Advanced Bioinformatics Tools in the Pharmacokinetic Profiles of Natural and Synthetic Compounds with Anti-Diabetic Activity
Abstract
:1. Brief Overview of Diabetes Types
2. Molecular Targets Involved in Diabetes Mellitus
3. Plants Involved in Diabetes Mellitus Management
4. Natural Compounds Involved in Diabetes Mellitus Management
5. Quantitative Structure–Activity Relationships (QSAR) Predicted Anti-Diabetic Activity
6. Molecular Docking and Molecular Dynamics Predicted Anti-Diabetic Activity
| Target | Compounds | Predicted Energy of Binding (kcal/mol) | Software Used | References |
| AR (PDB: ID:1US0 [15]) Organism: Homo sapiens | kaempferol | −10.034 | YASARA [125] | [71] |
| herbacetin | −9.623 | |||
| sorbifolin | −9.391 | |||
| IR (PDB: ID:1IR3 [16]) Organism: Homo sapiens | gossypetin | −8.429 | YASARA [125] | [71] |
| herbacetin | −8.165 | |||
| sorbifolin | −8.063 | |||
| SIRT6 (PDB ID: 3K35 [17]) Organism: Homo sapiens | gossypetin | −8.569 | YASARA [125] | [71] |
| herbacetin | −8.632 | |||
| kaempferol | −8.533 | |||
| sorbifolin | −8.697 | |||
| Target | Compound | Dock Score (-Potential of Mean Force) | Software Used | References |
| α-glucosidase (PDB 2ZE0 [126]) Organism: Geobacillus sp. HTA-462 | curcumin | −153 | LigandFit implemented in DS 2.5 (DS, Accelrys Software, San Diego, CA, USA) | [67] |
| antroquinonol | −180 | |||
| rutin | −159 | |||
| α-amylase (PDB 1HNY [127]) Organism: Homo sapiens | curcumin | −175 | LigandFit implemented in DS 2.5 (DS, Accelrys Software, San Diego, CA, USA) | [67] |
| 16-hydroxy-cleroda-3,13-dine-16,15-olide | −155 | |||
| docosanol | −154 | |||
| berberine | −142 | |||
| catechin | −135 | |||
| quercetin | −132 | |||
| rutin | −126 | |||
| Target | Compound | Docking Score (kcal/mol) | Software Used | References |
| Lysosomal α-glucosidase (PDB ID: 5KZX [128]) Organism: Homo sapiens | Isorutarine | −7.64 | Maestro 12.0 of Schrödinger LCC, New York, NY, USA | [117] |
| 2′Isopropylpsoralene | −6.64 | |||
| 4-hydroxy d-C-III | −6.45 | |||
| Target | Compound | Predicted Energy of Binding (kcal/mol) | Software Used | References |
| porcine pancreatic α-amylase (PDB ID: 1OSE [129]) Organism: Sus scrofa | Caffeoylquinic acid | −10.33 | Argus lab 4.0.1 [130] | [73] |
| O-Coumaroylquinic acid | −10.01 | |||
| Coumaroyl-Ohexoside | −9.75 | |||
| α-glucosidase (PDB ID: 3A4A [131]) Organism: Saccharomyces cerevisiae | Caffeoylquinic acid | −10.84 | Argus lab 4.0.1 [130] | |
| O-Coumaroylquinic acid | −10.65 | |||
| Coumaroyl-Ohexoside | −10.60 | |||
| Target | Compound | Binding Affinity (kcal/mol) | Software Used | References |
| human pancreatic α-amylase (PDB ID: 5E0F [132]) Organism: Homo sapiens | Ursolic acid | −9.8 | Autodock Vina 1.1.2 [133] | [74] |
| Oleanolic acid | −8.7 | |||
| Rosmarinic acid | −8.5 | |||
| human lysosomal acid α-glucosidase (PDB: 5NN8 [134]) Organism: Homo sapiens | Ursolic acid | −8.2 | ||
| Oleanolic acid | −8.2 | |||
| Rosmarinic acid | −8.2 | |||
| human pancreatic α-amylase (PDB: 5E0F [132]) Organism: Homo sapiens | Chlorogenic acid | −8.7 | Autodock Vina 1.1.2. [133] | [76] |
| Jasminoside A | −8.7 | |||
| Jasminoside F | −8.5 | |||
| human lysosomal acid α-glucosidase (PDB: 5NN8 [134]) Organism: Homo sapiens | Acarbose derived trisaccharide | −8.7 | ||
| Acarbose | −8.7 | |||
| Chlorogenic acid | −8.2 | |||
| Target | Compound | Predicted Energy of Binding (kcal/mol) | Software Used | References |
| porcine pancreatic α-amylase (PDB ID: 1OSE [129]) Organism: Sus scrofa | cryptochlorogenic acid | −9.860 | ArgusLab 4.0.1 [130] | [108] |
| feruloylquinic acid | −8.613 | |||
| neochlorogenic acid | −7.452 | |||
| α-glucosidase (PDB ID: 3A4A [131]) Organism: Saccharomyces cerevisiae | caffeoylquinic acid | −10.737 | ||
| neochlorogenic acid | −10.732 | |||
| cryptochlorogenic acid | −10.632 | |||
| Target | Compound | Docking Score | Software Used | References |
| AR (PDB ID: 3G5E [135]) Organism: Homo sapiens | (4Z,12Z)-cyclopentadeca-4, 12-dienone | −7.61 | GLIDE 5.0 of Schrödinger LCC, New York, NY, USA [136] | [109] |
| glucokinase (PDB ID: 4IXC [137]) Organism: Homo sapiens | −6.18 | |||
| PDK2 (PDB ID: 4MP2 [138]) Organism: Homo sapiens | −5.21 | |||
| PPARγ (PDB ID: 3DZY [139]) Organism: Homo sapiens | −7.57 | |||
| GSK-3 (PDB ID: 3F7Z [140]) Organism: Homo sapiens | −6.01 | |||
| 11β-HSD1 (PDB ID: 4K1L [141]) Organism: Homo sapiens | −7.85 | |||
| GFPT1 (PDB ID: 2ZJ4 [142]) Organism: Homo sapiens | −5.57 | |||
| Target | Compound | Docking Score (kcal/mol) | Software Used | References |
| α-glucosidase (predicted 3D structure) Organism: Saccharomyces cerevisiae | casticin | −8.452 | MOE, Chemical Computing Group, Monreal, Canada | [79] |
| negundoside | −7.923 | |||
| herbacetin rhamnoside | −7.369 | |||
| Target | Compound | S-Score | Software Used | References |
| IR (PDB: ID:1IR3 [16]) Organism: Homo sapiens | KDDGHL | −18.56 | MOE, Chemical Computing Group, Monreal, Canada | [59] |
| EPGGGG | −16.71 | |||
| TSEP | −15.66 | |||
| SGLT1 (PDB ID: 3DH4 [143]) Organism: Vibrio parahaemolyticus | ESIRD | −23.81 | ||
| DSRHR | −23.64 | |||
| RRKKV | −20.64 | |||
| dipeptidyl peptidase-IV (DPP (IV))(PDB ID: 4A5S [144]) Organism: Homo sapiens | PTRHM | −10.1067 | ||
| RRKKV | −9.9189 | |||
| KDDGHL | −9.4991 | |||
| GLUT2 (predicted 3D structure) | RRKKV | −10.5970 | ||
| RSIHEP | −10.5171 | |||
| ERFDSG | −9.6986 | |||
| Target | Compound | Binding Energy | Software Used | References |
| α-glucosidase (predicted 3D structure) | tocopherol | −7.7008 | MOE, Chemical Computing Group, Monreal, Canada | [80] |
