Solving the Puzzle: What Is the Role of Progestogens in Neovascularization?
Abstract
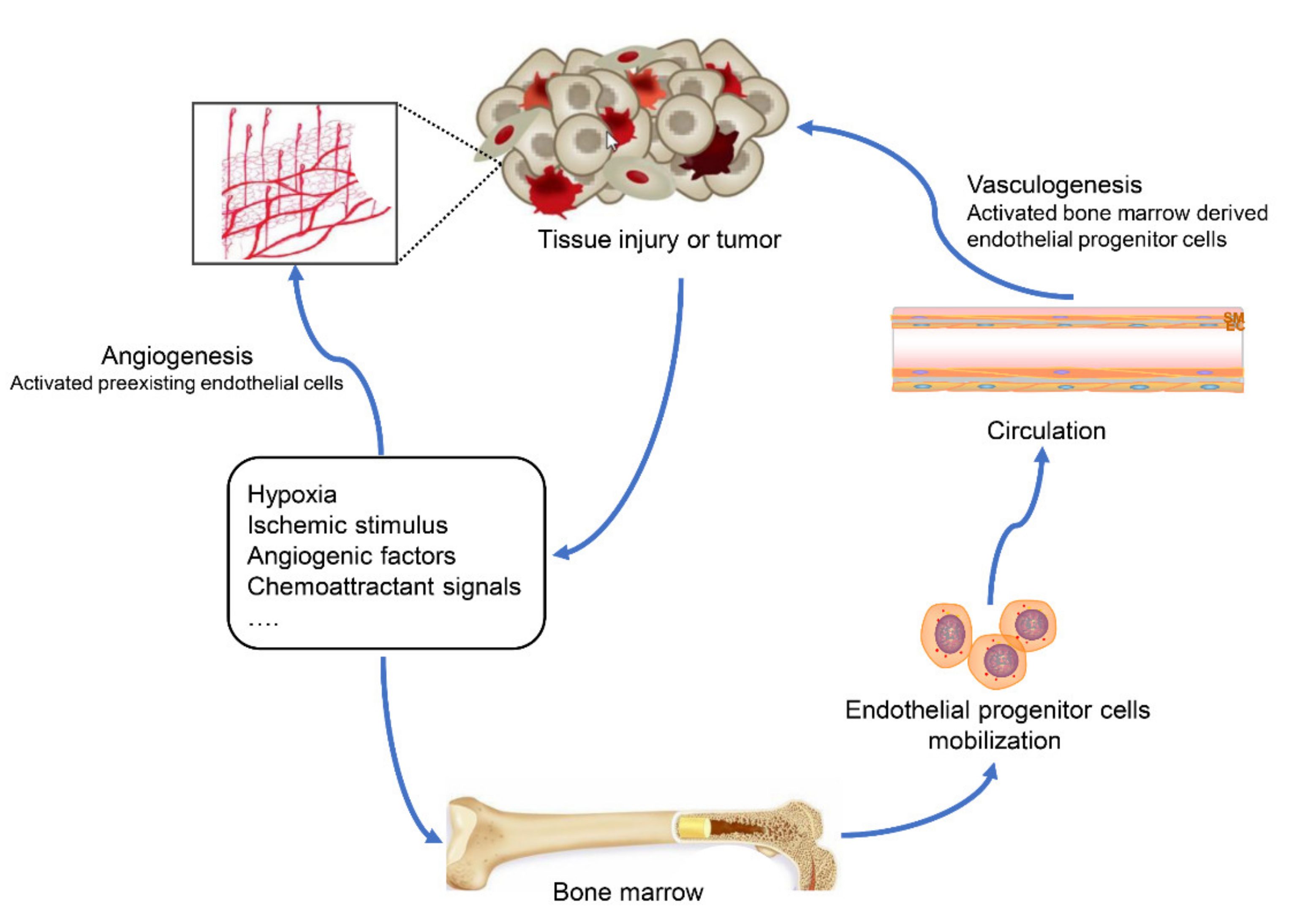
1. Introduction
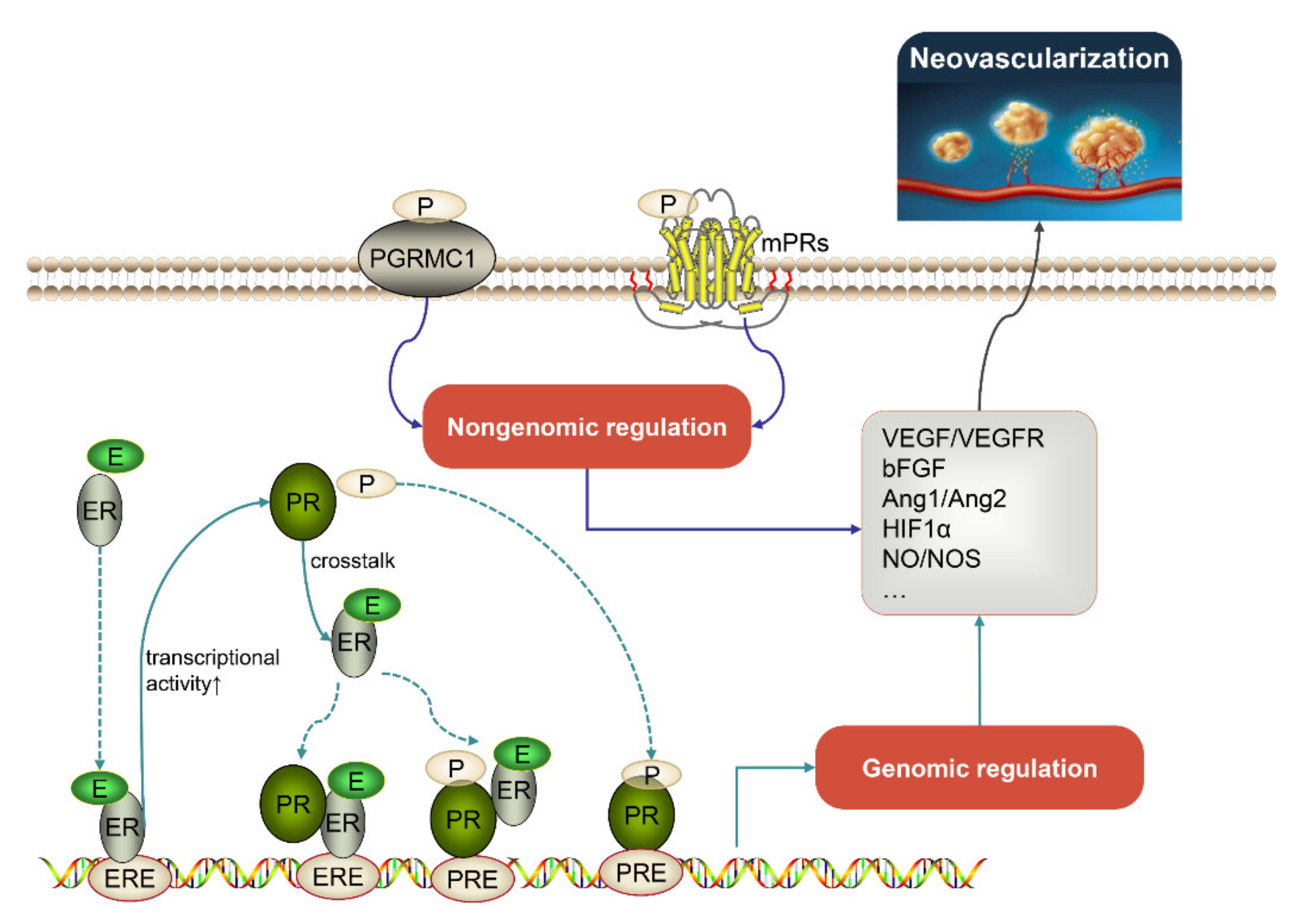
2. Downstream Factors Involved in Progestogen-Mediated Neovascularization
2.1. Vascular Endothelial Growth Factor (VEGF)
2.2. Basic Fibroblast Growth Factor (bFGF)
2.3. Platelet-Derived Endothelial Cell Growth Factor (PD-ECGF)
2.4. Angiopoietin (Ang)
2.5. Hypoxia Inducible Factor 1α (HIF1α)
2.6. Nitric Oxide Synthase (NOS)
2.7. Other Downstream Factors
3. Progestogen-Mediated Physiological Neovascularization
4. Progestogen-Mediated Pathological Neovascularization
4.1. Endometrial Cancer
4.2. Breast Cancer
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ribatti, D.; Pezzella, F. Overview on the Different Patterns of Tumor Vascularization. Cells 2021, 10, 639. [Google Scholar] [CrossRef] [PubMed]
- Demir, R.; Yaba, A.; Huppertz, B. Vasculogenesis and angiogenesis in the endometrium during menstrual cycle and implantation. Acta Histochem. 2010, 112, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; King, A.; Crombleholme, T.M.; Keswani, S.G. The Role of Endothelial Progenitor Cells in Postnatal Vasculogenesis: Implications for Therapeutic Neovascularization and Wound Healing. Adv. Wound Care 2013, 2, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M. Vasculogenesis: A crucial player in the resistance of solid tumours to radiotherapy. Br. J. Radiol. 2014, 87, 20130686. [Google Scholar] [CrossRef] [PubMed]
- Perrot-Applanat, M.; Ancelin, M.; Buteau-Lozano, H.; Meduri, G.; Bausero, P. Ovarian steroids in endometrial angiogenesis. Steroids 2000, 65, 599–603. [Google Scholar] [CrossRef]
- Bharti, J.N.; Rani, P.; Kamal, V.; Agarwal, P.N. Angiogenesis in Breast Cancer and its Correlation with Estrogen, Progesterone Receptors and other Prognostic Factors. J. Clin. Diagn. Res. 2015, 9, Ec05–Ec07. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Y.; Bay, B.H.; Aw, S.E.; Lin, V.C. A novel antiestrogenic mechanism in progesterone receptor-transfected breast cancer cells. J. Biol. Chem. 2005, 280, 17480–17487. [Google Scholar] [CrossRef]
- Taraborrelli, S. Physiology, production and action of progesterone. Acta Obstet. Gynecol. Scand. 2015, 94 (Suppl. S161), 8–16. [Google Scholar] [CrossRef]
- Singhal, H.; Greene, M.E.; Zarnke, A.L.; Laine, M.; Al Abosy, R.; Chang, Y.F.; Dembo, A.G.; Schoenfelt, K.; Vadhi, R.; Qiu, X.; et al. Progesterone receptor isoforms, agonists and antagonists differentially reprogram estrogen signaling. Oncotarget 2018, 9, 4282–4300. [Google Scholar] [CrossRef]
- Mohammed, H.; Russell, I.A.; Stark, R.; Rueda, O.M.; Hickey, T.E.; Tarulli, G.A.; Serandour, A.A.; Birrell, S.N.; Bruna, A.; Saadi, A.; et al. Progesterone receptor modulates ERα action in breast cancer. Nature 2015, 523, 313–317. [Google Scholar] [CrossRef]
- Daniel, A.R.; Gaviglio, A.L.; Knutson, T.P.; Ostrander, J.H.; D’Assoro, A.B.; Ravindranathan, P.; Peng, Y.; Raj, G.V.; Yee, D.; Lange, C.A. Progesterone receptor-B enhances estrogen responsiveness of breast cancer cells via scaffolding PELP1- and estrogen receptor-containing transcription complexes. Oncogene 2015, 34, 506–515. [Google Scholar] [CrossRef]
- Orshal, J.M.; Khalil, R.A. Gender, sex hormones, and vascular tone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R233–R249. [Google Scholar] [CrossRef]
- Edwards, D.P. Regulation of signal transduction pathways by estrogen and progesterone. Annu. Rev. Physiol. 2005, 67, 335–376. [Google Scholar] [CrossRef]
- Singhal, H.; Greene, M.E.; Tarulli, G.; Zarnke, A.L.; Bourgo, R.J.; Laine, M.; Chang, Y.F.; Ma, S.; Dembo, A.G.; Raj, G.V.; et al. Genomic agonism and phenotypic antagonism between estrogen and progesterone receptors in breast cancer. Sci. Adv. 2016, 2, e1501924. [Google Scholar] [CrossRef]
- Horwitz, K.B.; Sartorius, C.A. 90 YEARS OF PROGESTERONE: Progesterone and progesterone receptors in breast cancer: Past, present, future. J. Mol. Endocrinol. 2020, 65, T49–T63. [Google Scholar] [CrossRef]
- Trenti, A.; Tedesco, S.; Boscaro, C.; Trevisi, L.; Bolego, C.; Cignarella, A. Estrogen, Angiogenesis, Immunity and Cell Metabolism: Solving the Puzzle. Int. J. Mol. Sci. 2018, 19, 859. [Google Scholar] [CrossRef]
- Mints, M.; Jansson, M.; Sadeghi, B.; Westgren, M.; Uzunel, M.; Hassan, M.; Palmblad, J. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum. Reprod. 2008, 23, 139–143. [Google Scholar] [CrossRef]
- Bikfalvi, A. Angiogenesis: Molecular mechanisms of activation, promotion and maintenance. Off. J. Balk. Union Oncol. 2007, 12 (Suppl. S1), S59–S66. [Google Scholar]
- Krenning, G.; van Luyn, M.J.; Harmsen, M.C. Endothelial progenitor cell-based neovascularization: Implications for therapy. Trends Mol. Med. 2009, 15, 180–189. [Google Scholar] [CrossRef]
- Yu, P.; Li, S.; Zhang, Z.; Wen, X.; Quan, W.; Tian, Q.; Gao, C.; Su, W.; Zhang, J.; Jiang, R. Progesterone-mediated angiogenic activity of endothelial progenitor cell and angiogenesis in traumatic brain injury rats were antagonized by progesterone receptor antagonist. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef]
- Liu, L.H.; Lai, Y.; Linghu, L.J.; Liu, Y.F.; Zhang, Y. Effect of different concentrations of medroxy-progesterone acetate combined with 17β-estradiol on endothelial progenitor cells. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1790–1795. [Google Scholar]
- Ren, R.; Guo, J.; Shi, J.; Tian, Y.; Li, M.; Kang, H. PKM2 regulates angiogenesis of VR-EPCs through modulating glycolysis, mitochondrial fission, and fusion. J. Cell Physiol. 2020, 235, 6204–6217. [Google Scholar] [CrossRef] [PubMed]
- Shweiki, D.; Itin, A.; Neufeld, G.; Gitay-Goren, H.; Keshet, E. Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J. Clin. Investig. 1993, 91, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Lebovic, D.I.; Shifren, J.L.; Ryan, I.P.; Mueller, M.D.; Korn, A.P.; Darney, P.D.; Taylor, R.N. Ovarian steroid and cytokine modulation of human endometrial angiogenesis. Hum. Reprod. 2000, 15 (Suppl. S3), 67–77. [Google Scholar] [CrossRef] [PubMed]
- Ancelin, M.; Buteau-Lozano, H.; Meduri, G.; Osborne-Pellegrin, M.; Sordello, S.; Plouët, J.; Perrot-Applanat, M. A dynamic shift of VEGF isoforms with a transient and selective progesterone-induced expression of VEGF189 regulates angiogenesis and vascular permeability in human uterus. Proc. Natl. Acad. Sci. USA 2002, 99, 6023–6028. [Google Scholar] [CrossRef]
- Walter, L.M.; Rogers, P.A.; Girling, J.E. The role of progesterone in endometrial angiogenesis in pregnant and ovariectomised mice. Reproduction 2005, 129, 765–777. [Google Scholar] [CrossRef]
- Boroujeni, M.B.; Boroujeni, N.B.; Gholami, M. The effect of progesterone treatment after ovarian induction on endometrial VEGF gene expression and its receptors in mice at pre-implantation time. Iran. J. Basic Med. Sci. 2016, 19, 252–257. [Google Scholar]
