Abstract
Obesity is characterized by excessive accumulation of fat in the body, which is triggered by a body energy intake larger than body energy consumption. Due to complications such as cardiovascular diseases, type 2 diabetes (T2DM), obstructive pneumonia and arthritis, as well as high mortality, morbidity and economic cost, obesity has become a major health problem. The global prevalence of obesity, and its comorbidities is escalating at alarming rates, demanding the development of additional classes of therapeutics to reduce the burden of disease further. As a central energy sensor, the AMP-activated protein kinase (AMPK) has recently been elucidated to play a paramount role in fat synthesis and catabolism, especially in regulating the energy expenditure of brown/beige adipose tissue and the browning of white adipose tissue (WAT). This review discussed the role of AMPK in fat metabolism in adipose tissue, emphasizing its role in the energy expenditure of brown/beige adipose tissue and browning of WAT. A deeper understanding of the role of AMPK in regulating fat metabolism and energy expenditure can provide new insights into obesity research and treatment.
1. Introduction
Obesity is a disorder of nutrition and metabolism, which is triggered by genetic factors and environmental factors. Excessive accumulation of fat in the body is the main feature of obesity, which is caused by a body energy intake larger than body energy consumption. Obesity has become an increasingly severe global public health problem, due to its high incidence and the complexity of its related diseases, such as T2DM, liver steatosis, cardiovascular disease, stroke, dyslipidemia, hypertension, gallbladder problems, osteoarthritis, and certain types of cancer (endometrium, breast, ovary, prostate, liver, gallbladder, kidney, and colon) [1].
In mammalian cells, three different types of adipocytes are identified as white adipocytes, brown adipocytes, and beige adipocytes [2]. White adipocytes store excess energy in the form of triacylglycerol (TAG) and enjoy huge expansion capacity, so they are used to store energy to maintain the energy balance of the body. The excessive deposition of white adipocytes in WAT is implicated in obesity, insulin resistance, diabetes, and other metabolic diseases [3]. Contrary to white adipocytes, brown adipocytes are rich in mitochondria and are characterized by the high level of uncoupling protein 1 (UCP1) [4]. UCP1 can eliminate the membrane potential generated by mitochondria, increase oxygen consumption, and reduce ATP synthesis in the process of decoupling, and dissipate lipid to generate heat, which is an effective way to assist energy waste, thus producing a negative energy balance [5,6]. Thus, the energy expenditure will be increased by the activation of brown adipocytes. Recently, a beige adipocyte has been identified as the intermediate between BAT and WAT in mice and humans [7,8,9,10]. Theories behind the origin of beige adipocytes include (i) trans-differentiation from mature white adipocytes, (ii) existence of a distinct beige fat cell precursor, and (iii) differentiation from brown or white adipocyte precursors [5]. In the condition of chronic β-adrenergic stimuli or cold, WAT can demonstrate quite a few characteristics similar to brown adipose tissue, such as higher content of mitochondria and higher expression of UCP1, and this process is known as ‘browning’. The ‘browning’ WAT is called beige adipose tissue [11]. Beige fat is easily induced by various stimuli, such as chronic cold stimulation, exercise, and agonists of pro-adipogenic or pro-thermogenic transcription factors [12,13,14]. Considering the beneficial effects of BAT occurrence, remodeling white fat into thermogenesis-able beige fat has been considered as an attractive and promising therapeutics for the treatment of obesity and its related metabolic diseases.
AMPK plays a critical role in the regulation of adipose tissue metabolism. Current evidence confirms that AMPK activation is associated with lipogenesis/adipogenesis, fatty acid (FA) oxidation, BAT thermogenesis, and browning of WAT [15,16]. AMPK is a highly conserved and ubiquitously expressed serine/threonine protein kinase, which is a multiple heterotrimeric complexes and is made up of an α (isoforms α1 or α2) catalytic subunit, a regulatory and structurally crucial β (isoforms β1or β2) subunit, and a regulatory γ (isoforms γ1, γ2 or γ3) subunit [17]. The α (isoforms α1 or α2) catalytic subunit is comprised of an N-terminal kinase domain, an autoinhibitory domain, two regulatory-subunit-interacting domain, and a C-terminal β/γ subunits binding domain [18]. A catalytic phosphorylation site threonine-172 (Thr172) is contained on the N-terminus kinase domain of the α catalytic subunit. AMPK is activated when the Thr172 residue is phosphorylated, and this process can be induced by AMP [19] and a lot of upstream kinases, such as the calmodulin-dependent kinase kinases (CaMKKs) [20], the liver kinase B1(LKB1) [21], and transforming growth factor (TGF)-β-activated kinase-1 (TAK1) [22]. The β regulatory subunit is comprised of a glycogen-binding domain (GBD), a C-terminal scaffold domain, which promotes the combination of α and γ subunit. The β regulatory subunit is essential for maintaining the integrity of AMPK heterotrimer, and can regulate the reactivity of AMPK to the direct activators, such as A-769662 [23]. Four tandem cystathionine-beta-synthase (CBS) domains are contained in the γ subunit, and form the allosteric regulatory site of AMP/ATP on AMPK [24]. The compromised cellular energy status results in an increase in the proportion of cellular AMP: ATP, and AMP or ADP, can then bind to the CBS1 or CBS3 of the γ subunit, resulting in conformational change that activates AMPK via phosphorylation of the Thr172 site in the α subunit [25]. 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), an AMPK activator, which is an AMP mimetics, activates AMPK by interacting with γ subunit [26].
Indeed, AMPK activation is linked with a host of metabolic improvements and appears to play a vital role in mediating the beneficial effects of various pharmaceuticals/nutraceuticals [23,27,28]. In this review, in order to clarify the role of AMPK in adipose tissue, especially in regulating energy expenditure of brown/beige adipose tissue and browning of WAT, we summarize the recent studies of AMPK on fat catabolism, anabolism, and WAT browning process. Future considerations for studies examining the role of AMPK in fat metabolism and obesity are highlighted.
2. Mechanism of AMPK Improving Obesity
AMPK, a central regulator of cellular metabolism, mediates phosphorylation of target substrates and plays a paramount role in the regulation and maintenance of energy homeostasis, which is emerging as one of the most promising targets in the prevention and treatment of obesity based on its pivotal role in physiology and pathology [29,30]. Concerning the effect of AMPK on adipose tissue metabolism, we summarized four main mechanisms, including the role in lipogenesis/adipogenesis, lipolysis, energy expenditure, and browning of WAT.
2.1. The Role of AMPK in Lipogenesis/Adipogenesis
A paramount pathway of lipogenesis is the de novo synthesis (DNL), which is a complex and highly regulated metabolic pathway. In normal conditions, excess carbohydrate is converted into FA by DNL, and then the FA is then esterified into stored TAG, in the liver, and adipose tissue in the human body (Figure 1) [23]. In the cytosol, the acetyl-CoA is then converted to malonyl-CoA catalyzed by acetyl-CoA carboxylase (ACC, the first step in DNL), which is a speed limiting enzyme for FA synthesis [31]. The activity of ACC is inhibited by AMPK-mediated phosphorylation of ACC1 at Ser79 or ACC2 at the orthologous site Ser212, which reduces conversion of acetyl-CoA to malonyl-CoA and thus inhibits lipogenesis [15,32]. Therefore, AMPK activation inhibits FA synthesis by phosphorylating ACC at Ser79 to decrease the malonyl-CoA level. The ACC mutated mice have increased TAG accumulation when fed a normal chow diet compared with wide-type controls [31]. This is supported further by the observation that both fasting and exercise stimulate AMPK in rat adipose tissue with a concomitant reduction in malonyl-CoA, which may reflect inhibition of ACC [33]. After intravenous caffeine injection in rats, caffeine increased the phosphorylation of AMPK as well as ACC, and eventually increased glucose transport activity, and reduced the energy state [34]. These data indicate that removing AMPK phosphorylation of ACC leads to an increased TAG deposition through increasing FA DNL. Conversely, increasing AMPK activity or inhibiting ACC activity can improve TAG deposition by inhibiting FA synthesis (Figure 3).
