The Role of Matrix Metalloproteinases in Endometriosis: A Potential Target
Abstract
1. Introduction
2. The MMP Family
2.1. Classification of MMPs
2.2. Structure of MMPs
2.3. The Expression of MMPs (Natural MMP Inhibitors)
3. MMP Expression in Endometriosis
3.1. Summary of Clinical Studies
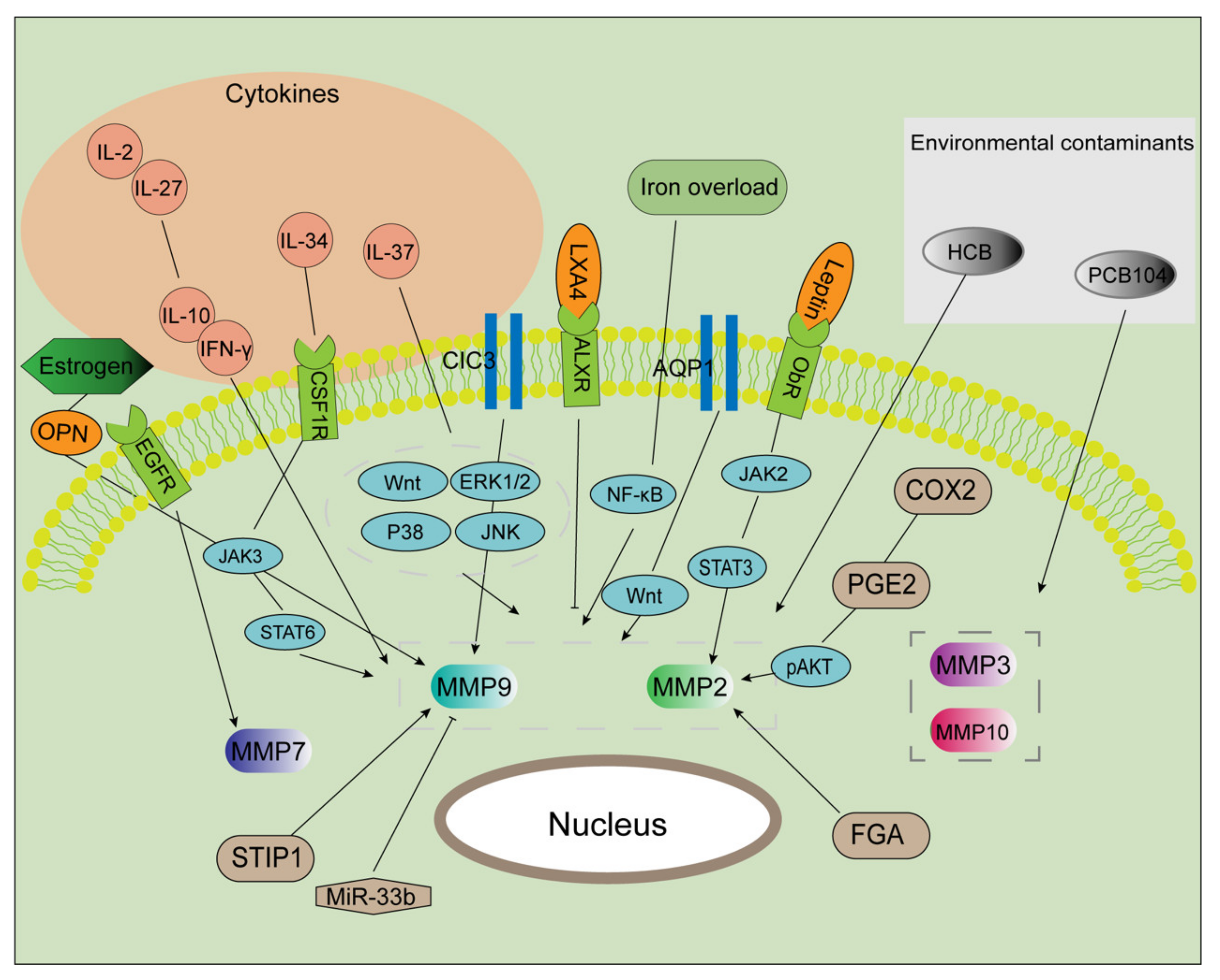
3.2. Complexity of MMP Regulation in Endometriosis
4. The Role of MMPs in the Pathophysiology of Endometriosis
4.1. MMPs at the Cellular Level
4.1.1. Migration and Invasion
4.1.2. Epithelial-Mesenchymal Transition
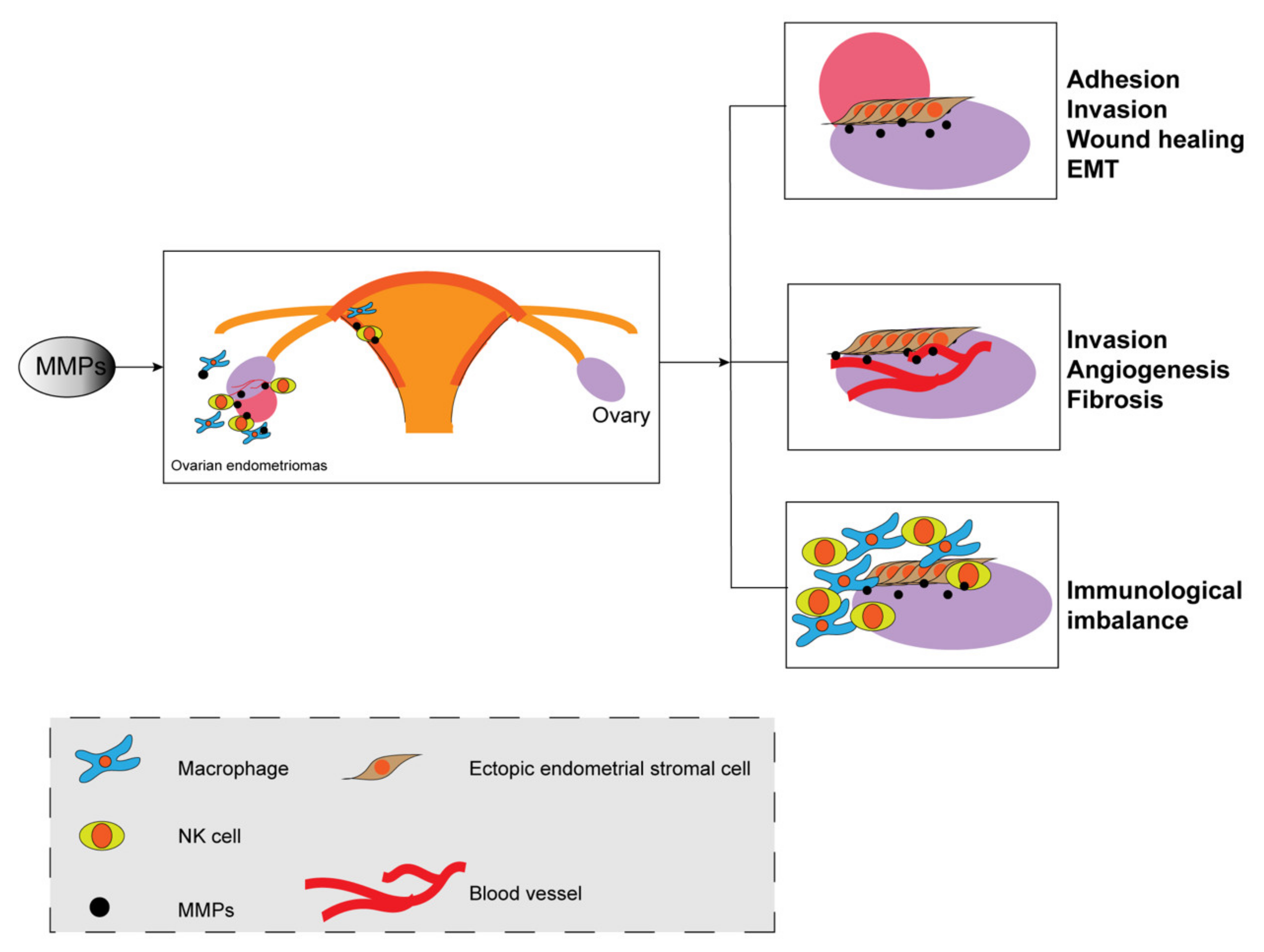
4.2. MMPs at the Tissue/Organism Level
4.2.1. Angiogenesis
4.2.2. Fibrosis
4.2.3. Immunological Imbalance
5. Potential Value of Inhibiting MMPs in Endometriosis
5.1. MMPs as Biomarkers in Endometriosis
5.2. MMPs as Therapeutic Targets
6. Summary and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samimi, M.; Pourhanifeh, M.H.; Mehdizadehkashi, A.; Eftekhar, T.; Asemi, Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. J. Cell. Physiol. 2019, 234, 19384–19392. [Google Scholar] [CrossRef] [PubMed]
- Farland, L.V.; Prescott, J.; Sasamoto, N.; Tobias, D.K.; Gaskins, A.J.; Stuart, J.J.; Carusi, D.A.; Chavarro, J.E.; Horne, A.W.; Rich-Edwards, J.W.; et al. Endometriosis and Risk of Adverse Pregnancy Outcomes. Obstet. Gynecol. 2019, 134, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Vigano, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef]
- Tsang, K.Y.; Cheung, M.C.; Chan, D.; Cheah, K.S. The developmental roles of the extracellular matrix: Beyond structure to regulation. Cell Tissue Res. 2010, 339, 93–110. [Google Scholar] [CrossRef]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Pitsos, M.; Kanakas, N. The role of matrix metalloproteinases in the pathogenesis of endometriosis. Reprod. Sci. 2009, 16, 717–726. [Google Scholar] [CrossRef]
- Kapoor, C.; Vaidya, S.; Wadhwan, V.; Kaur, G.; Pathak, A. Seesaw of matrix metalloproteinases (MMPs). J. Cancer Res. Ther. 2016, 12, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Balkowiec, M.; Maksym, R.B.; Wlodarski, P.K. The bimodal role of matrix metalloproteinases and their inhibitors in etiology and pathogenesis of endometriosis (Review). Mol. Med. Rep. 2018, 18, 3123–3136. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, N.; Jacobs-Cacha, C.; Mora-Gutierrez, J.M.; Vergara, A.; Orbe, J.; Soler, M.J. Matrix Metalloproteinases in Diabetic Kidney Disease. J. Clin. Med. 2020, 9, 472. [Google Scholar] [CrossRef]
- Stawowczyk, M.; Wellenstein, M.D.; Lee, S.B.; Yomtoubian, S.; Durrans, A.; Choi, H.; Narula, N.; Altorki, N.K.; Gao, D.; Mittal, V. Matrix Metalloproteinase 14 promotes lung cancer by cleavage of Heparin-Binding EGF-like Growth Factor. Neoplasia 2017, 19, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Riedl, R. Inhibitory Antibodies Designed for Matrix Metalloproteinase Modulation. Molecules 2019, 24, 2265. [Google Scholar] [CrossRef] [PubMed]
- Agren, M.S.; Mirastschijski, U.; Karlsmark, T.; Saarialho-Kere, U.K. Topical synthetic inhibitor of matrix metalloproteinases delays epidermal regeneration of human wounds. Exp. Dermatol. 2001, 10, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.L.; Nagano, T.; Nakamura, M.; Kumagai, N.; Mishima, H.; Nishida, T. Effect of galardin on collagen degradation by Pseudomonas aeruginosa. Exp. Eye Res. 1999, 69, 595–601. [Google Scholar] [CrossRef]
- Gatto, C.; Rieppi, M.; Borsotti, P.; Innocenti, S.; Ceruti, R.; Drudis, T.; Scanziani, E.; Casazza, A.M.; Taraboletti, G.; Giavazzi, R. BAY 12-9566, a novel inhibitor of matrix metalloproteinases with antiangiogenic activity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999, 5, 3603–3607. [Google Scholar]
- Lockhart, A.C.; Braun, R.D.; Yu, D.; Ross, J.R.; Dewhirst, M.W.; Humphrey, J.S.; Thompson, S.; Williams, K.M.; Klitzman, B.; Yuan, F.; et al. Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 586–593. [Google Scholar]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Zakiyanov, O.; Kalousova, M.; Zima, T.; Tesar, V. Matrix Metalloproteinases in Renal Diseases: A Critical Appraisal. Kidney Blood Press. Res. 2019, 44, 298–330. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.P.; Baird, S.K. Tissue inhibitor of matrix metalloproteinase-3 has both anti-metastatic and anti-tumourigenic properties. Clin. Exp. Metastasis 2020, 37, 69–76. [Google Scholar] [CrossRef]
- Fujii, T.; Duarte, S.; Lee, E.; Ke, B.; Busuttil, R.W.; Coito, A.J. Tissue Inhibitor of Metalloproteinase 3 Deficiency Disrupts the Hepatocyte E-Cadherin/beta-Catenin Complex and Induces Cell Death in Liver Ischemia/Reperfusion Injury. Liver Transplant. 2020, 26, 113–126. [Google Scholar] [CrossRef]
