Protease Activated Receptor 1 and Its Ligands as Main Regulators of the Regeneration of Peripheral Nerves
Abstract
:1. Introduction
2. Schwann Cells in the Physiology and Pathophysiology of Peripheral Nerves
3. Protease-Activated Receptor 1 (PAR1) General Features and Activation Mechanism
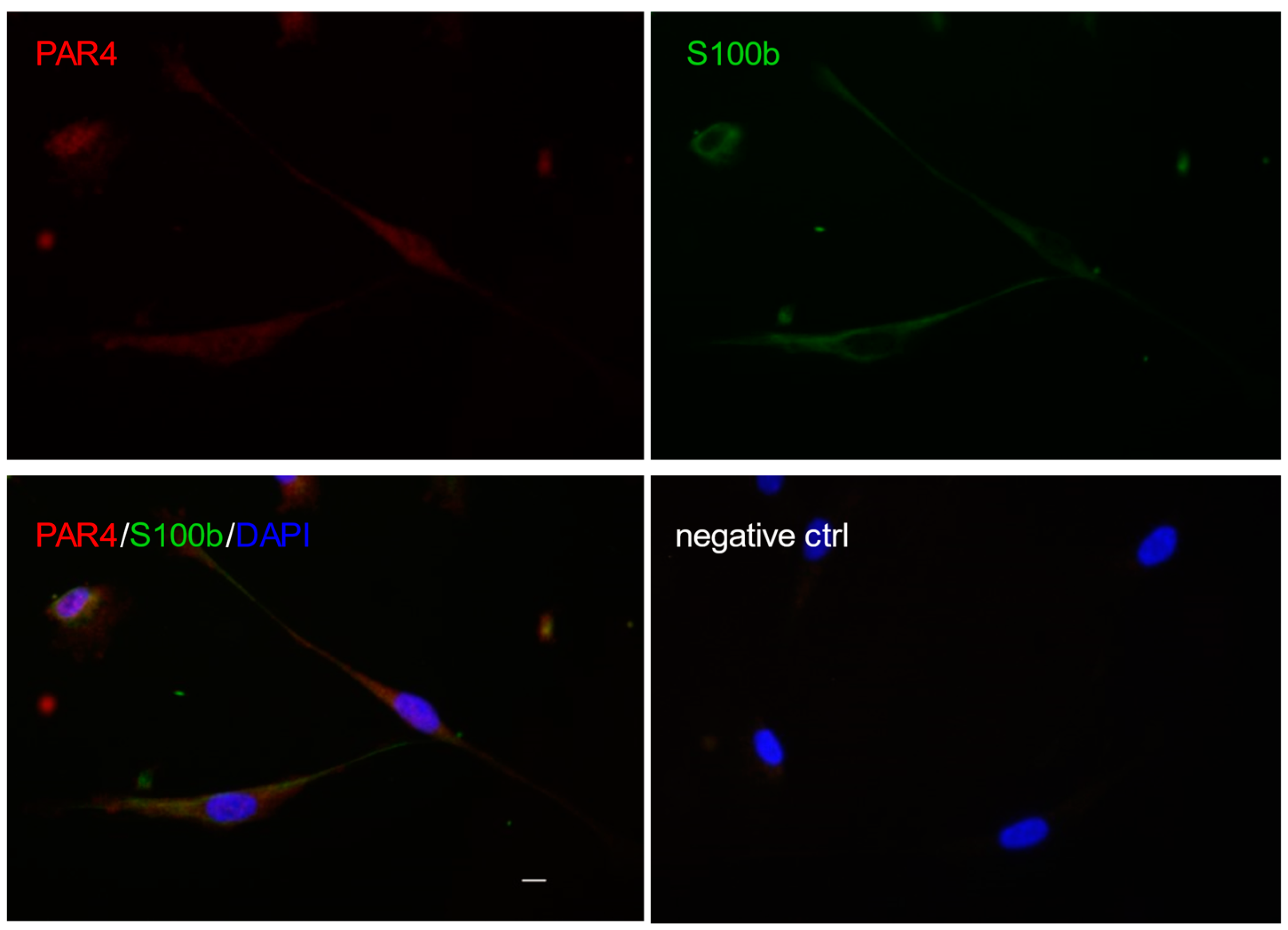
4. Protease-Activated Receptor 1 (PAR1)/Thrombin Axis in Peripheral Nerve Injury
5. Activated Factor X (FXa) in Peripheral Nerve Injury
6. Activated Factor VII (FVIIa) in Peripheral Nerve
7. Activated Protein C (APC) in Peripheral Nerve Injury
8. Plasmin in Peripheral Nerve Injury
9. Matrix Metalloproteinases (MMPs) in Peripheral Nerve Injury
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burnett, M.G.; Zager, E.L. Pathophysiology of Peripheral Nerve Injury: A Brief Review. Neurosurg. Focus 2004, 16, 1–7. [Google Scholar] [CrossRef]
- Grinsell, D.; Keating, C.P. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. BioMed Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siemionow, M.; Brzezicki, G. Chapter 8 Current Techniques and Concepts in Peripheral Nerve Repair. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 87, pp. 141–172. [Google Scholar] [CrossRef]
- Johnson, E.O.; Charchanti, A.; Soucacos, P.N. Nerve Repair: Experimental and Clinical Evaluation of Neurotrophic Factors in Peripheral Nerve Regeneration. Injury 2008, 39, 37–42. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Schmalbruch, H. Fiber Composition of the Rat Sciatic Nerve. Anat. Rec. 1986, 215, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Gera, O.; Shavit-Stein, E.; Bushi, D.; Harnof, S.; Shimon, M.B.; Weiss, R.; Golderman, V.; Dori, A.; Maggio, N.; Finegold, K.; et al. Thrombin and Protein C Pathway in Peripheral Nerve Schwann Cells. Neuroscience 2016, 339, 587–598. [Google Scholar] [CrossRef]
- Henderson, J.M.; Stein, S.F.; Kutner, M.; Wiles, M.-B.; Ansley, J.D.; Rudman, D. Analysis of Twenty-Three Plasma Proteins in Ascites The Depletion of Fibrinogen and Plasminogen. Ann. Surg. 1980, 192, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Gera, O.; Bushi, D.; Shimon, M.B.; Artan-Furman, A.; Harnof, S.; Maggio, N.; Dori, A.; Chapman, J.; Shavit-Stein, E. Local Regulation of Thrombin Activity by Factor Xa in Peripheral Nerve Schwann Cells. Neuroscience 2018, 371, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Balezina, O.P.; Gerasimenko, N.Y.; Dugina, T.N.; Strukova, S.M. Study of Neurotrophic Activity of Thrombin on the Model of Regenerating Mouse Nerve. Bull. Exp. Biol. Med. 2005, 139, 4–6. [Google Scholar] [CrossRef]
- Lee, P.; Spector, J.G.; Derby, A.; Roufa, D.G. Effects of Thrombin and Protease Nexin-1 on Peripheral Nerve Regeneration. Ann. Otol. Rhinol. Laryngol. 1998, 107, 61–69. [Google Scholar] [CrossRef]
- Shavit, E.; Beilin, O.; Korczyn, A.D.; Sylantiev, C.; Aronovich, R.; Drory, V.E.; Gurwitz, D.; Horresh, I.; Bar-Shavit, R.; Peles, E.; et al. Thrombin Receptor PAR-1 on Myelin at the Node of Ranvier: A New Anatomy and Physiology of Conduction Block. Brain 2008, 131, 1113–1122. [Google Scholar] [CrossRef] [Green Version]
- Pompili, E.; Fabrizi, C.; Somma, F.; Correani, V.; Maras, B.; Schininà, M.E.; Ciraci, V.; Artico, M.; Fornai, F.; Fumagalli, L. PAR1 Activation Affects the Neurotrophic Properties of Schwann Cells. Mol. Cell. Neurosci. 2017, 79, 23–33. [Google Scholar] [CrossRef]
- Kanno, H.; Pressman, Y.; Moody, A.; Berg, R.; Muir, E.M.; Rogers, J.H.; Ozawa, H.; Itoi, E.; Pearse, D.D.; Bunge, M.B. Combination of Engineered Schwann Cell Grafts to Secrete Neurotrophin and Chondroitinase Promotes Axonal Regeneration and Locomotion after Spinal Cord Injury. J. Neurosci. 2014, 34, 1838–1855. [Google Scholar] [CrossRef] [Green Version]
- Babetto, E.; Wong, K.M.; Beirowski, B. A Glycolytic Shift in Schwann Cells Supports Injured Axons. Nat. Neurosci. 2020, 23, 1215–1228. [Google Scholar] [CrossRef]
