Cellulose–Silver Composites Materials: Preparation and Applications
Abstract
:1. Introduction
2. Cellulose Forms
2.1. Cellulose Derivatives/Silver Nanocomposites
2.2. Cellulose Nanomaterials/Silver Nanocomposites
2.3. Bacterial Cellulose/Silver Nanocomposites
3. Silver Ions
4. Preparation Methods for Cellulose/Silver Nanocomposites
4.1. Using Mutual Solvents or One Post-Synthesis
4.2. In Situ Reduction
4.3. Electrospinning and Electrospraying
4.4. Additive Manufacturing
4.4.1. Electro Aided Dropping (EAD)
4.4.2. 3D Ink Jet Printing
5. Properties and Applications of Cellulose/Silver Composites
5.1. Drug Delivery
5.2. Wound Healing
5.3. Electric Conductivity
5.4. Thermal Conductivity
5.5. High-Performance Textiles
5.6. Photocatalytic Properties
5.7. Sensors
5.8. Food Packaging
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salama, A.; Hesemann, P. Recent Trends in Elaboration, Processing, and Derivatization of Cellulosic Materials Using Ionic Liquids. ACS Sustain. Chem. Eng. 2020, 8, 17893–17907. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in green polymer composites from lignin for multifunctional applications: A review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Abouzeid, R.E.; Khiari, R.; Salama, A.; Diab, M.; Beneventi, D.; Dufresne, A. In situ mineralization of nano-hydroxyapatite on bifunctional cellulose nanofiber/polyvinyl alcohol/sodium alginate hydrogel using 3D printing. Int. J. Biol. Macromol. 2020, 160, 538–547. [Google Scholar] [CrossRef]
- Hassan, H.; Salama, A.; El-ziaty, A.K.; El-sakhawy, M. New chitosan/silica/zinc oxide nanocomposite as adsorbent for dye removal. Int. J. Biol. Macromol. 2019, 131, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. Cellulose/silk fibroin assisted calcium phosphate growth: Novel biocomposite for dye adsorption. Int. J. Biol. Macromol. 2020, 165, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Hesemann, P. New N-guanidinium chitosan/silica ionic microhybrids as efficient adsorbent for dye removal from waste water. Int. J. Biol. Macromol. 2018, 111, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Hesemann, P. Synthesis of N-Guanidinium-chitosan/silica hybrid composites: Efficient adsorbents for anionic pollutants. J. Polym. Environ. 2018, 26, 1986–1997. [Google Scholar] [CrossRef]
- Chinthalapudi, N.; Kommaraju, V.V.D.; Kannan, M.K.; Nalluri, C.B.; Varanasi, S. Composites of cellulose nanofibers and silver nanoparticles for malachite green dye removal from water. Carbohydr. Polym. Technol. Appl. 2021, 2, 100098. [Google Scholar] [CrossRef]
- Salama, A.; El-Sakhawy, M.; Kamel, S. Carboxymethyl cellulose based hybrid material for sustained release of protein drugs. Int. J. Biol. Macromol. 2016, 93, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. Preparation of CMC-g-P(SPMA) super adsorbent hydrogels: Exploring their capacity for MB removal from waste water. Int. J. Biol. Macromol. 2018, 106, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. New sustainable hybrid material as adsorbent for dye removal from aqueous solutions. J. Colloid Interface Sci. 2017, 487, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Shukry, N.; El-Sakhawy, M. Carboxymethyl cellulose-g-poly(2-(dimethylamino) ethyl methacrylate) hydrogel as adsorbent for dye removal. Int. J. Biol. Macromol. 2015, 73, 72–75. [Google Scholar] [CrossRef]
- Belford, D.S.; Preston, R.D.; Cook, C.D.; Nevard, E.H. Timber Preservation by Copper Compounds. Nature 1957, 180, 1081–1083. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent Developments in Paper-Based Microfluidic Devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef]
- Salama, A. Functionalized hybrid materials assisted organic dyes removal from aqueous solutions. Environ. Nanotechnol. Monit. Manag. 2016, 6, 159–163. [Google Scholar] [CrossRef]
- Salama, A. Cellulose/calcium phosphate hybrids: New materials for biomedical and environmental applications. Int. J. Biol. Macromol. 2019, 127, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Van Rie, J.; Thielemans, W. Cellulose-gold nanoparticle hybrid materials. Nanoscale 2017, 9, 8525–8554. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S. Nanoporous Cellulose as Metal Nanoparticles Support. Biomacromolecules 2009, 10, 87–94. [Google Scholar] [CrossRef]
- Salama, A. Dicarboxylic cellulose decorated with silver nanoparticles as sustainable antibacterial nanocomposite material. Environ. Nanotechnol. Monit. Manag. 2017, 8, 228–232. [Google Scholar] [CrossRef]
- Pal, S.; Nisi, R.; Stoppa, M.; Licciulli, A. Silver-Functionalized Bacterial Cellulose as Antibacterial Membrane for Wound-Healing Applications. ACS Omega 2017, 2, 3632–3639. [Google Scholar] [CrossRef] [Green Version]
- Salama, A.; Etri, S.; Mohamed, S.A.A.; El-Sakhawy, M. Carboxymethyl cellulose prepared from mesquite tree: New source for promising nanocomposite materials. Carbohydr. Polym. 2018, 189, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Monier, M.; Abdel-Latif, D.A. Synthesis and characterization of ion-imprinted resin based on carboxymethyl cellulose for selective removal of UO₂2+. Carbohydr. Polym. 2013, 97, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Abou-Zeid, R.E.; Cruz-Maya, I.; Guarino, V. Soy protein hydrolysate grafted cellulose nanofibrils with bioactive signals for bone repair and regeneration. Carbohydr. Polym. 2019, 229, 115472. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Nagalakshmaiah, M.; El Kissi, N.; Dufresne, A. Ionic Compatibilization of Cellulose Nanocrystals with Quaternary Ammonium Salt and Their Melt Extrusion with Polypropylene. ACS Appl. Mater. Interfaces 2016, 8, 8755–8764. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmaiah, M.; Pignon, F.; El Kissi, N.; Dufresne, A. Surface adsorption of triblock copolymer (PEO–PPO–PEO) on cellulose nanocrystals and their melt extrusion with polyethylene. RSC Adv. 2016, 6, 66224–66232. [Google Scholar] [CrossRef]
