Emerging Role of Flavonoids as the Treatment of Depression
Abstract
1. Introduction
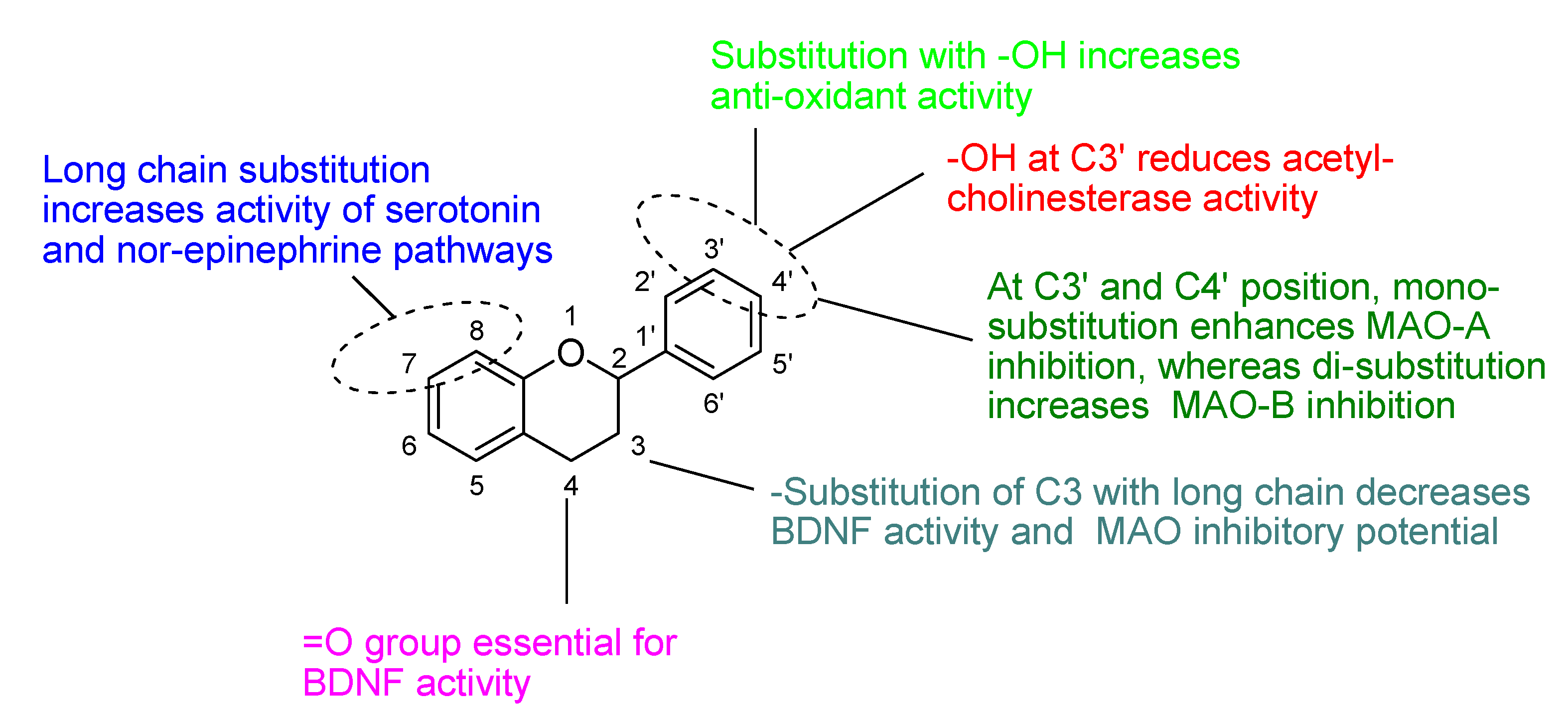
2. Flavonoids and Structure Activity Relationship
2.1. Flavones
Structure Activity Relationship (SAR) of Flavones
- The presence of ketonic group at C4 and ring B, C may be responsible for enhancement of BDNF level in the brains of mice.
- Attachment of hydroxyl group at C7 and long chain group at C8 resulting in increment of activity of serotonin and nor-epinephrine pathways.
- Hydroxyl group at C3′ and C4′ is necessary for increments of anti-oxidant potential and radical scavenging properties.
- At C3′ and C4′ position, mono-substitution enhances the selectivity towards MAO-A inhibition, whereas di-substitution increases the selectivity for MAO-B inhibition.
- Glycoside-O linkage at C7 abolished or reduces the MAO inhibitory potential.
- Acetate and methyl group at C7 and C8 decreased antioxidant potential of flavones.
2.2. Flavonols
SAR of Flavonols
- In flavonols, attachment at C3 with –OR, does not much affect the antidepressant potential.
- Substitution at C3 with hydroxyl group resulting in increase of brain levels of neurotransmitter, i.e., serotonin, dopamine, nor-epinephrine.
- The presence of ketonic group at C4 and ring B, C may be necessary for anti-depressant potential of flavonols.
- Substitution at –OR at C3 with long chain molecules (C6H11O5 or C6H11O6) resulting in decrease activation of hypothalamic-pituitary-adrenal axis, thereby decrease the release of ACTH.
- Substitution of –OR at C3 with long chain molecule may be responsible for decrease in BDNF activity of flavonols.
- Substitution of –OR with –OH group decreases the MAO inhibitory potential.
- Hydroxyl group at C3′ and C4′ is necessary for increment of antioxidant potential and radical scavenging property.
- Acetate and methyl group at C7 and C8 decreased antioxidant potential of flavones.
- Glycoside-O linkage at C7 abolished or reduces the MAO inhibitory potential.
2.3. Flavanones
SAR of Flavanones
- In flavanones, there is absence of double bond at C2 and C3 position; it means this bond is not necessary for antidepressant potential.
- Saturation of double bond at C2 and C3 position does not much affect the BDNF activity of flavanones.
- Saturation of double bond at C2 and C3 position reduces the MAO inhibitory potential.
- Substitution at C7 with O- (C12H21O9) may be responsible for selective interaction with kappa-opioid receptors.
- Glycoside-O linkage at C7 abolished or reduces the MAO inhibitory potential.
- Acetate and methyl group at C7 and C8 decreased antioxidant potential of flavonols.
- Hydroxyl group at C3′ and C4′ is necessary for an increment of antioxidant potential and radical scavenging property.
- Hydroxyl group at C3′ may be responsible for reduction in acetyl-cholinesterase activity.
2.4. Flavanonols
2.5. Flavanols
2.6. Others
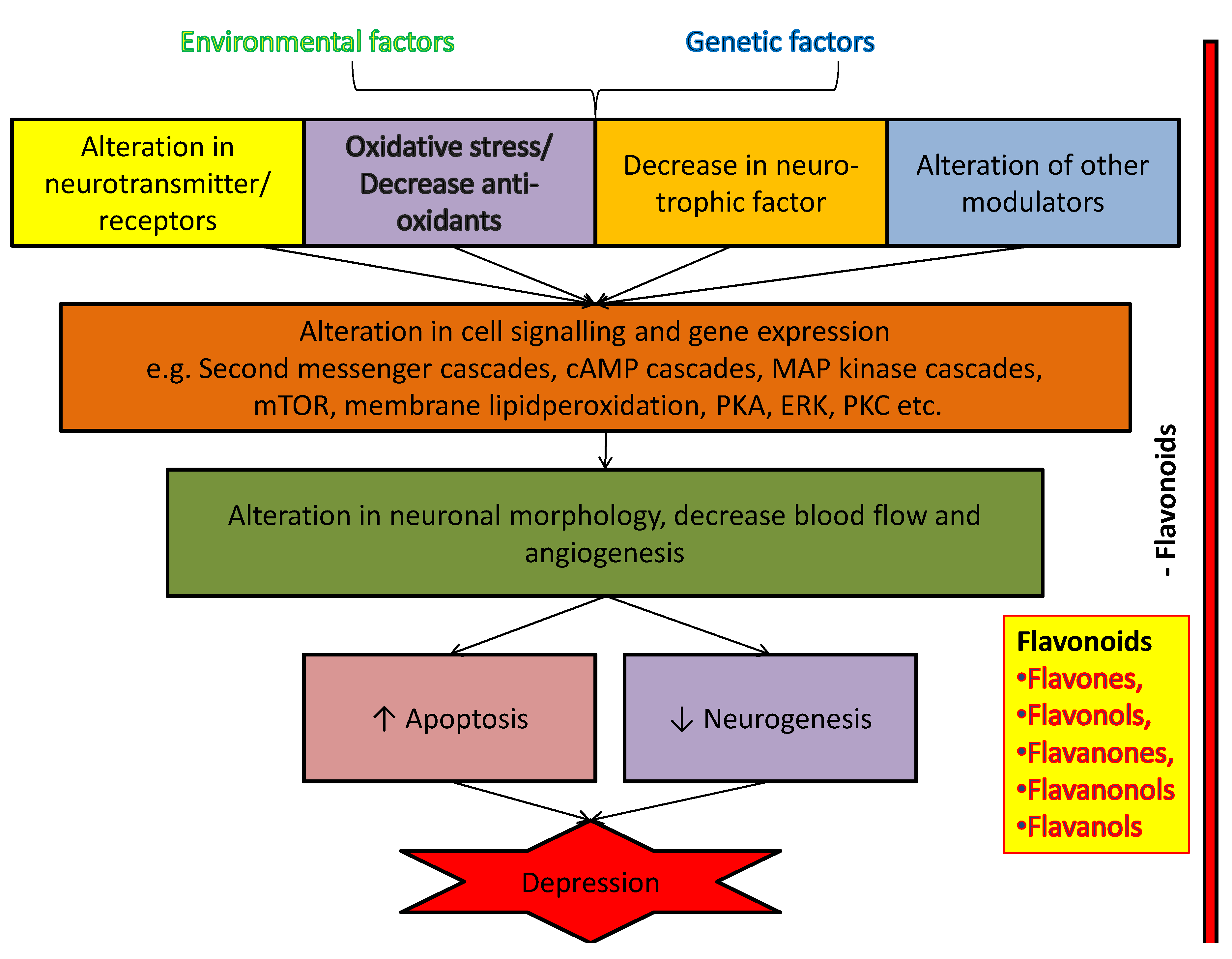
3. Possible Cellular and Molecular Mechanism of Anti-Depressant Action of Flavonoids
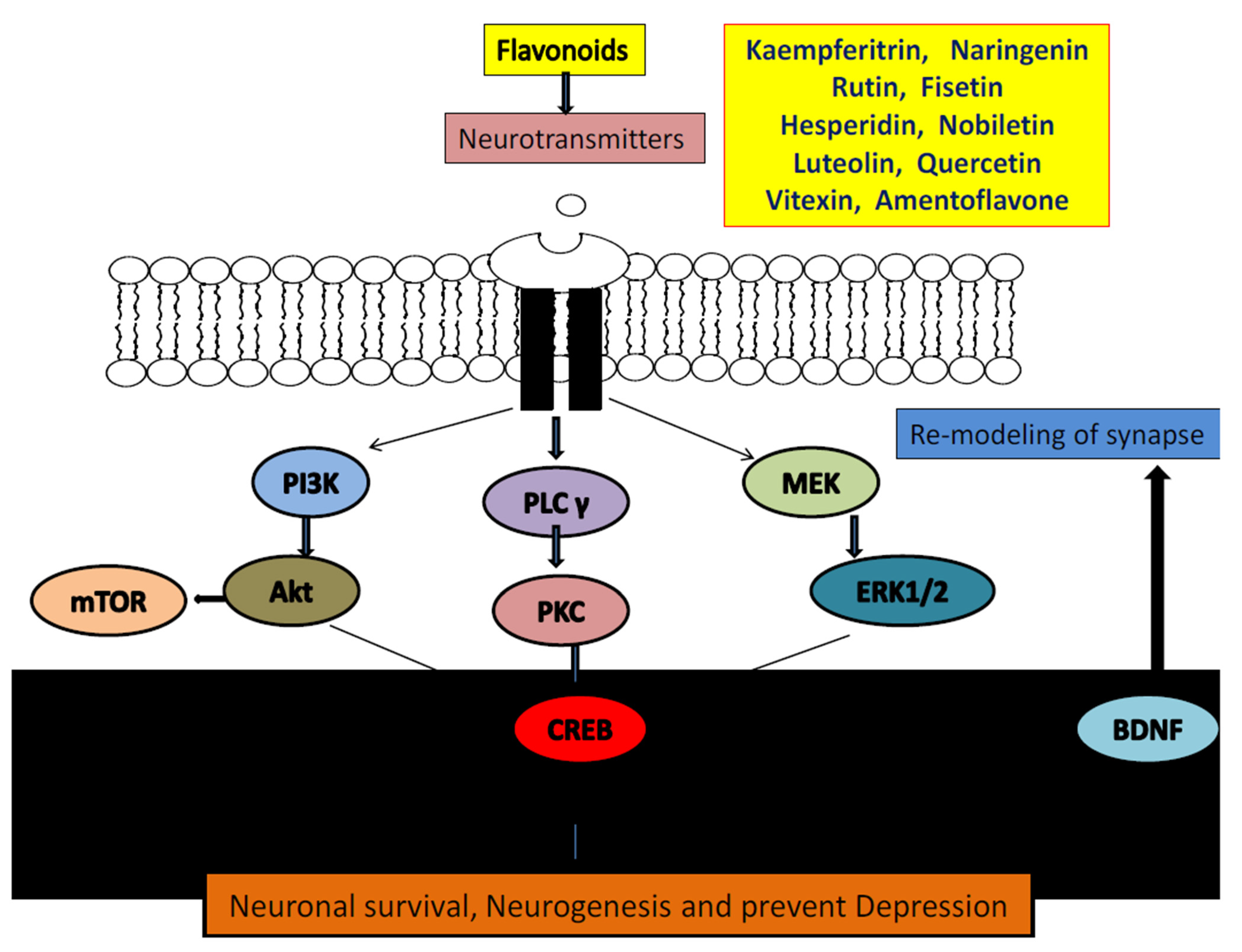
3.1. Flavonoids and Neurotransmitters
3.2. Flavonoids and Neurodegeneration
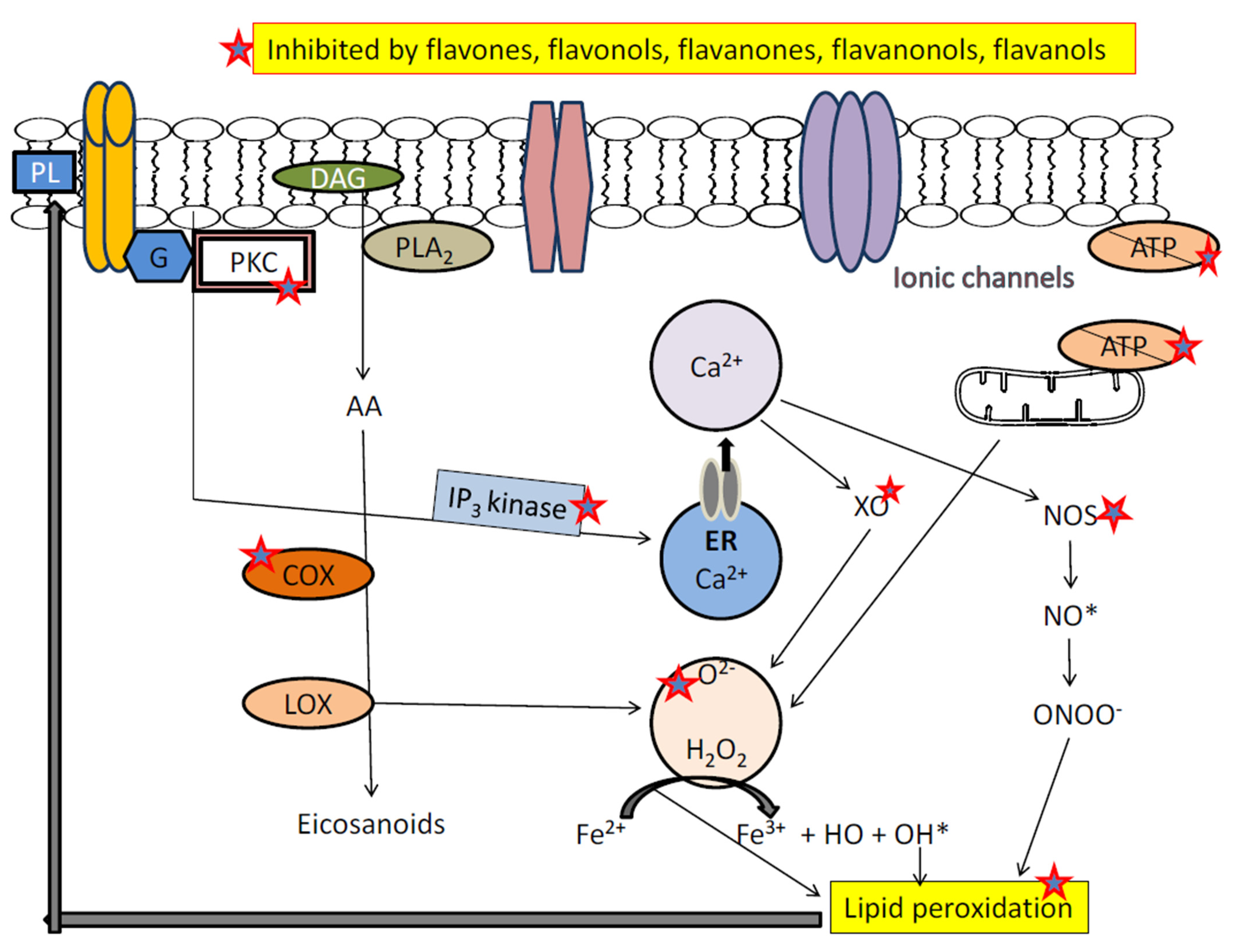
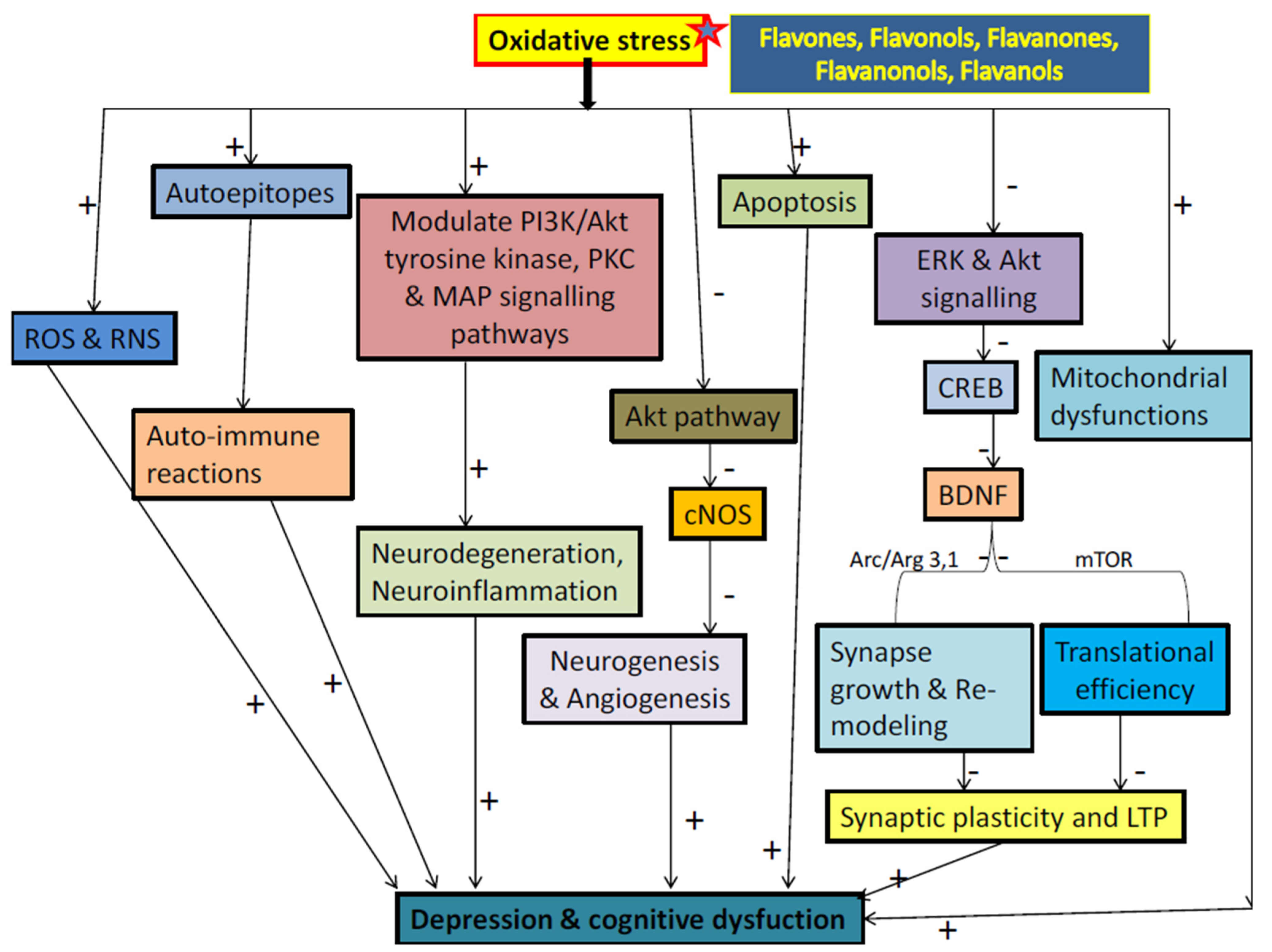
3.3. Flavonoids and Oxidative Stress
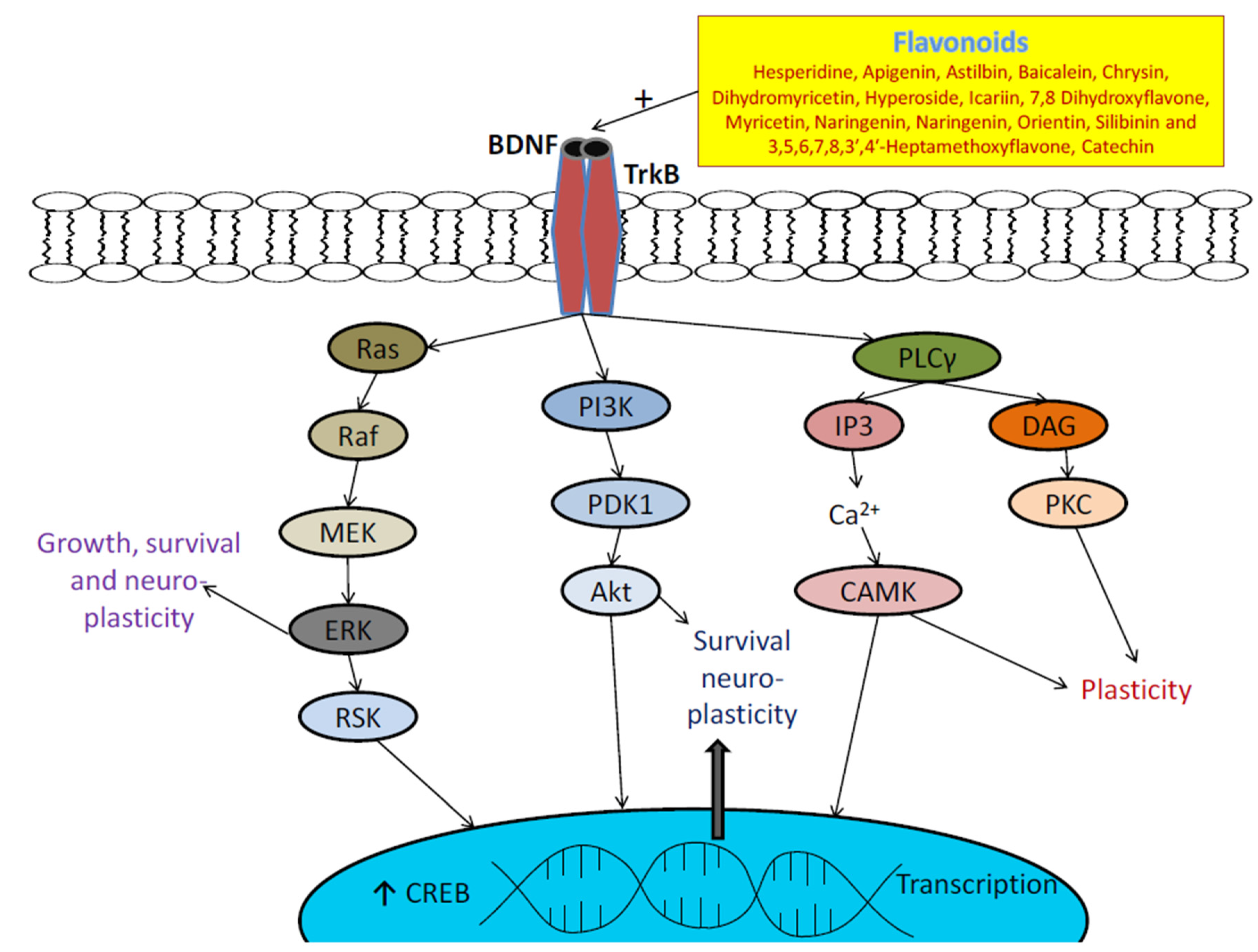
3.4. Flavonoids and BDNF Expression
4. Future Aspects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT | 5-Hydroxy tryptophan |
| MDD | Major depression disorder |
| MAO | Monoamino oxidase |
| D1 | Dopamine 1 |
| D2 | Dopamine 2 |
| COX | Cyclooxygenase |
| XO | Xanthine oxidase |
| NOS | Nitric oxide synthase |
| LOX | Lipooxygenase |
| IP3 | Inositol triphosphate |
| PKC | Phosphokinase C |
| BDNF | Brain derived neurotrophic factor |
| CREB | cAMP-response element binding protein |
| NO | Nitric oxidase |
| SOD | Superoxide dismutase |
| BCL-2 | B-cell lymphoma 2 |
| TrkB | Tropomyosin receptor kinase B |
References
- German-Ponciano, L.J.; Rosas-Sánchez, G.U.; Rivadeneyra-Domínguez, E.; Rodríguez-Landa, J.F. Advances in the preclinical study of some flavonoids as potential antidepressant agents. Scientifica 2018, 2963565, 1–14. [Google Scholar] [CrossRef]
- Kessler, R.C. The costs of depression. Psychiatr. Clin. N. Am. 2014, 35, 1–14. [Google Scholar] [CrossRef]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The effects of dietary improvement on symptoms of depression and anxiety: A meta-analysis of randomized controlled trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef]
- Xing, Y.; He, J.; Hou, J.; Lin, F.; Tian, J.; Kurihara, H. Gender differences in CMS and the effects of antidepressant venlafaxine in rats. Neurochem. Int. 2013, 63, 570–575. [Google Scholar] [CrossRef]
- Olivares-Nazario, M.; Fernández-Guasti, A.; Martínez-Mota, L. Age-related changes in the antidepressant-like effect of desipramine and fluoxetine in the rat forced-swim test. Behav. Pharm. 2016, 27, 22–28. [Google Scholar] [CrossRef]
- Harmer, C.J.; Duman, R.S.; Cowen, P.J. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry 2017, 4, 409–418. [Google Scholar] [CrossRef]
- LÓpez-Rubalcava, C.; Estrada-Camarena, E. Mexican medicinal plants with anxiolytic or antidepressant activity: Focus on preclinical research. J. Ethnopharmacol. 2016, 186, 377–391. [Google Scholar] [CrossRef]
- Machado, D.G.; Bettio, L.E.; Cunha, M.P.; Santos, A.R.; Pizzolatti, M.G.; Brighente, I.M.; Rodrigues, A.L. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur. J. Pharm. 2008, 587, 163–168. [Google Scholar] [CrossRef]
- Ji, J. Distinguishing subclinical (subthreshold) depression from the residual symptoms of major depression. Shanghai Arch. Psychiatry 2012, 24, 288–289. [Google Scholar]
- Arlington, V. Practice Guideline for the Treatment of Patients with Major Depressive Disorder, 3rd ed.; American Psychiatric Association: Washington, DC, USA, 2010. [Google Scholar]
- Sartorius, N.; Baghai, T.C.; Baldwin, D.S.; Barrett, B.; Brand, U.; Fleischhacker, W.; Goodwin, G.; Grunze, H.; Knapp, M.; Leonard, B.E.; et al. Antidepressant medications and other treatments of depressive disorders: A CINP Task Force report based on a review of evidence. Int. J. Neuropsychopharmacol. 2007, 10, S1–S207. [Google Scholar] [CrossRef] [PubMed]
- Singhal, M.; Manjula, M.; Vijay Sagar, K.J. Subclinical depression in Urban Indian adolescents: Prevalence, felt needs, and correlates. Indian J. Psychiatry 2016, 58, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.R.; Nuevo, R.; Chatterji, S.; Ayuso-Mateos, J.L. Definitions and factors associated with subthreshold depressive conditions: A systematic review. BMC Psychiatry 2012, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. Am. J. Psychiatry 2016, 173, 575–587. [Google Scholar] [CrossRef]
- Lande, R.G. Nutraceutical augmentation strategies for depression: A narrative review. J. Am. Osteopath Assoc. 2020, 120, 100–106. [Google Scholar]
- Rao, T.S.; Asha, M.R.; Ramesh, B.N.; Rao, K.S. Understanding nutrition, depression and mental illnesses. Indian J. Psychiatry 2008, 50, 77–82. [Google Scholar] [PubMed]
- Sarris, J. Clinical use of nutraceuticals in the adjunctive treatment of depression in mood disorders. Australas. Psychiatry 2017, 25, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.; O’Rourke, D.; Wurtman, J.J. Nutrient imbalances in depressive disorders: Possible brain mechanisms. Ann. N. Y. Acad. Sci. 1989, 575, 75–82. [Google Scholar] [CrossRef]
- Martínez-Cengotitabengoa, M.; González-Pinto, A. Nutritional supplements in depressive disorders. Actas Esp. Psiquiatr. 2017, 45, 8–15. [Google Scholar] [PubMed]
- Hoffmann, K.; Emons, B.; Brunnhuber, S.; Karaca, S.; Juckel, G. The role of dietary supplements in depression and anxiety—A narrative review. Pharmacopsychiatry 2019, 52, 261–279. [Google Scholar] [CrossRef]
- Targum, S.D.; Mischoulon, D. The status of nutraceuticals for the treatment of depression. Psychiatry 2009, 6, 46–48. [Google Scholar]
- Mishoulon, D.; Fava, M.F. Are nutritional supplements ready for prime time? J. Clin. Psychiatry 2008, 69, 1–2. [Google Scholar] [CrossRef]
- Gosavi, S.; Subramanian, M.; Reddy, R.; Shet, B.L. A study of prescription pattern of neutraceuticals, knowledge of the patients and cost in a tertiary care hospital. J. Clin. Diagn Res. 2016, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Srividya, A.R.; Venkatesh, N.; Vishnuvarthan, V.J. Neutraceutical as medicine. Int. J. Adv. Pharm. Res. 2010, 1, 132–133. [Google Scholar]
- Dureja, H.; Kaushik, D.; Kumar, V. Developments in neutraceuticals. Indian J. Pharm. 2003, 35, 363–364. [Google Scholar]
- Bhowmik, D.; Gopinath, H.; Kumar, P.B.; Duraivel, S.; Kumar, S.K.P. Nutraceutical—A bright scope and opportunity of Indian healthcare market. Pharma. Innov. J. 2013, 1, 29–41. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochem 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Survay, N.; Upadhyaya, C.P.; Kumar, B.; Young, K.E.; Yoon, D.-Y.; Park, S.-W. New Genera of Flavonols and Flavonol Derivatives as Therapeutic Molecules. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 1–18. [Google Scholar] [CrossRef]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoid.