- Lockwood, C.J.; Krikun, G.; Koo, A.B.; Kadner, S.; Schatz, F. Differential effects of thrombin and hypoxia on endometrial stromal and glandular epithelial cell vascular endothelial growth factor expression. J. Clin. Endocrinol. Metab. 2002, 87, 4280–4286. [Google Scholar] [CrossRef]
- Salmasi, S.; Sharifi, M.; Rashidi, B. Ovarian stimulation and exogenous progesterone affect the endometrial miR-16-5p, VEGF protein expression, and angiogenesis. Microvasc. Res. 2021, 133, 104074. [Google Scholar] [CrossRef]
- Salmasi, S.; Sharifi, M.; Rashidi, B. Evaluating the effect of ovarian stimulation and exogenous progesterone on CD31-positive cell density, VEGF protein, and miR-17-5p expression of endometrium immediately before implantation. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 133, 110922. [Google Scholar] [CrossRef]
- Cutini, P.H.; Massheimer, V.L. In vitro effects of progesterone and the synthetic progestin medroxyprogesterone acetate on vascular remodeling. Mol. Cell Endocrinol. 2019, 498, 110543. [Google Scholar] [CrossRef]
- Dingsheng, L.; Zengbing, L.; Dong, H. Favorable effects of progesterone on skin random flap survival in rats. Iran. J. Basic Med. Sci. 2016, 19, 1166–1170. [Google Scholar]
- Narimani, L.; Boroujeni, N.B.; Gholami, M.; Anbari, K.; Alavi, S.E.R.; Ahmadi, S.A.Y.; Boroujeni, M.B. Pre-Implantation Effects of Progesterone Administration on Ovarian Angiogenesis after Ovarian Stimulation: A Histological, Hormonal, and Molecular Analysis. JBRA Assist. Reprod. 2020, 24, 289–295. [Google Scholar] [CrossRef]
- Christensen, A.C.; Haresign, W.; Khalid, M. Progesterone exposure of seasonally anoestrous ewes alters the expression of angiogenic growth factors in preovulatory follicles. Theriogenology 2014, 81, 358–367. [Google Scholar] [CrossRef]
- Shimizu, T.; Miyamoto, A. Progesterone induces the expression of vascular endothelial growth factor (VEGF) 120 and Flk-1, its receptor, in bovine granulosa cells. Anim. Reprod. Sci. 2007, 102, 228–237. [Google Scholar] [CrossRef]
- Nichols, J.A.; Perego, M.C.; Schütz, L.F.; Hemple, A.M.; Spicer, L.J. Hormonal regulation of vascular endothelial growth factor A (VEGFA) gene expression in granulosa and theca cells of cattle1. J. Anim. Sci. 2019, 97, 3034–3045. [Google Scholar] [CrossRef]
- Trotter, A.; Kipp, M.; Schrader, R.M.; Beyer, C. Combined application of 17beta-estradiol and progesterone enhance vascular endothelial growth factor and surfactant protein expression in cultured embryonic lung cells of mice. Int. J. Pediatr. 2009, 2009, 170491. [Google Scholar] [CrossRef]
- Yuan, K.; Wing, L.C.; Lin, M.T. Pathogenetic Roles of Angiogenic Factors in Pyogenic Granulornas in Pregnancy Are Modulated by Female Sex Hormones. J. Periodontol. 2002, 73, 701–708. [Google Scholar] [CrossRef]
- Botelho, M.C.; Soares, R.; Alves, H. Progesterone in Breast Cancer Angiogenesis. SM J. Reprod. Health Infertil. 2015, 1, 1001. [Google Scholar]
- Fujimoto, J.; Toyoki, H.; Jahan, I.; Alam, S.M.; Sakaguchi, H.; Sato, E.; Tamaya, T. Sex steroid-dependent angiogenesis in uterine endometrial cancers. J. Steroid Biochem. Mol. Biol. 2005, 93, 161–165. [Google Scholar] [CrossRef]
- Hernández-Hernández, O.T.; González-García, T.K.; Camacho-Arroyo, I. Progesterone receptor and SRC-1 participate in the regulation of VEGF, EGFR and Cyclin D1 expression in human astrocytoma cell lines. J. Steroid Biochem. Mol. Biol. 2012, 132, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Garban, D.C.; Mah, V.; Alavi, M.; Maresh, E.L.; Chen, H.W.; Bagryanova, L.; Horvath, S.; Chia, D.; Garon, E.; Goodglick, L.; et al. Progesterone and estrogen receptor expression and activity in human non-small cell lung cancer. Steroids 2011, 76, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.; Herchenbach, D.; Pfisterer, J.; Breckwoldt, M. Effects of 17beta-estradiol and progesterone on interleukin-6 production and proliferation of human umbilical vein endothelial cells. Exp. Clin. Endocrinol. Diabetes 1998, 106, 334–339. [Google Scholar] [CrossRef]
- Okada, H.; Okamoto, R.; Tsuzuki, T.; Tsuji, S.; Yasuda, K.; Kanzaki, H. Progestins inhibit estradiol-induced vascular endothelial growth factor and stromal cell-derived factor 1 in human endometrial stromal cells. Fertil. Steril. 2011, 96, 786–791. [Google Scholar] [CrossRef]
- Al-Trad, B.; Ashankyty, I.M.; Alaraj, M. Progesterone ameliorates diabetic nephropathy in streptozotocin-induced diabetic Rats. Diabetol. Metab. Syndr. 2015, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Won, S.; Lee, J.H.; Wali, B.; Stein, D.G.; Sayeed, I. Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats: Involvement of the VEGF-MMP pathway. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2014, 34, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zuo, F.; Wang, Y.; Lu, H.; Yang, Q.; Wang, J. Progesterone Changes VEGF and BDNF Expression and Promotes Neurogenesis After Ischemic Stroke. Mol. Neurobiol. 2017, 54, 571–581. [Google Scholar] [CrossRef]
- Kim, M.; Park, H.J.; Seol, J.W.; Jang, J.Y.; Cho, Y.S.; Kim, K.R.; Choi, Y.; Lydon, J.P.; Demayo, F.J.; Shibuya, M.; et al. VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol. Med. 2013, 5, 1415–1430. [Google Scholar] [CrossRef]
- Hyder, S.M.; Chiappetta, C.; Stancel, G.M. Pharmacological and endogenous progestins induce vascular endothelial growth factor expression in human breast cancer cells. Int. J. Cancer 2001, 92, 469–473. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Kan, Z.; Zhang, B.; Yang, Z.; Chen, J.; Wang, D.; Wei, H.; Zhang, J.N.; Jiang, R. Progesterone increases circulating endothelial progenitor cells and induces neural regeneration after traumatic brain injury in aged rats. J. Neurotrauma 2012, 29, 343–353. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, Z.; Li, S.; Wen, X.; Quan, W.; Tian, Q.; Chen, J.; Zhang, J.; Jiang, R. Progesterone modulates endothelial progenitor cell (EPC) viability through the CXCL12/CXCR4/PI3K/Akt signalling pathway. Cell Prolif. 2016, 49, 48–57. [Google Scholar] [CrossRef]
- Kaya, H.S.; Hantak, A.M.; Stubbs, L.J.; Taylor, R.N.; Bagchi, I.C.; Bagchi, M.K. Roles of progesterone receptor A and B isoforms during human endometrial decidualization. Mol. Endocrinol. 2015, 29, 882–895. [Google Scholar] [CrossRef]
- Ichioka, M.; Mita, S.; Shimizu, Y.; Imada, K.; Kiyono, T.; Bono, Y.; Kyo, S. Dienogest, a synthetic progestin, down-regulates expression of CYP19A1 and inflammatory and neuroangiogenesis factors through progesterone receptor isoforms A and B in endometriotic cells. J. Steroid Biochem. Mol. Biol. 2015, 147, 103–110. [Google Scholar] [CrossRef]
- Lee, T.S.; Lin, J.J.; Huo, Y.N.; Lee, W.S. Progesterone Inhibits Endothelial Cell Migration Through Suppression of the Rho Activity Mediated by cSrc Activation. J. Cell. Biochem. 2015, 116, 1411–1418. [Google Scholar] [CrossRef]