- Fu, F.; Gu, J.; Cao, J.; Shen, R.; Liu, H.; Zhang, Y.; Liu, X.; Zhous, J. Reduction of Silver Ions Using an Alkaline Cellulose Dope: Straightforward Access to Ag/ZnO Decorated Cellulose Nanocomposite Film with Enhanced Antibacterial Activities. ACS Sustain. Chem. Eng. 2018, 6, 738–748. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Self-assembling of TEMPO Oxidized Cellulose Nanofibrils As Affected by Protonation of Surface Carboxyls and Drying Methods. ACS Sustain. Chem. Eng. 2016, 4, 1041–1049. [Google Scholar] [CrossRef]
- Maliyekkal, S.M.; Lisha, K.P.; Pradeep, T. A novel cellulose–manganese oxide hybrid material by in situ soft chemical synthesis and its application for the removal of Pb(II) from water. J. Hazard. Mater. 2010, 181, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; He, J. Cellulose as a Scaffold for Self-Assembly: From Basic Research to Real Applications. Langmuir 2016, 32, 12269–12282. [Google Scholar] [CrossRef]
- Zhu, C.; Xue, J.; He, J. Controlled In-Situ Synthesis of Silver Nanoparticles in Natural Cellulose Fibers Toward Highly Efficient Antimicrobial Materials. J. Nanosci. Nanotechnol. 2009, 9, 3067–3074. [Google Scholar] [CrossRef]
- Wu, M.; Kuga, S.; Huang, Y. Quasi-one-dimensional arrangement of silver nanoparticles templated by cellulose microfibrils. Langmuir 2008, 24, 10494–10497. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.; Wu, H.; Li, Q.; Hu, W.; Zhang, Z.; Huang, L.; Zhang, J.; Chen, D.; Deng, S.; et al. Graphene Oxide-IPDI-Ag/ZnO@Hydroxypropyl Cellulose Nanocomposite Films for Biological Wound-Dressing Applications. ACS Omega 2019, 4, 15373–15381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, S. Rapid synthesis of antimicrobial paper under microwave irradiation. Carbohydr. Polym. 2012, 90, 1538–1542. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-M.; Jia, N.; Ma, M.-G.; Zhang, Z.; Liu, Q.-H.; Sun, R.-C. Cellulose–silver nanocomposites: Microwave-assisted synthesis, characterization, their thermal stability, and antimicrobial property. Carbohydr. Polym. 2011, 86, 441–447. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Li, Z.; Wei, G.; Qiao, Y. Ag nanoparticles coated cellulose membrane with high infrared reflection, breathability and antibacterial property for human thermal insulation. J. Colloid Interface Sci. 2019, 535, 363–370. [Google Scholar] [CrossRef]
- Aladpoosh, R.; Montazer, M.; Samadi, N. In situ green synthesis of silver nanoparticles on cotton fabric using Seidlitzia rosmarinus ashes. Cellulose 2014, 21, 3755–3766. [Google Scholar] [CrossRef]
- Suwan, T.; Khongkhunthian, S.; Okonogi, S. Silver nanoparticles fabricated by reducing property of cellulose derivatives. Drug Discov. Ther. 2019, 13, 70–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gromovykh, T.I.; Vasil’kov, A.Y.; Sadykova, V.S.; Feldman, N.B.; Demchenko, A.G.; Lyundup, A.V.; Butenko, I.E.; Lutsenko, S.V. Creation of composites of bacterial cellulose and silver nanoparticles: Evaluation of antimicrobial activity and cytotoxicity. Int. J. Nanotechnol. 2019, 16, 408–420. [Google Scholar] [CrossRef]
- Uddin, K.M.A.; Lokanathan, A.R.; Liljeström, A.; Chen, X.; Rojas, O.J.; Laine, J. Silver nanoparticle synthesis mediated by carboxylated cellulose nanocrystals. Green Mater. 2014, 2, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Lokanathan, A.R.; Uddin, K.M.A.; Rojas, O.J.; Laine, J. Cellulose nanocrystal-mediated synthesis of silver nanoparticles: Role of sulfate groups in nucleation phenomena. Biomacromolecules 2014, 15, 373–379. [Google Scholar] [CrossRef]
- Stinson-Bagby, K.L.; Owens, J.; Rouffa, A.; Bortner, M.J.; Foster, E.J. Silver Nanoparticle Pulsed Synthesis and Attachment to Cellulose Nanocrystals. ACS Appl. Nano Mater. 2019, 2, 2317–2324. [Google Scholar] [CrossRef]
- Ma, P.; Jiang, L.; Yu, M.; Dong, W.; Chen, M. Green Antibacterial Nanocomposites from Poly(lactide)/Poly(butylene adipate-co-terephthalate)/Nanocrystal Cellulose-Silver Nanohybrids. ACS Sustain. Chem. Eng. 2016, 4, 6417–6426. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, H.; Gao, M.; Zhang, M.; Liu, P.; Liu, X. Cellulose nanocrystals/silver nanoparticles: In-situ preparation and application in PVA films. Holzforschung 2020, 74, 523–528. [Google Scholar] [CrossRef]
- Liu, H.; Song, J.; Shang, S.; Song, Z.; Wang, D. Cellulose Nanocrystal/Silver Nanoparticle Composites as Bifunctional Nanofillers within Waterborne Polyurethane. ACS Appl. Mater. Interfaces 2012, 4, 2413–2419. [Google Scholar] [CrossRef]
- Khamrai, M.; Banerjee, S.L.; Kundu, P.P. Modified bacterial cellulose based self-healable polyeloctrolyte film for wound dressing application. Carbohydr. Polym. 2017, 174, 580–590. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2013, 21, 1–30. [Google Scholar] [CrossRef]
