The Effect of Three-Month Vitamin D Supplementation on the Levels of Homocysteine Metabolism Markers and Inflammatory Cytokines in Sera of Psoriatic Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Issues
2.2. Study Design
2.3. Clinical and Demographic Characteristics
2.4. Biochemical Analyses
2.5. Measuring Techniques
2.6. Statistical Analyses
3. Results
3.1. Demographic Data in Psoriatic Patients
3.2. The Effect of Vitamin D Supplementation on Disease Severity in Psoriatic Patients
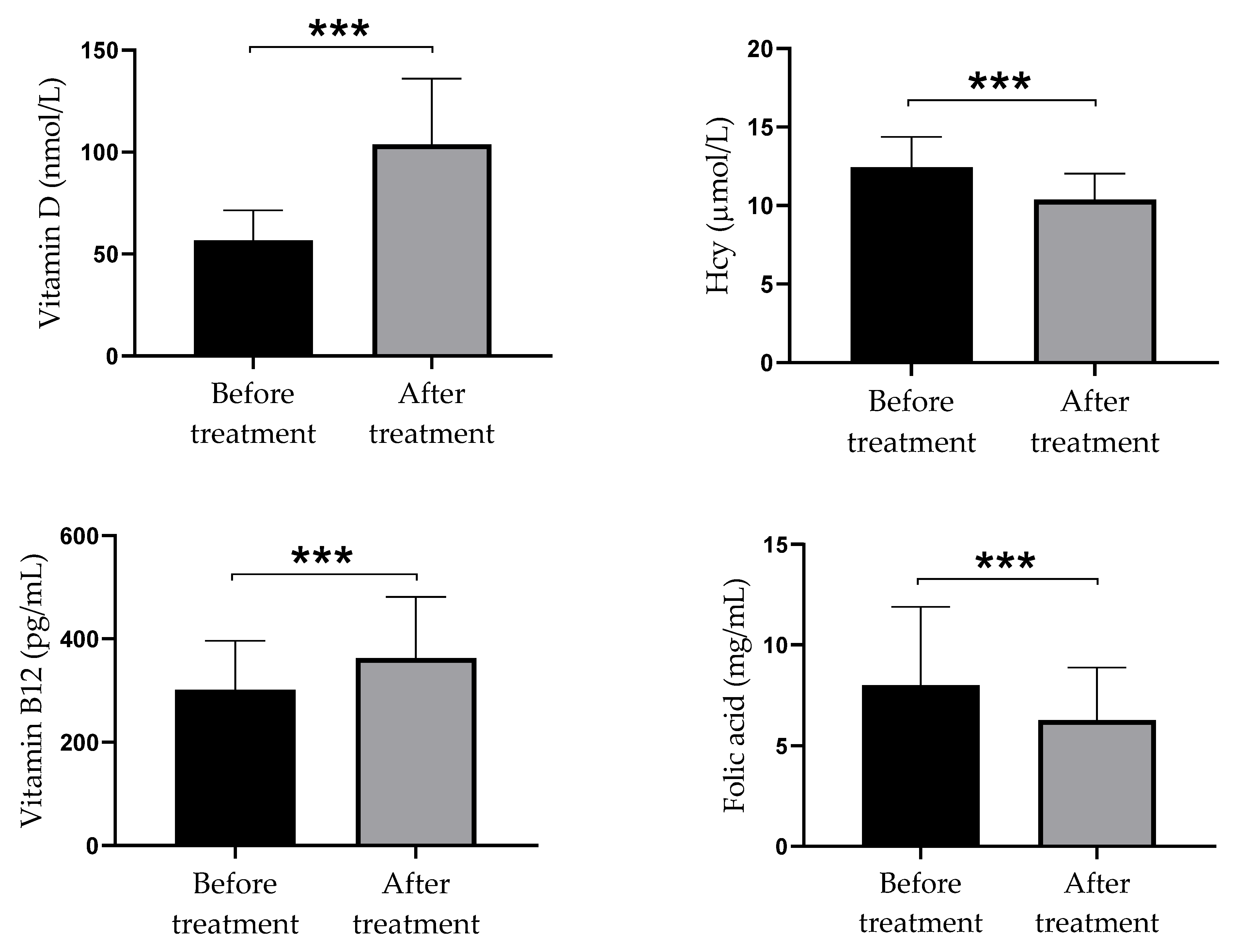
3.3. Serum Levels of Vitamin D, Homocysteine, VITAMIN B12, and Folate in Psoriatic Patients
3.4. Differences in Serum Levels of Vitamin D, Homocysteine, Vitamin B12, and Folate between Psoriatic Patients before and after Vitamin D Supplementation
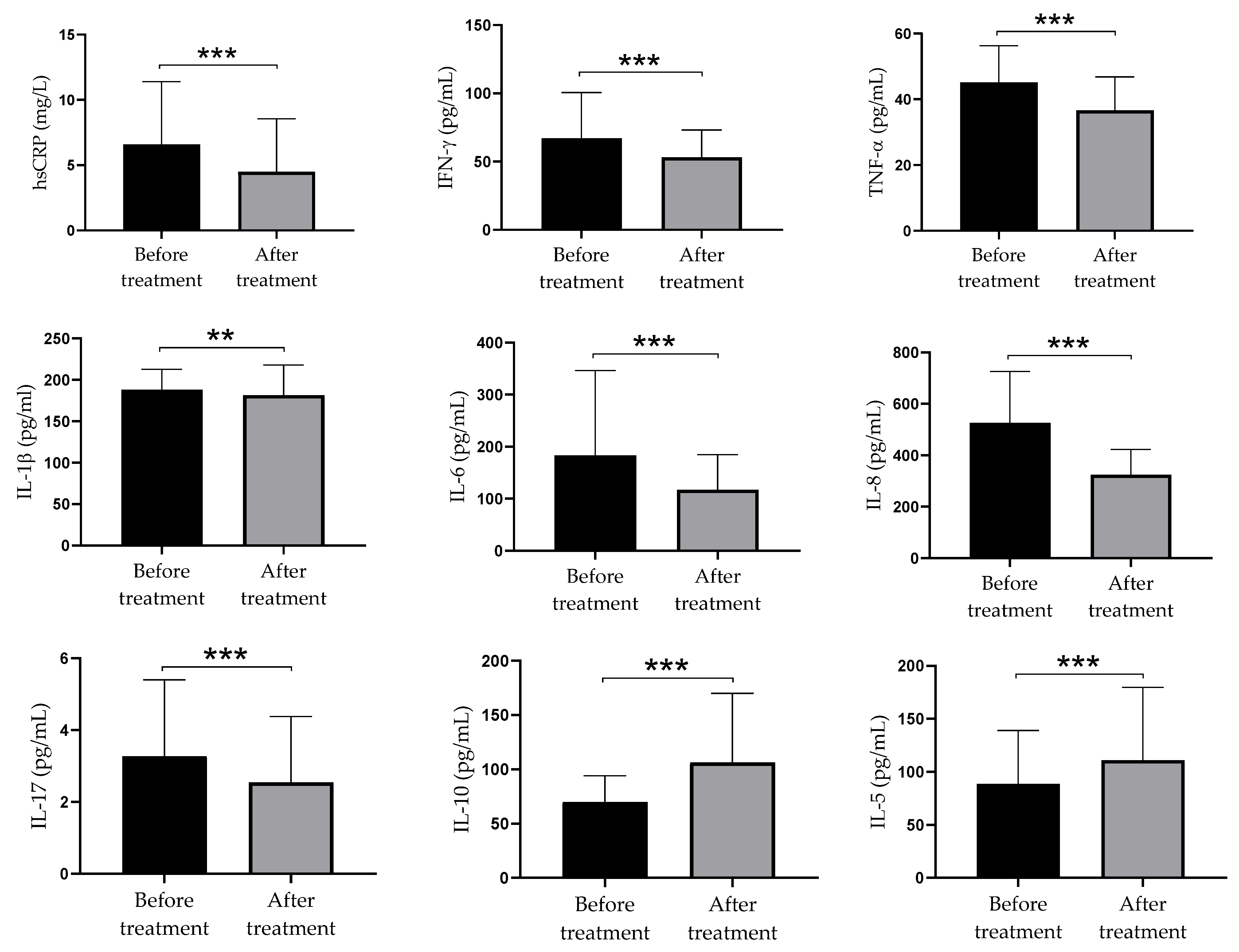
3.5. The Concentration of Pro-Inflammatory and Anti-Inflammatory Parameters in Serum of Psoriatic Patients before and after Supplementation with Vitamin D
3.6. Relationship between Serum Hcy and Pro-Inflammatory and Anti-Inflammatory Parameters in Serum of Psoriatic Patients before and after Supplementation with Vitamin D
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Satake, K.; Amano, T.; Okamoto, T. Calcipotriol and betamethasone dipropionate synergistically enhances the balance between regulatory and proinflammatory T cells in a murine psoriasis model. Sci. Rep. 2019, 9, 16322. [Google Scholar] [CrossRef]
- Petit, R.G.; Cano, A.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Severino, P.; Souto, E.B.; García, M.L.; Pujol, M.; et al. Psoriasis: From pathogenesis to pharmacological and nano-technological-based therapeutics. Int. J. Mol. Sci. 2021, 22, 4983. [Google Scholar] [CrossRef]
- Kusuba, N.; Kitoh, A.; Dainichi, T.; Honda, T.; Otsuka, A.; Egawa, G.; Nakajima, S.; Miyachi, Y.; Kabashima, K. Inhibition of IL-17–committed T cells in a murine psoriasis model by a vitamin D analogue. J. Allergy Clin. Immunol. 2018, 141, 972–981.e10. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the pathophysiology of inflammatory skin diseases. Skin Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.W.; Vender, R. Systemic role for vitamin D in the treatment of psoriasis and metabolic syndrome. Dermatol. Res. Pract. 2011, 2011, 276079. [Google Scholar] [CrossRef] [PubMed]
