- Burnett, A.L.; Goldstein, I.; Andersson, K.E.; Argiolas, A.; Christ, G.; Park, K.; Xin, Z.C. Future sexual medicine physiological treatment targets. J. Sex. Med. 2010, 7, 3269–3304. [Google Scholar] [CrossRef]
- Burnett, A.L.; Lowenstein, C.J.; Bredt, D.S.; Chang, T.S.; Snyder, S.H. Nitric oxide: A physiological mediator of penile erection. Science 1992, 257, 401–403. [Google Scholar] [CrossRef]
- Haning, H.; Niewöhner, U.; Bischoff, E. Phosphodiesterase type 5 (PDE5) inhibitors. Prog. Med. Chem. 2003, 41, 249–306. [Google Scholar] [CrossRef]
- Kass, D.A.; Champion, H.C.; Beavo, J.A. Phosphodiesterase Type 5: Expanding Roles in Cardiovascular Regulation. Circ. Res. 2007, 101, 1084–1095. [Google Scholar] [CrossRef]
- Burnett, A.L.; Nelson, R.J.; Calvin, D.C.; Liu, J.X.; Demas, G.E.; Klein, S.L.; Kriegsfeld, L.J.; Dawson, V.L.; Dawson, T.M.; Snyder, S.H. Nitric oxide-dependent penile erection in mice lacking neuronal nitric oxide synthase. Mol. Med. 1996, 2, 288–296. [Google Scholar] [CrossRef]
- Hurt, K.J.; Sezen, S.F.; Champion, H.C.; Crone, J.K.; Palese, M.A.; Huang, P.L.; Sawa, A.; Luo, X.; Musicki, B.; Snyder, S.H.; et al. Alternatively spliced neuronal nitric oxide synthase mediates penile erection. Proc. Natl. Acad. Sci. USA 2006, 103, 3440–3443. [Google Scholar] [CrossRef] [PubMed]
- Hurt, K.J.; Sezen, S.F.; Lagoda, G.F.; Musicki, B.; Rameau, G.A.; Snyder, S.H.; Burnett, A.L. Cyclic AMP-dependent phosphorylation of neuronal nitric oxyde synthase mediate penile erection. Proc. Natl. Acad. Sci. USA 2012, 109, 16624–16629. [Google Scholar] [CrossRef]
- Southam, E.; Garthwaite, J. Comparative effects of some nitric oxide donors on cyclic GMP levels in the rat cerebellar slices. Neurosci. Lett. 1991, 130, 107–111. [Google Scholar] [CrossRef]
- Southam, E.; Garthwaite, J. The nitric oxide-cyclic GMP signalling pathway in rat brain. Neuropharmacology 1993, 2, 1267–1277. [Google Scholar] [CrossRef]
- Garthwaite, J. NO as a multimodal transmitter in the brain: Discovery and current status. Br. J. Pharmacol. 2019, 176, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Vincent, S.R.; Kimura, H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience 1992, 46, 755–784. [Google Scholar] [CrossRef]
- Alonso, J.R.; Sanchez, F.; Arevalo, R.; Carretero, J.; Vazquez, R.; Aijon, J. Partial coexistence of NADPH-diaphorase and somatostatin in the rat hypothalamic paraventricular nucleus. Neurosci. Lett. 1992, 148, 101–104. [Google Scholar] [CrossRef]
- Torres, G.; Lee, S.; Rivier, C. Ontogeny of the rat hypothalamic nitric oxide synthase and colocalization with neuropeptides. Mol. Cell. Neurosci. 1993, 4, 155–163. [Google Scholar] [CrossRef]
- Sanchez, F.; Alonso, J.R.; Arevalo, R.; Blanco, E.; Aijon, J.; Vazquez, R. Coexistence of NADPH-diaphorase with vasopressin and oxytocin in the hypothalamic magnocellular neurosecretory nuclei of the rat. Cell Tissue Res. 1994, 276, 31–34. [Google Scholar] [CrossRef]
- Amir, S. Nitric oxide signalling in the hypothalamus. In Nitric Oxide in the Nervous System; Vincent, S., Ed.; Academic Press: London, UK, 1995; pp. 151–162. [Google Scholar]
- Buijs, R.M.; Geffard, M.; Pool, C.W.; Hoorneman, E.M. The dopaminergic innervation of the supraoptic and paraventricular nucleus. A light and electron microscopical study. Brain Res. 1984, 323, 65–74. [Google Scholar] [CrossRef]
- Lindvall, O.; Bjorklund, A.; Skagerberg, G. Selective histochemical demonstration of dopamine terminal systems in rat di- and telencephalon: New evidence for dopaminergic innervation of hypothalamic neurosecretory nuclei. Brain Res. 1984, 306, 19–30. [Google Scholar] [CrossRef]
- Van de Kar, L.D. Neuroendocrine pharmacology of serotoninergic (5-HT) neurons. Annu. Rev. Pharmacol. Toxicol. 1991, 31, 289–320. [Google Scholar] [CrossRef] [PubMed]
- Van den Pol, A.N. Glutamate and aspartate immunoreactivity in hypothalamic presynaptic axons. J. Neurosci. 1991, 11, 2087–2101. [Google Scholar] [CrossRef]
- Melis, M.R.; Argiolas, A. Nitric oxide synthase inhibitors prevent apomorphine-and oxytocin-induced penile erection and yawning in male rats. Brain Res. Bull. 1993, 32, 71–74. [Google Scholar] [CrossRef]
- Argiolas, A. Nitric oxide is a central mediator of penile erection. Neuropharmacology 1994, 3, 1339–1344. [Google Scholar] [CrossRef]
- Hull, E.M.; Lumley, L.A.; Matuszewich, L.; Dominguez, J.; Moses, J.; Lorrain, D.S. The role of nitric oxide in sexual function of male rats. Neuropharmacology 1994, 33, 1499–1504. [Google Scholar] [CrossRef]
- Lorrain, D.S.; Hull, E.M. Nitric oxide increases dopamine and serotonin release in the medial preoptic area. Neuroreport 1993, 5, 87–89. [Google Scholar] [CrossRef]
- Argiolas, A.; Melis, M.R. Neuromodulation of penile erection: An overview of the role of neurotransmitters and neuromodulators. Prog. Neurobiol. 1995, 47, 235–255. [Google Scholar] [CrossRef]
- Benelli, A.; Bertolini, A.; Poggioli, R.; Cavazzuti, E.; Calza, L.; Giardino, L.; Arletti, R. Nitric oxide is involved in male sexual behavior of rats. Eur. J. Pharmacol. 1995, 294, 505–510. [Google Scholar] [CrossRef]
- Sato, Y.; Christ, G.J.; Horita, H.; Adachi, H.; Suzuki, N.; Tsukamoto, T. The effects of alterations in nitric oxide levels in the paraventricular nucleus on copulatory behavior and reflexive erections in male rats. J. Urol. 1999, 162, 2182–2185. [Google Scholar] [CrossRef]
- Chen, K.K.; Chang, L.S. Involvement of L-arginine/nitric oxide pathway at the paraventricular nucleus of hypothalamus in central neural regulation of penile erection in the rat. Int. J. Impot. Res. 2002, 14, 139–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sato, Y.; Horita, H.; Kurohata, T.; Adachi, H.; Tsukamoto, T. Effect of the nitric oxide level in the medial preoptic area on male copulatory behavior in rats. Am. J. Physiol. 1998, 274, R243–R247. [Google Scholar] [CrossRef]
- Sato, Y.; Zhao, W.; Christ, G.J. Central modulation of the NO/cGMP pathway affects the MPOA-induced intracavernous pressure response. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R269–R278. [Google Scholar] [CrossRef]
- Chang, A.Y.; Chan, J.Y.; Chan, S.H. Differential contributions of nitric oxide synthase isoforms at hippocampal formation to negative feedback regulation of penile erection in the rat. Br. J. Pharmacol. 2002, 136, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Succu, S.; Sanna, F.; Cocco, C.; Melis, T.; Boi, A.; Ferri, G.L.; Argiolas, A. Oxytocin induces penile erection when injected into the ventral tegmental area of male rats: Role of nitric oxide and cyclic GMP. Eur. J. Neurosci. 2008, 28, 813–821. [Google Scholar] [CrossRef]