- Zhu, C.; Li, F.; Zhou, X.; Lin, L.; Zhang, T. Kombucha-synthesized bacterial cellulose: Preparation, characterization, and biocompatibility evaluation. J. Biomed. Mater. Res. Part A 2014, 102, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Laromaine, A.; Roig, A. Bacterial cellulose films: Influence of bacterial strain and drying route on film properties. Cellulose 2014, 21, 4455–4469. [Google Scholar] [CrossRef] [Green Version]
- Kucińska-Lipka, J.; Gubanska, I.; Janik, H. Bacterial cellulose in the field of wound healing and regenerative medicine of skin: Recent trends and future prospectives. Polym. Bull. 2015, 72, 2399–2419. [Google Scholar] [CrossRef]
- Branco da Cunha, C.; Klumpers, D.D.; Li, W.A.; Koshy, S.T.; Weaver, J.C.; Chaudhuri, O.; Mooney, D.J. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials 2014, 35, 8927–8936. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Tsuji, M.; Morimoto, M.; Saimoto, H. Synthesis of Silver Nanoparticles Templated by TEMPO-Mediated Oxidized Bacterial Cellulose Nanofibers. Biomacromolecules 2009, 10, 2714–2717. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Hu, W.; Shi, S.; Shen, W.; Zhang, X.; Wang, H. In situ synthesis of CdS nanoparticles on bacterial cellulose nanofibers. Carbohydr. Polym. 2009, 76, 509–512. [Google Scholar] [CrossRef]
- Wen, X.; Zheng, Y.; Wu, J.; Yue, L.; Wang, C.; Luan, J.; Wu, Z.; Wang, K. In vitro and in vivo investigation of bacterial cellulose dressing containing uniform silver sulfadiazine nanoparticles for burn wound healing. Prog. Nat. Sci. Mater. Int. 2015, 25, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Ghosh, A.K.; Sarkar, P.; Kundu, P.P. A Mussel Mimetic, Bioadhesive, Antimicrobial Patch Based on Dopamine-Modified Bacterial Cellulose/rGO/Ag NPs: A Green Approach toward Wound-Healing Applications. ACS Sustain. Chem. Eng. 2019, 7, 12083–12097. [Google Scholar] [CrossRef]
- Alonso-Díaz, A.; Floriach-Clark, J.; Fuentes, J.; Capellades, M.; Coll, N.S.; Laromaine, A. Enhancing Localized Pesticide Action through Plant Foliage by Silver-Cellulose Hybrid Patches. ACS Biomater. Sci. Eng. 2019, 5, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasim, M.; Mushtaq, M.; Khan, S.U.; Farooq, A.; Naeem, M.A.; Khan, M.R.; Salam, A.; Wei, Q. Development of bacterial cellulose nanocomposites: An overview of the synthesis of bacterial cellulose nanocomposites with metallic and metallic-oxide nanoparticles by different methods and techniques for biomedical applications. J. Ind. Text. 2020. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Y.; Vlahovic, B. A Review on Preparation and Applications of Silver-Containing Nanofibers. Nanoscale Res. Lett. 2016, 11, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Ferraria, A.M.; Boufi, S.; Battaglini, N.; Do Rego, A.M.B.; Reivilar, M. Hybrid systems of silver nanoparticles generated on cellulose surfaces. Langmuir 2010, 26, 1996–2001. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, H.K.; Lee, Y.M.; Kim, K.; Park, S.B. A practical procedure for producing silver nanocoated fabric and its antibacterial evaluation for biomedical applications. Chem. Commun. 2007, 2959–2961. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.G. A Pharmacological and Toxicological Profile of Silver as an Antimicrobial Agent in Medical Devices. Adv. Pharmacol. Sci. 2010, 2010, 910686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Li, S.; Yue, X.; Lu, W. Review of silver nanoparticles (AgNPs)-cellulose antibacterial composites. BioResources 2018, 13, 2150–2170. [Google Scholar] [CrossRef]
- Tran, C.D.; Prosenc, F.; Franko, M.; Benzi, G. One-Pot Synthesis of Biocompatible Silver Nanoparticle Composites from Cellulose and Keratin: Characterization and Antimicrobial Activity. ACS Appl. Mater. Interfaces 2016, 8, 34791–34801. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhao, N.; Zhang, X.; Xu, J. Cellulose/silver nanoparticles composite microspheres: Eco-friendly synthesis and catalytic application. Cellulose 2012, 19, 1239–1249. [Google Scholar] [CrossRef]
- Xu, H.; Xu, J.; Zhu, Z.; Liu, H.; Liu, S. In-Situ Formation of Silver Nanoparticles with Tunable Spatial Distribution at the Poly(N-isopropylacrylamide) Corona of Unimolecular Micelles. Macromolecules 2006, 39, 8451–8455. [Google Scholar] [CrossRef]
- Lu, Y.; Spyra, P.; Mei, Y.; Ballauff, M.; Pich, A. Composite Hydrogels: Robust Carriers for Catalytic Nanoparticles. Macromol. Chem. Phys. 2007, 208, 254–261. [Google Scholar] [CrossRef]
- Boroumand, M.N.; Montazer, M.; Simon, F.; Liesiene, J.; Šaponjic, Z.; Dutschk, V. Novel method for synthesis of silver nanoparticles and their application on wool. Appl. Surf. Sci. 2015, 346, 477–483. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yin, J.S.; Ma, E. Nanocrystalline Ag formed by low-temperature high-energy mechanical attrition. Nanostructured Mater. 1997, 8, 91–100. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr. Res. 2004, 339, 2627–2631. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.B.; Emam, H.E. Layer by layer assembly of nanosilver for high performance cotton fabrics. Fibers Polym. 2016, 17, 418–426. [Google Scholar] [CrossRef]
- Courrol, L.C.; de Oliveira Silva, F.R.; Gomes, L. A simple method to synthesize silver nanoparticles by photo-reduction. Colloids Surf. A Physicochem. Eng. Asp. 2007, 305, 54–57. [Google Scholar] [CrossRef]