| linoleic acid | −7.1746 | |||
| phytol | −7.0629 | |||
| Target | Compound | Binding Affinity (kcal/mol) | Software Used | References |
| α-glucosidase (PDB ID: 4J5T [145]) Organism: Saccharomyces cerevisiae S288C | phlorizin | −8.2 | AutoDock [133] | [81] |
| scandenin | −8.0 | |||
| pomiferin | −8.0 | |||
| DPP-4 (PDB ID: 2P8S [146]) Organism: Homo sapiens | phlorizin | −10.9 | ||
| pomiferin | −9.6 | |||
| mundulone and scandenin | −9.3 | |||
| IR (PDB: ID:1IR3 [16]) Organism: Homo sapiens | phlorizin | −7.0 | ||
| mundulone | −6.9 | |||
| pomiferin | −6.6 | |||
| Target | Compound | Docking Score (kcal/mol) | Software Used | References |
| GPDH (PDB ID: 1WPQ [147]) Organism: Homo sapiens | 2′,4′ dihydroxychalcone | −6.2652 | MOE, Chemical Computing Group, Monreal, Canada | [82] |
| compound 4 | −5.7992 | |||
| compound 3 | −5.6075 |
7. Anti-Diabetic Synthetic Compounds and Their Molecular Target Effects on BBB
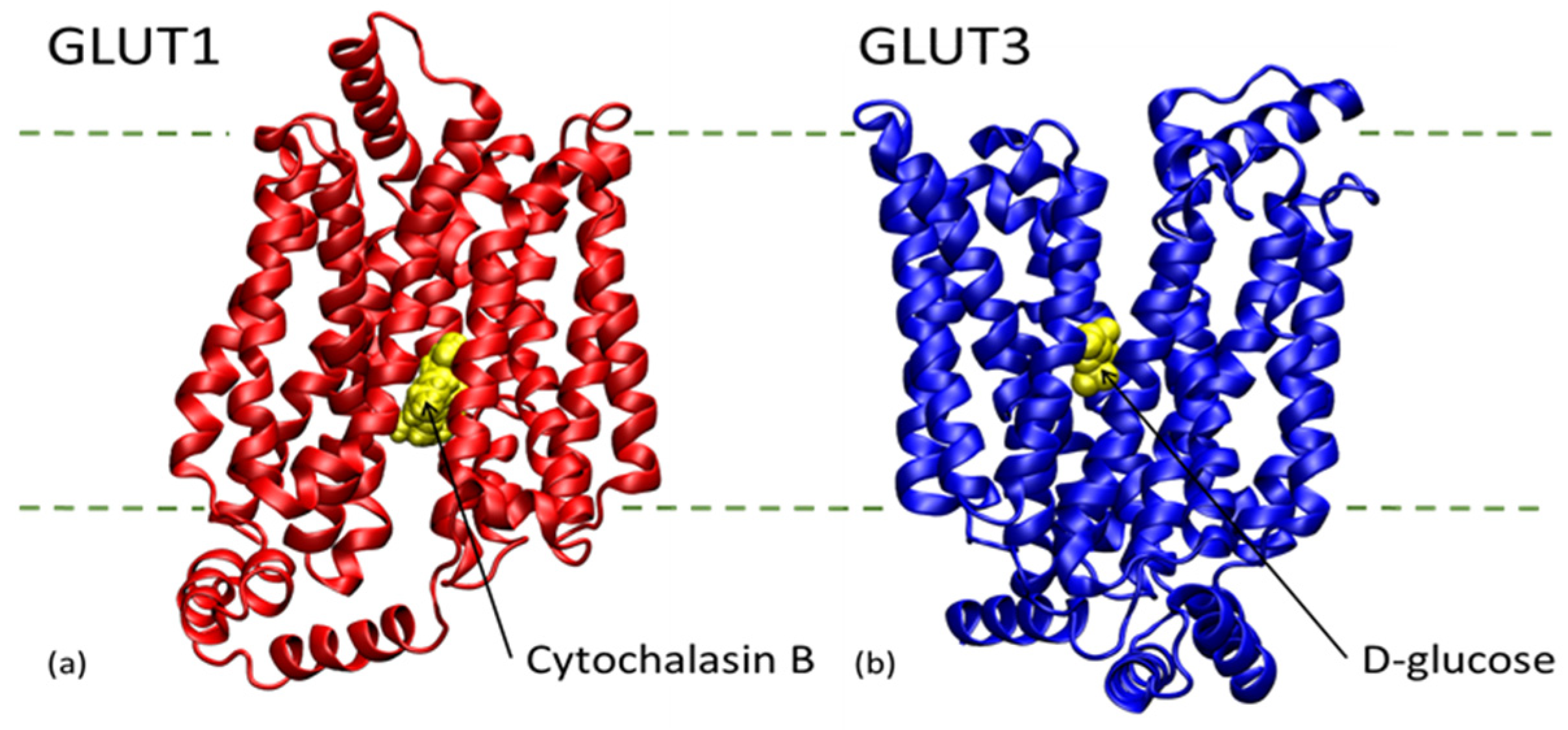

| Compound | pkCSM Numeric (log BBB) | admetSAR 2.0 BBB Probability | SMILES | Structure |
|---|---|---|---|---|
| propofol | 0.497 | +(0.99) | CC(C)C1=C(C(=CC=C1)C(C)C)O | 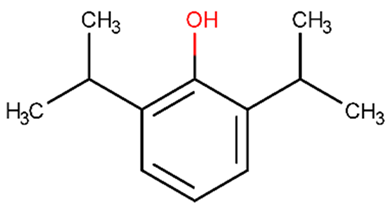 |
| TAK-242 | −0.715 | +(0.97) | CCOC(=O)C1=CCCCC1S(=O)(=O)NC2=C(C=C(C=C2)F)Cl | 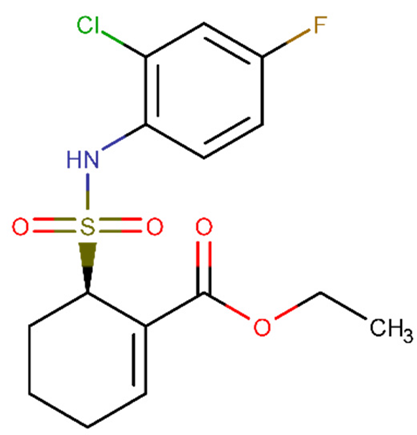 |
| U0126 | −0.967 | +(0.97) | C1=CC=C(C(=C1)N)SC(=C(C#N)C(=C(N)SC2=CC=CC=C2N)C#N)N | 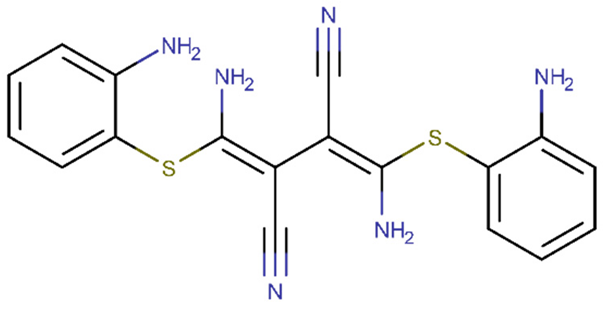 |
| Pyrrolidine dithiocarbamate | 0.041 | +(0.98) | C1CCN(C1)C(=S)S | 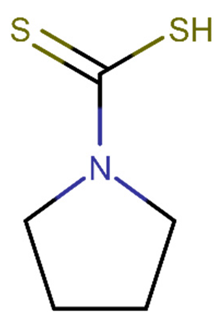 |
| APX3330 | −0.742 | +(0.91) | CCCCCCCCCC(=CC1=C(C(=O)C(=C(C1=O)OC)OC)C)C(=O)O | 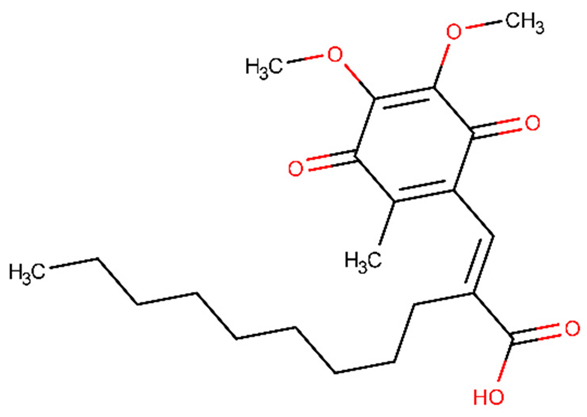 |
8. Natural Compounds That Prevent BBB Dysfunction in Diabetic Patients
9. Databases and Web-Servers of Anti-Diabetic Compounds
10. Blood Brain Barrier Permeability Prediction Web Services
| Compounds | pkCSM | admetSAR 2.0 | SMILES |
| gymnemic acid I, | −1.517 | +0.843 | CC=C(C)C(=O)OC1C(C2(C(CC1(C)C)C3=CCC4C5(CCC(C(C5CCC4(C3(CC2O)C)C)(C)CO)OC6C(C(C(C(O6)C(=O)O)O)O)O)C)COC(=O)C)O |
| gymnemic acid II, | −1.558 | +0.91 | CCC(C)C(=O)OC1C(C2(C(CC1(C)C)C3=CCC4C5(CCC(C(C5CCC4(C3(CC2O)C)C)(C)CO)OC6C(C(C(C(O6)C(=O)O)O)O)O)C)COC(=O)C)O |
| gymnemic acid III, | −1.652 | +0.91 | CCC(C)C(=O)OC1C(C2(C(CC1(C)C)C3=CCC4C5(CCC(C(C5CCC4(C3(CC2O)C)C)(C)CO)OC6C(C(C(C(O6)C(=O)O)O)O)O)C)CO)O |
| gymnemic IV, | −1.611 | +0.84 | CC=C(C)C(=O)OC1C(C2(C(CC1(C)C)C3=CCC4C5(CCC(C(C5CCC4(C3(CC2O)C)C)(C)CO)OC6C(C(C(C(O6)C(=O)O)O)O)O)C)CO)O |
| gymnemic acid, V, | −1.743 | +0.84 | CC=C(C)C(=O)OC1C(C2(C(CC1(C)C)C3=CCC4C5(CCC(C(C5CCC4(C3(CC2O)C)C)(C)CO)OC6C(C(C(C(O6)C(=O)O)O)O)O)C)CO)OC(=O)C(=CC)C |
| gymnemic VI, | −2.346 | −0.78 | CC=C(C)C(=O)OC1C(C2(C(CC1(C)C)C3=CCC4C5(CCC(C(C5CCC4(C3(CC2O)C)C)(C)CO)OC6C(C(C(C(O6)C(=O)O)O)OC7C(C(C(C(O7)CO)O)O)O)O)C)CO)O |
| gymnemic acid VII | −1.259 | +0.84 | CC1(CC2C3=CCC4C5(CCC(C(C5CCC4(C3(CC(C2(CC1O)CO)O)C)C)(C)CO)OC6C(C(C(C(O6)C(=O)O)O)O)O)C)C |
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, A. Type 1 Diabetes Mellitus. Nat. Rev. Dis. Primer 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 Diabetes Mellitus. Nat. Rev. Dis. Primer 2015, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, B.; Gulanick, M.; Lamendola, C. Risk Factors for Type 2 Diabetes Mellitus. J. Cardiovasc. Nurs. 2002, 16, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Yazdi, Z.S.; Beitelshees, A.L. Pharmacological Treatment of Hyperglycemia in Type 2 Diabetes. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L. Mechanisms in the Development of Type 2 Diabetes Mellitus. J. Cardiovasc. Nurs. 2002, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-Related Insulin Resistance. Available online: https://pubmed.ncbi.nlm.nih.gov/14679177/ (accessed on 12 October 2021).
- Geraldes, P.; King, G.L. Activation of Protein Kinase C Isoforms and Its Impact on Diabetic Complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagishi, S.; Imaizumi, T. Diabetic Vascular Complications: Pathophysiology, Biochemical Basis and Potential Therapeutic Strategy. Curr. Pharm. Des. 2005, 11, 2279–2299. [Google Scholar] [CrossRef]
- Neurodegenerative Disorders Associated with Diabetes Mellitus. Available online: https://pubmed.ncbi.nlm.nih.gov/15175861/ (accessed on 12 October 2021).
- American-Diabetes-Association. Standards of Medical Care in Diabetes. Diabetes Care 2020, 43, S1–S207. [Google Scholar]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.I.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing Mitochondrial Superoxide Production Blocks Three Pathways of Hyperglycaemic Damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Umpierrez, G.; Korytkowski, M. Diabetic Emergencies-Ketoacidosis, Hyperglycaemic Hyperosmolar State and Hypoglycaemia. Nat. Rev. Endocrinol. 2016, 12, 222–232. [Google Scholar] [CrossRef]
- Huber, J.D. Diabetes, Cognitive Function, and the Blood-Brain Barrier. Curr. Pharm. Des. 2008, 14, 1594–1600. [Google Scholar] [CrossRef]
- Borska, S.; Sopel, M.; Chmielewska, M.; Zabel, M.; Dziegiel, P. Quercetin as a Potential Modulator of P-Glycoprotein Expression and Function in Cells of Human Pancreatic Carcinoma Line Resistant to Daunorubicin. Molecules 2010, 15, 857–870. [Google Scholar] [CrossRef]
- Hyperglycemia-Driven Neuroinflammation Compromises BBB Leading to Memory Loss in Both Diabetes Mellitus (DM) Type 1 and Type 2 Mouse Models. Available online: https://pubmed.ncbi.nlm.nih.gov/29974394/ (accessed on 12 October 2021).