- Melis, M.R.; Sanna, F.; Succu, S.; Zarone, P.; Boi, A.; Argiolas, A. The role of oxytocin in the anticipatory and consummatory phases of male rat sexual behaviour. In Handbook of Oxytocin Research: Synthesis, Storage and Release, Actions and Drug Forms; Jastrow, H., Feuerbach, D., Eds.; Nova Publishers Inc.: New York, NY, USA, 2009; pp. 109–125. [Google Scholar]
- Melis, M.R.; Succu, S.; Sanna, F.; Boi, A.; Argiolas, A. Oxytocin injected into the ventral subiculum or the posteromedial cortical nucleus of the amygdala induces penile erection and increases extracellular dopamine levels in the nucleus accumbens of male rats. Eur. J. Neurosci. 2009, 30, 1349–1357. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Cocco, C.; Caboni, E.; Sanna, F.; Boi, A.; Ferri, G.L.; Argiolas, A. Oxytocin induces penile erection when injected into the ventral subiculum: Role of nitric oxide and glutamic acid. Neuropharmacology 2010, 58, 1153–1160. [Google Scholar] [CrossRef]
- Succu, S.; Sanna, F.; Argiolas, A.; Melis, M.R. Oxytocin injected into the hippocampal ventral subiculum induces penile erection in male rats by increasing glutamatergic neurotransmission in the ventral tegmental area. Neuropharmacology 2011, 61, 181–188. [Google Scholar] [CrossRef]
- Saito, S.; Kidd, G.J.; Trapp, B.D.; Dawson, T.M.; Bredt, D.S.; Wilson, D.A.; Traystman, R.J.; Snyder, S.H.; Hanley, D.F. Rat spinal cord neurons contain nitric oxide synthase. Neuroscience 1994, 3, 447–456. [Google Scholar] [CrossRef]
- Melis, M.R.; Argiolas, A. Central control of penile erection: A re-visitation of the role of oxytocin and its interaction with dopamine and glutamic acid in male rats. Neurosci. Biobehav. Rev. 2011, 35, 939–955. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.R.; Argiolas, A. Oxytocin, erectile function and sexual behaviour: Last discoveries and possible advances. Int. J. Mol. Sci. 2021, 22, 30376. [Google Scholar] [CrossRef]
- Argiolas, A.; Melis, M.R. The role of oxytocin and the paraventricular nucleus in the sexual behaviour of male mammals. Physiol. Behav. 2004, 83, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Argiolas, A.; Melis, M.R. Central control of penile erection: Role of the paraventricular nucleus of the hypothalamus. Prog. Neurobiol. 2005, 76, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Argiolas, A.; Melis, M.R. Neuropeptides and central control of sexual behavior from the past to the present: A review. Prog. Neurobiol. 2013, 108, 80–107. [Google Scholar] [CrossRef]
- Nelson, R.J.; Demas, G.E.; Huang, P.L.; Fishman, M.C.; Dawson, V.L.; Dawson, T.M.; Snyder, S.H. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature 1995, 378, 383–386. [Google Scholar] [CrossRef]
- Meisel, R.L.; Sachs, B.D. The physiology of male sexual behavior. In The Physiology of Reproduction; Knobil, E., Neil, J., Eds.; Raven Press: New York, NY, USA, 1994; pp. 3–96. [Google Scholar]
- Hull, E.M.; Meisel, R.L.; Sachs, B.D. Male sexual behavior. In Hormones, Brain and Behavior; Pfaff, D.W., Arnold, A.P., Etgen, A.M., Fahrbach, S.E., Rubin, R.T., Eds.; Academic Press: New York, NY, USA, 2002; pp. 3–137. [Google Scholar]
- Burns-Cusato, M.; Scordalakes, E.M.; Rissman, E.F. Of mice and missing data: What we know (and need to learn) about male sexual behavior. Physiol. Behav. 2004, 83, 217–232. [Google Scholar] [CrossRef]
- Allard, J.; Edmunds, N.J. Reflex penile erection in anesthetized mice: An exploratory study. Neuroscience 2008, 155, 283–290. [Google Scholar] [CrossRef]
- Andersson, K.E.; Wagner, G. Physiology of penile erection. Physiol. Rev. 1995, 75, 191–236. [Google Scholar] [CrossRef]
- McKenna, K.E. Central control of penile erection. Int. J. Impot. Res. 1998, 10 (Suppl. 1), 25–34. [Google Scholar]
- McKenna, K.E. Some proposals regarding the organization of the central nervous system control of penile erection. Neurosci. Biobehav. Rev. 2000, 24, 535–540. [Google Scholar] [CrossRef]
- Argiolas, A. Neuropeptides and sexual behaviour. Neurosci. Biobehav. Rev. 1999, 23, 1127–1142. [Google Scholar] [CrossRef]
- Sachs, B.D. Contextual approaches to the physiology and classification of erectile function, erectile dysfunction, and sexual arousal. Neurosci. Biobehav. Rev. 2000, 24, 541–560. [Google Scholar] [CrossRef]
- Giuliano, F.; Rampin, O. Central neural control of penile erection. Neurosci. Biobehav. Rev. 2000, 24, 517–533. [Google Scholar] [CrossRef]
- Giuliano, F.; Rampin, O. Neural control of erection. Physiol. Behav. 2004, 83, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol. Rev. 2011, 63, 811–859. [Google Scholar] [CrossRef]
- Chitaley, K.; Wingard, C.J.; Webb, R.C.; Branam, H.; Stopper, V.S.; Lewis, R.W.; Mills, T.M. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat. Med. 2001, 7, 119–122. [Google Scholar] [CrossRef]
- Giuliano, F.; Allard, J. Dopamine and sexual function. Int. J. Impot. Res. 2001, 13 (Suppl. 3), 18–28. [Google Scholar] [CrossRef]
- Melis, M.R.; Argiolas, A. Central oxytocinergic neurotransmission: A drug target for the therapy of psychogenic erectile dysfunction. Curr. Drugs Target 2003, 4, 55–66. [Google Scholar] [CrossRef]
- Heaton, J.P. Central neuropharmacological agents and mechanisms in erectile dysfunction: The role of dopamine. Neurosci. Biobehav. Rev. 2004, 24, 561–569. [Google Scholar] [CrossRef]
- Lue, T.; Tanagho, E. Physiology of erection and pharmacological management of impotence. J. Urol. 1987, 137, 829–836. [Google Scholar] [CrossRef]
- Argiolas, A.; Melis, M.R. The neuropharmacology of yawning. Eur. J. Pharmacol. 1998, 343, 1–16. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Mauri, A.; Argiolas, A. Nitric oxide production is increased in the paraventricular nucleus of the hypothalamus of male rats during non-contact penile erections and copulation. Eur. J. Neurosci. 1998, 10, 1968–1974. [Google Scholar] [CrossRef]
- Monaghan, D.T.; Bridges, R.J.; Cotman, C.W. The excitatory amino acid receptors: Their classes, pharmacology, and distinct properties in the function of the central nervous system. Ann. Rev. Pharmacol. Toxicol. 1989, 29, 365–398. [Google Scholar] [CrossRef]
- Melis, M.R.; Stancampiano, R.; Argiolas, A. Penile erection and yawning induced by paraventricular NMDA injection in male rats are mediated by oxytocin. Pharmacol. Biochem. Behav. 1994, 48, 203–207. [Google Scholar] [CrossRef]
- Melis, M.R.; Stancampiano, R.; Argiolas, A. Prevention by No-nitro-L-arginine methylester of apomorphine- and oxytocin-induced penile erection and yawning: Site of action in the brain. Pharmacol. Biochem. Behav. 1994, 48, 799–804. [Google Scholar] [CrossRef]