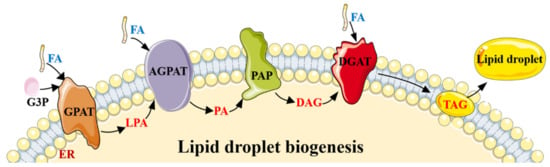
Figure 1.
The lipogenesis process by DNL. Lipid droplet biogenesis process. Glycerol-3-phosphate (G3P) and one molecule of activated FA generate lysophosphatidic acid (LPA), under the action of glycerol-3-phosphate acyltransferase (GPAT). LPA and another molecule of activated FA generate phosphatidic acid (PA) catalyzed by 1-acylglycerol-3-phosphate O-acyltransferase (AGPAT), and then PA is converted into diacylglycerol (DAG) by phosphatidate phosphatase (PAP). Then, diacylglycerol acyltransferase (DGAT) converts DAG and one molecule of activated FA into TAG. The whole process occurs in the endoplasmic reticulum (ER), and the generated TAG is released between the lipid bilayers of the ER membrane. When TAG is accumulated to a certain extent, new lipid droplets sprout from the ER and are released into the cytoplasm.
In the process of lipogenesis regulated by AMPK, another target of AMPK is sterol regulatory-element-binding protein-1c (SREBP-1c), which regulates the expression of various lipogenic genes, such as, ACC1, FA synthase (FAS), and stearoyl-CoA desaturase 1 (SCD1) [35]. SREBP-1c, the primary transcriptional regulator of fat lipogenesis, is inactivated after phosphorylation at Ser372 by AMPK, which downregulates lipogenic gene expression [35].
Preadipocytes differentiate into mature adipocytes mainly through two stages: the first stage is the cessation of cell division, and the withdrawal of cell division cycle; the other stage is characterized by cell growth stagnation, the beginning of differentiation, lipid droplet appearance, and eventually differentiate into mature adipocytes [36]. The process adipogenesis is accompanied by the elevated expression of the transcription factor PPARγ, which in turn can activate C/EBPα. PPARγ and C/EBPα accelerate cell differentiation in a positive feedback manner and induce the expression of quite a few adipocyte marker genes, such as adipocyte-specific FA-binding protein and FAS [37,38].
Activated AMPK has been proposed to inhibit proliferation in several cell types in different pathways. For example, AMPK has been demonstrated to inhibit cell proliferation via inhibition of rapamycin complex (mTOR) T-cell acute lymphoblastic leukemia [39], inhibit endothelial cell proliferation via elevation of p21 and p27 expression [40], and block the growth of the HepG2 cell line via phosphorylation of p53 [41], especially the role of AMPK in preadipocyte proliferation. A multitude of studies have shown that AMPK plays a paramount role in inhibiting adipogenesis via reducing the expression of C/EBPβ (which is essential for initiation of the adipogenic transcriptional cascade), and subsequent inhibition of PPARγ, C/EBPα and late adipogenic markers such as FAS, aP2, and SREBP-1c [38,42]. Recent studies have shown that the impact of AMPK on adipogenesis may be related to the WNT/β-catenin pathway regulated by AMPK [43]. AMPK activated by AICAR increases the expression of β-catenin and nuclear accumulation, with reduced expression of adipogenic genes, such as C/EBPβ, PPARγ, C/EBPα, FAS, aP2, and SREBP-1c in 3T3-L1 adipocytes. This is reversed with siRNA-mediated knockdown of β-catenin, providing a quintessential mechanism by which AMPK inhibits adipogenesis (Figure 3) [43,44].
2.2. The Role of AMPK in Lipolysis
In adipocytes, the process in which TAG is hydrolyzed into glycerol and FA is called lipolysis, which takes three stages to complete. In the first place, TAG is hydrolyzed to DAG and FA by adipose triglyceride lipase (ATGL, also known as desnutrin). Second, DAG is hydrolyzed to monoacylglycerol (MAG) and FA by hormone-sensitive lipase (HSL). Third, MAG is hydrolyzed to glycerol and FA by MAG lipase (MAGL) (Figure 2) [45]. Every reaction is tightly regulated, and AMPK plays a paramount role in lipolysis.
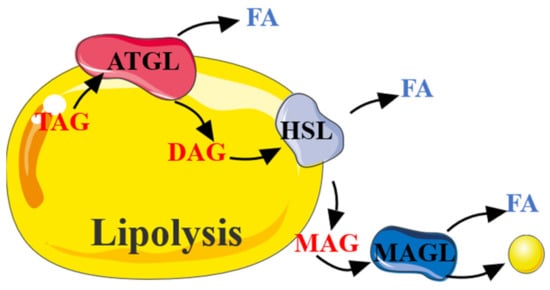
Figure 2.
The process of lipolysis. TAG was decomposed by continuous catalysis of ATGL, HSL, and MAGL to release FA.
The role of AMPK in lipolysis is controversial. Several studies report an anti-lipolytic effect of AMPK [46], whereas others suggest AMPK stimulates lipolysis [47,48]. This difference may be related to the tissue-specific function of the AMPK under different conditions. HSL is considered a speed-limiting enzyme for TAG hydrolysis, and the Ser563 and Ser660 sites of HSL can be phosphorylated to activate HSL for the lipolysis in adipose tissue, which is essential for the translocation of HSL to lipid droplets upon lipolysis stimulation. However, AMPK phosphorylated the Ser565 site of HSL and inhibited the phosphorylation at HSL Ser660 and Ser563, thereby reducing HSL activity and significantly inhibiting lipolysis in adipocytes [25]. Based on this study, it is considered that AMPK activation inhibits lipolysis (Figure 3).
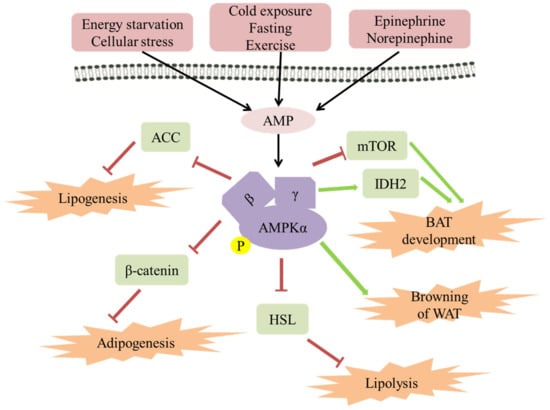
Figure 3.
The role of AMPK in regulating fat metabolism and energy expenditure in adipose tissue. AMPK has been activated by AMP in a starvation or stress state. It has been established that AMPK can be activated by cold exposure, fasting, and exercise, in a β-adrenaline-dependent manner. Activated AMPK leads to ATP production and a series of metabolic changes, for example, lipogenesis, adipogenesis, lipolysis, BAT energy expenditure, and browning of WAT.
However, new observations indicate that phosphorylation of HSL at Ser563 is not indispensable for the translocation of HSL to the surface of the lipid droplet [49]. While Ser565 is necessary for the translocation of HSL to the surface of the lipid droplet, because Ser565 is mutated into alanine, which leads to the inhibition of HSL translocation to the surface of the lipid droplet [49].
