Comparison of Two Alternative Procedures to Obtain Packed Red Blood Cells for β-Thalassemia Major Transfusion Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Blood Collection
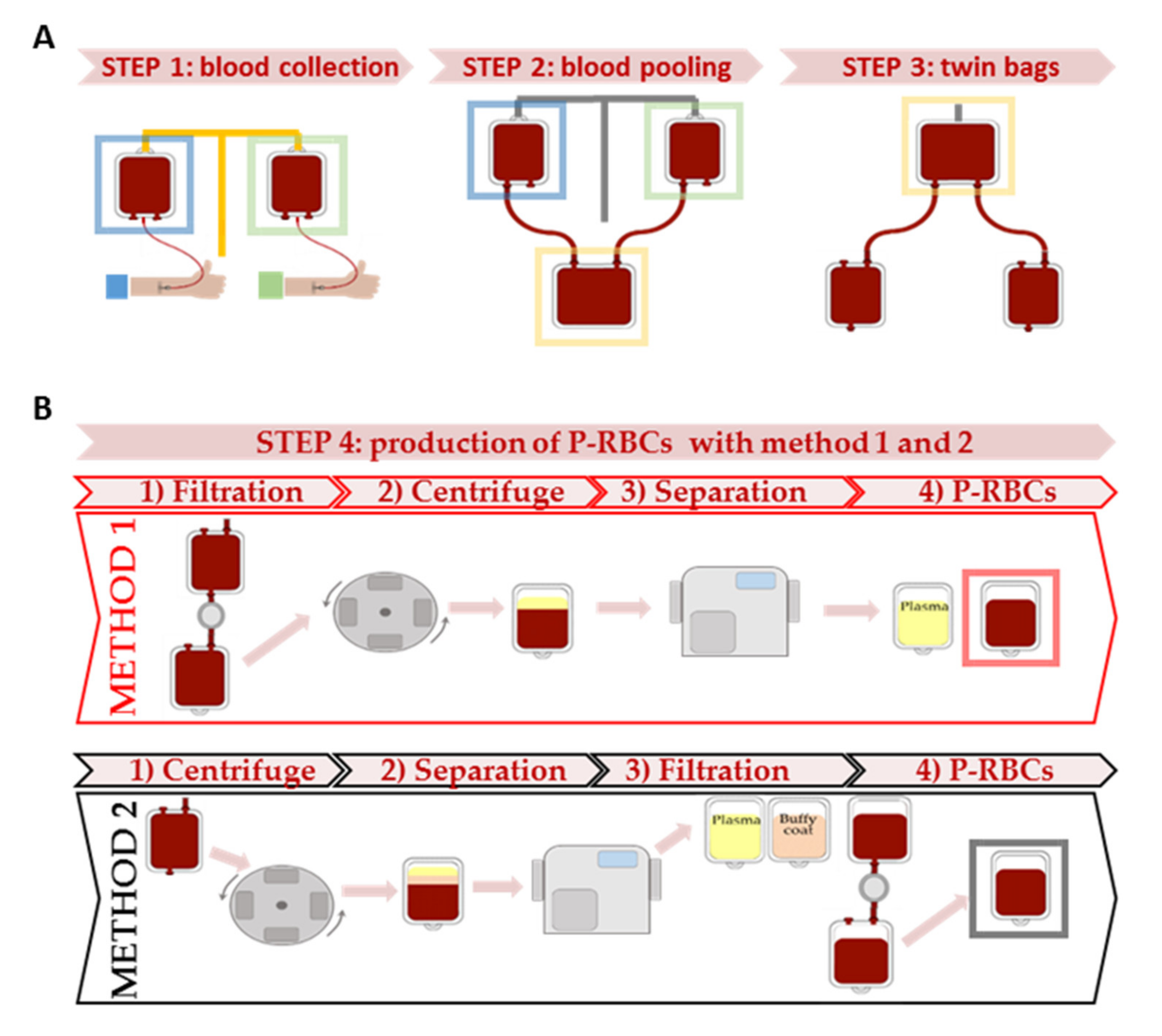
2.3. Packed RBCs and Samples Preparation
2.4. Volumes and Protein Content