- Melis, M.R.; Stancampiano, R.; Argiolas, A. Nitric oxide synthase inhibitors prevent N-methyl-D-aspartic acid-induced penile erection and yawning in male rats. Neurosci. Lett. 1994, 179, 9–12. [Google Scholar] [CrossRef]
- Stancampiano, R.; Melis, M.R.; Argiolas, A. Penile erection and yawning induced by 5HT1C receptor agonists in male rats: Relationship with dopaminergic and oxytocinergic transmission. Eur. J. Pharmacol. 1994, 261, 149–155. [Google Scholar] [CrossRef]
- Melis, M.R.; Stancampiano, R.; Argiolas, A. The role of nitric oxide in penile erection and yawning induced by 5-HT1C agonists in male rats. Naunyn-Schmiedeberg Arch. Pharmacol. 1995, 35, 439–446. [Google Scholar] [CrossRef]
- Poggioli, R.; Benelli, A.; Arletti, R.; Cavazzuti, E.; Bertolini, A. Nitric oxide is involved in the ACTH-induced behavioral syndrome. Peptides 1995, 16, 1263–1268. [Google Scholar] [CrossRef]
- Melis, M.R.; Stancampiano, R.; Lai, C.; Argiolas, A. Nitroglycerin-induced penile erection and yawning in male rats: Mechanism of action in the brain. Brain Res. Bull. 1995, 36, 527–531. [Google Scholar] [CrossRef]
- Argiolas, A.; Melis, M.R. Oxytocin-induced penile erection: Role of niric oxide. Adv. Exp. Med. Biol. 1995, 395, 247–255. [Google Scholar] [PubMed]
- Narayanan, K.; Spack, L.; McMillan, K.; Kilbourn, R.G.; Hayward, M.A.; Masters, B.S.; Griffith, O.W. S-alkyl-L-thiocitrullines. Potent stereoselective inhibitors of nitric oxide synthase with strong pressor activity in vivo. J. Biol. Chem. 1995, 270, 11103–11110. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Spano, M.S.; Locatelli, V.; Torsello, A.; Muller, E.E.; Deghenghi, R.; Argiolas, A. EP 60761 and EP 50885, two hexarelin anolgs, induce penile erection in male rats. Eur. J. Pharmacol. 2000, 404, 137–143. [Google Scholar] [CrossRef]
- Melis, M.R.; Spano, M.S.; Succu, S.; Locatelli, V.; Torsello, A.; Muller, E.E.; Deghenghi, R.; Argiolas, A. EP 60761- and EP 50885-induced penile erection: Structure-activity studies and comparison with apomorphine, oxytocin and N-methyl-D-aspartic acid. Int. J. Impot. Res. 2000, 12, 255–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Melis, M.R.; Succu, S.; Spano, M.S.; Torsello, A.; Locatelli, V.; Muller, E.E.; Deghenghi, R.; Argiolas, A. Penile erection induced by EP 80661 and other hexarelin peptide analogues: Role of paraventricular nitric oxide. Eur. J. Pharmacol. 2001, 411, 305–310. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Spano, M.S.; Deghenghi, R.; Argiolas, A. EP 91073 prevents EP 80661-induced penile erection: New evidence for the existence of specific EP peptide receptors mediating penile erection. Neuropharmacology 2001, 41, 254–262. [Google Scholar] [CrossRef]
- Succu, S.; Cocco, C.; Mascia, M.S.; Melis, T.; Melis, M.R.; Possenti, R.; Levi, A.; Ferri, G.L.; Argiolas, A. Pro-VGF-derived peptides induce penile erection in male rats: Possible involvement of oxytocin. Eur. J. Neurosci. 2004, 20, 3035–3040. [Google Scholar] [CrossRef]
- Succu, S.; Mascia, M.S.; Melis, T.; Sanna, F.; Melis, M.R.; Possenti, R.; Argiolas, A. Pro-VGF-derived peptides induce penile erection in male rats: Involvement of paraventricular nitric oxide. Neuropharmacology 2005, 49, 1017–1025. [Google Scholar] [CrossRef]
- Rinaldi-Carmona, M.; Barth, F.; Heaulme, M.; Shire, D.; Calandra, B.; Cong Martinez, S.; Maruani, J.; Neliat, G.; Caput, D.; Ferrara, P.; et al. SR 141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994, 350, 240–244. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Mascia, M.S.; Argiolas, A. Antagonism of cannabinoid CB1 receptors in the paraventricular nucleus of male rats induces penile erection. Neurosci. Lett. 2004, 359, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.R.; Succu, S.; Iannucci, U.; Argiolas, A. N-methyl-D-aspartic acid-induced penile erection and yawning: Role of hypothalamic paraventricular nitric oxide. Eur. J. Pharmacol. 1997, 328, 115–123. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Spano, M.S.; Argiolas, A. Effect of excitatory amino acid, dopamine, and oxytocin receptor antagonists on noncontact penile erections and paraventricular nitric oxide production in male rats. Behav. Neurosci. 2000, 114, 849–857. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Sanna, F.; Melis, T.; Mascia, M.S.; Enguehard-Gueiffier, C.; Hubner, H.; Gmeiner, P.; Gueiffier, A.; Argiolas, A. PIP3EA and PD-168077, two selective dopamine D4 receptor agonists, induce penile erection in male rats: Site and mechanism of action in the brain. Eur. J. Neurosci. 2006, 24, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Sanna, F.; Succu, S.; Melis, M.R.; Argiolas, A. Dopamine agonist-induced penile erection and yawning: Differential role of D2-like receptor subtypes and correlation with nitric oxide production in the paraventricular nucleus of the hypothalamus of male rats. Behav. Brain Res. 2012, 230, 355–364. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Mascia, M.S.; Sanna, F.; Melis, T.; Castelli, M.P.; Argiolas, A. The cannabinoid receptor antagonist SR-141716A induces penile erection in male rats: Involvement of paraventricular glutamic acid and nitric oxide. Neuropharmacology 2006, 50, 219–228. [Google Scholar] [CrossRef]
- Succu, S.; Mascia, M.S.; Sanna, F.; Melis, T.; Argiolas, A.; Melis, M.R. The cannabinoid CB1 receptor antagonist SR 141716A induces penile erection by increasing extra-cellular glutamic acid in the paraventricular nucleus of male rats. Behav. Brain Res. 2006, 169, 274–281. [Google Scholar] [CrossRef]
- Argiolas, A.; Melis, M.R.; Stancampiano, R.; Gessa, G.L. ω-Conotoxin prevents apomorphine- and oxytocin induced penile erection in male rats. Pharmacol. Biochem. Behav. 1990, 37, 253–257. [Google Scholar] [CrossRef]
- Melis, M.R.; Argiolas, A. Nitric oxide donors induce penile erection and yawning when injected in the central nervous system of male rats. Eur. J. Pharmacol. 1995, 294, 1–9. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Argiolas, A. Dopamine agonists increase nitric oxide production in the paraventricular nucleus of the hypothalamus in male rats: Correlation with penile erection and yawning. Eur. J. Neurosci. 1996, 8, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.R.; Spano, M.S.; Succu, S.; Argiolas, A. The oxytocin antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin reduces non-contact erections in male rats. Neurosci. Lett. 1999, 265, 171–174. [Google Scholar] [CrossRef]