- Germán, B.; Wei, R.; Hener, P.; Martins, C.; Ye, T.; Gottwick, C.; Yang, J.; Seneschal, J.; Boniface, K.; Li, M. Disrupting the IL-36 and IL-23/IL-17 loop underlies the efficacy of calcipotriol and corticosteroid therapy for psoriasis. JCI Insight 2019, 4, e123390. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Czerwińska, J.; Placek, W. The role of regulatory T cells and anti-inflammatory cytokines in psoriasis. Acta Dermatovenerol. Alp. Pannonica Adriat. 2018, 27, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, A.; Chabros, P.; Grywalska, E.; Pietrzak, D.; Kandzierski, G.; Wawrzycki, B.; Roliński, J.; Gawęda, K.; Krasowska, D. Serum concentration of interleukin 6 is related to inflammation and dyslipidemia in patients with psoriasis. Adv. Dermatol. Allergol. 2020, 37, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Trouba, K.J.; Hamadeh, H.K.; Amin, R.P.; Germolec, D.R. Oxidative stress and its role in skin disease. Antioxid. Redox Signal. 2002, 4, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Jindal, S.; Jindal, N. Psoriasis and cardiovascular diseases: A literature review to determine the causal relationship. Cureus 2018, 10, e2195. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Tessari, G.; Conti, A.; Piaserico, S.; Schianchi, S.; Peserico, A.; Giannetti, A.; Girolomoni, G. Prevalence of metabolic syndrome in patients with psoriasis: A hospital-based case control study. Br. J. Dermatol. 2007, 157, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Djuric, D.; Jakovljevic, V.; Zivkovic, V.; Srejovic, I. Homocysteine and homocysteine-related compounds: An overview of the roles in the pathology of the cardiovascular and nervous systems. Can. J. Physiol. Pharmacol. 2018, 96, 991–1003. [Google Scholar] [CrossRef]
- Brazzelli, V.; Grasso, V.; Fornara, L.; Moggio, E.; Gamba, G.; Villani, S.; Borroni, G. Homocysteine, vitamin B 12 and folic acid levels in psoriatic patients and correlation with disease severity. Int. J. Immunopathol. Pharmacol. 2010, 23, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Den Heijer, M.; Lewington, S.; Clarke, R. Homocysteine, MTHFR and risk of venous thrombosis: A meta-analysis of published epidemiological studies. J. Thromb. Haemost. 2005, 3, 292–299. [Google Scholar] [CrossRef]
- Ozden, H.K.; Polat, M.; Ozturk, S.; Bugdayci, G. Assessment of subclinical cardiac damage in chronic plaque psoriasis patients: A case control study. Arch. Med. Sci. Atheroscler. Dis. 2016, 7, e.126–e.132. [Google Scholar]
- Lin, X.; Meng, X.; Song, Z. Homocysteine and psoriasis. Biosci. Rep. 2019, 39, BSR20190867. [Google Scholar] [CrossRef] [PubMed]