2.5. Hematology Analysis
2.6. Leukocytes Quantification and Viability
2.7. Haemolysis and Erythrocytes Fragility
2.8. Cytokines Quantification
2.9. Electrolytes, Iron Metabolism and LDH Analysis
2.10. Proton Nuclear Magnetic Resonance (1H-NMR) Metabolomics Analysis
2.11. Statistical Analysis
3. Results
3.1. Study Population
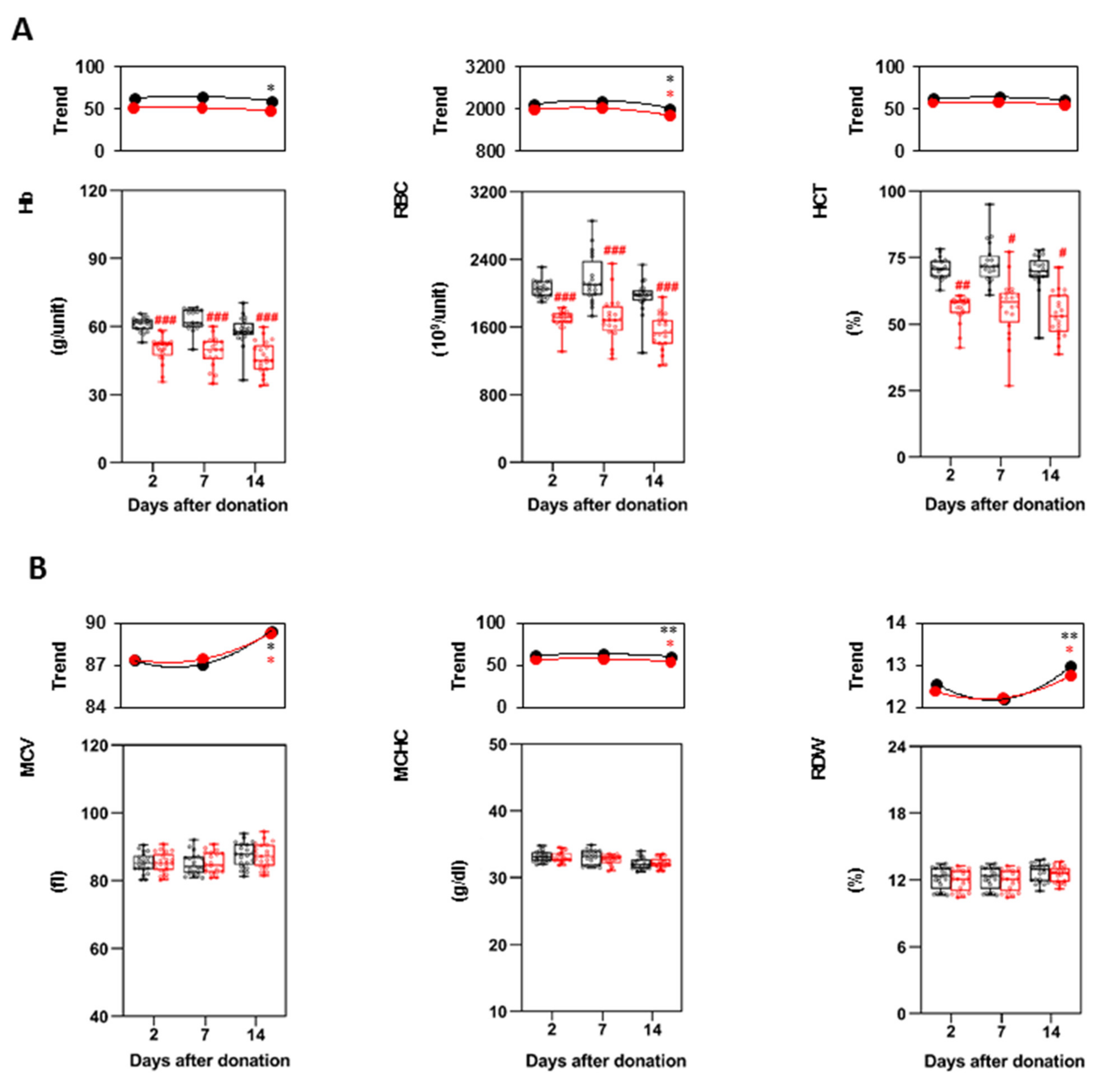
3.2. Characteristics of the P-RBCs Units and RBCs Indices
3.3. WBCs and PLTs
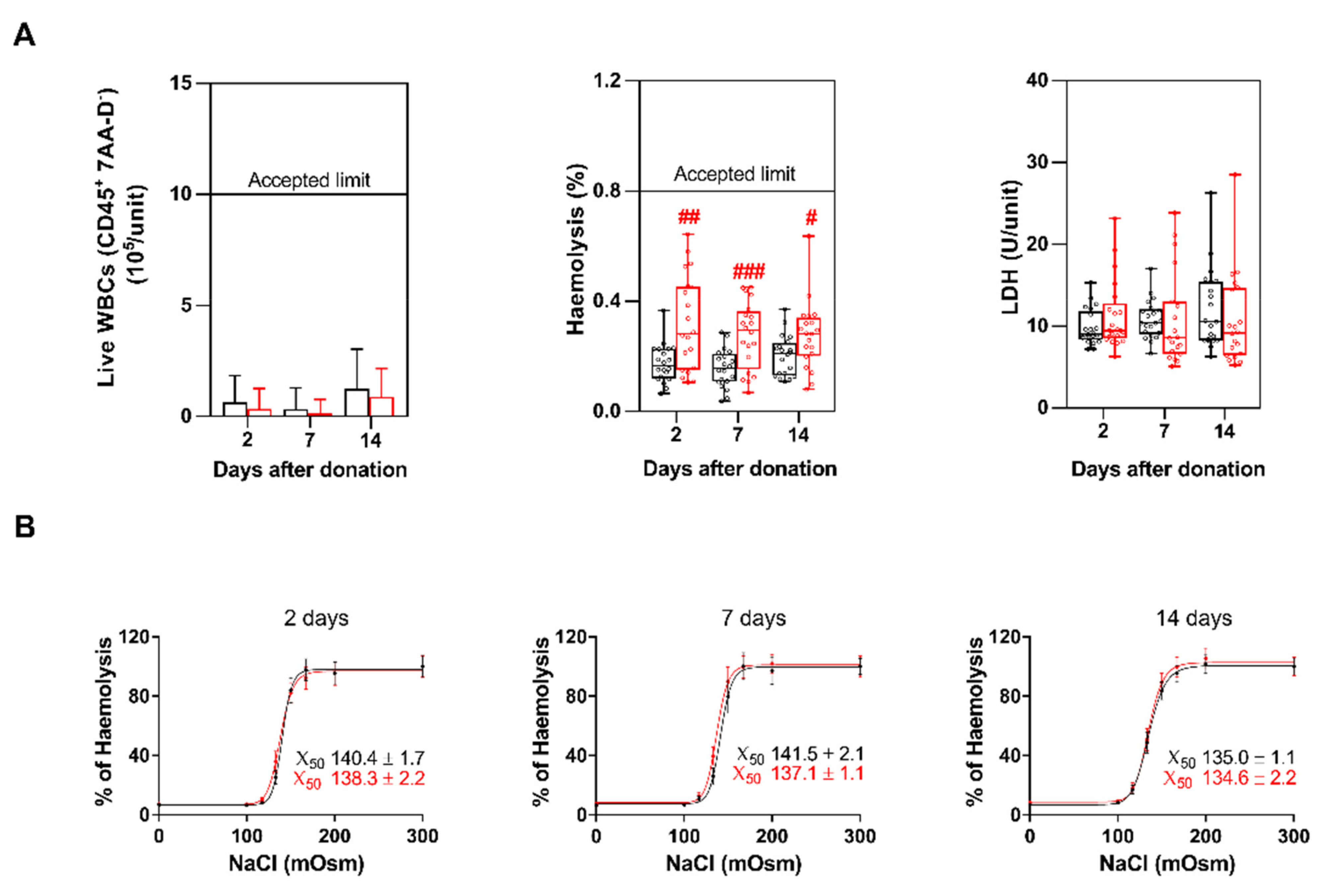
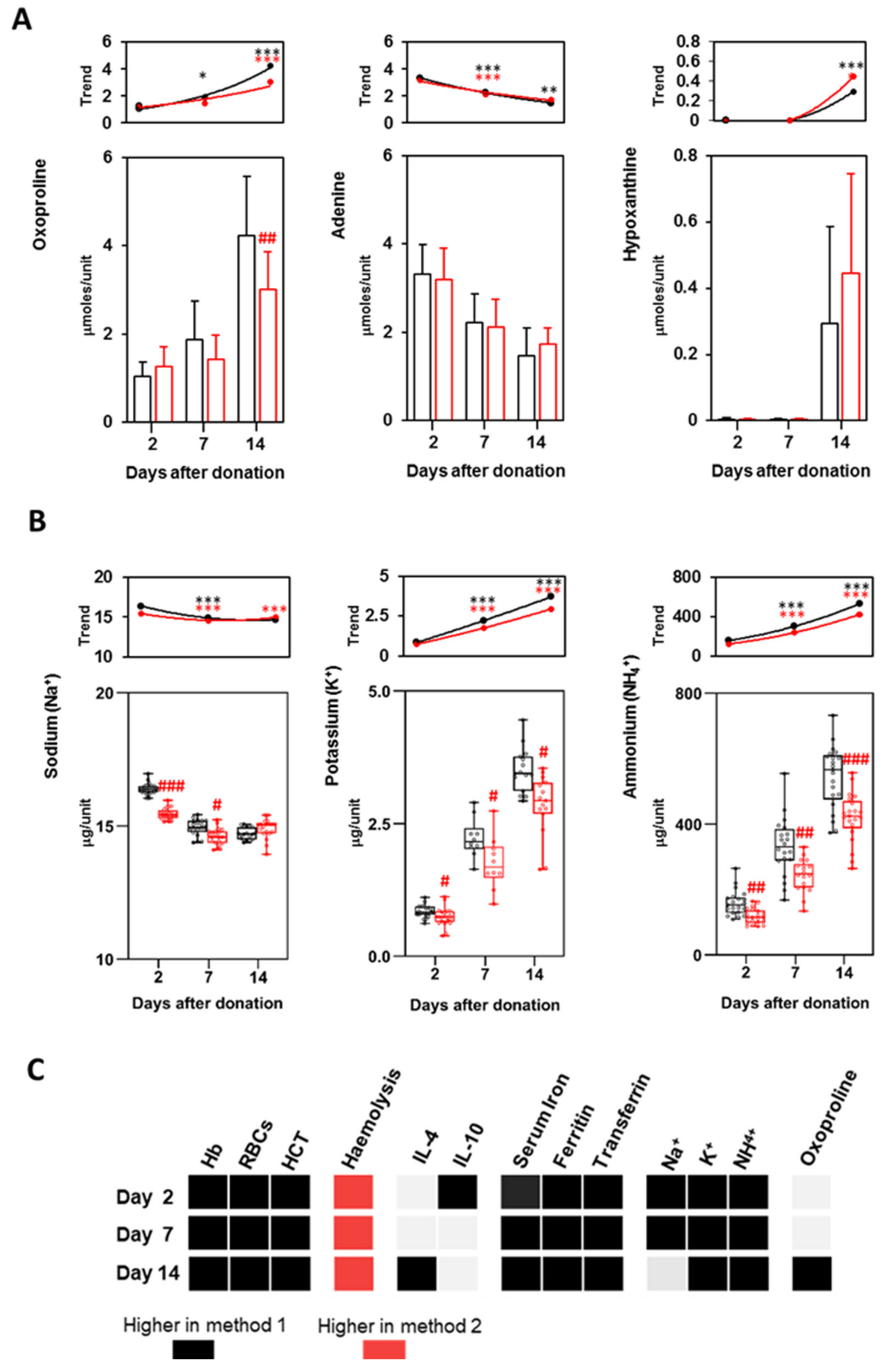
3.4. Haemolysis, LDH and Erythrocytes Fragility
3.5. Cytokines Content