- Melis, M.R.; Spano, M.S.; Succu, S.; Argiolas, A. Activation of GABAA receptors in the paraventricular nucleus of the hypothalamus reduces apomorphine-, NMDA- and oxytocin-induced penile erection and yawning in male rats. Neurosci. Lett. 2000, 281, 127–130. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Mascia, M.S.; Argiolas, A. The activation of γ-amino-butyric acid A receptors in the paraventricular nucleus of the hypothalamus reduces non-contact penile erections in male rats. Neurosci. Lett. 2001, 314, 123–126. [Google Scholar] [CrossRef]
- Succu, S.; Mascia, M.S.; Melis, T.; Melis, M.R.; Deghenghi, R.; Argiolas, A. Activation of GABAA and opioid receptors reduce penile erection induced by hexarelin peptides. Pharmacol. Biochem. Behav. 2003, 76, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.R.; Stancampiano, R.; Gessa, G.L.; Argiolas, A. Prevention by morphine of apomorphine- and oxytocin-induced penile erection: Site of action in the brain. Neuropsychopharmacology 1992, 6, 17–21. [Google Scholar]
- Melis, M.R.; Succu, S.; Argiolas, A. Prevention by morphine of N-methyl-D-aspartic acid induced penile erection and yawning: Involvement of nitric oxide. Brain Res. Bull. 1997, 44, 689–694. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Iannucci, U.; Argiolas, A. Morphine prevention of apomorphine- and oxytocin-induced penile erection and yawning: Involvement of nitric oxide. Naunyn-Schmiedeberg Arch. Pharmacol. 1997, 335, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Succu, S.; Mascia, M.S.; Melis, T.; Sanna, S.; Boi, A.; Melis, M.R.; Argiolas, A. Morphine reduces penile erection induced by the cannabinoid receptor antagonist SR 141617A in male rats: Role of paraventricular glutamic acid and nitric oxide. Neurosci. Lett. 2006, 404, 1–5. [Google Scholar] [CrossRef]
- Castelli, M.P.; Piras, A.P.; Melis, T.; Succu, S.; Sanna, F.; Melis, M.R.; Collu, B.S.; Ennas, M.G.; Diaz, G.; Mackie, K.; et al. Cannabinoid CB1 receptors in the paraventricular nucleus and central control of penile erection: Immunocytochemistry, autoradiography and behavioral studies. Neuroscience 2007, 147, 197–206. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Mascia, M.S.; Cortis, L.; Argiolas, A. Extracellular excitatory amino acids increase in the paraventricular nucleus of male rats during sexual activity: Main role of N-methyl-d-aspartic acid receptors in erectile function. Eur. J. Neurosci. 2004, 19, 2569–2575. [Google Scholar] [CrossRef]
- Melis, M.R.; Argiolas, A. Reduction of drug-induced yawning and penile erection and of noncontact erections in male rats by the activation of GABAA receptors in the paraventricular nucleus: Involvement of nitric oxide. Eur. J. Neurosci. 2002, 15, 852–860. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Spano, M.S.A.; Argiolas, A. Morphine injected into the paraventricular nucleus of the hypothalamus prevents non-contact erections and impairs copulation: Involvement of nitric oxide. Eur. J. Neurosci. 1999, 11, 1857–1864. [Google Scholar] [CrossRef]
- Argiolas, A.; Melis, M.R.; Mauri, A.; Gessa, G.L. Paraventricular nucleus lesion prevents yawning and penile erection induced by apomorphine and oxytocin, but not by ACTH in rats. Brain Res. 1987, 421, 349–352. [Google Scholar] [CrossRef]
- Bankowski, K.; Manning, M.; Seto, J.; Haldar, J.; Sawyer, W.H. Design and synthesis of potent in vivo antagonists of oxytocin. Int. J. Pept. Protein Res. 1980, 16, 382–391. [Google Scholar] [CrossRef]
- Argiolas, A.; Melis, M.R.; Vargiu, L.; Mauri, A.; Gessa, G.L. d(CH2)5Tyr(Me)2-Orn8-vasotocin, a potent oxytocin antagonist, antagonizes penile erection and yawning induced by oxytocin and apomorphine, but not by ACTH 1-24. Eur. J. Pharmacol. 1987, 134, 221–224. [Google Scholar] [CrossRef]
- Reynolds, I.J.; Wagner, J.A.; Snyder, S.H.; Thayer, S.A.; Olivera, B.M.; Miller, R.J. Brain voltage-sensitive calcium channel subtypes differentiated by omega-conotoxin fraction GVIA. Proc. Natl. Acad. Sci. USA 1986, 83, 8804–8807. [Google Scholar] [CrossRef] [PubMed]
- McCleskey, E.W.; Fox, A.P.; Feldman, D.H.; Cruz, L.J.; Olivera, B.M.; Tsien, R.W.; Yoshikami, D. ω-Conotoxin: Direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc. Natl. Acad. Sci. USA 1987, 84, 4327–4331. [Google Scholar] [CrossRef] [PubMed]
- Argiolas, A.; Melis, M.R.; Murgia, S.; Schiöth, H.B. ACTH- and a-MSH-induced grooming, stretching, yawning and penile erection in male rats: Site of action in the brain and role of melanocortin receptors. Brain Res. Bull. 2000, 51, 425–431. [Google Scholar] [CrossRef]
- Bertolini, A.; Gessa, G.L. Behavioural effects of ACTH and MSH peptides. J. Endocrinol. Inv. 1981, 4, 241–251. [Google Scholar] [CrossRef]
- Marson, L.; McKenna, K.E. A role for 5-hydroxytryptamine in descending inhibition of spinal sexual reflexes. Exp. Brain Res. 1991, 88, 313–320. [Google Scholar] [CrossRef]
- Marson, L.; List, M.S.; McKenna, K.E. Lesions of the nucleus paragigantocellularis alter ex copula penile reflexes. Brain Res. 1992, 592, 187–192. [Google Scholar] [CrossRef]
- Wagner, C.K.; Clemens, L.G. Neurophysin-containing pathway from the paraventricular nucleus of the hypothalamus to a sexually dimorphic motor nucleus in lumbar spinal cord. J. Comp. Neurol. 1993, 336, 106–116. [Google Scholar] [CrossRef]
- Dun, N.J.; Dun, S.L.; Förstermann, U. Nitric oxide synthase immunoreactivity in rat pontine medullary neurons. Neuroscience 1994, 59, 429–445. [Google Scholar] [CrossRef]
- Bush, P.A.; Aronson, W.J.; Buga, G.M.; Rajfer, J.; Ignarro, L.J. Nitric oxide is a potent relaxant of human and rabbit corpus cavernosum. J. Urol. 1992, 147, 1650–1655. [Google Scholar] [CrossRef]
- Holmquist, F.; Fridstrand, M.; Hedlund, H.; Andersson, K.E. Actions of 3-morpholino-sydnonimin (SIN-1) on rabbit isolated penile erectile tissue. J. Urol. 1993, 150, 1310–1315. [Google Scholar] [CrossRef]
- Wang, R.; Domer, F.R.; Sikka, S.C.; Kadowitz, P.J.; Hellstrom, W.J. Nitric oxide mediates penile erection in cats. J. Urol. 1994, 151, 234–237. [Google Scholar] [CrossRef]
- Hellstrom, W.J.; Monga, M.; Wang, R.; Domer, F.R.; Kadowitz, P.J.; Roberts, J.A. Penile erection in the primate: Induction with nitric-oxide donors. J. Urol. 1994, 151, 1723–1727. [Google Scholar] [CrossRef]
- Escrig, A.; Gonzalez-Mora, J.L.; Mas, M. Nitric oxide release in penile corpora cavernosa in a rat model of erection. J. Physiol. 1999, 516 Pt 1, 261–269. [Google Scholar] [CrossRef]
- Wegner, H.E.; Knispel, H.H.; Klän, R.; Miller, K. Efficacy of linsidomine chlorhydrate, a direct nitric oxide donor, in the treatment of human erectile dysfunction: Results of a double-blind cross over trial. Int. J. Impot. Res. 1995, 7, 233–237. [Google Scholar]