- Vanizor Kural, B.; Örem, A.; Çimşit, G.; Uydu, H.A.; Yandi, Y.E.; Alver, A. Plasma homocysteine and its relationships with atherothrombotic markers in psoriatic patients. Clin. Chim. Acta 2003, 332, 23–30. [Google Scholar] [CrossRef]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A Systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Mattozzi, C.; Paolino, G.; Salvi, M.; Macaluso, L.; Scarnò, M.; de Vita, G.; Calvieri, S.; Richetta, A.G. Correlation between plasmatic levels of vitamin D and PASI score. G. Ital. Dermatol. Venereol. 2018, 153, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Qayyum, R. The relationship between 25-hydroxyvitamin D and homocysteine in asymptomatic adults. J. Clin. Endocrinol. Metab. 2014, 99, 633–638. [Google Scholar] [CrossRef]
- Al-Bayyari, N.; Al-Zeidaneen, S.; Hamadneh, J. Vitamin D3 prevents cardiovascular diseases by lowering serum total homocysteine concentrations in overweight reproductive women: A randomized, placebo-controlled clinical trial. Nutr. Res. 2018, 59, 65–71. [Google Scholar] [CrossRef]
- Schmitt, J.; Wozel, G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology 2005, 210, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.; Zito, A.; Dentamaro, I.; Vestito, D.; Scicchitano, P.; Iacoviello, M.; De Pergola, G.; Devito, F. Vitamin D deficiency and cardiovascular diseases. G. Ital. Cardiol. 2015, 16, 16–20. [Google Scholar]
- McCullough, P.J.; McCullough, W.P.; Lehrer, D.; Travers, J.B.; Repas, S.J. Oral and topical vitamin D, sunshine, and UVB phototherapy safely control psoriasis in patients with normal pretreatment serum 25-hydroxyvitamin D concentrations: A literature review and discussion of health implications. Nutrients 2021, 13, 1511. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef]
- Kelly, P.J.; Rosand, J.; Kistler, J.P.; Shih, V.E.; Silveira, S.; Plomaritoglou, A.; Furie, K.L. Homocysteine, MTHFR 677C->T polymorphism, and risk of ischemic stroke: Results of a meta-analysis. Neurology 2002, 59, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Gisondi, P.; Radaeli, A.; Sala, R.; Calzavara Pinton, P.G.; Girolomoni, G. Plasma homocysteine and folate levels in patients with chronic plaque psoriasis: Hyperhomocysteinaemia and psoriasis. Br. J. Dermatol. 2006, 155, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, S.; Gül Kılıç, C.; Gönül, M.; Soylu, S.; Kılıç, A. Homocysteine, vitamin B12 and folic acid levels in psoriasis patients. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Uslu, M.; Şendur, N.; Şavk, E.; Karul, A.; Kozacı, D.; Gökbulut, C.; Karaman, G.; Kurt Ömürlü, İ. Blood homocysteine, folic acid, vitamin B12 and vitamin B6 levels in psoriasis patients. Turkderm 2017, 39, 92–97. [Google Scholar] [CrossRef]
