Characterization of the Biomechanical Properties of Skin Using Vibrational Optical Coherence Tomography: Do Changes in the Biomechanical Properties of Skin Stroma Reflect Structural Changes in the Extracellular Matrix of Cancerous Lesions?
Abstract
:1. Introduction
2. Methods
2.1. Subjects
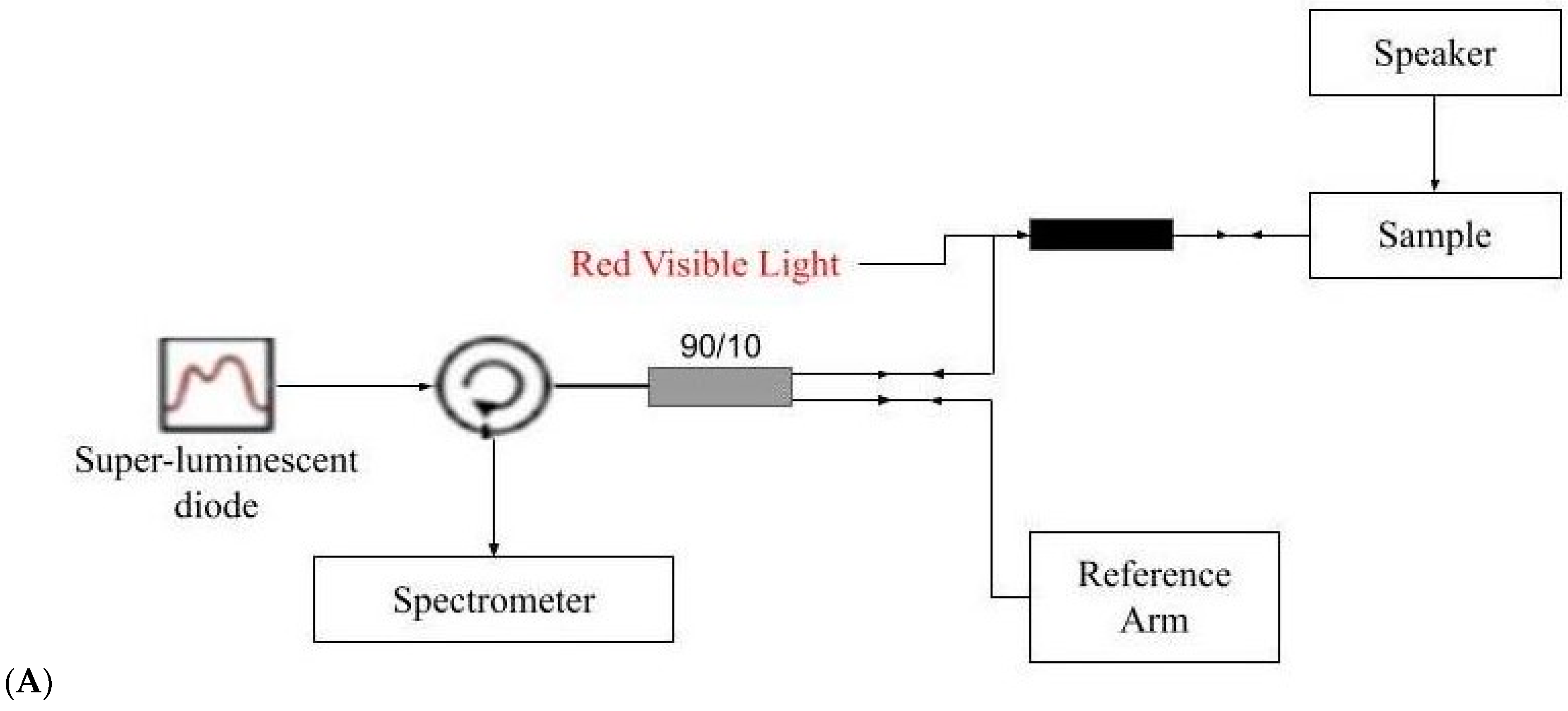
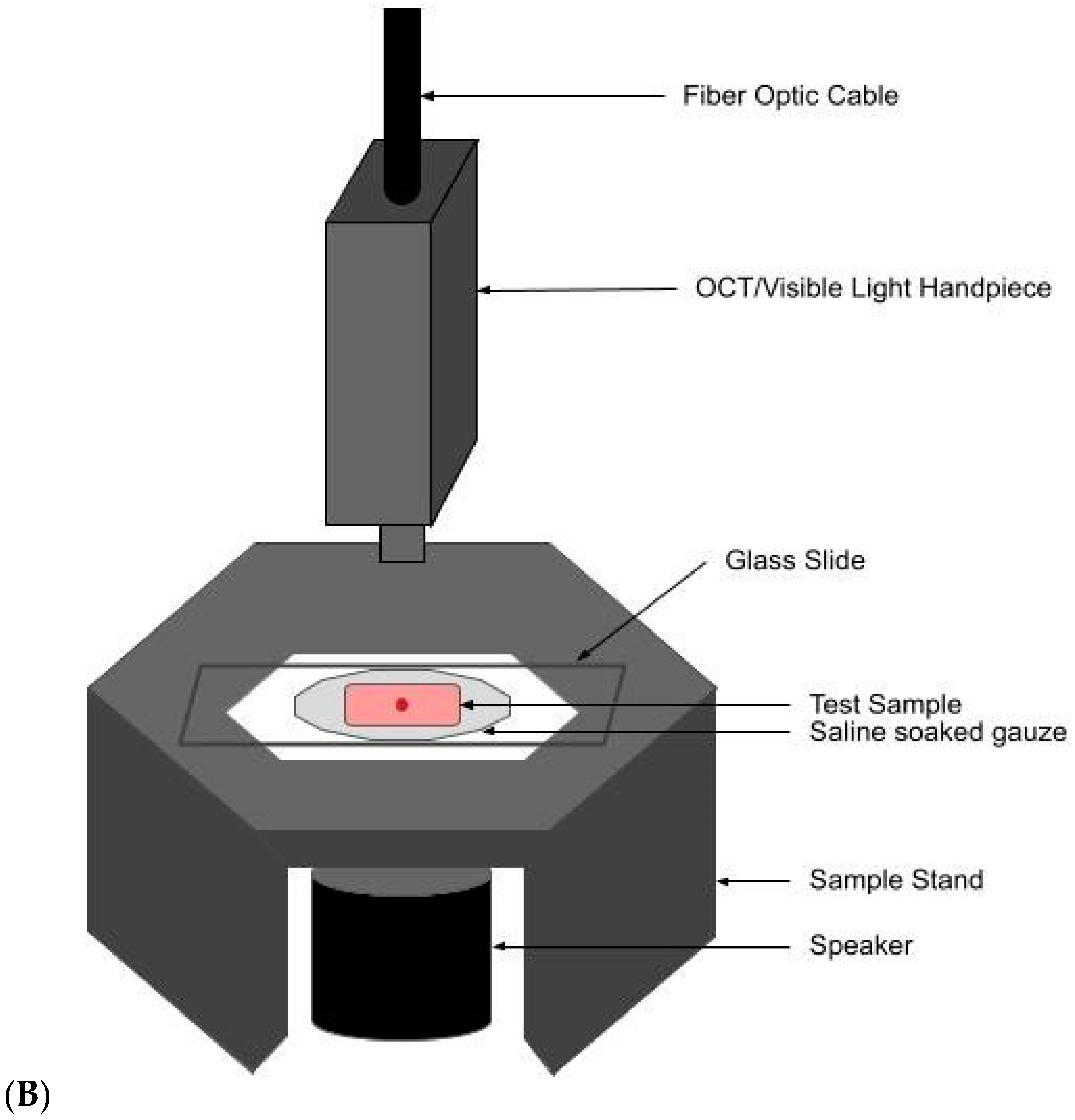
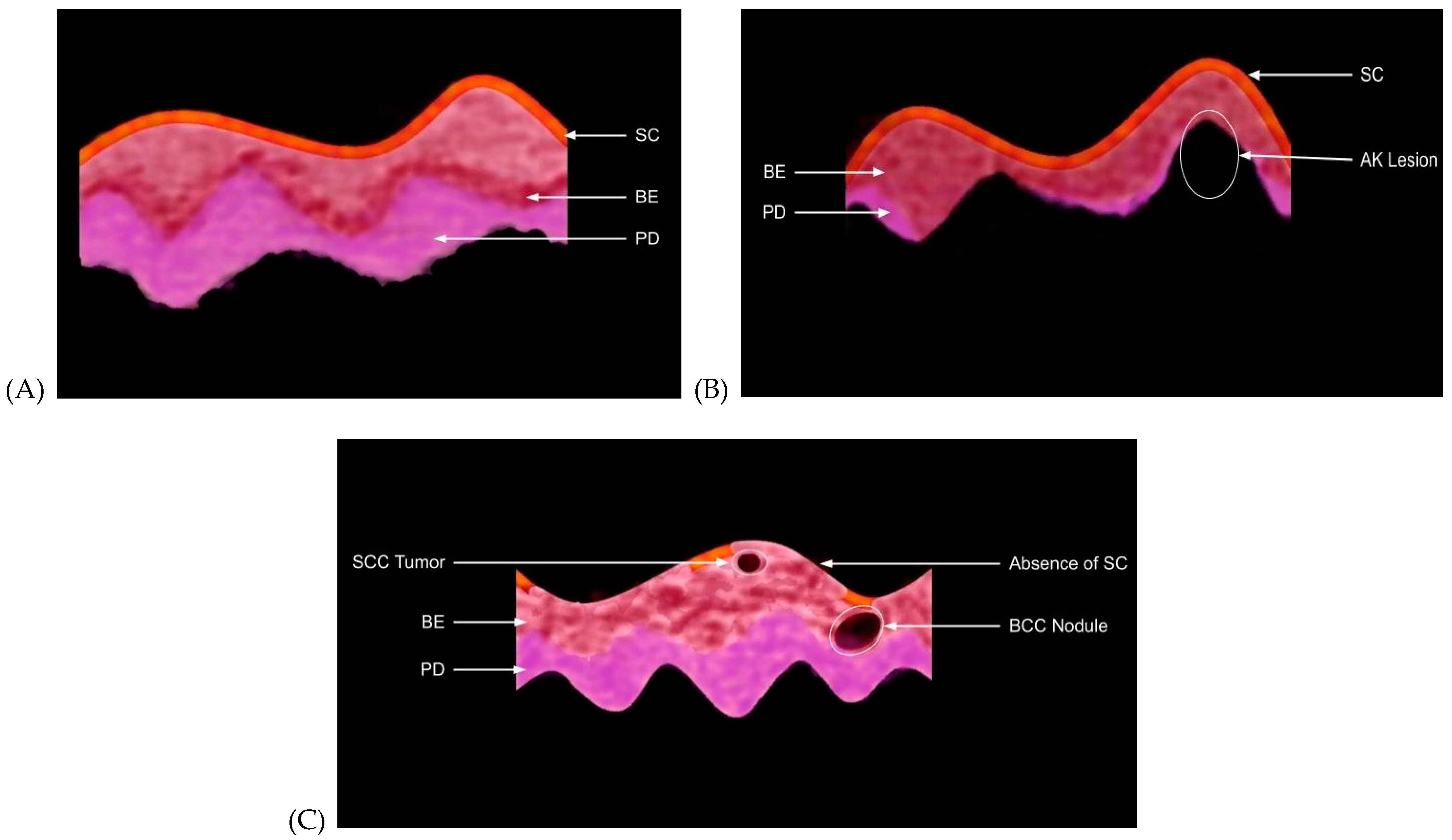
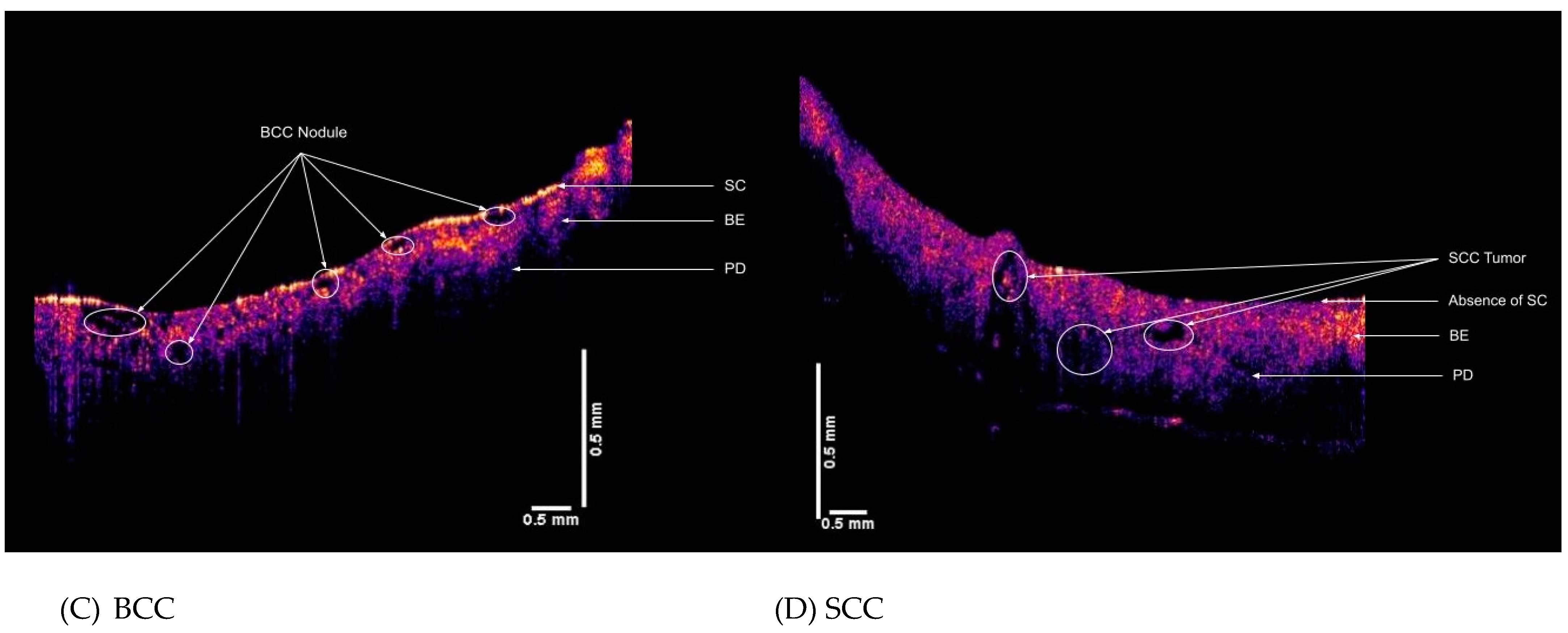
2.2. OCT Images and Scans of Pixel Intensity Versus Depth Measurements
2.3. Measurement of Resonant Frequency and the Elastic Modulus
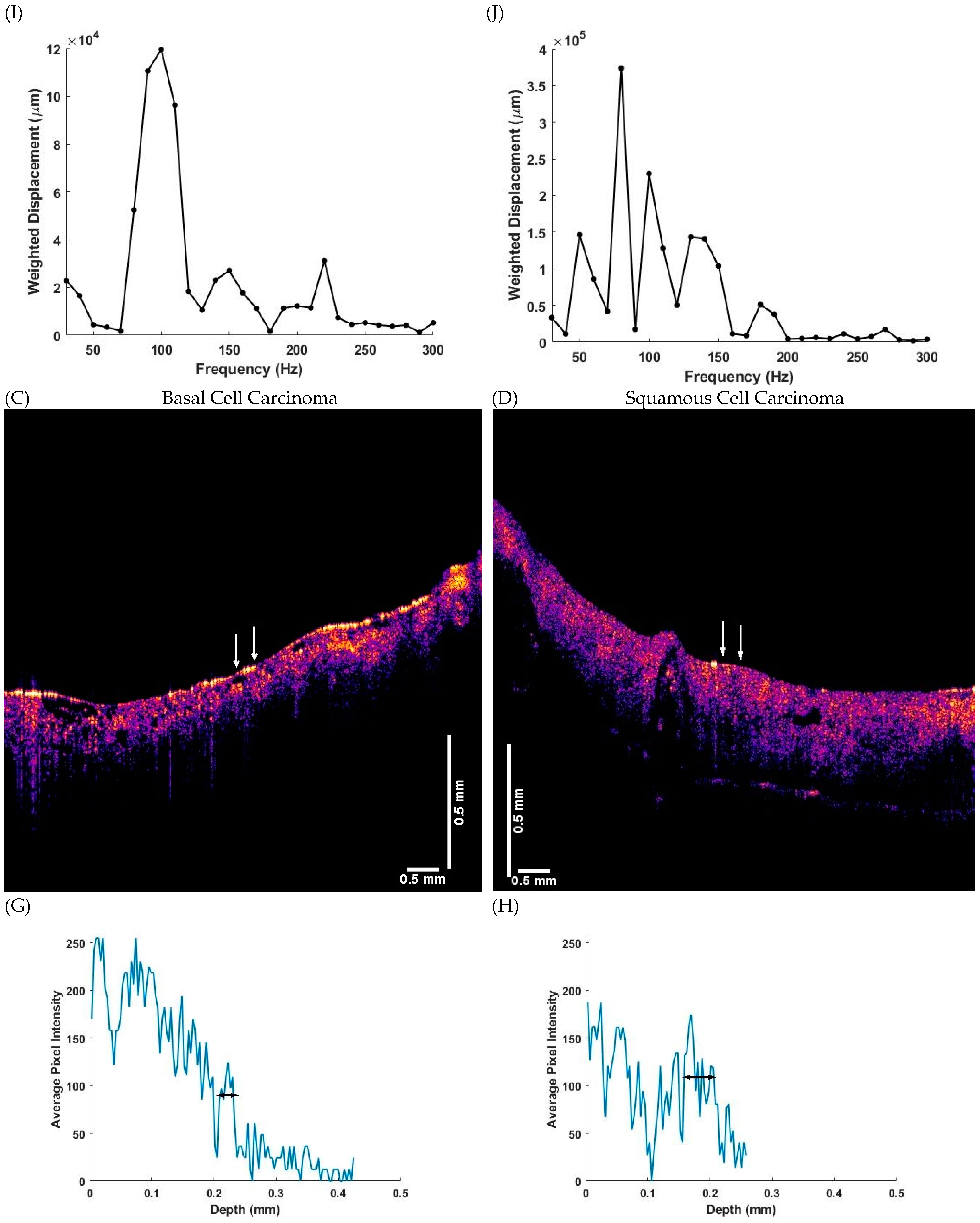
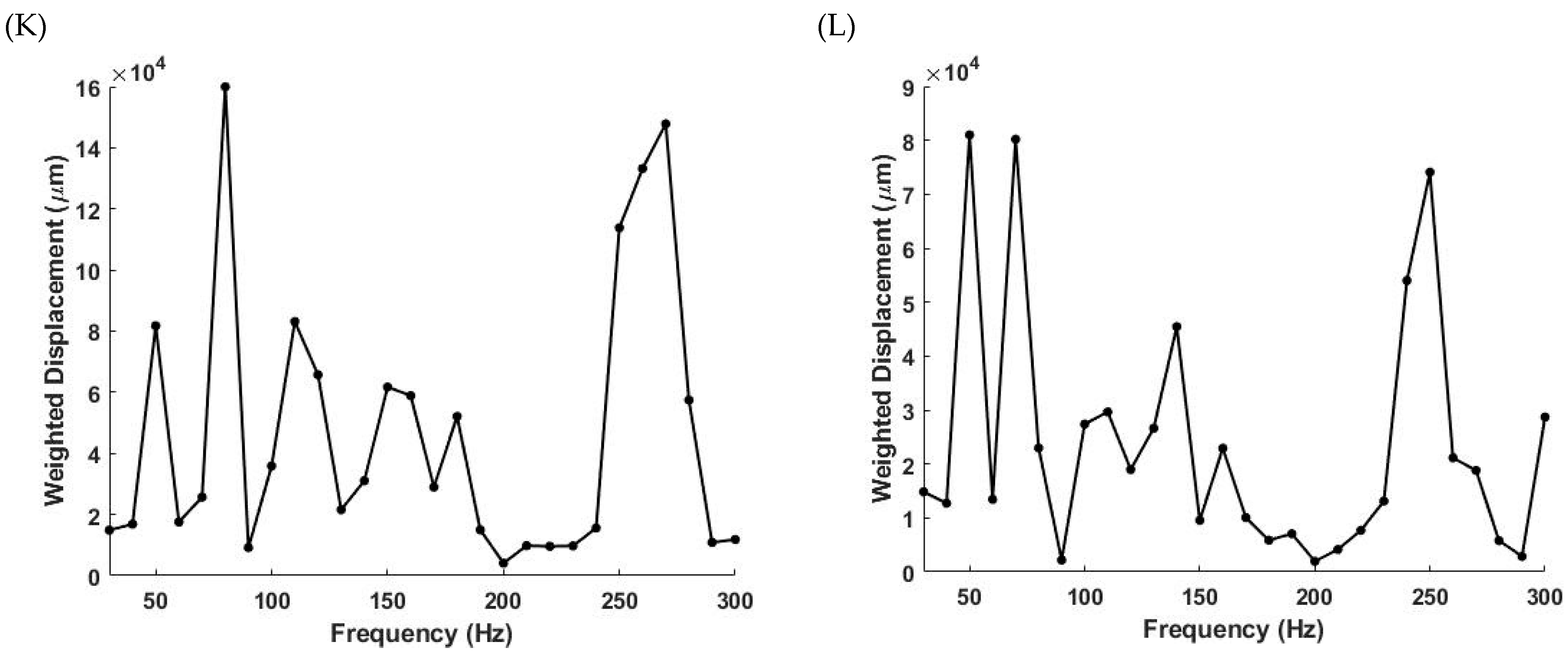
3. Results
4. Discussion
4.1. The 80 Hz Peak
4.2. The 130 Hz Peak
4.3. The 260 Hz Peak
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OCT | optical coherence tomography |
| VOCT | vibrational optical coherence tomography |
| H&E | hematoxylin and eosin |
| ECM | extracellular matrix |
| AK | actinic keratosis |
| BCC | basal cell carcinoma |
| SCC | squamous cell carcinoma |
| VM | vascular mimicry |
| YAP | yes-associated protein |
| TAZ | transcription activator with PDZ binding motif |
| EMT | epithelial–mesenchymal transition |
References
- Amercian Academy of Dermatology Association Website. Types of Skin Cancer. Available online: https://www.aad.org/public/diseases/skin-cancer/types/common (accessed on 7 September 2021).
- Skin Cancer 101. Skin cancers. Skin Cancer Found J. 2019, 37, 24–25. [Google Scholar]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.S. Prevalence of a history of skin cancer in 2007: Results of an incidence-based model. Arch. Dermatol. 2010, 146, 279–282. [Google Scholar] [CrossRef]
- Linos, E.; Katz, K.A.; Colsditz, G.A. Skin Cancer—The Importance of Prevention. JAMA Intern. Med. 2016, 176, 1435–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neville, J.A.; Welch, E.; Leffell, D.J. Management of nonmelanoma skin cancer in 2007. Nat. Clin. Pract. Oncol. 2007, 4, 462–469. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Weinstein, D.A.; Konda, S.; Coldiron, B.M. Use of skin biopsies among dermatologists. Dermatol. Surg. 2017, 43, 1348–1357. [Google Scholar] [CrossRef]