- Whalley, K. Glia: Schwann Cells Provide Life Support for Axons. Nat. Rev. Neurosci. 2014, 15, 698–699. [Google Scholar] [CrossRef]
- Cohen, C.C.H.; Popovic, M.A.; Klooster, J.; Weil, M.-T.; Möbius, W.; Nave, K.-A.; Kole, M.H.P. Saltatory Conduction along Myelinated Axons Involves a Periaxonal Nanocircuit. Cell 2020, 180, 311–322.e15. [Google Scholar] [CrossRef] [Green Version]
- Lim, B.C.; Rasband, M.N. Saltatory Conduction: Jumping to New Conclusions. Curr. Biol. 2020, 30, R326–R328. [Google Scholar] [CrossRef]
- Hursh, J.B. Conduction velocity and diameter of nerve fibers. Am. J. Physiol. Leg. Content 1939, 127, 131–139. [Google Scholar] [CrossRef]
- Rushton, W.A.H. A Theory of the Effects of Fibre Size in Medullated Nerve. J. Physiol. 1951, 115, 101–122. [Google Scholar] [CrossRef]
- Nave, K.-A.; Werner, H.B. Ensheathment and Myelination of Axons: Evolution of Glial Functions. Annu. Rev. Neurosci. 2021, 44, 197–219. [Google Scholar] [CrossRef]
- Le Douarin, N.M.; Smith, J. Development of the Peripheral Nervous System from the Neural Crest. Annu. Rev. Cell Biol. 1988, 4, 375–404. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.; Dulac, C.; Dupin, E.; Cameron-Curry, P. Glial Cell Lineages in the Neural Crest. Glia 1991, 4, 175–184. [Google Scholar] [CrossRef]
- Buchstaller, J.; Sommer, L.; Bodmer, M.; Hoffmann, R.; Suter, U.; Mantei, N. Efficient Isolation and Gene Expression Profiling of Small Numbers of Neural Crest Stem Cells and Developing Schwann Cells. J. Neurosci. 2004, 24, 2357–2365. [Google Scholar] [CrossRef]
- Webster, H.D. The Geometry of Peripheral Myelin Sheaths during Their Formation and Growth in Rat Sciatic Nerves. J. Cell Biol. 1971, 48, 348–367. [Google Scholar] [CrossRef] [PubMed]
- Webster, H.D.; Martin, R.; O’Connell, M.F. The Relationships between Interphase Schwann Cells and Axons before Myelination: A Quantitative Electron Microscopic Study. Dev. Biol. 1973, 32, 401–416. [Google Scholar] [CrossRef]
- Michailov, G.V.; Sereda, M.W.; Brinkmann, B.G.; Fischer, T.M.; Haug, B.; Birchmeier, C.; Role, L.; Lai, C.; Schwab, M.H.; Nave, K.-A. Axonal Neuregulin-1 Regulates Myelin Sheath Thickness. Science 2004, 304, 700–703. [Google Scholar] [CrossRef] [Green Version]
- Bosse, F.; Hasenpusch-Theil, K.; Küry, P.; Müller, H.W. Gene Expression Profiling Reveals That Peripheral Nerve Regeneration Is a Consequence of Both Novel Injury-Dependent and Reactivated Developmental Processes. J. Neurochem. 2006, 96, 1441–1457. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Pilch, K.S.; van der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef]
- Perry, V.H.; Tsao, J.W.; Fearn, S.; Brown, M.C. Radiation-Induced Reductions in Macrophage Recruitment Have Only Slight Effects on Myelin Degeneration in Sectioned Peripheral Nerves of Mice. Eur. J. Neurosci. 1995, 7, 271–280. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Belfiore, L.; Chu, T.-H.; Fleming, T.; Midha, R.; Biernaskie, J.; Schuurmans, C. Insights Into the Role and Potential of Schwann Cells for Peripheral Nerve Repair From Studies of Development and Injury. Front. Mol. Neurosci. 2020, 13, 608442. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Repair Schwann Cell and Its Function in Regenerating Nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagstaff, L.J.; Gomez-Sanchez, J.A.; Fazal, S.V.; Otto, G.W.; Kilpatrick, A.M.; Michael, K.; Wong, L.Y.; Ma, K.H.; Turmaine, M.; Svaren, J.; et al. Failures of Nerve Regeneration Caused by Aging or Chronic Denervation Are Rescued by Restoring Schwann Cell C-Jun. eLife 2021, 10, e62232. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Griffith, M.; Hantke, J.; Macias-Camara, N.; Azkargorta, M.; Aurrekoetxea, I.; et al. Schwann Cell Autophagy, Myelinophagy, Initiates Myelin Clearance from Injured Nerves. J. Cell Biol. 2015, 210, 153–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, M.; Badaut, J.; Thevenet, J.; Hirt, L. Activation of C-Jun in the Nuclei of Neurons of the CA-1 in Thrombin Preconditioning Occurs via PAR-1. J. Neurosci. Res. 2010, 88, 1338–1347. [Google Scholar] [CrossRef]