- Rom, S.; Heldt, N.A.; Gajghate, S.; Seliga, A.; Reichenbach, N.L.; Persidsky, Y. Hyperglycemia and Advanced Glycation End Products Disrupt BBB and Promote Occludin and Claudin-5 Protein Secretion on Extracellular Microvesicles. Sci. Rep. 2020, 10, 7274. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An Update on Natural Compounds in the Remedy of Diabetes Mellitus: A Systematic Review. J. Tradit. Complement. Med. 2017, 8, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Tzvetkov, N.T.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Gan, R.-Y.; Jozwik, A.; Grzybek, W.; Horbańczuk, J.O.; et al. Natural Products in Diabetes Research: Quantitative Literature Analysis. Nat. Prod. Res. 2020, 0, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Salim, B. Diabetes Mellitus and Its Treatment. Int. J. Diabetes Metab. 2005, 13, 111–134. [Google Scholar]
- Bhaskar, V.; Goldfine, I.D.; Bedinger, D.H.; Lau, A.; Kuan, H.F.; Gross, L.M.; Handa, M.; Maddux, B.A.; Watson, S.R.; Zhu, S.; et al. A Fully Human, Allosteric Monoclonal Antibody That Activates the Insulin Receptor and Improves Glycemic Control. Diabetes 2012, 61, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.; Kim, H.-S.; Lahusen, T.; Wang, R.-H.; Xu, X.; Gavrilova, O.; Jou, W.; Gius, D.; Deng, C.-X. SIRT6 Deficiency Results in Severe Hypoglycemia by Enhancing Both Basal and Insulin-Stimulated Glucose Uptake in Mice. J. Biol. Chem. 2010, 285, 36776–36784. [Google Scholar] [CrossRef] [Green Version]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic Instability and Aging-like Phenotype in the Absence of Mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef] [Green Version]
- Kuang, J.; Chen, L.; Tang, Q.; Zhang, J.; Li, Y.; He, J. The Role of Sirt6 in Obesity and Diabetes. Front. Physiol. 2018, 9, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose Reductase, Oxidative Stress, and Diabetic Mellitus. Front. Pharmacol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Etxeberria, U.; de la Garza, A.L.; Campión, J.; Martínez, J.A.; Milagro, F.I. Antidiabetic Effects of Natural Plant Extracts via Inhibition of Carbohydrate Hydrolysis Enzymes with Emphasis on Pancreatic Alpha Amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebovitz, H.E. ALPHA-GLUCOSIDASE INHIBITORS. Endocrinol. Metab. Clin. N. Am. 1997, 26, 539–551. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as α-Amylase and α-Glucosidase Inhibitors and Their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini-Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome Proliferator-Activated Receptor Targets for the Treatment of Metabolic Diseases. Mediators Inflamm. 2013, 2013, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, N.; Inagaki, N. Role of Sodium-Glucose Transporters in Glucose Uptake of the Intestine and Kidney. J. Diabetes Investig. 2012, 3, 352–353. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, K.; Hayashi, K.; Ito, S.; Hoshina, Y.; Sakai, M.; Yoshino, K.; Endo, K.; Fujitani, S.; Suzuki, T. Effects of SGLT2 Inhibitors on EGFR in Type 2 Diabetic Patients—the Role of Antidiabetic and Antihypertensive Medications. Hypertens. Res. 2021, 44, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Basu, A.K.; Mandal, B.; Mukhopadhyay, P.; Maity, A.; Chakraborty, S.; Devrabhai, P.K. 11β Hydroxysteroid Dehydrogenase – 1 Activity in Type 2 Diabetes Mellitus: A Comparative Study. BMC Endocr. Disord. 2019, 19, 15. [Google Scholar] [CrossRef]
- Elbein, S.C.; Zheng, H.; Jia, Y.; Chu, W.; Cooper, J.J.; Hale, T.; Zhang, Z. Molecular Screening of the Human Glutamine–Fructose-6-Phosphate Amidotransferase 1 (GFPT1) Gene and Association Studies with Diabetes and Diabetic Nephropathy. Mol. Genet. Metab. 2004, 82, 321–328. [Google Scholar] [CrossRef]
- Rocha, S.; Lucas, M.; Silva, V.L.M.; Gomes, P.M.O.; Silva, A.M.S.; Araújo, A.N.; Aniceto, N.; Guedes, R.C.; Corvo, M.L.; Fernandes, E.; et al. Pyrazoles as Novel Protein Tyrosine Phosphatase 1B (PTP1B) Inhibitors: An in Vitro and in Silico Study. Int. J. Biol. Macromol. 2021, 181, 1171–1182. [Google Scholar] [CrossRef]
- Giugliano, D.; Sportiello, L.; Capuano, A.; Maiorino, M.; Rossi, F.; Esposito, K. Dipeptidyl Peptidase-4 Inhibitors in Type 2 Diabetes Therapy – Focus on Alogliptin. Drug Des. Devel. Ther. 2013, 989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuhammad, A.; Taha, M.O. QSAR Studies in the Discovery of Novel Type-II Diabetic Therapies. Expert Opin. Drug Discov. 2016, 11, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.J.; St Jean, D.J.; Kurzeja, R.J.M.; Wahl, R.C.; Michelsen, K.; Cupples, R.; Chen, M.; Wu, J.; Sivits, G.; Helmering, J.; et al. Antidiabetic Effects of Glucokinase Regulatory Protein Small-Molecule Disruptors. Nature 2013, 504, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Toulis, K.A.; Nirantharakumar, K.; Pourzitaki, C.; Barnett, A.H.; Tahrani, A.A. Glucokinase Activators for Type 2 Diabetes: Challenges and Future Developments. Drugs 2020, 80, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vital, D.A.; Gonzalez de Mejia, E. Anthocyanins from Purple Corn Activate Free Fatty Acid-Receptor 1 and Glucokinase Enhancing in Vitro Insulin Secretion and Hepatic Glucose Uptake. PLOS ONE 2018, 13, e0200449. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Lin, S.; Myers, R.W.; Trujillo, M.E.; Pachanski, M.J.; Malkani, S.; Chen, H.-S.; Chen, Z.; Campbell, B.; Eiermann, G.J.; et al. Discovery of Orally Active Hepatoselective Glucokinase Activators for Treatment of Type II Diabetes Mellitus. Bioorg. Med. Chem. Lett. 2017, 27, 2063–2068. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.P.; Dahllöf, M.; Lundh, M.; Rasmussen, D.N.; Nielsen, M.D.; Billestrup, N.; Grunnet, L.G.; Mandrup-Poulsen, T. Histone Deacetylase (HDAC) Inhibition as a Novel Treatment for Diabetes Mellitus. Mol. Med. 2011, 17, 378–390. [Google Scholar] [CrossRef]
- Akone, S.H.; Ntie-Kang, F.; Stuhldreier, F.; Ewonkem, M.B.; Noah, A.M.; Mouelle, S.E.M.; Müller, R. Natural Products Impacting DNA Methyltransferases and Histone Deacetylases. Front. Pharmacol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Park, S.; Jeon, J.-H.; Min, B.-K.; Ha, C.-M.; Thoudam, T.; Park, B.-Y.; Lee, I.-K. Role of the Pyruvate Dehydrogenase Complex in Metabolic Remodeling: Differential Pyruvate Dehydrogenase Complex Functions in Metabolism. Diabetes Metab. J. 2018, 42, 270. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bhusal, A.; Kim, J.-H.; Jha, M.K.; Song, G.J.; Go, Y.; Jang, I.-S.; Lee, I.-K.; Suk, K. Astrocytic Pyruvate Dehydrogenase Kinase-2 Is Involved in Hypothalamic Inflammation in Mouse Models of Diabetes. Nat. Commun. 2020, 11, 5906. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, K.A.; Gajjar, A.K. CoMFA, CoMSIA and HQSAR Analysis of 3-Aryl-3-Ethoxypropanoic Acid Derivatives as GPR40 Modulators. Curr. Drug Discov. Technol. 2020, 17, 100–118. [Google Scholar] [CrossRef]
- Tsujihata, Y.; Ito, R.; Suzuki, M.; Harada, A.; Negoro, N.; Yasuma, T.; Momose, Y.; Takeuchi, K. TAK-875, an Orally Available G Protein-Coupled Receptor 40/Free Fatty Acid Receptor 1 Agonist, Enhances Glucose-Dependent Insulin Secretion and Improves Both Postprandial and Fasting Hyperglycemia in Type 2 Diabetic Rats. J. Pharmacol. Exp. Ther. 2011, 339, 228–237. [Google Scholar] [CrossRef]
- Thorens, B. GLUT2, Glucose Sensing and Glucose Homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Eldar-Finkelman, H.; Kaidanovich, O. The Role of Glycogen Synthase Kinase-3 in Insulin Resistance and Type 2 Diabetes. Expert Opin. Ther. Targets 2002, 6, 555–561. [Google Scholar] [CrossRef]
- Ishijima, S.; Takashima, T.; Ikemura, T.; Izutani, Y. Gymnemic Acid Interacts with Mammalian Glycerol-3-Phosphate Dehydrogenase. Mol. Cell. Biochem. 2008, 310, 203–208. [Google Scholar] [CrossRef]
- Saponins, Classification and Occurrence in the Plant Kingdom. ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0031942206006480?casa_token=N4YR7d_ankkAAAAA:yjLjMzrHAZKxlA_2S97bx6HsIllw9xC_NXMJurIhsnwO5Sqv6vGGy-_I-dInKmnAbIT9LlUD (accessed on 9 October 2021).