- Emiroglu, N.; Cengiz, F.P.; Kemerliz, F. The relationship between dermoscopy and histopathology of basal cell carcinoma. An. Bras. Dermatol. 2015, 90, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Weber, P.; Tschandl, P.; Sinz, C.; Kitler, H. Dermatoscopy of neoplastic skin lesions: Recent advances, updates, and revisions. Curr. Treat.Options Oncol. 2018, 19, 56. [Google Scholar] [CrossRef] [Green Version]
- Wolner, Z.J.; Yelomas, O.; Liopyris, K.; Rodgers, T.; Marchetti, M.M.; Marghoob, A.A. Enhancing skin cancer diagnosis with dermoscopy. Dermatol. Clin. 2017, 35, 417–437. [Google Scholar] [CrossRef]
- Privalle, A.; Havighurst, T.; Kim, K.M.; Bennett, D.D.; Xu, Y.G. Number of skin biopsies needed per malignancy: Comparing the use of skin biopsies among dermatologists and nondermatologist clinicians. J. Am. Acad. Dermatol. 2020, 82, 110–116. [Google Scholar] [CrossRef]
- Wernli, K.J.; Henrikson, N.B.; Morrison, C.C.; Nguyen, M.; Pocobelli, G.; Blasi, P.R. Screening for skin cancer in adults updated evidence report and systematic review for the US preventive services task force. JAMA 2016, 316, 436–447. [Google Scholar] [CrossRef]
- Wang, D.M.; Morgan, F.C.; Besaw, R.J.; Schmults, C.D. An ecological study of skin biopsies and skin cancer treatment procedures in the United States Medicare population, 2000 to 2015. J. Am. Acad. Dermatol. 2018, 78, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; Piccolo, V.; Lallas, A.; Jacomel, J.; Moscarella, E.; Alfano, R.; Argenziano, G. Dermoscopy of malignant tumours: What’s new. Dermatol. 2017, 233, 64–73. [Google Scholar] [CrossRef]