- Opdenakker, G.; Abu El-Asrar, A. Metalloproteinases mediate diabetes-induced retinal neuropathy and vasculopathy. Cell. Mol. Life Sci. 2019, 76, 3157–3166. [Google Scholar] [CrossRef]
- Rodgers, W.H.; Osteen, K.G.; Matrisian, L.M.; Navre, M.; Giudice, L.C.; Gorstein, F. Expression and localization of matrilysin, a matrix metalloproteinase, in human endometrium during the reproductive cycle. Am. J. Obstet. Gynecol. 1993, 168, 253–260. [Google Scholar] [CrossRef]
- Sui, X.; Li, Y.; Sun, Y.; Li, C.; Li, X.; Zhang, G. Expression and significance of autophagy genes LC3, Beclin1 and MMP-2 in endometriosis. Exp. Ther. Med. 2018, 16, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Szymanowski, K.; Mikolajczyk, M.; Wirstlein, P.; Dera-Szymanowska, A. Matrix metalloproteinase-2 (MMP-2), MMP-9, tissue inhibitor of matrix metalloproteinases (TIMP-1) and transforming growth factor-beta2 (TGF-beta2) expression in eutopic endometrium of women with peritoneal endometriosis. Ann. Agric. Environ. Med. 2016, 23, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Kodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Shabani Nashtaei, M.; Pazhohan, A.; Bahramrezai, M.; Berenjian, S.; Sobhani, A. The modulating effects of Resveratrol on the expression of MMP-2 and MMP-9 in endometriosis women: A randomized exploratory trial. Off. J. Int. Soc. Gynecol. Endocrinol. 2019, 35, 719–726. [Google Scholar] [CrossRef]
- Borghese, B.; Mondon, F.; Noël, J.C.; Fayt, I.; Mignot, T.M.; Vaiman, D.; Chapron, C. Gene expression profile for ectopic versus eutopic endometrium provides new insights into endometriosis oncogenic potential. Mol. Endocrinol. 2008, 22, 2557–2562. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.; Galleguillos, C.; Torres, M.; Sovino, H.; Fuentes, A.; Boric, M.A.; Johnson, M.C. Association between MMP1 and MMP9 activities and ICAM1 cleavage induced by tumor necrosis factor in stromal cell cultures from eutopic endometria of women with endometriosis. Reproduction 2009, 138, 837–847. [Google Scholar] [CrossRef]
- Shan, K.; Ying, W.; Jian-Hui, Z.; Wei, G.; Na, W.; Yan, L. The function of the SNP in the MMP1 and MMP3 promoter in susceptibility to endometriosis in China. Mol. Hum. Reprod. 2005, 11, 423–427. [Google Scholar] [CrossRef][Green Version]
- Borghese, B.; Chiche, J.D.; Vernerey, D.; Chenot, C.; Mir, O.; Bijaoui, G.; Bonaiti-Pellie, C.; Chapron, C. Genetic polymorphisms of matrix metalloproteinase 12 and 13 genes are implicated in endometriosis progression. Hum. Reprod. 2008, 23, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Laudanski, P.; Szamatowicz, J.; Ramel, P. Matrix metalloproteinase-13 and membrane type-1 matrix metalloproteinase in peritoneal fluid of women with endometriosis. Gynecol. Endocrinol. 2005, 21, 106–110. [Google Scholar] [CrossRef]
- Weigel, M.T.; Kramer, J.; Schem, C.; Wenners, A.; Alkatout, I.; Jonat, W.; Maass, N.; Mundhenke, C. Differential expression of MMP-2, MMP-9 and PCNA in endometriosis and endometrial carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 160, 74–78. [Google Scholar] [CrossRef]
- Ueda, M.; Yamashita, Y.; Takehara, M.; Terai, Y.; Kumagai, K.; Ueki, K.; Kanda, K.; Hung, Y.C.; Ueki, M. Gene expression of adhesion molecules and matrix metalloproteinases in endometriosis. Off. J. Int. Soc. Gynecol. Endocrinol. 2002, 16, 391–402. [Google Scholar] [CrossRef]
- Uzan, C.; Cortez, A.; Dufournet, C.; Fauvet, R.; Siffroi, J.P.; Daraï, E. Eutopic endometrium and peritoneal, ovarian and bowel endometriotic tissues express a different profile of matrix metalloproteinases-2, -3 and -11, and of tissue inhibitor metalloproteinases-1 and -2. Virchows Arch. 2004, 445, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Luddi, A.; Marrocco, C.; Governini, L.; Semplici, B.; Pavone, V.; Luisi, S.; Petraglia, F.; Piomboni, P. Expression of Matrix Metalloproteinases and Their Inhibitors in Endometrium: High Levels in Endometriotic Lesions. Int. J. Mol. Sci. 2020, 21, 2840. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.W.; Lee, J.Y.; Moon, H.S.; Hur, S.E.; Park, M.H.; Wen, Y.; Polan, M.L. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil. Steril. 2002, 78, 787–795. [Google Scholar] [CrossRef]
- Jana, S.; Chatterjee, K.; Ray, A.K.; DasMahapatra, P.; Swarnakar, S. Regulation of Matrix Metalloproteinase-2 Activity by COX-2-PGE2-pAKT Axis Promotes Angiogenesis in Endometriosis. PLoS ONE 2016, 11, e0163540. [Google Scholar] [CrossRef]