- Bolívar, S.; Navarro, X.; Udina, E. Schwann Cell Role in Selectivity of Nerve Regeneration. Cells 2020, 9, 2131. [Google Scholar] [CrossRef]
- Simón, D.; Martín-Bermejo, M.J.; Gallego-Hernández, M.T.; Pastrana, E.; García-Escudero, V.; García-Gómez, A.; Lim, F.; Díaz-Nido, J.; Avila, J.; Moreno-Flores, M.T. Expression of Plasminogen Activator Inhibitor-1 by Olfactory Ensheathing Glia Promotes Axonal Regeneration. Glia 2011, 59, 1458–1471. [Google Scholar] [CrossRef]
- Pompili, E.; Fabrizi, C.; Fornai, F.; Fumagalli, L. Role of the Protease-Activated Receptor 1 in Regulating the Function of Glial Cells within Central and Peripheral Nervous System. J. Neural Transm. 2019, 126, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Choi, C.-I.; Triplet, E.M.; Langley, M.R.; Kleppe, L.S.; Kim, H.N.; Simon, W.L.; Scarisbrick, I.A. Blocking the Thrombin Receptor Promotes Repair of Demyelinated Lesions in the Adult Brain. J. Neurosci. 2020, 40, 1483–1500. [Google Scholar] [CrossRef]
- Pompili, E.; Fabrizi, C. Thrombin in Peripheral Nerves: Friend or Foe? Neural Regen. Res. 2021, 16, 1223–1224. [Google Scholar] [CrossRef]
- Vu, T.K.; Hung, D.T.; Wheaton, V.I.; Coughlin, S.R. Molecular Cloning of a Functional Thrombin Receptor Reveals a Novel Proteolytic Mechanism of Receptor Activation. Cell 1991, 64, 1057–1068. [Google Scholar] [CrossRef]
- Nystedt, S.; Emilsson, K.; Wahlestedt, C.; Sundelin, J. Molecular Cloning of a Potential Proteinase Activated Receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 9208–9212. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, H.; Connolly, A.J.; Zeng, D.; Kahn, M.L.; Zheng, Y.W.; Timmons, C.; Tram, T.; Coughlin, S.R. Protease-Activated Receptor 3 Is a Second Thrombin Receptor in Humans. Nature 1997, 386, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.F.; Andersen, H.; Whitmore, T.E.; Presnell, S.R.; Yee, D.P.; Ching, A.; Gilbert, T.; Davie, E.W.; Foster, D.C. Cloning and Characterization of Human Protease-Activated Receptor 4. Proc. Natl. Acad. Sci. USA 1998, 95, 6642–6646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanc-Brude, O.P.; Archer, F.; Leoni, P.; Derian, C.; Bolsover, S.; Laurent, G.J.; Chambers, R.C. Factor Xa Stimulates Fibroblast Procollagen Production, Proliferation, and Calcium Signaling via PAR1 Activation. Exp. Cell Res. 2005, 304, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Schuepbach, R.A.; Riewald, M. Coagulation Factor Xa Cleaves Protease-Activated Receptor-1 and Mediates Signaling Dependent on Binding to the Endothelial Protein C Receptor. J. Thromb. Haemost. 2010, 8, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Sen, P.; Gopalakrishnan, R.; Kothari, H.; Keshava, S.; Clark, C.A.; Esmon, C.T.; Pendurthi, U.R.; Rao, L.V.M. Factor VIIa Bound to Endothelial Cell Protein C Receptor Activates Protease Activated Receptor-1 and Mediates Cell Signaling and Barrier Protection. Blood 2011, 117, 3199–3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuliopulos, A.; Covic, L.; Seeley, S.K.; Sheridan, P.J.; Helin, J.; Costello, C.E. Plasmin Desensitization of the PAR1 Thrombin Receptor: Kinetics, Sites of Truncation, and Implications for Thrombolytic Therapy. Biochemistry 1999, 38, 4572–4585. [Google Scholar] [CrossRef]