- Guan, L.P.; Liu, B.Y. Antidepressant-like effects and mechanisms of flavonoids and related analogues. Eur. J. Med. Chem. 2016, 121, 47–57. [Google Scholar] [CrossRef]
- Fernández, S.P.; Wasowski, C.; Loscalzo, L.M.; Granger, R.E.; Johnston, G.A.; Paladini, A.C.; Marder, M. Central nervous system depressant action of flavonoid glycosides. Eur. J. Pharmacol. 2006, 539, 168–176. [Google Scholar] [CrossRef]
- Martínez-Flórez, S.; González-Gallego, J.; Culebras, J.M.; Tuñón, M.J. Flavonoids: Properties and antioxidizing action. Nutr. Hosp. 2002, 17, 271–278. [Google Scholar]
- Paladini, A.C.; Marder, M.; Viola, H.; Wolfman, C.; Wasowski, C.; Medina, J.H. Flavonoids and the central nervous system: From forgotten factors to potent anxiolytic compounds. J. Pharm. Pharm. 1999, 51, 519–526. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharm. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef]
- Bakhtiari, M.; Panahi, Y.; Ameli, J.; Darvishi, B. Protective effects of flavonoids against Alzheimer’s disease-related neural dysfunctions. Biomed. Pharmacother. 2017, 93, 218–229. [Google Scholar] [CrossRef]
- Groot, H.; Rauen, U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam. Clin. Pharmacol. 1998, 12, 249–255. [Google Scholar] [CrossRef]
- Santos, E.L.; Maia, B.H.L.N.S.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, biosynthesis and chemical ecology. In Flavonoids—From Biosynthesis to Human Health; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 47–53. [Google Scholar] [CrossRef]
- Brodowska, K. Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Eur. J. Bio Res. 2017, 7, 108–123. [Google Scholar]
- Zhang, L.M.; Wang, H.L.; Zhao, N.; Chen, H.X.; Li, Y.F.; Zhang, Y.Z. Involvement of nitric oxide (NO) signaling pathway in the antidepressant action of the total flavonoids extracted fromXiaobuxin-Tang. Neurosci. Lett. 2014, 575, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.W.; Zhang, S.F.; Li, Z.H.; Han, F. 7,8- Dihydroxyflavone reverses the depressive symptoms in mouse chronic mild stress. Neurosci. Lett. 2016, 635, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, Q.; Xiao, G.; Li, J.; Luo, H.R.; Ye, K. O-methylated metabolite of 7,8-dihydroxyflavone activates TrkB receptor and displays antidepressant activity. Pharmacology 2013, 91, 185–200. [Google Scholar] [CrossRef]
- Kim, H.K.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Amentoflavone, a plant biflavone: A new potential anti-inflammatory agent. Arch. Pharm. Res. 1998, 21, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Ishola, I.; Chatterjee, M.; Tota, S.; Tadigopulla, N.; Adeyemi, O.O.; Palit, G.; Shukla, R. Antidepressant and anxiolytic effects of amentoflavone isolated from Cnestis ferruginea in mice. Pharmacol. Biochem. Behav. 2012, 103, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Nakazawa, T.; Yasuda, T.; Ueda, J.; Ohsawa, K. Antidepressant-like effects of apigenin and 2,4,5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull. 2003, 26, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Qin, T.; Qu, R.; Ma, S. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain. Behav. Brain Res. 2016, 296, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Guo, X.; Li, Y.; Yang, X.; Han, Y. Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice. Eur. J. Pharm. 2016, 774, 50–54. [Google Scholar] [CrossRef]
- Recalde-Gil, M.A.; Klein-Júnior, L.C.; Passos, C.D.S.; Salton, J.; Bordignon, S.A.L.; Monace, F.D.; Cechinel, V.; Teresinha Henriquesa, A. Monoamine oxidase inhibitory activity of biflavonoids from branches of Garcinia gardneriana (Clusiaceae). Nat. Prod. Commun. 2017, 12, 505–508. [Google Scholar] [CrossRef]
- Li, R.P.; Zhao, D.; Qu, R.; Fu, Q.; Ma, S.P. The effects of apigenin on lipopolysaccharide-induced depressive-like behavior in mice. Neurosci. Lett. 2015, 594, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, J.; Gu, J.; Xiong, Z.; Wang, F.; Wang, J.; Wang, W.; Chen, J. Baicalein improves cognitive deficits induced by chronic cerebral hypoperfusion in rats. Pharmacol. Biochem. Behav. 2007, 86, 423–430. [Google Scholar] [CrossRef]
- Shieh, D.E.; Liu, L.T.; Lin, C.C. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000, 20, 2861–2865. [Google Scholar] [PubMed]
- Xiong, Z.; Jiang, B.; Wu, P.-F.; Tian, J.; Shi, L.; Gu, J.; Hu, Z.-L.; Fu, H.; Wang, F.; Chen, J.-G. Antidepressant Effects of a Plant-Derived Flavonoid Baicalein Involving Extracellular Signal-Regulated Kinases Cascade. Biol. Pharm. Bull. 2011, 34, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.; Park, J.; Kim, S.-H.; Kwon, S.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.-H. Chronic Administration of Baicalein Decreases Depression-Like Behavior Induced by Repeated Restraint Stress in Rats. Korean J. Physiol. Pharmacol. 2013, 17, 393–403. [Google Scholar] [CrossRef]
- Li, Y.C.; Shen, J.D.; Li, J.; Wang, R.; Jiao, S.; Yi, L.T. Chronic treatment with baicalin prevents the chronic mild stress-induced depressive-like behavior: Involving the inhibition of cyclooxygenase-2 in rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 138–143. [Google Scholar] [CrossRef]
- Cho, H.; Yun, C.W.; Park, W.K.; Kong, J.Y.; Kim, K.S.; Park, Y.; Lee, S.; Kim, B.K. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharm. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef]
- Lapidot, T.; Walker, M.D.; Kanner, J. Antioxidant and prooxidant effects of phenolics on pancreatic β-cells in vitro. J. Agric. Food Chem. 2002, 50, 7220–7225. [Google Scholar] [CrossRef] [PubMed]
- Zarzecki, M.S.; Araujo, S.M.; Bortolotto, V.C.; Paula, M.T.; Jesse, C.R.; Prigol, M. Hypolipidemic action of chrysin on triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicol. Rep. 2014, 1, 200–208. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Giacomeli, R.; Del Fabbro, L.; da Silva Antunes, M.; de Gomes, M.G.; Goes, A.T.; Boeira, S.P.; Prigol, M.; et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+,K+- ATPase activity in the hippocampus and prefrontal cortex of mice: Antidepressant effect of chrysin. Neuroscience 2015, 289, 367–380. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; Gomes de Gomes, M.; Rossito Goes, A.T.; Souza, L.C.; Boeira, S.P. Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice. Chem. Biol. Interact. 2016, 260, 154–162. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.R.; Souza, L.C.; Giacomeli, R.; Antunes, M.; Luchese, C.; et al. Neurochemical factors associated with the antidepressant-like effect of flavonoid chrysin in chronically stressed mice. Eur. J. Pharm. 2016, 791, 284–296. [Google Scholar] [CrossRef]