- Huang, H.F.; Hong, L.H.; Tan, Y.; Sheng, J.Z. Matrix metalloproteinase 2 is associated with changes in steroid hormones in the sera and peritoneal fluid of patients with endometriosis. Fertil. Steril. 2004, 81, 1235–1239. [Google Scholar] [CrossRef]
- Paul, S.; Sharma, A.V.; Mahapatra, P.D.; Bhattacharya, P.; Reiter, R.J.; Swarnakar, S. Role of melatonin in regulating matrix metalloproteinase-9 via tissue inhibitors of metalloproteinase-1 during protection against endometriosis. J. Pineal Res. 2008, 44, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Bostanci Durmus, A.; Dincer Cengiz, S.; Yilmaz, H.; Candar, T.; Gursoy, A.Y.; Sinem Caglar, G. The levels of matrix metalloproteinase-9 and neutrophil gelatinase-associated lipocalin in different stages of endometriosis. J. Inst. Obstet. Gynaecol. 2019, 39, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Paul, S.; Swarnakar, S. Curcumin as anti-endometriotic agent: Implication of MMP-3 and intrinsic apoptotic pathway. Biochem. Pharmacol. 2012, 83, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Chen, P.; Liu, W. Down regulation of MiR-93 contributes to endometriosis through targeting MMP3 and VEGFA. Am. J. Cancer Res. 2015, 5, 1706–1717. [Google Scholar] [PubMed]
- Vallvé-Juanico, J.; López-Gil, C.; Ponomarenko, J.; Melnychuk, T.; Castellví, J.; Ballesteros, A.; Colás, E.; Gil-Moreno, A.; Santamaria Costa, X. External validation of putative biomarkers in eutopic endometrium of women with endometriosis using NanoString technology. J. Assist. Reprod. Genet. 2020, 37, 2981–2987. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Qi, L.; Xu, X.; Feng, Y.; Gong, X.; Aili, A.; Chen, Y.; Xue, Z.; Xue, J.; Tong, X. Analysis of differences in the transcriptomic profiles of eutopic and ectopic endometriums in women with ovarian endometriosis. PeerJ 2021, 9, e11045. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Jana, S.; DasMahapatra, P.; Swarnakar, S. EGFR-mediated matrix metalloproteinase-7 up-regulation promotes epithelial-mesenchymal transition via ERK1-AP1 axis during ovarian endometriosis progression. FASEB J. 2018, 32, 4560–4572. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Mogami, H.; Bou Nemer, L.; Word, L.; Rogers, D.; Miller, R.; Word, R.A. Endometrial stromal cell attachment and matrix homeostasis in abdominal wall endometriomas. Hum. Reprod. 2018, 33, 280–291. [Google Scholar] [CrossRef]
- Gaetje, R.; Holtrich, U.; Engels, K.; Kourtis, K.; Cikrit, E.; Kissler, S.; Rody, A.; Karn, T.; Kaufmann, M. Expression of membrane-type 5 matrix metalloproteinase in human endometrium and endometriosis. Gynecol. Endocrinol. 2007, 23, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.T.; Huang, Y.Q.; Wu, J.B.; Deng, Z.Q.; Wang, Y.; Lai, Z.Y.; Wang, H.B.; Sun, X.X.; Zhu, Y.L.; Du, M.M.; et al. Overexpression of chloride channel-3 is associated with the increased migration and invasion ability of ectopic endometrial cells from patients with endometriosis. Hum. Reprod. 2016, 31, 986–998. [Google Scholar] [CrossRef]
- Wang, H.S.; Tsai, C.L.; Chang, P.Y.; Chao, A.; Wu, R.C.; Chen, S.H.; Wang, C.J.; Yen, C.F.; Lee, Y.S.; Wang, T.H. Positive associations between upregulated levels of stress-induced phosphoprotein 1 and matrix metalloproteinase-9 in endometriosis/adenomyosis. PLoS ONE 2018, 13, e0190573. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.W.; Hong, L.; Xu, X.X.; Wang, Q.; Huang, J.L.; Jiang, L. Regulation of miR-33b on endometriosis and expression of related factors. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2027–2033. [Google Scholar] [PubMed]
- Chen, Y.; Li, H.; Cheng, H.Y.; Rui-Qiong, M.; Ye, X.; Cui, H.; Hong-Lan, Z.; Chang, X.H. Fibrinogen alpha chain is up-regulated and affects the pathogenesis of endometriosis. Reprod. Biomed. Online 2019, 39, 893–904. [Google Scholar] [CrossRef]
- Ahn, J.H.; Choi, Y.S.; Choi, J.H. Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol. Hum. Reprod. 2015, 21, 792–802. [Google Scholar] [CrossRef]
- Shu, C.; Shu, Y.; Gao, Y.; Chi, H.; Han, J. Inhibitory effect of AQP1 silencing on adhesion and angiogenesis in ectopic endometrial cells of mice with endometriosis through activating the Wnt signaling pathway. Cell Cycle 2019, 18, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, R.F.; Su, L.; Zhou, W.D.; Zhu, M.B.; Chen, Q.H. Lipoxin A4 regulates expression of the estrogen receptor and inhibits 17beta-estradiol induced p38 mitogen-activated protein kinase phosphorylation in human endometriotic stromal cells. Fertil. Steril. 2014, 102, 264–271. [Google Scholar] [CrossRef]