- Kayisli, U.A.; Luk, J.; Guzeloglu-Kayisli, O.; Seval, Y.; Demir, R.; Arici, A. Regulation of angiogenic activity of human endometrial endothelial cells in culture by ovarian steroids. J. Clin. Endocrinol. Metab. 2004, 89, 5794–5802. [Google Scholar] [CrossRef]
- Swiatek-De Lange, M.; Stampfl, A.; Hauck, S.M.; Zischka, H.; Gloeckner, C.J.; Deeg, C.A.; Ueffing, M. Membrane-initiated effects of progesterone on calcium dependent signaling and activation of VEGF gene expression in retinal glial cells. Glia 2007, 55, 1061–1073. [Google Scholar] [CrossRef]
- Neubauer, H.; Adam, G.; Seeger, H.; Mueck, A.O.; Solomayer, E.; Wallwiener, D.; Cahill, M.A.; Fehm, T. Membrane-initiated effects of progesterone on proliferation and activation of VEGF in breast cancer cells. Climacteric J. Int. Menopause Soc. 2009, 12, 230–239. [Google Scholar] [CrossRef]
- Peluso, J.J.; Liu, X.; Uliasz, T.; Pru, C.A.; Kelp, N.C.; Pru, J. PGRMC1/2 promotes luteal vascularization and maintains the primordial follicles of mice. Reproduction 2018, 156, 365–373. [Google Scholar] [CrossRef]
- Andrikopoulou, M.; Chatzistamou, I.; Gkilas, H.; Vilaras, G.; Sklavounou, A. Assessment of angiogenic markers and female sex hormone receptors in pregnancy tumor of the gingiva. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2013, 71, 1376–1381. [Google Scholar] [CrossRef]
- Meduri, G.; Bausero, P.; Perrot-Applanat, M. Expression of vascular endothelial growth factor receptors in the human endometrium: Modulation during the menstrual cycle. Biol. Reprod. 2000, 62, 439–447. [Google Scholar] [CrossRef]
- Ma, W.; Tan, J.; Matsumoto, H.; Robert, B.; Abrahamson, D.R.; Das, S.K.; Dey, S.K. Adult tissue angiogenesis: Evidence for negative regulation by estrogen in the uterus. Mol. Endocrinol. 2001, 15, 1983–1992. [Google Scholar] [CrossRef]
- Das, D.; Saikia, P.J.; Gowala, U.; Sarma, H.N. Cell Specific Expression of Vascular Endothelial Growth Factor Receptor-2 (Flk-1/KDR) in Developing Mice Embryo and Supporting Maternal Uterine Tissue during Early Gestation (D4-D7). Int. J. Fertil. Steril. 2021, 15, 148–157. [Google Scholar] [CrossRef]
- Liang, Y.; Hyder, S.M. Proliferation of endothelial and tumor epithelial cells by progestin-induced vascular endothelial growth factor from human breast cancer cells: Paracrine and autocrine effects. Endocrinology 2005, 146, 3632–3641. [Google Scholar] [CrossRef]
- Nayak, N.R.; Brenner, R.M. Vascular proliferation and vascular endothelial growth factor expression in the rhesus macaque endometrium. J. Clin. Endocrinol. Metab. 2002, 87, 1845–1855. [Google Scholar] [CrossRef]
- Nayak, N.R.; Critchley, H.O.; Slayden, O.D.; Menrad, A.; Chwalisz, K.; Baird, D.T.; Brenner, R.M. Progesterone withdrawal up-regulates vascular endothelial growth factor receptor type 2 in the superficial zone stroma of the human and macaque endometrium: Potential relevance to menstruation. J. Clin. Endocrinol. Metab. 2000, 85, 3442–3452. [Google Scholar] [CrossRef]
- Katoh, M. Therapeutics Targeting FGF Signaling Network in Human Diseases. Trends Pharmacol. Sci. 2016, 37, 1081–1096. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.; Hori, M.; Ichigo, S.; Hirose, R.; Sakaguchi, H.; Tamaya, T. Plausible novel therapeutic strategy of uterine endometrial cancer with reduction of basic fibroblast growth factor secretion by progestin and O-(chloroacetyl-carbamoyl) fumagillol (TNP-470; AGM-1470). Cancer Lett. 1997, 113, 187–194. [Google Scholar] [CrossRef]
- Mönckedieck, V.; Sannecke, C.; Husen, B.; Kumbartski, M.; Kimmig, R.; Tötsch, M.; Winterhager, E.; Grümmer, R. Progestins inhibit expression of MMPs and of angiogenic factors in human ectopic endometrial lesions in a mouse model. Mol. Hum. Reprod. 2009, 15, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.; Hori, M.; Ichigo, S.; Hirose, R.; Tamaya, T. Antiestrogenic compounds inhibit estrogen-induced expressions of basic fibroblast growth factor and its mRNA in well-differentiated endometrial cancer cells. Gen. Pharmacol. 1997, 28, 215–219. [Google Scholar] [CrossRef]
- Fujimoto, J.; Ichigo, S.; Sakaguchi, H.; Hirose, R.; Tamaya, T. Expression of platelet-derived endothelial cell growth factor and its mRNA in uterine endometrium during the menstrual cycle. Mol. Hum. Reprod. 1998, 4, 509–513. [Google Scholar] [CrossRef]
- Zhang, L.; Mackenzie, I.Z.; Rees, M.C.; Bicknell, R. Regulation of the expression of the angiogenic enzyme platelet-derived endothelial cell growth factor/thymidine phosphorylase in endometrial isolates by ovarian steroids and cytokines. Endocrinology 1997, 138, 4921–4930. [Google Scholar] [CrossRef]
- Aoki, I.; Fujimoto, J.; Tamaya, T. Effects of various steroids on platelet-derived endothelial cell growth factor (PD-ECGF) and its mRNA expression in uterine endometrial cancer cells. J. Steroid Biochem. Mol. Biol. 2003, 84, 217–222. [Google Scholar] [CrossRef]
- Zavarhei, M.D.; Bidgoli, S.A.; Ziyarani, M.M.; Shariatpanahi, M.; Ardalan, F.A. Progesterone receptor positive colorectal tumors have lower thymidine phosphorylase expression: An immunohistochemical study. Pak. J. Biol. Sci. 2007, 10, 4485–4489. [Google Scholar] [CrossRef][Green Version]
- Park, Y.G.; Choi, J.; Seol, J.W. Angiopoietin-2 regulated by progesterone induces uterine vascular remodeling during pregnancy. Mol. Med. Rep. 2020, 22, 1235–1242. [Google Scholar] [CrossRef]
- Krikun, G.; Critchley, H.; Schatz, F.; Wan, L.; Caze, R.; Baergen, R.N.; Lockwood, C.J. Abnormal uterine bleeding during progestin-only contraception may result from free radical-induced alterations in angiopoietin expression. Am. J. Pathol. 2002, 161, 979–986. [Google Scholar] [CrossRef]
- Hickey, M.; Krikun, G.; Kodaman, P.; Schatz, F.; Carati, C.; Lockwood, C.J. Long-term progestin-only contraceptives result in reduced endometrial blood flow and oxidative stress. J. Clin. Endocrinol. Metab. 2006, 91, 3633–3638. [Google Scholar] [CrossRef]
- Pan, X.Y.; Zhang, Z.H.; Wu, L.X.; Wang, Z.C. Effect of HIF-1a/VEGF signaling pathway on plasma progesterone and ovarian prostaglandin F2a secretion during luteal development of pseudopregnant rats. Genet. Mol. Res. 2015, 14, 8796–8809. [Google Scholar] [CrossRef]
- Song, G.; Kim, J.; Bazer, F.W.; Spencer, T.E. Progesterone and interferon tau regulate hypoxia-inducible factors in the endometrium of the ovine uterus. Endocrinology 2008, 149, 1926–1934. [Google Scholar] [CrossRef]
- Cattaneo, M.G.; Vanetti, C.; Decimo, I.; Di Chio, M.; Martano, G.; Garrone, G.; Bifari, F.; Vicentini, L.M. Sex-specific eNOS activity and function in human endothelial cells. Sci. Rep. 2017, 7, 9612. [Google Scholar] [CrossRef]
- Duda, D.G.; Fukumura, D.; Jain, R.K. Role of eNOS in neovascularization: NO for endothelial progenitor cells. Trends Mol. Med. 2004, 10, 143–145. [Google Scholar] [CrossRef]