In addition, unused FA liberated during lipolysis returns to adipocytes to be re-esterified into TAG, which creates an energy-consuming ‘futile cycle’ [50]. In fact, it has been estimated that about 40% FA liberated during lipolysis is re-esterified into TAG in human adipose tissue [51]. FA re-esterification needs to consume a lot of ATP, which leads to an increase in the AMP/ATP ratio, while a high AMP/ATP ratio subsequently activates AMPK. Therefore, AMPK may be indirectly activated by elevation of lipolysis due to an increase in the AMP/ATP ratio [52]. This is supported by the observations that mice lacking AMPKα1 enjoy smaller adipocytes with higher basal and β-adrenergic-stimulated lipolysis rates [53]. In fact, lipolysis has been significantly inhibited by the activation of AMPK both in primary adipocytes and in vivo, as reflected by the low FA content in serum [54]. However, with the prolongation of AICAR treatment time, AMPK activation increased lipolysis. This is supported by the observations that the release of glycerol either under basal or epinephrine-stimulated conditions is potently suppressed, and the output of FA under these conditions is initially reduced and then markedly elevated as the time of exposure of adipocytes to AICAR [54]. This phenomenon can be explained by that continuous activation of AMPK promotes the expression of ATGL, which converts ATG to DAG and FA, inhibits the activation of HSL, which then converts DTG to MAG and FA [54].
Taken together, these findings suggest that AMPK has both promoting and inhibiting effects on lipolysis through different time-dependent regulation of HSL, ATGL, and other enzymes, as well as changing the expression of genes promoting lipid utilization or storage in adipocytes. The overall impact of AMPK activation on lipolysis remains controversial, and future studies are needed to elucidate the effects of chronic AMPK activation on tissue specificity and systemic lipid metabolism, which may have crucial implications for the treatment of obesity.
2.3. The Role of AMPK in Energy Expenditure in Adipose Tissue
Obesity is characterized by excessive accumulation of fat in the body, which is caused by a body energy intake larger than body energy consumption. Therefore, the treatment of obesity is mainly focused on reducing the energy intake and increasing the energy output [55]. However, the therapeutic strategy that reduced the energy intake demonstrated strong side effects, and perhaps instead the therapeutic approach aimed at increasing energy expenditure will be an acceptable and attractive strategy to combat obesity [55,56,57]. Three different types of adipose tissue have existed in humans, and WAT is mainly used to store excess energy, while brown and beige adipose tissue is related to energy expenditure. Therefore, the strategy of increasing the thermogenic capacity of beige adipose tissue and brown adipose tissue may be the most effective and attractive treatment for obesity in humans [58,59].
As in a multitude of obese patients, AMPK activity is significantly impaired in a variety of obese animal models [60,61,62]. When stimulated by cold or β-adrenaline, the capability of adaptive thermogenesis and energy expenditure have been significantly attenuated in mice with adipose tissues AMPKα knockout [14]. The cold-induced thermogenic genes expression has been dramatically impaired in adipose tissues lacking AMPKα [14]. A study demonstrated that AMPK β1β2 adipose tissue-specific null (AMPKβ1β2-AKO) mice were generated and were found to develop an increase in high-fat diet (HFD)-induced insulin resistance and hepatic steatosis due to compromised BAT and WAT function [14,63]. Another study reported that adipose tissue-specific deletion of both AMPK α1 and α2 subunits (AMPKα1α2-AKO) are found to reduce lipolysis under basal conditions and increase lipolysis in adipose tissue treated with isoproterenol [15,64]. This indicates that AMPKα is required for adipose tissue thermogenesis and energy expenditure. Moreover, inguinal white adipocyte is promoted by adopting metabolic characteristics similar to brown adipocyte by AMPK activated by A-769662, which protects from HFD-induced obesity [14]. Collectively, these findings indicate that the inhibition of AMPK may lead to obesity and other related metabolic diseases, while activated AMPK may improve them.
Adipose tissue thermogenesis and energy expenditure are regulated by the expression of thermogenic genes, such as UCP-1, peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1α), which promote the development of brown and beige adipocyte [65]. Brown adipose tissue (BAT) consumes significant amounts of chemical energy through uncoupled respiration and thermogenesis mediated by the major thermogenic factor UCP-1 [66], which raises the interest in stimulating thermogenesis therapeutically for the treatment of metabolic diseases associated with obesity. PGC1α, another factor that regulates adipose tissue thermogenesis and energy expenditure, promotes the development of brown and beige adipocytes [65]. It is proved that the diabetes medication canagliflozin induces mitochondrial biogenesis and function through the AMPK-Sirt1-Pgc-1α signaling pathway, which directly increases cellular energy expenditure of adipocytes [67]. The role of AMPK alluded in brown adipogenesis is mainly focused on differentiation. CIDEA is involved in the degradation of the AMPKβ subunit through regulating the proteasome activity dependent on ubiquitin. When CIDEA expression is knocked out in mice, AMPK expression and activity are increased in BAT, and the mice accordingly had increased energy expenditure, and obesity triggered by an HFD was suppressed [68]. In addition, it has been recently confirmed that AMPK increased the development of brown adipocytes with a high abundance of UCP-1 [69]. Consistent with the phenotype observed when AMPKα activity is inhibited, brown adipocyte progenitor cells are profoundly decreased and brown adipogenesis is inhibited in AMPK deficient mice [70,71]. In general, there is growing evidence on the important role of AMPK as a promoter of the development of brown adipocytes.
Furthermore, UCP-2 and UCP-3 are also involved in the regulation of energy metabolism as UCP homologs. In animals with an HFD, exercise, and hibernation, the mRNA expression levels of UCP-2 and UCP-3 increased accordingly to promote glucose and fatty acid metabolism. The data from human genetic studies and measurements support the contribution of UCP2 and UCP3 to resting energy expenditure. Therefore, enhancing the expression of UCPs may increase energy expenditure and provide a defensive effect on disordered lipid metabolism and damage [72,73].The mammalian target of mTOR, an essential regulatory factor of cell growth, proliferation, and metabolism, the same as AMPK, plays a critical role in the process of preadipocyte differentiation [74]. AMPK activity is dependent on low-energy conditions, such as low ATP/AMP ratio, while mTOR is activated by diverse growth-positive signals, such as high ATP/AMP ratio [17,75]. mTOR promotes DNL through sterol responsive element binding protein (SREBP) transcription factors, promoting growth by promoting the transition of glucose metabolism from oxidative phosphorylation to glycolysis, and obese or HFD treated mice have more elevated mTOR signaling in many tissues, including the pancreas [76]. In fact, the mTOR activity is inhibited by AMPK through the phosphorylation of tuberous sclerosis complex 2 (TSC2), which is a critical regulatory protein of mTOR activity [77,78]. An early study demonstrates that the inhibition of mTOR by AMPK is essential for the differentiation of brown adipocytes in the early stage of differentiation [78]. In addition, it has been observed that brown adipose tissue and energy expenditure are increased, and the resistance to obesity induced by a high-fat diet is enhanced in mice with Raptor knockout in adipocytes [79]. However, persistent mTOR inhibition weakens the function of brown adipose tissue and increases fat accumulation [80,81,82]. These reveal a cross regulating role of the AMPK and mTOR pathway in the differentiation and function of brown adipocytes (Figure 3).
