Co-Evolution of Breast Milk Lipid Signaling and Thermogenic Adipose Tissue
Abstract
:1. The Lipid-Rich Breast Milk Is a Key Energy Source in Newborn Mammals
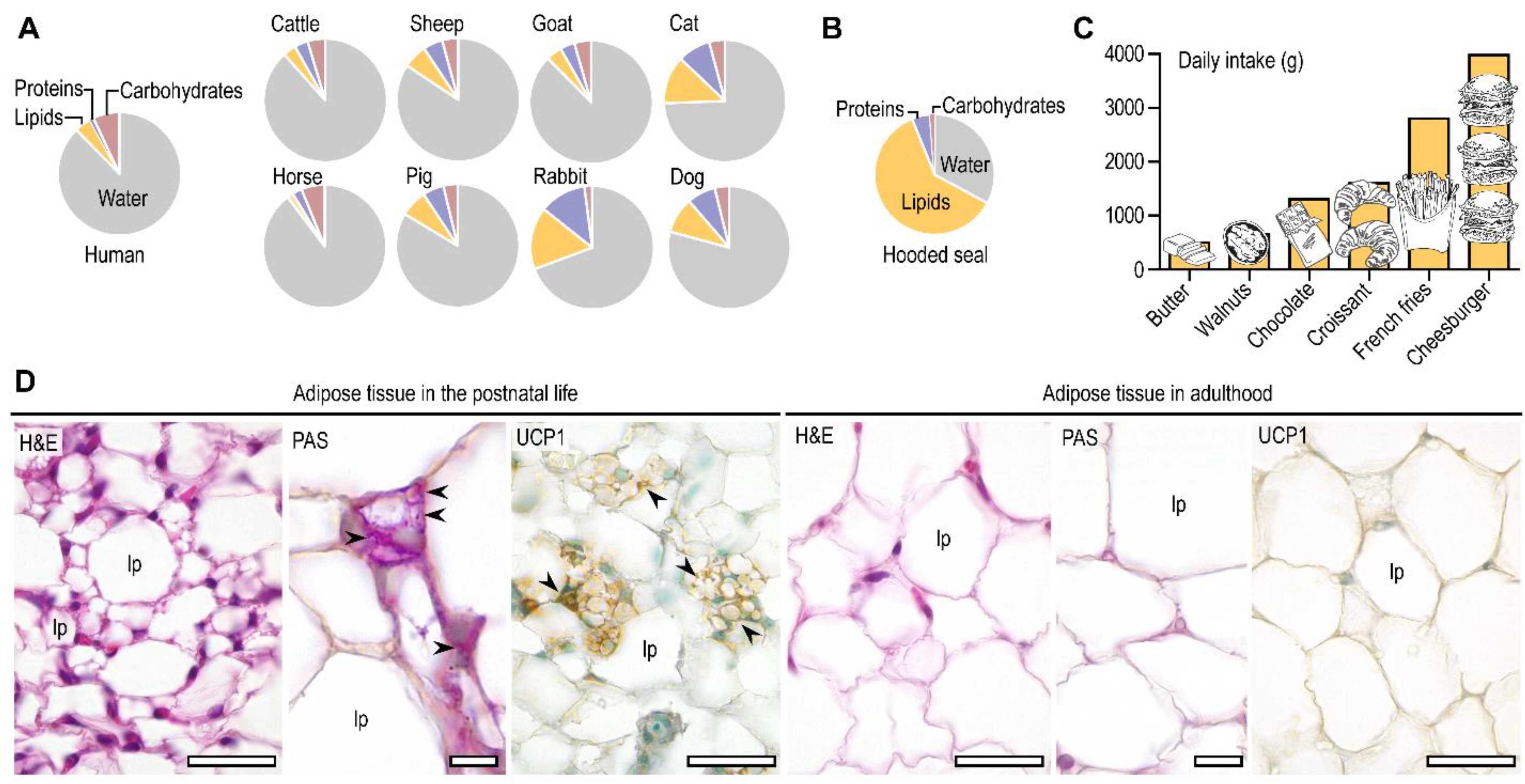
2. Fat Is a Relevant Metabolic Fuel after Birth
3. Dual Metabolic Roles of the Newborn Adipose Tissue: Fatty Acid Oxidation and Lipogenesis
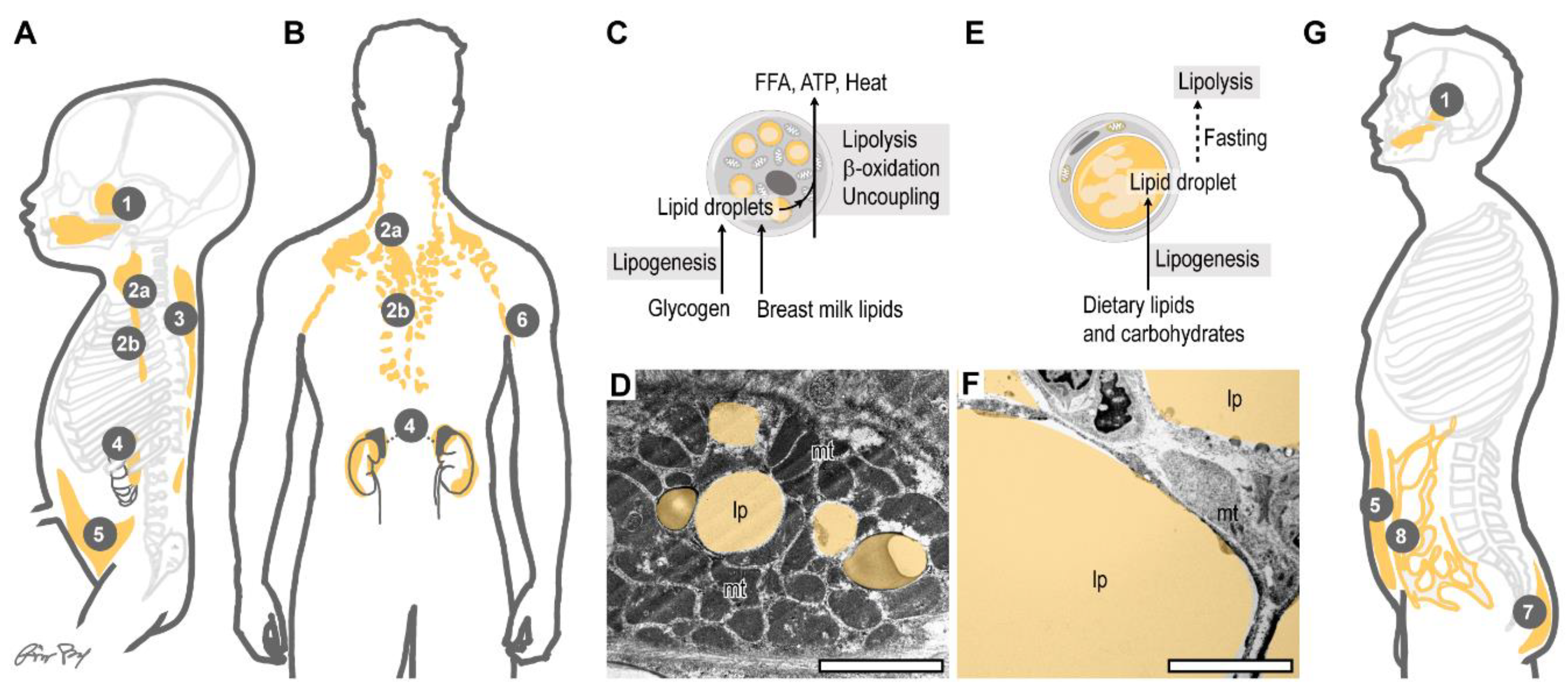
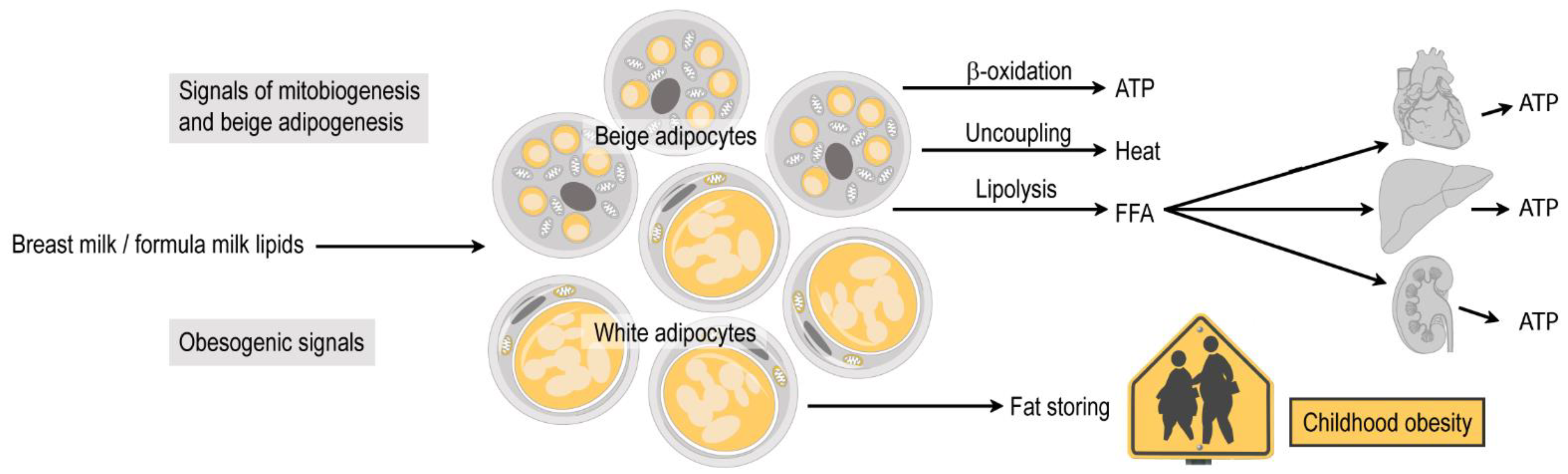
4. Infant Subcutaneous Fat: Brown, White or Beige?
5. Mitochondria: Indispensable Organelles for Fat Catabolism
6. Breast Milk Signals Are Necessary for Mitochondrial Metabolism
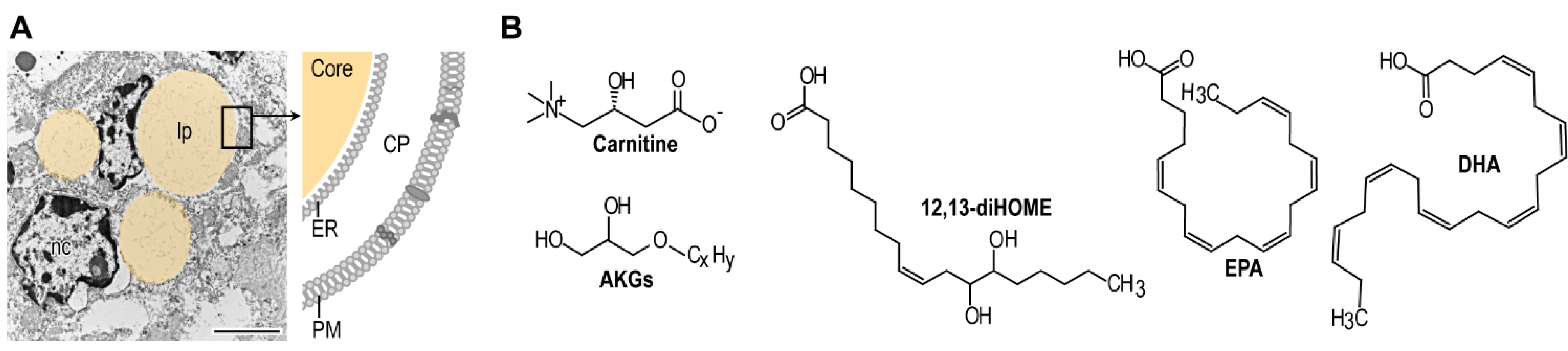
7. Evolution and Current Impact of Breastfeeding
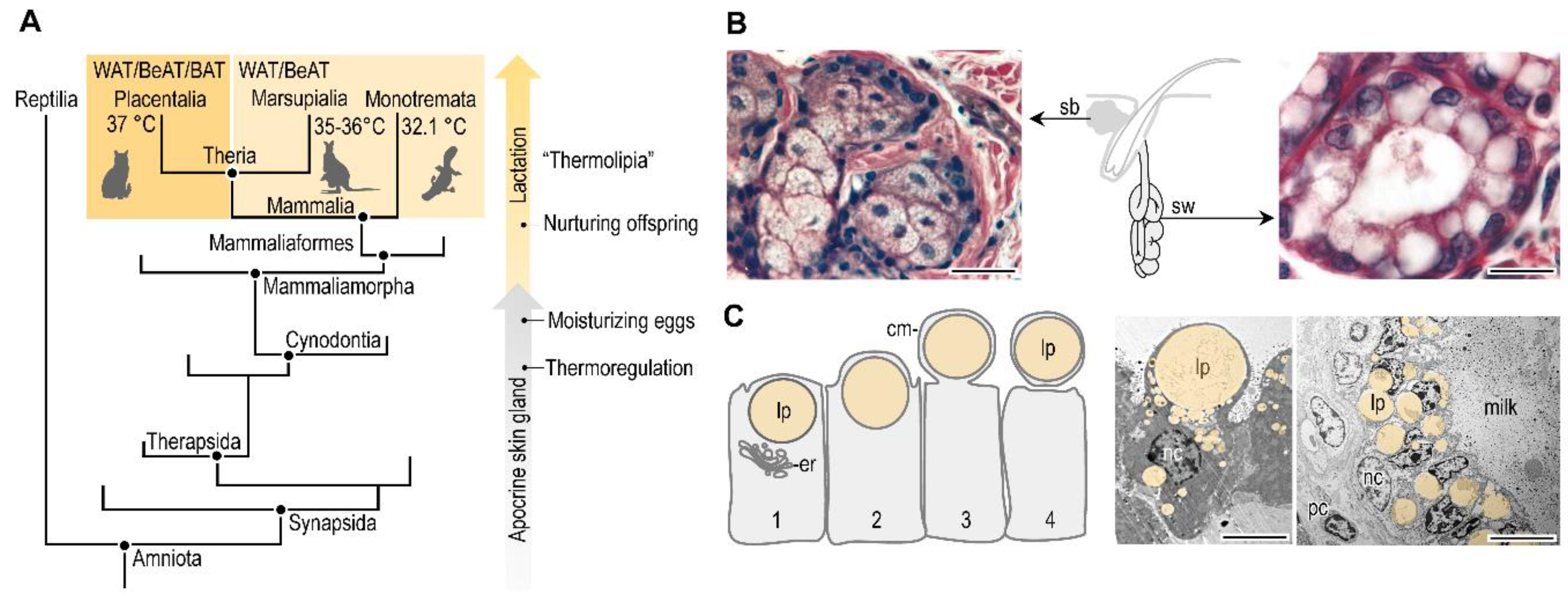
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera, E.; Amusquivar, E. Lipid metabolism in the fetus and the newborn. Diabetes Metab. Res. Rev. 2000, 16, 202–210. [Google Scholar] [CrossRef]
- Persson, B. Carbohydrate and Lipid Metabolism in the Newborn Infant. Acta Anaesthesiol. Scand. 1974, 18, 50–57. [Google Scholar] [CrossRef]
- Clària, J.; Nguyen, B.T.; Madenci, A.L.; Ozaki, C.K.; Serhan, C.N. Diversity of lipid mediators in human adipose tissue depots. Am. J. Physiol. Cell Physiol. 2013, 304, C1141–C1149. [Google Scholar] [CrossRef]
- Sullivan, E.M.; Pennington, E.R.; Green, W.D.; Beck, M.A.; Brown, D.A.; Shaikh, S.R. Mechanisms by Which Dietary Fatty Acids Regulate Mitochondrial Structure-Function in Health and Disease. Adv. Nutr. 2018, 9, 247–262. [Google Scholar] [CrossRef] [Green Version]
- Bensinger, S.J.; Tontonoz, P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 2008, 454, 470–477. [Google Scholar] [CrossRef]
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Boutens, L.; Stienstra, R. Adipose tissue macrophages: Going off track during obesity. Diabetologia 2016, 59, 879–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odegaard, J.I.; Ganeshan, K.; Chawla, A. Adipose Tissue Macrophages: “Amicus adipem?”. Cell Metab. 2013, 18, 767–768. [Google Scholar] [CrossRef] [Green Version]
- Nelson, A.J.; Stephenson, D.J.; Bone, R.N.; Cardona, C.L.; Park, M.A.; Tusing, Y.G.; Lei, X.; Kokotos, G.; Graves, C.L.; Mathews, C.E.; et al. Lipid mediators and biomarkers associated with type 1 diabetes development. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Knotts, T.A.; Chavez, J.A.; Wang, L.P.; Hoehn, K.L.; Summers, S.A. Lipid mediators of insulin resistance. Nutr. Rev. 2007, 65, S39–S46. [Google Scholar] [CrossRef]
- Yu, H.; Dilbaz, S.; Coßmann, J.; Hoang, A.C.; Diedrich, V.; Herwig, A.; Harauma, A.; Hoshi, Y.; Moriguchi, T.; Landgraf, K.; et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J. Clin. Investig. 2019, 129, 2485–2499. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B. Human Milk Lipids. Ann. Nutr. Metab. 2016, 69 (Suppl. S2). [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamosh, M. Protective function of proteins and lipids in human milk. Biol. Neonate 1998, 74, 163–176. [Google Scholar] [CrossRef]
- Skibiel, A.L.; Downing, L.M.; Orr, T.J.; Hood, W.R. The evolution of the nutrient composition of mammalian milks. J. Anim. Ecol. 2013, 82, 1254–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrzak-Fiećko, R.; Kamelska-Sadowska, A.M. The Comparison of Nutritional Value of Human Milk with Other Mammals’ Milk. Nutrients 2020, 12, 1404. [Google Scholar] [CrossRef]
- Hoang, A.C.; Yu, H.; Röszer, T. Transcriptional Landscaping Identifies a Beige Adipocyte Depot in the Newborn Mouse. Cells 2021, 10, 2368. [Google Scholar] [CrossRef]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef]
- Wolfs, D.; Lynes, M.D.; Tseng, Y.-H.; Pierce, S.; Bussberg, V.; Darkwah, A.; Tolstikov, V.; Narain, N.R.; Rudolph, M.C.; Kiebish, M.A.; et al. Brown fat-activating lipokine 12,13-diHOME in human milk is associated with infant adiposity. J. Clin. Endocrinol. Metab. 2020, 106, e943–e956. [Google Scholar] [CrossRef] [PubMed]
- Oftedal, O.T.; Bowen, W.D.; Boness, D.J. Energy Transfer by Lactating Hooded Seals and Nutrient Deposition in Their Pups during the Four Days from Birth to Weaning. Physiol. Zool. 1993, 66, 412–436. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Diabetes; WHO Press: Geneva, Switzerland, 2016. [Google Scholar]
- Dietz, W.H. Overweight in Childhood and Adolescence. New Engl. J. Med. 2004, 350, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J. The adiposity rebound in the 21st century children: Meaning for what? Korean J. Pediatr. 2018, 61, 375–380. [Google Scholar] [CrossRef]