- Sebastiano, M.; Momi, S.; Falcinelli, E.; Bury, L.; Hoylaerts, M.F.; Gresele, P. A Novel Mechanism Regulating Human Platelet Activation by MMP-2-Mediated PAR1 Biased Signaling. Blood 2017, 129, 883–895. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.E.; Kim, J.-M.; Jeong, S.K.; Jeon, J.E.; Yoon, H.-J.; Jeong, M.-K.; Lee, S.H. Protease-Activated Receptor-2 Mediates the Expression of Inflammatory Cytokines, Antimicrobial Peptides, and Matrix Metalloproteinases in Keratinocytes in Response to Propionibacterium Acnes. Arch. Derm. Res. 2010, 302, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, T.; Hirano, K.; Shintani, Y.; Nishimura, J.; Nakatsuka, A.; Kuga, H.; Takahashi, S.; Kanaide, H. Unproductive Cleavage and the Inactivation of Protease-Activated Receptor-1 by Trypsin in Vascular Endothelial Cells. Br. J. Pharm. 2003, 138, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Suidan, H.S.; Bouvier, J.; Schaerer, E.; Stone, S.R.; Monard, D.; Tschopp, J. Granzyme A Released upon Stimulation of Cytotoxic T Lymphocytes Activates the Thrombin Receptor on Neuronal Cells and Astrocytes. Proc. Natl. Acad. Sci. USA 1994, 91, 8112–8116. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.R.; Johnson, T.P.; Gnanapavan, S.; Giovannoni, G.; Wang, T.; Steiner, J.P.; Medynets, M.; Vaal, M.J.; Gartner, V.; Nath, A. Protease-Activated Receptor-1 Activation by Granzyme B Causes Neurotoxicity That Is Augmented by Interleukin-1β. J. Neuroinflammat. 2017, 14, 131. [Google Scholar] [CrossRef]
- Wang, T.; Lee, M.-H.; Choi, E.; Pardo-Villamizar, C.A.; Lee, S.B.; Yang, I.H.; Calabresi, P.A.; Nath, A. Granzyme B-Induced Neurotoxicity Is Mediated via Activation of PAR-1 Receptor and Kv1.3 Channel. PLoS ONE 2012, 7, e43950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, T.J.; Nannuru, K.C.; Singh, R.K. Cathepsin G Recruits Osteoclast Precursors via Proteolytic Activation of Protease-Activated Receptor-1. Cancer Res. 2009, 69, 3188–3195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nystedt, S.; Emilsson, K.; Larsson, A.K.; Strömbeck, B.; Sundelin, J. Molecular Cloning and Functional Expression of the Gene Encoding the Human Proteinase-Activated Receptor 2. Eur. J. Biochem. 1995, 232, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.; Perng, D.W.; Thabrew, H.; Compton, S.J.; Cairns, J.A.; McEuen, A.R.; Marthan, R.; Tunon De Lara, J.M.; Walls, A.F. Tryptase and Agonists of PAR-2 Induce the Proliferation of Human Airway Smooth Muscle Cells. J. Appl. Physiol. 2001, 91, 1372–1379. [Google Scholar] [CrossRef]
- Rothmeier, A.S.; Liu, E.; Chakrabarty, S.; Disse, J.; Mueller, B.M.; Østergaard, H.; Ruf, W. Identification of the Integrin-Binding Site on Coagulation Factor VIIa Required for Proangiogenic PAR2 Signaling. Blood 2018, 131, 674–685. [Google Scholar] [CrossRef]
- Morris, D.R.; Ding, Y.; Ricks, T.K.; Gullapalli, A.; Wolfe, B.L.; Trejo, J. Protease-Activated Receptor-2 Is Essential for Factor VIIa and Xa-Induced Signaling, Migration, and Invasion of Breast Cancer Cells. Cancer Res. 2006, 66, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Dömötör, E.; Bartha, K.; Machovich, R.; Adam-Vizi, V. Protease-Activated Receptor-2 (PAR-2) in Brain Microvascular Endothelium and Its Regulation by Plasmin and Elastase. J. Neurochem. 2002, 80, 746–754. [Google Scholar] [CrossRef]
- Mihara, K.; Ramachandran, R.; Saifeddine, M.; Hansen, K.K.; Renaux, B.; Polley, D.; Gibson, S.; Vanderboor, C.; Hollenberg, M.D. Thrombin-Mediated Direct Activation of Proteinase-Activated Receptor-2: Another Target for Thrombin Signaling. Mol. Pharm. 2016, 89, 606–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikonomopoulou, K.; Hansen, K.K.; Saifeddine, M.; Tea, I.; Blaber, M.; Blaber, S.I.; Scarisbrick, I.; Andrade-Gordon, P.; Cottrell, G.S.; Bunnett, N.W.; et al. Proteinase-Activated Receptors, Targets for Kallikrein Signaling. J. Biol. Chem. 2006, 281, 32095–32112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, R.; Schulze, B.; Krause, G.; Mayr, L.M.; Settmacher, U.; Henklein, P. Proteinase-Activated Receptors (PARs)—The PAR3 Neo-N-Terminal Peptide TFRGAP Interacts with PAR1. Regul. Pept. 2005, 125, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L.; Kuliopulos, A. Protease-Activated Receptor-4 Uses Dual Prolines and an Anionic Retention Motif for Thrombin Recognition and Cleavage. Biochem. J. 2003, 376, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Hollenberg, M.D.; Wallace, J.L. Thrombin-Induced Platelet Endostatin Release Is Blocked by a Proteinase Activated Receptor-4 (PAR4) Antagonist. Br. J. Pharm. 2001, 134, 701–704. [Google Scholar] [CrossRef] [Green Version]