Differentiation of brown adipose tissue regulated by AMPK may be related to DNA methylation and hydroxymethylation, which are closely associated with the cellular metabolic status and enzyme cofactors [83]. It is observed that brown adipose tissue mass, UCP-1 expression, and mitochondrial content are all lower in neonatal mice with AMPKα1 ablation. Interestingly, high levels of DNA methylation and low levels of hydroxymethylation are also observed in the Prdm16 promoter in these AMPKα1 deficient neonatal mice [71]. AMPKα1 ablation reduced isocitrate dehydrogenase 2 activity and cellular α-ketoglutarate levels, a key metabolite regulator for DNA demethylation mediated by ten-eleven translocation hydroxylases. Therefore, the differentiation of brown adipose tissue is related to the methylation of the Prdm16 promoter, which is associated with the increase of IDH2 activity induced by AMPK (Figure 3) [71].
The role of AMPK in adipose tissue can be further confirmed by the complete ablation of the AMPK alpha subunit or beta subunit in cultured adipocytes or mice adipose tissue. Since 2016, mice with the complete ablation of AMPK alpha1 and alpha2 subunits, or AMPK beta1 and beta2 subunits in adipose tissues, have been produced [14,63]. Mice with the complete ablation of AMPK alpha1 and alpha2 subunits (AMPKα AKO mice) were cold intolerant, and their inguinal WAT displayed impaired mitochondrial integrity and biogenesis, decreased energy expenditure and oxygen consumption, and reduced expression of thermogenesis-related genes upon cold exposure, such as UCP-1, CIDEA, Cox8b, Cox7a1, and Ppargc1a [14]. Mice with complete ablation of AMPK beta1 and beta2 subunits (AMPKβ AKO mice) were cold intolerant and resistant to β-adrenergic activation of BAT and browning of WAT [63]. These results demonstrate that AMPK plays a vital role in the browning process in inguinal WAT and regulates whole-body energy homeostasis, which suggests that the directly targeted activation of adipocyte AMPK is likely to be therapeutically viable. Further research reveals that the number of mitochondrion has been significantly affected by the ablation of adipocyte AMPK. The specific ablation of AMPK beta1 and beta2 subunits in adipose tissue, does not alter the mitochondrial number of BAT, but reduces the mitochondrial quality, which is characterized by large swollen mitochondrial and disrupted cristae in morphology [63]. Thus, the impact of the adipose tissue-specific ablation of AMPK on energy expenditure is implicated in the regulation of mitochondrial function by AMPK.
2.4. The Role of AMPK in Browning of WAT
Under the stimulation of certain factors, white adipocytes will be transformed into beige adipocytes, which is called ‘browning’. When these beige adipocytes are not activated, they exhibit properties similar to white adipocytes. Together with white adipocytes, they serve as an energy reservoir to release energy in the form of free FA and play an important role in glucose and other metabolisms. Once activated, beige adipocytes will acquire many dense mitochondria with high UCP1 expression and high thermogenic capacity similar to brown adipocytes for core thermoregulation, etc. [84].
In recent years, due to beige adipocytes having a heat-generating ability similar to brown adipocytes, there has been an increasing interest in the browning of fat. Although exercise, cold exposure, or beta-adrenergic have been considered as the most common inducers for fat browning, there are other inducible factors, such as, beta amino isobutyric acid, inflammatory stress, gamma amino butyric acid, hypoxia, PPARɣ agonists, JAK inhibition, and irisin (a cleavage product of Fndc5 gene) [8,85,86,87,88]. AMPK is a key regulator of brite cell formation in browning. Aliki et al. performed lentivirus-mediated short hairpin knockout and experiments with pharmacological inhibitors to prove that AMPK promotes the formation of UCP1-rich brown/beige adipocytes [69]. The change or disappearance of any subunit of AMPK will affect the appearance of brown/beige adipocytes, as shown through knock-down specific subunits. AMPKβ AKO mice enjoy an impaired browning ability to decrease mitochondrial content in the WAT, so only a small amount of beige fat can be produced [23]. Wu et al. found that a lack of adipocyte AMPKα induced thermogenesis and obesity under cold and over-nutrient conditions [14]. Consistent with these observations, numerous other studies have found that indirectly increasing AMPK activity in WAT also results in the browning of WAT and increases in energy expenditure, such as, folliculin, berberine, xanthohumol, myostatin, and liver kinase B1 [89,90,91,92,93]. In addition, the AMPK in the ventromedial nucleus of the hypothalamus (VMH) regulates thermogenesis by manipulating the sympathetic nerve firing to beige adipose tissue [94]. Phytol administration stimulates the browning of mice inguinal subcutaneous WAT, with an increased expression of brown adipocyte marker genes (UCP1, PRDM16, PGC1α, PDH, and Cyto C), which activated the AMPKα signaling pathway in mice inguinal subcutaneous WAT and 3T3-L1 cells [95,96]. Consistent with the result, the inhibition of AMPKα with Compound C (dorsomorphin, a selective AMPK inhibitor) abolished phytol-stimulated brown adipogenic differentiation and the formation of brown-like adipocytes [95,97]. These demonstrate that AMPK plays an important role in the browning of WAT. However, whether AMPK activation is a necessary and sufficient condition for browning phenotypes of white fat and the specific mechanism of AMPK activation inducing browning, are still not entirely clear. Future research should focus on improving the browning level of WAT and the particular mechanism of AMPK promoting the browning of WAT.
3. Other Functions of AMPK
In addition, besides the role in lipid metabolism, AMPK also regulates a variety of physiological metabolic processes, such as carbohydrate metabolism, protein metabolism, cell polarity, growth, apoptosis, and ferroptosis [98,99,100,101]. Correspondingly, the AMPK has been associated with a wide range of pathological conditions, such as, aging and longevity, obesity and metabolic syndrome, cardiovascular disease and reperfusion injury, cancer, dementia, neurogenesis, and stroke [98,102]. For aging and longevity, AMPK regulates the role of some downstream nutritional sensors, and ultimately controls the cell and physiological processes, such as, mTOR, the insulin/insulin-like growth factor signaling pathway (IIS), and the sirtuins, which directly or indirectly participates in the regulation of some signal, including AMPK, PGC1α, and mTOR, to delay cellular senescence by deacetylating some key proteins [103,104]. For cardiovascular disease and reperfusion injury, the activation of the AMPK signaling pathway is a protective mechanism in the ischemia/reperfusion injury, which might be associated with the reduction of protein synthesis triggered by the inactivation of eIF2α [105,106]. In addition, the activated AMPK elevates the level of glucose-regulated protein 78, reduces the expression of pro-apoptotic molecules CHOP and caspase 3, increases the ratio of Bcl2/Bax, and inhibit the ERS-induced apoptosis [107]. Activated AMPK could also inhibit cardiomyocyte apoptosis [108]. For cancer, AMPK is closely correlated to the tumor-suppressive functions of LKB1 and P53, consequently modulating the activity of cell survival signaling such as mTOR and Akt, leading to cell growth inhibition and cell cycle arrest [109,110,111]. Dementia, neurogenesis, and stroke are neurodegenerative disorders characterized by a progressive degeneration of nerve cells, eventually leading to disease. It has been reported that neurodegenerative disorders might be correlated with the overactivated AMPK [112,113,114]. At the systemic level, AMPK helps control appetite, energy expenditure, and substrate utilization in response to exercise, nutrients, and cytokines may be linked to an ocean of physiological processes. AMPK signaling may be altered in a number of disease conditions; however, despite the convincing associations between AMPK signaling under a number of different conditions and treatments, there is an urgent need for studies on the exact mechanism of AMPK.