- Mela, D.J.; Sacchetti, D.A. Sensory preferences for fats: Relationships with diet and body composition. Am. J. Clin. Nutr. 1991, 53, 908–915. [Google Scholar] [CrossRef]
- French, S.A.; Jejfery, R.W.; Folsom, A.R.; Williamson, D.F.; Byers, T. History of Intentional and Unintentional Weight Loss in a Population-Based Sample of Women Aged 55 to 69 Years. Obes. Res. 1995, 3, 163–170. [Google Scholar] [CrossRef]
- Dietz, W.H. Critical periods in childhood for the development of obesity. Am. J. Clin. Nutr. 1994, 59, 955–959. [Google Scholar] [CrossRef]
- Charney, E.; Goodman, H.C.; McBride, M.; Lyon, B.; Pratt, R. Childhood antecedents of adult obesity. Do chubby infants become obese adults? New Engl. J. Med. 1976, 295, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Fall, C. Developmental Origins of Cardiovascular Disease, Type 2 Diabetes and Obesity in Humans; Springer: Boston, MA, USA, 2006; Volume 573, pp. 8–28. [Google Scholar]
- Landgraf, K.; Rockstroh, D.; Wagner, I.V.; Weise, S.; Tauscher, R.; Schwartze, J.T.; Löffler, D.; Bühligen, U.; Wojan, M.; Till, H.; et al. Evidence of early alterations in adipose tissue biology and function and its association with obesity-related inflammation and insulin resistance in children. Diabetes 2015, 64, 1249–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geserick, M.; Vogel, M.; Gausche, R.; Lipek, T.; Spielau, U.; Keller, E.; Pfaffle, R.; Kiess, W.; Körner, A. Acceleration of BMI in early childhood and risk of sustained obesity. N. Engl. J. Med. 2018, 379, 1303–1312. [Google Scholar] [CrossRef]
- Rolland-Cachera, M.F.; Deheeger, M.; Bellisle, F.; Sempé, M.; Guilloud-Bataille, M.; Patois, E. Adiposity rebound in children: A simple indicator for predicting obesity. Am. J. Clin. Nutr. 1984, 39, 129–135. [Google Scholar] [CrossRef]
- Eriksson, J.G.; Forsén, T.; Tuomilehto, J.; Osmond, C.; Barker, D.J. Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. Diabetologia 2003, 46, 190–194. [Google Scholar] [CrossRef]
- Siervogel, R.M.; Roche, A.F.; Guo, S.M.; Mukherjee, D.; Chumlea, W.C. Patterns of change in weight/stature2 from 2 to 18 years: Findings from long-term serial data for children in the Fels longitudinal growth study. Int. J. Obes. 1991, 15, 479–485. [Google Scholar] [PubMed]
- Pietrobelli, A.; Agosti, M.; The MeNu Group. Nutrition in the First 1000 Days: Ten Practices to Minimize Obesity Emerging from Published Science. Int. J. Environ. Res. Public Health 2017, 14, 1491. [Google Scholar] [CrossRef] [Green Version]
- Carolan-Olah, M.; Duarte-Gardea, M.; Lechuga, J. A critical review: Early life nutrition and prenatal programming for adult disease. J. Clin. Nurs. 2015, 24, 3716–3729. [Google Scholar] [CrossRef]
- Lewis, D.S.; Bertrand, H.A.; McMahan, C.A.; McGill, H.C., Jr.; Carey, K.D.; Masoro, E.J. Preweaning food intake influences the adiposity of young adult baboons. J. Clin. Investig. 1986, 78, 899–905. [Google Scholar] [CrossRef] [Green Version]
- Dietz, W.H. Early Influences on Body Weight Regulation. In Regulation of Body Weight; Bouchard, C., Bray, G.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Uwaezuoke, S.N.; Eneh, C.I.; Ndu, I.K. Relationship between exclusive breastfeeding and lower risk of childhood obesity: A narrative review of published evidence. Clin. Med. Insights Pediatr. 2017, 11, 1179556517690196. [Google Scholar] [CrossRef] [PubMed]
- Bardanzellu, F.; Fanos, V.; Reali, A. “Omics” in Human Colostrum and Mature Milk: Looking to Old Data with New Eyes. Nutrients 2017, 9, 843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health 2014, 14, 1267. [Google Scholar] [CrossRef] [Green Version]