- Yendo, A.C.A.; de Costa, F.; Gosmann, G.; Fett-Neto, A.G. Production of Plant Bioactive Triterpenoid Saponins: Elicitation Strategies and Target Genes to Improve Yields. Mol. Biotechnol. 2010, 46, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. Phytochemical and Pharmacological Properties of Gymnema Sylvestre: An Important Medicinal Plant. BioMed Res. Int. 2014, 2014, e830285. [Google Scholar] [CrossRef] [Green Version]
- Zuñiga, L.Y.; González-Ortiz, M.; Martínez-Abundis, E. Effect of Gymnema Sylvestre Administration on Metabolic Syndrome, Insulin Sensitivity, and Insulin Secretion. J. Med. Food 2017, 20, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Sarker, M.M.R.; Ming, L.C.; Mohamed, I.N.; Zhao, C.; Sheikh, B.Y.; Tsong, H.F.; Rashid, M.A. Comprehensive Review on Phytochemicals, Pharmacological and Clinical Potentials of Gymnema Sylvestre. Front. Pharmacol. 2019, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Jung, J.; Jung, J.H.; Yoon, N.; Kang, S.S.; Roh, G.S.; Hahm, J.R. Hypoglycemic Efficacy and Safety of Momordica Charantia (Bitter Melon) in Patients with Type 2 Diabetes Mellitus. Complement. Ther. Med. 2020, 52, 102524. [Google Scholar] [CrossRef]
- Kasbia, G.S.; Arnason, J.T.; Imbeault, P. No Effect of Acute, Single Dose Oral Administration of Momordica Charantia Linn., on Glycemia, Energy Expenditure and Appetite: A Pilot Study in Non-Diabetic Overweight Men. J. Ethnopharmacol. 2009, 126, 127–133. [Google Scholar] [CrossRef]
- Chaturvedi, P. Antidiabetic Potentials of Momordica Charantia: Multiple Mechanisms Behind the Effects. J. Med. Food 2012, 15, 101–107. [Google Scholar] [CrossRef]
- Cortez-Navarrete, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; González-Ortiz, M.; Méndez-Del Villar, M. Momordica Charantia Administration Improves Insulin Secretion in Type 2 Diabetes Mellitus. J. Med. Food 2018, 21, 672–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, S.; Chaturvedi, P. Momordica Charantia Maintains Normal Glucose Levels and Lipid Profiles and Prevents Oxidative Stress in Diabetic Rats Subjected to Chronic Sucrose Load. J. Med. Food. 2010, 13, pp. 520–527. [CrossRef]
- Arif, R.; Ahmad, S.; Mustafa, G.; Mahrosh, H.S.; Ali, M.; Tahir ul Qamar, M.; Dar, H.R. Molecular Docking and Simulation Studies of Antidiabetic Agents Devised from Hypoglycemic Polypeptide-P of Momordica Charantia. BioMed Res. Int. 2021, 2021, e5561129. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Fenugreek Cultivation with Emphasis on Historical Aspects and Its Uses in Traditional Medicine and Modern Pharmaceutical Science. Mini Rev. Med. Chem. 2021, 21, 724–730. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Panax Ginseng and Panax Quinquefolius: From Pharmacology to Toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef]
- Zeng, M.; Pan, L.; Qi, S.; Cao, Y.; Zhu, H.; Guo, L.; Zhou, J. Systematic Review of Recent Advances in Pharmacokinetics of Four Classical Chinese Medicines Used for the Treatment of Cerebrovascular Disease. Fitoterapia 2013, 88, 50–75. [Google Scholar] [CrossRef]
- Leach, M.J.; Kumar, S. Cinnamon for Diabetes Mellitus. Cochrane Database Syst. Rev. 2012, 2012, CD007170. [Google Scholar] [CrossRef] [PubMed]
- Subash Babu, P.; Prabuseenivasan, S.; Ignacimuthu, S. Cinnamaldehyde--a Potential Antidiabetic Agent. Phytomed. Int. J. Phytother. Phytopharm. 2007, 14, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kim, C.S.; Cho, S.-H.; Chun, H.S.; Kim, J.-K.; Kim, D.K. The Effects of Angelica Decursiva Extract in the Inhibition of Cell Proliferation and in the Induction of Apoptosis in Osteogenic Sarcoma Cells. J. Med. Plants Res. 2009, 3, 241–245. [Google Scholar] [CrossRef]
- Zhao, D.; Islam, M.N.; Ahn, B.R.; Jung, H.A.; Kim, B.-W.; Choi, J.S. In Vitro Antioxidant and Anti-Inflammatory Activities of Angelica Decursiva. Arch. Pharm. Res. 2012, 35, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Jhong, C.-H.; Riyaphan, J.; Lin, S.-H.; Chia, Y.-C.; Weng, C.-F. Screening Alpha-Glucosidase and Alpha-Amylase Inhibitors from Natural Compounds by Molecular Docking in Silico. BioFactors Oxf. Engl. 2015, 41, 242–251. [Google Scholar] [CrossRef]
- Liao, Z.-C.; Jiang, X.; Tian, W.-J.; Lin, T.; Chen, H.-F. Chemical constituents from root of Angelica decursiva. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2017, 42, 2999–3003. [Google Scholar] [CrossRef]
- Fischer, E.; Charbonneau, H.; Tonks, N. Protein Tyrosine Phosphatases: A Diverse Family of Intracellular and Transmembrane Enzymes. Science 1991, 253, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Jannat, S.; Jung, H.A.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Coumarins from Angelica Decursiva Inhibit α-Glucosidase Activity and Protein Tyrosine Phosphatase 1B. Chem. Biol. Interact. 2016, 252, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, V.K.; Singh, A.K. Molecular Docking Analysis of Candidate Compounds derived from Medicinal Plants with Type 2 Diabetes Mellitus Targets. Bioinformation 2019, 15, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.-L.; Chan, K.-G.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Gynura Procumbens: An Overview of the Biological Activities. Front. Pharmacol. 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathiyaseelan, A.; Park, S.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.-H. Evaluation of Phytochemicals, Antioxidants, and Antidiabetic Efficacy of Various Solvent Fractions of Gynura Procumbens (Lour.) Merr. Process Biochem. 2021, 111, 51–62. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.; Mariadoss, A.V.A.; Sathiyaseelan, A.; Veeraraghavan, V.P.; Kim, S.; Wang, M.-H. Chemical Composition, Antioxidant, and Anti-Diabetic Activities of Ethyl Acetate Fraction of Stachys Riederi Var. Japonica (Miq.) in Streptozotocin-Induced Type 2 Diabetic Mice. Food Chem. Toxicol. 2021, 155, 112374. [Google Scholar] [CrossRef]
- Chen, L.; Li, M.; Yang, Z.; Tao, W.; Wang, P.; Tian, X.; Li, X.; Wang, W. Gardenia Jasminoides Ellis: Ethnopharmacology, Phytochemistry, and Pharmacological and Industrial Applications of an Important Traditional Chinese Medicine. J. Ethnopharmacol. 2020, 257, 112829. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.; Sathiyaseelan, A.; Kim, K.-N.; Cho, S.-H.; Mariadoss, A.V.A.; Wang, M.-H. Metabolite Profiling of Methanolic Extract of Gardenia Jaminoides by LC-MS/MS and GC-MS and Its Anti-Diabetic, and Anti-Oxidant Activities. Pharmaceuticals 2021, 14, 102. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Xue, F.; Nan, X.; Wang, H.; Hua, D.; Liu, J.; Yang, L.; Jiang, L.; Xiong, B. Nutritional Value, Bioactivity, and Application Potential of Jerusalem Artichoke (Helianthus Tuberosus L.) as a Neotype Feed Resource. Anim. Nutr. 2020, 6, 429–437. [Google Scholar] [CrossRef]
- Gill, B.S.; Mehra, R.; Navgeet; Kumar, S. Vitex Negundo and Its Medicinal Value. Mol. Biol. Rep. 2018, 45, 2925–2934. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Mumtaz, M.W.; Danish, M.; Rashid, U.; Mukhtar, H.; Irfan, A. Antidiabetic Functionality of Vitex Negundo L. Leaves Based on UHPLC-QTOF-MS/MS Based Bioactives Profiling and Molecular Docking Insights. Ind. Crops Prod. 2020, 152, 112445. [Google Scholar] [CrossRef]
- Sadiq, A.; Rashid, U.; Ahmad, S.; Zahoor, M.; AlAjmi, M.F.; Ullah, R.; Noman, O.M.; Ullah, F.; Ayaz, M.; Khan, I.; et al. Treating Hyperglycemia From Eryngium Caeruleum M. Bieb: In-Vitro α-Glucosidase, Antioxidant, in-Vivo Antidiabetic and Molecular Docking-Based Approaches. Front. Chem. 2020, 8, 1064. [Google Scholar] [CrossRef] [PubMed]
- Zabidi, N.A.; Ishak, N.A.; Hamid, M.; Ashari, S.E.; Mohammad Latif, M.A. Inhibitory Evaluation of Curculigo Latifolia on α-Glucosidase, DPP (IV) and in Vitro Studies in Antidiabetic with Molecular Docking Relevance to Type 2 Diabetes Mellitus. J. Enzyme Inhib. Med. Chem. 2021, 36, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sattar, E.; Shams, M.M.; Abd-Rabo, M.M.; Mahmoud, N.; Mahrous, E.A. Chemical and biological investigations of Limonium axillare reveal mechanistic evidence for its antidiabetic activity. PLoS ONE 2021, 16, e0255904. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A Major Update of the Phenol-Explorer Database to Incorporate Data on the Effects of Food Processing on Polyphenol Content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, T.; Mae, T.; Kishida, H.; Tsukagawa, M.; Mimaki, Y.; Kuroda, M.; Sashida, Y.; Takahashi, K.; Kawada, T.; Nakagawa, K.; et al. Curcuminoids and Sesquiterpenoids in Turmeric (Curcuma Longa L.) Suppress an Increase in Blood Glucose Level in Type 2 Diabetic KK-Ay Mice. J. Agric. Food Chem. 2005, 53, 959–963. [Google Scholar] [CrossRef]
- 2,4-Thiazolidinedione. Available online: https://go.drugbank.com/drugs/DB11898 (accessed on 6 October 2021).
- Docosanol. Available online: https://go.drugbank.com/drugs/DB00632 (accessed on 6 October 2021).