- Murad, F. Drugs used for the treatment of angina: Organic nitrates, calcium channels blockers and B-adrenergic antagonists. In Goodman and Gilman’s The Pharmacological Basis of Therapeutic; Rall, T.W., Nies, A.S., Taylor, T., Eds.; Pergamon Press: New York, NY, USA, 1990; pp. 764–783. [Google Scholar]
- Everett, G.M. Amy1 nitrite (“Poppers”) as an aphrodisiac. In Sexual Behavior—Pharmacology and Biochemistry; Sandler, M., Gessa, G.L., Eds.; Raven Press: New York, NY, USA, 1975; pp. 97–98. [Google Scholar]
- Gross, P.M.; Weaver, D.F.; Bowers, R.J.; Nag, S.; Ho, L.T.; Pang, J.J.; Espinosa, F.J. Neurotoxicity in conscious rats following intraventricular SNAP, a nitric oxide donor. Neuropharmacology 1994, 33, 915–924. [Google Scholar] [CrossRef]
- Ratnasooriya, W.D.; Jayakody, J.R.; Dharmasiri, M.G. Sodium nitroprusside impairs sexual competence of male rats. Hum. Exp. Toxicol. 2004, 23, 187–192. [Google Scholar] [CrossRef]
- Pauwels, B.; Boydens, C.; Brouckaert, P.; Van de Voorde, J. Oximes induce erection and are resistant to oxidative stress. J. Sex. Med. 2015, 12, 906–915. [Google Scholar] [CrossRef]
- Sahyoun, T.; Arrault, A.; Schneider, R. Amidoximes and Oximes: Synthesis, Structure, and Their Key Role as NO Donors. Molecules 2019, 24, 2470. [Google Scholar] [CrossRef]
- Hotta, Y.; Kataoka, T.; Mori, T.; Kimura, K. Review of a potential novel approach for erectile dysfunction: Light-controllable nitric oxide donors and nanoformulations. Sex. Med. Rev. 2020, 8, 297–302. [Google Scholar] [CrossRef]
- Farmer, M.; Yoon, H.; Goldstein, I. Future Targets for Female Sexual Dysfunction. J Sex. Med. 2016, 13, 1147–1165. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Araki, N.; Hamada, J.; Komatsumoto, S.; Shimazu, K.; Fukwchi, Y. A novel in vivo assay system for consecutive measurement of brain nitric oxide production combined with the microdialysis technique. Neurosci. Lett. 1984, 176, 165–168. [Google Scholar] [CrossRef]
- Melis, M.R.; Succu, S.; Iannucci, U.; Argiolas, A. Oxytocin increases nitric oxide production in the paraventricular nucleus of the hypothalamus of male rats: Correlation with penile erection and yawning. Regul. Pept. 1997, 69, 105–111. [Google Scholar] [CrossRef]
- Succu, S.; Sanna, F.; Melis, T.; Boi, A.; Argiolas, A.; Melis, M.R. Stimulation of dopamine receptors in the paraventricular nucleus of the hypothalamus of male rats induces penile erection and increases extra-cellular dopamine in the nucleus accumbens: Involvement of central oxytocin. Neuropharmacology 2007, 52, 1034–1043. [Google Scholar] [CrossRef]
- Melis, M.R.; Argiolas, A.; Gessa, G.L. Apomorphine-induced penile erection and yawning: Site of action in brain. Brain Res. 1987, 415, 98–104. [Google Scholar] [CrossRef]
- Sanna, F.; Succu, S.; Hübner, H.; Gmeiner, P.; Argiolas, A.; Melis, M.R. Dopamine D2-like receptor agonists induce penile erection in male rats: Differential role of D2, D3 and D4 receptors in the paraventricular nucleus of the hypothalamus. Behav. Brain Res. 2011, 225, 169–176. [Google Scholar] [CrossRef]
- Woodruff, G.N.; Foster, A.C.; Gill, R.; Kemp, J.A.; Wong, E.H.; Iversen, L.L. The interaction between MK-801 and receptors for N-methyl-D-aspartate: Functional consequences. Neuropharmacology 1987, 26, 903–909. [Google Scholar] [CrossRef]
- Torsello, A.; Locatelli, V.; Melis, M.R.; Succu, S.; Spano, M.S.; Deghenghi, R.; Muller, E.E.; Argiolas, A. Differential orexigenic effects of hexarelin and its analogs in the rat hypothalamus: Indication for multiple growth hormone secretagogue receptor subtypes. Neuroendocrinology 2000, 72, 327–332. [Google Scholar] [CrossRef]
- Baskerville, T.A.; Douglas, A.J. Interaction between dopamine and oxytocin in the control of sexual behaviour. Prog. Brain Res. 2008, 170, 277–289. [Google Scholar] [CrossRef]
- Baskerville, T.A.; Allard, J.; Wayman, C.; Douglas, A.J. Dopamine-oxytocin interaction in penile erection. Eur. J. Neurosci. 2009, 30, 2151–2164. [Google Scholar] [CrossRef]
- Sanna, F.; Bratzu, J.; Argiolas, A.; Melis, M.R. Oxytocin induces penile erection and yawning when injected into the bed nucleus of the stria terminalis: Involvement of glutamic acid, dopamine, and nitric oxide. Horm. Behav. 2017, 96, 52–61. [Google Scholar] [CrossRef]
- Bratzu, J.; Bharatiya, R.; Manca, E.; Cocco, C.; Argiolas, A.; Melis, M.R.; Sanna, F. Oxytocin induces penile erection and yawning when injected into the bed nucleus of the stria terminalis: A microdialysis and immunohistochemical study. Behav. Brain Res. 2019, 375, 112147. [Google Scholar] [CrossRef] [PubMed]
- Yells, D.P.; Hendricks, S.E.; Prendergast, M.A. Lesions of the nucleus paragigantocellularis: Effects on mating behavior in male rats. Brain Res. 1992, 596, 73–79. [Google Scholar] [CrossRef]
- Szele, F.G.; Murphy, D.L.; Garrick, N.A. Effects of fenfluramine, m-chlorophenylpiperazine, and other serotonin related agonists and antagonists on penile erection in nonhuman primates. Life Sci. 1988, 43, 1297–1304. [Google Scholar] [CrossRef]
- Foreman, M.M.; Hall, J.L.; Love, R.I. The role of 5-I-IT, receptor in the regulation of sexual response of male rats. Life Sci. 1989, 45, 1263–1270. [Google Scholar] [CrossRef]
- Foreman, M.M.; Hall, J.L.; Love, R.L. Effects of fenfluramine and para-chloroamphetamine on sexual behavior in male rats. Psychopharmacology 1992, 107, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J. Nitric oxide as the physiological mediator of penile erection. J. NIH Res. 1992, 4, 59–62. [Google Scholar]
- Gruetter, C.A.; Gruetter, D.J.; Lyon, J.E.; Kadowitz, P.J.; Ignarro, L.J. Relationship between cyclic guanosine 3’:5’monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: Effects of methylene blue and methemoglobin. J. Pharmacol. Exp. Ther. 1981, 219, 181–186. [Google Scholar]
- Mulsch, A.; Luckhoff, A.; Pohl, U.; Busse, R.; Bassenge, E. LY 83583 (6- anilino-5,8_quinolinedione) blocks nitrovasodilator-induced cyclic GMP increases and inhibition of platelet activation. Naunyn-Schmiedeberg Arch. Pharmacol. 1988, 340, 119–125. [Google Scholar] [CrossRef]
- Melis, M.R.; Melis, T.; Cocco, C.; Succu, S.; Sanna, F.; Pillolla, G.; Boi, A.; Ferri, G.L.; Argiolas, A. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur. J. Neurosci. 2007, 26, 1026–1035. [Google Scholar] [CrossRef]
- Ding, J.D.; Burette, A.; Nedvetsky, P.I.; Schmidt, H.H.; Weinberg, R.J. Distribution of soluble guanylyl cyclase in the rat brain. J. Comp. Neurol. 2004, 472, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.M.; Rickard, N.S. New perspectives on the mechanisms through which nitric oxide may affect learning and memory processes. Neurosci. Biobehav. Rev. 2007, 31, 413–425. [Google Scholar] [CrossRef]