4. Advances in AMPK Targeted Drugs
More and more AMPK targeted drugs are currently appearing in front of the public, but due to various challenges, such as the complexity of different combinations of AMPK subunit isoforms in tissues and cells of different species, the phosphorylation events of AMPK activation and the safety of AMPK activation are still unclear [115], as currently only one has been approved by the regulatory authorities to enter the market and more are in clinical trials. Metformin, launched in 1959, has become an excellent first-line pharmacologic treatment for type 2 diabetes in most patients. Unlike most modern drugs, metformin is derived from natural products used in herbal medicine, rather than designed for specific pathways or mechanisms [116]. One of its prominent therapeutic effects is the inhibition of liver gluconeogenesis. The most widely studied mechanism of the drug is the activation of the signal kinase AMPK, so as to achieve the main therapeutic effect of inhibiting hepatic gluconeogenesis. In addition, studies have shown that metformin exerts its anti-obesity effects by increasing mitochondrial biogenesis, reducing fatty acid intake, and stimulating brown fat metabolism to produce heat [117]. Therefore, the fixed-dose combination of metformin and sibutramine has undergone a phase III clinical study in Silanes to treat obesity. However, because sibutramine may increase the risk of heart disease and stroke among users, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency suspended the sale and use of all weight-reducing drugs containing sibutramine in the European Union in 2010, so there have been few reports on this study recently. Fluoxetine, a drug under preclinical study and mainly used to treat various depression, has been proved to have remarkable antiproliferative activity and induce autophagy death in breast cancer cells. The mechanism of its autophagy death is related to the inhibition of eEF2K and the activation of the AMPK-mTOR-ULK complex axis [118]. Metformin combined with fluoxetine can enhance the antidepressant effect of fluoxetine, and its mechanism may be related to the activation of AMPK and cyclic adenosine monophosphate response element binding protein (CREB) [119]. Although many AMPK-related drugs have not yet been marketed due to various challenges, they still have huge therapeutic potential. Finally, the summary of other AMPK-related drugs is shown in Table 1.

Table 1.
AMPK targeted drugs in obesity therapy.
5. Conclusions
AMPK is present in all tissues as αβγ heterotrimer, which is regulated by multiple genes encoding each of the subunits (α1, α2, β1, β2, γ1, γ2, γ3) with differential tissue-specific expression and activity. AMPK regulates cellular energy metabolism through direct effects on gene transcription and key metabolic enzymes. There is now clear and compelling evidence that AMPK plays an important role in regulating adipose tissue metabolism, especially in regulating the energy expenditure of brown adipose tissue and beige adipose tissue.
However, there are still quite a few important issues that require further study. Firstly, AMPK integrates a multitude of inputs and acts on an ocean of different substrates, and is a central energy sensor in vivo, which is involved in the regulation of multiple metabolic pathways. Determining the predominant effectors of AMPK mediated browning of WAT and non-shivering thermogenesis will be an issue for future studies. Secondly, whether beige adipocytes can contribute to whole-body energy expenditure significantly in vivo remains controversial, particularly as accumulating evidence exists that the thermogenic contribution might be insignificant [88,120]. Furthermore, as studies have shown that AMPK exerts opposite effects on the central nervous system and peripheral metabolism, activation of the system is not the best strategy to treat obesity, so site-specific operations on AMPK are necessary. In addition, beige fat might enjoy a different physiological function than thermogenesis. This is supported by the fact that it can be induced by exercise [121]. The role of AMPK in the browning of adipocytes remains to be studied further.
Author Contributions
Q.W., L.Z. and Y.L. participated in the conception and design of the study. Q.W. and J.S. consulted the relevant literature and wrote the manuscript. M.L. and Y.Z. performed the literature research, participated in writing of the manuscript related to the AMPK targeted drugs. L.Z. and Y.L. revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (No. 81803573, 81870591), China Postdoctoral Science Foundation (No. 2018M640672), Key R&D and Promotion Projects in Henan Province (Nos. 202102310155, 212102310874 and 212102311019), College Students Innovative Entrepreneurial Training Plan Program (No. 202110475083). College Students Innovative Entrepreneurial Training Plan Program (No. 202110475083).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Perez-Campos, E.; Mayoral, L.P.-C.; Andrade, G.M.; Mayoral, E.P.-C.; Huerta, T.H.; Canseco, S.P.; Canales, F.J.R.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Santiago, A.D.P.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What We Talk About When We Talk About Fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Liu, J.; Shan, T. New Roles of Lkb1 in Regulating Adipose Tissue Development and Thermogenesis. J. Cell. Physiol. 2017, 232, 2296–2298. [Google Scholar] [CrossRef]
- Seale, P.; Lazar, M.A. Brown Fat in Humans: Turning up the Heat on Obesity. Diabetes 2009, 58, 1482–1484. [Google Scholar] [CrossRef] [Green Version]
- Kaisanlahti, A.; Glumoff, T. Browning of white fat: Agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidossis, L.S.; Porter, C.; Saraf, M.K.; Børsheim, E.; Radhakrishnan, R.S.; Chao, T.; Ali, A.; Chondronikola, M.; Mlcak, R.; Finnerty, C.C.; et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015, 22, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Sharp, L.Z.; Shinoda, K.; Ohno, H.; Scheel, D.W.; Tomoda, E.; Ruiz, L.; Hu, H.; Wang, L.; Pavlova, Z.; Gilsanz, V.; et al. Human BAT Possesses Molecular Signatures That Resemble Beige/Brite Cells. PLoS ONE 2012, 7, e49452. [Google Scholar] [CrossRef]
- Lidell, M.E.; Betz, M.J.; Leinhard, O.D.; Heglind, M.; Elander, L.; Slawik, M.; Mussack, T.; Nilsson, D.; Romu, T.; Nuutila, P.; et al. Evidence for two types of brown adipose tissue in humans. Nat. Med. 2013, 19, 631–634. [Google Scholar] [CrossRef] [Green Version]
- Nedergaard, J.; Cannon, B. The Browning of White Adipose Tissue: Some Burning Issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef] [Green Version]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, T.; Sakai, J.; Kajimura, S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat. Rev. Mol. Cell Biol. 2016, 17, 480–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef] [PubMed]
- López, M. EJE PRIZE 2017: Hypothalamic AMPK: A golden target against obesity? Eur. J. Endocrinol. 2017, 176, R235–R246. [Google Scholar] [CrossRef]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef]
- Carling, D.; Mayer, F.V.; Sanders, M.J.; Gamblin, S. AMP-activated protein kinase: Nature’s energy sensor. Nat. Chem. Biol. 2011, 7, 512–518. [Google Scholar] [CrossRef]