- Gomides, L.F.; Duarte, I.D.; Ferreira, R.G.; Perez, A.C.; Francischi, J.N.; Klein, A. Proteinase-Activated Receptor-4 Plays a Major Role in the Recruitment of Neutrophils Induced by Trypsin or Carrageenan during Pleurisy in Mice. Pharmacology 2012, 89, 275–282. [Google Scholar] [CrossRef]
- Sambrano, G.R.; Huang, W.; Faruqi, T.; Mahrus, S.; Craik, C.; Coughlin, S.R. Cathepsin G Activates Protease-Activated Receptor-4 in Human Platelets. J. Biol. Chem. 2000, 275, 6819–6823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlin, S.R. How the Protease Thrombin Talks to Cells. Proc. Natl. Acad. Sci. USA 1999, 96, 11023–11027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.N.; Ramachandran, R.; Yau, M.-K.; Suen, J.Y.; Fairlie, D.P.; Hollenberg, M.D.; Hooper, J.D. Structure, Function and Pathophysiology of Protease Activated Receptors. Pharmacol. Ther. 2011, 130, 248–282. [Google Scholar] [CrossRef]
- Wolfe, B.L.; Trejo, J. Clathrin-Dependent Mechanisms of G Protein-Coupled Receptor Endocytosis. Traffic 2007, 8, 462–470. [Google Scholar] [CrossRef]
- Trejo, J.; Hammes, S.R.; Coughlin, S.R. Termination of Signaling by Protease-Activated Receptor-1 Is Linked to Lysosomal Sorting. Proc. Natl. Acad. Sci. USA 1998, 95, 13698–13702. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Nieman, M.T. The Domino Effect Triggered by the Tethered Ligand of the Protease Activated Receptors. Thromb. Res. 2020, 196, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Chandrabalan, A.; Ramachandran, R. Molecular Mechanisms Regulating Proteinase-Activated Receptors (PARs). FEBS J. 2021, 288, 2697–2726. [Google Scholar] [CrossRef] [PubMed]
- Suo, Z.; Wu, M.; Ameenuddin, S.; Anderson, H.E.; Zoloty, J.E.; Citron, B.A.; Andrade-Gordon, P.; Festoff, B.W. Participation of Protease-Activated Receptor-1 in Thrombin-Induced Microglial Activation. J. Neurochem. 2002, 80, 655–666. [Google Scholar] [CrossRef]
- Naldini, A.; Bernini, C.; Pucci, A.; Carraro, F. Thrombin-Mediated IL-10 up-Regulation Involves Protease-Activated Receptor (PAR)-1 Expression in Human Mononuclear Leukocytes. J. Leukoc. Biol. 2005, 78, 736–744. [Google Scholar] [CrossRef]
- Niessen, F.; Schaffner, F.; Furlan-Freguia, C.; Pawlinski, R.; Bhattacharjee, G.; Chun, J.; Derian, C.K.; Andrade-Gordon, P.; Rosen, H.; Ruf, W. Dendritic Cell PAR1-S1P3 Signalling Couples Coagulation and Inflammation. Nature 2008, 452, 654–658. [Google Scholar] [CrossRef]
- Fabrizi, C.; Pompili, E.; Panetta, B.; Nori, S.L.; Fumagalli, L. Protease-Activated Receptor-1 Regulates Cytokine Production and Induces the Suppressor of Cytokine Signaling-3 in Microglia. Int. J. Mol. Med. 2009, 24, 367–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Metcalf, M.; Bunnett, N.W. Biased Signaling of Protease-Activated Receptors. Front. Endocrinol. 2014, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Liu, A.P.; Smith, T.H.; Trejo, J. Cofactoring and Dimerization of Proteinase-Activated Receptors. Pharm. Rev. 2013, 65, 1198–1213. [Google Scholar] [CrossRef] [Green Version]
- Pompili, E.; Ciraci, V.; Leone, S.; De Franchis, V.; Familiari, P.; Matassa, R.; Familiari, G.; Tata, A.M.; Fumagalli, L.; Fabrizi, C. Thrombin Regulates the Ability of Schwann Cells to Support Neuritogenesis and to Maintain the Integrity of the Nodes of Ranvier. Eur. J. Histochem. 2020, 64, 3109. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, I.V.; Ma, J.Y.; Citron, B.A.; Ratzlaff, K.T.; Gregory, E.J.; Akaaboune, M.; Festoff, B.W. Neural Thrombin and Protease Nexin I Kinetics after Murine Peripheral Nerve Injury. J. Neurochem. 1996, 67, 2188–2199. [Google Scholar] [CrossRef]