- Sanna, F.; Succu, S.; Boi, A.; Melis, M.R.; Argiolas, A. Phosphodiesterase type 5 inhibitors facilitate noncontact erections in male rats: Site of action in the brain and mechanism of action. J. Sex. Med. 2009, 6, 2680–2689. [Google Scholar] [CrossRef]
- Murad, F.; Mittal, C.K.; Arnold, W.P.; Katsuki, S.; Kimura, H. Guanylate cyclase: Activation by azide, nitrocompounds, nitric oxide and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv. Cycl. Nucleotide Res. 1978, 9, 145–158. [Google Scholar]
- Bitran, D.; Hull, E.M. Pharmacological analysis of male sexual behavior. Neurosci. Biobehav. Rev. 1987, 11, 365–389. [Google Scholar] [CrossRef]
- Caggiula, A.R.; Shaw, D.H.; Antelman, M.; Edwards, D.J. Interactive effects of brain catecholamines and variations in sexual and non-sexual arousal on copulatory behavior of male rats. Brain Res. 1976, 111, 321–336. [Google Scholar] [CrossRef]
- Okabe, S.; Kitano, K.; Nagasawa, M.; Mogi, K.; Kikusui, T. Testosterone inhibits facilitating effects of parenting experience on parental behavior and the oxytocin neural system in mice. Physiol. Behav. 2013, 118, 159–164. [Google Scholar] [CrossRef]
- Floody, O.R. Processes regulating the initiation and postejaculatory resumption of copulatory behavior in male hamsters. Behav. Process. 2014, 105, 79–84. [Google Scholar] [CrossRef]
- Gray, G.D.; Dewsbury, A.D. A quantitative description of copulatory behavior in prairie voles (Microtus ochrogaster). Brain Behav. Evol. 1973, 8, 426–452. [Google Scholar] [CrossRef]
- Nitsch, F.; Stueckle, S.; Stahl, D.; Zinner, D. Copulation patterns in captive hamadryas baboons: A quantitative analysis. Primates 2011, 52, 373–383. [Google Scholar] [CrossRef]
- Balthazart, J.; Ball, G. Topography in the preoptic region: Differential regulation of appetitive and consummatory male sexual behaviors. Front. Neuroendocr. 2007, 28, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Rauceo, S.; Harding, C.F.; Maldonado, A.; Gaysinkaya, L.; Tulloch, I.; Rodriguez, E. Dopaminergic modulation of reproductive behavior and activity in male zebra finches. Behav. Brain Res. 2008, 187, 133–139. [Google Scholar] [CrossRef][Green Version]
- Caggiula, A.R.; Herndon, J.G., Jr.; Scanlon, R.; Greenstone, D.; Bradshaw, W.; Sharp, D. Dissociation of active from immobility components of sexual behavior in female rats by central 6-hydroxydopamine: Implications for CA involvement in sexual behavior and sensorimotor responsiveness. Brain Res. 1979, 172, 505–520. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Gorzalka, B.B. Opioids and sexual behavior. Neurosci. Biobehav. Rev. 1987, 11, 1–34. [Google Scholar] [CrossRef]
- Bialy, M.; Beck, J.; Abramczyk, P.; Trzebski, A.; Przybylski, J. Sexual behavior in male rats after nitric oxide synthesis inhibition. Physiol. Behav. 1996, 60, 139–143. [Google Scholar] [CrossRef]
- Dominguez, J.M.; Hull, E.M. Dopamine, the medial preoptic area, and male sexual behavior. Physiol. Behav. 2005, 86, 356–368. [Google Scholar] [CrossRef]
- Dominguez, J.M.; Hull, E.M. Serotonin impairs copulation and attenuates ejaculation-induced glutamate activity in the medial preoptic area. Behav. Neurosci. 2010, 124, 554–557. [Google Scholar] [CrossRef]
- Hull, E.M.; Dominguez, J.M. Getting his act together: Roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Res. 2006, 1126, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Will, R.G.; Hull, E.M.; Dominguez, J.M. Influences of dopamine and glutamate in the medial preoptic area on male sexual behavior. Pharmacol. Biochem. Behav. 2014, 121, 115–123. [Google Scholar] [CrossRef]
- Lorrain, D.S.; Matuszewich, L.; Howard, R.V.; Du, J.; Hull, E.M. Nitric oxide promotes medial preoptic dopamine release during male rat copulation. Neuroreport 1996, 8, 31–34. [Google Scholar] [CrossRef]
- Dominguez, J.M.; Muschamp, J.W.; Schmich, J.M.; Hull, E.M. Nitric oxide mediates glutamate-evoked dopamine release in the medial preoptic area. Neuroscience 2004, 125, 203–210. [Google Scholar] [CrossRef]
- Sato, S.M.; Hull, E.M. The nitric oxide-guanosine 3′,5′-cyclic monophosphate pathway regulates dopamine efflux in the medial preoptic area and copulation in male rats. Neuroscience 2006, 139, 417–428. [Google Scholar] [CrossRef]
- Hull, E.M.; Du, J.; Lorrain, D.S.; Matuszewich, L. Extracellular dopamine in the medial preoptic area: Implications for sexual motivation and hormonal control of copulation. J. Neurosci. 1995, 15, 7465–7471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dominguez, J.; Riolo, J.V.; Xu, Z.; Hull, E.M. Regulation by the medial amygdala of copulation and medial preoptic dopamine release. J. Neurosci. 2001, 21, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Lagoda, G.; Muschamp, J.W.; Vigdorchik, A.; Hull, E.M. A nitric oxide synthesis inhibitor in the medial preoptic area inhibits copulation and stimulus sensitization in male rats. Behav. Neurosci. 2004, 118, 1317–1323. [Google Scholar] [CrossRef]
- Ma, X.; Reyna, A.; Mani, S.K.; Matzuk, M.M.; Kumar, T.R. Impaired male sexual behavior in activin receptor type II knockout mice. Biol. Reprod. 2005, 73, 1182–1190. [Google Scholar] [CrossRef][Green Version]
- Jean, A.; Bonnet, P.; Liere, P.; Mhaouty-Kodja, S.; Hardin-Pouzet, H. Revisiting medial preoptic area plasticity induced in male mice by sexual experience. Sci. Rep. 2017, 7, 17846. [Google Scholar] [CrossRef]
- Brenman, J.E.; Bredt, D.S. Synaptic signaling by nitric oxide. Curr. Opin. Neurobiol. 1997, 7, 374–378. [Google Scholar] [CrossRef]
- Zheng, H.; Bidasee, K.R.; Mayhan, W.G.; Patel, K.P. Lack of central nitric oxide triggers erectile dysfunction in diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1158–R1164. [Google Scholar] [CrossRef] [PubMed]
- Normandin, J.J.; Murphy, A.Z. Somatic genital reflexes in rats with a nod to humans: Anatomy, physiology, and the role of the social neuropeptides. Horm. Behav. 2011, 59, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, D.W.; Schwartz-Giblin, S.; Mccarthy, M.M.; Kow, L.M. Cellular and molecular mechanisms of female reproductive behaviors. In The Physiology of Reproduction, 2nd ed.; Knobil, E., Neil, J.D., Eds.; Raven Press: NewYork, NY, USA, 1994; pp. 107–220. [Google Scholar]
- Jennings, K.J.; de Lecea, L. Neural and hormonal control of sexual behavior. Endrocrinology 2020, 161, bqaa150. [Google Scholar] [CrossRef] [PubMed]
- Kow, L.M.; Pfaff, D.W. Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behav. Brain Res. 1998, 92, 169–180. [Google Scholar] [CrossRef]