- Sato-Berrú, R.; Redón, R.; Vázquez-Olmos, A.; Saniger, J.M. Silver nanoparticles synthesized by direct photoreduction of metal salts. Application in surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 376–380. [Google Scholar] [CrossRef]
- Fujii, Y.; Imagawa, K.; Omura, T.; Suzuki, T.; Minami, H. Preparation of Cellulose/Silver Composite Particles Having a Recyclable Catalytic Property. ACS Omega 2020, 5, 1919–1926. [Google Scholar] [CrossRef] [Green Version]
- Wendler, F.; Konkin, A.; Heinze, T. Studies on the stabilization of modified Lyocell solutions. Macromol. Symp. 2008, 262, 72–84. [Google Scholar] [CrossRef]
- Fadakar Sarkandi, A.; Montazer, M.; Harifi, T.; Mahmoudi Rad, M. Innovative preparation of bacterial cellulose/silver nanocomposite hydrogels: In situ green synthesis, characterization, and antibacterial properties. J. Appl. Polym. Sci. 2021, 138, 49824. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Fouda, A. Green approach for one-pot synthesis of silver nanorod using cellulose nanocrystal and their cytotoxicity and antibacterial assessment. Int. J. Biol. Macromol. 2018, 106, 784–792. [Google Scholar] [CrossRef]
- Hanif, Z.; Khan, Z.A.; Choi, D.; La, M.; Park, S.J. One-pot synthesis of silver nanoparticle deposited cellulose nanocrystals with high colloidal stability for bacterial contaminated water purification. J. Environ. Chem. Eng. 2021, 9, 105535. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Xiao, S.L.; Shi, S.Q.; Cai, L.P. A one-pot synthesis and characterization of antibacterial silver nanoparticle-cellulose film. Polymers 2020, 12, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haslinger, S.; Ye, Y.; Rissanen, M.; Hummel, M.; Sixta, H. Cellulose Fibers for High-Performance Textiles Functionalized with Incorporated Gold and Silver Nanoparticles. ACS Sustain. Chem. Eng. 2020, 8, 649–658. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Kim, I.S.; Ni, Q.Q. A comparative study on synthesis of AgNPs on cellulose nanofibers by thermal treatment and DMF for antibacterial activities. Mater. Sci. Eng. C 2019, 98, 1179–1195. [Google Scholar] [CrossRef]
- Li, R.; He, M.; Li, T.; Zhang, L. Preparation and properties of cellulose/silver nanocomposite fibers. Carbohydr. Polym. 2015, 115, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.W.; Kim, I.S.; Ni, Q.Q. Cellulose acetate nanofibers embedded with AgNPs anchored TiO 2 nanoparticles for long term excellent antibacterial applications. Carbohydr. Polym. 2019, 207, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.W.; Jo, Y.K.; Lee, H.; Oh, S.G.; Hwang, D.S.; Khatri, Z.; Kim, I.S. Antibacterial efficacy of poly(vinyl alcohol) composite nanofibers embedded with silver-anchored silica nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1121–1128. [Google Scholar] [CrossRef]
- Spagnol, C.; Fragal, E.H.; Pereira, A.G.B.; Nakamura, C.V.; Muniz, E.C.; Follmann, H.D.M.; Rubira, A.F. Cellulose nanowhiskers decorated with silver nanoparticles as an additive to antibacterial polymers membranes fabricated by electrospinning. J. Colloid Interface Sci. 2018, 531, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, N.; Yamin, A.; Hamid, S.A.; Khodir, W.K.W.A.; Guarino, V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. J. Funct. Biomater. 2021, 12, 59. [Google Scholar] [CrossRef]
- Štular, D.; Savio, E.; Simončič, B.; Šobak, M.; Jerman, I.; Poljanšek, I.; Ferri, A.; Tomšič, B. Multifunctional antibacterial and ultraviolet protective cotton cellulose developed by in situ biosynthesis of silver nanoparticles into a polysiloxane matrix mediated by sumac leaf extract. Appl. Surf. Sci. 2021, 563, 150361. [Google Scholar] [CrossRef]
- Ravindran, A.; Mani, V.; Chandrasekaran, N.; Mukherjee, A. Selective colorimetric sensing of cysteine in aqueous solutions using silver nanoparticles in the presence of Cr3+. Talanta 2011, 85, 533–540. [Google Scholar] [CrossRef]
- Phan, D.N.; Dorjjugder, N.; Khan, M.Q.; Saito, Y.; Taguchi, G.; Lee, H.; Mukai, Y.; Kim, I.S. Synthesis and attachment of silver and copper nanoparticles on cellulose nanofibers and comparative antibacterial study. Cellulose 2019, 26, 6629–6640. [Google Scholar] [CrossRef]
- Alahmadi, N.S.; Betts, J.W.; Heinze, T.; Kelly, S.M.; Koschella, A.; Wadhawan, J.D. Synthesis and antimicrobial effects of highly dispersed, cellulose-stabilized silver/cellulose nanocomposites. RSC Adv. 2018, 8, 3646–3656. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, S.; Wang, Z.; Yao, Q.; Fang, S.; Zhou, X.; Yuan, X.; Xie, J. The in situ synthesis of silver nanoclusters inside a bacterial cellulose hydrogel for antibacterial applications. J. Mater. Chem. B 2020, 8, 4846–4850. [Google Scholar] [CrossRef]
- Ozturk, O.; Oter, O.; Yildirim, S.; Subasi, E.; Ertekin, K.; Celik, E.; Temel, H. Tuning oxygen sensitivity of ruthenium complex exploiting silver nanoparticles. J. Lumin. 2014, 155, 191–197. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, C.; Zhang, F.; Monty, J.; Linhardt, R.J.; Simmons, T.J. Can natural fibers be a silver bullet? Antibacterial cellulose fibers through the covalent bonding of silver nanoparticles to electrospun fibers. Nanotechnology 2016, 27, 055102. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Shabir, N.; Vasita, R.; Jadhav, A.H.; Sheikh, F.A. Regenerated cellulose nanofibers from cellulose acetate: Incorporating hydroxyapatite (HAp) and silver (Ag) nanoparticles (NPs), as a scaffold for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111547. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Lakshmi, G.B.V.S.; Sri, S.; Chauhan, D.; Chakraborty, A.; Singh, S.; Solanki, P.R. Potential of electrospun cellulose acetate nanofiber mat integrated with silver nanoparticles from Azadirachta indica as antimicrobial agent. J. Polym. Res. 2020, 27, 350. [Google Scholar] [CrossRef]