- Morgensen, M.; Gregor, B.E.; Jemec, M.D. Diagnosis of nonmelanoma skin cancer/keratinocyte carcinoma: A review of dianostic accuracy of nonmelanoma skin cancer diagnostic test and technologies. Amer. Soc. Dermatol. Surg. 2007, 33, 1158–1174. [Google Scholar]
- Lallas, A.; Apalla, Z.; Argenziano, G.; Logo, C.; Moscarella, E.; Specchio, F.; Raucci, M.; Zalaudek, I. The dermoscopic universe of basal cell carcinoma. Dermatol. Pract. Concept. 2013, 4, 11–24. [Google Scholar]
- Reinehr, C.P.H.; Bakos, R.M. Actinic keratoses: Review of clinical, dermoscopic, and therapeutic aspects. An. Bras. Dermatol. 2019, 94, 637–657. [Google Scholar] [CrossRef]
- Combalia, A.; Carrera, C. Squamous Cell Carcinoma: An Update on Diagnosis and Treatment. Dermatol. Pract. Concept. 2020, 10, e2020066. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Shah, R.G.; Richard, M.; Benedetto, D. Comparative “virtual biopsies” of normal skin and skin lesions using vibrational optical coherence tomography. Skin Res. Technol. 2019, 25, 743–749. [Google Scholar] [CrossRef]
- Silver, F.H.; Shah, R.G.; Richard, M.; Benedetto, D. Use of Vibrational Optical Coherence Tomography to Image and Characterize a Squamous Cell Carcinoma. J. Derm. Res. Ther. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Coutts, L.V.; Miller, N.R.; Harland, C.C.; Bamber, J.C. Feasibility of skin surface elastography by tracking skin surface topography. J. Biomed. Opt. 2013, 18, 121513. [Google Scholar] [CrossRef]
- Shah, K.; Weissleder, R. Molecular optical imaging: Applications leading to the development of present day therapeutics. NeuroRx 2005, 2, 215–225. [Google Scholar] [CrossRef]
- Low, G. General review of magnetic resonance elastography. World J. Radiol. 2016, 8, 7101217. [Google Scholar] [CrossRef]
- Venkatesh, S.K.; Ehman, R.L. Magnetic Resonance Elastography; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–137. ISBN 978-1-4939-15. [Google Scholar]
- Kennedy, B.F.; Kennedy, K.M.; Sampson, D.D. A review of optical coherence elastography: Fundamentals, techniques and prospects. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 272–288. [Google Scholar] [CrossRef]