- Ishisaka, M.; Kakefuda, K.; Yamauchi, M.; Tsuruma, K.; Shimazawa, M.; Tsuruta, A.; Hara, H. Luteolin shows an antidepressant-like effect via suppressing endoplasmic reticulum stress. Bio Pharm Bull. 2011, 34, 1481–1486. [Google Scholar] [CrossRef]
- de la Peña, J.B.; Kim, C.A.; Lee, H.L.; Yoon, S.Y.; Kim, H.J.; Hong, E.Y.; Kim, G.H.; Ryu, J.H.; Lee, Y.S.; Kim, K.M.; et al. Luteolin mediates the antidepressant-like effects of Cirsium japonicum in mice, possibly through modulation of the GABA-A receptor. Arch. Pharm. Res. 2014, 37, 263–269. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Yamakuni, T.; Hashimoto, M.; Haque, A.M.; Shido, O.; Mimaki, Y.; Sashida, Y.; Ohizumi, Y. Nobiletin restoring β-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer’s disease model rats. Neurosci. Lett. 2006, 400, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Xu, H.L.; Feng, J.; Zhan, X.; Zhou, L.P.; Cui, C.C. Involvement of monoaminergic systems in the antidepressant-like effect of nobiletin. Physio. Behav. 2011, 102, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Ling, A.P.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A review on medicinal properties of orientin. Adv. Pharm. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, N.; Ren, J.; Wu, Y.; Wang, S.T.; Huang, X.F.; Yu, Y. Orientin improves depression like behaviour and BDNF in chronic stressed mice. Mol. Nutr. Food Res. 2015, 59, 1130–1142. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.E.; Cao, J.; Zeng, G.; Shen, C.; Li, Y.; Zhou, M.; Chen, Y.; Pu, W.; Potters, L.; et al. Vitexins, nature-derived lignin compounds, induces apoptosis and suppress tumor growth. Clin. Cancer Res. 2009, 15, 5161–5169. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, Z.; Cheng, K.-W.; Shan, F.; Ren, G.-X.; Chen, F.; Wang, M. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem. 2008, 106, 475–481. [Google Scholar] [CrossRef]
- Prabhakar, M.; Bano, H.; Kumar, I.; Shamsi, M.; Khan, S. Pharmacological investigations on vitexin. Planta Med. 1981, 43, 396–403. [Google Scholar] [CrossRef]
- Gorzalczany, S.; Marrassini, C.; Miño, J.; Acevedo, C.; Ferraro, G. Antinociceptive activity of ethanolic extract and isolated compounds of Urtica circularis. J. Ethnopharmacol. 2011, 134, 733–738. [Google Scholar] [CrossRef]
- Kim, J.; Lee, I.; Seo, J.; Jung, M.; Kim, Y.; Yim, N.; Bae, K. Vitexin, orientin and other flavonoids from Spirodela polyrhiza inhibit adipogenesis in 3T3-L1 cells. Phytother. Res. 2010, 24, 1543–1548. [Google Scholar] [CrossRef]
- Afifi, F.U.; Abu-Dahab, R. Phytochemical screening and biological activities of Eminium spiculatum (Blume) Kuntze (family Araceae). Nat. Prod. Res. 2012, 26, 878–882. [Google Scholar] [CrossRef]
- Li, H.; Song, F.; Xing, J.; Tsao, R.; Liu, Z.; Liu, S. Screening and structural characterization of α-glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LCDAD- MSn and SORI-CID FTICR MS. J. Am. Soc. Mass Spectrom. 2009, 20, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; El-Shafae, A.M.; Al-Taweel, A.; Fawzy, G.A.; Malik, A.; Afza, N.; Latif, M.; Iqbal, L. Antioxidant and urease inhibitory C-glycosylflavonoids from Celt. Afr. J. Asian Nat. Prod. Res. 2011, 13, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Can, O.D.; Ozkay, U.D.; Uçel, U.I. Anti-depressantlike effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. Eur. J. Pharm. 2013, 699, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Ogawa, M.; Amakura, Y.; Yoshimura, M.; Okuyama, S.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone reduces interleukin-4 production in the spleen cells of mice. Biomed. Res. 2016, 37, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Morita, M.; Miyoshi, K.; Nishigawa, Y.; Kaji, M.; Sawamoto, A.; Terugo, T.; Toyoda, N.; Makihata, N.; Amakura, Y.; et al. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a citrus flavonoid, on protection against memory impairment and neuronal cell death in a global cerebral ischemia mouse model. Neurochem. Int. 2014, 70, 30–38. [Google Scholar] [CrossRef]
- Sawamoto, A.; Okuyama, S.; Yamamoto, K.; Amakura, Y.; Yoshimura, M.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a citrus flavonoid, ameliorates corticosterone-induced depression-like behavior and restores brain-derived neurotrophic factor expression, neurogenesis, and neuroplasticity in the hippocampus. Molecules 2016, 21, 541. [Google Scholar] [CrossRef]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Brash, D.E.; Havre, P.A. New careers for antioxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 13969–13971. [Google Scholar] [CrossRef]
- Zhen, L.; Zhu, J.; Zhao, X.; Huang, W.; An, Y.; Li, S.; Du, X.; Lin, M.; Wang, Q.; Xu, Y.; et al. The antidepressant-like effect of fisetin involves the serotonergic and noradrenergic system. Behav. Brain Res. 2012, 228, 359–366. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, X.; Zhang, X.; Chen, Z.; Xu, L.; Chen, L.; Wang, G.; Pan, J. The effects of fisetin on lipopolysaccharide-induced depressive-like behavior in mice. Metab Brain Dis. 2016, 31, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Lu, J.; Shi, H.; Gong, S.; Wang, Y.; Hamdy, R.C.; Chua, B.H.L.; Yang, L.; Xu, X. Fisetin provides antidepressant effects by activating the tropomyosin receptor kinase B signal pathway in mice. J. Neurochem. 2017, 143, 561–568. [Google Scholar] [CrossRef]
- Park, J.Y.; Han, X.; Piao, M.J.; Oh, M.C.; Fernando, P.M.; Kang, K.A.; Ryu, Y.S.; Jung, U.; Kim, I.G.; Hyun, J.W. Hyperoside induces endogenous antioxidant system to alleviate oxidative stress. J. Cancer Prev. 2016, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Liu, C.; Pan, F.; Shi, D.; Zhang, Y. Antidepressant-like effect of hyperoside isolated from Apocynum venetum leaves: Possible cellular mechanisms. Phytomedicine 2012, 19, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.S.; Stolz, E.D.; Betti, A.H.; Stein, A.C.; Schripsema, J.; Poser, G.L.; Rates, S.M. The anti-immobility effect of hyperoside on the forced swimming test in rats is mediated by the D2-like receptors activation. Planta Med. 2011, 77, 334–339. [Google Scholar] [CrossRef]
- Butterweck, V.; Hegger, M.; Winterhoff, H. Flavonoids of St. John’sWort reduce HPAaxis function in the rat. Planta Med. 2004, 70, 1008–1011. [Google Scholar] [CrossRef]
- Li, H.F.; Guan, X.Y.; Yang, W.Z.; Liu, K.D.; Ye, M.; Sun, C.; Lu, S.; Guo, D.A. Antioxidant flavonoids from Epimedium wushanense. Fitoterapia 2012, 83, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, C.; Wu, X.; Liu, F.; Du, Y.; Sun, J.; Tao, J.; Dong, J. Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience 2015, 294, 193–205. [Google Scholar] [CrossRef]