- Rito, A.I.; Buoncristiano, M.; Spinelli, A.; Salanave, B.; Kunešová, M.; Hejgaard, T.; García Solano, M.; Fijałkowska, A.; Sturua, L.; Hyska, J.; et al. Association between Characteristics at Birth, Breastfeeding and Obesity in 22 Countries: The WHO European Childhood Obesity Surveillance Initiative—COSI 2015/2017. Obes. Facts 2019, 12, 226–243. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Qiao, Y.; Zhao, P.; Li, W.; Katzmarzyk, P.T.; Chaput, J.-P.; Fogelholm, M.; Kuriyan, R.; Lambert, E.V.; Maher, C.; et al. Breastfeeding and childhood obesity: A 12-country study. Matern. Child Nutr. 2020, 16, e12984. [Google Scholar] [CrossRef]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Smith, G.D.; Cook, D.G. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am. J. Clin. Nutr. 2006, 84, 1043–1054. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Quinn, S.; Dwyer, T.; Ponsonby, A.L.; Jones, G. Maternal diet, breastfeeding and adolescent body composition: A 16-year prospective study. Eur. J. Clin. Nutr. 2012, 66, 1329–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenbauer, J.; Herzig, P.; Giani, G. Early infant feeding and risk of type 1 diabetes mellitus—A nationwide population-based case-control study in pre-school children. Diabetes Metab. Res. Rev. 2008, 24, 211–222. [Google Scholar] [CrossRef]
- English, S.; Wright, I.; Ashburn, V.; Ford, G.; Caramaschi, D. Prenatal anxiety, breastfeeding and child growth and puberty: Linking evolutionary models with human cohort studies. Ann. Hum. Biol. 2020, 47, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Monte, W.C. Infant formula ingestion is associated with the development of diabetes in the BB/Wor rat. Life Sci. 2000, 66, 1501–1507. [Google Scholar] [CrossRef]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A.; et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, L.; Li, M.; Lam, S.M.; Wang, G.; Wu, Y.; Zhang, H.; Niu, C.; Zhang, X.; Liu, X.; et al. Microbiota Depletion Impairs Thermogenesis of Brown Adipose Tissue and Browning of White Adipose Tissue. Cell Rep. 2019, 26, 2720–2737. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, S.A.; Birch, L.L. Infant dietary experience and acceptance of solid foods. Pediatrics 1994, 93, 271–277. [Google Scholar] [PubMed]
- Ventura, A.K. Does Breastfeeding Shape Food Preferences? Links to Obesity. Ann. Nutr. Metab. 2017, 70 (Suppl. S3), 8–15. [Google Scholar] [CrossRef]
- Van Aerde, J.E.; Wilke, M.S.; Feldman, M.; Clandinin, M.T. Chapter 40—Accretion of Lipid in the Fetus and Newborn. In Fetal and Neonatal Physiology, 3rd ed.; Polin, R.A., Fox, W.W., Abman, S.H., Eds.; W.B. Saunders: London, UK, 2004; pp. 388–404. [Google Scholar]
- Whyte, R.K.; Bayley, H.S. Energy Metabolism of the Newborn Infant. In Advances in Nutritional Research; Draper, H.H., Ed.; Springer: Boston, MA, USA, 1990; pp. 79–108. [Google Scholar]
- Farkas, V.; Kelenyi, G.; Sandor, A. A dramatic accumulation of glycogen in the brown adipose tissue of rats following recovery from cold exposure. Arch. Biochem. Biophys. 1999, 365, 54–61. [Google Scholar] [CrossRef]
- Novak, M.; Monkus, E.; Pardo, V. Human neonatal subcutaneous adipose tissue. Function and ultrastructure. Biol. Neonate 1971, 19, 306–321. [Google Scholar] [CrossRef]
- Mayeuf-Louchart, A.; Lancel, S.; Sebti, Y.; Pourcet, B.; Loyens, A.; Delhaye, S.; Duhem, C.; Beauchamp, J.; Ferri, L.; Thorel, Q.; et al. Glycogen Dynamics Drives Lipid Droplet Biogenesis during Brown Adipocyte Differentiation. Cell Rep. 2019, 29, 1410–1418. [Google Scholar] [CrossRef]
- Patel, D.; Kalhan, S. Glycerol Metabolism and Triglyceride-Fatty Acid Cycling in the Human Newborn: Effect of Maternal Diabetes and Intrauterine Growth Retardation. Pediatric Res. 1992, 31, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furse, S.; Billing, G.; Snowden, S.; Smith, J.; Goldberg, G.; Koulman, A. Relationship between the lipid composition of maternal plasma and infant plasma through breast milk. Metabolomics 2019, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Insull, W., Jr.; Hirsch, J.; James, T.; Ahrens, E.H., Jr. The fatty acids of human milk. II. Alterations produced by manipulation of caloric balance and exchange of dietary fats. J. Clin. Investig. 1959, 38, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, M. Lingual lipase and fat digestion in the neonatal period. J. Pediatric Gastroenterol. Nutr. 1983, 2 (Suppl. S1), S236–S241. [Google Scholar] [CrossRef]
- Sinclair, J.C. Temperature Regulation And Energy Metabolism in the Newborn (Monographs in Neonatalogy); Grune & Stratton: Florence, KY, USA, 1978. [Google Scholar]
- Stevens, E.E.; Patrick, T.E.; Pickler, R. A History of Infant Feeding. J. Perinat. Educ. 2009, 18, 32–39. [Google Scholar] [CrossRef]
- Stave, U. Perinatal Physiology; Plenum Medical Company: New York, NY, USA; London, UK, 1970. [Google Scholar]
- Hahn, P.; Greenberg, R.; Dobiášová, M.; Drahota, Z. Triglyceride synthesis from various precursors in adipose tissue of the rat during development. Can. J. Biochem. 1968, 46, 735–741. [Google Scholar] [CrossRef]