- Campàs, O.; Hammoto, T.; Hasso, S.; Sperling, R.A.; O’Connell, D.O.; Bischof, A.G.; Maas, R.; Weitz, D.A.; Mahadevan, L.; Ingber, D.E. Quantifying cell-generated mechanical forces within living embryonic tissues. Nat. Methods 2014, 11, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.F.; McLaughlin, R.A.; Kennedy, K.M.; Chin, L.; Curatolo, A.; Tien, A.; Latham, B.; Saunders, C.M.; Sampson, D.D. Optical coherence micro-elastography: Mechanical-Contrast imaging of tissue microstructure. Biomed. Opt. Express 2014, 5, 2113–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Larin, K.V. Optical coherence elastography for tissue characterization: A review. J. Biophotonics 2015, 176, 279–302. [Google Scholar] [CrossRef] [Green Version]
- Drakonaki, E.E.; Allen, G.M.; Wilson, D.J. Ultrasound elastography for musculoskeletal applications. Br. J. Radiol. 2012, 85, 1435–1445. [Google Scholar] [CrossRef] [Green Version]
- Dasgeb, B.; Morris, M.A.; Mehregan, D.; Siegel, E.L. Quantified ultrasound elastography in the assessment of cutaneous carcinoma. Br. J. Radiol. 2015, 88, 19–21. [Google Scholar] [CrossRef] [Green Version]
- Silver, F.H.; Kelkar, N.; Desmukh, T.; Horvath, I.; Shah, R.G. Mechano-Vibrational Spectroscopy of Tissues and Materials Using Vibrational Optical Coherence Tomography: A New Non-Invasive and Non-Destructive Technique. Recent Prog. Mater. 2020, 2, 1–16. [Google Scholar] [CrossRef]
- Silver, F.H.; Horvath, I.; Kelkar, N.; Desmukh, T.; Shah, R. In vivo biomechanical analysis of human tendon using vibrational optical coherence tomograph: Preliminary results. J. Clin. Cases Rep. 2021, 4, 12–19. [Google Scholar]
- Silver, F.H. Measurement of Mechanical Properties of Natural and Engineered Implants. Adv. Tissue Eng. Regen. Med. Open Access 2016, 1, 20–25. [Google Scholar] [CrossRef]