- Wu, R.; Zhou, W.; Chen, S.; Shi, Y.; Su, L.; Zhu, M.; Chen, Q.; Chen, Q. Lipoxin A4 suppresses the development of endometriosis in an ALX receptor-dependent manner via the p38 MAPK pathway. Br. J. Pharmacol. 2014, 171, 4927–4940. [Google Scholar] [CrossRef]
- Wu, R.F.; Huang, Z.X.; Ran, J.; Dai, S.J.; Lin, D.C.; Ng, T.W.; Chen, Q.X.; Chen, Q.H. Lipoxin A4 Suppresses Estrogen-Induced Epithelial-Mesenchymal Transition via ALXR-Dependent Manner in Endometriosis. Reprod. Sci. 2018, 25, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.M.; Lai, Z.Z.; Ha, S.Y.; Yang, H.L.; Liu, L.B.; Wang, Y.; Shi, J.W.; Ruan, L.Y.; Ye, J.F.; Wu, J.N.; et al. IL-2 and IL-27 synergistically promote growth and invasion of endometriotic stromal cells by maintaining the balance of IFN-γ and IL-10 in endometriosis. Reproduction 2020, 159, 251–260. [Google Scholar] [CrossRef]
- Lin, K.; Ma, J.; Peng, Y.; Sun, M.; Xu, K.; Wu, R.; Lin, J. Autocrine Production of Interleukin-34 Promotes the Development of Endometriosis through CSF1R/JAK3/STAT6 signaling. Sci. Rep. 2019, 9, 16781. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yu, K.; Jiang, Z.; Xue, M. IL-37 affects the occurrence and development of endometriosis by regulating the biological behavior of endometrial stromal cells through multiple signaling pathways. Biol. Chem. 2018, 399, 1325–1337. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, C.; Chen, H.; Nian, Y.; Bai, Z.; Ha, C. The involvement of osteopontin and matrix metalloproteinase- 9 in the migration of endometrial epithelial cells in patients with endometriosis. Reprod. Biol. Endocrinol. 2015, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Choi, Y.S.; Choi, J.H. Iron-Storage Protein Ferritin Is Upregulated in Endometriosis and Iron Overload Contributes to a Migratory Phenotype. Biomedicines 2020, 8, 454. [Google Scholar] [CrossRef]
- Chiappini, F.; Sanchez, M.; Miret, N.; Cocca, C.; Zotta, E.; Ceballos, L.; Pontillo, C.; Bilotas, M.; Randi, A. Exposure to environmental concentrations of hexachlorobenzene induces alterations associated with endometriosis progression in a rat model. Food Chem. Toxicol. 2019, 123, 151–161. [Google Scholar] [CrossRef]
- Hu, T.; Yao, M.; Fu, X.; Chen, C.; Wu, R. Polychlorinated biphenyl 104 promotes migration of endometrial stromal cells in endometriosis. Toxicol. Lett. 2018, 290, 19–28. [Google Scholar] [CrossRef]
- Stejskalová, A.; Fincke, V.; Nowak, M.; Schmidt, Y.; Borrmann, K.; von Wahlde, M.K.; Schäfer, S.D.; Kiesel, L.; Greve, B.; Götte, M. Collagen I triggers directional migration, invasion and matrix remodeling of stroma cells in a 3D spheroid model of endometriosis. Sci. Rep. 2021, 11, 4115. [Google Scholar] [CrossRef] [PubMed]
- Rydlova, M.; Holubec, L., Jr.; Ludvikova, M., Jr.; Kalfert, D.; Franekova, J.; Povysil, C.; Ludvikova, M. Biological activity and clinical implications of the matrix metalloproteinases. Anticancer Res. 2008, 28, 1389–1397. [Google Scholar] [PubMed]
- Thakur, V.; Bedogni, B. The membrane tethered matrix metalloproteinase MT1-MMP at the forefront of melanoma cell invasion and metastasis. Pharmacol. Res. 2016, 111, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xiong, Y.; Jin, L.; Zhang, M.; Huang, L.; Mao, Y.; Zhou, C.; Qiao, Y.; Zhang, Y. Bisphenol A Exposure Enhances Endometrial Stromal Cell Invasion and Has a Positive Association with Peritoneal Endometriosis. Reprod. Sci. 2020, 27, 704–712. [Google Scholar] [CrossRef]
- Chiappini, F.; Bastón, J.I.; Vaccarezza, A.; Singla, J.J.; Pontillo, C.; Miret, N.; Farina, M.; Meresman, G.; Randi, A. Enhanced cyclooxygenase-2 expression levels and metalloproteinase 2 and 9 activation by Hexachlorobenzene in human endometrial stromal cells. Biochem. Pharmacol. 2016, 109, 91–104. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Zhou, W.H.; Wang, L.; He, Y.Y.; Li, D.J. Combination of estrogen and dioxin is involved in the pathogenesis of endometriosis by promoting chemokine secretion and invasion of endometrial stromal cells. Hum. Reprod. 2008, 23, 1614–1626. [Google Scholar] [CrossRef][Green Version]