- Festoff, B.W.; Citron, B.A. Thrombin and the Coag-Inflammatory Nexus in Neurotrauma, ALS, and Other Neurodegenerative Disorders. Front. Neurol. 2019, 10, 59. [Google Scholar] [CrossRef]
- Court, F.A.; Sherman, D.L.; Pratt, T.; Garry, E.M.; Ribchester, R.R.; Cottrell, D.F.; Fleetwood-Walker, S.M.; Brophy, P.J. Restricted Growth of Schwann Cells Lacking Cajal Bands Slows Conduction in Myelinated Nerves. Nature 2004, 431, 191–195. [Google Scholar] [CrossRef]
- Burda, J.E.; Radulovic, M.; Yoon, H.; Scarisbrick, I.A. Critical Role for PAR1 in Kallikrein 6-Mediated Oligodendrogliopathy. Glia 2013, 61, 1456–1470. [Google Scholar] [CrossRef]
- Kalderon, N. Migration of Schwann Cells and Wrapping of Neurites in Vitro: A Function of Protease Activity (Plasmin) in the Growth Medium. Proc. Natl. Acad. Sci. USA 1979, 76, 5992–5996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajadi, E.; Aliaghaei, A.; Farahni, R.M.; Rashidiani-Rashidabadi, A.; Raoofi, A.; Sadeghi, Y.; Bagheri, M.; Ilkhani, S.; Abdollahifar, M.-A. Tissue Plasminogen Activator Loaded PCL Nanofibrous Scaffold Promoted Nerve Regeneration After Sciatic Nerve Transection in Male Rats. Neurotox. Res. 2021, 39, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Klimovich, P.S.; Semina, E.V.; Karagyaur, M.N.; Rysenkova, K.D.; Sysoeva, V.Y.; Mironov, N.A.; Sagaradze, G.D.; Az’muko, A.A.; Popov, V.S.; Rubina, K.A.; et al. Urokinase Receptor Regulates Nerve Regeneration through Its Interaction with A5β1-Integrin. Biomed. Pharm. 2020, 125, 110008. [Google Scholar] [CrossRef]
- Liu, H.; Kim, Y.; Chattopadhyay, S.; Shubayev, I.; Dolkas, J.; Shubayev, V.I. Matrix Metalloproteinase Inhibition Enhances the Rate of Nerve Regeneration In Vivo by Promoting Dedifferentiation and Mitosis of Supporting Schwann Cells. J. Neuropathol. Exp. Neurol. 2010, 69, 386–395. [Google Scholar] [CrossRef]
- Willis Fox, O.; Preston, R.J.S. Molecular Basis of Protease-activated Receptor 1 Signaling Diversity. J. Thromb. Haemost. 2020, 18, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Muscella, A.; Vetrugno, C.; Cossa, L.G.; Marsigliante, S. TGF-Β1 Activates RSC96 Schwann Cells Migration and Invasion through MMP-2 and MMP-9 Activities. J. Neurochem. 2020, 153, 525–538. [Google Scholar] [CrossRef]
- Lehmann, H.C.; Köhne, A.; Bernal, F.; Jangouk, P.; Meyer Zu Hörste, G.; Dehmel, T.; Hartung, H.-P.; Previtali, S.C.; Kieseier, B.C. Matrix Metalloproteinase-2 Is Involved in Myelination of Dorsal Root Ganglia Neurons. Glia 2009, 57, 479–489. [Google Scholar] [CrossRef]
- Muir, D. Differences in Proliferation and Invasion by Normal, Transformed and NF1 Schwann Cell Cultures Are Influenced by Matrix Metalloproteinase Expression. Clin. Exp. Metastasis 1995, 13, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.; Frump, D.; Lin, M.; Caiozzo, V.J.; Mozaffar, T.; Steward, O.; Gupta, R. Matrix Metalloproteinase 3 Deletion Preserves Denervated Motor Endplates after Traumatic Nerve Injury. Ann. Neurol. 2013, 73, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Tengara, S.; Kamo, A.; Ogawa, H.; Takamori, K. Matrix Metalloproteinase-8 Is Involved in Dermal Nerve Growth: Implications for Possible Application to Pruritus from in Vitro Models. J. Investig. Derm. 2011, 131, 2105–2112. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, S.; Myers, R.R.; Janes, J.; Shubayev, V. Cytokine Regulation of MMP-9 in Peripheral Glia: Implications for Pathological Processes and Pain in Injured Nerve. Brain Behav. Immun. 2007, 21, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Shubayev, V.I.; Angert, M.; Dolkas, J.; Campana, W.M.; Palenscar, K.; Myers, R.R. TNFalpha-Induced MMP-9 Promotes Macrophage Recruitment into Injured Peripheral Nerve. Mol. Cell Neurosci. 2006, 31, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Remacle, A.G.; Hullugundi, S.K.; Dolkas, J.; Angert, M.; Chernov, A.V.; Strongin, A.Y.; Shubayev, V.I. Acute- and Late-Phase Matrix Metalloproteinase (MMP)-9 Activity Is Comparable in Female and Male Rats after Peripheral Nerve Injury. J. Neuroinflammat. 2018, 15, 89. [Google Scholar] [CrossRef] [Green Version]