- Silver, F.H.; Deshmukh, T.; Benedetto, D.; Kelkar, N. Mechano-vibrational spectroscopy of skin: Are changes in collagen and vascular tissue components early signs of basal cell carcinoma formation? Skin Res. Technol. 2020, 27, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.G.; Devore, D.; Pierce, M.C.; Silver, F.H. Vibrational analysis of implants and tissues: Calibration and mechanical spectroscopy of multi-component materials. J. Biomed. Mater. Res.—Part A 2017, 105, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.G.; Silver, F.H. Viscoelastic Behavior of Tissues and Implant Materials: Estimation of the Elastic Modulus and Viscous Contribution Using Optical Coherence Tomography and Vibrational Analysis. J. Biomed. Technol. Res. 2017, 3, 105–109. [Google Scholar]
- Silver, F.H.; Seehra, G.P.; Freeman, J.W.; DeVore, D. Viscoelastic properties of young and old dermis: A proposed molecular mechnism for elastic energy storgae in collagen ad and elastin. J. Appl. Polym. Sci. 2002, 86, 1978–1985. [Google Scholar] [CrossRef]
- Samani, A.; Zubovits, J.; Plewes, D. Elastic moduli of normal and pathological humanbreast tissues: An inversion-technique-based investigation of 169 samples. Phys. Med. Biol. 2007, 52, 1565–1576. [Google Scholar] [CrossRef]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2016, 7, 1120–1134. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-H.; Lin, H.-K.; Lin, I.-H.; Chiou, Y.-W.; Chen, H.-W.; Liu, C.-Y.; Harn, H.I.-C.; Chiu, W.-T.; Wang, Y.-K.; Shen, M.-R.; et al. Mechanical phenotype of cancer cells: Cell softening and loss of stiffness sensing. Oncotarget 2015, 6, 20946–20958. [Google Scholar] [CrossRef] [Green Version]
- Lekka, M. Discrimination between normal and cancerous cells using AFM. Bionanoscience 2016, 6, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Odell, I.D.; Flavell, R.A.; Silver, F.H. Vibrational optical coherence tomography detects unique skin fibrotic states: Preliminary results of animal and human studies. J. Am. Acad. Dematol. 2020, 85, 780–782. [Google Scholar] [CrossRef]