- Wu, X.; Wu, J.; Xia, S.; Li, B.; Dong, J. Icaritin opposes the development of social aversion after defeat stress via increases of GR mRNA and BDNF mRNA in mice. Behav. Brain Res. 2013, 256, 602–608. [Google Scholar] [CrossRef]
- Wei, K.; Xu, Y.; Zhao, Z.; Wu, X.; Du, Y.; Sun, J.; Yi, T.; Dong, J.; Liu, B. Icariin alters the expression of glucocorticoid receptor, FKBP5 and SGK1 in rat brains following exposure to chronic mild stress. Int. J. Mol. Med. 2016, 38, 337–344. [Google Scholar] [CrossRef]
- Gong, M.J.; Han, B.; Wang, S.M.; Liang, S.W.; Zou, Z.J. Icariin reverses corticosterone-induced depression-like behavior, decrease in hippocampal brain-derived neurotrophic factor (BDNF) andmetabolic network disturbances revealed by NMR based metabonomics in rats. J. Pharm. Biomed. Anal. 2016, 123, 63–73. [Google Scholar] [CrossRef]
- Scheggi, S.; Marandino, A.; Del Monte, D.; DE Martino, L.; Pelliccia, T.; Fusco, M.D.R.; Petenatti, E.M.; Gambarana, C.; De Feo, V. The protective effect of Hypericum connatum on stress-induced escape deficit in rat is related to its flavonoid content. Pharm. Bio. 2016, 54, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Yamaguchi, S.; Shirai, M.; Miyoshi, M.; Moon, J.H.; Oshima, S.; Inakuma, T.; Tsushida, T.; Kato, Y. Protection by quercetin and quercetin 3-O-beta-D-glucuronide of peroxynitrite-induced antioxidant consumption in human plasma low-density lipoprotein. Free Radic Res. 2001, 35, 925–931. [Google Scholar] [CrossRef]
- Cid-Ortega, S.; Monroy-Rivera, J.A. Extraction of kaempferol and its glycosides using supercritical fluids from plant sources: A review. Food Tech. Biotechnol. 2018, 56, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Cassani, J.; Dorantes-Barrón, A.; Novales, L.; Real, G.; Estrada-Reyes, R. Anti-depressant-like effect of kaempferitrin isolated from Justicia spicigera Schltdl (Acanthaceae) in two behavior models in mice: Evidence for the involvement of the serotonergic system. Molecules 2014, 19, 21442–21461. [Google Scholar] [CrossRef]
- Rashid, M.I.; Fareed, M.I.; Rashid, H.; Aziz, H.; Ehsan, N.; Khalid, S.; Ghaffar, I.; Ali, R.; Gul, A.; Hakeem, K.R. Flavonoids and Their Biological Secrets. Plant. Hum. Health 2019, 2, 579–605. [Google Scholar]
- Park, S.; Sim, Y.; Han, P.; Lee, J.; Suh, H. Antidepressantlike effect of kaempferol and quercitirin, isolated from Opuntia ficus-indica var.Saboten. Exp. Neurobiol. 2010, 19, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.-X.; Lang, J.-L.; Song, Y.-Y.; Wu, Y.-Z.; Lv, M.-H.; Zhao, X.; Liu, Y.-H.; Xu, C.-Y. Studies on anti-depressant activity of four flavonoids isolated from Apocynum venetum Linn (Apocynaceae) leaf in mice. Trop. J. Pharm. Res. 2015, 14, 2269–2277. [Google Scholar] [CrossRef]
- Park, K.S.; Chong, Y.; Kim, M.K. Myricetin: Biological activity related to human health. Appl. Biol. Chem. 2016, 59, 259–269. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. 2020, 20, 241–246. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, G.; Cui, L.; Wang, Q. Myricetin attenuates depressant-like behavior in mice subjected to repeated restraint stress. Int. J. Mol. Sci. 2015, 16, 28377–28385. [Google Scholar] [CrossRef]
- Domitrović, R.; Rashed, K.; Cvijanović, O.; Vladimir-Knežević, S.; Škoda, M.; Višnić, A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem. Biol. Interact. 2015, 230, 21–29. [Google Scholar] [CrossRef]
- Meyer, E.; Mori, M.A.; Campos, A.C.; Andreatini, R.; Guimarães, F.S.; Milani, H.; de Oliveira, R.M.W. Myricitrin induces antidepressant-like effects and facilitates adult neurogenesis in mice. Behav. Brain Res. 2017, 316, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrition 2016, 8, 167–172. [Google Scholar] [CrossRef]
- Demir, E.A.; Gergerlioglu, H.S.; Oz, M. Antidepressant like effects of quercetin in diabetic rats are independent of hypothalamic-pituitary-adrenal axis. Acta Neuropsychiatr. 2016, 28, 23–30. [Google Scholar] [CrossRef]
- Rinwa, P.; Kumar, A. Quercetin suppress microglial neuroinflammatory response and induce antidepressent-like effect in olfactory bulbectomized rats. Neuroscience 2013, 255, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Arasu, M.V.; Park, C.H.; Park, S.U. An up-to-date review of rutin and its biological and pharmacological activities. Excli. J. 2015, 14, 59–63. [Google Scholar]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Tech. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.-X.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A therapeutic agent for obesity. Drug Des. Dev. 2019, 13, 3855–3866. [Google Scholar] [CrossRef]
- Hajialyani, M.; Farzaei, M.H.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a neuroprotective agent: A review of animal and clinical evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef]
- Mao-Qiang, M.; Yang, B.; Elias, P.M. Benefits of hesperidin for cutaneous functions. Evid. Based Complement. Altern. Med. 2019, 2019, 2676307. [Google Scholar]
- Nardarajah, D. Hesperidin-A short Review. Res. J. Pharm. Technol. 2014, 7, 78–80. [Google Scholar]
- El-Marasy, S.A.; Abdallah, H.M.I.; El-Shenawy, S.M.; El-Khatib, A.S.; El-Shabrawy, O.A.; Kenawy, S.A. Anti-depressant effect of hesperidin in diabetic rats. Can. J. Physiol. Pharm. 2014, 92, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Filho, C.B.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.; Souza, L.C.; Boeira, S.P.; Jesse, C.R. Kappa-opioid receptors mediate the antidepressant-like activity of hesperidin in the mouse forced swimming test. Eur. J. Pharm. 2013, 698, 286–291. [Google Scholar] [CrossRef]
- Souza, L.C.; de Gomes, M.G.; Goes, A.T.; Del Fabbro, L.; Carlos Filho, B.; Boeira, S.P.; Jesse, C.R. Evidence for the involvement of the serotonergic 5-HT(1A) receptors in the antidepressant-like effect caused by hesperidin in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Liou, S.S.; Hong, T.Y.; Liu, I.M. Hesperidin prevents high glucose-induced damage of retinal pigment epithelial cells. Planta Med. 2018, 84, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-F.; Chen, S.-M.; Chen, X.-M.; Mu, R.-H.; Wang, S.-S.; Geng, D.; Liu, Q.; Yi, L.-T. ERK-dependent brain-derived neurotrophic factor regulation by hesperidin in mice exposed to chronic mild stress. Brain Res. Bull. 2016, 124, 40–47. [Google Scholar] [CrossRef]