- Gowans, G.J.; Hawley, S.A.; Ross, F.A.; Hardie, D.G. AMP Is a True Physiological Regulator of AMP-Activated Protein Kinase by Both Allosteric Activation and Enhancing Net Phosphorylation. Cell Metab. 2013, 18, 556–566. [Google Scholar] [CrossRef] [Green Version]
- Woods, A.; Dickerson, K.; Heath, R.; Hong, S.-P.; Momcilovic, M.; Johnstone, S.R.; Carlson, M.; Carling, D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005, 2, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G. The LKB1-AMPK Pathway—Friend or Foe in Cancer? Cancer Cell 2013, 23, 131–132. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Shen, X.; Shen, F.; Zhong, W.; Wu, H.; Liu, S.; Lai, J. TAK1 activates AMPK-dependent cell death pathway in hydrogen peroxide-treated cardiomyocytes, inhibited by heat shock protein-70. Mol. Cell. Biochem. 2013, 377, 35–44. [Google Scholar] [CrossRef]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, B.; Heath, R.; Saiu, P.; Leiper, F.C.; Leone, P.; Jing, C.; Walker, P.A.; Haire, L.; Eccleston, J.F.; Davis, C.T.; et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature 2007, 449, 496–500. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Zhai, A.; Zhang, B.; Tian, G. AMPK-Mediated Regulation of Lipid Metabolism by Phosphorylation. Biol. Pharm. Bull. 2018, 41, 985–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, J.E.; Brocklehurst, K.J.; Marley, A.E.; Carey, F.; Carling, D.; Beri, R.K. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994, 353, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Hawley, S.A.; Fullerton, M.D.; Ross, F.A.; Schertzer, J.D.; Chevtzoff, C.; Walker, K.J.; Peggie, M.W.; Zibrova, D.; Green, K.A.; Mustard, K.J.; et al. The Ancient Drug Salicylate Directly Activates AMP-Activated Protein Kinase. Science 2012, 336, 918–922. [Google Scholar] [CrossRef] [Green Version]
- Um, J.-H.; Park, S.-J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. AMP-Activated Protein Kinase-Deficient Mice Are Resistant to the Metabolic Effects of Resveratrol. Diabetes 2009, 59, 554–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.-M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.-P.; O’Neill, H.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, A.K.; Ruderman, N.B. Malonyl-CoA and AMP-activated protein kinase: An expanding part-nership. Mol. Cell. Biochem. 2003, 253, 65–70. [Google Scholar] [CrossRef]
- Park, H.; Kaushik, V.K.; Constant, S.; Prentki, M.; Przybytkowski, E.; Ruderman, N.; Saha, A. Coordinate Regulation of Malonyl-CoA Decarboxylase, sn-Glycerol-3-phosphate Acyltransferase, and Acetyl-CoA Carboxylase by AMP-activated Protein Kinase in Rat Tissues in Response to Exercise. J. Biol. Chem. 2002, 277, 32571–32577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loureiro, L.M.R.; Reis, C.E.G.; Da Costa, T.H.M. Effects of Coffee Components on Muscle Glycogen Recovery: A Systematic Review. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J.; et al. AMPK Phosphorylates and Inhibits SREBP Activity to Attenuate Hepatic Steatosis and Atherosclerosis in Diet-Induced Insulin-Resistant Mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowherd, R.M.; Lyle, R.E.; McGehee, R.E., Jr. Molecular regulation of adipocyte differentiation. Semin. Cell Dev. Biol. 1999, 10, 3–10. [Google Scholar] [CrossRef]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogene-sis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Liu, W.; Liu, F.; Ji, A.; Li, Y. The Orphan Nuclear Receptor 4A1: A Potential New Therapeutic Target for Metabolic Diseases. J. Diabetes Res. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Grimaldi, C.; Chiarini, F.; Tabellini, G.; Ricci, F.; Tazzari, P.L.; Battistelli, M.; Falcieri, E.; Bortul, R.; Melchionda, F.; Iacobucci, I.; et al. AMP-dependent kinase/mammalian target of rapamycin complex 1 signaling in T-cell acute lymphoblastic leukemia: Therapeutic implications. Leukemia 2011, 26, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Peyton, K.J.; Liu, X.-M.; Yu, Y.; Yates, B.; Durante, W. Activation of AMP-Activated Protein Kinase Inhibits the Proliferation of Human Endothelial Cells. J. Pharmacol. Exp. Ther. 2012, 342, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Imamuraab, K.; Oguraa, T.; Kishimotoa, A.; Kaminishib, M.; Esumi, H. Cell Cycle Regulation via p53 Phosphorylation by a 5′-AMP Activated Protein Kinase Activator, 5-Aminoimidazole- 4-Carboxamide-1-β-d-Ribofuranoside, in a Human Hepatocellular Carcinoma Cell Line. Biochem. Biophys. Res. Commun. 2001, 287, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Habinowski, S.A.; Witters, L.A. The Effects of AICAR on Adipocyte Differentiation of 3T3-L1 Cells. Biochem. Biophys. Res. Commun. 2001, 286, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Lee, H.; Kang, R.; Bae, S. AICAR, an activator of AMPK, inhibits adipogenesis via the WNT/β-catenin pathway in 3T3-L1 adipocytes. Int. J. Mol. Med. 2011, 28, 65–71. [Google Scholar] [CrossRef]
- Bijland, S.; Mancini, S.J.; Salt, I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013, 124, 491–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducharme, N.A.; Bickel, P.E. Minireview: Lipid Droplets in Lipogenesis and Lipolysis. Endocrinology 2008, 149, 942–949. [Google Scholar] [CrossRef] [Green Version]
- Anthony, N.M.; Gaidhu, M.P.; Ceddia, R.B. Regulation of Visceral and Subcutaneous Adipocyte Lipolysis by Acute AICAR-induced AMPK Activation. Obesity 2009, 17, 1312–1317. [Google Scholar] [CrossRef]
- Koh, H.-J.; Hirshman, M.F.; He, H.; Li, Y.; Manabe, Y.; Balschi, J.A.; Goodyear, L.J. Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem. J. 2007, 403, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.; Mu, J.; Birnbaum, M.J. Role of AMP-activated Protein Kinase in Cyclic AMP-dependent Lipolysis In 3T3-L1 Adipocytes. J. Biol. Chem. 2003, 278, 43074–43080. [Google Scholar] [CrossRef] [Green Version]
- Su, C.-L.; Sztalryd, C.; Contreras, J.A.; Holm, C.; Kimmel, A.R.; Londos, C. Mutational Analysis of the Hormone-sensitive Lipase Translocation Reaction in Adipocytes. J. Biol. Chem. 2003, 278, 43615–43619. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.; Arch, J.R.; Newsholme, E.A. Effects of hormones on the rate of the triacylglycerol/fatty acid substrate cycle in adipocytes and epididymal fat pads. FEBS Lett. 1982, 146, 327–330. [Google Scholar] [CrossRef] [Green Version]
- Reshef, L.; Olswang, Y.; Cassuto, H.; Blum, B.; Croniger, C.M.; Kalhan, S.; Tilghman, S.M.; Hanson, R.W. Glyceroneogenesis and the Triglyceride/Fatty Acid Cycle. J. Biol. Chem. 2003, 278, 30413–30416. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, M.-S.; Miyoshi, H.; Souza, S.C.; Cacicedo, J.M.; Saha, A.; Greenberg, A.S.; Ruderman, N. AMP-activated Protein Kinase Is Activated as a Consequence of Lipolysis in the Adipocyte: Potential mechanism and physiological relevance. J. Biol. Chem. 2008, 283, 16514–16524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daval, M.; Diot-Dupuy, F.; Bazin, R.; Hainault, I.; Viollet, B.; Vaulont, S.; Hajduch, E.; Ferre, P.; Foufelle, F. Anti-lipolytic Action of AMP-activated Protein Kinase in Rodent Adipocytes. J. Biol. Chem. 2005, 280, 25250–25257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaidhu, M.P.; Fediuc, S.; Anthony, N.M.; So, M.; Mirpourian, M.; Perry, R.L.; Ceddia, R.B. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: Novel mechanisms integrating HSL and ATGL. J. Lipid Res. 2009, 50, 704–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, C.S.; Lecoultre, V.; Ravussin, E. Brown Adipose Tissue. Circulation 2012, 125, 2782–2791. [Google Scholar] [CrossRef] [Green Version]
