Gut-Derived Endotoxin and Telomere Length Attrition in Adults with and without Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometry and Blood Collection
2.3. Biochemical Analyses
2.4. Telomere Length (TL) Measurements
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Participants
3.2. Telomeric Length, Glycemic Profile, and Pro-Inflammatory Markers
3.3. Associations between Telomere Length and Endotoxin
3.4. Significant Predictors of TL
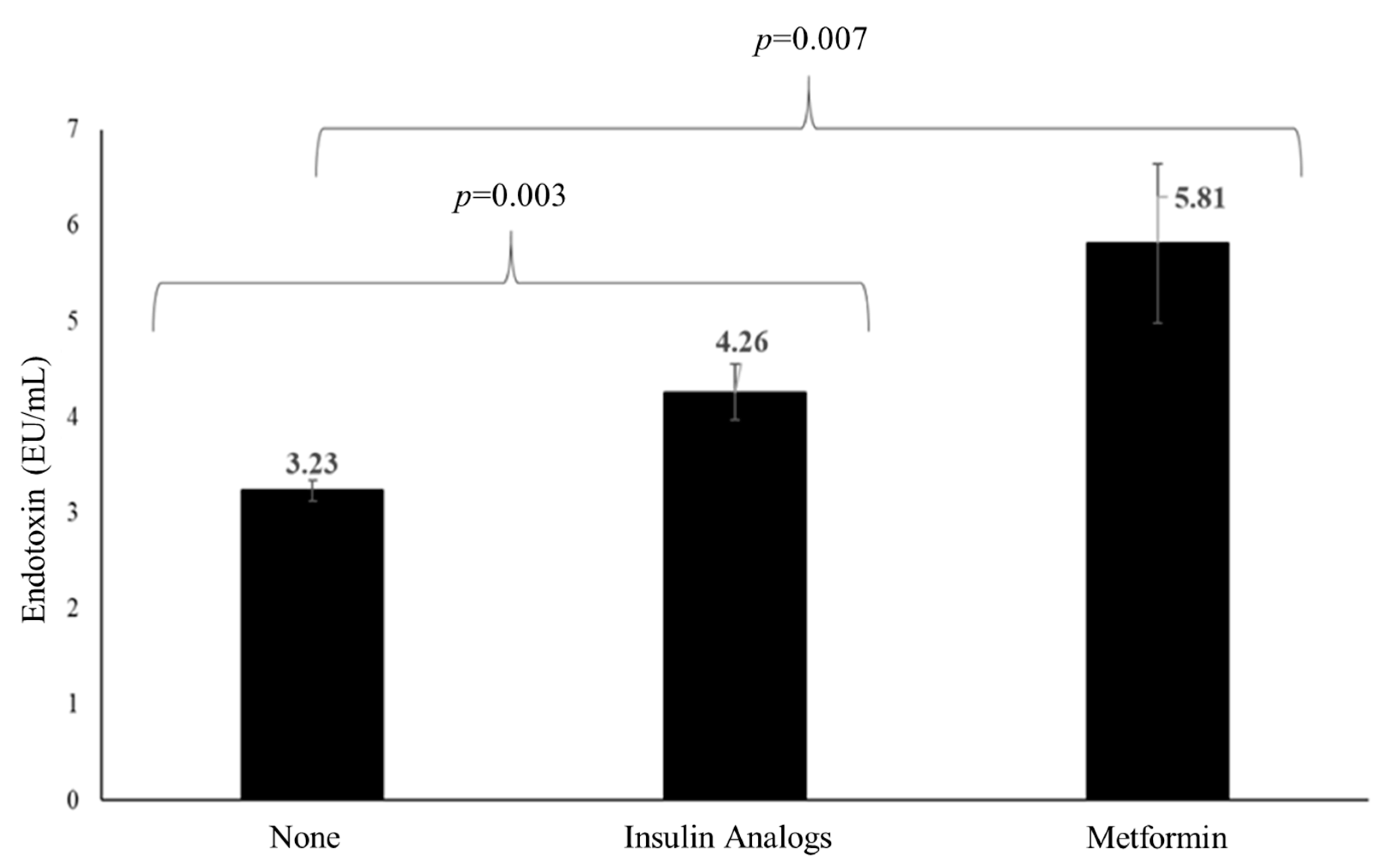
3.5. Effects of Medications on TL and Endotoxin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ozougwu, J.; Obimba, K.; Belonwu, C.; Unakalamba, C. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013, 4, 46–57. [Google Scholar] [CrossRef]
- Robert, A.A.; Al Dawish, M.A. The worrying trend of diabetes mellitus in Saudi Arabia: An urgent call to action. Curr. Diabetes Rev. 2020, 16, 204–210. [Google Scholar] [CrossRef]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, M.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef]
- Ahima, R.S. Connecting obesity, aging and diabetes. Nat. Med. 2009, 15, 996–997. [Google Scholar] [CrossRef] [PubMed]
- Al-Disi, D.A.; Al-Daghri, N.M.; Khan, N.; Alfadda, A.A.; Sallam, R.M.; Alsaif, M.; Sabico, S.; Tripathi, G.; McTernan, P.G. Postprandial Effect of a High-Fat Meal on Endotoxemia in Arab Women with and without Insulin-Resistance-Related Diseases. Nutrients 2015, 7, 6375–6389. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Ortiz, B.; Albarrán-Tamayo, F.; Arenas-Aranda, D.; Benítez-Bribiesca, L.; Malacara-Hernández, J.; Martínez-Garza, S.; Hernández-González, M.; Solorio, S.; Garay-Sevilla, M.; Mora-Villalpando, C. Telomere length and type 2 diabetes in males, a premature aging syndrome. Aging Male 2012, 15, 54–58. [Google Scholar] [CrossRef]
- Tamura, Y.; Takubo, K.; Aida, J.; Araki, A.; Ito, H. Telomere attrition and diabetes mellitus. Geriatr. Gerontol. Int. 2016, 16, 66–74. [Google Scholar] [CrossRef]
- Wang, J.; Dong, X.; Cao, L.; Sun, Y.; Qiu, Y.; Zhang, Y.; Cao, R.; Covasa, M.; Zhong, L. Association between telomere length and diabetes mellitus: A meta-analysis. J. Int. Med Res. 2016, 44, 1156–1173. [Google Scholar] [CrossRef]
- Cheng, F.; Carroll, L.; Joglekar, M.V.; Januszewski, A.S.; Wong, K.K.; Hardikar, A.A.; Jenkins, A.J.; Ma, R.C. Diabetes, metabolic disease, and telomere length. Lancet Diabetes Endocrinol. 2020, 9, 117–126. [Google Scholar] [CrossRef]