- San Keskin, N.O.; Deniz, F.; Nazir, H. Anti microbial corrosion properties of electrospun cellulose acetate nanofibers containing biogenic silver nanoparticles for copper coatings. RSC Adv. 2020, 10, 39901–39908. [Google Scholar] [CrossRef]
- Wang, S.; Sun, J.; Jia, Y.; Yang, L.; Wang, N.; Xianyu, Y.; Chen, W.; Li, X.; Cha, R.; Jiang, X. Nanocrystalline Cellulose-Assisted Generation of Silver Nanoparticles for Nonenzymatic Glucose Detection and Antibacterial Agent. Biomacromolecules 2016, 17, 2472–2478. [Google Scholar] [CrossRef] [PubMed]
- Musino, D.; Rivard, C.; Landrot, G.; Novales, B.; Rabilloud, T.; Capron, I. Hydroxyl groups on cellulose nanocrystal surfaces form nucleation points for silver nanoparticles of varying shapes and sizes. J. Colloid Interface Sci. 2021, 584, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Pourreza, N.; Golmohammadi, H.; Naghdi, T.; Yousefi, H. Green in-situ synthesized silver nanoparticles embedded in bacterial cellulose nanopaper as a bionanocomposite plasmonic sensor. Biosens. Bioelectron. 2015, 74, 353–359. [Google Scholar] [CrossRef]
- Guarino, V.; Altobelli, R.; Cirillo, V.; Cummaro, A.; Ambrosio, L. Additive electrospraying: A route to process electrospun scaffolds for controlled molecular release. Polym. Adv. Technol. 2015, 26, 1359–1369. [Google Scholar] [CrossRef]
- Zuppolini, S.; Maya, I.C.; Diodato, L.; Guarino, V.; Borriello, A.; Ambrosio, L. Self-associating cellulose-graft-poly(ε-caprolactone) to design nanoparticles for drug release. Mater. Sci. Eng. C 2020, 108, 110385. [Google Scholar] [CrossRef]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antibacterial surfaces prepared by electrospray coating of photocatalytic nanoparticles. Chem. Eng. J. 2018, 334, 1108–1118. [Google Scholar] [CrossRef]
- Guarino, V.; Cruz-Maya, I.; Altobelli, R.; Abdul Khodir, W.K.; Ambrosio, L.; Alvarez Pèrez, M.A.; Flores, A.A. Electrospun polycaprolactone nanofibres decorated by drug loaded chitosan nano-reservoirs for antibacterial treatments. Nanotechnology 2017, 28, 505103. [Google Scholar] [CrossRef]
- Guarino, V.; Cirillo, V.; Altobelli, R.; Ambrosio, L. Polymer-based platforms by electric field-assisted techniques for tissue engineering and cancer therapy. Expert Rev. Med Devices 2015, 12, 113–129. [Google Scholar] [CrossRef]
- Giachini, P.A.G.S.; Gupta, S.S.; Wang, W.; Wood, D.; Yunusa, M.; Baharlou, E.; Sitti, M.; Menges, A. Additive manufacturing of cellulose-based materials with continuous, multidirectional stiffness gradients. Sci. Adv. 2020, 6, eaay0929. [Google Scholar] [CrossRef] [Green Version]
- Markstedt, K.; Escalante, A.; Toriz, G.; Gatenholm, P. Biomimetic Inks Based on Cellulose Nanofibrils and Cross-Linkable Xylans for 3D Printing. ACS Appl. Mater. Interfaces 2017, 9, 40878–40886. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Mohan, T.; Dobaj Štiglic, A.; Beaumont, M.; Konnerth, J.; Gürer, F.; Makuc, D.; Maver, U.; Gradišnik, L.; Plavec, J.; Kargl, R.; et al. Generic Method for Designing Self-Standing and Dual Porous 3D Bioscaffolds from Cellulosic Nanomaterials for Tissue Engineering Applications. ACS Appl. Bio Mater. 2020, 3, 1197–1209. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Zhang, X.; Yang, P.; Långvik, O.; Wang, X.; Zhang, Y.; Cheng, F.; Osterberg, M.; Willfor, S.; Xu, C. Surface Engineered Biomimetic Inks Based on UV Cross-Linkable Wood Biopolymers for 3D Printing. ACS Appl. Mater. Interfaces 2019, 11, 12389–12400. [Google Scholar] [CrossRef] [Green Version]
- Sayem, A.S.M.; Shahariar, H.; Haider, J. An Overview on the Opportunities for 3D Printing With Biobased Materials. Encycl. Renew. Sustain. Mater. 2020, 2, 839–847. [Google Scholar] [CrossRef]
- Dalton, P.D. Melt electrowriting with additive manufacturing principles. Curr. Opin. Biomed. Eng. 2017, 2, 49–57. [Google Scholar] [CrossRef]
- Robinson, T.M.; Hutmacher, D.W.; Dalton, P.D. The Next Frontier in Melt Electrospinning: Taming the Jet. Adv. Funct. Mater. 2019, 29, 1904664. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Bronner, T.; Yamamoto, M.; Yamane, H. Regeneration of cellulose dissolved in ionic liquid using laser-heated melt-electrospinning. Carbohydr. Polym. 2018, 201, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Lintinen, K.; Luiro, S.; Figueiredo, P.; Sakarinen, E.; Mousavi, Z.; Seitsonen, J.; Riviere, G.N.S.; Mattinen, U.; Niemela, M.; Tammela, P.; et al. Antimicrobial Colloidal Silver–Lignin Particles via Ion and Solvent Exchange. ACS Sustain. Chem. Eng. 2019, 7, 15297–15303. [Google Scholar] [CrossRef] [Green Version]
- Mohan, D.; Teong, Z.K.; Bakir, A.N.; Sajab, M.S.; Kaco, H. Extending Cellulose-Based Polymers Application in Additive Manufacturing Technology: A Review of Recent Approaches. Polymers 2020, 12, 1876. [Google Scholar] [CrossRef]