- Riyaphan, J.; Jhong, C.-H.; Lin, S.-R.; Chang, C.-H.; Tsai, M.-J.; Lee, D.-N.; Sung, P.-J.; Leong, M.K.; Weng, C.-F. Hypoglycemic Efficacy of Docking Selected Natural Compounds against α-Glucosidase and α-Amylase. Molecules 2018, 23, 2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaleshkumar, K.; Rajaram, R.; Gayathri, N.; Sivasudha, T.; Arun, G.; Archunan, G.; Gulyás, B.; Padmanabhan, P. Muscle Extract of Arothron Immaculatus Regulates the Blood Glucose Level and the Antioxidant System in High-Fat Diet and Streptozotocin Induced Diabetic Rats. Bioorganic Chem. 2019, 90, 103072. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Tzeng, Y.-M. Review of Pharmacological Effects of Antrodia Camphorata and Its Bioactive Compounds. Evid.-Based Complement. Altern. Med. ECAM 2011, 2011, 212641. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.Y.; Sulake, R.S.; Huang, P.-K.; Shih, H.-Y.; Sie, H.-W.; Lai, Y.-K.; Chen, C.; Weng, C.F. Synthetic (+)-Antroquinonol Exhibits Dual Actions against Insulin Resistance by Triggering AMP Kinase and Inhibiting Dipeptidyl Peptidase IV Activities. Br. J. Pharmacol. 2015, 172, 38–49. [Google Scholar] [CrossRef] [Green Version]
- Kamarudin, M.N.A.; Sarker, M.M.R.; Kadir, H.A.; Ming, L.C. Ethnopharmacological Uses, Phytochemistry, Biological Activities, and Therapeutic Applications of Clinacanthus Nutans (Burm. f.) Lindau: A Comprehensive Review. J. Ethnopharmacol. 2017, 206, 245–266. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sharfaraz, A.; Dutta, A.; Ahsan, A.; Masud, M.A.; Ahmed, I.A.; Goh, B.H.; Urbi, Z.; Sarker, M.M.R.; Ming, L.C. A Review of Ethnobotany, Phytochemistry, Antimicrobial Pharmacology and Toxicology of Nigella sativa L. Biomed. Pharmacother. 2021, 143, 112182. [Google Scholar] [CrossRef]
- Rutin. Available online: https://go.drugbank.com/drugs/DB01698 (accessed on 6 October 2021).
- Ragheb, S.R.; El Wakeel, L.M.; Nasr, M.S.; Sabri, N.A. Impact of Rutin and Vitamin C Combination on Oxidative Stress and Glycemic Control in Patients with Type 2 Diabetes. Clin. Nutr. ESPEN 2020, 35, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A. Mechanisms of Antidiabetic Effects of Flavonoid Rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Berberine. Available online: https://go.drugbank.com/drugs/DB04115 (accessed on 6 October 2021).
- Yin, J.; Gao, Z.; Liu, D.; Liu, Z.; Ye, J. Berberine Improves Glucose Metabolism through Induction of Glycolysis. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E148–E156. [Google Scholar] [CrossRef] [Green Version]
- Pan, G.; Wang, G.; Sun, J.; Huang, Z.; Zhao, X.; Gu, Y.; Liu, X. Inhibitory action of berberine on glucose absorption. Yao Xue Xue Bao 2003, 38, 911–914. [Google Scholar]
- Epigallo Catechin Gallate: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB16120 (accessed on 7 October 2021).
- Proença, C.; Oliveira, A.; Freitas, M.; Ribeiro, D.; Sousa, J.L.C.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. Structural Specificity of Flavonoids in the Inhibition of Human Fructose 1,6-Bisphosphatase. J. Nat. Prod. 2020, 83, 1541–1552. [Google Scholar] [CrossRef]
- Veeramani, C.; Alsaif, M.A.; Al-Numair, K.S. Herbacetin, a Flaxseed Flavonoid, Ameliorates High Percent Dietary Fat Induced Insulin Resistance and Lipid Accumulation through the Regulation of Hepatic Lipid Metabolizing and Lipid-Regulating Enzymes. Chem. Biol. Interact. 2018, 288, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory Kinetics and Mechanism of Kaempferol on α-Glucosidase. Food Chem. 2016, 190, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kumar Tekade, R.; Kalia, K. Kaempferol in Ameliorating Diabetes-Induced Fibrosis and Renal Damage: An in Vitro and in Vivo Study in Diabetic Nephropathy Mice Model. Phytomed. Int. J. Phytother. Phytopharm. 2020, 76, 153235. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent Progress Regarding Kaempferol for the Treatment of Various Diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef] [Green Version]
- Forid, M.S.; Rahman, M.A.; Aluwi, M.F.F.M.; Uddin, M.N.; Roy, T.G.; Mohanta, M.C.; Huq, A.M.; Amiruddin Zakaria, Z. Pharmacoinformatics and UPLC-QTOF/ESI-MS-Based Phytochemical Screening of Combretum Indicum against Oxidative Stress and Alloxan-Induced Diabetes in Long–Evans Rats. Molecules 2021, 26, 4634. [Google Scholar] [CrossRef]
- Gao, Q.; Jeon, S.J.; Jung, H.A.; Lee, H.E.; Park, S.J.; Lee, Y.; Lee, Y.; Ko, S.Y.; Kim, B.; Choi, J.S.; et al. Nodakenin Enhances Cognitive Function and Adult Hippocampal Neurogenesis in Mice. Neurochem. Res. 2015, 40, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Park, S.; Saravanakumar, K.; Sathiyaseelan, A.; Wang, M.-H. Ethyl Acetate Fraction of Helianthus Tuberosus L. Induces Anti-Diabetic, and Wound-Healing Activities in Insulin-Resistant Human Liver Cancer and Mouse Fibroblast Cells. Antioxidants 2021, 10, 99. [Google Scholar] [CrossRef]
- Natarajan, A.; Sugumar, S.; Bitragunta, S.; Balasubramanyan, N. Molecular Docking Studies of (4Z, 12Z)-Cyclopentadeca-4, 12-Dienone from Grewia Hirsuta with Some Targets Related to Type 2 Diabetes. BMC Complement. Altern. Med. 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udrea, A.-M. Computational approaches of new perspectives in the treatment of depression during pregnancy. Farmacia 2018, 66, 680–687. [Google Scholar] [CrossRef]
- Avram, S.; Stan, M.S.; Udrea, A.M.; Buiu, C.; Boboc, A.A.; Mernea, M. 3D-ALMOND-QSAR Models to Predict the Antidepressant Effect of Some Natural Compounds. Pharmaceutics 2021, 13, 1449. [Google Scholar] [CrossRef]
- Avram, S.; Buiu, C.; Duda-Seiman, D.; Duda-Seiman, C.; Borcan, F.; Mihailescu, D. Evaluation of the Pharmacological Descriptors Related to the Induction of Antidepressant Activity and Its Prediction by QSAR/QRAR Methods. Mini-Rev. Med. Chem. 2012, 12, 467–476. [Google Scholar] [CrossRef]
- Avram, S.; Milac, A.-L.; Mihailescu, D. 3D-QSAR Study Indicates an Enhancing Effect of Membrane Ions on Psychiatric Drugs Targeting Serotonin Receptor 5-HT1A. Mol. Biosyst. 2012, 8, 1418. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.C.; Dhanjal, J.K.; Malik, V.; Radhakrishnan, N.; Jayakanthan, M.; Sundar, D. Quantitative Structure-Activity Relationship (QSAR): Modeling Approaches to Biological Applications. In Encyclopedia of Bioinformatics and Computational Biology; Ranganathan, S., Gribskov, M., Nakai, K., Schönbach, C., Eds.; Academic Press: Oxford, UK, 2019; pp. 661–676. ISBN 978-0-12-811432-2. [Google Scholar]
- Izadpanah, E.; Riahi, S.; Abbasi-Radmoghaddam, Z.; Gharaghani, S.; Mohammadi-Khanaposhtanai, M. A Simple and Robust Model to Predict the Inhibitory Activity of α-Glucosidase Inhibitors through Combined QSAR Modeling and Molecular Docking Techniques. Mol. Divers. 2021, 25, 1811–1825. [Google Scholar] [CrossRef] [PubMed]
- Jamil, W.; Shaikh, J.; Yousuf, M.; Taha, M.; Khan, K.M.; Shah, S.A.A. Synthesis, Anti-Diabetic and in Silico QSAR Analysis of Flavone Hydrazide Schiff Base Derivatives. J. Biomol. Struct. Dyn. 2021, 1–16. [Google Scholar] [CrossRef]
- Maurya, A.K.; Mulpuru, V.; Mishra, N. Discovery of Novel Coumarin Analogs against the α-Glucosidase Protein Target of Diabetes Mellitus: Pharmacophore-Based QSAR, Docking, and Molecular Dynamics Simulation Studies. ACS Omega 2020, 5, 32234–32249. [Google Scholar] [CrossRef]
- Xu, J.; Huang, S.; Luo, H.; Li, G.; Bao, J.; Cai, S.; Wang, Y. QSAR Studies on Andrographolide Derivatives as α-Glucosidase Inhibitors. Int. J. Mol. Sci. 2010, 11, 880–895. [Google Scholar] [CrossRef] [Green Version]
- Ghamali, M.; Chtita, S.; Hmamouchi, R.; Adad, A.; Bouachrine, M.; Lakhlifi, T. The Inhibitory Activity of Aldose Reductase of Flavonoid Compounds: Combining DFT and QSAR Calculations. J. Taibah Univ. Sci. 2016, 10, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Tozar, T.; Santos Costa, S.; Udrea, A.-M.; Nastasa, V.; Couto, I.; Viveiros, M.; Pascu, M.L.; Romanitan, M.O. Anti-Staphylococcal Activity and Mode of Action of Thioridazine Photoproducts. Sci. Rep. 2020, 10, 18043. [Google Scholar] [CrossRef]
- Udrea, A.-M.; Dinache, A.; Pagès, J.-M.; Pirvulescu, R.A. Quinazoline Derivatives Designed as Efflux Pump Inhibitors: Molecular Modeling and Spectroscopic Studies. Molecules 2021, 26, 2374. [Google Scholar] [CrossRef] [PubMed]
- Udrea, A.-M.; Mernea, M.; Buiu, C.; Avram, S. Scutellaria Baicalensis Flavones as Potent Drugs against Acute Respiratory Injury during SARS-CoV-2 Infection: Structural Biology Approaches. Processes 2020, 8, 1468. [Google Scholar] [CrossRef]
- Nistorescu, S.; Gradisteanu Pircalabioru, G.; Udrea, A.-M.; Simon, A.; Pascu, M.L.; Chifiriuc, M.-C. Laser-Irradiated Chlorpromazine as a Potent Anti-Biofilm Agent for Coating of Biomedical Devices. Coatings 2020, 10, 1230. [Google Scholar] [CrossRef]
- Udrea, A.-M.; Avram, S.; Nistorescu, S.; Pascu, M.-L.; Romanitan, M.O. Laser Irradiated Phenothiazines: New Potential Treatment for COVID-19 Explored by Molecular Docking. J. Photochem. Photobiol. B 2020, 211, 111997. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Vriend, G. YASARA View—Molecular Graphics for All Devices—from Smartphones to Workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef] [Green Version]
- Shirai, T.; Hung, V.S.; Morinaka, K.; Kobayashi, T.; Ito, S. Crystal Structure of GH13 α-Glucosidase GSJ from One of the Deepest Sea Bacteria. Proteins Struct. Funct. Bioinforma. 2008, 73, 126–133. [Google Scholar] [CrossRef]
- Brayer, G.D.; Luo, Y.; Withers, S.G. The Structure of Human Pancreatic α -Amylase at 1.8 Å Resolution and Comparisons with Related Enzymes. Protein Sci. 1995, 4, 1730–1742. [Google Scholar] [CrossRef] [PubMed]
- Bank, R.P.D. RCSB PDB—5KZX: Crystal Structure of Human GAA. Available online: https://www.rcsb.org/structure/5KZX (accessed on 30 June 2021).