- Veening, J.G.; Coolen, L.M.; Gerrits, P.O. Neural mechanisms of female sexual behavior in the rat; comparison with male ejaculatory control. Pharmacol. Biochem. Behav. 2014, 121, 16–30. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Flanagan-Cato, L.M.; Blaustein, J.D. Female sexual behavior. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T., Zeleznik, A., Eds.; Academic Press: New York, NY, USA, 2015; pp. 2287–2370. [Google Scholar]
- Rettori, V.; Belova, N.; Dees, W.L.; Nyberg, C.L.; Gimeno, M.; McCann, S.M. Role of nitric oxide in the control of luteinizing hormone-releasing hormone release in vivo and in vitro. Proc. Natl. Acad. Sci. USA 1993, 90, 10130–10134. [Google Scholar] [CrossRef]
- Mani, S.K.; Allen, J.M.; Rettori, V.; McCann, S.M.; O’Malley, B.W.; Clark, J.H. Nitric oxide mediates sexual behavior in female rats. Proc. Natl. Acad. Sci. USA 1994, 91, 6468–6472. [Google Scholar] [CrossRef]
- McCann, S.M.; Haens, G.; Mastronardi, C.; Walczewska, A.; Karanth, S.; Rettori, V.; Yu, W.H. The role of nitric oxide (NO) in control of LHRH release that mediates gonadotropin release and sexual behavior. Curr. Pharm. Des. 2003, 9, 381–390. [Google Scholar] [CrossRef]
- Chu, H.P.; Etgen, A.M. A potential role of cyclic GMP in the regulation of lordosis behavior of female rats. Horm. Behav. 1997, 32, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Rachman, I.M.; Unnerstall, J.R.; Pfaff, D.W.; Cohen, R.S. Regulation of neuronal nitric oxide synthase mRNA in lordosis-relevant neurons of the ventromedial hypothalamus following short-term estrogen treatment. Brain Res. Mol. Brain Res. 1998, 59, 105–108. [Google Scholar] [CrossRef]
- Hellier, V.; Brock, O.; Candlish, M.; Desroziers, E.; Aoki, M.; Mayer, C.; Piet, R.; Herbison, A.; Colledge, W.H.; Prévot, V.; et al. Female sexual behavior in mice is controlled by kisspeptin neurons. Nat. Commun. 2018, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Bentefour, Y.; Bakker, J. Kisspeptin signaling and nNOS neurons in the VMHvl modulate lordosis behavior but not mate preference in female mice. Neuropharmacology 2021, 198, 108762. [Google Scholar] [CrossRef]
- Meerts, S.H.; Park, J.H.; Sekhawat, R. Sexual experience modulates partner preference and mPOA nitric oxide synthase in female rats. Behav. Neurosci. 2016, 130, 490–499. [Google Scholar] [CrossRef]
- Ferrini, M.G.; Magee, T.R.; Vernet, D.; Rajfer, J.; González-Cadavid, N.F. Penile neuronal nitric oxide synthase and its regulatory proteins are present in hypothalamic and spinal cord regions involved in the control of penile erection. J. Comp. Neurol. 2003, 458, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chu, S.; Qian, T.; Chen, J.; Zhang, J. Ginsenoside Rg1 improves male copulatory behavior via nitric oxide/cyclic guanosine monophosphate pathway. J. Sex. Med. 2010, 7, 743–750. [Google Scholar] [CrossRef]
- Yeh, K.Y.; Wu, C.H.; Tsai, Y.F. Noncontact erection is enhanced by Ginkgo biloba treatment in rats: Role of neuronal NOS in the paraventricular nucleus and sacral spinal cord. Psychopharmacology 2012, 222, 439–446. [Google Scholar] [CrossRef]
- Huang, A.C.; Wu, J.M.; Chang, Y.H.; Dubey, N.K.; Chiu, A.W.; Yeh, C.Y.; Tsai, T.H.; Yeh, K.Y. Neuronal nitric oxide synthase activity mediates Lycium barbarum polysaccharides-enhanced sexual performance without stimulating noncontact erection in rats. Psychopharmacology 2019, 236, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.; Pecoraro, S.; Sarnacchiaro, P.; Silvani, M.; Antonini, G. The daily therapy with L-arginine 2500 mg and tadalafil 5 mg in combination and in monotherapy for the treatment of erectile dysfunction: A prospective, randomized multicentre study. Sex. Med. 2020, 8, 178–185. [Google Scholar] [CrossRef]
- Swanson, L.W.; Sawchenko, P.E. Hypothalamic integration: Organization of the paraventricular and supraoptic nuclei. Ann. Rev. Neurosci. 1983, 6, 269–324. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y. Estradiol-sensitive projection neurons in the female rat preoptic area. Front. Neurosci. 2015, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.R.; Argiolas, A. Role of central nitric oxide in the control of penile erection and yawning. Prog. Neuropsychopharmacol. Biol. Psychiatry 1997, 21, 899–922. [Google Scholar] [CrossRef]
| Penile Erection Induced by | L-NAME | Effect on Penile Erection L-NMMA | D-NMMA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Given | Given | Given | |||||||
| i.p. | i.c.v | PVN | i.p. | i.c.v. | PVN | i.p. | i.c.v. | PVN | |
| Dopamine agonists | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | == | == | == |
| Oxytocin | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | == | == | == |
| NMDA | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | == | == | == |
| 5-HT1C agonists | ↓ | ↓ | == | ↓ | ↓ | == | == | == | == |
| ACTH 1-24 | ↓ | ↓ | == | ↓ | ↓ | == | == | == | == |
| Hexarelin peptides | n.a. | n.a. | ↓ | n.a. | n.a. | ↓ | n.a. | n.a. | == |
| VGF peptides | n.a. | n.a. | ↓ | n.a. | n.a. | ↓ | n.a. | n.a. | == |
| SR 141716A | n.a. | n.a. | ↓ | n.a. | n.a. | ↓ | n.a. | n.a. | == |
| Penile Erection | ||
|---|---|---|
| NO Donor Given | i.c.v. | Into the PVN |
| l-arginine | == | ↑ |
| d-arginine | == | == |
| Nitroglycerin | ↑ | ↑ |
| Sodium nitroprusside | == | ↑ |
| Isoamyl nitrite | ↑ | n.a. |
| Hydroxylamine | ↑ | n.a. |
| S-nitroso-D-acetyl-penicillamine | n.a. | n.a. |
| PVN Pretreatment | PVN Treatment | NO Production | Penile Erection |
|---|---|---|---|
| Vehicle/Drug/Peptide | Drug/Peptide | ||
| L-NAME/SMTC | DA agonists | ↓ | ↓ |
| Haloperidol | DA agonists | ↓ | ↓ |
| SCH 23390 | DA agonists | == | == |
| Oxy-Ant | apomorphine | == | == |
| OXY-Ant i.c.v. | apomorphine | == | ↓ |
| (+)MK-801 | apomorphine | == | == |
| L-NAME/SMTC | oxytocin | ↓ | ↓ |
| Haloperidol | oxytocin | == | == |
| Oxy-Ant | oxytocin | ↓ | ↓ |
| Oxy-Ant i.c.v. | oxytocin | ↓ | ↓ |
| (+)MK-801 | oxytocin | == | == |
| L-NAME/SMTC | NMDA | ↓ | ↓ |
| Haloperidol | NMDA | == | == |
| Oxy-Ant | NMDA | == | == |
| Oxy-Ant i.c.v. | NMDA | == | ↓ |
| (+)MK-801 | NMDA | ↓ | ↓ |
| L-NAME | VGF 588-617 | ↓ | ↓ |
| Cis-flupenthixol | VGF 588-617 | n.a. | == |
| Oxy-Ant | VGF 588-617 | == | == |
| Oxy-Ant i.c.v. | VGF 588-617 | == | ↓ |
| (+)MK-801 | VGF 588-617 | n.a. | == |
| L-NAME | EP 80661 | ↓ | ↓ |
| Cys-flupenthixol | EP 80661 | n.a. | == |
| Oxy-Ant | EP 80661 | == | == |
| Oxy-Ant i.c.v. | EP 80661 | == | ↓ |
| (+)MK-801 | EP 80661 | n.a. | == |
| PVN Treatment | NO Production | Non-Contact Erections | Copulation |
|---|---|---|---|
| L-NAME | ↓ | ↓ | ↓ |
| Muscimol | ↓ | ↓ | ↓ |
| Baclofen | == | == | == |
| Morphine | ↓ | ↓ | ↓ |
| U-69,593 | == | == | == |
| Methylene Blue | == | == | == |
| Hemoglobin | == | == | == |
| Oxy-Ant | == | == | == |
| Oxy-Ant i.c.v. | == | ↓ | ↓ |