- Pala, M.; Bhattacharyac, S.; Kalyanc, G.; Hazrac, S. Cadherin profiling for therapeutic interventions in Epithelial Mesenchymal Transition (EMT) and tumorigenesis. Exp. Cell Res. 2018, 368, 137–148. [Google Scholar] [CrossRef]
- Silver, F.H. Viewpoint: Mechanotransduction-The Relationship between Gravity, Cells and Tensile loading in Extracellular Matrix. Biocell 2021, in press. [Google Scholar]
- Silver, F.H.; Siperko, L.M.; Seehra, G.P. Mechanobiology of force transduction in dermis. Skin Res. Technol. 2002, 8, 1–21. [Google Scholar]
- Angulo-Urarte, A.; Tanne Van Der Wal, T.; Huveneers, S. Cell–Cell junctions as sensors and transducers of mechanical forces. Biochim. Biophy. Acta—Biomembr. 2020, 1862, 183316. [Google Scholar] [CrossRef]
- Ayollo, D.V.; Zhitnyak, I.Y.; Vasiliev, J.M.; Gloushankova, N.A. Rearrangements of the Actin Cytoskeleton and E-Cadherin–Based Adherens Junctions Caused by Neoplasic Transformation Change Cell–Cell Interactions. PLoS ONE 2009, 4, e8027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazan, R.B.; Phillips, G.R.; Qiao, R.F.; Norton, L.; Aaronson, S.A. Exogenous Expression of N-Cadherin in Breast Cancer Cells Induces Cell Migration, Invasion, and Metastasis Exogenous Expression of N-Cadherin in Breast Cancer Cells Induces Cell Migration, Invasion, and Metastasis. J. Cell Biol. 2000, 148, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Casal, J.I.; Bartolomé, R.A. Beyond N-Cadherin, Relevance of Cadherins 5, 6 and 17 in Cancer Progression and Metastasis. Int. J. Mol. Sci. 2019, 20, 3373. [Google Scholar] [CrossRef] [Green Version]
- Silver, F.H.; Kelkar, N.; Deshmukh, T. In Vivo Non-Invasive Analysis of the Mechanical Properties of Vessel Walls using Vibrational Optical Coherence Tomography. J. Cardio Res. Rep. 2021, 5. [Google Scholar] [CrossRef]
- Farnsworth, R.M.; Lackmann, M.; Achen, M.G.; Stacker, S.A. Vascular remodeling in cancer. Oncogene 2014, 33, 3496–3505. [Google Scholar] [CrossRef] [Green Version]