- Gilles, C.; Astier, J.-P.; Marchis-Mouren, G.; Cambillau, C.; Payan, F. Crystal Structure of Pig Pancreatic Alpha-Amylase Isoenzyme II, in Complex with the Carbohydrate Inhibitor Acarbose. Eur. J. Biochem. 1996, 238, 561–569. [Google Scholar] [CrossRef]
- Thompson, M.A. Molecular Docking Using ArgusLab, an Efficient Shape-Based Search Algorithm and the a Score Scoring Function. Philadelphia 2004, 172, 42. [Google Scholar]
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Crystal Structures of Isomaltase from Saccharomyces Cerevisiae and in Complex with Its Competitive Inhibitor Maltose: Crystal Structure of Isomaltase. FEBS J. 2010, 277, 4205–4214. [Google Scholar] [CrossRef]
- Bank, R.P.D. RCSB PDB—5E0F: Human Pancreatic Alpha-Amylase in Complex with Mini-Montbretin A. Available online: https://www.rcsb.org/structure/5E0F (accessed on 2 November 2021).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Roig-Zamboni, V.; Cobucci-Ponzano, B.; Iacono, R.; Ferrara, M.C.; Germany, S.; Bourne, Y.; Parenti, G.; Moracci, M.; Sulzenbacher, G. Structure of Human Lysosomal Acid α-Glucosidase–a Guide for the Treatment of Pompe Disease. Nat. Commun. 2017, 8, 1111. [Google Scholar] [CrossRef] [Green Version]
- Van Zandt, M.C.; Doan, B.; Sawicki, D.R.; Sredy, J.; Podjarny, A.D. Discovery of [3-(4,5,7-Trifluoro-Benzothiazol-2-Ylmethyl)-Pyrrolo[2,3-b]Pyridin-1-Yl]Acetic Acids as Highly Potent and Selective Inhibitors of Aldose Reductase for Treatment of Chronic Diabetic Complications. Bioorg. Med. Chem. Lett. 2009, 19, 2006–2008. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Bank, R.P.D. RCSB PDB—4IXC: Crystal Structure of Human Glucokinase in Complex with a Small Molecule Activator. Available online: https://www.rcsb.org/structure/4IXC (accessed on 2 November 2021).
- Tso, S.-C.; Qi, X.; Gui, W.-J.; Wu, C.-Y.; Chuang, J.L.; Wernstedt-Asterholm, I.; Morlock, L.K.; Owens, K.R.; Scherer, P.E.; Williams, N.S.; et al. Structure-Guided Development of Specific Pyruvate Dehydrogenase Kinase Inhibitors Targeting the ATP-Binding Pocket. J. Biol. Chem. 2014, 289, 4432–4443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, V.; Huang, P.; Hamuro, Y.; Raghuram, S.; Wang, Y.; Burris, T.P.; Rastinejad, F. Structure of the Intact PPAR-γ–RXR-α Nuclear Receptor Complex on DNA. Nature 2008, 456, 350–356. [Google Scholar] [CrossRef]
- Saitoh, M.; Kunitomo, J.; Kimura, E.; Hayase, Y.; Kobayashi, H.; Uchiyama, N.; Kawamoto, T.; Tanaka, T.; Mol, C.D.; Dougan, D.R.; et al. Design, Synthesis and Structure–Activity Relationships of 1,3,4-Oxadiazole Derivatives as Novel Inhibitors of Glycogen Synthase Kinase-3β. Bioorg. Med. Chem. 2009, 17, 2017–2029. [Google Scholar] [CrossRef]
- Böhme, T.; Engel, C.K.; Farjot, G.; Güssregen, S.; Haack, T.; Tschank, G.; Ritter, K. 1,1-Dioxo-5,6-Dihydro-[4,1,2]Oxathiazines, a Novel Class of 11ß-HSD1 Inhibitors for the Treatment of Diabetes. Bioorg. Med. Chem. Lett. 2013, 23, 4685–4691. [Google Scholar] [CrossRef]
- Nakaishi, Y.; Bando, M.; Shimizu, H.; Watanabe, K.; Goto, F.; Tsuge, H.; Kondo, K.; Komatsu, M. Structural Analysis of Human Glutamine:Fructose-6-Phosphate Amidotransferase, a Key Regulator in Type 2 Diabetes. FEBS Lett. 2009, 583, 163–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faham, S.; Watanabe, A.; Besserer, G.M.; Cascio, D.; Specht, A.; Hirayama, B.A.; Wright, E.M.; Abramson, J. The Crystal Structure of a Sodium Galactose Transporter Reveals Mechanistic Insights into Na + /Sugar Symport. Science 2008, 321, 810–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, J.M.; Clark, D.E.; Dunsdon, S.J.; Fenton, G.; Fillmore, A.; Harris, N.V.; Higgs, C.; Hurley, C.A.; Krintel, S.L.; MacKenzie, R.E.; et al. Novel Heterocyclic DPP-4 Inhibitors for the Treatment of Type 2 Diabetes. Bioorg. Med. Chem. Lett. 2012, 22, 1464–1468. [Google Scholar] [CrossRef]
- Barker, M.K.; Rose, D.R. Specificity of Processing α-Glucosidase I Is Guided by the Substrate Conformation. J. Biol. Chem. 2013, 288, 13563–13574. [Google Scholar] [CrossRef] [Green Version]
- Biftu, T.; Scapin, G.; Singh, S.; Feng, D.; Becker, J.W.; Eiermann, G.; He, H.; Lyons, K.; Patel, S.; Petrov, A.; et al. Rational Design of a Novel, Potent, and Orally Bioavailable Cyclohexylamine DPP-4 Inhibitor by Application of Molecular Modeling and X-Ray Crystallography of Sitagliptin. Bioorg. Med. Chem. Lett. 2007, 17, 3384–3387. [Google Scholar] [CrossRef]
- Ou, X.; Ji, C.; Han, X.; Zhao, X.; Li, X.; Mao, Y.; Wong, L.-L.; Bartlam, M.; Rao, Z. Crystal Structures of Human Glycerol 3-Phosphate Dehydrogenase 1 (GPD1). J. Mol. Biol. 2006, 357, 858–869. [Google Scholar] [CrossRef]
- Sharma, P.; Joshi, T.; Joshi, T.; Chandra, S.; Tamta, S. Molecular Dynamics Simulation for Screening Phytochemicals as α-Amylase Inhibitors from Medicinal Plants. J. Biomol. Struct. Dyn. 2021, 39, 6524–6538. [Google Scholar] [CrossRef]
- Maurus, R.; Begum, A.; Williams, L.K.; Fredriksen, J.R.; Zhang, R.; Withers, S.G.; Brayer, G.D. Alternative Catalytic Anions Differentially Modulate Human α-Amylase Activity and Specificity. Biochemistry 2008, 47, 3332–3344. [Google Scholar] [CrossRef]
- Yadav, A.; Sharma, S.; Desk, S. Curcumin and Pipernonaline to Curb Diabetes the Natural Way: A Molecular Modeling, Docking and Dynamics Simulation Study. J. Comput. Chem. Mol. Model. 2020, 4. [Google Scholar]
- de Groot, J.C.; Weidner, C.; Krausze, J.; Kawamoto, K.; Schroeder, F.C.; Sauer, S.; Büssow, K. Structural Characterization of Amorfrutins Bound to the Peroxisome Proliferator-Activated Receptor γ. J. Med. Chem. 2013, 56, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- In Silico Screening and Identification of Natural Compound Sophoraflavanone G as Potential Human Sodium-Glucose Cotransporter 2 Inhibitor. Biointerface Res. Appl. Chem. 2021, 11, 14173–14184. [CrossRef]
- Thakuria, B.; Laskar, S.; Adhikari, S. A Bioinformatics-Based Investigation to Screen and Analyze the Bioactivity of Piper Longum Linn. Compounds as a Ground-Breaking Hostile to Antidiabetic Activity. Pharmacogn. Mag. 2020, 16, 199. [Google Scholar] [CrossRef]
- Hosfield, D.J.; Wu, Y.; Skene, R.J.; Hilgers, M.; Jennings, A.; Snell, G.P.; Aertgeerts, K. Conformational Flexibility in Crystal Structures of Human 11beta-Hydroxysteroid Dehydrogenase Type I Provide Insights into Glucocorticoid Interconversion and Enzyme Regulation. J. Biol. Chem. 2005, 280, 4639–4648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bank, R.P.D. RCSB PDB—2V4M: The Isomerase Domain of Human Glutamine-Fructose-6-Phosphate Transaminase 1 (GFPT1) in Complex with Fructose 6-Phosphate. Available online: https://www.rcsb.org/structure/2V4M (accessed on 6 October 2021).