- Crelin, E.S. Anatomy of the Newborn: An Atlas; Lea & Febiger: Philadelphia, PA, USA, 1969. [Google Scholar]
- Tostevin, P.; Ellis, H. The buccal pad of fat: A review. Clin. Anat. (New York N.Y.) 1995, 8, 403–406. [Google Scholar] [CrossRef]
- Novak, M.; Penn, D.; Monkus, E. Regulation of lipolysis in human neonatal adipose tissue. Effects of alteration in carbohydrate metabolism. Biol. Neonate 1973, 22, 451–467. [Google Scholar] [CrossRef]
- Novak, M.; Monkus, E. Metabolism of Subcutaneous Adipose Tissue in the Immediate Postnatal Period of Human Newborns. 1. Developmental Changes in Lipolysis and Glycogen Content. Pediatric Res. 1972, 6, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawkins, M.J.R.; Scopes, J.W. Non-shivering Thermogenesis and Brown Adipose Tissue in the Human New-born Infant. Nature 1965, 206, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Bligh, J. Thermogenesis. Nature 1971, 234, 53–54. [Google Scholar] [CrossRef]
- Heim, T.; Kellermayer, M.; Dani, M. Thermal conditions and the mobilization of lipids from brown and white adipose tissue in the human neonate. Acta Paediatr. Acad. Sci. Hung. 1968, 9, 109–120. [Google Scholar] [PubMed]
- Verduci, E.; Calcaterra, V.; Di Profio, E.; Fiore, G.; Rey, F.; Magenes, V.C.; Todisco, C.F.; Carelli, S.; Zuccotti, G.V. Brown Adipose Tissue: New Challenges for Prevention of Childhood Obesity. A Narrative Review. Nutrients 2021, 13, 1450. [Google Scholar] [CrossRef]
- Hahn, P.; Novak, M. Development of brown and white adipose tissue. J. Lipid Res. 1975, 16, 79–91. [Google Scholar] [CrossRef]
- Clara, M. Entwicklungsgeschichte des Menschen; Georg Thieme Verlag: Leipzig, Germany, 1955. [Google Scholar]
- Leitner, B.P.; Huang, S.; Brychta, R.J.; Duckworth, C.J.; Baskin, A.S.; McGehee, S.; Tal, I.; Dieckmann, W.; Gupta, G.; Kolodny, G.M.; et al. Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl. Acad. Sci. 2017, 114, 8649–8654. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.H.; Wu, T.W.; Yin, L.; Kim, M.S.; Chia, J.M.; Perkins, T.G.; Gilsanz, V. MRI detection of brown adipose tissue with low fat content in newborns with hypothermia. Magn. Reson. Imaging 2014, 32, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Bigornia, S.J.; Farb, M.G.; Mott, M.M.; Hess, D.T.; Carmine, B.; Fiscale, A.; Joseph, L.; Apovian, C.M.; Gokce, N. Relation of depot-specific adipose inflammation to insulin resistance in human obesity. Nutr. Diabetes 2012, 2, e30. [Google Scholar] [CrossRef] [Green Version]
- Hull, D. The structure and function of brown adipose tissue. Br. Med. Bull. 1966, 22, 92–96. [Google Scholar] [CrossRef]
- Junqueira, L.C. Adipose Tissue, in: Basic Histology, 5th ed.; Lange Medical Publications: Los Altos, CA, USA, 1986. [Google Scholar]
- Röszer, T. The invertebrate midintestinal gland (“hepatopancreas”) is an evolutionary forerunner in the integration of immunity and metabolism. Cell Tissue Res. 2014, 358, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Röszer, T.; Kiss-Toth, E.D. FMRF-amide is a glucose-lowering hormone in the snail Helix aspersa. Cell Tissue Res. 2014, 358, 371–383. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Ann. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Braus, H. Anatomie des Menschen: Ein Lehrbuch für Studierende und Ärzte (Band 1): Bewegungsapparat; Springer: Berlin/Heidelberg, Germany, 1921; Volume 3. [Google Scholar]
- Colleluori, G.; Perugini, J.; Barbatelli, G.; Cinti, S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev. Endocr. Metab. Disord. 2021, 22, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Vernon, R.G.; Pond, C.M. Adaptations of Maternal Adipose Tissue to Lactation. J. Mammary Gland Biol. Neoplasia 1997, 2, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Bligh, J. Hibernation. In Temperature Regulation in Mammals and Other Vertebrates; Bigh, J., Ed.; American Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1973. [Google Scholar]
- Cinti, S. Adipose Organ Development and Remodeling. Compr. Physiol. 2018, 8, 1357–1431. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [Green Version]
- Kozak, L.P. The genetics of brown adipocyte induction in white fat depots. Front Endocrinol (Lausanne) 2011, 2, 64. [Google Scholar] [CrossRef] [Green Version]