- Harte, A.L.; Da Silva, N.F.; Miller, M.A.; Cappuccio, F.P.; Kelly, A.; O’Hare, J.P.; Barnett, A.H.; Al-Daghri, N.M.; Al-Attas, O.; Alokail, M.; et al. Telomere length attrition, a marker of biological senescence, is inversely correlated with triglycerides and cholesterol in South Asian males with type 2 diabetes mellitus. Exp. Diabetes Res. 2012, 2012, 895185. [Google Scholar] [CrossRef]
- Chan, W.; Bosch, J.A.; Phillips, A.C.; Chin, S.H.; Antonysunil, A.; Inston, N.; Moore, S.; Kaur, O.; McTernan, P.G.; Borrows, R. The Associations of Endotoxemia with Systemic Inflammation, Endothelial Activation, and Cardiovascular Outcome in Kidney Transplantation. J. Ren. Nutr. 2018, 28, 13–27. [Google Scholar] [CrossRef]
- Harte, A.L.; Varma, M.C.; Tripathi, G.; McGee, K.C.; Al-Daghri, N.M.; Al-Attas, O.S.; Sabico, S.; O’Hare, J.P.; Ceriello, A.; Saravanan, P.; et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care 2012, 35, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Gkranias, N.; Li, K.; Salpea, K.D.; Parkar, M.; Orlandi, M.; Suvan, J.E.; Eng, H.L.; Taddei, S.; Patel, K. Association between short leukocyte telomere length, endotoxemia, and severe periodontitis in people with diabetes: A cross-sectional survey. Diabetes Care 2014, 37, 1140–1147. [Google Scholar] [CrossRef]
- Ly, N.P.; Litonjua, A.; Gold, D.R.; Celedón, J.C. Gut microbiota, probiotics, and vitamin d: Interrelated exposures influencing allergy, asthma, and obesity? J. Allergy Clin. Immunol. 2011, 127, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- de la Escalera, L.M.; Kyrou, I.; Vrbikova, J.; Hainer, V.; Sramkova, P.; Fried, M.; Piya, M.K.; Kumar, S.; Tripathi, G.; McTernan, P.G. Impact of gut hormone fgf-19 on type-2 diabetes and mitochondrial recovery in a prospective study of obese diabetic women undergoing bariatric surgery. BMC Med. 2017, 15, 1–9. [Google Scholar]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ahmed Ansari, M.G.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Yakout, S.; Alnaami, A.M.; Alokail, M.S.; McTernan, P.G. Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naïve T2DM patients: A randomized clinical trial. J. Transl. Med. 2017, 15, 249. [Google Scholar] [CrossRef]
- Zeng, J.B.; Liu, H.B.; Ping, F.; Li, W.; Li, Y.X. Insulin treatment affects leukocyte telomere length in patients with type 2 diabetes: 6-year longitudinal study. J. Diabetes Its Complicat. 2019, 33, 363–367. [Google Scholar] [CrossRef]
- Fasching, C.L. Telomere length measurement as a clinical biomarker of aging and disease. Crit. Rev. Clin. Lab. Sci. 2018, 55, 443–465. [Google Scholar] [CrossRef]