- Pan, H.; Zhang, P.; Li, J.R.; Wang, H.; Jin, M.F.; Feng, C.; Huang, H.F. c-Fos-Regulated Matrix Metalloproteinase-9 Expression is Involved in 17β-Estradiol-Promoted Invasion of Human Endometrial Stromal Cell. Curr. Mol. Med. 2016, 16, 266–275. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, W.; Li, N.; Liu, H.; He, H.; Du, Y.; Zhang, Z.; Liu, Y. Estrogen stabilizes hypoxia-inducible factor 1α through G protein-coupled estrogen receptor 1 in eutopic endometrium of endometriosis. Fertil. Steril. 2017, 107, 439–447. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, L.; Xiong, Y.; Liu, H.; Liu, Y. Hypoxia Promotes Invasion of Endometrial Stromal Cells via Hypoxia-Inducible Factor 1α Upregulation-Mediated β-Catenin Activation in Endometriosis. Reprod. Sci. 2016, 23, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Xiong, W.; Zhang, L.; Xiong, Y.; Li, N.; He, H.; Du, Y.; Liu, Y. Hypoxia-inducible factor-1α promotes endometrial stromal cells migration and invasion by upregulating autophagy in endometriosis. Reproduction 2017, 153, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Xiong, W.; Zhang, L.; Du, Y.; Liu, Y.; Xiong, X. Long non-coding RNA MALAT1 mediates hypoxia-induced pro-survival autophagy of endometrial stromal cells in endometriosis. J. Cell Mol. Med. 2019, 23, 439–452. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Deng, L.; Yang, S.; Li, M.; Long, S.; Chen, S.; Lin, F.; Xiao, L. Decreased MT1-MMP in gastric cancer suppressed cell migration and invasion via regulating MMPs and EMT. Tumour Biol. 2015, 36, 6883–6889. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Luo, J.; Li, L.; Li, S.; Yang, L.; Pan, H.; Liu, Q.; Qin, H.; Chen, C.; Feng, J. Gab2 facilitates epithelial-to-mesenchymal transition via the MEK/ERK/MMP signaling in colorectal cancer. J. Exp. Clin. Cancer Res. 2016, 35, 5. [Google Scholar] [CrossRef]
- Vos, M.C.; Hollemans, E.; Ezendam, N.; Feijen, H.; Boll, D.; Pijlman, B.; van der Putten, H.; Klinkhamer, P.; van Kuppevelt, T.H.; van der Wurff, A.A.; et al. MMP-14 and CD44 in Epithelial-to-Mesenchymal Transition (EMT) in ovarian cancer. J. Ovarian Res. 2016, 9, 53. [Google Scholar] [CrossRef]
- Chen, C.M.; Lin, C.L.; Chiou, H.L.; Hsieh, S.C.; Lin, C.L.; Cheng, C.W.; Hung, C.H.; Tsai, J.P.; Hsieh, Y.H. Loss of endothelial cell-specific molecule 1 promotes the tumorigenicity and metastasis of prostate cancer cells through regulation of the TIMP-1/MMP-9 expression. Oncotarget 2017, 8, 13886–13897. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nie, J.; Zhou, Q.; Liu, W.; Zhu, F.; Chen, W.; Mao, H.; Luo, N.; Dong, X.; Yu, X. Downregulation of Par-3 expression and disruption of Par complex integrity by TGF-beta during the process of epithelial to mesenchymal transition in rat proximal epithelial cells. Biochim. Biophys. Acta 2008, 1782, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Karamanou, K.; Franchi, M.; Vynios, D.; Brézillon, S. Epithelial-to-mesenchymal transition and invadopodia markers in breast cancer: Lumican a key regulator. Semin. Cancer Biol. 2020, 62, 125–133. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Munoz-Felix, J.M.; Pedrosa, A.R.; Hodivala-Dilke, K.M. “Splitting the matrix”: Intussusceptive angiogenesis meets MT1-MMP. EMBO Mol. Med. 2020, 12, e11663. [Google Scholar] [CrossRef]
- Mazor, R.; Alsaigh, T.; Shaked, H.; Altshuler, A.E.; Pocock, E.S.; Kistler, E.B.; Karin, M.; Schmid-Schonbein, G.W. Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells. J. Biol. Chem. 2013, 288, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.K.; Ishii, G.; Saito, S.; Yano, K.; Hoshino, A.; Suzuki, T.; Ochiai, A. Degradation of soluble VEGF receptor-1 by MMP-7 allows VEGF access to endothelial cells. Blood 2009, 113, 2363–2369. [Google Scholar] [CrossRef]
- Sang, Q.X. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998, 8, 171–177. [Google Scholar] [CrossRef]
- Sood, D.; Cairns, D.M.; Dabbi, J.M.; Ramakrishnan, C.; Deisseroth, K.; Black, L.D., 3rd; Santaniello, S.; Kaplan, D.L. Functional maturation of human neural stem cells in a 3D bioengineered brain model enriched with fetal brain-derived matrix. Sci. Rep. 2019, 9, 17874. [Google Scholar] [CrossRef]
- Noriega-Guerra, H.; Freitas, V.M. Extracellular Matrix Influencing HGF/c-MET Signaling Pathway: Impact on Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3300. [Google Scholar] [CrossRef]
- Vigano, P.; Candiani, M.; Monno, A.; Giacomini, E.; Vercellini, P.; Somigliana, E. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 2018, 33, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kendziorski, J.A.; Belcher, S.M. Strain-specific induction of endometrial periglandular fibrosis in mice exposed during adulthood to the endocrine disrupting chemical bisphenol A. Reprod. Toxicol. 2015, 58, 119–130. [Google Scholar] [CrossRef]