| PVN Pretreatment | PVN Treatment | NO Production | Penile Erection |
|---|---|---|---|
| Vehicle/Drug/Peptide | Drug/Peptide | ||
| Muscimol | DA agonists | ↓ | ↓ |
| Baclofen | DA agonists | == | == |
| Morphine | DA agonists | ↓ | ↓ |
| U-69,593 | DA agonists | == | == |
| Muscimol | oxytocin | ↓ | ↓ |
| Baclofen | oxytocin | == | == |
| Morphine | oxytocin | ↓ | ↓ |
| U-69,593 | oxytocin | == | == |
| Muscimol | NMDA | ↓ | ↓ |
| Baclofen | NMDA | == | == |
| Morphine | NMDA | ↓ | ↓ |
| U-69,593 | NMDA | n.a. | == |
| Muscimol | VGF 588-617 | ↓ | ↓ |
| Baclofen | VGF 588-617 | n.a. | == |
| Morphine | VGF 588-617 | n.a. | ↓ |
| U-69,593 | VGF 588-617 | n.a. | == |
| Muscimol | EP 80661 | ↓ | ↓ |
| Baclofen | EP 80661 | n.a. | == |
| Morphine | EP 80661 | ↓ | ↓ |
| U-69,593 | EP 80661 | n.a. | == |
| Brain Area | NO Synthesis Site | Mechanism of Action |
|---|---|---|
| PVN | Cell bodies of oxytocinergic neurons projecting to extrahypohalamic brain areas and spinal cord | Activation of central oxytocin neurotransmission apparently by acting as intracellular messenger and with a mechanism that does not involve guanylate cyclase yet to be identified. |
| Medial preoptic area | Medial preoptic cells yet to be identified | Activation of dopamine release by insert hypothalamic dopaminergic neurons by acting as a retrograde messenger with a mechanism that involves the activation of guanylate cyclase. |
| Ventromedial nucleus of the hypothalamus | Neurons yet to be identified | Activation of the lordosis response by a still unknown mechanism. |
| Ventral tegmental area | Cell bodies of mesolimbic/mesocortical dopaminergic neurons | Activation of mesolimbic dopaminergic neurons projecting to the nucleus accumbens and medial prefrontal cortex by acting as intracellular messenger with a mechanism that involves the activation of guanylate cyclase. |
| Ventral subiculum of the hippocampus | Glutamatergic neurons of the subiculum | Activation of glutamatergic neurons projecting directly or indirectly (through the medial prefrontal cortex) to the ventral tegmental area in order to activate mesolimbic dopaminergic neurons by acting as intercellular messenger with a mechanism involving guanylate cyclase. |
| Posteromedial cortical nucleus of the amygdala | Glutamatergic neurons of the amygdalar nucleus | Activation of glutamatergic neurons projecting directly or indirectly (through the medial prefrontal cortex) to the ventral tegmental area in order to activate mesolimbic dopaminergic neurons by acting as intercellular messenger, possibly with a mechanism involving guanylate cyclase. |
| Bed nucleus of the stria terminalis | Glutamatergic neurons of the bed nucleus | Activation of glutamatergic neurons projecting to the PVN and other extrahypothalamic brain areas mediating erectile function. It is still unknown if NO acts as intercellular or intracellular messenger and if guanylate cyclase is involved or not. |
| Ventral medulla (nucleus paragigantocellularis | 5-HT cell bodies of neurons projecting to the spinal cord (L2-S2)? | Activation of 5HT neurons projecting from the ventral medulla to the spinal tract L2-S2, with a still-unknown mechanism? |
| Spinal cord (L2-S2) | Spinal neurons of the L2-S2 spinal tract | Activation of spinal neurons projecting to the pelvic plexuses and pudendal nerves that reach the penis to control cavernous corpora relaxation and the muscles at the basis of the penis involved in penile reflexes and reflex erection. |
| Penile Erection | ||||
|---|---|---|---|---|
| i.c.v. Pretreatment | PVN Pretreatment | NO Donor Given | i.c.v | PVN |
| Vehicle | Vehicle | |||
| Drug | Drug | |||
| Peptide | Peptide | |||
| Saline | d-arginine | == | ||
| Saline | d-arginine | == | ||
| Saline | l-arginine | == | ||
| Saline | l-arginine | ↑ | ||
| Oxy-Ant | l-arginine | ↓ | ||
| Oxy-Ant | l-arginine | ↑ | ||
| L-NAME | l-arginine | ↓ | ||
| L-NAME | l-arginine | ↓ | ||
| Vehicle | Nitroglycerin | ↑ | ||
| Vehicle | Nitroglycerin | ↑ | ||
| Oxy-Ant | Nitroglycerin | ↓ | ||
| Oxy-Ant | Nitroglycerin | ↑ | ||
| L-NAME | Nitroglycerin | ↑ | ||
| L-NAME | Nitroglycerin | ↑ | ||
| vehicle | SNP | ↑ | ||
| Oxy-Ant | SNP | ↓ | ||
| Oxy-Ant | SNP | ↑ | ||
| L-NAME | SNP | ↑ | ||
| L-NAME | SNP | ↑ | ||
| Vehicle | Isoamyl nitrite | ↑ | ||
| Oxy-Ant | Isoamyl nitrite | ↓ | ||
| L-NAME | Isoamyl nitrite | ↑ | ||
| Vehicle | Hydroxylamine | ↑ | ||
| Oxy-Ant | Hydroxylamine | ↓ | ||
| L-NAME | Hydroxylamine | ↑ | ||
| Drug, Peptide, NO Donor into the PVN | Penile Erection | NO Production | ||||||
|---|---|---|---|---|---|---|---|---|
| GC Inhibitors | Hemoglobin | GC Inhibitors | Hemoglobin | |||||
| Given | Given | |||||||
| i.c.v. | PVN | i.c.v. | PVN | i.c.v. | PVN | i.c.v. | PVN | |
| DA agonists | ↓ | == | == | == | == | == | ↓ | ↓ |
| Oxytocin | ↓ | == | == | == | == | == | ↓ | ↓ |
| NMDA | ↓ | == | == | == | == | == | ↓ | ↓ |
| EP 80661 | ↓ | == | == | == | n.a. | n.a. | ↓ | ↓ |
| VGF 588-617 | ↓ | == | == | == | n.a. | n.a. | ↓ | ↓ |
| 5-HT1C agonists | ↓ | == | == | == | n.a. | n.a. | ↓ | ↓ |
| NO donors | ↓ | == | == | == | n.a. | n.a. | ↓ | ↓ |
| Oxytocin Injected in the | ||||
|---|---|---|---|---|
| Pretreatment | Ventral Tegmental Area | Ventral Subiculum | ||
| Penile Erection | NO Production | Penile Erection | NO Production | |
| L-NAME | ↓ | ↓ | ↓ | ↓ |
| Oxy-Ant | ↓ | ↓ | ↓ | ↓ |
| ODQ | ↓ | == | n.a. | n.a. |
| Haemoglobin | == | ↓ | ↓ | ↓ |
| MPOA Treatment | Dopamine | Copulation |
|---|---|---|
| l-arginine | ↑ | ↑ |
| L-NAME | ↓ | ↓ |
| D-NAME | ↑ | ↑ |
| L-NAME+l-Arginine | ↓ | ↓ |
| SNP | ↑ | ↑ |
| ODQ | ↓ | ↓ |
| ODQ+SNP | ↓ | ↓ |
| 8-Bromo-cGMP | ↑ | ↑ |
| L-NMMA+8-Bromo-cGMP | ↑ | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melis, M.R.; Argiolas, A. Erectile Function and Sexual Behavior: A Review of the Role of Nitric Oxide in the Central Nervous System. Biomolecules 2021, 11, 1866. https://doi.org/10.3390/biom11121866
Melis MR, Argiolas A. Erectile Function and Sexual Behavior: A Review of the Role of Nitric Oxide in the Central Nervous System. Biomolecules. 2021; 11(12):1866. https://doi.org/10.3390/biom11121866
Chicago/Turabian StyleMelis, Maria Rosaria, and Antonio Argiolas. 2021. "Erectile Function and Sexual Behavior: A Review of the Role of Nitric Oxide in the Central Nervous System" Biomolecules 11, no. 12: 1866. https://doi.org/10.3390/biom11121866
APA StyleMelis, M. R., & Argiolas, A. (2021). Erectile Function and Sexual Behavior: A Review of the Role of Nitric Oxide in the Central Nervous System. Biomolecules, 11(12), 1866. https://doi.org/10.3390/biom11121866