- Donato, F.; de Gomes, M.G.; Goes, A.T.; Filho, C.B.; Del Fabbro, L.; Antunes, M.S.; Souza, L.C.; Boeira, S.P.; Jesse, C.R. Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: Possible role of l-arginine-NO-cGMP pathway and BDNF levels. Brain Res. Bull. 2014, 104, 19–26. [Google Scholar] [CrossRef]
- González-Cortazar, M.; Maldonado-Abarca, A.M.; Jiménez-Ferrer, E.; Marquina, S.; Ventura-Zapata, E.; Zamilpa, A.; Tortoriello, J.; Herrera-Ruiz, M. Isosakuranetin-5-O-rutinoside: A new flavanone with antidepressant activity isolated from Salvia elegans vahl. Molecules 2013, 18, 13260–13270. [Google Scholar] [CrossRef]
- Wang, W.; Hu, X.; Zhao, Z.; Liu, P.; Hu, Y.; Zhou, J.; Zhou, D.; Wang, Z.; Guo, D.; Guo, H. Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1179–1184. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Roghani, M. Effect of naringenin on intracerebroventricular streptozotocin-induced cognitive deficits in rat: A behavioral analysis. Pharmacology 2006, 78, 193–197. [Google Scholar] [CrossRef]
- Olsen, H.T.; Stafford, G.I.; Staden, J.V.; Christensen, S.B.; Jäger, A.K. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. J. Ethnopharmacol. 2008, 117, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Muthaiah, V.P.; Venkitasamy, L.; Michael, F.M.; Chandrasekar, K.; Venkatachalam, S. Neuroprotective role of naringenin on carbaryl induced neurotoxicity in mouse neuroblastoma cells. J. Pharm. Pharm. 2013, 4, 192–197. [Google Scholar]
- Yi, L.-T.; Liu, B.-B.; Li, J.; Luo, L.; Liu, Q.; Geng, D.; Tang, Y.; Xia, Y.; Wu, D. BDNF signaling is necessary for the antidepressant-like effect of naringenin. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Li, C.F.; Zhan, X.; Cui, C.C.; Xiao, F.; Zhou, L.P.; Xie, Y. Involvement of monoaminergic system in the antidepressant-like effect of the flavonoid naringenin in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1223–1228. [Google Scholar] [CrossRef]
- Yi, L.-T.; Li, J.; Li, H.-C.; Su, D.-X.; Quan, X.-B.; He, X.-C.; Wang, X.-H. Antidepressant-like behavioral, neurochemical and neuroendocrine effects of naringenin in the mouse repeated tail suspension test. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 175–181. [Google Scholar] [CrossRef]
- Suseem, S.R.; Joseph, D. The myth and the fact on naringin—Areview. Res. J. Pharm. Tech. 2019, 12, 367–374. [Google Scholar] [CrossRef]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic potential of naringin: An overview. Pharm Biol 2016, 54, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, M.; Jangra, A.; Mishra, M.; Sharma, Y.; Ahmed, S.; Ghosh, P.; Kumar, V.; Vohora, D.; Khanam, R. Naringin and sertraline ameliorate doxorubicin-induced behavioral deficits throughmodulation of serotonin level and mitochondrial complexes protection pathway in rat hippocampus. Neurochem. Res. 2016, 41, 2352–2366. [Google Scholar] [CrossRef] [PubMed]
- Naqinezhad, A.; Nabavi, S.; Nabavi, S.; Ebrahimzadeh, M. Antioxidant and antihemolytic activities of flavonoid rich fractions of Artemisia tschernieviana Besser. Eur. Rev. Med. Pharm. Sci. 2012, 16, 88–94. [Google Scholar]
- Petacci, F.; Freitas, S.S.; Brunetti, I.L.; Khalil, N.M. Inhibition of peroxidase activity and scavenging of reactive oxygen species by astilbin isolated from Dimorphandra mollis (Fabaceae, Caesalpinioideae). Biol. Res. 2010, 43, 63–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lv, Q.Q.; Wu, W.J.; Guo, X.L.; Liu, R.L.; Yang, Y.P.; Zhou, D.S.; Zhang, J.X.; Liu, J.Y. Antidepressant activity of astilbin: Involvement of monoaminergic neurotransmitters and BDNF signal pathway. Biol. Pharm. Bull. 2014, 37, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Yan, P.; Zhu, L.; Yang, H.; Zhao, Y.; Kirby, B.P.; Waddington, J.L.; Zhen, X. Dihydromyricetin exerts a rapid antidepressant-like effect in association with enhancement of BDNF expression and inhibition of neuroinflammation. Psychopharmacology 2018, 235, 233–244. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Lee, S.A.; Hong, S.S.; Lee, K.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Monoamine oxidase inhibitory components from the roots of Sophora flavescens. Arch. Pharmacal. Res. 2005, 28, 190–194. [Google Scholar] [CrossRef]
- Javelot, H.; Messaoudi, M.; Jacquelin, C.; Violle, N.; Bisson, J.F.; Nejdi, A.; Rozan, P.; Desor, D. Antidepressant-like properties of cocoa’s polyphenols-The role of flavanoids and flavanols on depression. Agro. Food Ind. Hi-Tech. 2009, 20, 19–21. [Google Scholar]
- Zafir, A.; Ara, A.; Banu, N. In vivo antioxidant status: A putative target of antidepressant action. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 220–228. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.G.; Fang, D.; Le, W.D. (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J. Neurosci. Res. 2008, 78, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Dixit, N.; Baboota, S.; Kohli, K.; Ahmad, S.; Ali, J. Silymarin: A review of pharmacological aspects and bioavailability enhancement approaches. Indian J. Pharm. 2007, 39, 172–179. [Google Scholar] [CrossRef]
- Thakare, V.N.; Aswar, M.K.; Kulkani, Y.P.; Patil, R.R.; Patel, B.M. Silymarin ameliorates experimentally induced depressive like behavior in rats: Involvement of hippocampal BDNF signaling, inflammatory cytokines and oxidative stress response. Physio. Behav. 2017, 179, 401–410. [Google Scholar] [CrossRef]
- Hritcu, L.; Ionita, R.; Postu, P.A.; Gupta, G.K.; Turkez, H.; Lima, T.C.; Carvalho, C.U.S.; de Sousa, D. Antidepressant Flavonoids and Their Relationship with Oxidative Stress. Oxidative Med. Cell. Longev. 2017, 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-H.; Kim, S.-K.; Lee, S.-Y.; Jang, C.-G. Flavonoids as therapeutic candidates for emotional disorders such as anxiety and depression. Arch. Pharmacal Res. 2020, 43, 1128–1143. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. Flavonoids and brain health: Multiple effects underpinned by common mechanisms. Genes Nutr. 2009, 4, 243–250. [Google Scholar] [CrossRef]
- Hasler, G. Pathophysiology of depression: Do we have any solid evidence of interest to clinicians? World J. Psychiatry 2010, 9, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.J. Relationship of neurotransmitters to the symptoms of major depressive disorder. J. Clin. Psychiatry 2008, 69, 4–7. [Google Scholar]
- Aan het Rot, M.; Mathew, S.J.; Charney, D.S. Neurobiological mechanisms in major depressive disorder. CMAJ 2009, 180, 305–313. [Google Scholar] [CrossRef]
- Brigitta, B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin. Neurosci. 2002, 4, 7–20. [Google Scholar] [PubMed]