- Holmbeck, K.; Bianco, P.; Caterina, J.; Yamada, S.; Kromer, M.; Kuznetsov, S.A.; Mankani, M.; Robey, P.G.; Poole, A.R.; Pidoux, I.; et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 1999, 99, 81–92. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Canis, M.; Pouly, J.L.; Darcha, C. Soft matrices inhibit cell proliferation and inactivate the fibrotic phenotype of deep endometriotic stromal cells in vitro. Hum. Reprod. 2016, 31, 541–553. [Google Scholar] [CrossRef]
- Vallve-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update 2019, 25, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Sciezynska, A.; Komorowski, M.; Soszynska, M.; Malejczyk, J. NK Cells as Potential Targets for Immunotherapy in Endometriosis. J. Clin. Med. 2019, 8, 1468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.J.; Yang, H.L.; Shao, J.; Mei, J.; Chang, K.K.; Zhu, R.; Li, M.Q. Anti-inflammatory cytokines in endometriosis. Cell. Mol. Life Sci. 2019, 76, 2111–2132. [Google Scholar] [CrossRef] [PubMed]
- Oosterlynck, D.J.; Meuleman, C.; Waer, M.; Vandeputte, M.; Koninckx, P.R. The natural killer activity of peritoneal fluid lymphocytes is decreased in women with endometriosis. Fertil. Steril. 1992, 58, 290–295. [Google Scholar] [CrossRef]
- Rossi, G.R.; Trindade, E.S.; Souza-Fonseca-Guimaraes, F. Tumor Microenvironment-Associated Extracellular Matrix Components Regulate NK Cell Function. Front. Immunol. 2020, 11, 73. [Google Scholar] [CrossRef]
- Shiraishi, K.; Mimura, K.; Kua, L.F.; Koh, V.; Siang, L.K.; Nakajima, S.; Fujii, H.; Shabbir, A.; Yong, W.P.; So, J.; et al. Inhibition of MMP activity can restore NKG2D ligand expression in gastric cancer, leading to improved NK cell susceptibility. J. Gastroenterol. 2016, 51, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.F.; Faure, M.; Long, E.O. LFA-1 contributes an early signal for NK cell cytotoxicity. J. Immunol. 2004, 173, 3653–3659. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gomez, E.; Vazquez-Martinez, E.R.; Reyes-Mayoral, C.; Cruz-Orozco, O.P.; Camacho-Arroyo, I.; Cerbon, M. Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis. Front. Endocrinol. 2019, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Shoji, Y.; Wu, M.C.; Chuang, P.C.; Lin, C.C.; Huang, M.F.; Tsai, S.J. Suppression of matrix metalloproteinase-9 by prostaglandin E(2) in peritoneal macrophage is associated with severity of endometriosis. Am. J. Pathol. 2005, 167, 1061–1069. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, M.; Yang, Q.; Liu, W.; Geng, H.; Pan, L.; Wang, L.; Ge, R.; Ji, L.; Cui, S.; et al. Chronic Hypoxia-Induced Microvessel Proliferation and Basal Membrane Degradation in the Bone Marrow of Rats Regulated through the IL-6/JAK2/STAT3/MMP-9 Pathway. BioMed Res. Int. 2020, 2020, 9204708. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, J.; Ding, X.; Sun, Y.; Cui, Z.; Liu, W. Tanshinone I Inhibits IL-1beta-Induced Apoptosis, Inflammation And Extracellular Matrix Degradation In Chondrocytes CHON-001 Cells And Attenuates Murine Osteoarthritis. Drug Des. Dev. Ther. 2019, 13, 3559–3568. [Google Scholar] [CrossRef]
- Meola, J.; Rosa e Silva, J.C.; Dentillo, D.B.; da Silva, W.A., Jr.; Veiga-Castelli, L.C.; Bernardes, L.A.; Ferriani, R.A.; de Paz, C.C.; Giuliatti, S.; Martelli, L. Differentially expressed genes in eutopic and ectopic endometrium of women with endometriosis. Fertil. Steril. 2010, 93, 1750–1773. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, H.; Aguiar, V.G.; Paz, C.C.; Tanus-Santos, J.E.; Penna, I.A.; Navarro, P.A. Increased circulating MMP-2 levels in infertile patients with moderate and severe pelvic endometriosis. Reprod. Sci. 2013, 20, 557–562. [Google Scholar] [CrossRef]
- Cho, Y.J.; Kim, N.H.; Jeong, K.A.; Lee, J.Y.; Moon, H.S.; Kim, H.L.; Chung, H.W. Association between MMP-2 and TIMP-2 gene polymorphisms and advanced-stage endometriosis in Korean women. Am. J. Reprod. Immunol. 2013, 69, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, R.; Jiang, Q.; Li, Z.; Wu, R. Expression of cellular adherent and invasive molecules in recurrent ovarian endometriosis. J. Int. Med. Res. 2020, 48, 300060520971993. [Google Scholar] [CrossRef]
- Gambadauro, P.; Carli, V.; Hadlaczky, G. Depressive symptoms among women with endometriosis: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 220, 230–241. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Alfaraj, S.; Yong, P.; Casper, R. New developments in the medical treatment of endometriosis. Fertil. Steril. 2017, 107, 555–565. [Google Scholar] [CrossRef]