- Rupnow, H.L.; Phernetton, T.M.; Shaw, C.E.; Modrick, M.L.; Bird, I.M.; Magness, R.R. Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1699–H1705. [Google Scholar] [CrossRef]
- You, Y.; Tan, W.; Guo, Y.; Luo, M.; Shang, F.F.; Xia, Y.; Luo, S. Progesterone promotes endothelial nitric oxide synthase expression through enhancing nuclear progesterone receptor-SP-1 formation. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H341–H348. [Google Scholar] [CrossRef]
- Cutini, P.H.; Campelo, A.E.; Massheimer, V.L. Differential regulation of endothelium behavior by progesterone and medroxyprogesterone acetate. J. Endocrinol. 2014, 220, 179–193. [Google Scholar] [CrossRef]
- Simoncini, T.; Caruso, A.; Garibaldi, S.; Fu, X.D.; Giretti, M.S.; Baldacci, C.; Scorticati, C.; Fornari, L.; Mannella, P.; Genazzani, A.R. Activation of nitric oxide synthesis in human endothelial cells using nomegestrol acetate. Obstet. Gynecol. 2006, 108, 969–978. [Google Scholar] [CrossRef]
- Oishi, A.; Takahashi, K.; Ohmichi, M.; Mochizuki, Y.; Inaba, N.; Kurachi, H. Role of glucocorticoid receptor in the inhibitory effect of medroxyprogesterone acetate on the estrogen-induced endothelial nitric oxide synthase phosphorylation in human umbilical vein endothelial cells. Fertil. Steril. 2011, 95, 1168–1170. [Google Scholar] [CrossRef]
- Houshdaran, S.; Chen, J.C.; Vallvé-Juanico, J.; Balayan, S.; Vo, K.C.; Smith-McCune, K.; Greenblatt, R.M.; Irwin, J.C.; Giudice, L.C. Progestins Related to Progesterone and Testosterone Elicit Divergent Human Endometrial Transcriptomes and Biofunctions. Int. J. Mol. Sci. 2020, 21, 2625. [Google Scholar] [CrossRef]
- Truong, T.H.; Lange, C.A. Deciphering Steroid Receptor Crosstalk in Hormone-Driven Cancers. Endocrinology 2018, 159, 3897–3907. [Google Scholar] [CrossRef]
- Pang, Y.; Dong, J.; Thomas, P. Progesterone increases nitric oxide synthesis in human vascular endothelial cells through activation of membrane progesterone receptor-α. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E899–E911. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Grunstein, J.; Tejada, M.; Peale, F.; Frantz, G.; Liang, W.C.; Bai, W.; Yu, L.; Kowalski, J.; Liang, X.; et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004, 23, 2800–2810. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.; Guerreiro, S.; Botelho, M. Elucidating progesterone effects in breast cancer: Cross talk with PDGF signaling pathway in smooth muscle cell. J. Cell. Biochem. 2007, 100, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Iruela-Arispe, M.L.; Porter, P.; Bornstein, P.; Sage, E.H. Thrombospondin-1, an inhibitor of angiogenesis, is regulated by progesterone in the human endometrium. J. Clin. Investig. 1996, 97, 403–412. [Google Scholar] [CrossRef]
- Hyder, S.M.; Liang, Y.; Wu, J.; Welbern, V. Regulation of thrombospondin-1 by natural and synthetic progestins in human breast cancer cells. Endocr. Relat. Cancer 2009, 16, 809–817. [Google Scholar] [CrossRef]
- Arroyo, J.A.; Winn, V.D. Vasculogenesis and angiogenesis in the IUGR placenta. Semin. Perinatol. 2008, 32, 172–177. [Google Scholar] [CrossRef]
- Hyder, S.M.; Stancel, G.M. Regulation of angiogenic growth factors in the female reproductive tract by estrogens and progestins. Mol. Endocrinol. 1999, 13, 806–811. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Faramarzi, M. Endometrial thickness and pregnancy outcome after intrauterine insemination. Fertil. Steril. 2007, 88, 432–437. [Google Scholar] [CrossRef]
- Jabbour, H.N.; Kelly, R.W.; Fraser, H.M.; Critchley, H.O. Endocrine regulation of menstruation. Endocr. Rev. 2006, 27, 17–46. [Google Scholar] [CrossRef]
- Di Renzo, G.C.; Giardina, I.; Clerici, G.; Brillo, E.; Gerli, S. Progesterone in normal and pathological pregnancy. Horm. Mol. Biol. Clin. Investig. 2016, 27, 35–48. [Google Scholar] [CrossRef]
- Shifren, J.L.; Tseng, J.F.; Zaloudek, C.J.; Ryan, I.P.; Meng, Y.G.; Ferrara, N.; Jaffe, R.B.; Taylor, R.N. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: Implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J. Clin. Endocrinol. Metab. 1996, 81, 3112–3118. [Google Scholar] [CrossRef]
- Greb, R.R.; Heikinheimo, O.; Williams, R.F.; Hodgen, G.D.; Goodman, A.L. Vascular endothelial growth factor in primate endometrium is regulated by oestrogen-receptor and progesterone-receptor ligands in vivo. Hum. Reprod. 1997, 12, 1280–1292. [Google Scholar] [CrossRef]
- Matsubara, K.; Abe, E.; Matsubara, Y.; Kameda, K.; Ito, M. Circulating endothelial progenitor cells during normal pregnancy and pre-eclampsia. Am. J. Reprod. Immunol. 2006, 56, 79–85. [Google Scholar] [CrossRef]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef]
- Matsubara, Y.; Matsubara, K. Estrogen and progesterone play pivotal roles in endothelial progenitor cell proliferation. Reprod. Biol. Endocrinol. RB E 2012, 10, 2. [Google Scholar] [CrossRef]
- Gambino, L.S.; Wreford, N.G.; Bertram, J.F.; Dockery, P.; Lederman, F.; Rogers, P.A. Angiogenesis occurs by vessel elongation in proliferative phase human endometrium. Hum. Reprod. 2002, 17, 1199–1206. [Google Scholar] [CrossRef]
- Abberton, K.M.; Healy, D.L.; Rogers, P.A. Smooth muscle alpha actin and myosin heavy chain expression in the vascular smooth muscle cells surrounding human endometrial arterioles. Hum. Reprod. 1999, 14, 3095–3100. [Google Scholar] [CrossRef]
- Abberton, K.M.; Taylor, N.H.; Healy, D.L.; Rogers, P.A. Vascular smooth muscle cell proliferation in arterioles of the human endometrium. Hum. Reprod. 1999, 14, 1072–1079. [Google Scholar] [CrossRef]
- Kohnen, G.; Campbell, S.; Jeffers, M.D.; Cameron, I.T. Spatially regulated differentiation of endometrial vascular smooth muscle cells. Hum. Reprod. 2000, 15, 284–292. [Google Scholar] [CrossRef]
- Girling, J.E.; Lederman, F.L.; Walter, L.M.; Rogers, P.A. Progesterone, but not estrogen, stimulates vessel maturation in the mouse endometrium. Endocrinology 2007, 148, 5433–5441. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.W.; Dunlap, K.A.; Frank, J.W.; Erikson, D.W.; White, B.G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A. Effects of long-term progesterone on developmental and functional aspects of porcine uterine epithelia and vasculature: Progesterone alone does not support development of uterine glands comparable to that of pregnancy. Reproduction 2010, 140, 583–594. [Google Scholar] [CrossRef]
- Wen, L.; Chen, L.H.; Li, H.Y.; Chang, S.P.; Liao, C.Y.; Tsui, K.H.; Sung, Y.J.; Chao, K.C. Roles of estrogen and progesterone in endometrial hemodynamics and vascular endothelial growth factor production. J. Chin. Med. Assoc. 2009, 72, 188–193. [Google Scholar] [CrossRef]
- Tan, W.; Chen, L.; Guo, L.; Ou, X.; Xie, D.; Quan, S. Relationship between macrophages in mouse uteri and angiogenesis in endometrium during the peri-implantation period. Theriogenology 2014, 82, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Ramathal, C.Y.; Bagchi, I.C.; Taylor, R.N.; Bagchi, M.K. Endometrial decidualization: Of mice and men. Semin. Reprod. Med. 2010, 28, 17–26. [Google Scholar] [CrossRef]