- Castro-Barquero, S.; Lamuela-Raventós, R.M.; Doménech, M.; Estruch, R. Relationship between Mediterranean Dietary Polyphenol Intake and Obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Li, B.; Wang, H.; Zou, X.; Gao, R.; Zhang, W.; Shu, T.; Zhao, H.; Liu, B.; Wang, J. Asthma alle-viates obesity in males through regulating metabolism and energy expenditure. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 350–359. [Google Scholar] [CrossRef]
- Saito, M.; Okamatsu-Ogura, Y.; Matsushita, M.; Watanabe, K.; Yoneshiro, T.; Nio-Kobayashi, J.; Iwanaga, T.; Miyagawa, M.; Kameya, T.; Nakada, K.; et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes 2009, 58, 1526–1531. [Google Scholar] [CrossRef] [Green Version]
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef] [Green Version]
- Ruderman, N.B.; Xu, J.X.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Gauthier, M.-S.; O’Brien, E.L.; Bigornia, S.; Mott, M.; Cacicedo, J.M.; Xu, X.J.; Gokce, N.; Apovian, C.; Ruderman, N. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem. Biophys. Res. Commun. 2011, 404, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Gauthier, M.-S.; Hess, D.; Apovian, C.; Cacicedo, J.M.; Gokce, N.; Farb, M.; Valentine, R.J.; Ruderman, N. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J. Lipid Res. 2012, 53, 792–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottillo, E.P.; Desjardins, E.M.; Crane, J.; Smith, B.K.; Green, A.; Ducommun, S.; Henriksen, T.; Rebalka, I.A.; Razi, A.; Sakamoto, K.; et al. Lack of Adipocyte AMPK Exacerbates Insulin Resistance and Hepatic Steatosis through Brown and Beige Adipose Tissue Function. Cell Metab. 2016, 24, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-J.; Tang, T.; Abbott, M.; Viscarra, J.A.; Wang, Y.; Sul, H.S. AMPK Phosphorylates Desnutrin/ATGL and Hormone-Sensitive Lipase To Regulate Lipolysis and Fatty Acid Oxidation within Adipose Tissue. Mol. Cell. Biol. 2016, 36, 1961–1976. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, E.M.; Steinberg, G.R. Emerging Role of AMPK in Brown and Beige Adipose Tissue (BAT): Implications for Obesity, Insulin Resistance, and Type 2 Diabetes. Curr. Diabetes Rep. 2018, 18, 80. [Google Scholar] [CrossRef]
- Fenzl, A.; Kiefer, F.W. Brown adipose tissue and thermogenesis. Horm. Mol. Biol. Clin. Investig. 2014, 19. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Q.; Li, Y.; Tang, Q.; Wu, T.; Chen, L.; Pu, S.; Zhao, Y.; Zhang, G.; Huang, C.; et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte 2020, 9, 484–494. [Google Scholar] [CrossRef]
- Qi, J.; Gong, J.; Zhao, T.; Zhao, J.; Lam, P.; Ye, J.; Li, J.Z.; Wu, J.; Zhou, H.-M.; Li, P. Downregulation of AMP-activated protein kinase by Cidea-mediated ubiquitination and degradation in brown adipose tissue. EMBO J. 2008, 27, 1537–1548. [Google Scholar] [CrossRef] [Green Version]
- Perdikari, A.; Kulenkampff, E.; Rudigier, C.; Neubauer, H.; Luippold, G.; Redemann, N.; Wolfrum, C. A high-throughput, image-based screen to identify kinases involved in brown adipocyte development. Sci. Signal. 2017, 10, eaaf5357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, Q.; Zhang, L.; Liang, X.; Sun, X.; Wang, B.; Chen, Y.; Zhu, M.; Du, M. AMPKα1 deficiency suppresses brown adipogenesis in favor of fibrogenesis during brown adipose tissue development. Biochem. Biophys. Res. Commun. 2017, 491, 508–514. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, X.; Sun, X.; Zhang, L.; Fu, X.; Rogers, C.J.; Berim, A.; Zhang, S.; Wang, S.; Wang, B.; et al. AMPK/α-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab. 2016, 24, 542–554. [Google Scholar] [CrossRef] [Green Version]
- Ricquier, D.; Bouillaud, F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem. J. 2000, 345, 161–179. [Google Scholar] [CrossRef]
- Matsuda, J.; Hosoda, K.; Itoh, H.; Son, C.; Doi, K.; Tanaka, T.; Fukunaga, Y.; Inoue, G.; Nishimura, H.; Yoshimasa, Y.; et al. Cloning of rat uncoupling protein-3 and uncoupling protein-2 cDNAs: Their gene expression in rats fed high-fat diet. FEBS Lett. 1997, 418, 200–204. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, W.; Liu, F.; Wang, Q.; Song, M.; Yu, Q.; Tang, K.; Teng, T.; Wu, D.; Wang, X.; et al. IMCA Induces Ferroptosis Mediated by SLC7A11 through the AMPK/mTOR Pathway in Colorectal Cancer. Oxidative Med. Cell. Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Fernández-Veledo, S.; Vázquez-Carballo, A.; Vila-Bedmar, R.; Ceperuelo-Mallafré, V.; Vendrell, J. Role of energy- and nutrient-sensing kinases AMP-activated Protein Kinase (AMPK) and Mammalian Target of Rapamycin (mTOR) in Adipocyte Differentiation. IUBMB Life 2013, 65, 572–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976, Erratum in Cell 2017, 169, 361– 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Vila-Bedmar, R.; Lorenzo, M.; Fernández-Veledo, S. Adenosine 5′-Monophosphate-Activated Protein Kinase-Mammalian Target of Rapamycin Cross Talk Regulates Brown Adipocyte Differentiation. Endocrinology 2010, 151, 980–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polak, P.; Cybulski, N.; Feige, J.N.; Auwerx, J.; Rüegg, M.A.; Hall, M.N. Adipose-Specific Knockout of raptor Results in Lean Mice with Enhanced Mitochondrial Respiration. Cell Metab. 2008, 8, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.M.; Sato, M.; Dallner, O.S.; Sandström, A.L.; Pisani, D.F.; Chambard, J.-C.; Amri, E.-Z.; Hutchinson, D.S.; Bengtsson, T. Glucose uptake in brown fat cells is dependent on mTOR complex 2–promoted GLUT1 translocation. J. Cell Biol. 2014, 207, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Labbé, S.M.; Mouchiroud, M.; Caron, A.; Secco, B.; Freinkman, E.; Lamoureux, G.; Gélinas, Y.; LeComte, R.; Bossé, Y.; Chimin, P.; et al. mTORC1 is Required for Brown Adipose Tissue Recruitment and Metabolic Adaptation to Cold. Sci. Rep. 2016, 6, 37223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, J.M.; Csikasz, R.I.; Dehvari, N.; Lu, L.; Sandström, A.; Öberg, A.I.; Nedergaard, J.; Stone-Elander, S.; Bengtsson, T. β 3 -Adrenergically induced glucose uptake in brown adipose tissue is independent of UCP1 presence or activity: Mediation through the mTOR pathway. Mol. Metab. 2017, 6, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; E Wellen, K.; Rabinowitz, J.D. Metabolic control of methylation and acetylation. Curr. Opin. Chem. Biol. 2015, 30, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Vaart, J.; Boon, M.; Houtkooper, R. The Role of AMPK Signaling in Brown Adipose Tissue Activation. Cells 2021, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Moisan, A.; Lee, Y.-K.; Zhang, J.D.; Hudak, C.S.; Meyer, C.A.; Prummer, M.; Zoffmann, S.; Truong, H.H.; Ebeling, M.; Kiialainen, A.; et al. White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nature 2014, 17, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of White Fat: Novel Insight Into Factors, Mechanisms, and Therapeutics. J. Cell. Physiol. 2016, 232, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; AlOmar, S.Y. Oxygen Deprivation and the Cellular Response to Hypoxia in Adipocytes––Perspectives on White and Brown Adipose Tissues in Obesity. Front. Endocrinol. 2015, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Zhang, Z.; Zheng, H.; Xiong, S.; Su, Y.; Ma, X.; Yi, C. Browning of Human Subcutaneous Adipose Tissue after Its Transplantation in Nude Mice. Plast. Reconstr. Surg. 2018, 142, 392–400. [Google Scholar] [CrossRef]