- Azad, T.; Ghahremani, M.; Yang, X. The Role of YAP and TAZ in Angiogenesis and Vascular Mimicry. Cells 2019, 8, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winslow, S.; Linsquest, K.E.; Edsjo, A.; Larsson, C. The expression pattern of matrix-producing tumor stroma is of prognostic importance in breast cancer. BMC Cancer 2016, 16, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanangelt, K.M.H.; Green, A.R.; Heemskerk, I.M.F. The prognostic value of the tumor-stroma ratio is most discriminative in patients with grade III or triple-negative breast cancer. Int. J. Cancer 2020, 146, 2296–2304. [Google Scholar] [CrossRef] [Green Version]
- del Pozo Martin, Y.; Park, D.; Ramachandran, A.; Ombrato, L.; Calvo, F.; Chakravarty, P.; Spencer-Dere, B.; Derzsi, S.; Hill, C.S.; Sahai, E.; et al. Mesenchymal Cancer Cell-Stroma Crosstalk Promotes Niche Activation, Epithelial Reversion, and Metastatic Colonization. Cell Rep. 2015, 13, 2456–2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silver, F.H.; Shah, R. “Virtual Biopsies” of Normal Skin and Thermal and Chemical Burn Wounds. Adv. Skin Wound Care 2020, 33, 1–6. [Google Scholar] [CrossRef]


| Parameter | Normal Skin | AK | BCC | SCC |
|---|---|---|---|---|
| Sample Size | 59 | 7 | 55 | 46 |
| Average (SD) | 4.48 × 104 (4.73 × 104) | 1.431 × 105 (1.179 × 105) | 9.08 × 104 (9.73 × 104) | 9.10 × 104 (9.79 × 104) |
| Normal Skin | NA | 0.035 | 7.74 × 10−3 | 0.01 |
| AK | NA | 0.148 | 0.15 | |
| BCC | NA | 0.49 |
| Normal Skin | AK | BCC | SCC | |
|---|---|---|---|---|
| Average (SD) | 9.48 × 104 (1.920 × 105) | 3.60 × 103 (9.54 × 103) | 1.283 × 105 (1.407 × 105) | 7.88 × 104 (1.009 × 105) |
| Normal Skin | NA | 0.049 | 0.274 | 0.38 |
| AK | NA | 1.24 × 10−8 | 5.22 × 10−6 | |
| BCC | NA | 0.021 |
| Normal Skin | AK | BCC | SCC | |
|---|---|---|---|---|
| Average (SD) | 0.30 (0.28) | 0.88 (0.56) | 1.160 (0.89) | 1.064 (0.77) |
| Normal Skin | NA | 0.022 | 1.62 × 10−8 | 1.36 × 10−7 |