- Chen, X.; Jin, X.; Liu, L.; Man, C.W.; Huang, J.; Wang, C.C.; Zhang, S.; Li, T.C. Differential expression of vascular endothelial growth factor angiogenic factors in different endometrial compartments in women who have an elevated progesterone level before oocyte retrieval, during in vitro fertilization-embryo transfer treatment. Fertil. Steril. 2015, 104, 1030–1036. [Google Scholar] [CrossRef]
- Douglas, N.C.; Tang, H.; Gomez, R.; Pytowski, B.; Hicklin, D.J.; Sauer, C.M.; Kitajewski, J.; Sauer, M.V.; Zimmermann, R.C. Vascular endothelial growth factor receptor 2 (VEGFR-2) functions to promote uterine decidual angiogenesis during early pregnancy in the mouse. Endocrinology 2009, 150, 3845–3854. [Google Scholar] [CrossRef]
- Kapiteijn, K.; Koolwijk, P.; van der Weiden, R.M.; van Nieuw Amerongen, G.; Plaisier, M.; van Hinsbergh, V.W.; Helmerhorst, F.M. Human embryo-conditioned medium stimulates in vitro endometrial angiogenesis. Fertil. Steril. 2006, 85 (Suppl. S1), 1232–1239. [Google Scholar] [CrossRef]
- Manolea, M.M.; Dijmărescu, A.L.; Popescu, F.C.; Novac, M.B.; DiŢescu, D. Evaluation of the implantation site morphology in spontaneous abortion. Rom. J. Morphol. Embryol. 2015, 56, 125–131. [Google Scholar]
- Lydon, J.P.; DeMayo, F.J.; Funk, C.R.; Mani, S.K.; Hughes, A.R.; Montgomery, C.A., Jr.; Shyamala, G.; Conneely, O.M.; O’Malley, B.W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995, 9, 2266–2278. [Google Scholar] [CrossRef]
- Johannisson, E.; Oberholzer, M.; Swahn, M.L.; Bygdeman, M. Vascular changes in the human endometrium following the administration of the progesterone antagonist RU 486. Contraception 1989, 39, 103–117. [Google Scholar] [CrossRef]
- Slayden, O.D.; Zelinski-Wooten, M.B.; Chwalisz, K.; Stouffer, R.L.; Brenner, R.M. Chronic treatment of cycling rhesus monkeys with low doses of the antiprogestin ZK 137 316: Morphometric assessment of the uterus and oviduct. Hum. Reprod. 1998, 13, 269–277. [Google Scholar] [CrossRef]
- Fang, Z.; Yang, S.; Lydon, J.P.; DeMayo, F.; Tamura, M.; Gurates, B.; Bulun, S.E. Intact progesterone receptors are essential to counteract the proliferative effect of estradiol in a genetically engineered mouse model of endometriosis. Fertil. Steril. 2004, 82, 673–678. [Google Scholar] [CrossRef]
- Duran, C.L.; Abbey, C.A.; Bayless, K.J. Establishment of a three-dimensional model to study human uterine angiogenesis. Mol. Hum. Reprod. 2018, 24, 74–93. [Google Scholar] [CrossRef]
- Rashidi, B.; Mardani, M.; Karizbodagh, M.P. Evaluation of Progesterone and Ovulation-stimulating Drugs on the Glandular Epithelium and Angiogenesis in Mice. Adv. Biomed. Res. 2017, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Subakir, S.B.; Hadisaputra, W.; Handoyo, A.E.; Affandi, B. Endometrial angiogenic response in Norplant users. Hum. Reprod. 1996, 11 (Suppl. S2), 51–55. [Google Scholar] [CrossRef] [PubMed]
- Goodger, A.M.; Rogers, P.A.; Affandi, B. Endometrial endothelial cell proliferation in long-term users of subdermal levonorgestrel. Hum. Reprod. 1994, 9, 1647–1651. [Google Scholar] [CrossRef]
- Vázquez, F.; Rodríguez-Manzaneque, J.C.; Lydon, J.P.; Edwards, D.P.; O’Malley, B.W.; Iruela-Arispe, M.L. Progesterone regulates proliferation of endothelial cells. J. Biol. Chem. 1999, 274, 2185–2192. [Google Scholar] [CrossRef]
- Hsu, S.P.; Ho, P.Y.; Juan, S.H.; Liang, Y.C.; Lee, W.S. Progesterone inhibits human endothelial cell proliferation through a p53-dependent pathway. Cell. Mol. Life Sci. 2008, 65, 3839–3850. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.P.; Lee, W.S. Progesterone receptor activation of extranuclear signaling pathways in regulating p53 expression in vascular endothelial cells. Mol. Endocrinol. 2011, 25, 421–432. [Google Scholar] [CrossRef]
- Kim, I.D.; Ahn, K.H.; Lee, S.; Hong, S.C.; Kim, S.H.; Kim, T. Effect of ovariectomy, 17-beta estradiol, and progesterone on histology and estrogen receptors of bladder in female partial bladder outlet obstruction rat model. J. Obstet. Gynaecol. Res. 2013, 39, 1259–1267. [Google Scholar] [CrossRef]
- Katayama, Y.; Uchino, J.; Chihara, Y.; Tamiya, N.; Kaneko, Y.; Yamada, T.; Takayama, K. Tumor Neovascularization and Developments in Therapeutics. Cancers 2019, 11, 316. [Google Scholar] [CrossRef]
- Troisi, J.; Sarno, L.; Landolfi, A.; Scala, G.; Martinelli, P.; Venturella, R.; Di Cello, A.; Zullo, F.; Guida, M. Metabolomic Signature of Endometrial Cancer. J. Proteome Res. 2018, 17, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Bodurka, D.C.; Sun, C.C.; Levenback, C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: A literature review. Gynecol. Oncol. 2004, 95, 133–138. [Google Scholar] [CrossRef]
- Lee, I.I.; Maniar, K.; Lydon, J.P.; Kim, J.J. Akt regulates progesterone receptor B-dependent transcription and angiogenesis in endometrial cancer cells. Oncogene 2016, 35, 5191–5201. [Google Scholar] [CrossRef]
- Hyder, S.M.; Murthy, L.; Stancel, G.M. Progestin regulation of vascular endothelial growth factor in human breast cancer cells. Cancer Res. 1998, 58, 392–395. [Google Scholar]
- Fujimoto, J.; Sakaguchi, H.; Hirose, R.; Ichigo, S.; Tamaya, T. Progestins suppress estrogen-induced expression of vascular endothelial growth factor (VEGF) subtypes in uterine endometrial cancer cells. Cancer Lett. 1999, 141, 63–71. [Google Scholar] [CrossRef]
- Mohr, P.E.; Wang, D.Y.; Gregory, W.M.; Richards, M.A.; Fentiman, I.S. Serum progesterone and prognosis in operable breast cancer. Br. J. Cancer 1996, 73, 1552–1555. [Google Scholar] [CrossRef]
- Fu, X.D.; Russo, E.; Zullino, S.; Genazzani, A.R.; Simoncini, T. Sex steroids and breast cancer metastasis. Horm. Mol. Biol. Clin. Investig. 2010, 3, 383–389. [Google Scholar] [CrossRef]
- Wu, J.; Brandt, S.; Hyder, S.M. Ligand- and cell-specific effects of signal transduction pathway inhibitors on progestin-induced vascular endothelial growth factor levels in human breast cancer cells. Mol. Endocrinol. 2005, 19, 312–326. [Google Scholar] [CrossRef]
- Thomassen, M.; Tan, Q.; Kruse, T.A. Gene expression meta-analysis identifies metastatic pathways and transcription factors in breast cancer. BMC Cancer 2008, 8, 394. [Google Scholar] [CrossRef]
- Kougioumtzi, A.; Tsaparas, P.; Magklara, A. Deep sequencing reveals new aspects of progesterone receptor signaling in breast cancer cells. PLoS ONE 2014, 9, e98404. [Google Scholar] [CrossRef]
- Lee, O.; Choi, M.R.; Christov, K.; Ivancic, D.; Khan, S.A. Progesterone receptor antagonism inhibits progestogen-related carcinogenesis and suppresses tumor cell proliferation. Cancer Lett. 2016, 376, 310–317. [Google Scholar] [CrossRef]