- Shin, Y.-A. How does obesity and physical activity affect aging?: Focused on telomere as a biomarker of aging. J. Obes. Metab. Syndr. 2019, 28, 92. [Google Scholar] [CrossRef]
- Ledda, C.; Loreto, C.; Rapisarda, V. Telomere length as a biomarker of biological aging in shift workers. Appl. Sci. 2020, 10, 2764. [Google Scholar] [CrossRef]
- Al-Attas, O.S.; Al-Daghri, N.M.; Alokail, M.S.; Alfadda, A.; Bamakhramah, A.; Sabico, S.; Pritlove, D.; Harte, A.; Tripathi, G.; McTernan, P.G. Adiposity and insulin resistance correlate with telomere length in middle-aged Arabs: The influence of circulating adiponectin. Eur. J. Endocrinol. 2010, 163, 601–607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gardner, J.P.; Li, S.; Srinivasan, S.R.; Chen, W.; Kimura, M.; Lu, X.; Berenson, G.S.; Aviv, A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation 2005, 111, 2171–2177. [Google Scholar] [CrossRef]
- Tzanetakou, I.P.; Katsilambros, N.L.; Benetos, A.; Mikhailidis, D.P.; Perrea, D.N. “Is obesity linked to aging?”: Adipose tissue and the role of telomeres. Ageing Res. Rev. 2012, 11, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Postigo, M.; Oliva-Olivera, W.; Coin-Aragüez, L.; Ramos-Molina, B.; Giraldez-Perez, R.M.; Lhamyani, S.; Alcaide-Torres, J.; Perez-Martinez, P.; El Bekay, R.; Cardona, F. Metabolic endotoxemia promotes adipose dysfunction and inflammation in human obesity. Am. J. Physiol. -Endocrinol. Metab. 2019, 316, E319–E332. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Yousef, M.; Sabico, S.L.; Chrousos, G.P. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): A decade of an epidemic. BMC Med. 2011, 9, 76. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Clerici, M.; Al-Attas, O.; Forni, D.; Alokail, M.S.; Alkharfy, K.M.; Sabico, S.; Mohammed, A.K.; Cagliani, R.; Sironi, M. A nonsense polymorphism (R392X) in TLR5 protects from obesity but predisposes to diabetes. J. Immunol. 2013, 190, 3716–3720. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Wani, K.; Alnaami, A.M.; Sabico, S.; Al-Ajlan, A.; Chrousos, G.P.; Alokail, M.S. Sensitivity of various adiposity indices in identifying cardiometabolic diseases in Arab adults. Cardiovasc. Diabetol. 2015, 14, 101. [Google Scholar] [CrossRef]
- Matthews, D.; Hosker, J.; Rudenski, A.; Naylor, B.; Treacher, D.; Turner, R. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Raschenberger, J.; Heydon, E.E.; Tsimikas, S.; Haun, M.; Mayr, A.; Weger, S.; Witztum, J.L.; Butterworth, A.S.; Willeit, J. Leucocyte telomere length and risk of type 2 diabetes mellitus: New prospective cohort study and literature-based meta-analysis. PLoS ONE 2014, 9, e112483. [Google Scholar]