- Kumar, G.P.; Khanum, F. Neuroprotective potential of phytochemicals. Pharm. Rev. 2012, 6, 81–90. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress and the central nervous system. J. Pharm. Exp. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Kuhad, A. Mitochondrial dysfunction in depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Gates, K.S. An overview of chemical processes that damage cellular DNA: Spontaneous hydrolysis, alkylation, and reactions with radicals. Chem Res. Toxicol. 2009, 22, 1747–1760. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic. Biol. Med. 2006, 40, 376–387. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharm. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural. Regen Res. 2013, 8, 2003–2014. [Google Scholar]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Afanasév, I. Signaling of reactive oxygen and nitrogen species in Diabetes mellitus. Oxid. Med. Cell Longev. 2010, 3, 361–373. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Closa, D.; Folch-Puy, E. Oxygen free radicals and the systemic inflammatory response. IUBMB Life 2004, 56, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71–77. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective effect of antioxidants in the brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Qian, S.Y.; Buettner, G.R. Iron and free radical oxidations in cell membranes. Cell. Mol. Biol. 2000, 46, 657–662. [Google Scholar] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.C.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mishra, A.; Pandey, A.K. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement. Altern. Med. 2013, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Phenolic content, reducing power and membrane protective activities of Solanum xanthocarpum root extracts. Vegetos 2013, 26, 301–307. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, A.; Pandey, A.K. Calotropis procera root extract has capability to combat free radical mediated damage. ISRN Pharm. 2013, 2013, 691372. [Google Scholar] [CrossRef]
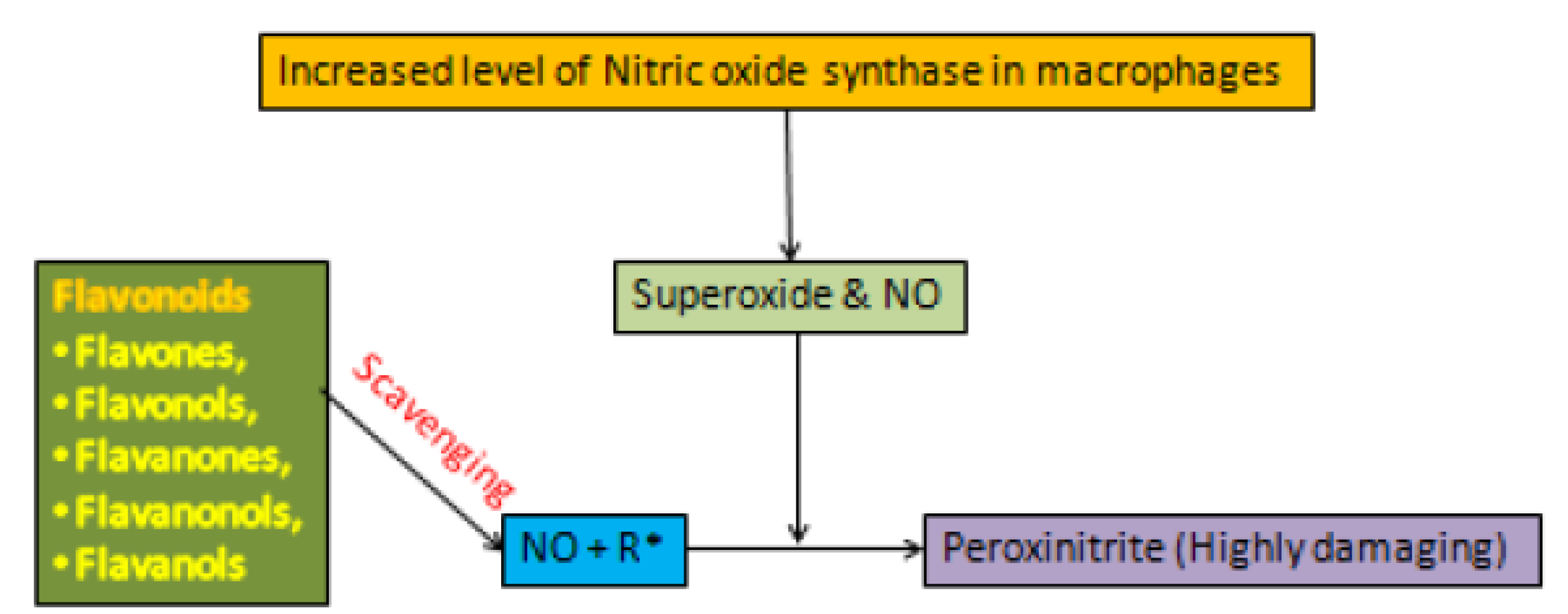
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed]
- Bloodsworth, A.; O’Donnell, V.B.; Freeman, B.A. Nitric oxide regulation of free radical- and enzyme-mediated lipid and lipoprotein oxidation. Arter. Thromb. Vasc. Biol. 2000, 20, 1707–1715. [Google Scholar] [CrossRef]
- Tiedge, M.; Lortz, S.; Munday, R.; Lenzen, S. Complementary action of antioxidant enzymes in the protection of bioengineered insulin-producing RINm5F cells against the toxicity of reactive oxygen species. Diabetes 1998, 47, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. Med. J. 2018, 54, 287–293. [Google Scholar] [CrossRef]
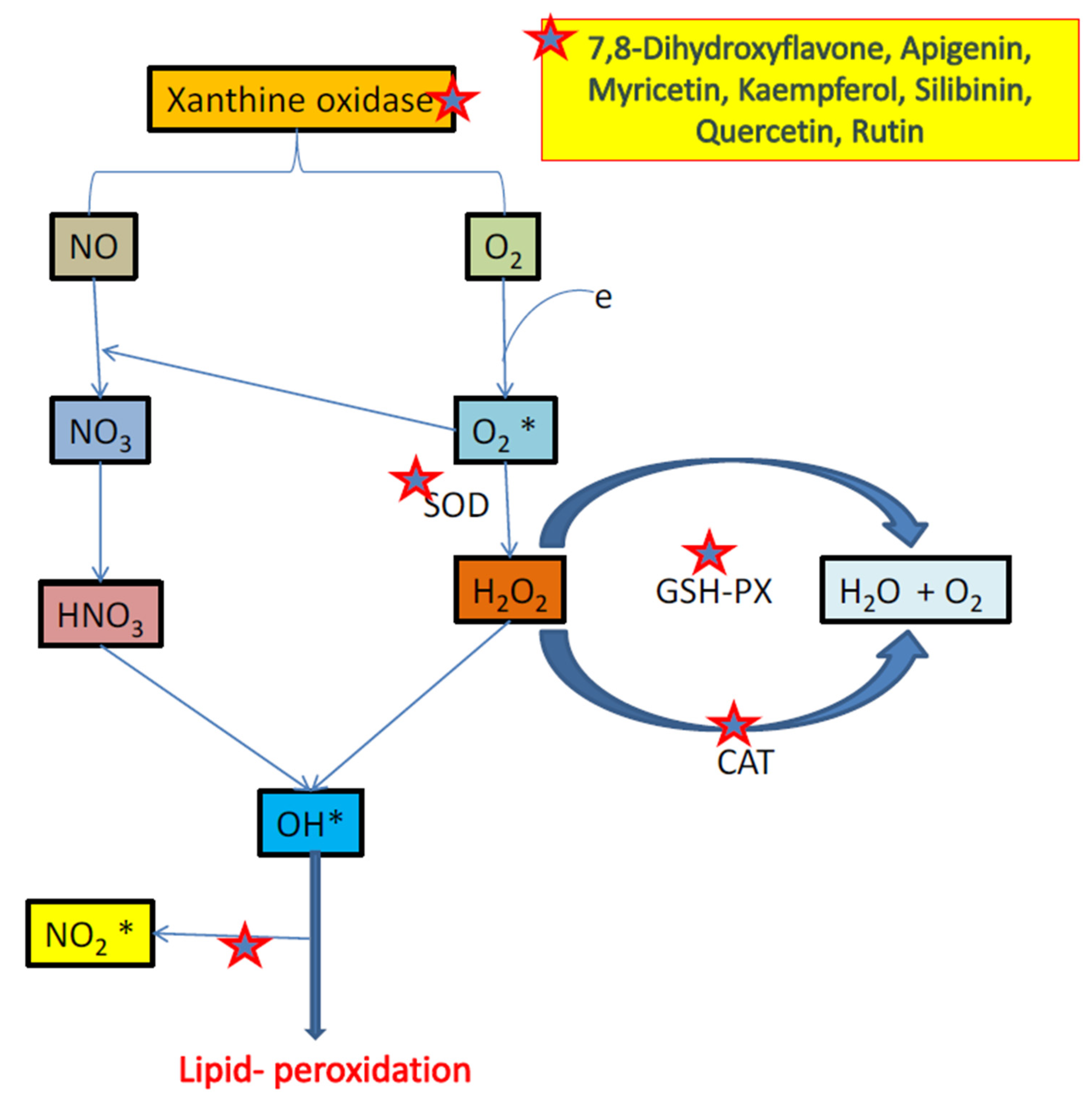
- Pacher, P.; Nivorozhkin, A.; Szabó, C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharm. Rev. 2006, 58, 87–114. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.M.; Camara, S.; Tatschner, T.; Frangou, S.; Sheldrick, A.J.; Riederer, P.; Grünblatt, E. Increased xanthine oxidase in the thalamus and putamen in depression. World J. Biol. Psychiatry 2010, 11, 314–320. [Google Scholar] [CrossRef]
- Herken, H.; Gurel, A.; Selek, S.; Armutcu, F.; Ozen, M.E.; Bulut, M.; Kap, O.; Yumru, M.; Savas, H.A.; Akyol, O. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: Impact of antidepressant treatment. Arch. Med. Res. 2007, 38, 247–252. [Google Scholar] [CrossRef]
- Vaváková, M.; Ďuračková, Z.; Trebatická, J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid. Med. Cell Longev. 2015, 2015, 898393. [Google Scholar] [CrossRef]
- Kostić, D.A.; Dimitrijević, D.S.; Stojanović, G.S.; Palić, I.R.; Đorđević, A.S.; Ickovski, J.D. Xanthine oxidase: Isolation, assays of activity, and inhibition. J. Chem. 2015, 2015, 294858. [Google Scholar] [CrossRef]
- Hudaib, M.M.; Tawaha, K.A.; Mohammad, M.K.; Assaf, A.M.; Issa, A.Y.; Alali, F.Q.; Aburjai, T.A.; Bustanji, Y.K. Xanthine oxidase inhibitory activity of the methanolic extracts of selected Jordanian medicinal plants. Pharmacogn. Mag. 2011, 7, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Iio, M.; Ono, Y.; Kai, S.; Fukumoto, M. Effects of flavonoids on xanthine oxidation as well as on cytochrome c reduction by milk xanthine oxidase. J. Nutr. Sci. Vitam. 1986, 32, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Lee, Y.J.; Lu, F.J.; Chiang, H.C. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993, 13, 2165–2170. [Google Scholar]
- Pauff, J.M.; Hille, R. Inhibition studies of bovine xanthine oxidase by luteolin, silibinin, quercetin, and curcumin. J. Nat. Prod. 2009, 72, 725–731. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Y.; Kuang, S.; Yang, Y.; Tian, X.; Ma, J.; Mai, S.; Xue, L.; Yang, J. Cyclooxygenase-2 Signalling Pathway in the Cortex is Involved in the Pathophysiological Mechanisms in the Rat Model of Depression. Sci. Rep. 2017, 7, 488. [Google Scholar] [CrossRef] [PubMed]
- Müller, N. COX-2 inhibitors as antidepressants and antipsychotics: Clinical evidence. Curr. Opin. Investig. Drugs 2010, 11, 31–42. [Google Scholar]
- Sethi, R.; Gómez-Coronado, N.; Walker, A.J.; Robertson, O.D.; Agustini, B.; Berk, M.; Dodd, S. Neurobiology and therapeutic potential of cyclooxygenase-2 (cox-2) inhibitors for inflammation in neuropsychiatric disorders. Front. Psychiatry 2019, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Schwarz, M.J. COX-2 inhibition in schizophrenia and major depression. Curr. Pharm. Des. 2008, 14, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.; Kondev, V.; Bedse, G.; Baldi, R.; Marcus, D.; Patel, S. Cyclooxygenase-2 inhibition reduces anxiety-like behavior and normalizes enhanced amygdala glutamatergic transmission following chronic oral corticosterone treatment. Neurobiol. Stress 2019, 11, 100190. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharm. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Nagahara, A.H.; Tuszynski, M.H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011, 10, 209–219. [Google Scholar] [CrossRef]
- Liu, P.Z.; Nusslock, R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef]
- Dwivedi, Y. Brain-derived neurotrophic factor: Role in depression and suicide. Neuropsychiatr. Dis. Treat. 2009, 5, 433–449. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, Y.K. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010, 7, 231–235. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Z.Y. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharm. Sin. 2011, 32, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Razzoli, M.; Domenici, E.; Carboni, L.; Rantamaki, T.; Lindholm, J.; Castrén, E.; Arban, R. A role for BDNF/TrkB signaling in behavioral and physiological consequences of social defeat stress. Genes Brain Behav. 2011, 10, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Nabeshima, T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharm. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef]