- Zhou, H.; Singh, H.; Parsons, Z.D.; Lewis, S.M.; Bhattacharya, S.; Seiner, D.R.; LaButti, J.N.; Reilly, T.J.; Tanner, J.J.; Gates, K.S. The Biological Buffer Bicarbonate/CO2 Potentiates H2O2-Mediated Inactivation of Protein Tyrosine Phosphatases. J. Am. Chem. Soc. 2011, 133, 15803–15805. [Google Scholar] [CrossRef] [Green Version]
- Hiramatsu, H.; Kyono, K.; Higashiyama, Y.; Fukushima, C.; Shima, H.; Sugiyama, S.; Inaka, K.; Yamamoto, A.; Shimizu, R. The Structure and Function of Human Dipeptidyl Peptidase IV, Possessing a Unique Eight-Bladed β-Propeller Fold. Biochem. Biophys. Res. Commun. 2003, 302, 849–854. [Google Scholar] [CrossRef]
- Pautsch, A.; Stadler, N.; Löhle, A.; Rist, W.; Berg, A.; Glocker, L.; Nar, H.; Reinert, D.; Lenter, M.; Heckel, A.; et al. Crystal Structure of Glucokinase Regulatory Protein. Biochemistry 2013, 52, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Vo, T.H.; Tran, N.; Nguyen, D.; Le, L. An in Silico Study on Antidiabetic Activity of Bioactive Compounds in Euphorbia Thymifolia Linn. SpringerPlus 2016, 5, 1359. [Google Scholar] [CrossRef] [Green Version]
- Saeed, M.; Shoaib, A.; Tasleem, M.; Alabdallah, N.M.; Alam, M.J.; Asmar, Z.E.; Jamal, Q.M.S.; Bardakci, F.; Alqahtani, S.S.; Ansari, I.A.; et al. Assessment of Antidiabetic Activity of the Shikonin by Allosteric Inhibition of Protein-Tyrosine Phosphatase 1B (PTP1B) Using State of Art: An In Silico and In Vitro Tactics. Molecules 2021, 26, 3996. [Google Scholar] [CrossRef]
- Puius, Y.A.; Zhao, Y.; Sullivan, M.; Lawrence, D.S.; Almo, S.C.; Zhang, Z.Y. Identification of a Second Aryl Phosphate-Binding Site in Protein-Tyrosine Phosphatase 1B: A Paradigm for Inhibitor Design. Proc. Natl. Acad. Sci. USA 1997, 94, 13420–13425. [Google Scholar] [CrossRef] [Green Version]
- Lauffer, B.E.L.; Mintzer, R.; Fong, R.; Mukund, S.; Tam, C.; Zilberleyb, I.; Flicke, B.; Ritscher, A.; Fedorowicz, G.; Vallero, R.; et al. Histone Deacetylase (HDAC) Inhibitor Kinetic Rate Constants Correlate with Cellular Histone Acetylation but Not Transcription and Cell Viability. J. Biol. Chem. 2013, 288, 26926–26943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scafuri, B.; Bontempo, P.; Altucci, L.; De Masi, L.; Facchiano, A. Molecular Docking Simulations on Histone Deacetylases (HDAC)-1 and -2 to Investigate the Flavone Binding. Biomedicines 2020, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Mast, H.; Thompson, J.L.; Lee, S.H.; Mohr, J.P.; Sacco, R.L. Hypertension and Diabetes Mellitus as Determinants of Multiple Lacunar Infarcts. Stroke 1995, 26, 30–33. [Google Scholar] [CrossRef]
- Prasad, S.; Sajja, R.K.; Naik, P.; Cucullo, L. Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. J. Pharmacovigil. 2014, 2, 125. [Google Scholar] [CrossRef]
- Yan, T.; Venkat, P.; Chopp, M.; Zacharek, A.; Yu, P.; Ning, R.; Qiao, X.; Kelley, M.R.; Chen, J. APX3330 Promotes Neurorestorative Effects after Stroke in Type One Diabetic Rats. Aging Dis. 2018, 9, 453. [Google Scholar] [CrossRef] [Green Version]
- Yorulmaz, H.; Kaptan, E.; Seker, F.B.; Oztas, B. Type 1 Diabetes Exacerbates Blood-Brain Barrier Alterations during Experimental Epileptic Seizures in an Animal Model. Cell Biochem. Funct. 2015, 33, 285–292. [Google Scholar] [CrossRef]
- Duelli, R.; Kuschinsky, W. Brain Glucose Transporters: Relationship to Local Energy Demand. Physiology 2001, 16, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, M.S.; Nandini, C.D. Influence of Quercetin, Naringenin and Berberine on Glucose Transporters and Insulin Signalling Molecules in Brain of Streptozotocin-Induced Diabetic Rats. Biomed. Pharmacother. 2017, 94, 605–611. [Google Scholar] [CrossRef]
- Di Dedda, C.; Vignali, D.; Piemonti, L.; Monti, P. Pharmacological Targeting of GLUT1 to Control Autoreactive T Cell Responses. Int. J. Mol. Sci. 2019, 20, 4962. [Google Scholar] [CrossRef] [Green Version]
- Macheda, M.L.; Rogers, S.; Best, J.D. Molecular and Cellular Regulation of Glucose Transporter (GLUT) Proteins in Cancer. J. Cell. Physiol. 2005, 202, 654–662. [Google Scholar] [CrossRef] [PubMed]
- JUNG, C.; AL, R. Cytochalasin B binding sites and glucose transport carrier in human erythrocyte ghosts. J. Biol. Chem. 1977, 252, 5456–5463. [Google Scholar] [CrossRef]
- Kapoor, K.; Finer-Moore, J.S.; Pedersen, B.P.; Caboni, L.; Waight, A.; Hillig, R.C.; Bringmann, P.; Heisler, I.; Müller, T.; Siebeneicher, H.; et al. Mechanism of Inhibition of Human Glucose Transporter GLUT1 Is Conserved between Cytochalasin B and Phenylalanine Amides. Proc. Natl. Acad. Sci. USA 2016, 113, 4711–4716. [Google Scholar] [CrossRef] [Green Version]
- Custódio, T.F.; Paulsen, P.A.; Frain, K.M.; Pedersen, B.P. Structural Comparison of GLUT1 to GLUT3 Reveal Transport Regulation Mechanism in Sugar Porter Family. Life Sci. Alliance 2021, 4. [Google Scholar] [CrossRef] [PubMed]
- Libby, C.J.; Gc, S.; Benavides, G.A.; Fisher, J.L.; Williford, S.E.; Zhang, S.; Tran, A.N.; Gordon, E.R.; Jones, A.B.; Tuy, K.; et al. A Role for GLUT3 in Glioblastoma Cell Invasion That Is Not Recapitulated by GLUT1. Cell Adhes. Migr. 2021, 15, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Sun, P.; Yan, C.; Ke, M.; Jiang, X.; Xiong, L.; Ren, W.; Hirata, K.; Yamamoto, M.; Fan, S.; et al. Molecular Basis of Ligand Recognition and Transport by Glucose Transporters. Nature 2015, 526, 391–396. [Google Scholar] [CrossRef]
- Hoffman, W.H.; Cudrici, C.D.; Boodhoo, D.; Tatomir, A.; Rus, V.; Rus, H. Intracerebral Matrix Metalloproteinase 9 in Fatal Diabetic Ketoacidosis. Exp. Mol. Pathol. 2019, 108, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, N.; Irudayanathan, F.J.; Nangia, S. Computational Nanoscopy of Tight Junctions at the Blood–Brain Barrier Interface. Int. J. Mol. Sci. 2019, 20, 5583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Sun, X.; Shen, X.; Lu, Y.; Wang, J.; Sun, Z.; Miao, C.; Chen, J. Propofol Attenuates TNF-α-Induced MMP-9 Expression in Human Cerebral Microvascular Endothelial Cells by Inhibiting Ca2+/CAMK II/ERK/NF-ΚB Signaling Pathway. Acta Pharmacol. Sin. 2019, 40, 1303–1313. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Chen, Z.; Yu, M.; Li, J.; Dong, H.; Li, N.; Ding, X.; Ge, Y.; Liu, C.; et al. TLR4-Mediated Hippocampal MMP/TIMP Imbalance Contributes to the Aggravation of Perioperative Neurocognitive Disorder in Db/Db Mice. Neurochem. Int. 2020, 140, 104818. [Google Scholar] [CrossRef] [PubMed]
- Elkins, P.A.; Ho, Y.S.; Smith, W.W.; Janson, C.A.; D’Alessio, K.J.; McQueney, M.S.; Cummings, M.D.; Romanic, A.M. Structure of the C-Terminally Truncated Human ProMMP9, a Gelatin-Binding Matrix Metalloproteinase. Acta Crystallogr. D Biol. Crystallogr. 2002, 58, 1182–1192. [Google Scholar] [CrossRef] [Green Version]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.-S.; Lee, H.; Lee, J.-O. The Structural Basis of Lipopolysaccharide Recognition by the TLR4–MD-2 Complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Claudin-5 AlphaFold Structure Prediction. Available online: https://alphafold.ebi.ac.uk/entry/O00501 (accessed on 12 October 2021).
- Irudayanathan, F.J.; Wang, X.; Wang, N.; Willsey, S.R.; Seddon, I.A.; Nangia, S. Self-Assembly Simulations of Classic Claudins—Insights into the Pore Structure, Selectivity, and Higher Order Complexes. J. Phys. Chem. B 2018, 122, 7463–7474. [Google Scholar] [CrossRef]
- Irudayanathan, F.J.; Wang, N.; Wang, X.; Nangia, S. Architecture of the Paracellular Channels Formed by Claudins of the Blood–Brain Barrier Tight Junctions. Ann. N. Y. Acad. Sci. 2017, 1405, 131–146. [Google Scholar] [CrossRef]
- Sang, H.; Qiu, Z.; Cai, J.; Lan, W.; Yu, L.; Zhang, H.; Li, M.; Xie, Y.; Guo, R.; Ye, R.; et al. Early Increased Bradykinin 1 Receptor Contributes to Hemorrhagic Transformation After Ischemic Stroke in Type 1 Diabetic Rats. Transl. Stroke Res. 2017, 8, 597–611. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Fan, Z.; Chen, Q.; Chen, J.; Sun, Y.; Jiang, X.; Xiao, Q. Soluble Epoxide Hydrolase Inhibitor Protects against Blood-Brain Barrier Dysfunction in a Mouse Model of Type 2 Diabetes via the AMPK/HO-1 Pathway. Biochem. Biophys. Res. Commun. 2020, 524, 354–359. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. AdmetSAR 2.0: Web-Service for Prediction and Optimization of Chemical ADMET Properties. Bioinforma. Oxf. Engl. 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- PkCSM—Pharmacokinetics. Available online: http://biosig.unimelb.edu.au/pkcsm/ (accessed on 1 October 2021).
- AdmetSAR. Available online: http://lmmd.ecust.edu.cn/admetsar2/ (accessed on 1 October 2021).