- Rosa, E.C.C.C.; Dos Santos, R.R.C.; Fernandes, L.F.A.; Neves, F.d.A.R.; Coelho, M.S.; Amato, A.A. Leukocyte telomere length correlates with glucose control in adults with recently diagnosed type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 135, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Bonfigli, A.R.; Spazzafumo, L.; Prattichizzo, F.; Bonafè, M.; Mensà, E.; Micolucci, L.; Giuliani, A.; Fabbietti, P.; Testa, R.; Boemi, M. Leukocyte telomere length and mortality risk in patients with type 2 diabetes. Oncotarget 2016, 7, 50835. [Google Scholar] [CrossRef]
- Demissie, S.; Levy, D.; Benjamin, E.J.; Cupples, L.A.; Gardner, J.P.; Herbert, A.; Kimura, M.; Larson, M.G.; Meigs, J.B.; Keaney, J.F. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the framingham heart study. Aging Cell 2006, 5, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M. Gender and telomere length: Systematic review and meta-analysis. Exp. Gerontol. 2014, 51, 15–27. [Google Scholar] [CrossRef]
- Nawrot, T.S.; Staessen, J.A.; Gardner, J.P.; Aviv, A. Telomere length and possible link to x chromosome. Lancet 2004, 363, 507–510. [Google Scholar] [CrossRef]
- Borrás, C.; Sastre, J.; García-Sala, D.; Lloret, A.; Pallardó, F.V.; Viña, J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free. Radic. Biol. Med. 2003, 34, 546–552. [Google Scholar] [CrossRef]
- Vina, J.; Borras, C.; Gambini, J.; Sastre, J.; Pallardo, F.V. Why females live longer than males: Control of longevity by sex hormones. Sci. Aging Knowl. Environ. SAGE KE 2005, 2005, pe17. [Google Scholar] [CrossRef]
- Baruah, M. C-reactive protein (crp) and markers of oxidative stress in acute myocardial infarction. In Clinical Significance of C-reactive Protein; Springer: Berlin/Heidelberg, Germany, 2020; pp. 95–115. [Google Scholar]
- Farzaneh-Far, R.; Cawthon, R.M.; Na, B.; Browner, W.S.; Schiller, N.B.; Whooley, M.A. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: Data from the heart and soul study. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Li, S.-H.; Szmitko, P.E.; Fedak, P.W.; Verma, S. C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2476–2482. [Google Scholar] [CrossRef] [PubMed]
- McTernan, C.L.; McTernan, P.G.; Harte, A.L.; Levick, P.; Barnett, A.H.; Kumar, S. Resistin-The link between central obesity and type 2 diabetes. Lancet 2002, 35, 46–47. [Google Scholar] [CrossRef]
- McTernan, P.G.; McTernan, C.L.; Chetty, R.; Jenner, K.; Fisher, F.M.; Lauer, M.N.; Crocker, J.; Barnett, A.H.; Kumar, S. Increased Resistin Gene and Protein Expression in Human Abdominal Adipose Tissue. J. Clin. Endocrinol. Metab. 2002, 87, 2407–2410. [Google Scholar] [CrossRef]