- Arkadash, V.; Yosef, G.; Shirian, J.; Cohen, I.; Horev, Y.; Grossman, M.; Sagi, I.; Radisky, E.S.; Shifman, J.M.; Papo, N. Development of High Affinity and High Specificity Inhibitors of Matrix Metalloproteinase 14 through Computational Design and Directed Evolution. J. Biol. Chem. 2017, 292, 3481–3495. [Google Scholar] [CrossRef] [PubMed]
- Ager, E.I.; Kozin, S.V.; Kirkpatrick, N.D.; Seano, G.; Kodack, D.P.; Askoxylakis, V.; Huang, Y.; Goel, S.; Snuderl, M.; Muzikansky, A.; et al. Blockade of MMP14 activity in murine breast carcinomas: Implications for macrophages, vessels, and radiotherapy. J. Natl. Cancer Inst. 2015, 107, djv017. [Google Scholar] [CrossRef]
- Goffin, L.; Fagagnini, S.; Vicari, A.; Mamie, C.; Melhem, H.; Weder, B.; Lutz, C.; Lang, S.; Scharl, M.; Rogler, G.; et al. Anti-MMP-9 Antibody: A Promising Therapeutic Strategy for Treatment of Inflammatory Bowel Disease Complications with Fibrosis. Inflamm. Bowel Dis. 2016, 22, 2041–2057. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Starodub, A.; Sharma, S.; Berlin, J.; Patel, M.; Wainberg, Z.A.; Chaves, J.; Gordon, M.; Windsor, K.; Brachmann, C.B.; et al. Andecaliximab/GS-5745 Alone and Combined with mFOLFOX6 in Advanced Gastric and Gastroesophageal Junction Adenocarcinoma: Results from a Phase I Study. Clin. Cancer Res. 2018, 24, 3829–3837. [Google Scholar] [CrossRef]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef] [PubMed]
- Falardeau, P.; Champagne, P.; Poyet, P.; Hariton, C.; Dupont, E. Neovastat, a naturally occurring multifunctional antiangiogenic drug, in phase III clinical trials. Semin. Oncol. 2001, 28, 620–625. [Google Scholar] [CrossRef]
- Latreille, J.; Batist, G.; Laberge, F.; Champagne, P.; Croteau, D.; Falardeau, P.; Levinton, C.; Hariton, C.; Evans, W.K.; Dupont, E. Phase I/II trial of the safety and efficacy of AE-941 (Neovastat) in the treatment of non-small-cell lung cancer. Clin. Lung Cancer 2003, 4, 231–236. [Google Scholar] [CrossRef]
- Sharpe-Timms, K.L.; Zimmer, R.L.; Jolliff, W.J.; Wright, J.A.; Nothnick, W.B.; Curry, T.E. Gonadotropin-releasing hormone agonist (GnRH-a) therapy alters activity of plasminogen activators, matrix metalloproteinases, and their inhibitors in rat models for adhesion formation and endometriosis: Potential GnRH-a-regulated mechanisms reducing adhesion formation. Fertil. Steril. 1998, 69, 916–923. [Google Scholar] [CrossRef]
- Woo, J.H.; Ahn, J.H.; Jang, D.S.; Choi, J.H. Effect of Dehydrocostus Lactone Isolated from the Roots of Aucklandia lappa on the Apoptosis of Endometriotic Cells and the Alternative Activation of Endometriosis-Associated Macrophages. Am. J. Chin. Med. 2019, 47, 1289–1305. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; He, Z.; Zhang, G.; Lang, J. Effects of cisplatin and letrozole on surgically induced endometriosis and comparison of the two medications in a rat model. Eur. J. Pharm. Sci. 2016, 93, 132–140. [Google Scholar] [CrossRef]
- Hu, F.; Hu, Y.; Peng, F. Synergistic and protective effect of atorvastatin and amygdalin against histopathological and biochemical alterations in Sprague-Dawley rats with experimental endometriosis. AMB Express 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, J.; Zhang, Y.; Sun, W.; Li, Z.; Wang, Q.; Xu, X.; Li, C.; Li, P. Anti-endometriosis Mechanism of Jiawei Foshou San Based on Network Pharmacology. Front. Pharmacol. 2018, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Sirohi, V.K.; Gupta, K.; Dwivedi, A. Naringenin ameliorates progression of endometriosis by modulating Nrf2/Keap1/HO1 axis and inducing apoptosis in rats. J. Nutr. Biochem. 2019, 70, 215–226. [Google Scholar] [CrossRef]
- Wei, X.; Shao, X. Nobiletin alleviates endometriosis via down-regulating NF-kappaB activity in endometriosis mouse model. Biosci. Rep. 2018, 38, BSR20180470. [Google Scholar] [CrossRef]
- Machado, D.E.; Rodrigues-Baptista, K.C.; Alessandra-Perini, J.; Soares de Moura, R.; Santos, T.A.; Pereira, K.G.; Marinho da Silva, Y.; Souza, P.J.; Nasciutti, L.E.; Perini, J.A. Euterpe oleracea Extract (Acai) Is a Promising Novel Pharmacological Therapeutic Treatment for Experimental Endometriosis. PLoS ONE 2016, 11, e0166059. [Google Scholar] [CrossRef]
- Samartzis, E.P.; Fink, D.; Stucki, M.; Imesch, P. Doxycycline reduces MMP-2 activity and inhibits invasion of 12Z epithelial endometriotic cells as well as MMP-2 and -9 activity in primary endometriotic stromal cells in vitro. Reprod. Biol. Endocrinol. 2019, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Woo, J.H.; Kim, H.M.; Oh, M.S.; Jang, D.S.; Choi, J.H. Anti-Endometriotic Effects of Pueraria Flower Extract in Human Endometriotic Cells and Mice. Nutrients 2017, 9, 212. [Google Scholar] [CrossRef]