- Bligh, J. Engineering Models of Mammalian Thermoregulation. In Temperature Regulation in Mammals and Other Vertebrates; Bigh, J., Ed.; American Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1973. [Google Scholar]
- Finlin, B.S.; Zhu, B.; Confides, A.L.; Westgate, P.M.; Harfmann, B.D.; Dupont-Versteegden, E.E.; Kern, P.A. Mast Cells Promote Seasonal White Adipose Beiging in Humans. Diabetes 2017, 66, 1237–1246. [Google Scholar] [CrossRef] [Green Version]
- Gaudry, M.J.; Jastroch, M.; Treberg, J.R.; Hofreiter, M.; Paijmans, J.L.A.; Starrett, J.; Wales, N.; Signore, A.V.; Springer, M.S.; Campbell, K.L. Inactivation of thermogenic UCP1 as a historical contingency in multiple placental mammal clades. Sci. Adv. 2017, 3, e1602878. [Google Scholar] [CrossRef] [Green Version]
- Wright, T.; Davis, R.W.; Pearson, H.C.; Murray, M.; Sheffield-Moore, M. Skeletal muscle thermogenesis enables aquatic life in the smallest marine mammal. Science 2021, 373, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Young, T.K. Obesity, Central Fat Patterning, and Their Metabolic Correlates among the Inuit of the Central Canadian Arctic. Hum. Biol. 1996, 68, 245–263. [Google Scholar] [PubMed]
- Stothers, J.K. Head insulation and heat loss in the newborn. Arch. Dis. Child. 1981, 56, 530–534. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Gurmaches, J.; Guertin, D.A. Adipocyte lineages: Tracing back the origins of fat. Biochim Biophys Acta 2014, 1842, 340–351. [Google Scholar] [CrossRef] [Green Version]
- Billon, N.; Iannarelli, P.; Monteiro, M.C.; Glavieux-Pardanaud, C.; Richardson, W.D.; Kessaris, N.; Dani, C.; Dupin, E. The generation of adipocytes by the neural crest. Development 2007, 134, 2283–2292. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.; Xu, L.; Chen, X.; Han, W.; Ruan, C.; Li, J.; Cai, C.; Ye, M.; Gao, P. Neural Crest Cells Differentiate Into Brown Adipocytes and Contribute to Periaortic Arch Adipose Tissue Formation. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1629–1644. [Google Scholar] [CrossRef] [Green Version]
- Lidell, M.E.; Betz, M.J.; Leinhard, O.D.; Heglind, M.; Elander, L.; Slawik, M.; Mussack, T.; Nilsson, D.; Romu, T.; Nuutila, P.; et al. Evidence for two types of brown adipose tissue in humans. Nat. Med. 2013, 19, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Hao, G.; Shao, M.; Nham, K.; An, Y.; Wang, Q.; Zhu, Y.; Kusminski, C.M.; Hassan, G.; Gupta, R.K.; et al. An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metab. 2018, 27, 252–262. [Google Scholar] [CrossRef] [Green Version]
- Carobbio, S.; Rosen, B.; Vidal-Puig, A. Adipogenesis: New insights into brown adipose tissue differentiation. J. Mol. Endocrinol. 2013, 51, T75–T85. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Cannon, B.; de Jong, J.M.A.; Fischer, A.W.; Nedergaard, J.; Petrovic, N. Human brown adipose tissue: Classical brown rather than brite/beige? Exp. Physiol. 2020, 105, 1191–1200. [Google Scholar] [CrossRef]
- Sharp, L.Z.; Shinoda, K.; Ohno, H.; Scheel, D.W.; Tomoda, E.; Ruiz, L.; Hu, H.; Wang, L.; Pavlova, Z.; Gilsanz, V.; et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE 2012, 7, e49452. [Google Scholar] [CrossRef]
- Symonds, M.E.; Lomax, M.A. Maternal and environmental influences on thermoregulation in the neonate. Proc. Nutr. Soc. 2007, 51, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockstroh, D.; Landgraf, K.; Wagner, I.V.; Gesing, J.; Tauscher, R.; Lakowa, N.; Kiess, W.; Bühligen, U.; Wojan, M.; Till, H.; et al. Direct evidence of brown adipocytes in different fat depots in children. PLoS ONE 2015, 10, e0117841. [Google Scholar] [CrossRef] [PubMed]
- Gilsanz, V.; Hu, H.H.; Kajimura, S. Relevance of brown adipose tissue in infancy and adolescence. Pediatric Res. 2013, 73, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symonds, M.E.; Bloor, I.; Ojha, S.; Budge, H. The placenta, maternal diet and adipose tissue development in the newborn. Ann. Nutr. Metab. 2017, 70, 232–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.; Park, H.S.; Kim, J.; Jang, Y.J.; Kim, J.-H.; Lee, Y.; Heo, Y. Depot-specific UCP1 expression in human white adipose tissue and its association with obesity-related markers. Int. J. Obes. 2020, 44, 697–706. [Google Scholar] [CrossRef]
- Von Liebig, J. Origin of fat in domesticated anmals. In Animal Chemistry: Or Organic Chemistry in Its Application to Physiology and Pathology. Justus Liebig; Edited from the Author’s Manuscript by William Gregory with Additions, Notes, and Corrections by Gregory and John W. Webster; John Owen: Cambridge, UK, 1842. [Google Scholar]