- Choe, J.-Y.; Bae, J.; Jung, H.-Y.; Park, S.-H.; Lee, H.-J.; Kim, S.-K. Serum resistin level is associated with radiographic changes in hand osteoarthritis: Cross-sectional study. Jt. Bone Spine 2012, 79, 160–165. [Google Scholar] [CrossRef]
- Al-Attas, O.S.; Al-Daghri, N.M.; Al-Rubeaan, K.; da Silva, N.F.; Sabico, S.L.; Kumar, S.; McTernan, P.G.; Harte, A.L. Changes in endotoxin levels in T2DM subjects on anti-diabetic therapies. Cardiovasc. Diabetol. 2009, 8, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Aoki, M.; Yamada, K. Leukocyte Telomere Length and Serum Levels of High-Molecular-Weight Adiponectin and Dehydroepiandrosterone-Sulfate Could Reflect Distinct Aspects of Longevity in Japanese Centenarians. Gerontol. Geriatr. Med. 2017, 3, 2333721417696672. [Google Scholar] [CrossRef]
- Ohman-Hanson, R.A.; Cree-Green, M.; Kelsey, M.M.; Bessesen, D.H.; Sharp, T.A.; Pyle, L.; Pereira, R.I.; Nadeau, K.J. Ethnic and Sex Differences in Adiponectin: From Childhood to Adulthood. J. Clin. Endocrinol. Metab. 2016, 101, 4808–4815. [Google Scholar] [CrossRef]
- Bonneau, G.A.; Pedrozo, W.R.; Berg, G. Adiponectin and waist circumference as predictors of insulin-resistance in women. Diabetes Metab. Syndr. Clin. Res. Rev. 2014, 8, 3–7. [Google Scholar] [CrossRef]
- Wasim, H.; Al-Daghri, N.M.; Chetty, R.; McTernan, P.G.; Barnett, A.; Kumar, S. Relationship of serum adiponectin and resistin to glucose intolerance and fat topography in south-asians. Cardiovasc. Diabetol. 2006, 5, 1–5. [Google Scholar] [CrossRef]
- Khalangot, M.; Krasnienkov, D.; Vaiserman, A. Telomere length in different metabolic categories: Clinical associations and modification potential. Exp. Biol. Med. 2020, 245, 1115–1121. [Google Scholar] [CrossRef]
- Gurung, R.L.; Liu, S.; Liu, J.J.; Chan, S.M.; Moh, M.C.; Ang, K.; Tang, W.E.; Sum, C.F.; Tavintharan, S.; Lim, S.C. Ethnic disparities in relationships of obesity indices with telomere length in asians with type 2 diabetes. J. Diabetes 2019, 11, 386–393. [Google Scholar] [CrossRef] [PubMed]
- El Mouzan, M.I.; Foster, P.J.; Al Herbish, A.S.; Al Salloum, A.A.; Al Omer, A.A.; Qurachi, M.M.; Kecojevic, T. Prevalence of overweight and obesity in saudi children and adolescents. Ann. Saudi Med. 2010, 30, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, T.A.; Meireles, G.S.; Dias, A.T.; Aires, R.; Porto, M.L.; Gava, A.L.; Vasquez, E.C.; Pereira, T.M.C.; Campagnaro, B.P.; Meyrelles, S.S. Increased ros production and DNA damage in