- Petzold, T.; Thienel, M.; Dannenberg, L.; Mourikis, P.; Helten, C.; Ayhan, A.; M’Pembele, R.; Achilles, A.; Trojovky, K.; Konsek, D.; et al. Rivaroxaban Reduces Arterial Thrombosis by Inhibition of FXa-Driven Platelet Activation via Protease Activated Receptor-1. Circ. Res. 2020, 126, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Kondreddy, V.; Pendurthi, U.R.; Xu, X.; Griffin, J.H.; Rao, L.V.M. FVIIa (Factor VIIa) Induces Biased Cytoprotective Signaling in Mice Through the Cleavage of PAR (Protease-Activated Receptor)-1 at Canonical Arg41 (Arginine41) Site. Arter. Thromb. Vasc. Biol. 2020, 40, 1275–1288. [Google Scholar] [CrossRef] [Green Version]
- Wojtukiewicz, M.Z.; Hempel, D.; Sierko, E.; Tucker, S.C.; Honn, K.V. Endothelial Protein C Receptor (EPCR), Protease Activated Receptor-1 (PAR-1) and Their Interplay in Cancer Growth and Metastatic Dissemination. Cancers 2019, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Wozniak, J.M.; Grimsey, N.J.; Girada, S.; Patwardhan, A.; Molinar-Inglis, O.; Smith, T.H.; Lapek, J.D.; Gonzalez, D.J.; Trejo, J. Phosphoproteomic Analysis of Protease-Activated Receptor-1 Biased Signaling Reveals Unique Modulators of Endothelial Barrier Function. Proc. Natl. Acad. Sci. USA 2020, 117, 5039–5048. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Sinha, R.K.; Burnier, L.; Bouwens, E.A.; Griffin, J.H. Biased Agonism of Protease-Activated Receptor 1 by Activated Protein C Caused by Noncanonical Cleavage at Arg46. Blood 2012, 120, 5237–5246. [Google Scholar] [CrossRef] [Green Version]
- Soh, U.J.K.; Trejo, J. Activated Protein C Promotes Protease-Activated Receptor-1 Cytoprotective Signaling through β-Arrestin and Dishevelled-2 Scaffolds. Proc. Natl. Acad. Sci. USA 2011, 108, E1372–E1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Rocha, M.; Avila, J.; Armas-Portela, R. Tissue-Type Plasminogen Activator (TPA) Is the Main Plasminogen Activator Associated with Isolated Rat Nerve Growth Cones. Neurosci. Lett. 1994, 180, 123–126. [Google Scholar] [CrossRef]
- Siconolfi, L.B.; Seeds, N.W. Mice Lacking TPA, UPA, or Plasminogen Genes Showed Delayed Functional Recovery after Sciatic Nerve Crush. J. Neurosci. 2001, 21, 4348–4355. [Google Scholar] [CrossRef] [PubMed]
- Siconolfi, L.B.; Seeds, N.W. Mice Lacking Tissue Plasminogen Activator and Urokinase Plasminogen Activator Genes Show Attenuated Matrix Metalloproteases Activity after Sciatic Nerve Crush. J. Neurosci. Res. 2003, 74, 430–434. [Google Scholar] [CrossRef]
- Hantaï, D.; Rao, J.S.; Festoff, B.W. Rapid Neural Regulation of Muscle Urokinase-like Plasminogen Activator as Defined by Nerve Crush. Proc. Natl. Acad. Sci. USA 1990, 87, 2926–2930. [Google Scholar] [CrossRef] [Green Version]
- Akassoglou, K.; Kombrinck, K.W.; Degen, J.L.; Strickland, S. Tissue Plasminogen Activator-Mediated Fibrinolysis Protects against Axonal Degeneration and Demyelination after Sciatic Nerve Injury. J. Cell Biol. 2000, 149, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Demestre, M.; Wells, G.M.; Miller, K.M.; Smith, K.J.; Hughes, R.A.C.; Gearing, A.J.; Gregson, N.A. Characterisation of Matrix Metalloproteinases and the Effects of a Broad-Spectrum Inhibitor (BB-1101) in Peripheral Nerve Regeneration. Neuroscience 2004, 124, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, D.H.; Sung, J.; Yang, H.; Park, J.W.; Youn, I. Electrical Stimulation Accelerates Neurite Regeneration in Axotomized Dorsal Root Ganglion Neurons by Increasing MMP-2 Expression. Biochem. Biophys. Res. Commun. 