- Liu, W.; Kinnefors, A.; Boström, M.; Rask-Andersen, H. Expression of TrkB and BDNF in human cochlea-an immunohistochemical study. Cell Tissue Res. 2011, 345, 213–221. [Google Scholar] [CrossRef]
- Jin, W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s disease. J. Clin. Med. 2020, 9, 257. [Google Scholar] [CrossRef]
- Ginsberg, S.D.; Malek-Ahmadi, M.H.; Alldred, M.J.; Chen, Y.; Chen, K.; Chao, M.V.; Counts, S.E.; Mufson, E.J. Brain-derived neurotrophic factor (BDNF) and TrkB hippocampal gene expression are putative predictors of neuritic plaque and neurofibrillary tangle pathology. Neurobiol. Dis. 2019, 132, 104540. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Wang, Y.; Sun, Z. The relationships between stress, mental disorders, and epigenetic regulation of BDNF. Int. J. Mol. Sci. 2020, 21, 1375. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. Animal models of depression: Molecular perspectives. Curr. Top. Behav. Neurosci. 2011, 7, 121–147. [Google Scholar]
- Phillips, C. Brain-derived neurotrophic factor, depression, and physical activity: Making the neuroplastic connection. Neural. Plast. 2017, 2017, 7260130. [Google Scholar] [CrossRef]
- Björkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef]
- Zhou, C.; Zhong, J.; Zou, B.; Fang, L.; Chen, J.-J.; Deng, X.; Zhang, L.; Zhao, X.; Qu, Z.; Lei, Y.; et al. Meta-analyses of comparative efficacy of antidepressant medications on peripheral BDNF concentration in patients with depression. PLoS ONE 2017, 12, e0172270. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A.; Singh, D. Dietary flavonoids interaction with CREB-BDNF pathway: An unconventional approach for comprehensive management of epilepsy. Curr. Neuropharmacol. 2019, 17, 1158–1175. [Google Scholar] [CrossRef] [PubMed]
- Neshatdoust, S.; Saunders, C.; Castle, S.M.; Vauzour, D.; Williams, C.; Butler, L.; Lovegrove, J.A.; Spencer, J.P. High-flavonoid intake induces cognitive improvements linked to changes in serum brain-derived neurotrophic factor: Two randomised, controlled trials. Nutr. Healthy Aging 2016, 4, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, I.; Silva, L.M.; Corrêa da Silva, J.A.; Steimbach, V.M.; De Souza, M.M. Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharm. Biochem. Behav. 2015, 136, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.S.; Jesse, C.R.; Ruff, J.R.; Espinosa, D.D.O.; Gomes, N.S.; Altvater, E.E.T.; Donato, F.; Giacomeli, R.; Boeira, S.P. Hesperidin reverses cognitive and depressive disturbances induced by olfactory bulbectomy in mice by modulating hippocampal neurotrophins and cytokine levels and acetylcholinesterase activity. Eur. J. Pharmacol. 2016, 789, 411–420. [Google Scholar] [CrossRef] [PubMed]
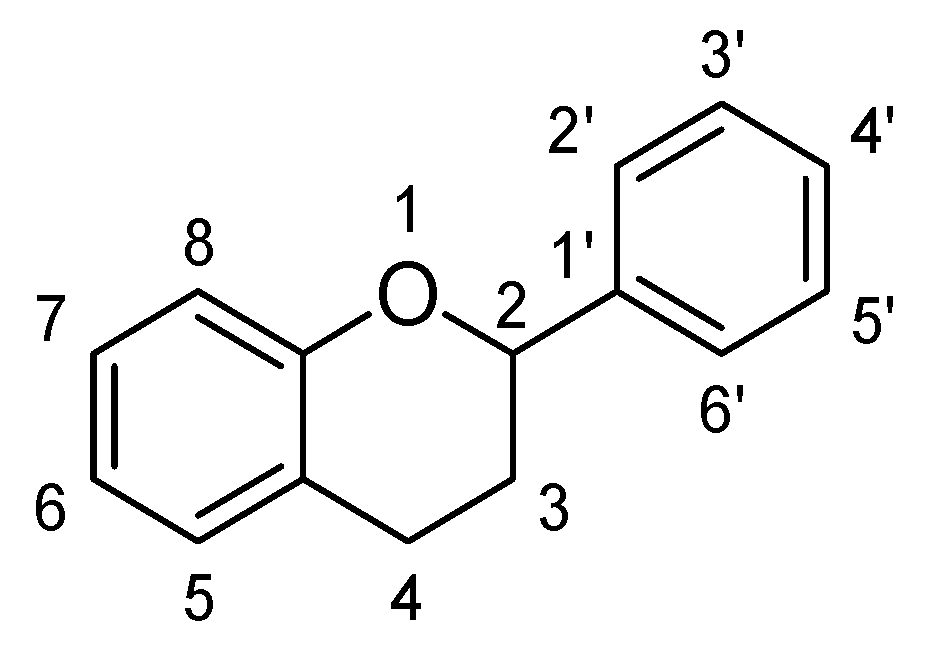
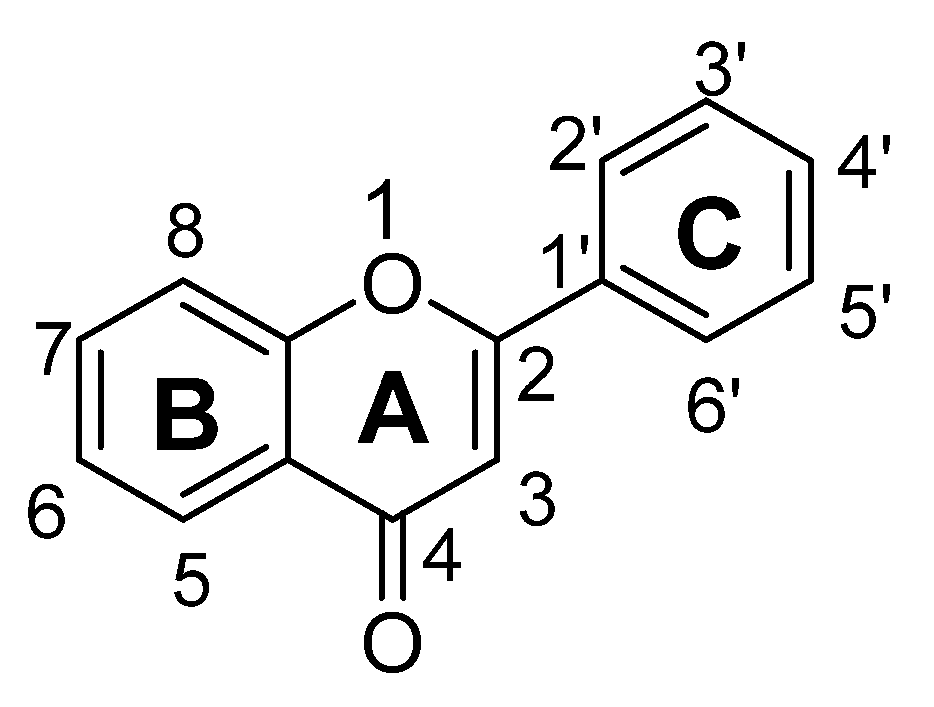
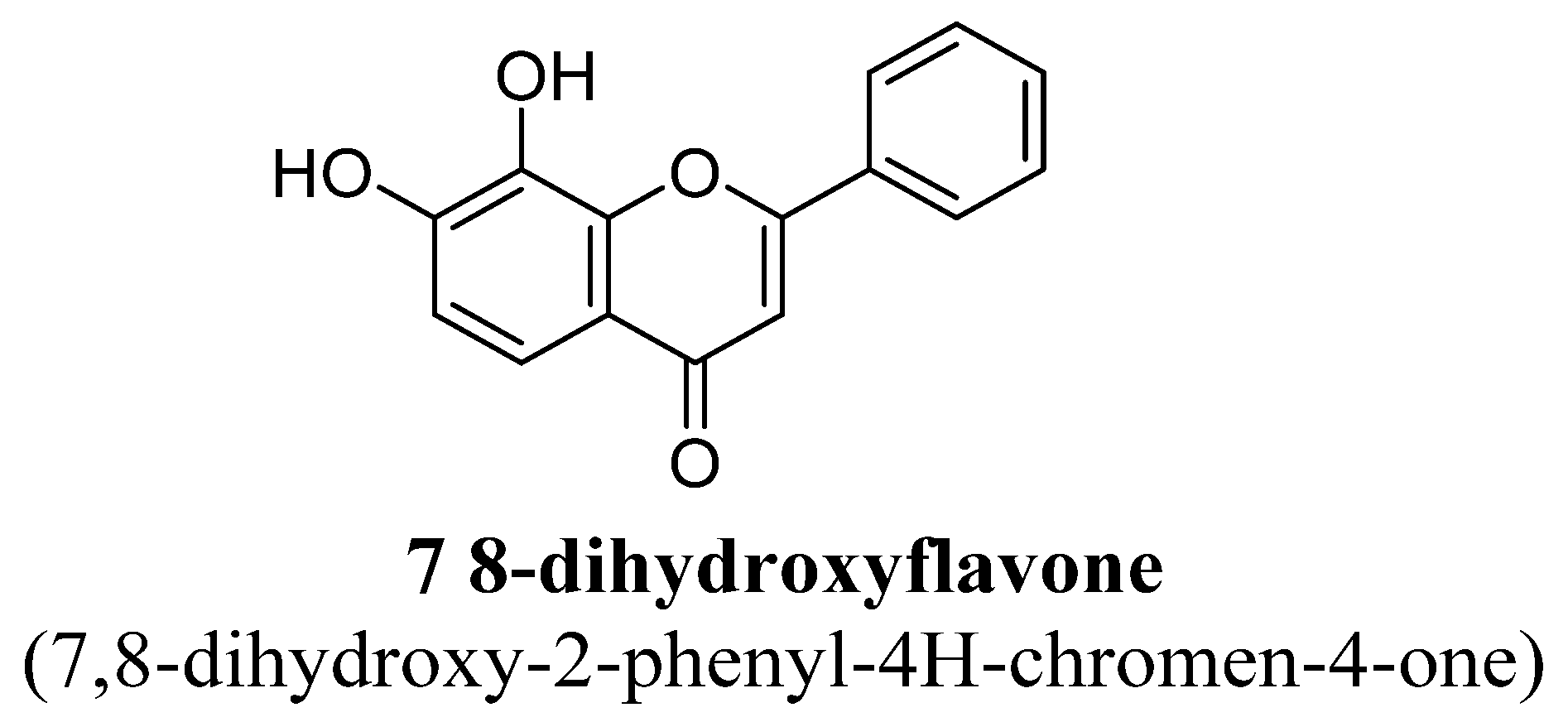
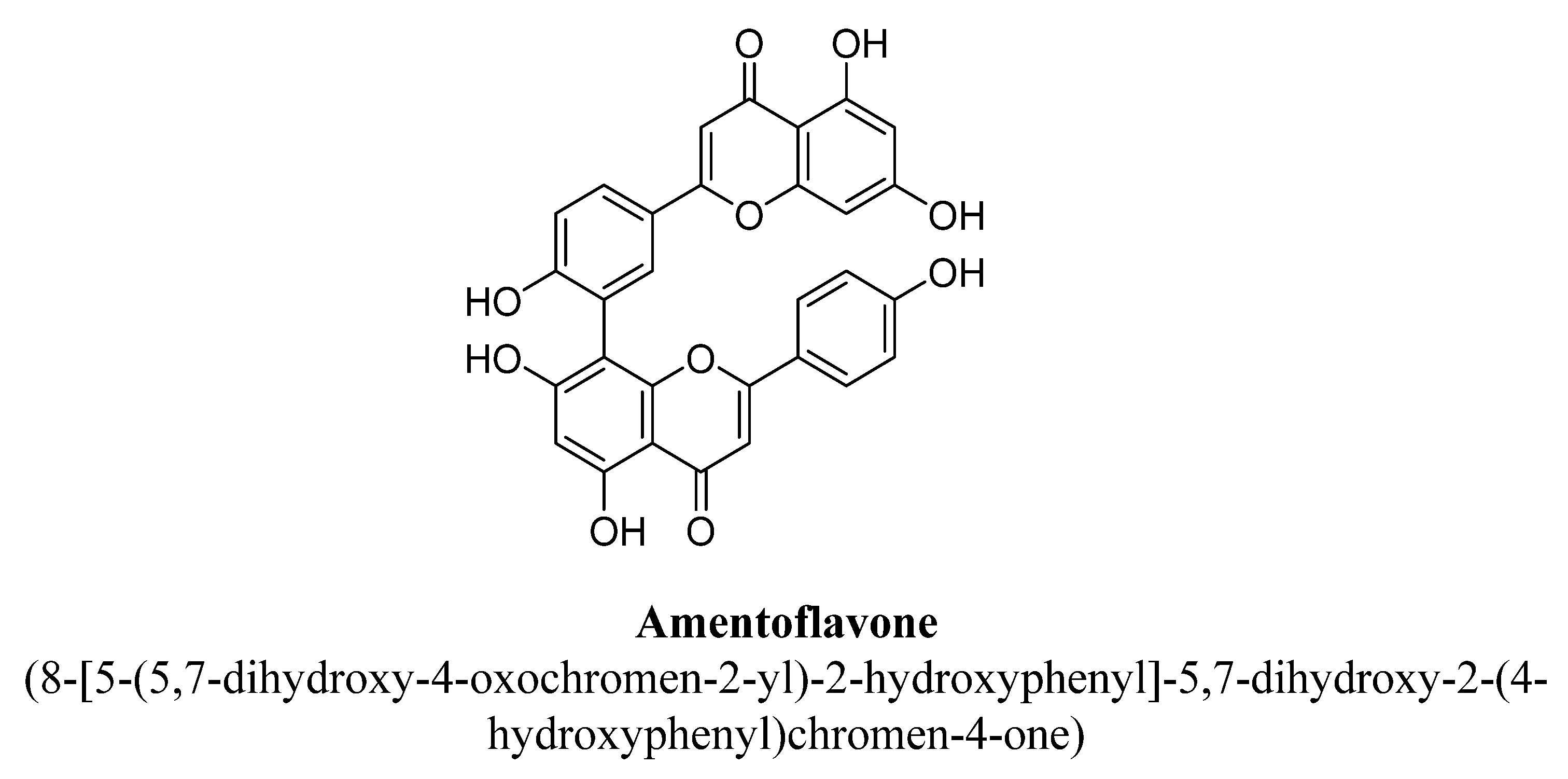
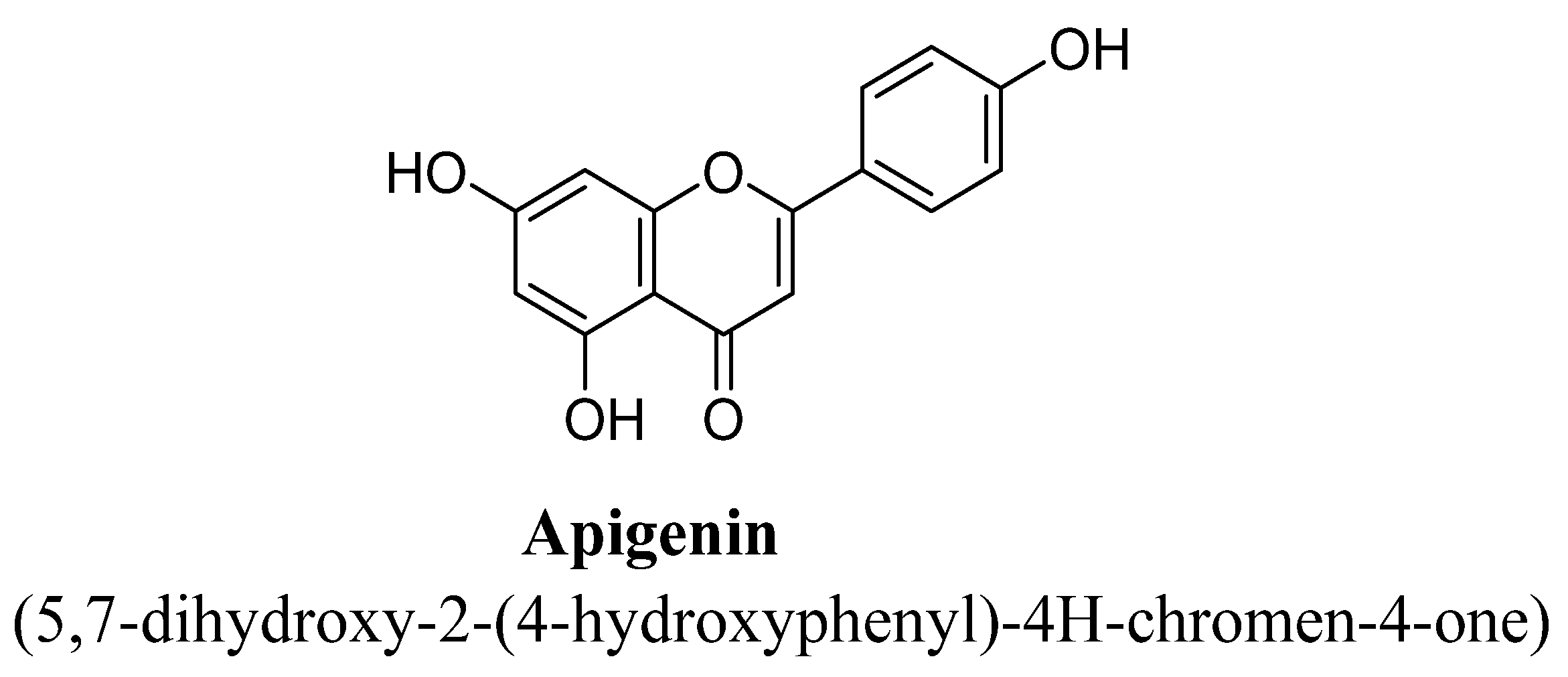
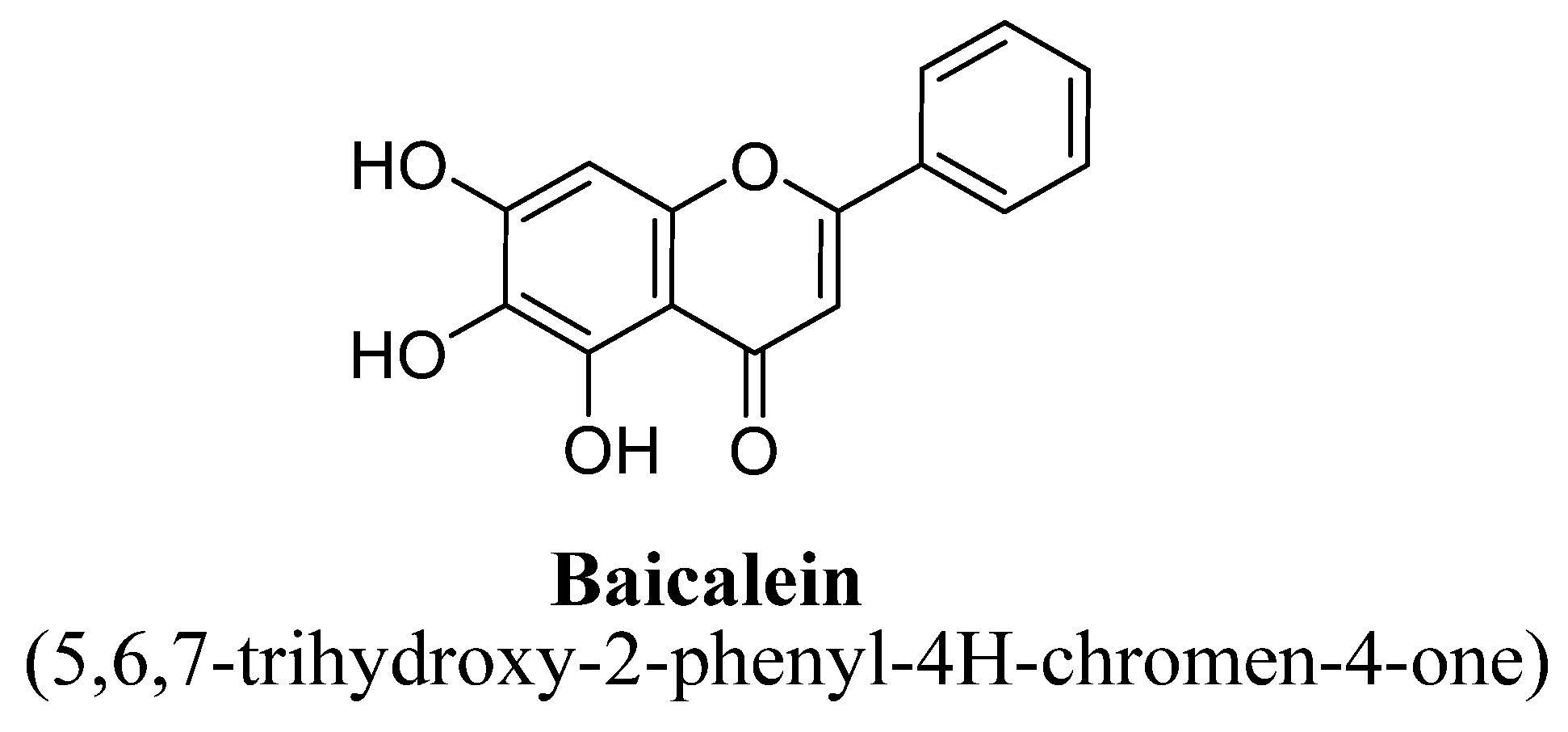
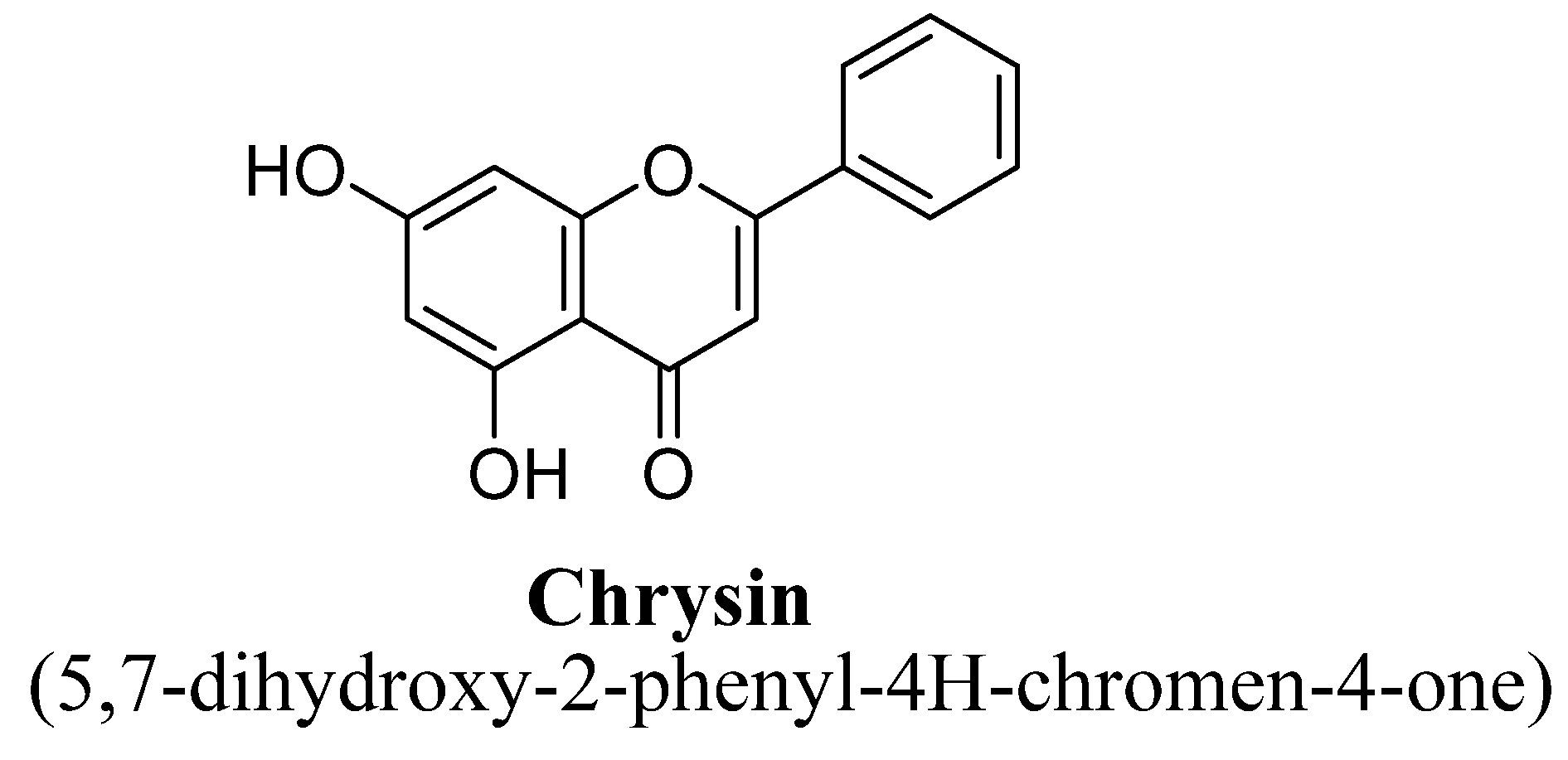
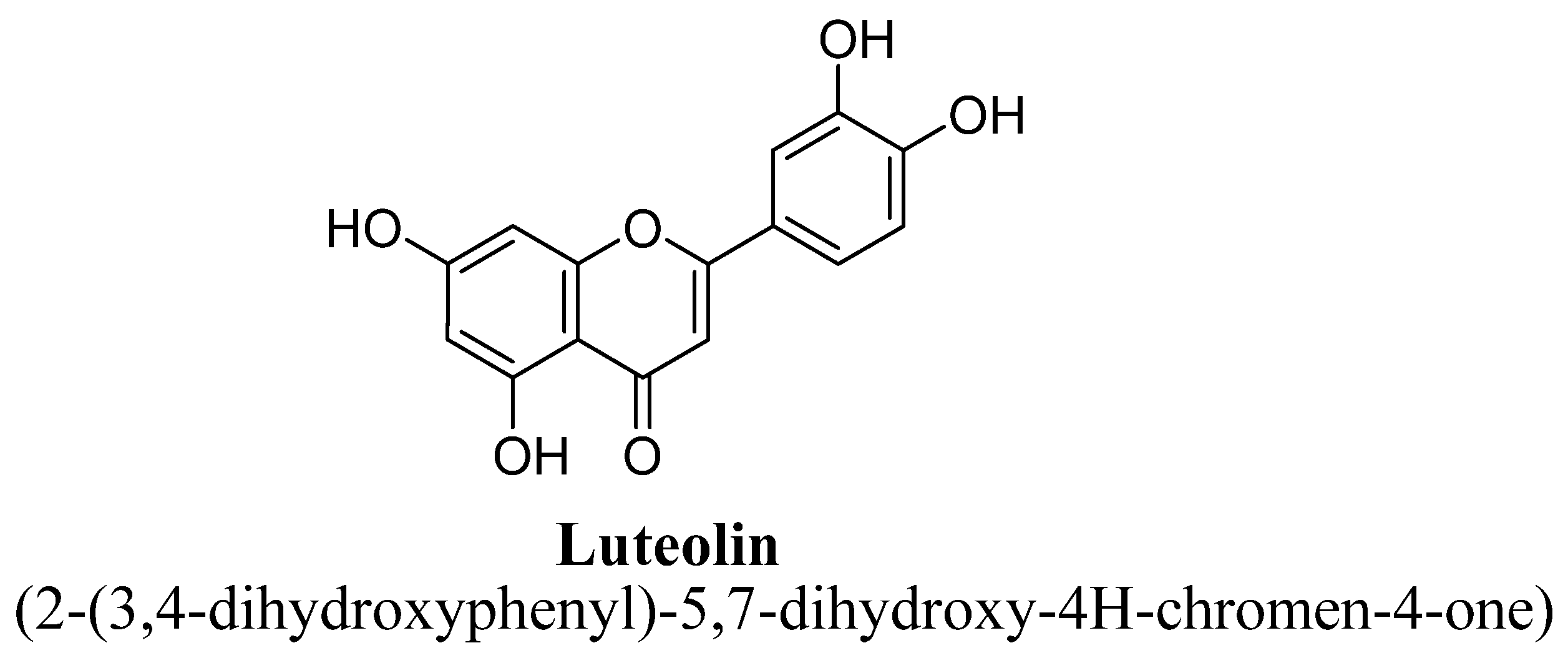
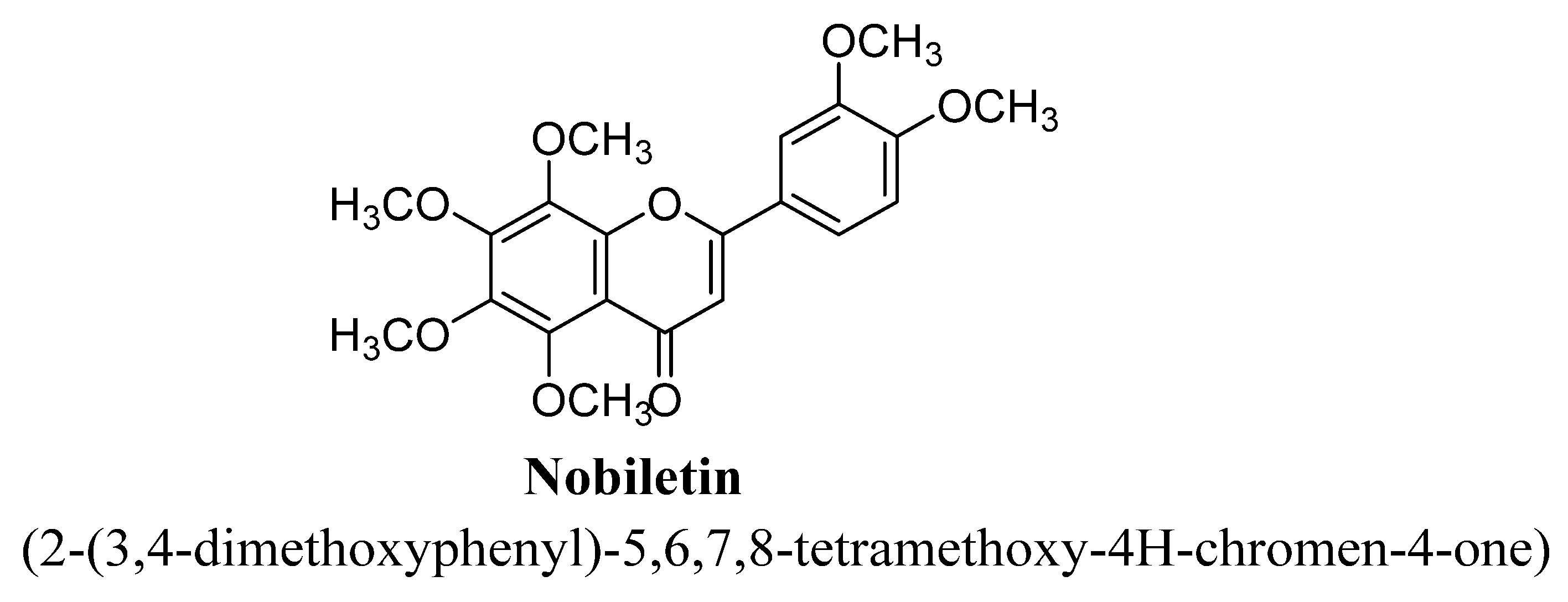
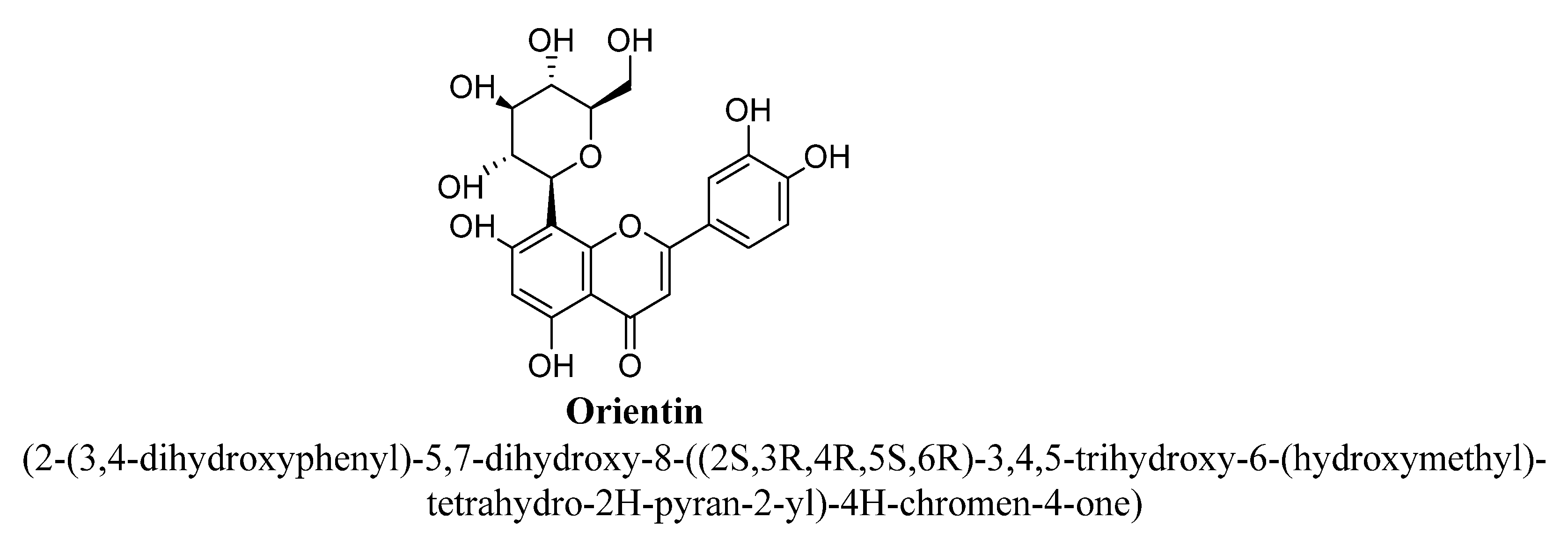

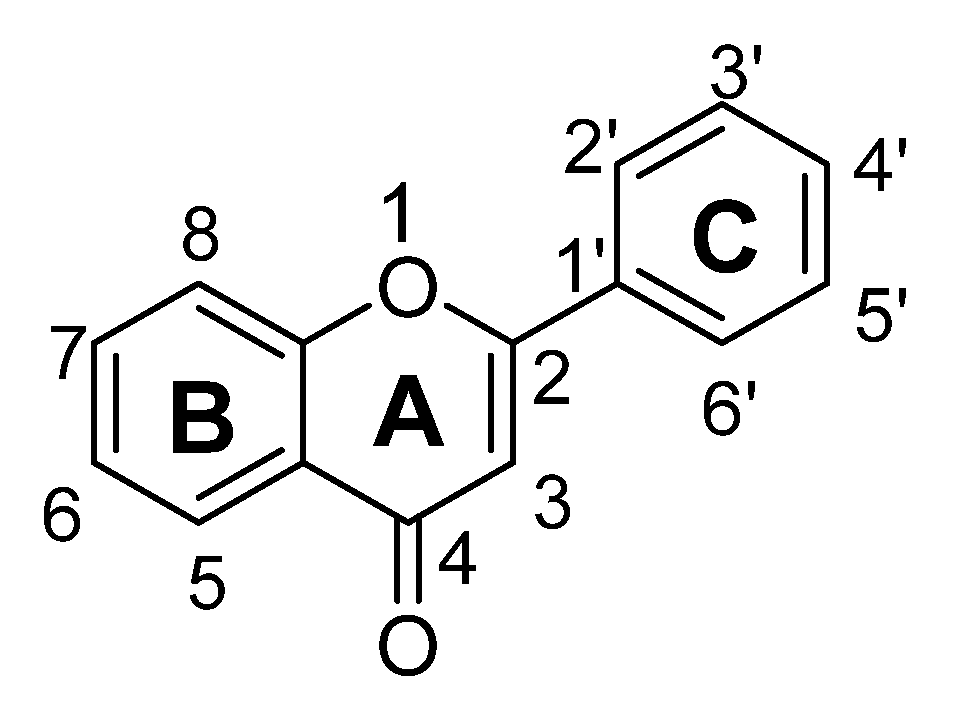
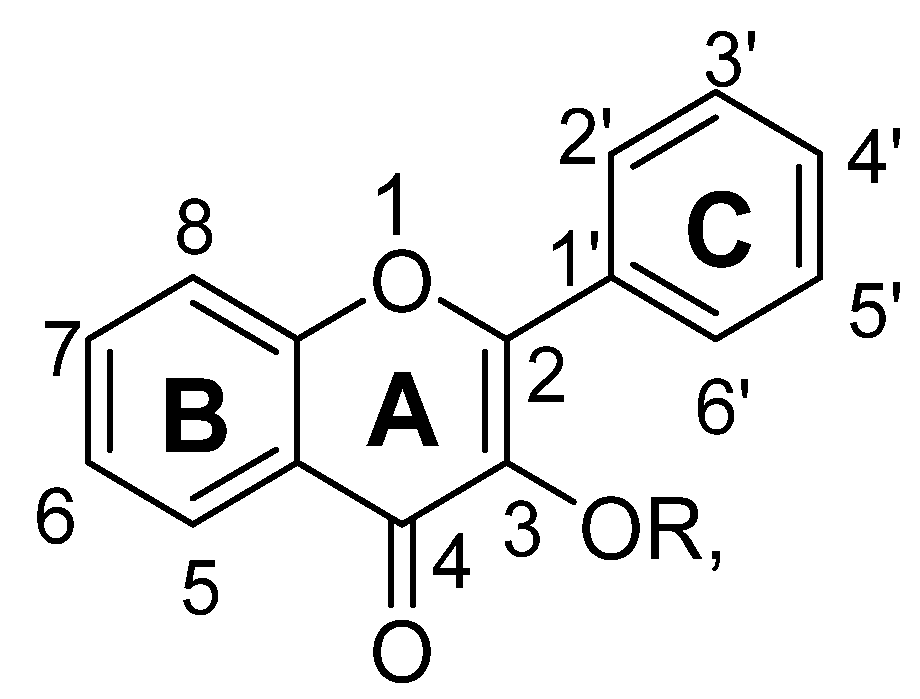

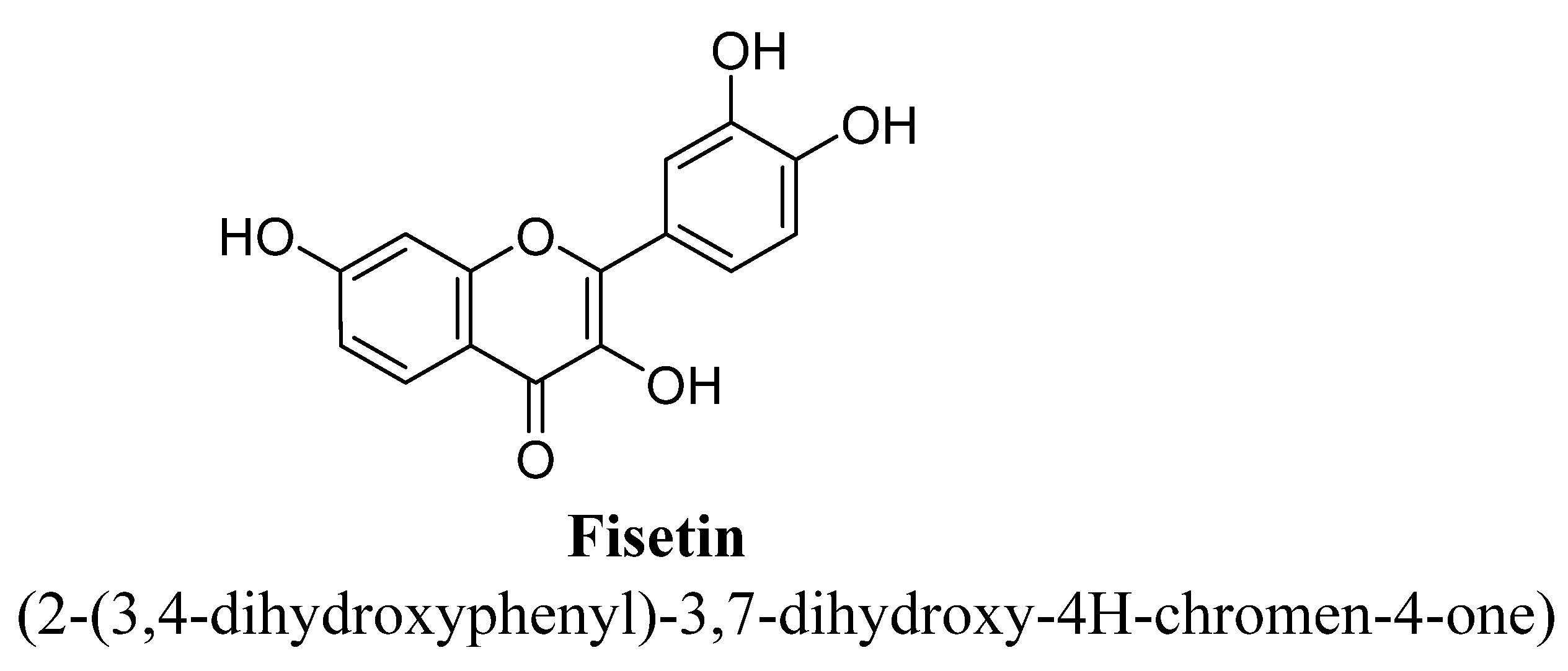
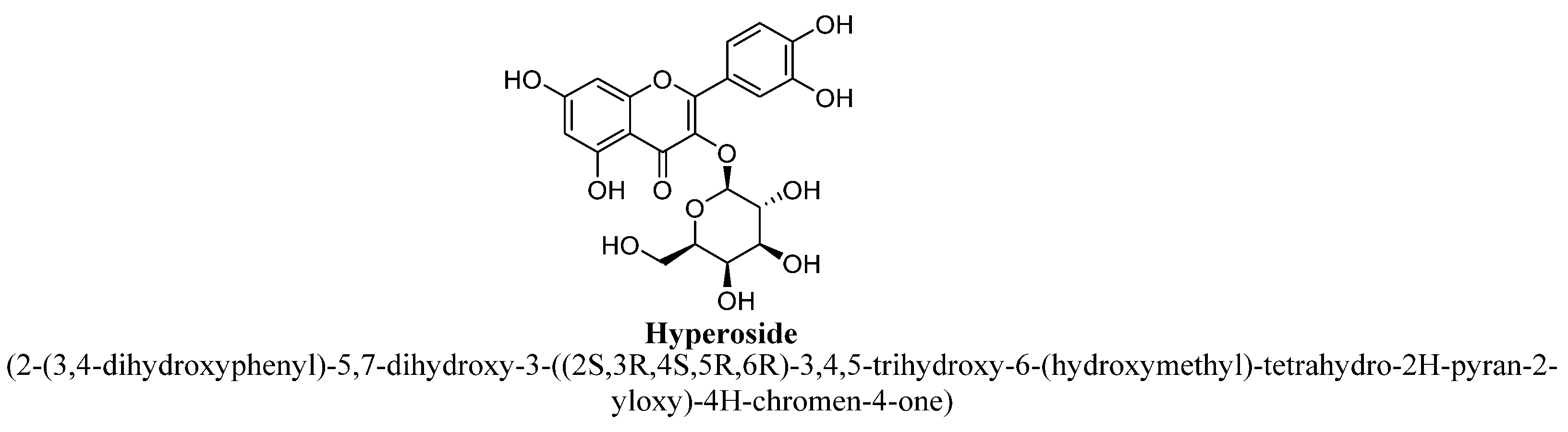

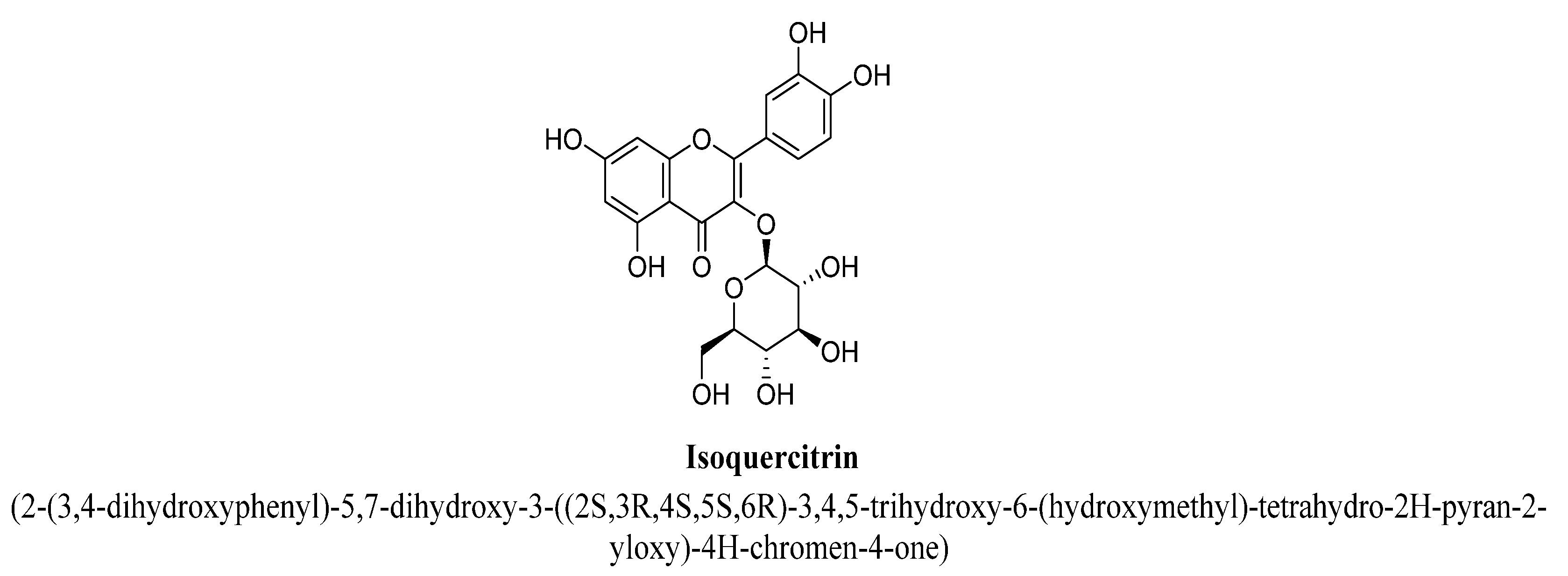
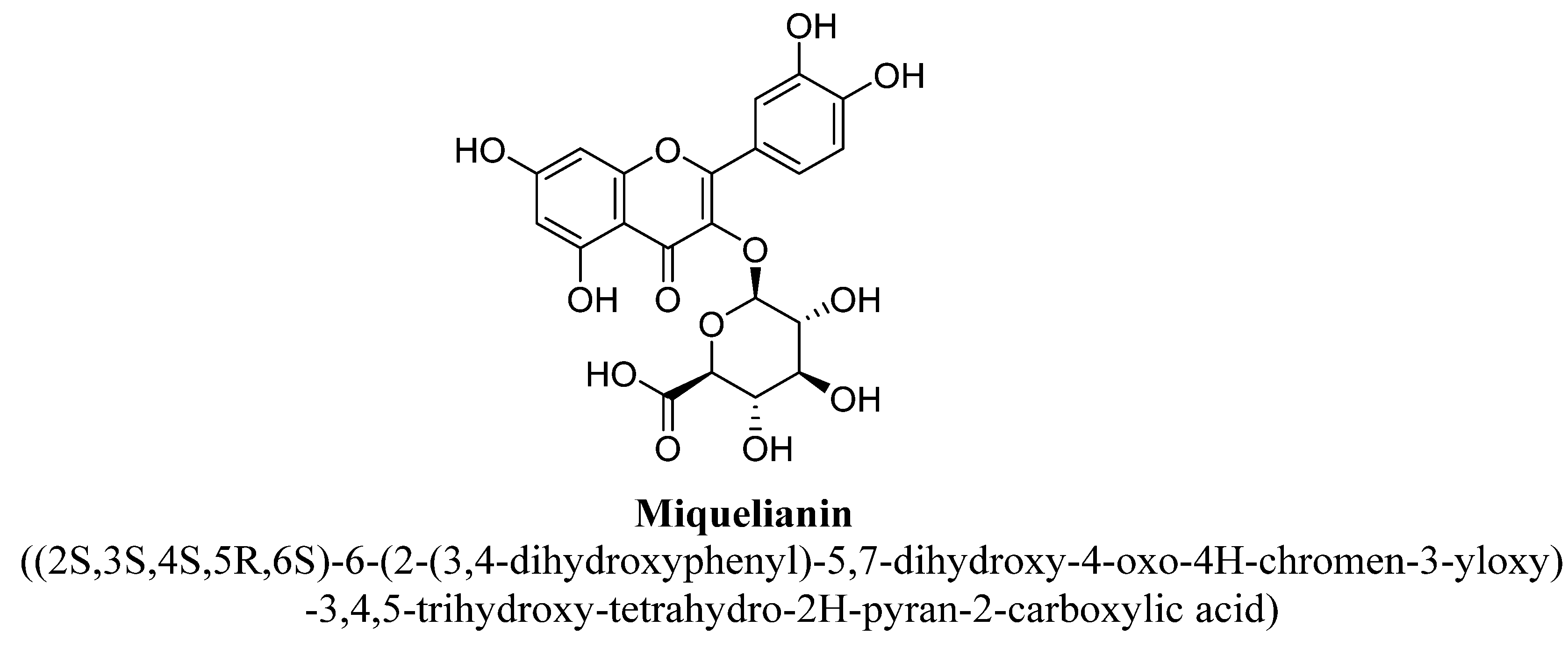
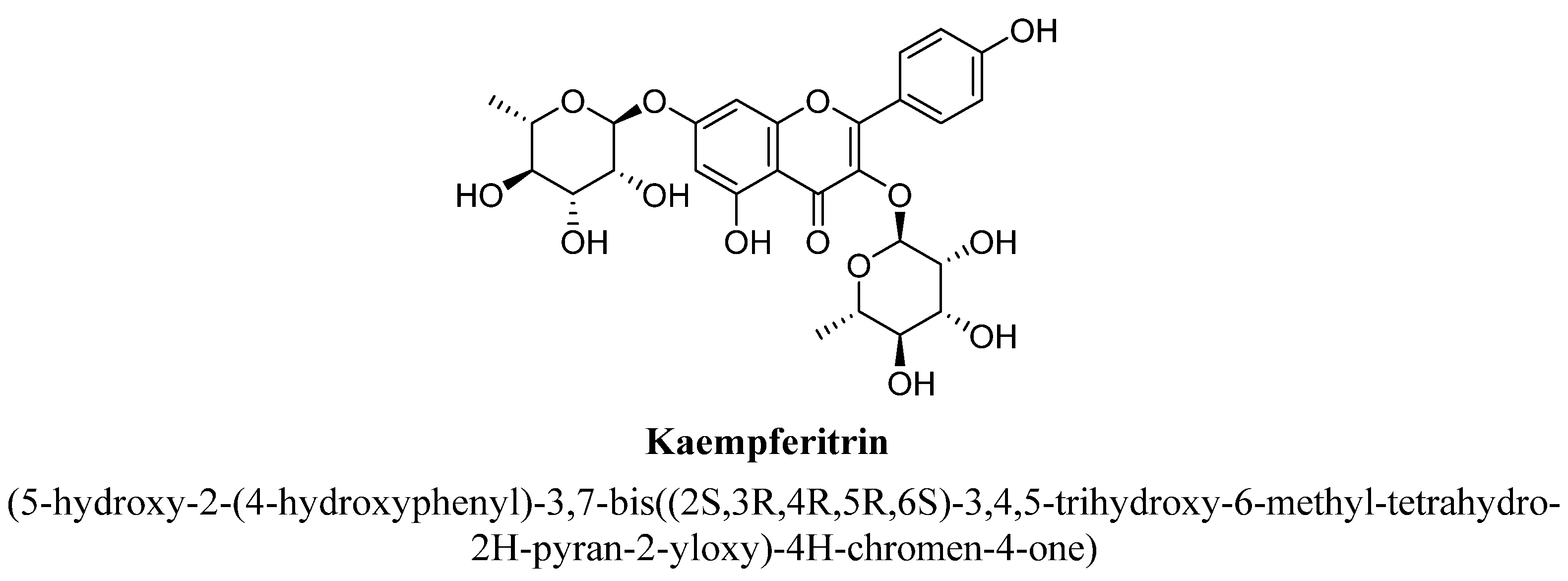
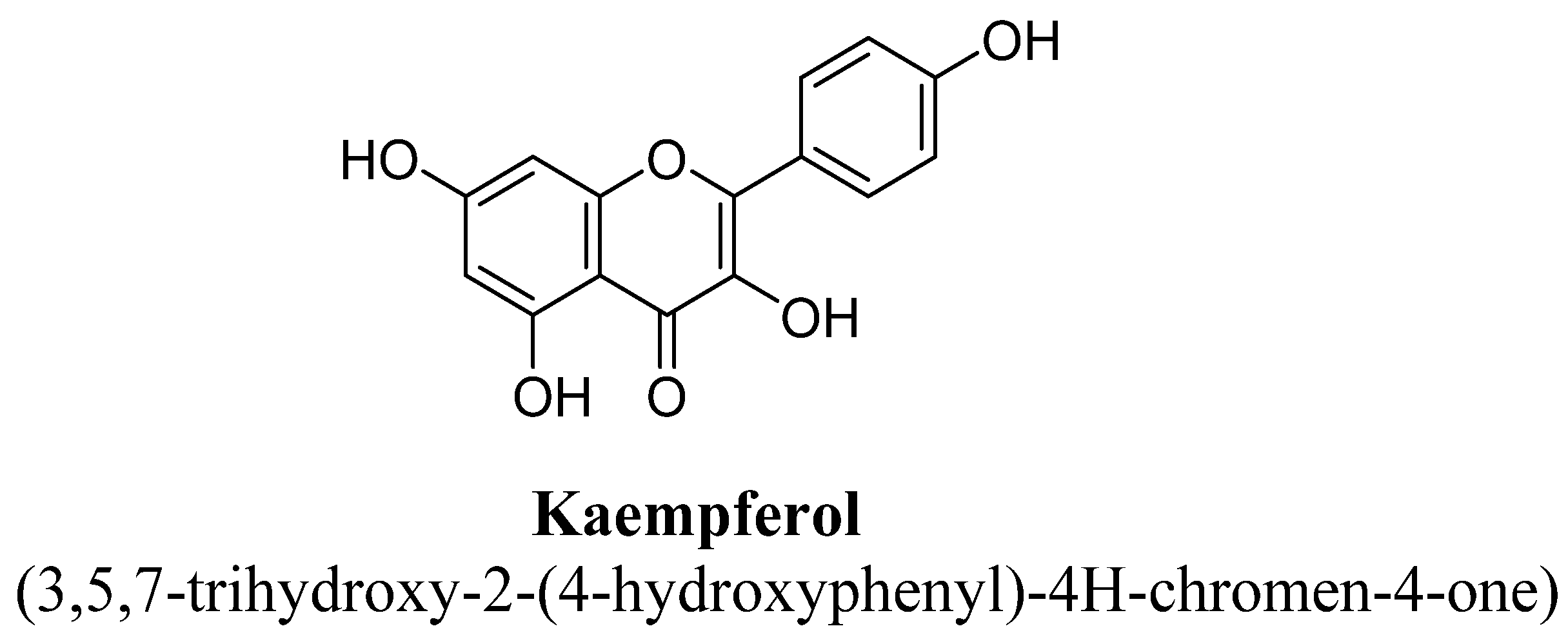
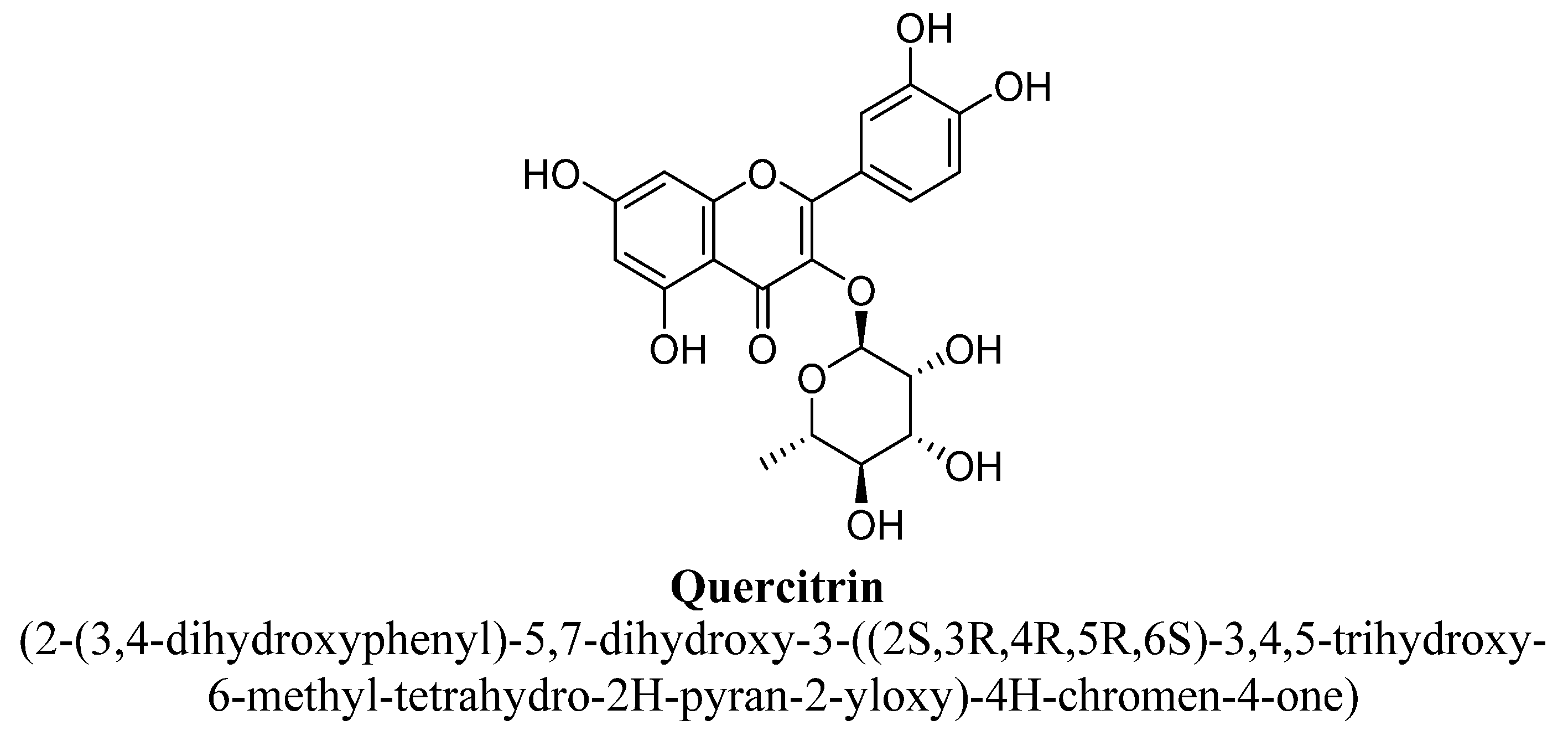
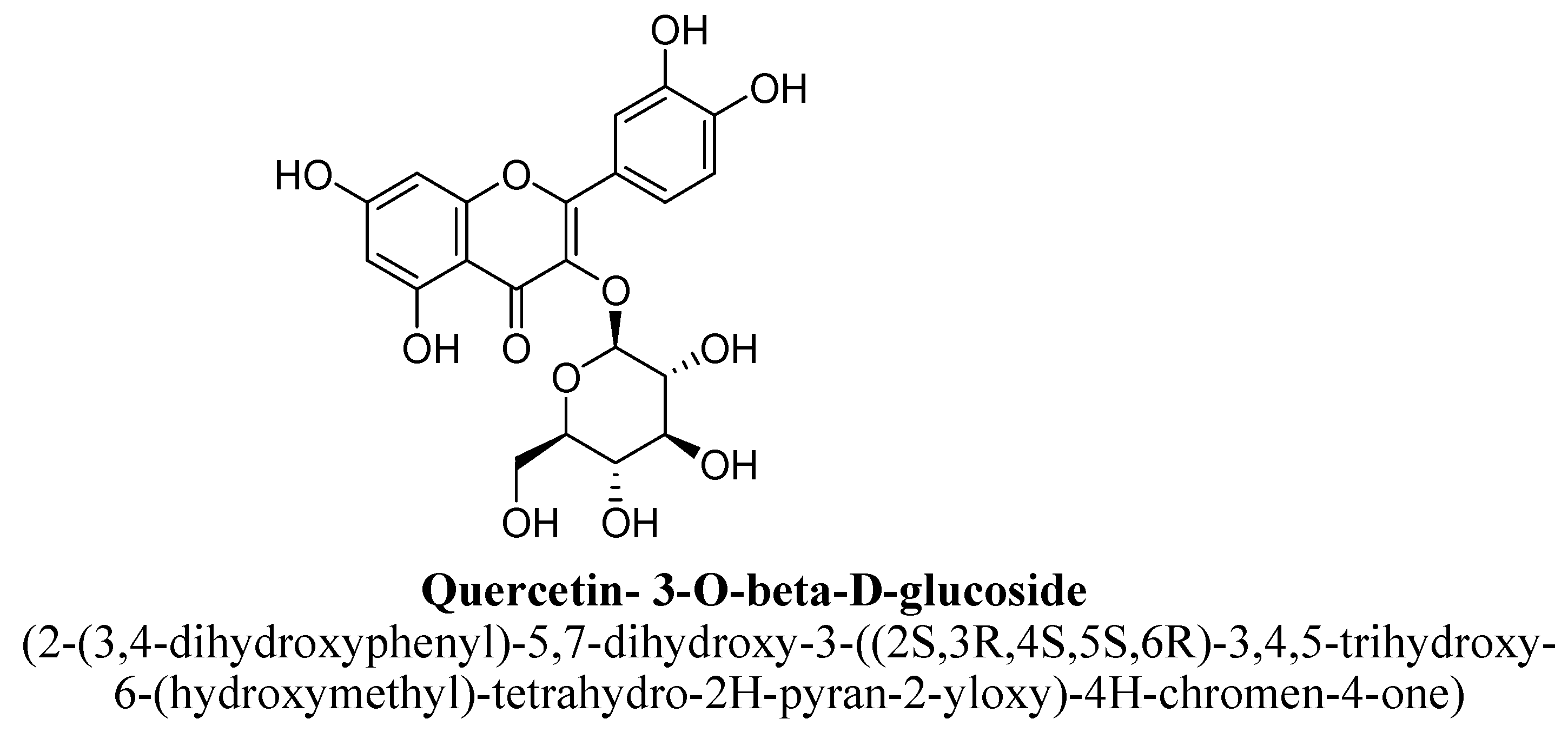
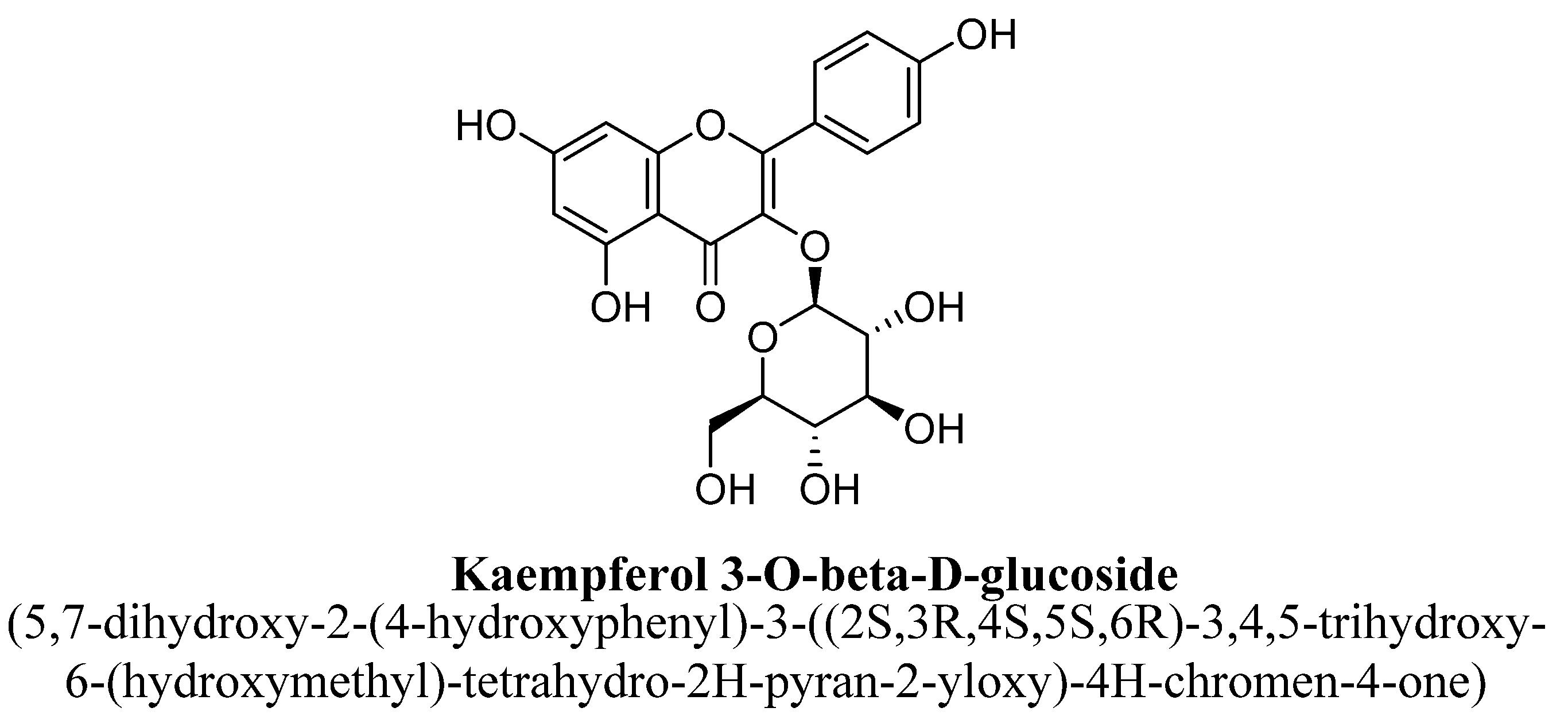
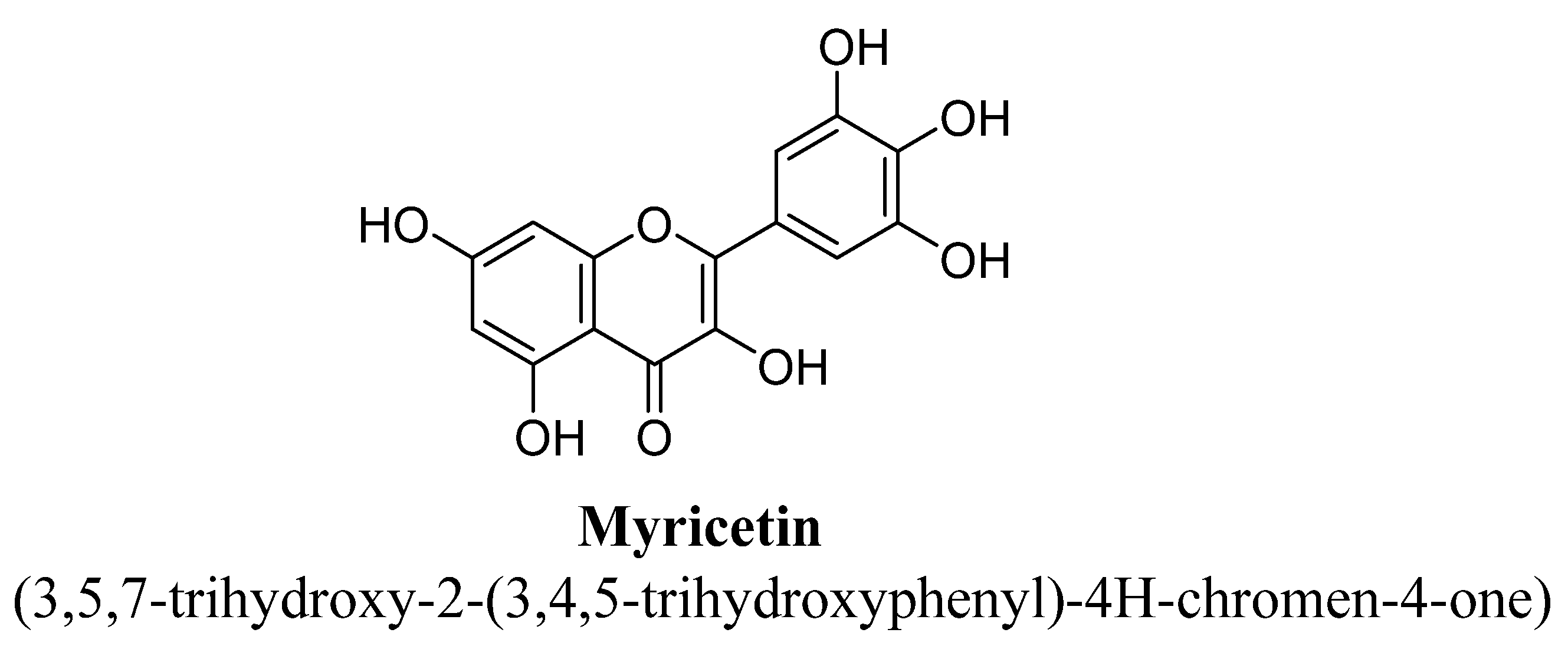
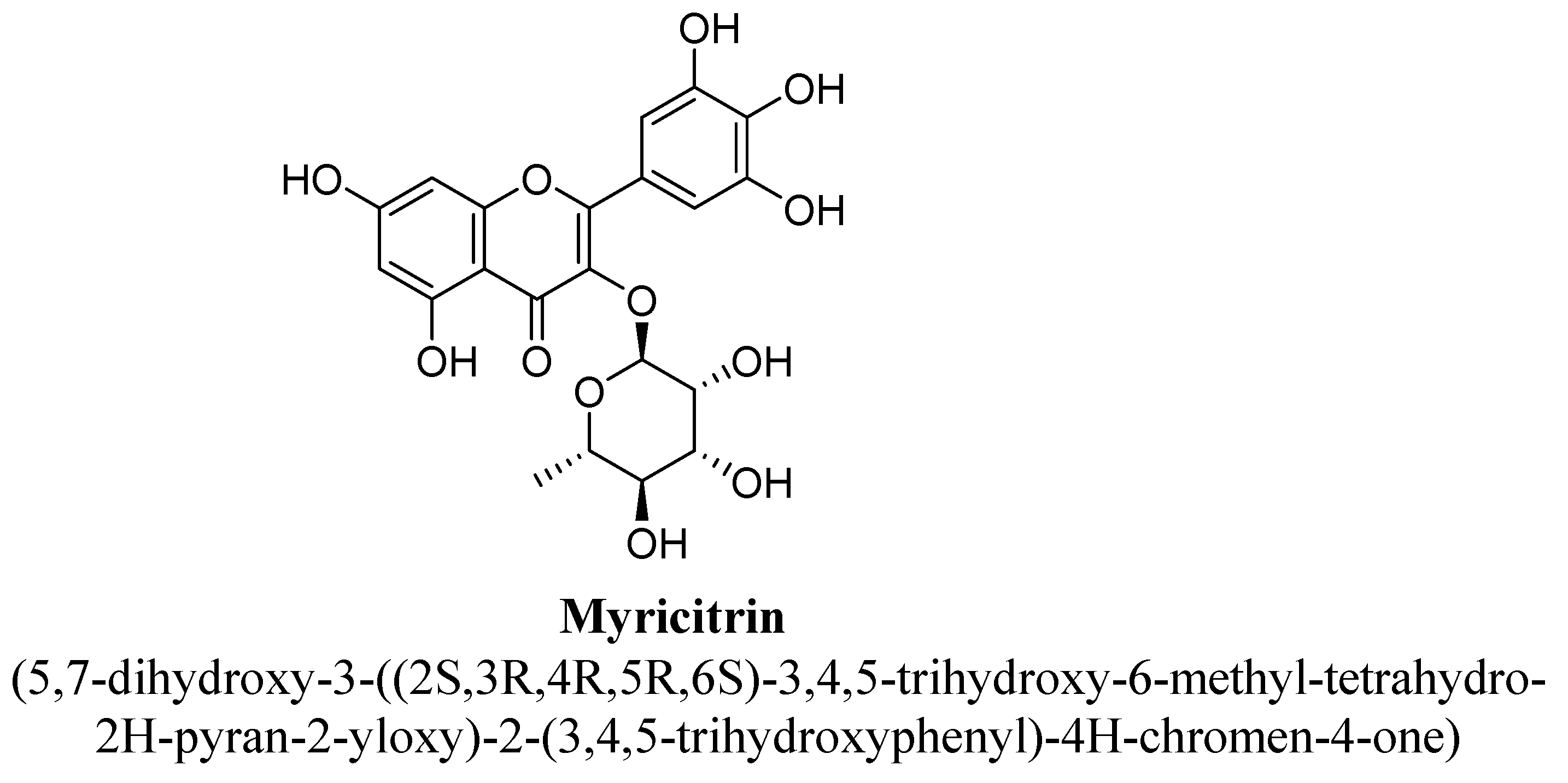
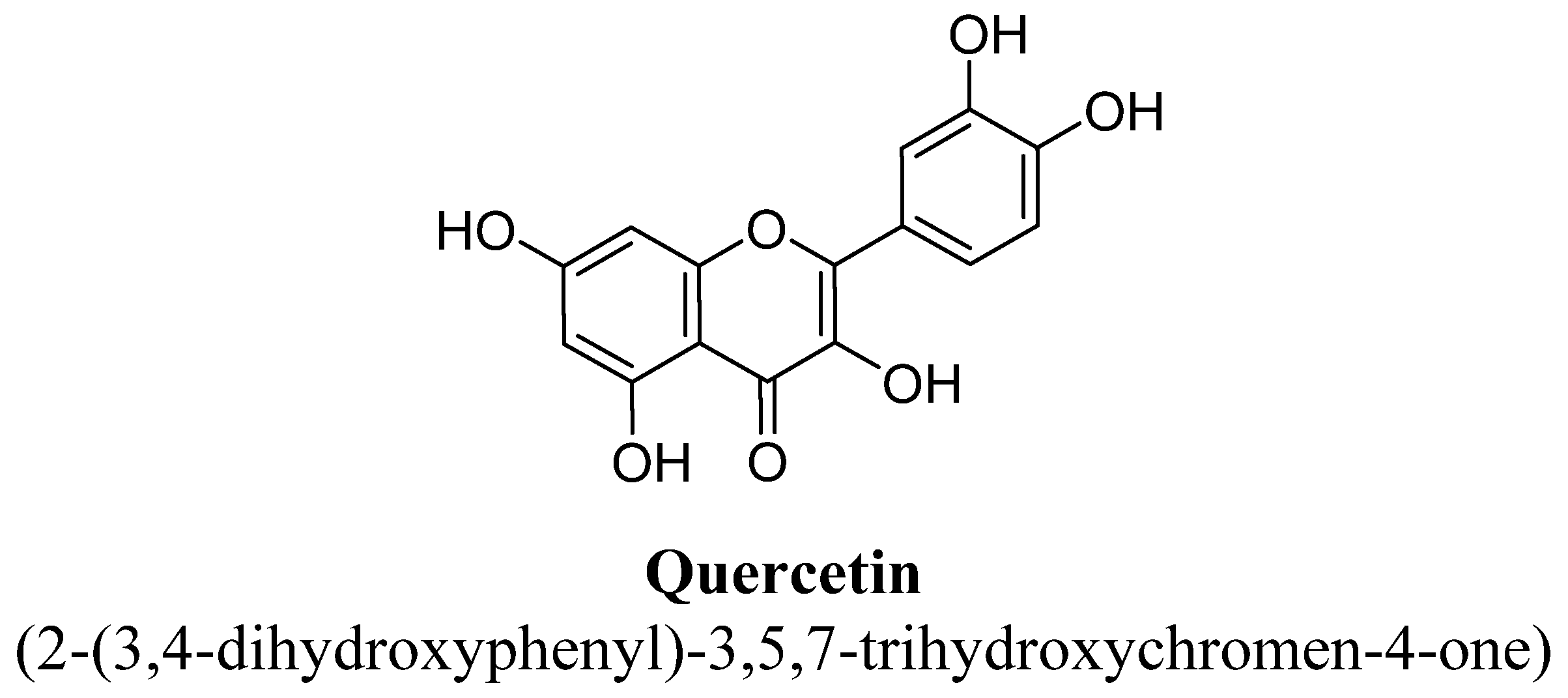
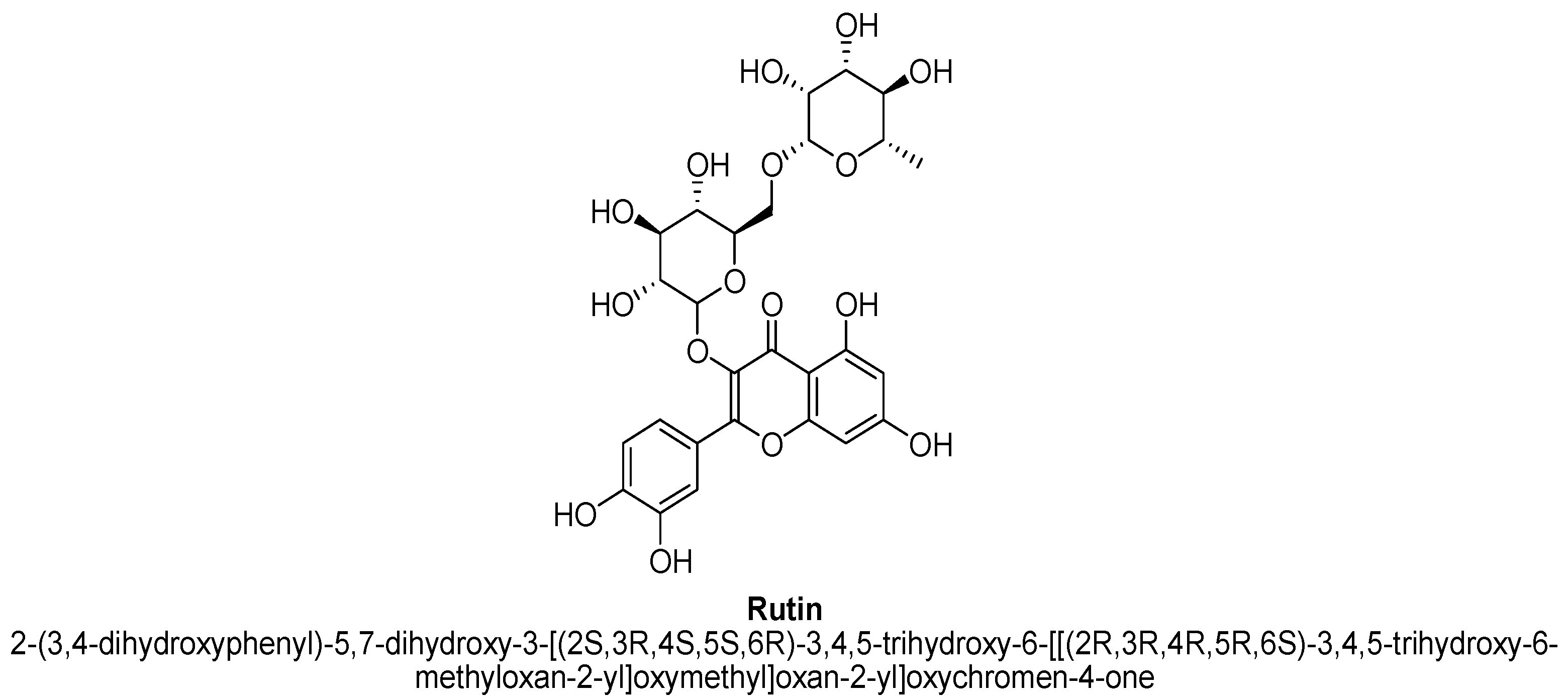
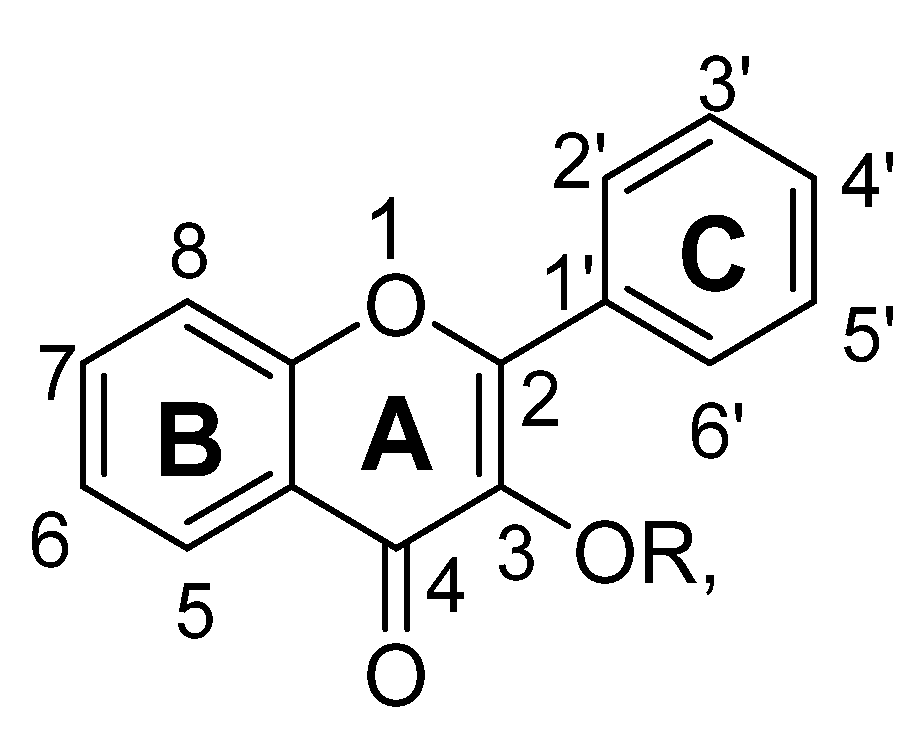
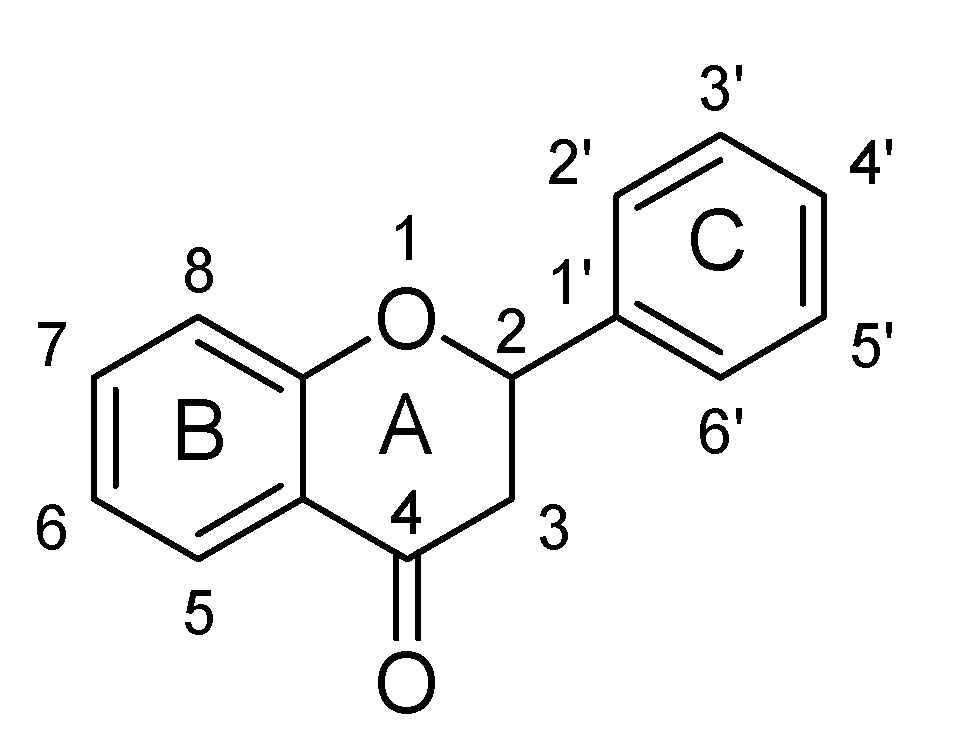

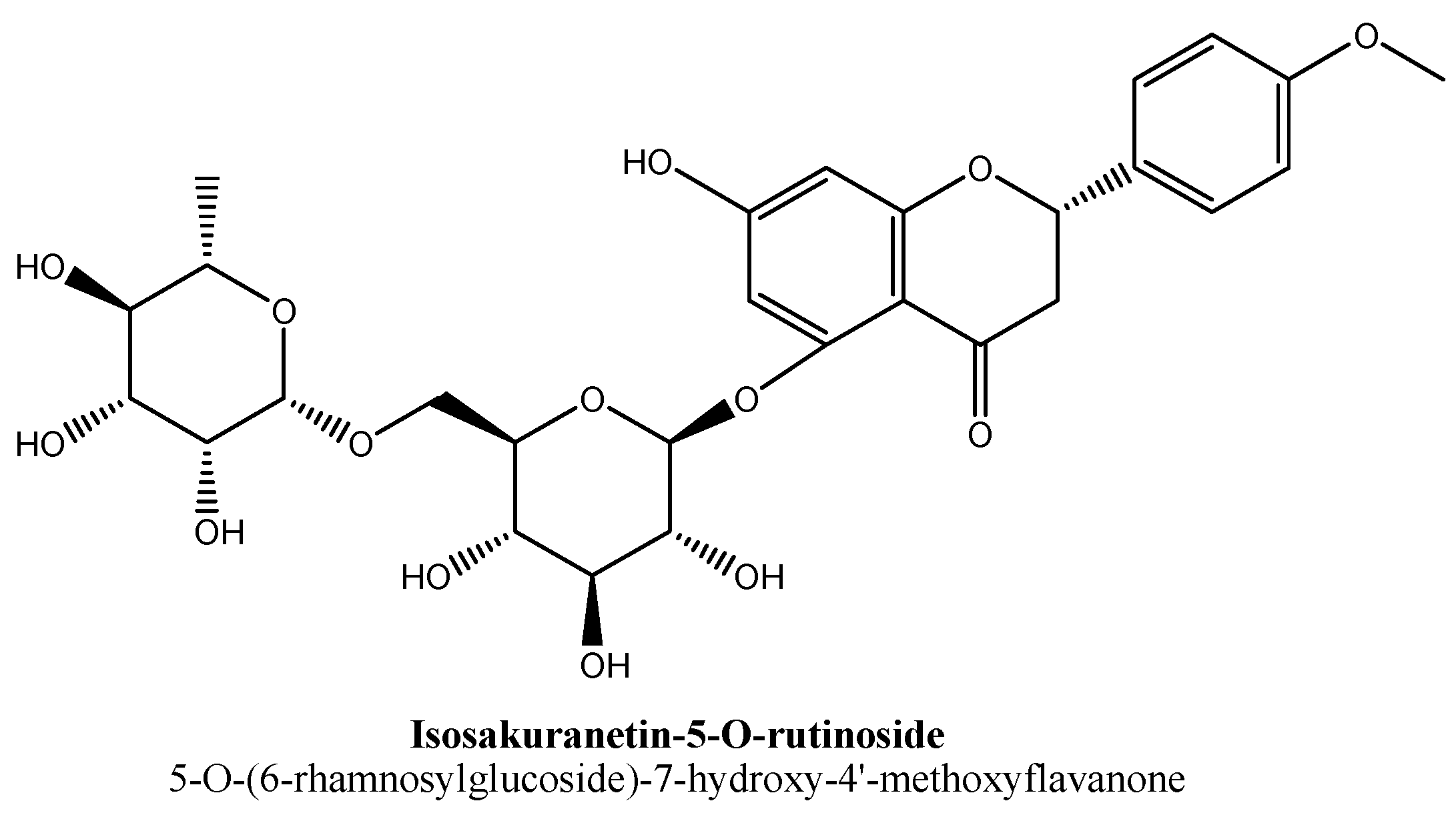
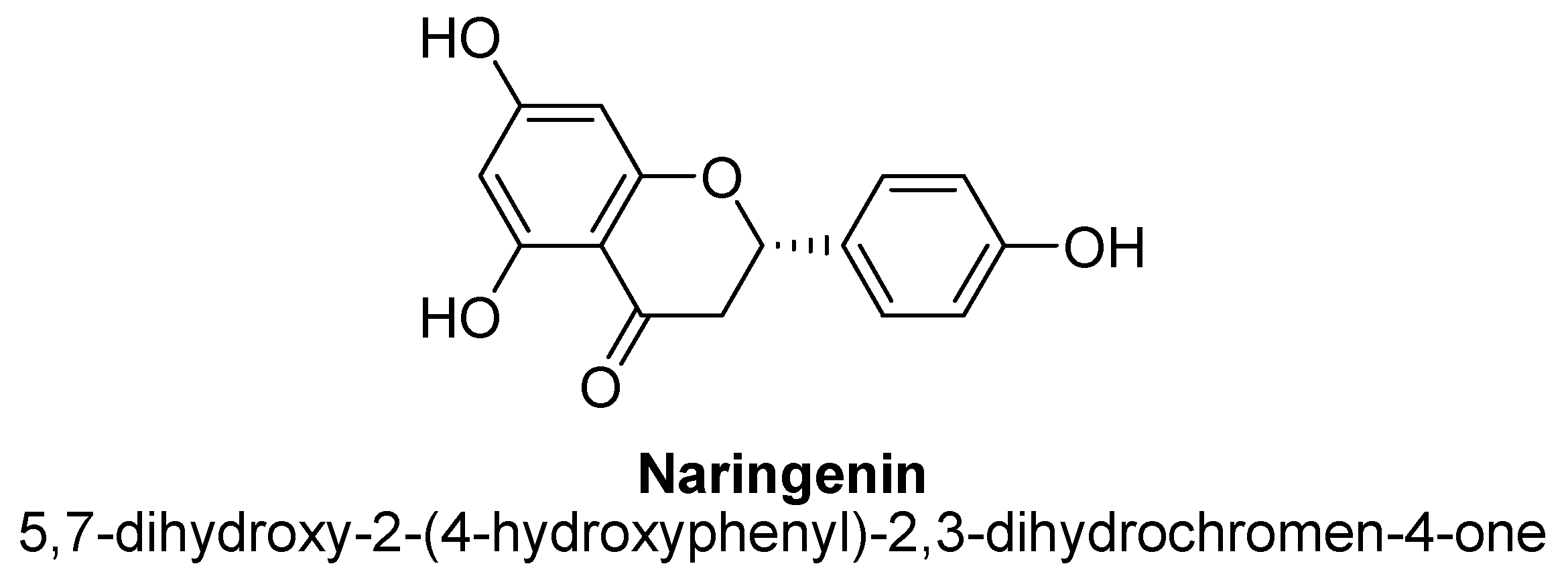
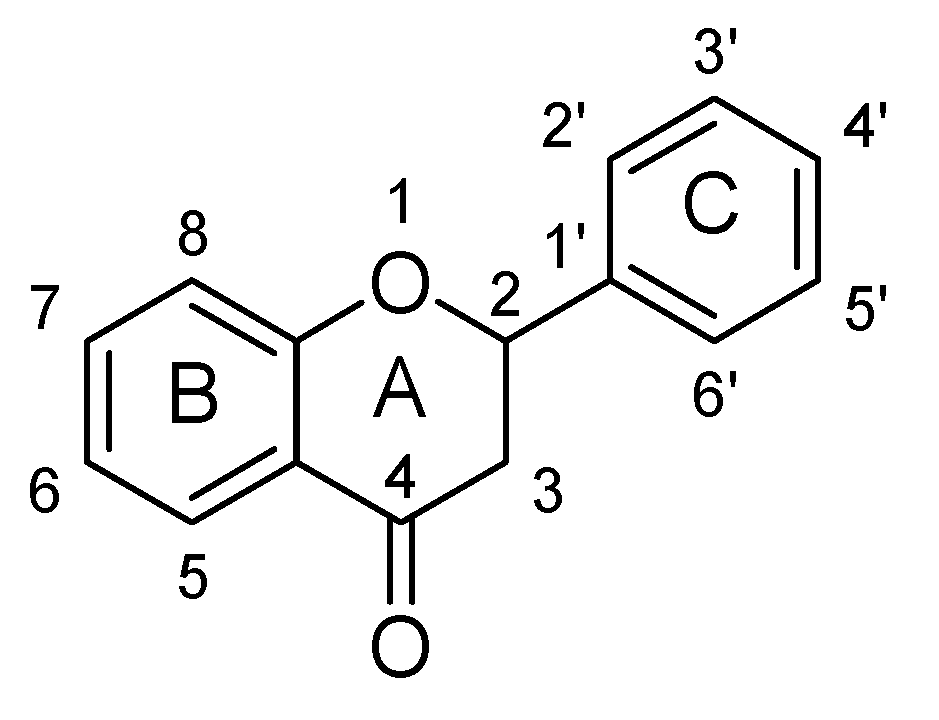
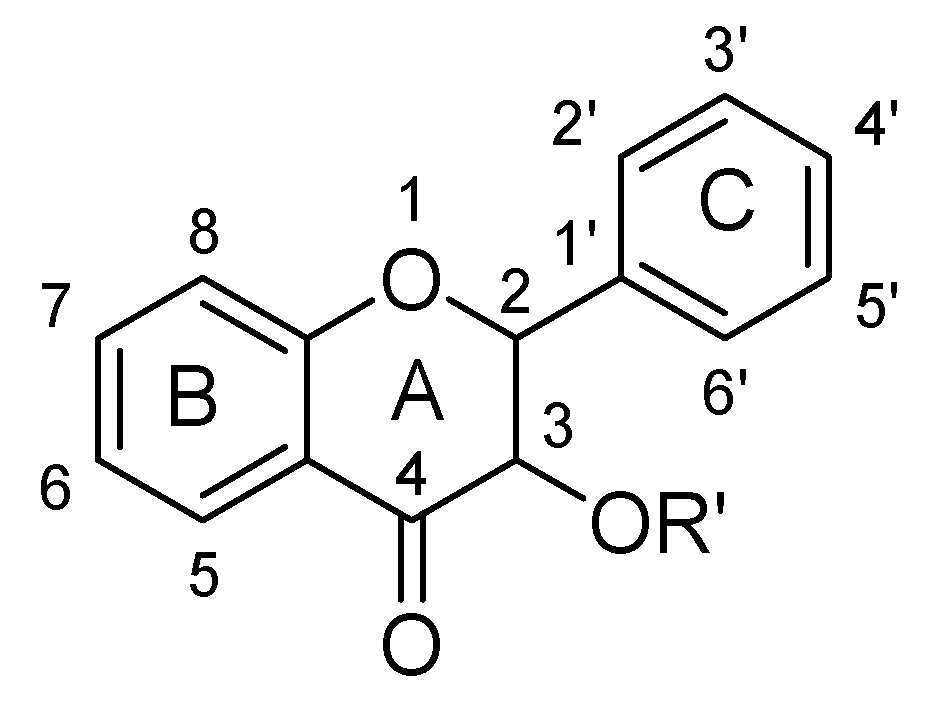
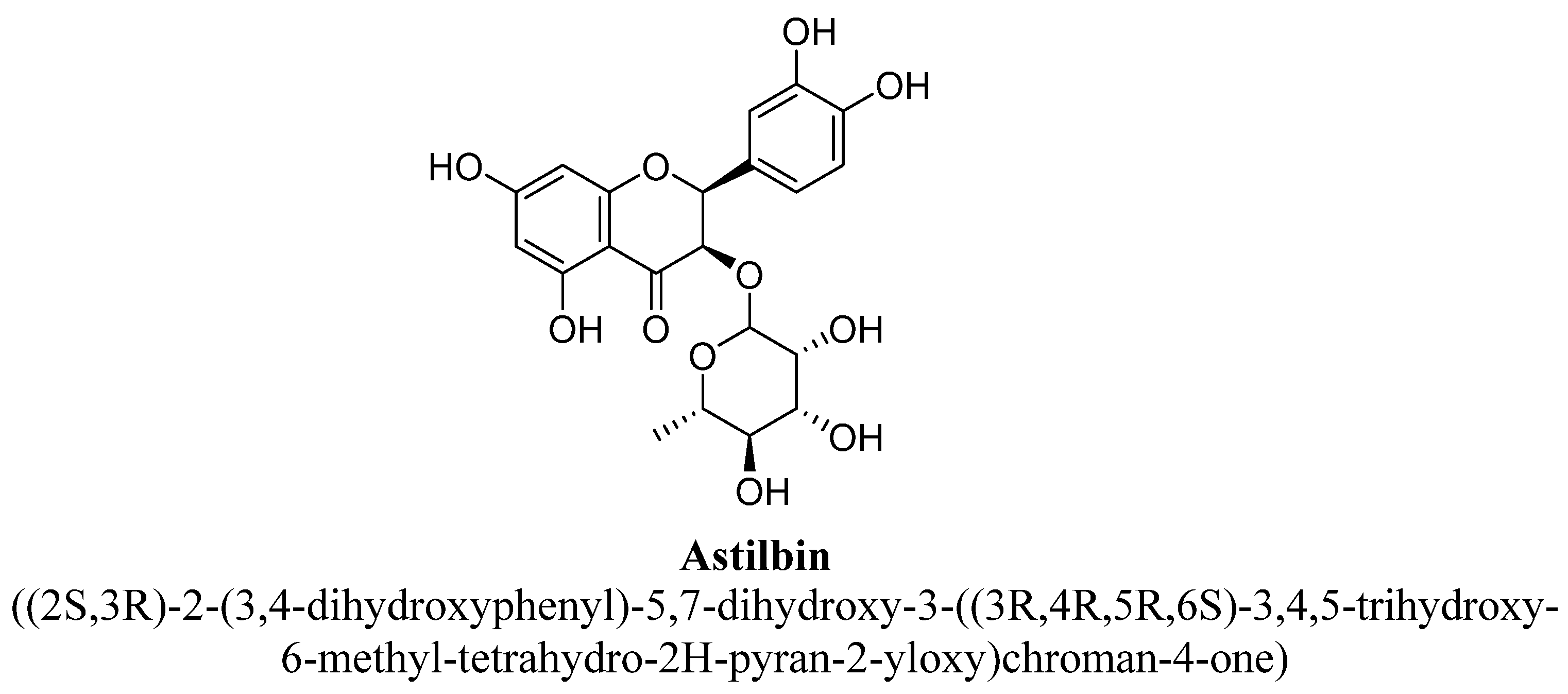
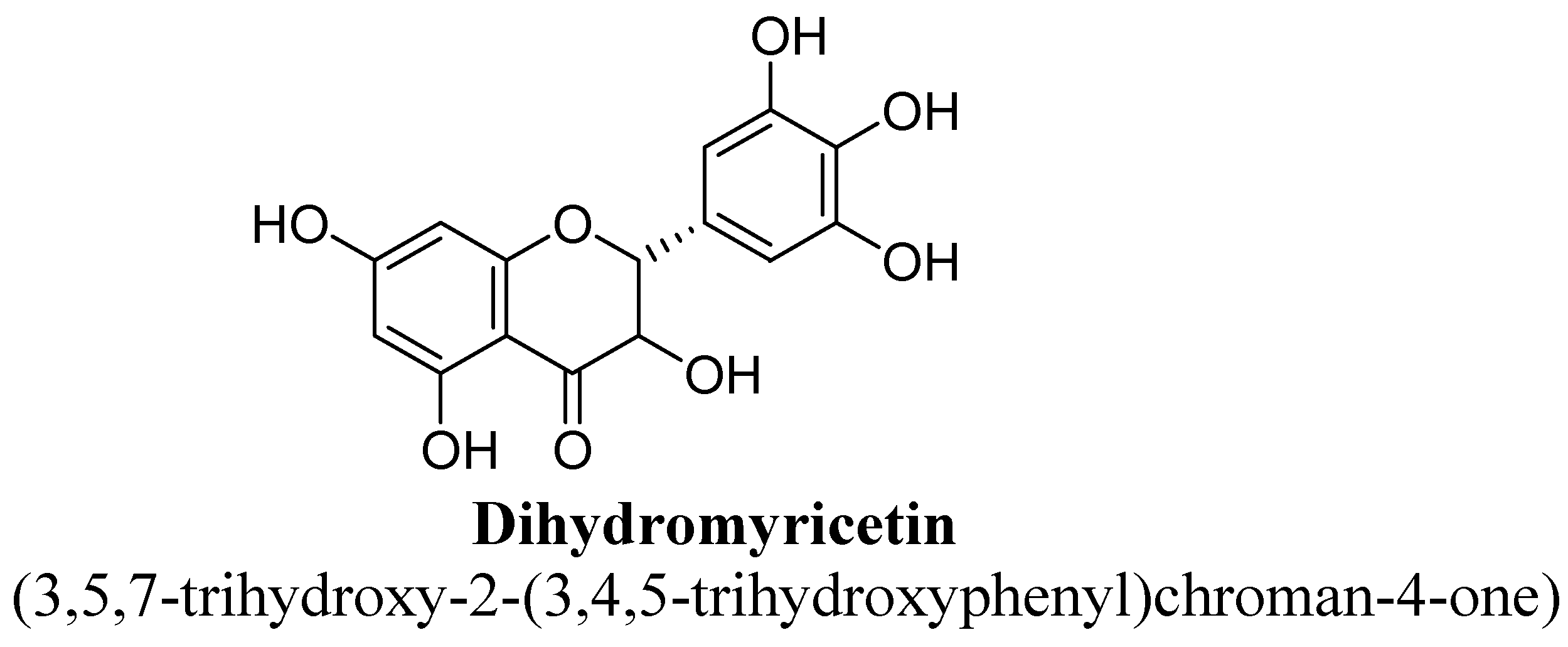
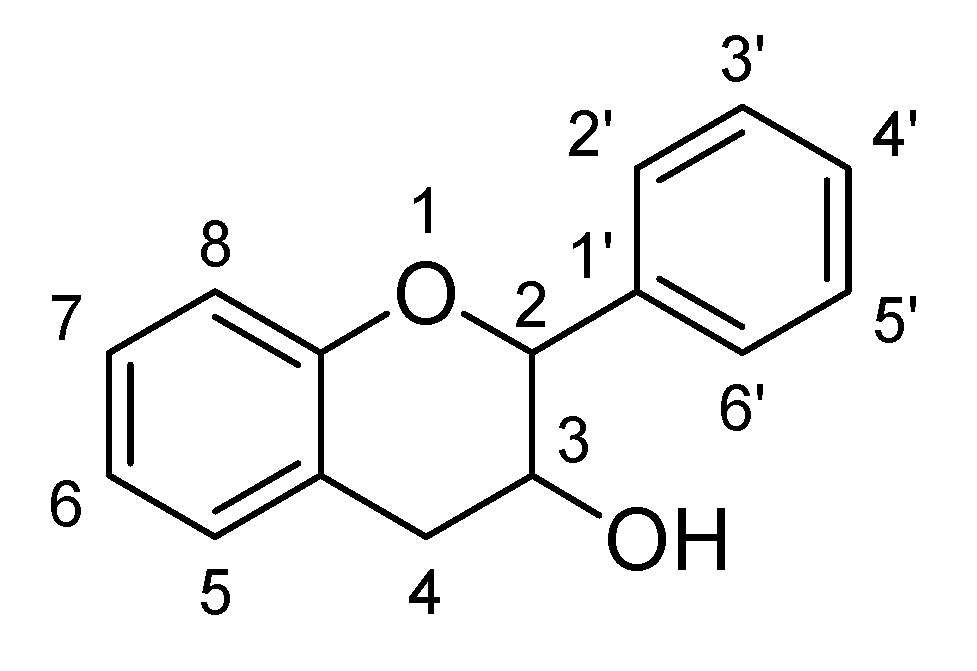
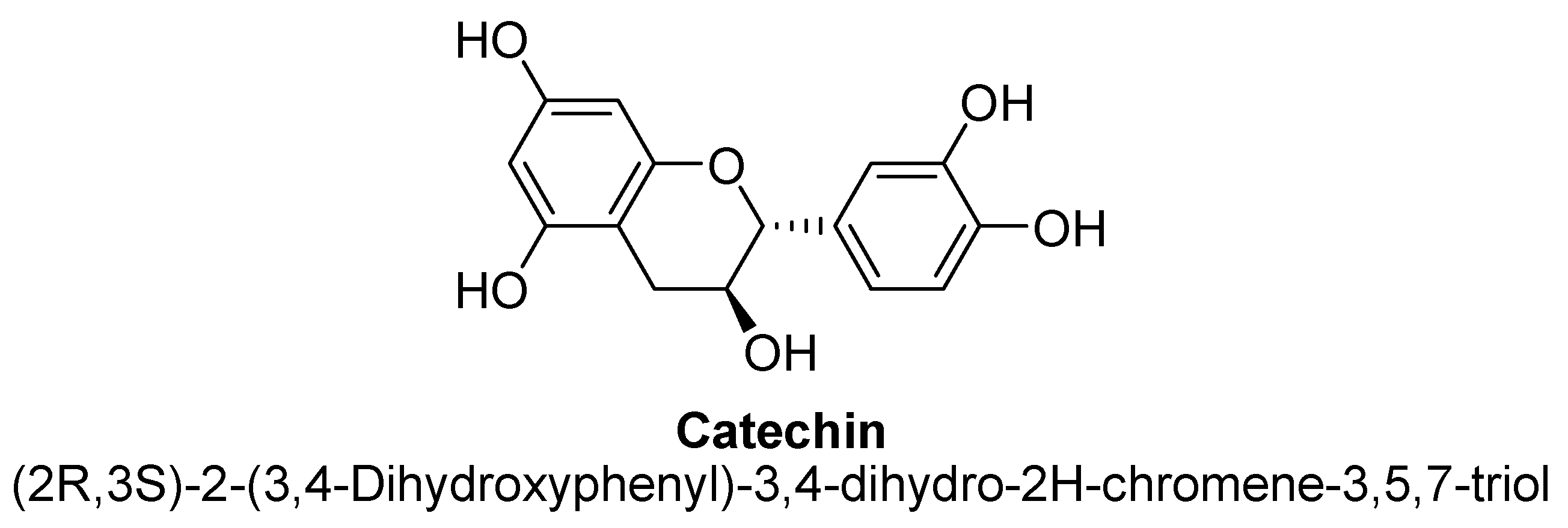
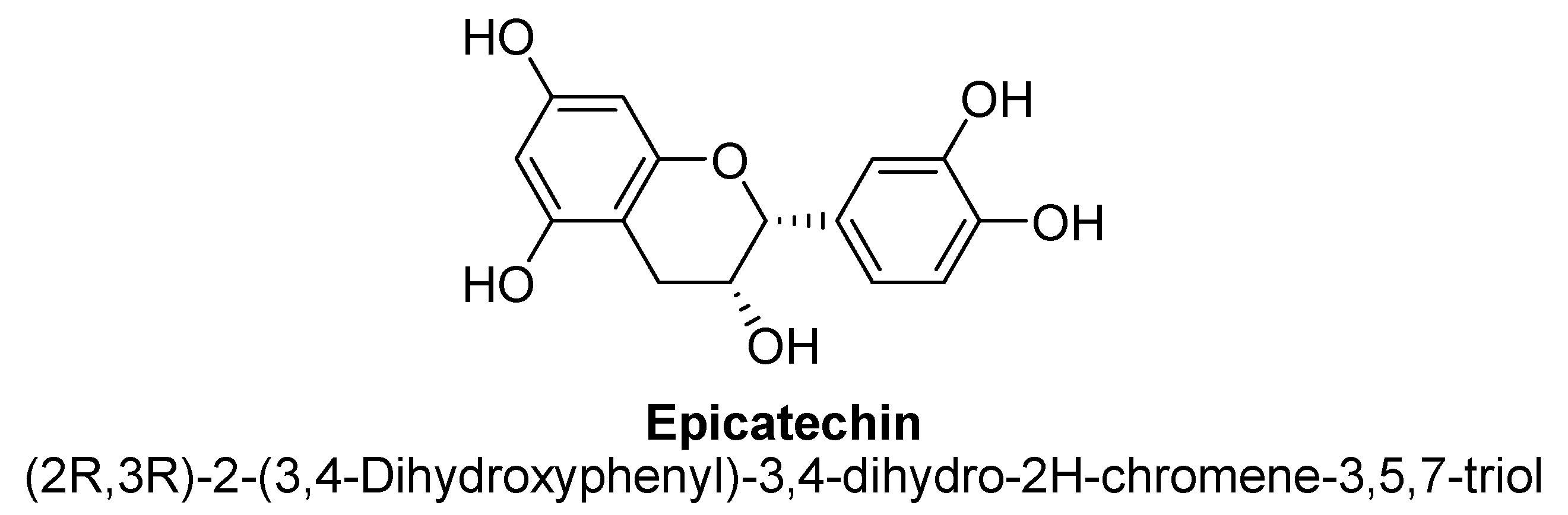
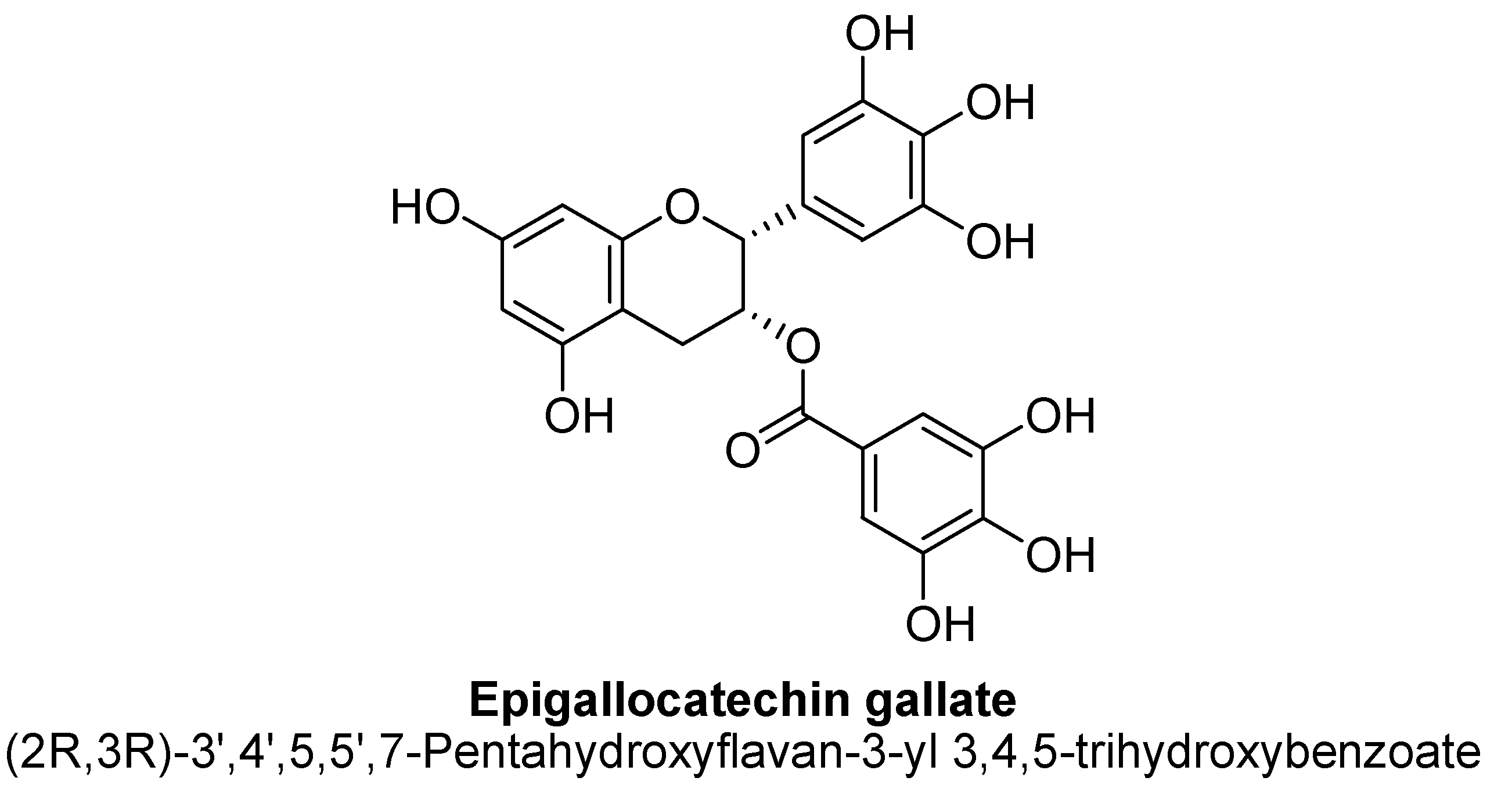
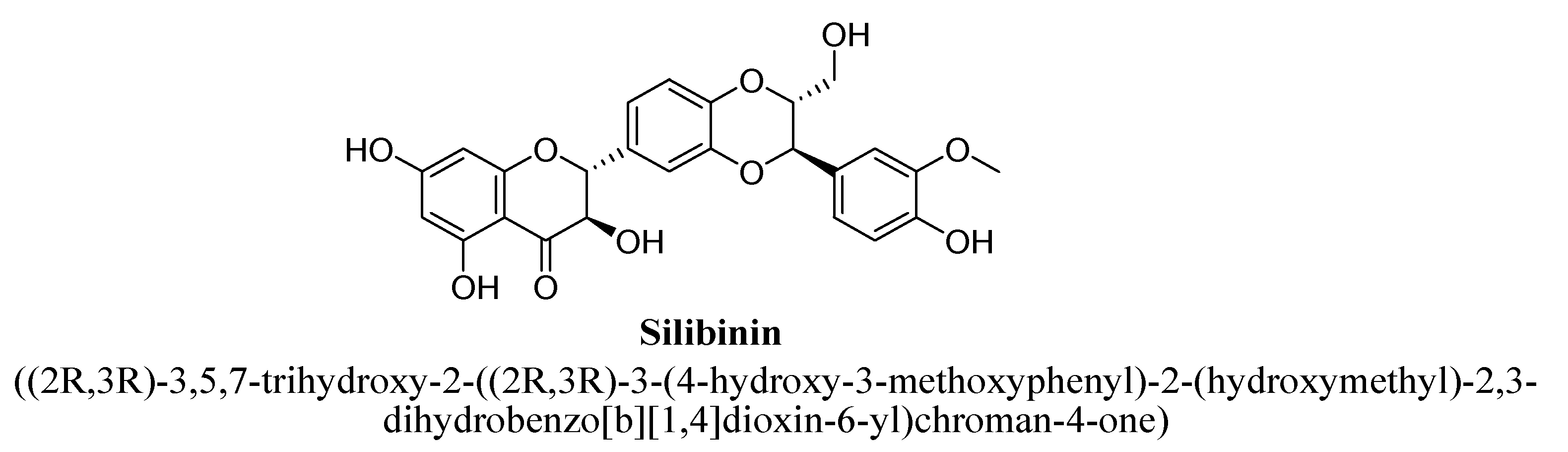
| Sr. No. | Flavones | C5 | C6 | C7 | C8 | C3′ | C4′ |
|---|---|---|---|---|---|---|---|
| 1. | 7,8-Dihydroxyflavone | -OH | -OH | ||||
| 2. | Amentoflavone | -OH | -OH | -C15H12O5 | -OH | ||
| 3. | Apigenin | -OH | -OH | -OH | |||
| 4. | Baicalein | -OH | -OH | -OH | |||
| 5. | Chrysin | -OH | -OH | ||||
| 6. | Luteolin | -OH | -OH | -OH | -OH | ||
| 7. | Nobiletin | -OCH3 | -OCH3 | -OCH3 | -OCH3 | -OCH3 | |
| 8. | Orientin | -OH | -OH | -C6H11O5 | -OH | -OH | |
| 9. | Vitexin | -OH | -OH | -C6H11O5 | -OH |
| Sr. No. | Flavonols | R | C5 | C6 | C7 | C8 | C3′ | C4′ | C5′ |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 3,5,6,7,8,3′,4′-Heptamethoxyflavone | -CH3 | -OCH3 | -OCH3 | -OCH3 | -OCH3 | -OCH3 | -OCH3 | |
| 2. | Fisetin | H | OH | OH | OH | ||||
| 3. | Hyperoside | (C6H11O5) | -OH | -OH | -OH | -OH | |||
| 4. | Icariin | H | OH | -O(C6H11O5) | C5H9 | OCH3 | |||
| 5. | Isoquercitrin | -(C6H11O5) | OH | OH | OH | OH | |||
| 6. | Kaempferitrin | (C6H11O4) | -OH | -O(C6H11O4) | -OH | ||||
| 7. | Kaempferol | H | OH | OH | OH | ||||
| 8. | Kaempferol-3-O-β-D-glucose | -(C6H11O5) | OH | OH | OH | ||||
| 9. | Miquelianin | -(C6H9O6) | OH | OH | OH | OH | |||
| 10. | Myricetin | H | OH | OH | OH | OH | OH | ||
| 11. | Myricitrin | -(C6H11O4) | OH | OH | OH | OH | OH | ||
| 12. | Quercetin | H | OH | OH | OH | OH | |||
| 13. | Quercetin-3-O-β-D-glucose | -(C6H11O5) | OH | OH | OH | OH | |||
| 14. | Quercitrin | -(C6H11O4) | OH | OH | OH | OH | |||
| 15. | Rutin | -(C12H21O9) | OH | OH | OH | OH |
| Sr. No. | Flavanone | C5 | C7 | C3′ | C4′ |
|---|---|---|---|---|---|
| 1. | Hesperidin | -OH | -O(C12H21O9) | -OH | -OCH3 |
| 2. | Isosakuranetin-5-O-rutinoside | -O(C12H21O9) | -OH | -OCH3 | |
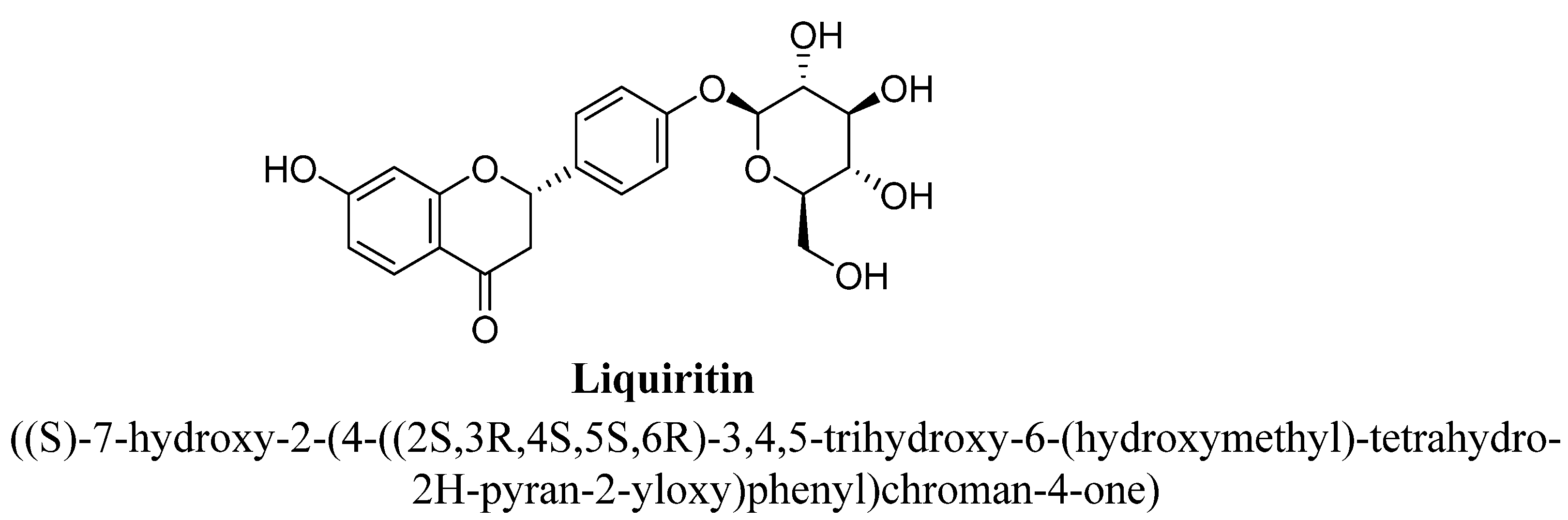
| 3. | Liquiritin | -OH | -O(C6H11O5) | ||
| 4. | Naringenin | -OH | -OH | -OH | |
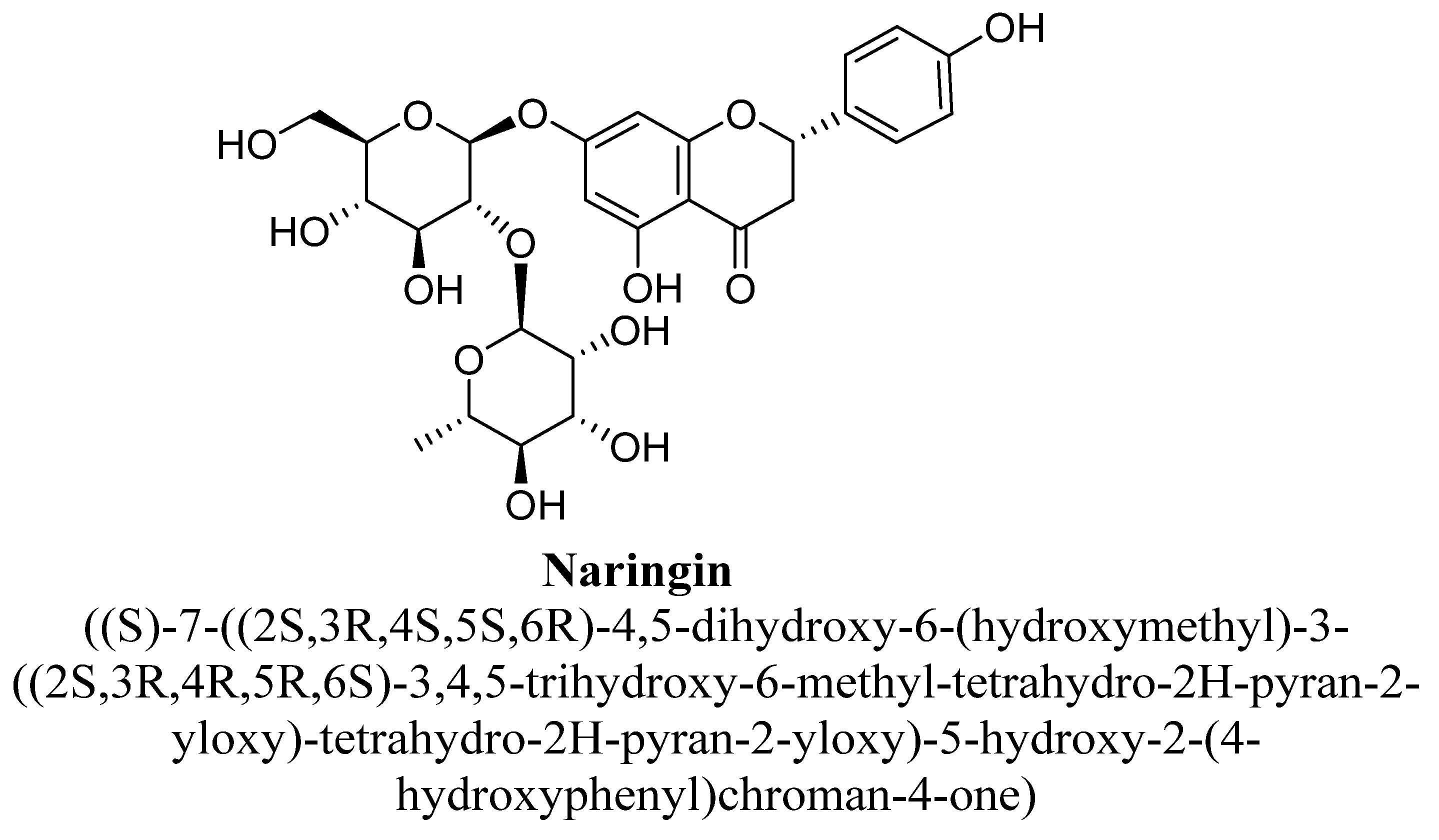
| 5. | Naringin | -OH | -O(C12H20O9) |