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of Anisotropic Hydrogels and Their Applications. Angew. Chem. Int. Ed. 2018, 57, 2532–2543. [Google Scholar] [CrossRef]
- Liverani, L.; Guarino, V.; La Carrubba, V.; Boccaccini, A.R. Porous Biomaterials and Scaffolds for Tissue Engineering. Encycl. Biomed. Eng. 2019, 188–202. [Google Scholar] [CrossRef]
- Fujisaki, Y.; Koga, H.; Nakajima, Y.; Nakata, M.; Tsuji, H.; Yamamoto, T.; Kurita, T.; Nogi, M.; Shimidzu, N. Transparent Nanopaper-Based Flexible Organic Thin-Film Transistor Array. Adv. Funct. Mater. 2014, 24, 1657–1663. [Google Scholar] [CrossRef]
- Dias, O.A.T.; Konar, S.; Leão, A.L.; Yang, W.; Tjong, J.; Sain, M. Current State of Applications of Nanocellulose in Flexible Energy and Electronic Devices. Front. Chem. 2020, 8, 420. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Kim, C.; Nogi, M.; Suganuma, K. Electrically conductive lines on cellulose nanopaper for flexible electrical devices. Nanoscale 2013, 5, 9289–9295. [Google Scholar] [CrossRef] [PubMed]
- Nogi, M.; Komoda, N.; Otsuka, K.; Suganuma, K. Foldable nanopaper antennas for origami electronics. Nanoscale 2013, 5, 4395–4399. [Google Scholar] [CrossRef]
- Koga, H.; Saito, T.; Kitaoka, T.; Nogi, M.; Suganuma, K.; Isogai, A. Transparent, Conductive, and Printable Composites Consisting of TEMPO-Oxidized Nanocellulose and Carbon Nanotube. Biomacromolecules 2013, 14, 1160–1165. [Google Scholar] [CrossRef]
- Hoeng, F.; Bras, J.; Gicquel, E.; Krosnicki, G.; Denneulin, A. Inkjet printing of nanocellulose–silver ink onto nanocellulose coated cardboard. RSC Adv. 2017, 7, 15372–15381. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.D.; Choi, J.S.; Kim, B.S.; Chan Choi, Y.; Cho, Y.W. Piezoelectric inkjet printing of polymers: Stem cell patterning on polymer substrates. Polymer 2010, 51, 2147–2154. [Google Scholar] [CrossRef]
- Altobelli, R.; Guarino, V.; Ambrosio, L. Micro- and nanocarriers by electrofludodynamic technologies for cell and molecular therapies. Process. Biochem. 2016, 51, 2143–2154. [Google Scholar] [CrossRef]
- Guarino, V.; Altobelli, R.; Caputo, T.; Ambrosio, L.; Caserta, S.; Calcagnile, P.; Demitri, C. Mono- and Bi-Phasic Cellulose Acetate Micro-Vectors for Anti-Inflammatory Drug Delivery. Pharmaceutics 2019, 11, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarino, V.; Caputo, T.; Calcagnile, P.; Altobelli, R.; Demitri, C.; Ambrosio, L. Core/shell cellulose-based microspheres for oral administration of Ketoprofen Lysinate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.A.; Ivanova, O.S. 3D printing of multifunctional nanocomposites. Nano Today 2013, 8, 119–120. [Google Scholar] [CrossRef]
- Siqueira, G.; Kokkinis, D.; Libanori, R.; Hausmann, M.K.; Gladman, A.S.; Neels, A.; Tingaut, P.; Zimmermann, T.; Lewis, J.A.; Studart, A.R. Cellulose Nanocrystal Inks for 3D Printing of Textured Cellular Architectures. Adv. Funct. Mater. 2017, 27, 1604619. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Wang, Y.; Ray, U.; Zhu, S.; Dai, J.; Chen, C.; Fu, K.; Jang, S.-H.; Henderson, D.; et al. Cellulose-Nanofiber-Enabled 3D Printing of a Carbon-Nanotube Microfiber Network. Small Methods 2017, 1, 1700222. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Mietner, J.B.; Jiang, X.; Edlund, U.; Saake, B.; Navarro, J.R.G. 3D printing of a bio-based ink made of cross-linked cellulose nanofibrils with various metal cations. Sci. Rep. 2021, 11, 6461. [Google Scholar] [CrossRef]
- Daia, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Nia, Y. 3D printing using plant-derived cellulose and its derivatives: A review. Carbohydr. Polym. 2019, 203, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Chinga-Carrasco, G. Potential and Limitations of Nanocelluloses as Components in Biocomposite Inks for Three-Dimensional Bioprinting and for Biomedical Devices. Biomacromolecules 2018, 19, 701–711. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules. 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Yang, G.-H.; Lee, J.-H.; Kim, G.-H. 3D bioprinting and its in vivo applications. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Jenni, L.; Panu, L.; Antti, P.; Riitta, M.; Sni, M.-K.; Tatu, P.; Heikki, P.; Inger, V.-L.; Pekka, U.; Vesa, H.P. D-Printable Bioactivated Nanocellulose-Alginate Hydrogels. ACS Appl. Mater. Interfaces 2017, 9, 21959–21970. [Google Scholar]
- Martinez Avila, H.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 2016, 1, 22–35. [Google Scholar] [CrossRef]
- Bergonzi, C.; Remaggi, G.; Graiff, C.; Bergamonti, L.; Potenza, M.; Ossiprandi, M.C.; Zanotti, I.; Bernini, F.; Bettini, R.; Elviri, L. Three-Dimensional (3D) Printed Silver Nanoparticles/Alginate/Nanocrystalline Cellulose Hydrogels: Study of the Antimicrobial and Cytotoxicity Efficacy. Nanomaterials 2020, 10, 844. [Google Scholar] [CrossRef]
- Moon, J.Y.; Lee, J.; Hwang, T.I.; Park, C.H.; Kim, C.S. A Multifunctional, One-Step Gas Foaming Strategy for Antimicrobial Silver Nanoparticle-Decorated 3D Cellulose Nanofiber Scaffolds. Carbohydr. Polym. 2021, 273, 118603. [Google Scholar] [CrossRef]
- Information, S. Synthesis of Natural Cellulose-Templated TiO2/Ag Nanosponge Composites and Photocatalytic Properties. Appl. Mater. Interfaces 2012, 4, 2781–2787. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Mei, C.; Li, Y.; Duan, G.; Agarwal, S.; Greiner, A.; Ma, C.; Jiang, S. Wood-Inspired Anisotropic Cellulose Nano fibril Composite Sponges for Multifunctional Applications. ACS Sustain. Chem. Eng. 2020, 3, 3346–3354. [Google Scholar] [CrossRef]