monocytes are biomarkers of aging and atherosclerosis. Biol. Res. 2018, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; McTernan, P.G.; Harte, A.L.; Silva, N.F.; Strazzullo, P.; Alberti, K.G.; Kumar, S.; Cappuccio, F.P. Ethnic and sex differences in circulating endotoxin levels: A novel marker of atherosclerotic and cardiovascular risk in a British multi-ethnic population. Atherosclerosis 2009, 203, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Gubbels Bupp, M.R.; Potluri, T.; Fink, A.L.; Klein, S.L. The Confluence of Sex Hormones and Aging on Immunity. Front. Immunol. 2018, 9, 1269. [Google Scholar] [CrossRef]
- Aulock, S.v.; Deininger, S.; Draing, C.; Gueinzius, K.; Dehus, O.; Hermann, C. Gender difference in cytokine secretion on immune stimulation with lps and lta. J. Interferon Cytokine Res. 2006, 26, 887–892. [Google Scholar] [CrossRef]
| Parameters | All | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ND Control | T2DM | p-Value | ND Control | T2DM | p-Value | ND Control | T2DM | p-Value | |
| N | 417 | 387 | 183 | 202 | 234 | 185 | |||
| Obese (%) | 207 (54.5) | 195 (52.4) | 0.85 | 67 (39.4) | 73 (36.1) | 0.57 | 140 (66.7) | 122 (67.8) | 0.47 |
| Hypertensive (%) | 129 (30.9) | 165 (42.6) | 0.001 | 53 (29.0) | 77 (38.1) | 0.07 | 76 (32.5) | 88 (47.6) | 0.002 |
| Insulin Analogs (%) | - | 85 (10.6) | - | 48 (12.5) | - | 37 (8.8) | |||
| Metformin (%) | - | 23 (2.9) | - | 14 (3.6) | - | 9 (2.1) | |||
| Statins (%) | - | 17 (2.1) | - | 13 (3.4) | - | 4 (1.0) | |||
| Antihypertensive (%) | 11 (2.6) | 31 (8.0) | <0.001 | 5 (2.7) | 16 (7.9) | <0.001 | 6 (2.6) | 15 (8.1) | <0.001 |
| Aspirin (%) | 4 (0.2) | 32 (8.3) | <0.001 | 3 (1.5) | 23 (12.6) | <0.001 | 1 (0.4) | 9 (4.9) | <0.001 |
| Parameters | All | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ND Control | T2DM | p-Value | ND Control | T2DM | p-Value | ND Control | T2DM | p-Value | |
| N | 417 | 387 | 183 | 202 | 234 | 185 | |||
| Age (years) | 55.7 ± 7.4 | 58.7 ± 8.1 | <0.001 | 55.9 ± 8.4 | 60.9 ± 8.4 | <0.001 | 55.5 ± 6.5 | 56.3 ± 6.9 | 0.26 |
| BMI (kg/m2) | 30.9 ± 5.6 | 30.6 ± 5.4 | 0.52 | 28.5 ± 4.7 | 29.1 ± 4.8 | 0.27 | 32.8 ± 5.5 | 32.3 ± 5.5 | 0.36 |
| WHR | 0.94 ± 0.1 | 0.96 ± 0.1 | <0.001 | 0.96 ± 0.07 | 1.0 ± 0.06 | <0.001 | 0.91 ± 0.09 | 0.91 ± 0.07 | 0.96 |
| SBP (mmHg) | 125.1 ± 14.3 | 132.4 ± 14.5 | <0.001 | 125.9 ± 13.9 | 132.3 ± 12.9 | <0.001 | 124.5 ± 14.5 | 132.4 ± 15.9 | <0.001 |
| DBP (mmHg) | 77.9 ± 9.6 | 80.2 ± 8.7 | 0.001 | 79.8 ± 7.8 | 81.2 ± 6.0 | 0.09 | 76.4 ± 10.5 | 79.3 ± 10.6 | 0.007 |