- Hoffmann-Ostenhof, O. Intermediary Metabolism; Van Nostrand Reinhold Co.: New York, NY, USA, 1987. [Google Scholar]
- De Robertis, E.D.P.; Nowinski, W.W.; Saez, A.F. Morphlogy of the mitochondria. In Cell Bilogy, 4th ed.; W. B. Saunders: London, UK, 1965. [Google Scholar]
- Aherne, W.; Hull, D. Brown adipose tissue and heat production in the newborn infant. J. Pathol. Bacteriol. 1966, 91, 223–234. [Google Scholar] [CrossRef]
- Hawdon, J.M.; Ward Platt, M.P.; Aynsley-Green, A. Patterns of metabolic adaptation for preterm and term infants in the first neonatal week. Arch. Dis. Child. 1992, 67, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Maniscalco, M.W.; Warshaw, J.B. Cellular Energy Metabolism During Fetal and Perinatal Development. In Temperature Regulation and Energy Metabolism in the Newborn., Sinclair, C.J., Ed.; Grune & Stratton: New York, NY, USA; San Francisco, CA, USA; London, UK, 1978. [Google Scholar]
- De Oliveira, M.R.; Nabavi, S.F.; Nabavi, S.M.; Jardim, F.R. Omega-3 polyunsaturated fatty acids and mitochondria, back to the future. Trends Food Sci. Technol. 2017, 67, 76–92. [Google Scholar] [CrossRef]
- Hartvigsen, K.; Ravandi, A.; Harkewicz, R.; Kamido, H.; Bukhave, K.; Holmera, G.; Kuksis, A. 1-O-alkyl-2-(omega-oxo)acyl-sn-glycerols from shark oil and human milk fat are potential precursors of PAF mimics and GHB. Lipids 2006, 41, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Saghatelian, A.; Chong, L.W.; Zhang, C.L.; Cravatt, B.F.; Evans, R.M. Maternal PPAR gamma protects nursing neonates by suppressing the production of inflammatory milk. Genes Dev. 2007, 21, 1895–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Elten, T.M.; van Rossem, L.; Wijga, A.H.; Brunekreef, B.; de Jongste, J.C.; Koppelman, G.H.; Smit, H.A. Breast milk fatty acid composition has a long-term effect on the risk of asthma, eczema, and sensitization. Allergy 2015, 70, 1468–1476. [Google Scholar] [CrossRef]
- De la Garza Puentes, A.; Martí Alemany, A.; Chisaguano, A.M.; Montes Goyanes, R. The Effect of Maternal Obesity on Breast Milk Fatty Acids and Its Association with Infant Growth and Cognition-The PREOBE Follow-Up. Nutrients 2019, 11, 2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, M.C.; Young, B.E. Early infant adipose deposition is positively associated with the n-6 to n-3 fatty acid ratio in human milk independent of maternal BMI. Int. J. Obes. 2017, 41, 510–517. [Google Scholar] [CrossRef] [Green Version]
- Fan, R.; Koehler, K.; Chung, S. Adaptive thermogenesis by dietary n-3 polyunsaturated fatty acids: Emerging evidence and mechanisms. Biochim. Biophys Acta Mol. Cell Biol. Lipids 2019, 1864, 59–70. [Google Scholar] [CrossRef]
- Félix-Soriano, E.; Sáinz, N.; Gil-Iturbe, E.; Collantes, M.; Fernández-Galilea, M.; Castilla-Madrigal, R.; Ly, L.; Dalli, J.; Moreno-Aliaga, M.J. Changes in brown adipose tissue lipid mediator signatures with aging, obesity, and DHA supplementation in female mice. FASEB J. 2021, 35, e21592. [Google Scholar] [CrossRef]
- Hauner, H.; Much, D.; Vollhardt, C.; Brunner, S.; Schmid, D.; Sedlmeier, E.M.; Heimberg, E.; Schuster, T.; Zimmermann, A.; Schneider, K.T.; et al. Effect of reducing the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: An open-label randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 383–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Much, D.; Brunner, S.; Vollhardt, C.; Schmid, D.; Sedlmeier, E.-M.; Brüderl, M.; Heimberg, E.; Bartke, N.; Boehm, G.; Bader, B.L.; et al. Breast milk fatty acid profile in relation to infant growth and body composition: Results from the INFAT study. Pediatric Res. 2013, 74, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Cunnane, S.C.; Ryan, M.A.; Nadeau, C.R.; Bazinet, R.P.; Musa-Veloso, K.; McCloy, U. Why is carbon from some polyunsaturates extensively recycled into lipid synthesis? Lipids 2003, 38, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, A.; Hirasawa, A.; Poulain-Godefroy, O.; Bonnefond, A.; Hara, T.; Yengo, L.; Kimura, I.; Leloire, A.; Liu, N.; Iida, K.; et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 2012, 483, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Gila-Diaz, A.; Carrillo, G.H.; Singh, P. Specialized Pro-Resolving Lipid Mediators in Neonatal Cardiovascular Physiology and Diseases. Antioxidants 2021, 10, 933. [Google Scholar] [CrossRef]