- Yan, M.; Audet-Walsh, E.; Manteghi, S.; Dufour, C.R.; Walker, B.; Baba, M.; St-Pierre, J.; Giguère, V.; Pause, A. Chronic AMPK activation via loss of FLCN induces functional beige adipose tissue through PGC-1α/ERRα. Genes Dev. 2016, 30, 1034–1046. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, H.; Li, B.; Meng, X.; Wang, J.; Zhang, Y.; Yao, S.; Ma, Q.; Jin, L.; Yang, J.; et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014, 5, 5493. [Google Scholar] [CrossRef] [Green Version]
- Kirkwood, J.S.; Legette, L.L.; Miranda, C.L.; Jiang, Y.; Stevens, J.F. A Metabolomics-driven Elucidation of the Anti-obesity Mechanisms of Xanthohumol. J. Biol. Chem. 2013, 288, 19000–19013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, P.; Xue, J.; Wu, Z.; Wang, H.; Han, J.; Liang, H.; Tian, D. Liver kinase B1 induces browning phenotype in 3 T3-L1 adipocytes. Gene 2018, 682, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Hu, F. AMPK in the Ventromedial Nucleus of the Hypothalamus: A Key Regulator for Thermogenesis. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef]
- Zhang, F.; Ai, W.; Hu, X.; Meng, Y.; Yuan, C.; Su, H.; Wang, L.; Zhu, X.; Gao, P.; Shu, G.; et al. Phytol stimulates the browning of white adipocytes through the activation of AMP-activated protein kinase (AMPK) α in mice fed high-fat diet. Food Funct. 2018, 9, 2043–2050. [Google Scholar] [CrossRef]
- Kang, N.H.; Mukherjee, S.; Min, T.; Kang, S.C.; Yun, J.W. Trans-anethole ameliorates obesity via induction of browning in white adipocytes and activation of brown adipocytes. Biochimie 2018, 151, 1–13. [Google Scholar] [CrossRef]
- Liu, X.; Chhipa, R.R.; Nakano, I.; Dasgupta, B. The AMPK Inhibitor Compound C Is a Potent AMPK-Independent Antiglioma Agent. Mol. Cancer Ther. 2014, 13, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Angin, Y.; Beauloye, C.; Horman, S.; Bertrand, L. Regulation of Carbohydrate Metabolism, Lipid Metabolism, and Protein Metabolism by AMPK. In AMP-Activated Protein Kinase; Experientia Supplementum; Cordero, M., Viollet, B., Eds.; Springer: Cham, Switzerland, 2016; Volume 107, pp. 23–43. [Google Scholar] [CrossRef]
- Ghosh, P. The stress polarity pathway: AMPK ’GIV’-es protection against metabolic insults. Aging 2017, 9, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Villanueva-Paz, M.; Cotán, D.; Garrido-Maraver, J.; Oropesa-Ávila, M.; de la Mata, M.; Delgado-Pavón, A.; de Lavera, I.; Alcocer-Gómez, E.; Álvarez-Córdoba, M.; Sánchez-Alcázar, J.A. AMPK Regulation of Cell Growth, Apoptosis, Autophagy, and Bioenergetics. Exp. Suppl. 2016, 107, 45–71. [Google Scholar] [CrossRef]
- Tian, Z.; Liang, G.; Cui, K.; Liang, Y.; Wang, Q.; Lv, S.; Cheng, X.; Zhang, L. Insight Into the Prospects for RNAi Therapy of Cancer. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Burkewitz, K.; Weir, H.J.M.; Mair, W.B. AMPK as a Pro-longevity Target. Exp. Suppl. 2016, 107, 227–256. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhu, W.; Li, Y.; Xin, P.; Li, J.; Liu, M.; Li, J.; Redington, A.N.; Wei, M. Apelin-13 protects the heart against ischemia-reperfusion injury through inhibition of ER-dependent apoptotic pathways in a time-dependent fashion. Am. J. Physiol. Circ. Physiol. 2011, 301, H1471–H1486. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Lu, Y.; Liu, D.; Zhang, W.; Liu, J.; Tang, H.; Zhu, Y. Apigenin-7- O -β- d -(-6″- p -coumaroyl)-glucopyranoside pretreatment attenuates myocardial ischemia/reperfusion injury via activating AMPK signaling. Life Sci. 2018, 203, 246–254. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Chen, T.-P.; Wang, Y.-C.; Lin, Y.-M.; Fang, S.-W. AMP-Activated Protein Kinase Activation during Cardioplegia-Induced Hypoxia/Reoxygenation Injury Attenuates Cardiomyocytic Apoptosis via Reduction of Endoplasmic Reticulum Stress. Mediat. Inflamm. 2010, 2010, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.-B.; Wu, X.-Y.; Gu, J.-H.; Guo, Q.-T.; Shen, W.-X.; Lu, P. Activation of AMP-Activated Protein Kinase Contributes to Doxorubicin-Induced Cell Death and Apoptosis in Cultured Myocardial H9c2 Cells. Cell Biophys. 2011, 60, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Liu, P.; Xie, X. AMPK and Cancer. Exp. Suppl. 2016, 107, 203–226. [Google Scholar] [PubMed]
- Zhang, L.; Liu, W.; Wang, Q.; Li, Q.; Wang, H.; Wang, J.; Teng, T.; Chen, M.; Ji, A.; Li, Y. New Drug Candidate Targeting the 4A1 Orphan Nuclear Receptor for Medullary Thyroid Cancer Therapy. Molecules 2018, 23, 565. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [Green Version]
- Domise, M.; Vingtdeux, V. AMPK in Neurodegenerative Diseases. Exp. Suppl. 2016, 107, 153–177. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, C.; Dietrich, M.O.; Metzger, F.; Loetscher, H.; Torres-Aleman, I. Disturbed Cross Talk between Insulin-Like Growth Factor I and AMP-Activated Protein Kinase as a Possible Cause of Vascular Dysfunction in the Amyloid Precursor Protein/Presenilin 2 Mouse Model of Alzheimer’s Disease. J. Neurosci. 2007, 27, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, D.; Mitchelhill, K.I.; Gao, G.; Widmer, J.; Michell, B.J.; Teh, T.; House, C.M.; Fernandez, C.S.; Cox, T.; Witters, L.A.; et al. Mammalian AMP-activated Protein Kinase Subfamily. J. Biol. Chem. 1996, 271, 611–614. [Google Scholar] [CrossRef] [Green Version]
- Olivier, S.; Foretz, M.; Viollet, B. Promise and challenges for direct small molecule AMPK activators. Biochem. Pharmacol. 2018, 153, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Agius, L.; Ford, B.E.; Chachra, S.S. The Metformin Mechanism on Gluconeogenesis and AMPK Activation: The Metabolite Perspective. Int. J. Mol. Sci. 2020, 21, 3240. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, L.; Zhao, Y.; Jiang, Y.; Chen, L.; Yu, Y.; Ouyang, L. Fluoxetine induces autophagic cell death via eEF2K-AMPK-mTOR-ULK complex axis in triple negative breast cancer. Cell Prolif. 2017, 51, e12402. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Zhang, J.; Hong, L.; Huang, W.; Dai, X.; Ye, Q.; Chen, X. Metformin ameliorates stress-induced depression-like behaviors via enhancing the expression of BDNF by activating AMPK/CREB-mediated histone acetylation. J. Affect. Disord. 2019, 260, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.; Mittag, J. Breaking BAT: Can browning create a better white? J. Endocrinol. 2016, 228, R19–R29. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like Is a Hormone that Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).