- Bai, F.; Zheng, W.; Dong, Y.; Wang, J.; Garstka, M.A.; Li, R.; An, J.; Ma, H. Serum levels of adipokines and cytokines in psoriasis patients: A systematic review and meta-analysis. Oncotarget 2018, 9, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Priyadarssini, M.; Divya Priya, D.; Indhumathi, S.; Rajappa, M.; Chandrashekar, L.; Thappa, D.M. Immunophenotyping of T cells in the peripheral circulation in psoriasis. Br. J. Biomed. Sci. 2016, 73, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Dyring-Andersen, B.; Bonefeld, C.M.; Bzorek, M.; Løvendorf, M.B.; Lauritsen, J.P.H.; Skov, L.; Geisler, C. The vitamin D analogue calcipotriol reduces the frequency of CD8 + IL-17 + T cells in psoriasis lesions. Scand. J. Immunol. 2015, 82, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Fujiyama, T.; Ito, T.; Umayahara, T.; Ikeya, S.; Tatsuno, K.; Funakoshi, A.; Hashizume, H.; Tokura, Y. Topical application of a vitamin D3 analogue and corticosteroid to psoriasis plaques decreases skin infiltration of TH17 cells and their ex vivo expansion. J. Allergy Clin. Immunol. 2016, 138, 517–528.e5. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Ghoreschi, K. The role of IL-4 in psoriasis. Expert Rev. Clin. Immunol. 2017, 13, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Onderdijk, A.J.; Baerveldt, E.M.; Kurek, D.; Kant, M.; Florencia, E.F.; Debets, R.; Prens, E.P. IL-4 Downregulates IL-1β and IL-6 and induces GATA3 in psoriatic epidermal cells: Route of action of a Th2 cytokine. J. Immunol. 2015, 195, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Thomas, P.; Breit, S.; Dugas, M.; Mailhammer, R.; van Eden, W.; van der Zee, R.; Biedermann, T.; Prinz, J.; Mack, M.; et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat. Med. 2003, 9, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Daly, L.; Robinson, K.; Naughten, E.; Cahalane, S.; Fowler, B.; Graham, I. Hyperhomocysteinemia: An indepedent risk factor for cardiovascular disease. N. Engl. J. Med. 1991, 324, 1149–1155. [Google Scholar] [CrossRef]