- Wu, C.N.; Fuh, S.C.; Lin, S.P.; Lin, Y.Y.; Chen, H.Y.; Liu, J.M.; Cheng, K.C. TEMPO-Oxidized Bacterial Cellulose Pellicle with Silver Nanoparticles for Wound Dressing. Biomacromolecules 2018, 19, 544–554. [Google Scholar] [CrossRef]
- Li, J.; Kang, L.; Wang, B.; Chen, K.; Tian, X.; Ge, Z.; Zeng, J.; Xu, J.; Gao, W. Controlled Release and Long-Term Antibacterial Activity of Dialdehyde Nanofibrillated Cellulose/Silver Nanoparticle Composites. ACS Sustain. Chem. Eng. 2019, 7, 1146–1158. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, C.; Guan, L.; Zhang, W.; Gu, J. Green Synthesis of Cellulose Nanofibrils Decorated with Ag Nanoparticles and Their Application in Colorimetric Detection of l -Cysteine. ACS Sustain. Chem. Eng. 2020, 8, 12713–12721. [Google Scholar] [CrossRef]
- Lee, T.W.; Lee, S.E.; Jeong, Y.G. Highly Effective Electromagnetic Interference Shielding Materials based on Silver Nanowire/Cellulose Papers. ACS Appl. Mater. Interfaces 2016, 8, 13123–13132. [Google Scholar] [CrossRef]
- Das, D.; Senapati, S.; Nanda, K.K. “Rinse, Repeat”: An Efficient and Reusable SERS and Catalytic Platform Fabricated by Controlled Deposition of Silver Nanoparticles on Cellulose Paper. ACS Sustain. Chem. Eng. 2019, 7, 14089–14101. [Google Scholar] [CrossRef] [Green Version]
- Gu, R.; Yun, H.; Chen, L.; Wang, Q.; Huang, X. Regenerated Cellulose Films with Amino-Terminated Hyperbranched Polyamic Anchored Nanosilver for Active Food Packaging. ACS Appl. Bio Mater. 2020, 3, 602–610. [Google Scholar] [CrossRef] [Green Version]
- Mandal, B.; Rameshbabu, A.P.; Soni, S.R.; Ghosh, A.; Dhara, S.; Pal, S. In Situ Silver Nanowire Deposited Cross-Linked Carboxymethyl Cellulose: A Potential Transdermal Anticancer Drug Carrier. ACS Appl. Mater. Interfaces 2017, 9, 36583–36595. [Google Scholar] [CrossRef]
- Sheikh, E.S.; Sheikh, E.S.; Fetterolf, D.E. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int. Wound J. 2014, 11, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef]
- Plumed-Ferrer, C.; Wright AVon Lindfors, T.; Xu, C.; Latonen, R. Biocomposites of Nano fi brillated Cellulose, Polypyrrole, and Silver Nanoparticles with Electroconductive and Antimicrobial Properties. Biomacromolecules 2014, 15, 3655–3663. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, M.; Chen, X.; Xu, F. Facile Template Synthesis of Microfibrillated Cellulose/Polypyrrole/Silver Nanoparticles Hybrid Aerogels with Electrical Conductive and Pressure Responsive Properties. ACS Sustain. Chem. Eng. 2015, 3, 3346–3354. [Google Scholar] [CrossRef]
- Yu, A.; Ramesh, P.; Sun, X.; Bekyarova, E.; Itkis, M.E.; Haddon, R.C. Enhanced Thermal Conductivity in a Hybrid Graphite Nanoplatelet—Carbon Nanotube Filler for Epoxy Composites. Adv. Mater. 2008, 20, 4740–4744. [Google Scholar] [CrossRef]
- Park, W.; Hu, J.; Jauregui, L.A.; Ruan, X.; Chen, Y.P. Electrical and thermal conductivities of reduced graphene oxide/polystyrene composites. Appl. Phys. Lett. 2014, 104, 113101. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Feng, J. Highly Thermally Conductive Composite Films Based on Nanofibrillated Cellulose in Situ Coated with a Small Amount of Silver Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 24193–24200. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Sun, L.; Jiang, W.; Lynch, V.M. Antimicrobial regenerated cellulose/nano-silver fiber without leaching. J. Bioact. Compat. Polym. 2015, 30, 17–33. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Marques, P.A.A.P.; Neto, C.P.; Trindade, T.; Daina, S.; Sadocco, P. Antibacterial activity of nanocomposites of silver and bacterial or vegetable cellulosic fibers. Acta Biomater. 2009, 5, 2279–2289. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of Localized Surface Plasmon Resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.S.; Park, J.E.; Lee, J. Properties and antimicrobial efficacy of cellulose fiber coated with silver nanoparticles and 3-mercaptopropyltrimethoxysilane (3-MPTMS). J. Appl. Polym. Sci. 2011, 119, 2261–2267. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Siqueira, M.C.; Coelho, G.F.; de Moura, M.R.; Bresolin, J.D.; Hubinger, S.Z.; Marconcini, J.M.; Mattoso, L.H. Evaluation of antimicrobial activity of silver nanoparticles for carboxymethylcellulose film applications in food packaging. J. Nanosci. Nanotechnol. 2014, 14, 5512–5517. [Google Scholar] [CrossRef] [PubMed]








| Cellulose Source | Preparation Method | Properties | Applications | Ref. |
|---|---|---|---|---|
| Cellulose | Chemical reduction | Ag/ZnO decorated cellulose nanocomposite | Rapid sterilization and eradication | [82] |
| Synthesis of silver nanoparticles-covered three-dimensional cellulose | 3D cellulose-Ag scaffold | Tissue engineering and other relevant applications | [142] | |
| Surface sol−gel method | TiO2/Ag nanosponges containing uniform dispersion of silver nanoparticles | Photocatalysts | [143] | |
| Cellulose fibers | In situ biosynthesis of Ag NPs by sumac leaf extract as reducing and stabilising agent | Face-centered cubic Ag NPs with size of 52 to 105 nm | Ag NP improved the durability of the coating | [83] |