| Total Cholesterol | 5.04 ± 1.1 | 5.07 ± 1.2 | 0.64 | 5.1 ± 1.2 | 4.9 ± 1.1 | 0.28 | 4.9 ± 0.9 | 5.2 ± 1.2 | 0.06 |
| HDL Cholesterol | 1.0 ± 0.3 | 0.99 ± 0.3 | 0.88 | 0.84 ± 0.3 | 0.92 ± 0.3 | 0.004 | 1.1 ± 0.3 | 1.1 ± 0.2 | 0.18 |
| LDL Cholesterol | 3.2 ± 0.9 | 3.1 ± 0.9 | 0.46 | 3.4 ± 1.0 | 3.1 ± 0.9 | 0.02 | 3.1 ± 0.8 | 3.2 ± 0.9 | 0.22 |
| LDL/HDL ratio | 3.7 ± 2.5 | 3.5 ± 1.5 | 0.13 | 4.4 ± 2.1 | 3.7 ± 1.6 | <0.001 | 3.2 ± 2.6 | 3.2 ± 1.3 | 0.86 |
| Triglycerides # | 1.5 (1.2–2.1) | 1.7 (1.3–2.3) | 0.002 | 1.7 (1.3–2.3) | 1.7 (1.2–2.3) | 0.61 | 1.4 (1.1–2.0) | 1.7 (1.3–2.4) | <0.001 |
| Parameters | All | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ND Control | T2DM | p-Value | ND Control | T2DM | p-Value | ND Control | T2DM | p-Value | |
| N | 417 | 387 | 234 | 185 | 183 | 202 | |||
| TL (BP) | 5880 ± 1464 | 5554 ± 1346 | <0.001 | 5830 ± 1705 | 5435 ± 1380 | 0.02 | 5912.3 ± 1290 | 5686 ± 199 | 0.08 |
| Glucose (mmol/L) | 5.7 ± 0.9 | 10.2 ± 3.1 | <0.001 | 5.8 ± 0.9 | 10.0 ± 3.0 | <0.001 | 5.6 ± 0.8 | 10.3 ± 3.2 | <0.001 |
| Insulin (μU/mL) | 11.1 (6.9–16.3) | 10.1 (6.5–16.1) | 0.14 | 10.3 (6.8–16.8) | 9.7 (6.3–16.0) | 0.812 | 11.8 (7.2–16.3) | 10.4 (6.5–16.1) | 0.087 |
| HOMA-IR | 2.8 (1.7–4.5) | 4.2 (2.7–7.6) | <0.001 | 2.5 (1.5–4.6) | 3.9 (2.7–7.6) | <0.001 | 2.9 (1.9–4.4) | 4.3 (2.7–7.4) | <0.001 |
| Endotoxin (IU/mL) | 3.0 (1.8–4.8) | 2.5 (1.7–4.1) | 0.03 | 2.6 (1.7–4.1) | 2.5 (1.7–4.6) | 0.56 | 3.2 (1.8–4.9) | 2.4 (1.8–3.8) | 0.03 |
| Adiponectin (ug/mL) | 13.5 (5.4–24.9) | 13.9 (8.5–20.2) | 0.38 | 3.4 (1.0–13.1) | 11.8 (8–17) | <0.001 | 18.6 (11.9–34) | 15.8 (10.1–25) | 0.002 |
| Resistin (ng/mL) | 27.3 (2.7–114) | 28.4 (20–41) | 0.97 | 2.2 (1.2–10.1) | 25.7 (18.2–38) | <0.001 | 96.9 (31.4–168) | 30.9 (21.2–43) | <0.001 |
| PAI-1 (ng/mL) | 6.9 (0.3–10.4) | 10.1 (7.4–12.7) | <0.001 | 0.3 (0.1–4.4) | 10.1 (7.4–17) | <0.001 | 9.4 (6.8–12.4) | 10.0 (7.6–12.7) | 0.048 |
| Leptin (ng/mL) | 16.6 (6.4–32.2) | 14.7 (8.3–26.4) | 0.56 | 10.7 (4.7–23.8) | 10.8 (6.2–16.7) | 0.95 | 22.3 (10.5–37) | 20.9 (12.4–33) | 0.99 |
| ANG-II (pg/mL) | 0.9 (0.4–1.2) | 0.2 (0.1–0.4) | <0.001 | 1.0 (0.6–1.3) | 0.2 (0.1–0.5) | <0.001 | 0.20 (0.08–0.3) | 0.21 (0.1–0.4) | 0.18 |
| TNF-α (pg/mL) | 2.4 (1.3–6.1) | 1.3 (0.9–2.1) | <0.001 | 3.7 (1.5–7.2) | 1.3 (0.9–1.9) | <0.001 | 2.0 (1.3–3.4) | 1.4 (0.9–2.1) | <0.001 |
| CRP (ug/mL) | 2.0 (0.8–4.5) | 2.9 (0.9–6.0) | 0.06 | 2.8 (0.9–6.5) | 3.0 (1.5–6.0) | 0.55 | 1.5 (0.7–2.5) | 2.8 (0.2–5.6) | 0.02 |
| Parameters | All | All | Males | Females | |||
|---|---|---|---|---|---|---|---|
| ND Control | T2DM | ND Control | T2DM | ND Control | T2DM | ||