- McIlroy, S.P.; Dynan, K.B.; Lawson, J.T.; Patterson, C.C.; Passmore, A.P. Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke 2002, 33, 2351–2356. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, M.K.; Philippou, H.; Stubbs, P.J.; Adami, A.; Amersey, R.; Noble, M.M.; Lane, D.A. Relationships between homocysteine, factor VIIa, and thrombin generation in acute coronary syndromes. Circulation 2000, 101, 372–377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.Z.; Maruyama, K.; Ono, I.; Iwatsuki, K.; Kaneko, F. Regulatory effects of 1, 25-dihydroxyvitamin D3 and a novel vitamin D3 analogue MC903 on secretion of interleukin-1 alpha (IL-1α) and IL-8 by normal human keratinocytes and a human squamous cell carcinoma cell line (HSC-1). J. Dermatol. Sci. 1994, 7, 24–31. [Google Scholar] [CrossRef]
- Giannoni, M.; Consales, V.; Campanati, A.; Ganzetti, G.; Giuliodori, K.; Postacchini, V.; Liberati, G.; Azzaretto, L.; Vichi, S.; Guanciarossa, F.; et al. Homocysteine plasma levels in psoriasis patients: Our experience and review of the literature. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mild (PASI < 10) n = 15 | Moderate (PASI 10–19) n = 14 | Severe (PASI ≥ 20) n = 11 | Total n = 40 | p Value | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 5 (33.3) | 8 (57.1) | 5 (45.5) | 18 (45.0) | 0.479 b | |
| Female | 10 (66.7) | 6 (42.9) | 6 (54.5) | 22 (55.0) | ||
| Age (year) | 49.13 ± 15.14 | 47.0 ± 18.25 | 44.55 ± 11.06 | 47.13 ± 15.10 | 0.755 a | |
| BMI (kg/m2) | 25.72 ± 2.66 | 25.79 ± 2.88 | 27.97 ± 1.35 | 26.36 ± 2.60 | 0.051 a | |
| Disease duration (years) | 14.67 ± 11.16 | 12.79 ± 10.32 | 14.36 ± 15.20 | 13.93 ± 11.84 | 0.908 a | |
| Smoking status | ||||||
| Smoker | 9 (60.0) | 10 (71.4) | 8 (72.7) | 27 (67.5) | 0.760 c | |
| Nonsmoker | 6 (40.0) | 4 (28.6) | 3 (27.3) | 13 (32.5) | ||
| Family history of psoriasis | ||||||
| positive | 8 (53.3) | 7 (50.0) | 6 (54.5) | 21 (52.5) | 0.972 b | |
| negative | 7 (46.7) | 7 (50.0) | 5 (45.5) | 19 (47.5) | ||
| Beginning of disease | ||||||
| early | 7 (46.7) | 8 (57.1) | 5 (45.5) | 20 (50.0) | 0.852 b | |
| late | 8 (53.3) | 6 (42.9) | 6 (54.5) | 20 (50.0) | ||
| Disease Severity by PASI | Before Vitamin D Supplementation | After Vitamin D Supplementation | p Value |
|---|---|---|---|
| Mild form of disease | 15 (37.5) | 25 (62.5) | <0.001 |
| Moderate form of disease | 14 (35.0) | 10 (25.0) | <0.001 |
| Severe form of disease | 11 (27.5) | 5 (12.5) | <0.001 |
| Biochemical Markers | Before Vitamin D Supplementation | After Vitamin D Supplementation | p Value | |
|---|---|---|---|---|
| Vitamin D | ||||
| Low vitamin D < 75.0 ng/mL | 40 (100) | 8 (20.0) | 0.001 | |
| Normal vitamin D | 0 (0) | 32 (80.0) | ||
| Hcy | ||||