| AK | NA | 0.132 | 0.21 | |
| BCC | NA | 0.28 |
| Normal Skin | AK | BCC | SCC |
|---|---|---|---|
| 0.30 (0.38) | 0.81 (0.54) | 3.38 (0.69) | 1.186 (0.85) |
| NA | 0.02 | 1.04 × 10−6 | 8.82 × 10−7 |
| NA | 0.13 | 0.07 | |
| NA | 0.26 |
| Normal Skin | AK | BCC | SCC | |
|---|---|---|---|---|
| Average (SD) | 0.36 (0.36) | 0.122 (0.33) | 2.27 (4.55) | 1.574 (1.401) |
| Normal Skin | NA | 0.057 | 1.89 × 10−3 | 1.50 × 10−6 |
| AK | NA | 5.70 × 10−4 | 1.60 × 10−7 | |
| BCC | NA | 0.144 |
| Normal Skin | AK | BCC | SCC | |
|---|---|---|---|---|
| Average (SD) | 0.89 (1.094) | 0.49 (0.23) | 0.88 (0.65) | 0.88 (0.56) |
| Normal Skin | NA | 0.08 | 0.43 | 0.43 |
| AK | NA | 0.004 | 0.004 | |
| BCC | NA | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silver, F.H.; Kelkar, N.; Deshmukh, T.; Ritter, K.; Ryan, N.; Nadiminti, H. Characterization of the Biomechanical Properties of Skin Using Vibrational Optical Coherence Tomography: Do Changes in the Biomechanical Properties of Skin Stroma Reflect Structural Changes in the Extracellular Matrix of Cancerous Lesions? Biomolecules 2021, 11, 1712. https://doi.org/10.3390/biom11111712
Silver FH, Kelkar N, Deshmukh T, Ritter K, Ryan N, Nadiminti H. Characterization of the Biomechanical Properties of Skin Using Vibrational Optical Coherence Tomography: Do Changes in the Biomechanical Properties of Skin Stroma Reflect Structural Changes in the Extracellular Matrix of Cancerous Lesions? Biomolecules. 2021; 11(11):1712. https://doi.org/10.3390/biom11111712
Chicago/Turabian StyleSilver, Frederick H., Nikita Kelkar, Tanmay Deshmukh, Kelly Ritter, Nicole Ryan, and Hari Nadiminti. 2021. "Characterization of the Biomechanical Properties of Skin Using Vibrational Optical Coherence Tomography: Do Changes in the Biomechanical Properties of Skin Stroma Reflect Structural Changes in the Extracellular Matrix of Cancerous Lesions?" Biomolecules 11, no. 11: 1712. https://doi.org/10.3390/biom11111712