- Kim, J.H.; Yang, Y.I.; Ahn, J.H.; Lee, J.G.; Lee, K.T.; Choi, J.H. Deer (Cervus elaphus) antler extract suppresses adhesion and migration of endometriotic cells and regulates MMP-2 and MMP-9 expression. J. Ethnopharmacol. 2012, 140, 391–397. [Google Scholar] [CrossRef]
- Kiykac Altinbas, S.; Tapisiz, O.L.; Cavkaytar, S.; Simsek, G.; Oguztuzun, S.; Goktolga, U. Is montelukast effective in regression of endometrial implants in an experimentally induced endometriosis model in rats? Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 184, 7–12. [Google Scholar] [CrossRef]
- Jana, S.; Rudra, D.S.; Paul, S.; Snehasikta, S. Curcumin delays endometriosis development by inhibiting MMP-2 activity. Indian J. Biochem. Biophys. 2012, 49, 342–348. [Google Scholar]
- Miyashita, M.; Koga, K.; Izumi, G.; Sue, F.; Makabe, T.; Taguchi, A.; Nagai, M.; Urata, Y.; Takamura, M.; Harada, M.; et al. Effects of 1,25-Dihydroxy Vitamin D3 on Endometriosis. J. Clin. Endocrinol. Metab. 2016, 101, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
| Classification | MMPs | Location | Change (Endometriosis. vs. Control) | Reference |
|---|---|---|---|---|
| Collagenases | MMP1 | Eutopic endometrium Peripheral blood | up down | [28] [29] |
| MMP13 | Ectopic endometrium Peritoneal fluid | up down | [30] [31] | |
| Gelatinases | MMP2 | Ectopic endometrium Eutopic endometrium Peripheral blood Peritoneal fluid | up up down ns up up | [24,32,33,34,35] [36] [37] [25,34] [24,38] [24,38] |
| MMP9 | Ectopic endometrium Eutopic endometrium Peripheral blood | up ns down up | [32,33,39] [25] [28] [40] | |
| Stromelysins | MMP3 | Ectopic endometrium Eutopic endometrium Peripheral blood | up down ns | [34,35,41,42] [34] [29] |
| MMP10 | Ectopic endometrium | up | [35] | |
| MMP11 | Ectopic endometrium Eutopic endometrium | up down | [43,44] [34] | |
| Matrilysins | MMP7 | Ectopic endometrium Peripheral blood | up up | [43,45,46] [45] |
| MMP26 | Ectopic endometrium | up | [27] | |
| Membrane-type MMPs | MT1-MMP | Ectopic endometrium Eutopic endometrium Peritoneal fluid | up up down | [33,37] [36] [31] |
| MT5-MMP | Eutopic endometrium | up | [47] | |
| Other MMPs | MMP12 | Ectopic endometrium | up | [30] |
| MMP23 | Ectopic endometrium | up | [27] |
| Drug(s) | In Vivo or In Vitro | Species or Cell Type | MMP | Function | Reference |
|---|---|---|---|---|---|
| GnRH-a | In vivo | Rats | MMPs | Inhibit invasion | [118] |
| Atorvastatin and amygdalin | In vivo | Rats | MMP2, MMP9 | Inhibit invasion | [121] |
| Jiawei Foshou San | In vivo | Rats | MMP2, MMP9 | Inhibit invasion | [122] |
| Naringeni | In vivo | Rats | MMP2, MMP9 | Inhibit invasion | [123] |
| Nobiletin | In vivo | Mice | MMP1, MMP3 | Inhibit invasion | [124] |
| Euterpe oleracea extract | In vivo | Rats | MMP9 | Inhibit invasion | [125] |
| Doxycycline | In vitro | 12Z epithelial endometriotic cells, human endometriotic stromal cells | MMP2, MMP9 | Inhibit invasion | [126] |
| Pueraria flower extract | In vitro and in vivo | Human endometriotic cells and mice | MMP2, MMP9 | Inhibit invasion | [127] |
| Cervus elaphus | In vitro | Human endometriotic cells | MMP2, MMP9 | Inhibit invasion | [128] |
| Cisplatin and letrozole | In vivo | Rats | MMP2 | Inhibit angiogenesis | [120] |
| Dehydrocostus Lactone | In vitro | Human macrophages | MMP2, MMP9 | Inhibit activation | [119] |
| Resveratrol | In vivo | Human (endometrial tissue, fluid, and serum) | MMP2, MMP9 | Anti-inflammatory | [26] |
| Montelukast | In vivo | Rats | MMP2 | Decrease the area of lesions | [129] |
| Curcumin | In vivo | Mice | MMP2,TIMP2, MT1-MMP | Decrease the area of lesions | [130] |
| 1,25-Dihydroxy Vitamin D3 | In vitro | Human endometriotic stromal cells | MMP2, MMP9 | May inhibit invasion | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, J.; Ye, J.; Li, M.; Zhu, Z. The Role of Matrix Metalloproteinases in Endometriosis: A Potential Target. Biomolecules 2021, 11, 1739. https://doi.org/10.3390/biom11111739
Ke J, Ye J, Li M, Zhu Z. The Role of Matrix Metalloproteinases in Endometriosis: A Potential Target. Biomolecules. 2021; 11(11):1739. https://doi.org/10.3390/biom11111739
Chicago/Turabian StyleKe, Junya, Jiangfeng Ye, Mingqing Li, and Zhiling Zhu. 2021. "The Role of Matrix Metalloproteinases in Endometriosis: A Potential Target" Biomolecules 11, no. 11: 1739. https://doi.org/10.3390/biom11111739
APA StyleKe, J., Ye, J., Li, M., & Zhu, Z. (2021). The Role of Matrix Metalloproteinases in Endometriosis: A Potential Target. Biomolecules, 11(11), 1739. https://doi.org/10.3390/biom11111739