- Carroll, C.E.; Liang, Y.; Benakanakere, I.; Besch-Williford, C.; Hyder, S.M. The anticancer agent YC-1 suppresses progestin-stimulated VEGF in breast cancer cells and arrests breast tumor development. Int. J. Oncol. 2013, 42, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Richer, J.; Horwitz, K.B.; Hyder, S.M. Progestin-dependent induction of vascular endothelial growth factor in human breast cancer cells: Preferential regulation by progesterone receptor B. Cancer Res. 2004, 64, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.L.; Day, C.N.; Hoskin, T.L.; Habermann, E.B.; Boughey, J.C. Adolescents and Young Adults with Breast Cancer have More Aggressive Disease and Treatment Than Patients in Their Forties. Ann. Surg. Oncol. 2019, 26, 3920–3930. [Google Scholar] [CrossRef] [PubMed]
- Vameşu, S. Angiogenesis and progesterone receptor status in primary breast cancer patients: An analysis of 158 needle core biopsies. Rom. J. Morphol. Embryol. 2007, 48, 267–274. [Google Scholar]
- Uchida, M.; Tsuboi, H.; Yamaji, T.; Murata, N.; Kohno, T.; Sugino, E.; Hibino, S.; Shimamura, M.; Oikawa, T. Inhibition by 9alpha-fluoromedoroxyprogesterone acetate (FMPA) against mammary carcinoma induced by dimethylbenz[a]anthracene in rats and angiogenesis in the rabbit cornea-comparison with medroxyprogesterone acetate (MPA). Cancer Lett. 2000, 154, 63–69. [Google Scholar] [CrossRef]
- Pietras, R.J.; Márquez, D.C.; Chen, H.W.; Tsai, E.; Weinberg, O.; Fishbein, M. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids 2005, 70, 372–381. [Google Scholar] [CrossRef]
- Tan, J.; Paria, B.C.; Dey, S.K.; Das, S.K. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology 1999, 140, 5310–5321. [Google Scholar] [CrossRef]
- Mirkin, S.; Archer, D.F. Effects of levonorgestrel, medroxyprogesterone acetate, norethindrone, progesterone, and 17beta-estradiol on thrombospondin-1 mRNA in Ishikawa cells. Fertil. Steril. 2004, 82, 220–222. [Google Scholar] [CrossRef]
- Classen-Linke, I.; Alfer, J.; Krusche, C.A.; Chwalisz, K.; Rath, W.; Beier, H.M. Progestins, progesterone receptor modulators, and progesterone antagonists change VEGF release of endometrial cells in culture. Steroids 2000, 65, 763–771. [Google Scholar] [CrossRef]
- Mangal, R.K.; Wiehle, R.D.; Poindexter, A.N., 3rd; Weigel, N.L. Differential expression of uterine progesterone receptor forms A and B during the menstrual cycle. J. Steroid Biochem. Mol. Biol. 1997, 63, 195–202. [Google Scholar] [CrossRef]
- Wang, H.; Critchley, H.O.; Kelly, R.W.; Shen, D.; Baird, D.T. Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol. Hum. Reprod. 1998, 4, 407–412. [Google Scholar] [CrossRef]
- Jacobsen, B.M.; Richer, J.K.; Schittone, S.A.; Horwitz, K.B. New human breast cancer cells to study progesterone receptor isoform ratio effects and ligand-independent gene regulation. J. Biol. Chem. 2002, 277, 27793–27800. [Google Scholar] [CrossRef]
- Heryanto, B.; Rogers, P.A. Regulation of endometrial endothelial cell proliferation by oestrogen and progesterone in the ovariectomized mouse. Reproduction 2002, 123, 107–113. [Google Scholar] [CrossRef]
- Li, Y.; Adur, M.K.; Kannan, A.; Davila, J.; Zhao, Y.; Nowak, R.A.; Bagchi, M.K.; Bagchi, I.C.; Li, Q. Progesterone Alleviates Endometriosis via Inhibition of Uterine Cell Proliferation, Inflammation and Angiogenesis in an Immunocompetent Mouse Model. PLoS ONE 2016, 11, e0165347. [Google Scholar] [CrossRef]
- Shao, R.; Cao, S.; Wang, X.; Feng, Y.; Billig, H. The elusive and controversial roles of estrogen and progesterone receptors in human endometriosis. Am. J. Transl. Res. 2014, 6, 104–113. [Google Scholar]
- Benakanakere, I.; Besch-Williford, C.; Schnell, J.; Brandt, S.; Ellersieck, M.R.; Molinolo, A.; Hyder, S.M. Natural and synthetic progestins accelerate 7,12-dimethylbenz[a]anthracene-initiated mammary tumors and increase angiogenesis in Sprague-Dawley rats. Clin. Cancer Res. 2006, 12, 4062–4071. [Google Scholar] [CrossRef]
- Finlay-Schultz, J.; Gillen, A.E.; Brechbuhl, H.M.; Ivie, J.J.; Matthews, S.B.; Jacobsen, B.M.; Bentley, D.L.; Kabos, P.; Sartorius, C.A. Breast Cancer Suppression by Progesterone Receptors Is Mediated by Their Modulation of Estrogen Receptors and RNA Polymerase III. Cancer Res. 2017, 77, 4934–4946. [Google Scholar] [CrossRef]
- Valadez-Cosmes, P.; Vázquez-Martínez, E.R.; Cerbón, M.; Camacho-Arroyo, I. Membrane progesterone receptors in reproduction and cancer. Mol. Cell Endocrinol. 2016, 434, 166–175. [Google Scholar] [CrossRef]
- Ruan, X.; Cai, G.; Wei, Y.; Gu, M.; Zhang, Y.; Zhao, Y.; Mueck, A.O. Association of circulating Progesterone Receptor Membrane Component-1 (PGRMC1) with breast tumor characteristics and comparison with known tumor markers. Menopause 2020, 27, 183–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, X.; Willibald, M.; Seeger, H.; Fehm, T.; Neubauer, H.; Mueck, A.O. May progesterone receptor membrane component 1 (PGRMC1) predict the risk of breast cancer? Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2016, 32, 58–60. [Google Scholar] [CrossRef]
- Asperger, H.; Stamm, N.; Gierke, B.; Pawlak, M.; Hofmann, U.; Zanger, U.M.; Marton, A.; Katona, R.L.; Buhala, A.; Vizler, C.; et al. Progesterone receptor membrane component 1 regulates lipid homeostasis and drives oncogenic signaling resulting in breast cancer progression. Breast Cancer Res. 2020, 22, 75. [Google Scholar] [CrossRef]
- Pedroza, D.A.; Subramani, R.; Tiula, K.; Do, A.; Rashiraj, N.; Galvez, A.; Chatterjee, A.; Bencomo, A.; Rivera, S.; Lakshmanaswamy, R. Crosstalk between progesterone receptor membrane component 1 and estrogen receptor α promotes breast cancer cell proliferation. Lab. Investig. 2021, 101, 733–744. [Google Scholar] [CrossRef]
- Hewitt, S.C.; Korach, K.S. Progesterone action and responses in the alphaERKO mouse. Steroids 2000, 65, 551–557. [Google Scholar] [CrossRef]
- Giulianelli, S.; Vaqué, J.P.; Soldati, R.; Wargon, V.; Vanzulli, S.I.; Martins, R.; Zeitlin, E.; Molinolo, A.A.; Helguero, L.A.; Lamb, C.A.; et al. Estrogen receptor alpha mediates progestin-induced mammary tumor growth by interacting with progesterone receptors at the cyclin D1/MYC promoters. Cancer Res. 2012, 72, 2416–2427. [Google Scholar] [CrossRef]
- Schneck, H.; Ruan, X.; Seeger, H.; Cahill, M.A.; Fehm, T.; Mueck, A.O.; Neubauer, H. Membrane-receptor initiated proliferative effects of dienogest in human breast cancer cells. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2013, 29, 160–163. [Google Scholar] [CrossRef]
- Susa, T.; Ikaga, R.; Kajitani, T.; Iizuka, M.; Okinaga, H.; Tamamori-Adachi, M.; Okazaki, T. Wild-type and specific mutant androgen receptor mediates transcription via 17β-estradiol in sex hormone-sensitive cancer cells. J. Cell Physiol. 2015, 230, 1594–1606. [Google Scholar] [CrossRef]
- Sato, B. Can an autocrine loop explain sex-hormone-dependent tumor growth? A brief overview. Oncology 1999, 57 (Suppl. S2), 3–6. [Google Scholar] [CrossRef]