| N (M/F) | 804 | 417 (183/234) | 387 (202/185) | 183 | 202 | 234 | 185 |
| Age (years) | 0.24 ** | ||||||
| WHR | −0.16 * | ||||||
| Glucose (mmol/L) | 0.20 ** | 0.24 ** | |||||
| Total Cholesterol | −0.08 * | −0.10 * | |||||
| HDL Cholesterol | −0.08 * | ||||||
| LDL/HDL ratio | −0.13 * | ||||||
| Triglycerides # | 0.15 * | ||||||
| HOMA-IR | 0.14 * | 0.20 * | |||||
| Endotoxin (IU/mL) | −0.17 ** | −0.27 ** | −0.18 ** | −0.24 ** | −0.29 ** | ||
| Adiponectin (ug/mL) | 0.18 * | 0.23 * | |||||
| Resistin (ng/mL) | 0.15 * | 0.20 * | |||||
| PAI-1 (ng/mL) | 0.18 * | 0.25 ** | 0.32** | 0.27 ** | 0.25 * | ||
| TNF-α (pg/mL) | −0.15 * | ||||||
| CRP (ug/mL) | −0.14 * | −0.15 * | −0.25 ** | ||||
| Parameters | N | Significant Predictors of TL |
|---|---|---|
| All Participants | 804 | Endotoxin, Triglycerides, BMI R2 = 0.18; p < 0.001 |
| T2DM | 387 | Endotoxin, Glucose R2 = 0.21; p = 0.002 |
| ND Control | 417 | Glucose, BMI R2 = 0.56; p < 0.001 |
| Male T2DM | 185 | Endotoxin R2 = 0.19; p = 0.007 |
| Male ND Control | 235 | BMI and Glucose R2 = 0.76; p = 0.001 |
| Female T2DM | 202 | HbA1c R2 = 0.68; p = 0.14 |
| Female ND Control | 183 | Glucose R2 = 0.13; p = 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Daghri, N.M.; Abdi, S.; Sabico, S.; Alnaami, A.M.; Wani, K.A.; Ansari, M.G.A.; Khattak, M.N.K.; Khan, N.; Tripathi, G.; Chrousos, G.P.; et al. Gut-Derived Endotoxin and Telomere Length Attrition in Adults with and without Type 2 Diabetes. Biomolecules 2021, 11, 1693. https://doi.org/10.3390/biom11111693
Al-Daghri NM, Abdi S, Sabico S, Alnaami AM, Wani KA, Ansari MGA, Khattak MNK, Khan N, Tripathi G, Chrousos GP, et al. Gut-Derived Endotoxin and Telomere Length Attrition in Adults with and without Type 2 Diabetes. Biomolecules. 2021; 11(11):1693. https://doi.org/10.3390/biom11111693
Chicago/Turabian StyleAl-Daghri, Nasser M., Saba Abdi, Shaun Sabico, Abdullah M. Alnaami, Kaiser A. Wani, Mohammed G. A. Ansari, Malak Nawaz Khan Khattak, Nasiruddin Khan, Gyanendra Tripathi, George P. Chrousos, and et al. 2021. "Gut-Derived Endotoxin and Telomere Length Attrition in Adults with and without Type 2 Diabetes" Biomolecules 11, no. 11: 1693. https://doi.org/10.3390/biom11111693
APA StyleAl-Daghri, N. M., Abdi, S., Sabico, S., Alnaami, A. M., Wani, K. A., Ansari, M. G. A., Khattak, M. N. K., Khan, N., Tripathi, G., Chrousos, G. P., & McTernan, P. G. (2021). Gut-Derived Endotoxin and Telomere Length Attrition in Adults with and without Type 2 Diabetes. Biomolecules, 11(11), 1693. https://doi.org/10.3390/biom11111693