| Cellulose nanofibers | Thermal treatment and DMF as reducing agents | Good distribution of AgNPs on cellulose nanofibers | Antimicrobial activities | [77] |
| Decoration with AgNPs via ultraviolet radiation and copper nanoparticles via chemical reduction | The metal release related to the contents of copper or silver | Superior bactericidal activity | [85] | |
| Directional freeze-drying | Silver nanowires | Anisotropic 3D composite sponge | [144] | |
| Celluose nanocrystals | Nucleation of silver nanoparticles | Mediators for silver nanoparticles preparation with good size distribution | [43] | |
| Cellulose acetate nanofibers | In situ synthesis of silver nanoparticles followed by electrospinning technique | Dense and compact entangled nanofibers | An efficient anticorrosive material | [92] |
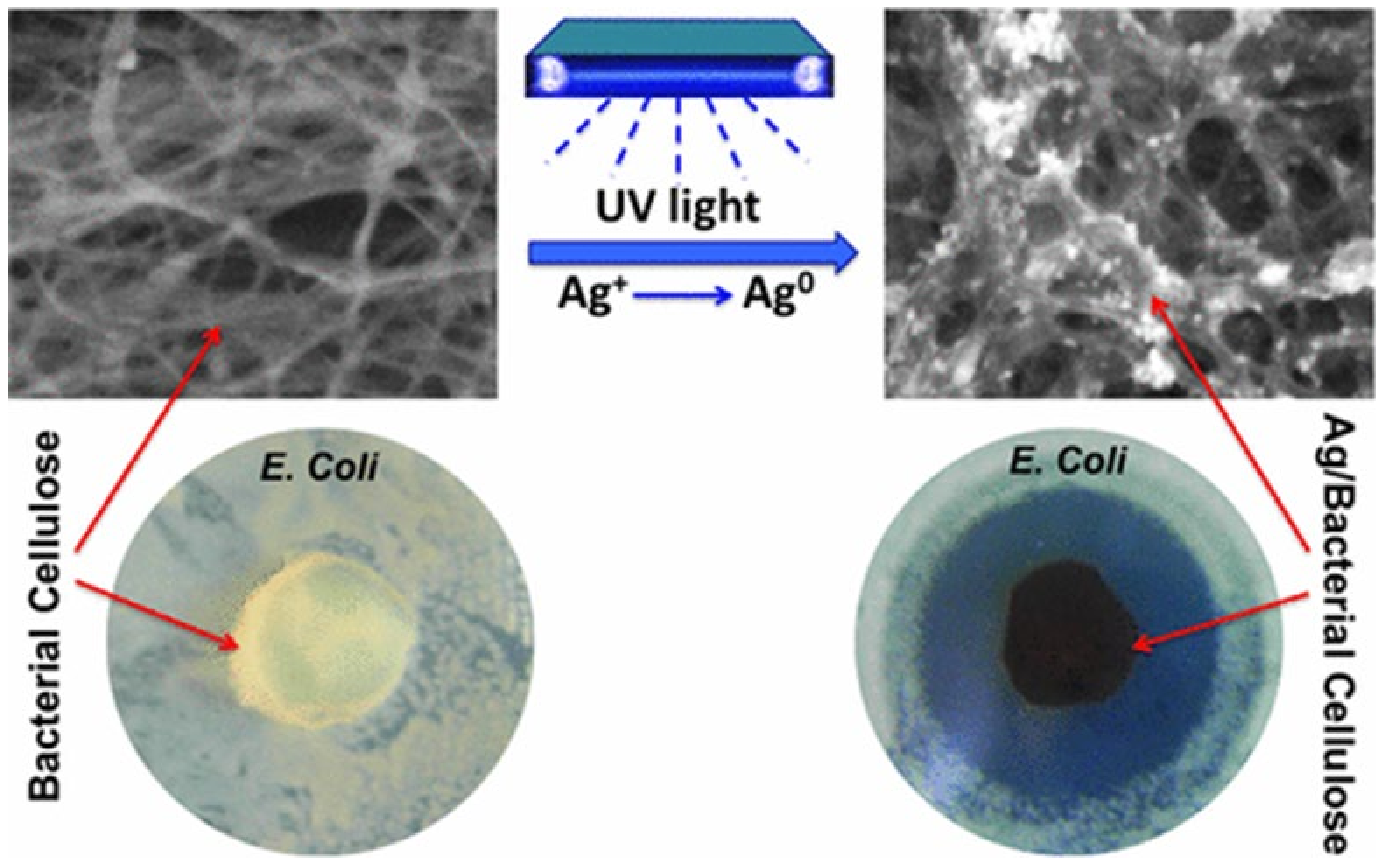
| Bacterial cellulose | UV light irradiation | AgNPs with narrow size distribution along with some aggregate | Antimicrobial membrane for wound-healing treatment | [20] |
| Hydrogel. In situ reduction of Ag NPs | Homogeneous distribution of Ag NPs inside BC hydrogel | Broad-spectrum antimicrobial performance | [87] | |
| Nanocrystals. Chemical reduction of Ag+ ions | High metallic Ag content ranging from 88% to 97% | Food packaging, paints, or surface treatment | [94] | |
| Silver nanoparticles ~16.5 nm were thermal reduction | In situ synthesized on TEMPO oxidized bacterial cellulose nanofiber surfaces by | Wound dressing | [145] | |
| Oxidized bacterial cellulose | Ion-exchange followed by thermal reduction | Controlled size distribution | [54] | |
| Dicarboxylic cellulose | In situ immobilization of silver nanoparticles | Uniform silver nanoparticles with 15 nm size. | Dicarboxylic cellulose/silver nanocomposite | [19] |
| Oxidized cellulose microfibrils containing aldehyde groups | Silver mirror reaction | Particle size ranged from 5 to 25 nm | Materials had an electric conductivity of approximately 5 S/cm | [34] |
| Dialdehyde nanofobrillated cellulose | In situ immobilization of silver nanoparticles | Silver nanoparticles (~31.07 nm) were fabricated and uniformly anchored | Controlled release and long-term antibacterial | [146] |
| Hydroxypropyl cellulose. | Silver-coated zinc oxide nanoparticles by solution blending | Multifunctional composite films | Accelerated wound-healing, antibacterial properties | [35] |
| TEMPO-oxidized cellulose nanofibrils | Silver nanoparticles diameter range of 8−25 nm | In situ reduction to form CNF/silver nanoparticle Suspention | Selective detection of cysteine | [147] |
| Cellulose ultrathin films grafted by N,N′-carbonyldiimidazole | In situ immobilization of silver nanoparticles | Higher silver density regions | Enable controlled electrical conductivity of cellulose surfaces | [61] |
| Cellulose pulp | Hydrothermal in situ reduction followed by dry-jet wet-spun | Homogenous distributed silver among the fiber cross section | Yellow fabrics | [76] |
| Cellulose paper | The addition of various cellulose derivatives suppresses aggregation of Ag NPs during reduction | The concentration of Ag NPs is proportional to the initial silver salt concentration | Enhanced antibacterial activity of the cotton fibers | [86] |
| Dip-coating technique | Silver nanowire | Cellulose/silver nanowires papers | [148] | |
| Filter paper | Silver nanoparticles | Reduction and immobilization | Catalyst for or 4-nitrophenol reduction, and to emphasize its duality as a SERS substrate | [149] |
| Cellulose nanowhiskers | Chemical reduction | Homogeneous AgNPs | Antimicrobial activity and biomedical applications | [81] |
| Electrospun cellulose acetate nanofiber | Electrospun nanomats of cellulose acetate with the incorporation of Ag NPs | Green synthesized silver nanoparticles (3–8 nm) | Activity towards biofilms, healthcare, and design of antimicrobial nanomat and wound dressing | [91] |
| Porous cellulose | Ion exchange of carboxylate groups to Ag cations followed by the reduction | Composite cellulose/Ag particles | Catalysis | [78] |
| Porous cellulose particles | Solvent-releasing method: silver cation exchange reduction reaction using the carboxylate groups | Composite cellulose/Ag particles | Catalysis | [124] |
| Cellulose/Keratin | One-Pot Synthesis | 27 ± 2 for Ag0 and 9 ± 1 nm for Ag+ | Blends containing either Ag+ or Ag0 | [65] |
| Regenerated cellulose | Hyperbranched polyamide-amine/silver nanoparticles | In situ | Food packaging | [150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, A.; Abouzeid, R.E.; Owda, M.E.; Cruz-Maya, I.; Guarino, V. Cellulose–Silver Composites Materials: Preparation and Applications. Biomolecules 2021, 11, 1684. https://doi.org/10.3390/biom11111684
Salama A, Abouzeid RE, Owda ME, Cruz-Maya I, Guarino V. Cellulose–Silver Composites Materials: Preparation and Applications. Biomolecules. 2021; 11(11):1684. https://doi.org/10.3390/biom11111684
Chicago/Turabian StyleSalama, Ahmed, Ragab E. Abouzeid, Medhat E. Owda, Iriczalli Cruz-Maya, and Vincenzo Guarino. 2021. "Cellulose–Silver Composites Materials: Preparation and Applications" Biomolecules 11, no. 11: 1684. https://doi.org/10.3390/biom11111684
APA StyleSalama, A., Abouzeid, R. E., Owda, M. E., Cruz-Maya, I., & Guarino, V. (2021). Cellulose–Silver Composites Materials: Preparation and Applications. Biomolecules, 11(11), 1684. https://doi.org/10.3390/biom11111684