- Guzeloglu Kayisli, O.; Kayisli, U.A.; Basar, M.; Semerci, N.; Schatz, F.; Lockwood, C.J. Progestins Upregulate FKBP51 Expression in Human Endometrial Stromal Cells to Induce Functional Progesterone and Glucocorticoid Withdrawal: Implications for Contraceptive- Associated Abnormal Uterine Bleeding. PLoS ONE 2015, 10, e0137855. [Google Scholar] [CrossRef]
- Piasecka, D.; Braun, M.; Kitowska, K.; Mieczkowski, K.; Kordek, R.; Sadej, R.; Romanska, H. FGFs/FGFRs-dependent signalling in regulation of steroid hormone receptors-implications for therapy of luminal breast cancer. J. Exp. Clin. Cancer Res. 2019, 38, 230. [Google Scholar] [CrossRef]
- Pan, C.; Liu, Y.P.; Li, Y.F.; Hu, J.X.; Zhang, J.P.; Wang, H.M.; Li, J.; Xu, L.C. Effects of cypermethrin on the ligand-independent interaction between androgen receptor and steroid receptor coactivator-1. Toxicology 2012, 299, 160–164. [Google Scholar] [CrossRef]
- Robertson, S.; Rohwer, J.M.; Hapgood, J.P.; Louw, A. Impact of glucocorticoid receptor density on ligand-independent dimerization, cooperative ligand-binding and basal priming of transactivation: A cell culture model. PLoS ONE 2013, 8, e64831. [Google Scholar] [CrossRef]
- Zhang, G.; Yanamala, N.; Lathrop, K.L.; Zhang, L.; Klein-Seetharaman, J.; Srinivas, H. Ligand-independent antiapoptotic function of estrogen receptor-beta in lung cancer cells. Mol. Endocrinol. 2010, 24, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Mitani, Y.; Lin, S.H.; Pytynia, K.B.; Ferrarotto, R.; El-Naggar, A.K. Reciprocal and Autonomous Glucocorticoid and Androgen Receptor Activation in Salivary Duct Carcinoma. Clin. Cancer Res. 2020, 26, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.B.; Janowski, B.A.; Chen, C.C.; Mendelson, C.R. Progesterone receptor inhibits aromatase and inflammatory response pathways in breast cancer cells via ligand-dependent and ligand-independent mechanisms. Mol. Endocrinol. 2008, 22, 1812–1824. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, X.; Lu, X.; Xie, M.; He, B.; He, S.; You, S.; Chen, Q. Progesterone/Org inhibits lung adenocarcinoma cell growth via membrane progesterone receptor alpha. Thorac. Cancer 2020, 11, 2209–2223. [Google Scholar] [CrossRef]

| Reference | Progestogens | Object | Effect | Concentration |
|---|---|---|---|---|
| [133] | P4 | Human breast cancer cells T47-D | VEGF ↑ | 10−8 mol/L |
| [39] | P4 | Human breast cancer cells MCF7 | VEGF ↑ | - |
| [141] | P4 MPA NET Norgestrel | Human breast cancer cells T47-D | VEGF ↑ | 10−8 mol/L |
| [49] | P4 | Human breast cancer cells T47-D | VEGF ↑ | 10−8 mol/L |
| [142] | P4 MPA MGA | Human breast cancer cells T47-D | VEGF ↑ (PR-B cell) P4-PR-B-induced VEGF ↓ (PR-A cell) | 10−8 mol/L |
| [90] | P4 | Human breast cancer cells MCF7 | Platelet-derived growth factor A ↑ VEGF ↑ | 10−8 mol/L |
| [137] | P4 MPA | Human breast cancer cells BT-474 and T47-D | VEGF ↑ | 10−8 mol/L |
| [92] | P4 MPA MGA | Human breast cancer cells T47-D and BT-474 | Thrombospondin-1 ↑ | P4: 10−9 mol/L M MPA: 10−8 mol/L MGA: 10−8 mol/L |
| [63] | P4 MPA | Human breast cancer cells T47-D, BT-474, HCC-1428 and MDAMB-231 Human umbilical vein endothelial cells | VEGF ↑ | P4: 10−8 mol/L MPA: 10−8 mol/L |
| [133] | MPA Norgestrel NET, Norethynodrel | Human breast cancer cells T47-D | VEGF ↑ | MPA: 10−8 mol/L Norgestrel: 10−8 mol/L NET: 10−8 mol/L Norethynodrel: 10−8 mol/L |
| [57] | Membrane-impermeable P4 conjugate | Human breast cancer cells MCF7 | VEGF mRNA↑ | 10−6 mol/L |
| [72] | P4 MPA | Human Ishikawa cells | PD-ECGF ↑ | 10−8 mol/L |
| [67,69] | P4 | Human Ishikawa cells | Basic fibroblast growth factor ↓ | 10−8 mol/L |
| [148] | P4 MPA LNG NET | Human Ishikawa cells | Thrombospondin-1 ↑ | 10−8 to 10−6 mol/L |
| [109] | P4 | Human Ishikawa cells Human endometrial epithelial cells | VEGF ↑ | 10−8 mol/L |
| [71] | P4 | Human endometrial epithelial cells Human endometrial stromal cells | PD-ECGF ↑ (stromal cells P4 treatment) PD-ECGF ↑ (epithelial cells under P4+ TGFβ1 treatment) | P4: 5 × 10−8 mol/L TGFβ1: 10 ng/mL |
| [55] | P4 | Human endometrial endothelial cells | Angiogenic capacity and vascular tube formation ↑ | 10−10 mol/L |
| [74] | P4 | Human uterine microvascular endothelial cells | Angiopoietin-2 ↑ | 10−5 mol/L |
| [132] | R5020 | Human uterine microvascular endothelial cells incubated with conditioned media from Ishikawa cells treated by R5020 | Invasion ↓ | 10−8 mol/L |
| [91] | P4 | Human endometrial stromal cells | Thrombospondin-1 ↑ | 10−8 to 5×10−8 mol/L |
| [75] | MPA | Human endometrial stromal cells | Angiopoietin-1 ↑ | 10−7 mol/L |
| [53] | P4 Dienogest | Human endometriotic epithelial cells | VEGF ↓ | P4: 10−7 mol/L Dienogest: 10−7 mol/L |
| [44] | P4 MPA NET LNG DNG | Human endometrial stromal cells | VEGF ↓ | P4: 10−7 mol/L MPA: 10−9 to 10−7 mol/L NET: 10−9 to 10−7 mol/L LNG: 10−9 to 10−7 mol/L DNG: 10−9 to 10−7 mol/L |
| [149] | MPA | Human endometrial epithelial cells Human endometrial fibroblasts | VEGF ↓ (fibroblast) VEGF ↑ (epithelial cell) | 10−6 mol/L |
| [98] | MPA | Human endometrial stomal cells | VEGF ↑ | 10−7 mol/L |
| [86] | LNG NETA | Human endometrial stromal fibroblasts | VEGF ↓ | LNG: 5.47×10−7 mol/L NETA: 0.29×10−6 mol/L |
| [54] | P4 | Human umbilical vein endothelial cells | Migration ↓ | 0.5 ×10−8 to 10−6 mol/L |
| [125,126] | P4 | Human umbilical vein endothelial cells | G0/G1 arrest | 0.5 ×10−6 mol/L |
| [82,88] | P4 | Human umbilical vein endothelial cells | Endothelial nitric oxide synthase ↑ | 2 ×10−8 mol/L |
| [124] | P4 | Human dermal endothelial cells | Arrest endothelial cell cycle in G1 | 0.5 × 10−8 mol/L |
| [46] | P4 | Immortalized mouse brain endothelial cells | tPA-induced VEGF↓ | P4: 2× 10−7 mol/L (tPA: 20 μg/mL) |
| [102] | P4 | Human endothelial progenitor cells | Proliferation ↑ | 10−7 mol/L |
| [20,51] | P4 | Endothelial progenitor cells (rats) | Angiogenic potential ↑ (10−9 mol/L) Angiogenic potential ↓ (10−7 mol/L) | 10−7to 10−9 mol/L |
| [31,83] | P4 MPA | Endothelial cells (rats) | P4-induced VEGF ↑ P4-induced nitric oxide synthase ↑ P4 and MPA promote capillary-like tube formation↑ | P4: 10−9 to 10−7 mol/L MPA: 10−9 to 10−7 mol/L |
| [84] | P4 MPA | Human endothelial cells | P4-induced endothelial nitric oxide synthase ↑ MPA-induced endothelial nitric oxide synthase ↓ | P4: 10−8 mol/L MPA: 10−8 mol/L |
| [36] | P4 | Granulosa cells from large follicles | VEGFA mRNA ↑ | 300 ng/mL (9.5×10−7 mol/L) |
| [35] | P4 | Ovary bovine granulosa cells (cows) | VEGF120 ↑ (10ng/mL) HIF1α ↑ (10ng/mL) VEGFR2 ↑ (10ng/mL) VEGF120 ↓ (100ng/mL) HIF1α ↓ (100ng/mL) VEGFR2 ↓ (100ng/mL) | 10 ng/mL (3.18 ×10−8 mol/L) 100 ng/mL (3.18×10−7 mol/L) |
| [42] | P4 MPA | Human lung cancer A549 | VEGF ↑ | P4: 10−8 mol/L MPA: 10−8 mol/L |
| [41] | P4 | Human astrocytoma D54 cell line | VEGF ↑ | 10−8 mol/L |
| [37] | P4 | Human fibroblasts and alveolar cells type II (mice) | VEGF ↑ (no significance) | 10–8 mol/L |