- Kam, A.; Li, K.M.; Razmovski-Naumovski, V.; Nammi, S.; Chan, K.; Li, Y.; Li, G.Q. The Protective Effects of Natural Products on Blood-Brain Barrier Breakdown. Curr. Med. Chem. 2012, 19, 1830–1845. [Google Scholar] [CrossRef]
- Mamo, J.C.; Lam, V.; Brook, E.; Mooranian, A.; Al-Salami, H.; Fimognari, N.; Nesbit, M.; Takechi, R. Probucol Prevents Blood-Brain Barrier Dysfunction and Cognitive Decline in Mice Maintained on pro-Diabetic Diet. Diab. Vasc. Dis. Res. 2019, 16, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, Y.-H.; Chen, K.-H.; Kuo, P.-C.; Pao, C.-C.; Chen, J.-K. Neurodegeneration in Streptozotocin-Induced Diabetic Rats Is Attenuated by Treatment with Resveratrol. Neuroendocrinology 2013, 98, 116–127. [Google Scholar] [CrossRef]
- Yi, L.; Jin, X.; Chen, C.-Y.; Fu, Y.-J.; Zhang, T.; Chang, H.; Zhou, Y.; Zhu, J.-D.; Zhang, Q.-Y.; Mi, M.-T. Chemical Structures of 4-Oxo-Flavonoids in Relation to Inhibition of Oxidized Low-Density Lipoprotein (LDL)-Induced Vascular Endothelial Dysfunction. Int. J. Mol. Sci. 2011, 12, 5471–5489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.-N.; Liu, L.-B.; Xue, Y.-X.; Wang, P. Effects of Insulin Combined with Idebenone on Blood-Brain Barrier Permeability in Diabetic Rats. J. Neurosci. Res. 2015, 93, 666–677. [Google Scholar] [CrossRef]
- Taïlé, J.; Patché, J.; Veeren, B.; Gonthier, M.-P. Hyperglycemic Condition Causes Pro-Inflammatory and Permeability Alterations Associated with Monocyte Recruitment and Deregulated NFκB/PPARγ Pathways on Cerebral Endothelial Cells: Evidence for Polyphenols Uptake and Protective Effect. Int. J. Mol. Sci. 2021, 22, 1385. [Google Scholar] [CrossRef]
- Unno, K.; Pervin, M.; Nakagawa, A.; Iguchi, K.; Hara, A.; Takagaki, A.; Nanjo, F.; Minami, A.; Nakamura, Y. Blood-Brain Barrier Permeability of Green Tea Catechin Metabolites and Their Neuritogenic Activity in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and Its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, H.-J.; Kornmann, F.; Fuhrmann, G.F. The Inhibitory Effects of Flavonoids and Antiestrogens on the Glut1 Glucose Transporter in Human Erythrocytes. Chem. Biol. Interact. 2003, 146, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Vlachodimitropoulou, E.; Sharp, P.A.; Naftalin, R.J. Quercetin–Iron Chelates Are Transported via Glucose Transporters. Free Radic. Biol. Med. 2011, 50, 934–944. [Google Scholar] [CrossRef]
- Yousof Ali, M.; Jung, H.A.; Choi, J.S. Anti-Diabetic and Anti-Alzheimer’s Disease Activities of Angelica Decursiva. Arch. Pharm. Res. 2015, 38, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Li, X.; He, L.; Zheng, Y.; Lu, H.; Li, J.; Zhong, L.; Tong, R.; Jiang, Z.; Shi, J.; et al. Antidiabetic Potential of Flavonoids from Traditional Chinese Medicine: A Review. Am. J. Chin. Med. 2019, 47, 933–957. [Google Scholar] [CrossRef]
- Azevedo, C.; Correia-Branco, A.; Araújo, J.R.; Guimarães, J.T.; Keating, E.; Martel, F. The Chemopreventive Effect of the Dietary Compound Kaempferol on the MCF-7 Human Breast Cancer Cell Line Is Dependent on Inhibition of Glucose Cellular Uptake. Nutr. Cancer 2015, 67, 504–513. [Google Scholar] [CrossRef]
- Gunnink, L.K.; Alabi, O.D.; Kuiper, B.D.; Gunnink, S.M.; Schuiteman, S.J.; Strohbehn, L.E.; Hamilton, K.E.; Wrobel, K.E.; Louters, L.L. Curcumin Directly Inhibits the Transport Activity of GLUT1. Biochimie 2016, 125, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Xia, M.; Ye, Z.; Shi, Y.; Zhou, L.; Hua, Y. Curcumin Improves Diabetes Mellitus-associated Cerebral Infarction by Increasing the Expression of GLUT1 and GLUT3. Mol. Med. Rep. 2018, 17, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Peeyush, K.T.; Gireesh, G.; Jobin, M.; Paulose, C.S. Neuroprotective Role of Curcumin in the Cerebellum of Streptozotocin-Induced Diabetic Rats. Life Sci. 2009, 85, 704–710. [Google Scholar] [CrossRef]
- Kumar, V.; Sachan, R.; Rahman, M.; Sharma, K.; Al-Abbasi, F.A.; Anwar, F. Prunus Amygdalus Extract Exert Antidiabetic Effect via Inhibition of DPP-IV: In-Silico and in-Vivo Approaches. J. Biomol. Struct. Dyn. 2021, 39, 4160–4174. [Google Scholar] [CrossRef]
- Chen, Q.; Mo, R.; Wu, N.; Zou, X.; Shi, C.; Gong, J.; Li, J.; Fang, K.; Wang, D.; Yang, D.; et al. Berberine Ameliorates Diabetes-Associated Cognitive Decline through Modulation of Aberrant Inflammation Response and Insulin Signaling Pathway in DM Rats. Front. Pharmacol. 2017, 8, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cok, A.; Plaisier, C.; Salie, M.J.; Oram, D.S.; Chenge, J.; Louters, L.L. Berberine Acutely Activates the Glucose Transport Activity of GLUT1. Biochimie 2011, 93, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- Ni, D.; Ai, Z.; Munoz-Sandoval, D.; Suresh, R.; Ellis, P.R.; Yuqiong, C.; Sharp, P.A.; Butterworth, P.J.; Yu, Z.; Corpe, C.P. Inhibition of the Facilitative Sugar Transporters (GLUTs) by Tea Extracts and Catechins. FASEB J. 2020, 34, 9995–10010. [Google Scholar] [CrossRef] [PubMed]
- Slavic, K.; Derbyshire, E.T.; Naftalin, R.J.; Krishna, S.; Staines, H.M. Comparison of Effects of Green Tea Catechins on Apicomplexan Hexose Transporters and Mammalian Orthologues. Mol. Biochem. Parasitol. 2009, 168, 113–116. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, C.S.; Choi, H.Y.; Yune, T.Y. Ginseng Extracts, GS-KG9 and GS-E3D, Prevent Blood-Brain Barrier Disruption and Thereby Inhibit Apoptotic Cell Death of Hippocampal Neurons in Streptozotocin-Induced Diabetic Rats. Nutrients 2020, 12, 2383. [Google Scholar] [CrossRef]
- Khatoon, A.; Rashid, I.; Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Pathak, N.; Mir, S.S.; Ahmad, K.; Hussain, T.; Srivastava, P. ADNCD: A Compendious Database on Anti-Diabetic Natural Compounds Focusing on Mechanism of Action. 3 Biotech 2018, 8, 361. [Google Scholar] [CrossRef]
- Madariaga-Mazón, A.; Naveja, J.J.; Medina-Franco, J.L.; Noriega-Colima, K.O.; Martinez-Mayorga, K. DiaNat-DB: A Molecular Database of Antidiabetic Compounds from Medicinal Plants. RSC Adv. 2021, 11, 5172–5178. [Google Scholar] [CrossRef]
- Pérez-Sánchez, H.; den-Haan, H.; Peña-García, J.; Lozano-Sánchez, J.; Martínez Moreno, M.E.; Sánchez-Pérez, A.; Muñoz, A.; Ruiz-Espinosa, P.; Pereira, A.S.P.; Katsikoudi, A.; et al. DIA-DB: A Database and Web Server for the Prediction of Diabetes Drugs. J. Chem. Inf. Model. 2020, 60, 4124–4130. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, L.; Lv, M.; Pei, R.; Li, P.; Pei, Z.; Wang, Y.; Su, W.; Xie, X.-Q. AlzPlatform: An Alzheimer’s Disease Domain-Specific Chemogenomics Knowledgebase for Polypharmacology and Target Identification Research. J. Chem. Inf. Model. 2014, 54, 1050–1060. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Patiyal, S.; Dhall, A.; Sharma, N.; Raghava, G.P.S. B3Pred: A Random-Forest-Based Method for Predicting and Designing Blood–Brain Barrier Penetrating Peptides. Pharmaceutics 2021, 13, 1237. [Google Scholar] [CrossRef] [PubMed]
- Shaker, B.; Yu, M.-S.; Song, J.S.; Ahn, S.; Ryu, J.Y.; Oh, K.-S.; Na, D. LightBBB: Computational Prediction Model of Blood–Brain-Barrier Penetration Based on LightGBM. Bioinformatics 2021, 37, 1135–1139. [Google Scholar] [CrossRef]
- Carpenter, T.S.; Kirshner, D.A.; Lau, E.Y.; Wong, S.E.; Nilmeier, J.P.; Lightstone, F.C. A Method to Predict Blood-Brain Barrier Permeability of Drug-Like Compounds Using Molecular Dynamics Simulations. Biophys. J. 2014, 107, 630–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thai, N.Q.; Theodorakis, P.E.; Li, M.S. Fast Estimation of the Blood–Brain Barrier Permeability by Pulling a Ligand through a Lipid Membrane. J. Chem. Inf. Model. 2020, 60, 3057–3067. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udrea, A.M.; Gradisteanu Pircalabioru, G.; Boboc, A.A.; Mares, C.; Dinache, A.; Mernea, M.; Avram, S. Advanced Bioinformatics Tools in the Pharmacokinetic Profiles of Natural and Synthetic Compounds with Anti-Diabetic Activity. Biomolecules 2021, 11, 1692. https://doi.org/10.3390/biom11111692
Udrea AM, Gradisteanu Pircalabioru G, Boboc AA, Mares C, Dinache A, Mernea M, Avram S. Advanced Bioinformatics Tools in the Pharmacokinetic Profiles of Natural and Synthetic Compounds with Anti-Diabetic Activity. Biomolecules. 2021; 11(11):1692. https://doi.org/10.3390/biom11111692
Chicago/Turabian StyleUdrea, Ana Maria, Gratiela Gradisteanu Pircalabioru, Anca Andreea Boboc, Catalina Mares, Andra Dinache, Maria Mernea, and Speranta Avram. 2021. "Advanced Bioinformatics Tools in the Pharmacokinetic Profiles of Natural and Synthetic Compounds with Anti-Diabetic Activity" Biomolecules 11, no. 11: 1692. https://doi.org/10.3390/biom11111692
APA StyleUdrea, A. M., Gradisteanu Pircalabioru, G., Boboc, A. A., Mares, C., Dinache, A., Mernea, M., & Avram, S. (2021). Advanced Bioinformatics Tools in the Pharmacokinetic Profiles of Natural and Synthetic Compounds with Anti-Diabetic Activity. Biomolecules, 11(11), 1692. https://doi.org/10.3390/biom11111692