2019, 508, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.A.; Muir, D. MMP-2 and MMP-9 Increase the Neurite-Promoting Potential of Schwann Cell Basal Laminae and Are Upregulated in Degenerated Nerve. Mol. Cell Neurosci. 2000, 16, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Driscoll, H.E.; Newton, V.L.; Gardiner, N.J. Matrix Metalloproteinase-2 Is Downregulated in Sciatic Nerve by Streptozotocin Induced Diabetes and/or Treatment with Minocycline: Implications for Nerve Regeneration. Exp. Neurol. 2014, 261, 654–665. [Google Scholar] [CrossRef]
- Parks, W.C.; Wilson, C.L.; López-Boado, Y.S. Matrix Metalloproteinases as Modulators of Inflammation and Innate Immunity. Nat. Rev. Immunol. 2004, 4, 617–629. [Google Scholar] [CrossRef]
- Fang, X.; Chen, J.; Wang, W.; Feng, G.; Li, X.; Zhang, X.; Zhang, Y.; Zhang, J.; Xu, Z.; Tai, J.; et al. Matrix Metalloproteinase 9 (MMP9) Level and MMP9 -1562C>T in Patients with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis of Case-Control Studies. Sleep Med. 2020, 67, 110–119. [Google Scholar] [CrossRef]


| PAR1 Activating Protease | SC Cultures | Peripheral Nerve | Cleavage Site |
|---|---|---|---|
| Thrombin | Low levels, increased release of neurotrophic factors [13] | Low levels, enhanced regeneration [10] | Canonical |
| High levels, decreased SC neurotrophic activity [80] | High levels, reduced regeneration [11] | ||
| FXa | Increased release of thrombin in a Schwannoma cell line [9] | Inhibition of FXa restores motor function after injury [9] | Canonical |
| FVIIa | Expressed in a Schwannoma cell line [9] | Expressed at the nodes of Ranvier [9] | Canonical |
| APC/EPCR | EPCR expression in a Schwannoma cell line [7] | EPCR increased expression after crush injury [7] | Canonical and noncanonical |
| Plasmin | Increase SC migration and wrapping of nerve fibers [85] | tPA and uPA promote nerve regeneration after injury [86,87] | Canonical and noncanonical |
| MMPs | Inhibit SC proliferation [88] | Inhibit nerve regeneration [89] | Canonical and noncanonical |
| MMP2 | Stimulation of SC migration [90] Enhancement of myelination in SC/DRG co-cultures [91] | Noncanonical | |
| MMP3 | Inhibition of SC proliferation [92] | In MMP3 KO mice, NMJ are preserved [93] | Noncanonical |
| MMP8 | Localized at growth cones in nerve fibers [94] | Canonical | |
| MMP9 | Inhibition of proliferation and trophic activity of SCs [95,96] Stimulation of SC migration [90] | Upregulated after nerve injury [97] | Canonical and noncanonical |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pompili, E.; De Franchis, V.; Giampietri, C.; Leone, S.; De Santis, E.; Fornai, F.; Fumagalli, L.; Fabrizi, C. Protease Activated Receptor 1 and Its Ligands as Main Regulators of the Regeneration of Peripheral Nerves. Biomolecules 2021, 11, 1668. https://doi.org/10.3390/biom11111668
Pompili E, De Franchis V, Giampietri C, Leone S, De Santis E, Fornai F, Fumagalli L, Fabrizi C. Protease Activated Receptor 1 and Its Ligands as Main Regulators of the Regeneration of Peripheral Nerves. Biomolecules. 2021; 11(11):1668. https://doi.org/10.3390/biom11111668
Chicago/Turabian StylePompili, Elena, Valerio De Franchis, Claudia Giampietri, Stefano Leone, Elena De Santis, Francesco Fornai, Lorenzo Fumagalli, and Cinzia Fabrizi. 2021. "Protease Activated Receptor 1 and Its Ligands as Main Regulators of the Regeneration of Peripheral Nerves" Biomolecules 11, no. 11: 1668. https://doi.org/10.3390/biom11111668
APA StylePompili, E., De Franchis, V., Giampietri, C., Leone, S., De Santis, E., Fornai, F., Fumagalli, L., & Fabrizi, C. (2021). Protease Activated Receptor 1 and Its Ligands as Main Regulators of the Regeneration of Peripheral Nerves. Biomolecules, 11(11), 1668. https://doi.org/10.3390/biom11111668