| [38] | P4 | Human pyogenic granuloma (during pregnancy) Human monocyte-like U937 cells | VEGF ↑ Basic fibroblast growth factor ↑ | 100ng/mL (plus 5mg/mL LPS) |
| [56] | Membrane-impermeable P4 conjugate | Retinal glial cells (porcine) | VEGF ↑ | 10−7 mol/L |
| Reference | Treatment | Object | Effect |
|---|---|---|---|
| [26] | P4 Estrogen priming + P4 | Endometrium endothelial cell (mice) | P4 promoting VEGF Estrogen priming inhibits P4-induced angiogenesis |
| [141] | MPA | Mammary tumors (rats) | CD34-positive blood vessels ↑ |
| [27] | P4 | Uterine (mice) | VEGF ↑ VEGFR1 ↓ VEGFR2 ↓ |
| [61] | P4 ↓P4 + E2 | Uterine (mice) | VEGF ↑ and VEGFR2 ↑ (P4 treatment) ↓VEGF ↑ (P4+E2 treatment) |
| [132] | P4 | Endometrial cancer (mice) | Angiogenesis ↓ |
| [33] | P4 | Ovarian tissue (mice) | VEGF ↑ |
| [48] | P4 | Uterus (mice) | VEGFA ↑ VEGFR2 ↑ |
| [153] | P4 + E2 | Endometrial endothelial cells (male mice) | Epithelial and endothelial cell proliferation ↓ (E2 10ng + P4) Endothelial cell proliferation ↑ (E2 100ng + P4) |
| [121] | P4 | Endometrium (mice) | Angiogenesis ↓ |
| [107] | P4 | Endometrium (mice) | Endometrial vascular maturation ↑ |
| [108] | P4 | Endometrium (pig) | Angiogenesis ↑ |
| [68] | P4 Dydrogesterone Dihydrodydrogesterone | Human ectopic endometrial lesions (mice) | Basic fibroblast growth factor ↓ (P4 or dydrogesterone treatment) VEGF ↓ (dydrogesterone or dihydrodydrogesterone treatment) |
| [154] | P4 | Endometriosis (mice) | Angiogenesis ↓ |
| [119] | P4 | Ectopic uterine tissue (mice) | The size of ectopic uterine tissues ↓ (P4 treatment) The size of ectopic uterine tissues ↑ (E2 treatment) P4 inhibits E2 effects |
| [64] | P4 | Endometrium endometrial stroma (macaques) | VEGF ↓ in stroma of the superficial endometrial zones |
| [46] | tPA + P4 | Brain tissue (rats) | Inhibiting tPA-induced VEGF |
| [81] | P4 P4 + E2 | Uterine artery endothelium (sheep) | Endothelial nitric oxide synthase ↑ (P4 or P4 + E2 treatment) |
| [145] | MPA FMPA | Corneal assays (rabbits) | Angiogenesis ↓ |
| [38] | P4 P4 + E2 | Air ponch granuloma (mice) | VEGF ↑ (P4 or P4 + E2 treatment) |
| [45] | P4 | Diabetic nephropathy model (rats) | VEGF ↓ |
| [32] | P4 | Skin flap (rats) | VEGF ↑ |
| [127] | P4 | Bladder detrusor (rats) | Blood vessel density ↓ |
| [124] | P4 | Re-endothelialization assays (rats) | Re-endothelialization of injured aortae ↓ |
| [47] | P4 | Brains (rats) | VEGF ↓ No change in microvessel density |
| [20,50] | P4 | Brains (rats) | Circulating endothelial pro-genitor cells ↑ Vessel density ↑ |
| [29,30] | P4 | Uterine (rats) | VEGF ↑ Endothelial cell density ↑ miR-16-5p ↑ miR-17-5p ↑ |
| [78] | P4 | Uterus (ovines) | VEGFA ↑ HIF1A ↑ HIF2A ↑ |
| [140] | P4 MPA | Mammary glands (mice) | Average number of small sized blood vessels ↑ (MPA treatment, but not P4) |
| [156] | P4 MPA | Mammary tumor (rats) | VEGF ↑ in basal cell (P4 treatment) VEGF ↑ in epithelial and stromal cells (MPA treatment) |
| Reference | Treatment | Object | Effect | Concentration |
|---|---|---|---|---|
| [134] | E2 priming + P4 E2 priming + MPA E2 priming + 17a-hydroxyprogesterone | Human Ishikawa cells | Inhibiting E2-induced VEGF | E2: 10−8 mol/L P4: 10−6 mol/L MPA: 10−6 mol/L 17a-hydroxyprogesterone: 10−6 mol/L |
| [67,69] | E2 priming + P4 E2 priming + MPA E2 priming +17a-hydroxyprogesterone | Human Ishikawa cells | Inhibiting E2-induced basic fibroblast growth factor | E2: 10−8 mol/L P4: 10−8 mol/L MPA: 10−6 mol/L 17a-hydroxyprogesterone: 10−6 mol/L |
| [72] | E2 priming + P4 E2 priming + MPA E2 priming + 17a-hydroxyprogesterone | Human Ishikawa cells | Promoting E2-induced PD-ECGF (E2 priming + P4, E2 priming + 17a-hydroxyprogesterone, but not E2 priming +MPA) | E2: 10−8 mol/L P4: 10−8 mol/L MPA: 10−6 mol/L 17a-hydroxyprogesterone: 10−6 mol/L |
| [142] | P4 + E2 | Human breast cancer cells T47-D | VEGF ↑ (PR-B cells) No effect on VEGF expression (PR-A cells) | P4: 10−8 mol/L E2: 10−6 mol/L |
| [35] | P4 + E2 | Ovary granulosa cells(cows) | VEGF120 ↓ VEGFR2 ↓ | P4: 10 ng/mL E2: 1 ng/mL |
| [37] | P4 + E2 | Lung fibroblasts and alveolar cells type II (mice) | VEGF ↑ | E2: 10–8 to 10–6 mol/L P4: 10–8 to 10–6 mol/L |
| [38] | P4 + E2 | Human monocyte-like U937 cells | VEGF ↑ | E2: 20ng/mL P4: 100ng/mL (pretreated by LPS 5mg/mL) |
| [42] | P4 + E2 E2 priming + MPA | Human lung cancer A549 | VEGF ↑ Promoting E2-induced VEGF | P4: 10−8 mol/L MPA: 10−8 mol/L E2: 10−8 mol/L |
| [86] | P4 + E2 LNG + E2 NETA + E2 | Human endometrial stromal fibroblasts | VEGFA mRNA ↓ | P4: 10−6 mol/L LNG: 5.47×10−7 mol/L NETA: 0.294×10−6 mol/L E2: 10−8 mol/L |
| [25] | P4 + E2 | Human endometrial stromal cells | VEGF189 mRNA ↑ | E2: 10−8 mol/L P4: 10−6 mol/L (pretreated by EGF 20 ng/mL) |
| [98] | E2 priming +MPA | Human endometrial stromal cells | Promoting E2-induced VEGF | E2: 10−8 mol/L MPA: 10−7 mol/L |
| [44] | E2 priming + P4 E2 priming + MPA E2 priming + NET E2 priming + LNG E2 priming + dienogest | Human endometrial stromal cells | Inhibiting E2-induced VEGF | P4: 10−7 mol/L MPA: 10−9 to 10−7 mol/L NET: 10−9 to 10−7 mol/L LNG: 10−9 to 10−7 mol/L DNG: 10−9 to 10−7 mol/L |
| [28] | MPA + E2 | Human endometrial stromal cells | Thrombin ↑ VEGF ↑ | E2: 10−8 mol/L MPA: 10−7 mol/L |
| [24] | E2 priming + MPA | Human endometrial stromal cells | Promoting E2-induced VEGF | P4: 10−7 mol/L E2: 10−8 mol/L |
| [149] | E2 priming + MPA | Human endometrial epithelial cells Human endometrial fibroblasts | Inhibiting E2-induced VEGF (fibroblasts) Promoting E2-induced VEGF (epithelial) | MPA: 10−6 mol/L E2: 10−8 mol/L |
| [120] | P4 + E2 | Human uterine microvascular endothelial cells | Invasion ↑ | E2: 10−10 to 10−8 mol/L P4: 10−8 to 10−6 mol/L |
| [21] | MPA + E2 | Endothelial progenitor cells (human) | Partially inhibiting E2-induced proliferation (MPA < 10−5 mol/L) Significantly inhibiting E2-induced proliferation (MPA > 10−4 mol/L) | E2: 10−8 mol/L MPA: 10−5 to 10−4 mol/L |
| [55] | P4 + E2 | Human endometrial endothelial cell | Proliferation ↑ | E2: 10−8 mol/L P4: 10−8 mol/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.; Xiao, J.; Chen, Q. Solving the Puzzle: What Is the Role of Progestogens in Neovascularization? Biomolecules 2021, 11, 1686. https://doi.org/10.3390/biom11111686
Xia Z, Xiao J, Chen Q. Solving the Puzzle: What Is the Role of Progestogens in Neovascularization? Biomolecules. 2021; 11(11):1686. https://doi.org/10.3390/biom11111686
Chicago/Turabian StyleXia, Zhi, Jian Xiao, and Qiong Chen. 2021. "Solving the Puzzle: What Is the Role of Progestogens in Neovascularization?" Biomolecules 11, no. 11: 1686. https://doi.org/10.3390/biom11111686
APA StyleXia, Z., Xiao, J., & Chen, Q. (2021). Solving the Puzzle: What Is the Role of Progestogens in Neovascularization? Biomolecules, 11(11), 1686. https://doi.org/10.3390/biom11111686