| Sr. No. | Flavanonols | R’ | C5 | C6 | C3′ | C4′ | C5′ |
|---|---|---|---|---|---|---|---|
| 1. | Astilbin | -O(C6H11O4) | -OH | -OH | -OH | -OH | |
| 2. | Dihydromyricetin | -H | -OH | -OH | -OH | -OH | -OH |
| Flavones | ||||||
|---|---|---|---|---|---|---|
| Isolated Bioactive Flavonoids | Doses | Route | Animal Species | Treatment Duration | Mechanism of Actions | References |
| 7,8-Dihydroxyflavone | 1, 3, and 10 mg/kg | Intra-gastric | Male Swiss mice | 60 min before test |
| [41] |
| 10 and 20 mg/kg | Intraperitoneal | Male C57BL/6 mice | 28 days |
| [42] | |
| 5 mg/kg | Oral | Male C57BL/6 mice | 21 days |
| [43] | |
| Amentoflavone | 6.25, 12.5, 25, or 50 mg/kg | Oral | Male Swiss albino mice | 3 days |
| [45] |
| Apigenin | 12.5 and 25 mg/kg | I.p. | Male ddY mice | 1 h |
| [47] |
| 20 mg/kg | I.g. | Male Sprague–Dawley rats | 21 days |
| [48] | |
| 20 and 40mg/kg | Oral | Male ICR mice | 21 days |
| [49] | |
| 25, 50 mg/kg | I.p. | Male ICR mice | 7 days |
| [51] | |
| Baicalein | 1, 2, or 4 mg/kg | I.p. | Male Kunming mice | 7 and 21 days |
| [54] |
| 10, 20, and 40 mg/kg | I.p. | Male Sprague–Dawley rats | 14 days |
| [55] | |
| 10, 20, or 40 mg/kg | Oral | Male Wistar rats | 35 days |
| [56] | |
| Chrysin | 5 and 20 mg/kg | Oral | Female C57B/6J mice | 28 days |
| [60] |
| 5 and 20 mg/kg | Oral | Male C57B/6J mice | 14 days |
| [61] | |
| 5 and 20 mg/kg | Oral | Female C57B/6J mice | 28 days |
| [62] | |
| Luteolin | 50 mg/kg | Oral | Male ICR mice | 23 days |
| [63] |
| 5 or 10 mg/kg | Oral | Male ICR mice | 30 min before test |
| [64] | |
| Nobiletin | 20, 50, or 100 mg/kg | Oral | Male ICR mice | After 60 min |
| [66] |
| Orientin | 20 and 40 mg/kg | Oral | Male Kunming mice | 21 days |
| [68] |
| Vitexin | 10,20 and 30 mg/kg | Oral | Male BALB/c mice | 60 min before test |
| [77] |
| Flavonols | ||||||
| 3,5,6,7,8,3′,4′-Heptamethoxyflavone | 50 mg/kg | S.c. | C57BL/6 mice | 25 days |
| [80] |
| Fisetin | 10 or 20 mg/kg | Oral | Male ICR mice | 4 days |
| [83] |
| 20, 40, or 80 mg/kg | Oral | Male ICR mice | 7 days |
| [84] | |
| 5 mg/kg | Oral | Male ICR mice | 21 days |
| [85] | |
| Hyperoside | 2.5, 5, and 10 μg/mL | I.p. | PC12 cell line | 4 h |
| [87] |
| 10, 20, or 40 mg/kg | I.p. | Male CF1 mice | 14 days |
| [88] | |
| 0.6 mg/kg | Oral | Male CD rats | 14–56 days |
| [89] | |
| Icariin | 20 and 40 mg/kg | Oral | Male Sprague–Dawley rats | 35days |
| [91] |
| 5 and 10 mg/kg | Oral | Male C57BL/6J mice | 28 days |
| [92] | |
| 20 and 40 mg/kg | Oral | Male Sprague–Dawley rats | 35 days |
| [93] | |
| 60 mg/kg | Oral | Male Sprague–Dawley rats | 21 days |
| [94] | |
| Isoquercitrin | 0.6 mg/kg | Oral | Male CD rats | 14–56 days |
| [89] |
| 2.5 mg/kg | Oral | Male Sprague–Dawley rats | 14 days |
| [95] | |
| Kaempferitrin | 1, 5, 10, or 20 mg/kg | Oral | Male Swiss Webster mice | 4 days |
| [98] |
| Kaempferol | 30 mg/kg/day | Oral | Male ICR mice | 14 days |
| [100] |
| 0.35 mM/kg | I.p. | Male Swiss mice | 60 min prior to the test |
| [101] | |
| Kaempferol-3-O-β-D-glucose | 0.35 mM/kg | I.p. | Male Swiss mice | 60 min prior to the test |
| [101] |
| Miquelianin | 0.6 mg/kg | Oral | Male CD rats | 14 days |
| [89] |
| Myricetin | 50 mg/kg | I.p. | Male C57BL/6 mice | 21 days |
| [104] |
| Myricitrin | 10 mg/kg | I.p. | Male Balb/C mice | 21 days |
| [106] |
| Quercetin | 2.5, 5, 10, 20 and 40 mg/kg | Oral | Male Sprague–Dawley rats | 14 days |
| [95] |
| 0.35 mM/kg | I.p. | Male Swiss mice | 60 min prior to the test |
| [101] | |
| 50 or 100 mg/kg | I.p. | Male Wistar rats | 21 days |
| [108] | |
| 40 and 80 mg/Kg | Oral | Male olfactory bulbectomy rats | 14 days |
| [109] | |
| 25 mg/kg | Oral | Female Swiss mice | 14 days |
| [211] | |
| Quercetin- 3-O-β-D-glucose | 0.35 mM/kg | I.p. | Male Swiss mice | 60 min prior to the test |
| [101] |
| Quercitrin | 30 mg/kg/day | Oral | Male ICR mice | 14 days |
| [100] |
| Rutin | 0.3, 1, 3, 10 mg/kg | Oral | Male Swiss mice | 4 days |
| [8] |
| 5 and 10 mg/kg | Oral | Male Sprague–Dawley rats | 14 days |
| [95] | |
| Flavanones | ||||||
| Hesperidin | 25, 50, or 100 mg/kg | Oral | Male albino Wistar rats | 21 days |
| [117] |
| 0.01, 0.1, 0.3, and 1 mg/kg | I.p | Male Swiss mice | 21 days |
| [118] | |
| 0.01, 0.1, 0.3, and 1 mg/kg | I.p. | Male Swiss mice | 21 days |
| [119] | |
| 25 and 50 mg/kg | Oral | Male ICR mice | 21 days |
| [121] | |
| 0.01, 0.1, 0.3, and 1 mg/kg | I.p. | Male Swiss mice | 21 days |
| [122] | |
| 0.4, 4, 8, 16, and 32 mg/kg | Oral | Male imprinting control region mice | After 1 h |
| [119] | |
| 50 mg/kg | Oral | Male C57BL/6 mice | 13 days |
| [217] | |
| Isosakuranetin-5-O-rutinoside | 15 and 30 mg/kg | Oral | Male ICR mice | 24, 18, and 1 h before test |
| [123] |
| Liquiritin | 10, 20 and 40 mg/kg | G.i. | Male mice | 30 min before Sample |
| [124] |
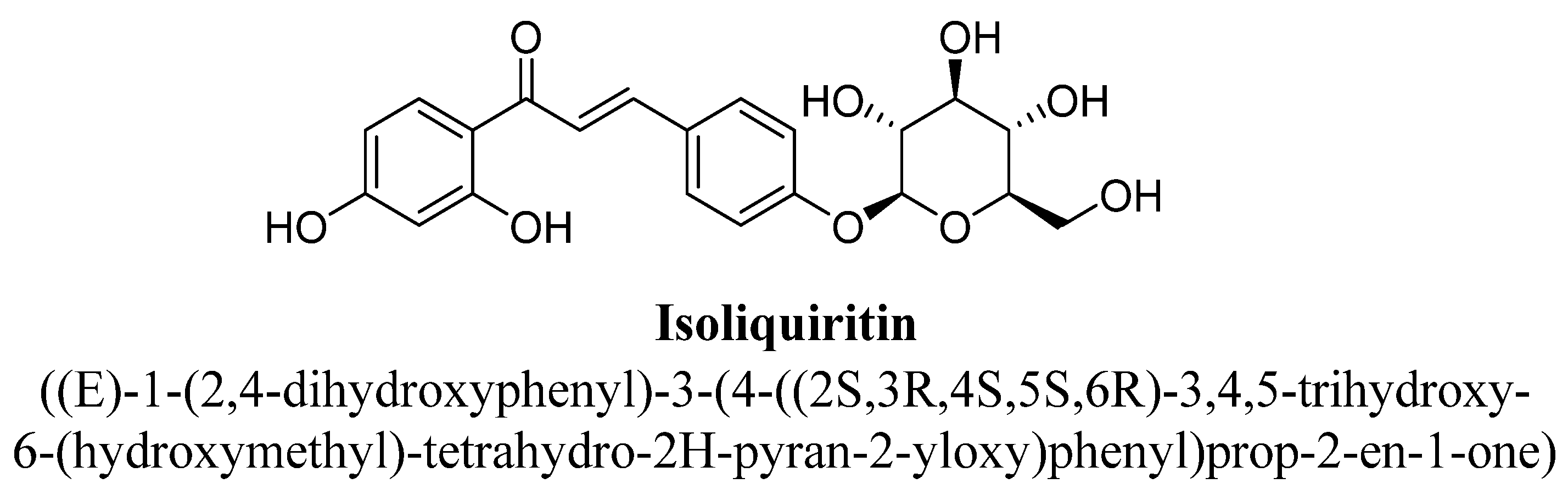
| Isoliquiritin | 10, 20 and 40 mg/kg | G.i. | Male mice | 30 min before sample |
| [124] |
| Naringenin | 5, 10 and 20 mg/kg | Oral | Male ICR mice | 14 days |
| [130] |
| 20 mg/kg | Oral | Male ICR mice | 21 days |
| [128] | |
| Naringin | 50 and 100 mg/kg | I.p. | Male Wistar rats | 14 days |
| [133] |
| Flavanonols | ||||||
| Astilbin | 10, 20, or 40 mg/kg | I.p. | Male C57BL/6J mice | 21 days |
| [136] |
| Dihydromyricetin | 10 and 20 mg/kg | I.p. | Male C57BL/6J mice | 7 days |
| [137] |
| Flavanols | ||||||
| Catechin | 88.6 and 58.9 µM | - | Wistar male rat | - |
| [139] |
| Epicatechin | 88.6 and 58.9 µM | - | Wistar male rat | - |
| [139] |
| Epigallocatechin gallate | 500 ng/mL | - | Sprague–Dawley rat brains | 24 h |
| [141] |
| Other flavonoids | ||||||
| Silibinin | 100 and 200 mg/kg | Oral | Either sex Wistar rats | 14 days |
| [143,144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pannu, A.; Sharma, P.C.; Thakur, V.K.; Goyal, R.K. Emerging Role of Flavonoids as the Treatment of Depression. Biomolecules 2021, 11, 1825. https://doi.org/10.3390/biom11121825
Pannu A, Sharma PC, Thakur VK, Goyal RK. Emerging Role of Flavonoids as the Treatment of Depression. Biomolecules. 2021; 11(12):1825. https://doi.org/10.3390/biom11121825
Chicago/Turabian StylePannu, Arzoo, Prabodh Chander Sharma, Vijay Kumar Thakur, and Ramesh K. Goyal. 2021. "Emerging Role of Flavonoids as the Treatment of Depression" Biomolecules 11, no. 12: 1825. https://doi.org/10.3390/biom11121825
APA StylePannu, A., Sharma, P. C., Thakur, V. K., & Goyal, R. K. (2021). Emerging Role of Flavonoids as the Treatment of Depression. Biomolecules, 11(12), 1825. https://doi.org/10.3390/biom11121825