- Wu, J.; Gouveia-Figueira, S.; Domellöf, M.; Zivkovic, A.M.; Nording, M.L. Oxylipins, endocannabinoids, and related compounds in human milk: Levels and effects of storage conditions. Prostaglandins Other Lipid Mediat. 2016, 122, 28–36. [Google Scholar] [CrossRef]
- Dieckmann, S.; Maurer, S.; Fromme, T.; Colson, C.; Virtanen, K.A.; Amri, E.-Z.; Klingenspor, M. Fatty Acid Metabolite Profiling Reveals Oxylipins as Markers of Brown but Not Brite Adipose Tissue. Front. Endocrinol. (Lausanne) 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Vasan, S.K.; Noordam, R.; Gowri, M.S.; Neville, M.J.; Karpe, F.; Christodoulides, C. The proposed systemic thermogenic metabolites succinate and 12,13-diHOME are inversely associated with adiposity and related metabolic traits: Evidence from a large human cross-sectional study. Diabetologia 2019, 62, 2079–2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watschinger, K.; Werner, E.R. Orphan enzymes in ether lipid metabolism. Biochimie 2013, 95, 59–65. [Google Scholar] [CrossRef] [Green Version]
- McCartney, C.A.; Bull, I.D.; Yan, T.; Dewhurst, R.J. Assessment of archaeol as a molecular proxy for methane production in cattle. J. Dairy Sci. 2013, 96, 1211–1217. [Google Scholar] [CrossRef] [Green Version]
- Try, K. The presence of the hydrocarbons pristane and phytane in human adipose tissue and the occurrence of normal amounts in patients with Refsum’s disease. Scand. J. Clin. Lab. Investig. 1967, 19, 385–387. [Google Scholar] [CrossRef]
- Le Bon, A.M.; Cravedi, J.P.; Tulliez, J.E. Disposition and metabolism of pristane in rat. Lipids 1988, 23, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ai, W.; Hu, X.; Meng, Y.; Yuan, C.; Su, H.; Wang, L.; Zhu, X.; Gao, P.; Shu, G.; et al. Phytol stimulates the browning of white adipocytes through the activation of AMP-activated protein kinase (AMPK) α in mice fed high-fat diet. Food Funct. 2018, 9, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Bollongino, R.; Burger, J.; Powell, A.; Mashkour, M.; Vigne, J.-D.; Thomas, M.G. Modern Taurine Cattle Descended from Small Number of Near-Eastern Founders. Mol. Biol. Evol. 2012, 29, 2101–2104. [Google Scholar] [CrossRef]
- Rokosz, M. History of the Aurochs (Bos Taurus Primigenius) in Poland. Anim. Genet. Resour. Inf. 2011, 16, 5–12. [Google Scholar] [CrossRef]
- Hewelt-Belka, W.; Garwolinska, D.; Mlynarczyk, M.; Kot-Wasik, A. Comparative Lipidomic Study of Human Milk from Different Lactation Stages and Milk Formulas. Nutrients 2020, 12, 2165. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Stremmel, W.; Weiskirchen, R.; John, S.M.; Schmitz, G. Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development. Biomolecules 2021, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Townsend, S.; Caubilla Barron, J.; Loc-Carrillo, C.; Forsythe, S. The presence of endotoxin in powdered infant formula milk and the influence of endotoxin and Enterobacter sakazakii on bacterial translocation in the infant rat. Food Microbiol. 2007, 24, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.; Manger, P.R.; Rubidge, B.S. Palaeoneurological clues to the evolution of defining mammalian soft tissue traits. Sci. Rep. 2016, 6, 25604. [Google Scholar] [CrossRef] [Green Version]
- Oftedal, O. The Mammary Gland and Its Origin During Synapsid Evolution. J. Mammary Gland Biol. Neoplasia 2002, 7, 225–252. [Google Scholar] [CrossRef]
- Blackburn, D.G. Evolutionary origins of the mammary gland. Mammal Rev. 1991, 21, 81–96. [Google Scholar] [CrossRef]
- Satoh, K.; Ginsburg, E.; Vonderhaar, B.K. Msx-1 and Msx-2 in mammary gland development. J. Mammary Gland Biol. Neoplasia 2004, 9, 195–205. [Google Scholar] [CrossRef]
- Parodi, P.W.; Griffiths, M. A comparison of the positional distribution of fatty acids in milk triglycerides of the extant monotremes platypus (Ornithorhynchus anatinus) and echidna (Tachyglossus aculeatus). Lipids 1983, 18, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.A.; Neumann, M.; Grant, T.R.; Griffiths, M. Fatty acids of the milk and food of the platypus (Ornithorhynchus anatinus). Lipids 1988, 23, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Stannard, H.J.; Miller, R.D.; Old, J.M. Marsupial and monotreme milk-a review of its nutrient and immune properties. PeerJ 2020, 8, e9335. [Google Scholar] [CrossRef]