| Normal Hcy | 15 (37.5) | 33 (82.5) | 0.001 | |
| High Hcy ˃ 12.0 µmol/L | 25 (62.5) | 7 (17.5) | ||
| Vitamin B12 | ||||
| Low vitamin B12 < 211.0 ng/mL | 5 (12.5) | 3 (7.5) | 0.157 | |
| Normal vitamin B12 | 35 (87.5) | 37 (92.5) | ||
| Folate | ||||
| Low Folate < 3.89 µmol/L | 3 (7.5) | 7 (17.5) | 0.102 | |
| Normal Folate | 37 (92.5) | 33 (82.5) | ||
| Pro-Inflammatory and Anti-Inflammatory Parameters | Before Vitamin D Supplementation | After Vitamin D Spplementation |
|---|---|---|
| r (p) | r (p) | |
| hsCRP | −0.146 (0.374) | −0.147 (0.373) |
| IFN-γ | 0.148 (0.370) | 0.089 (0.588) |
| TNF-α | −0.046 (0.779) | −0.045 (0.784) |
| IL-1β | 0.078 (0.639) | −0.338 (0.035) |
| IL-6 | 0.153 (0.354) | −0.047 (0.776) |
| IL-8 | −0.091 (0.580) | −0.374 (0.019) |
| IL-17 | 0.266 (0.102) | 0.117 (0.479) |
| IL-10 | −0.106 (0.522) | 0.046 (0.780) |
| IL-5 | 0.183 (0.264) | 0.096 (0.561) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prtina, A.; Rašeta Simović, N.; Milivojac, T.; Vujnić, M.; Grabež, M.; Djuric, D.; Stojiljković, M.P.; Soldat Stanković, V.; Čolić, M.J.; Škrbić, R. The Effect of Three-Month Vitamin D Supplementation on the Levels of Homocysteine Metabolism Markers and Inflammatory Cytokines in Sera of Psoriatic Patients. Biomolecules 2021, 11, 1865. https://doi.org/10.3390/biom11121865
Prtina A, Rašeta Simović N, Milivojac T, Vujnić M, Grabež M, Djuric D, Stojiljković MP, Soldat Stanković V, Čolić MJ, Škrbić R. The Effect of Three-Month Vitamin D Supplementation on the Levels of Homocysteine Metabolism Markers and Inflammatory Cytokines in Sera of Psoriatic Patients. Biomolecules. 2021; 11(12):1865. https://doi.org/10.3390/biom11121865
Chicago/Turabian StylePrtina, Alma, Nela Rašeta Simović, Tatjana Milivojac, Milorad Vujnić, Milkica Grabež, Dragan Djuric, Miloš P. Stojiljković, Valentina Soldat Stanković, Miodrag J. Čolić, and Ranko Škrbić. 2021. "The Effect of Three-Month Vitamin D Supplementation on the Levels of Homocysteine Metabolism Markers and Inflammatory Cytokines in Sera of Psoriatic Patients" Biomolecules 11, no. 12: 1865. https://doi.org/10.3390/biom11121865
APA StylePrtina, A., Rašeta Simović, N., Milivojac, T., Vujnić, M., Grabež, M., Djuric, D., Stojiljković, M. P., Soldat Stanković, V., Čolić, M. J., & Škrbić, R. (2021). The Effect of Three-Month Vitamin D Supplementation on the Levels of Homocysteine Metabolism Markers and Inflammatory Cytokines in Sera of Psoriatic Patients. Biomolecules, 11(12), 1865. https://doi.org/10.3390/biom11121865