- Del Río, R.; Dip Pérez, E.; Marín Gabriel, M.Á.; The Neo-COVID-19 Research Group. Multi-centre study showed reduced compliance with the World Health Organization recommendations on exclusive breastfeeding during COVID-19. Acta Paediatr. 2020. ahead of print. [Google Scholar] [CrossRef]
- Guttman, N.; Zimmerman, D.R. Low-income mothers’ views on breastfeeding. Social Sci. Med. (1982) 2000, 50, 1457–1473. [Google Scholar] [CrossRef]
- Roberts, C.; Manchester, K. Metabolic and Endocrine Disease. In the Archaeology of Disease; The History Press: Stroud, UK, 2010; pp. 221–250. [Google Scholar]
- Stantis, C.; Schutkowski, H.; Sołtysiak, A. Reconstructing breastfeeding and weaning practices in the Bronze Age Near East using stable nitrogen isotopes. Am. J. Phys. Anthropol. 2020, 172, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Waters-Rist, A.L.; Bazaliiskii, V.I.; Weber, A.W.; Katzenberg, M.A. Infant and child diet in Neolithic hunter-fisher-gatherers from Cis-Baikal, Siberia: Intra-long bone stable nitrogen and carbon isotope ratios. Am. J. Phys. Anthr. 2011, 146, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Howcroft, R.; Eriksson, G.; Lidén, K. Conformity in diversity? Isotopic investigations of infant feeding practices in two iron age populations from Southern Öland, Sweden. Am. J. Phys. Anthr. 2012, 149, 217–230. [Google Scholar] [CrossRef]
- Tsutaya, T.; Shimomi, A.; Nagaoka, T.; Sawada, J.; Hirata, K.; Yoneda, M. Infant feeding practice in medieval Japan: Stable carbon and nitrogen isotope analysis of human skeletons from Yuigahama-minami. Am. J. Phys. Anthr. 2015, 156, 241–251. [Google Scholar] [CrossRef]
- Shakespeare, W. Shakespeare’s Tragedy of Romeo and Juliet, with Preface and Glossary by Israel Gollancz; J. M. Dent & Sons: London, UK, 1912. [Google Scholar]
- Hayward, J.S.; Lisson, P.A. Evolution of brown fat: Its absence in marsupials and monotremes. Can. J. Zool. 1992, 70, 171–179. [Google Scholar] [CrossRef]
- Jastroch, M.; Withers, K.W.; Taudien, S.; Frappell, P.B.; Helwig, M.; Fromme, T.; Hirschberg, V.; Heldmaier, G.; McAllan, B.M.; Firth, B.T.; et al. Marsupial uncoupling protein 1 sheds light on the evolution of mammalian nonshivering thermogenesis. Physiol. Genom. 2008, 32, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röszer, T. M2 Macrophages in the Integument and in the Musculoskeletal System. In The M2 Macrophage, Röszer, T.; Springer International Publishing: Midtown Manhattan, NY, USA, 2020; pp. 133–151. [Google Scholar]
- Tare, M.; Parkington, H.C.; Morley, R. Vitamin D in Pregnancy and Offspring Health. In Early Life Origin of Health and Disease; Wintour, E.M., Owens, J.A., Eds.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Twiss, K.C. The Archaeology of Food: Identity, Politics, and Ideology in the Prehistoric and Historic Past; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Lempainen, J.; Tauriainen, S.; Vaarala, O.; Makela, M.; Honkanen, H.; Marttila, J.; Veijola, R.; Simell, O.; Hyoty, H.; Knip, M.; et al. Interaction of enterovirus infection and cow’s milk-based formula nutrition in type 1 diabetes-associated autoimmunity. Diabetes Metab. Res. Rev. 2012, 28, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef] [Green Version]
- Galante, L.; Milan, A.M.; Reynolds, C.M.; Cameron-Smith, D.; Vickers, M.H.; Pundir, S. Sex-Specific Human Milk Composition: The Role of Infant Sex in Determining Early Life Nutrition. Nutrients 2018, 10, 1194. [Google Scholar] [CrossRef] [Green Version]
- Shah, B.; Tombeau Cost, K.; Fuller, A.; Birken, C.S.; Anderson, L.N. Sex and gender differences in childhood obesity: Contributing to the research agenda. BMJ Nutr. Prev. Health 2020, 3, 387. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Röszer, T. Co-Evolution of Breast Milk Lipid Signaling and Thermogenic Adipose Tissue. Biomolecules 2021, 11, 1705. https://doi.org/10.3390/biom11111705
Röszer T. Co-Evolution of Breast Milk Lipid Signaling and Thermogenic Adipose Tissue. Biomolecules. 2021; 11(11):1705. https://doi.org/10.3390/biom11111705
Chicago/Turabian StyleRöszer, Tamás. 2021. "Co-Evolution of Breast Milk Lipid Signaling and Thermogenic Adipose Tissue" Biomolecules 11, no. 11: 1705. https://doi.org/10.3390/biom11111705
APA StyleRöszer, T. (2021). Co-Evolution of Breast Milk Lipid Signaling and Thermogenic Adipose Tissue. Biomolecules, 11(11), 1705. https://doi.org/10.3390/biom11111705