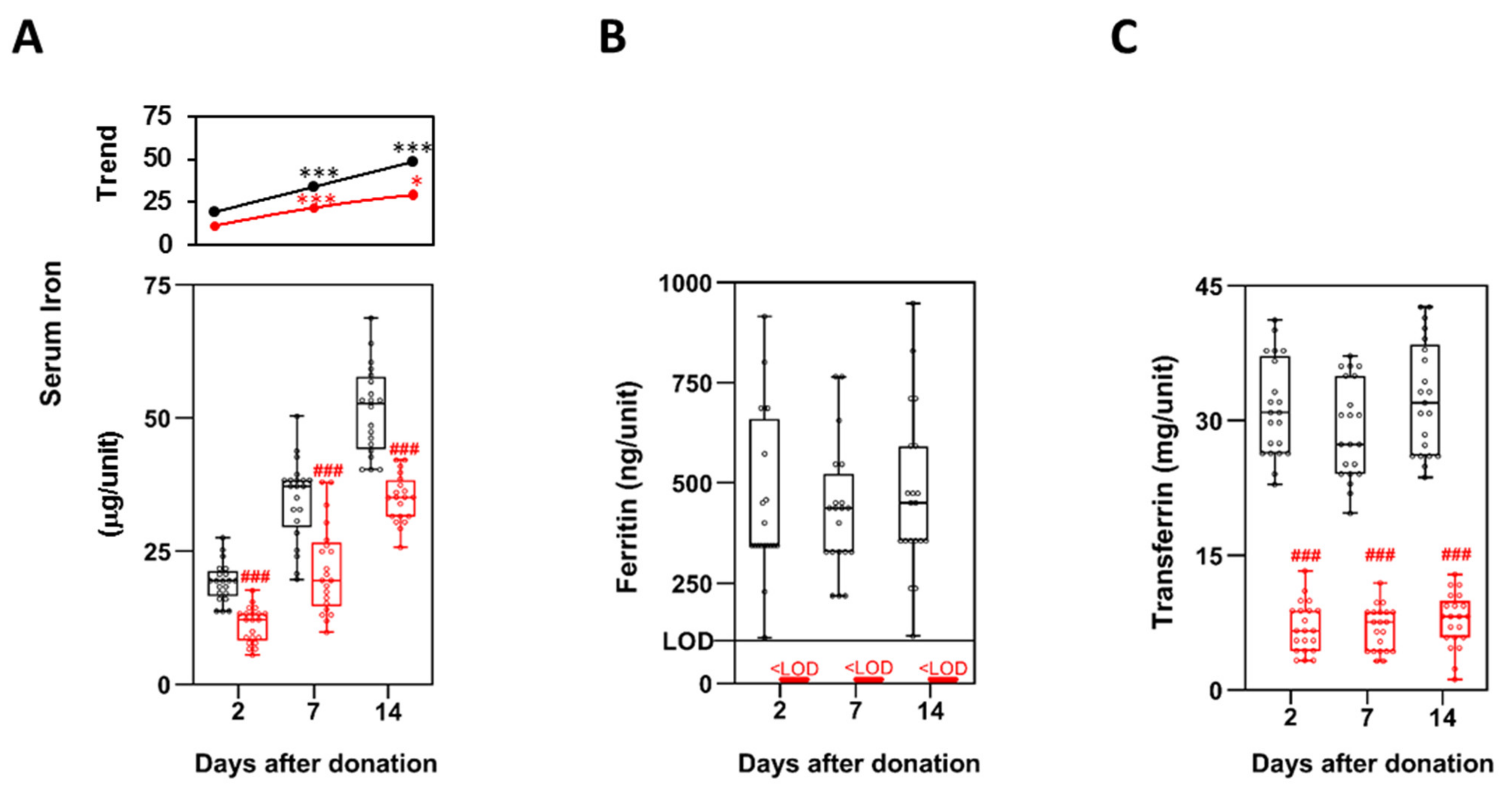
3.6. Iron Metabolism
3.7. Metabolites and Electrolytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Origa, R. β-Thalassemia. Genet. Med. 2017, 19, 609–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, F.T.; Sayani, F.; Trompeter, S.; Drasar, E.; Piga, A. Challenges of blood transfusions in β-thalassemia. Blood Rev. 2019, 37, 100588. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.A.; Walters, M.C.; Kwiatkowski, J.; Rasko, J.E.; Ribeil, J.-A.; Hongeng, S.; Magrin, E.; Schiller, G.J.; Payen, E.; Semeraro, M.; et al. Gene Therapy in Patients with Transfusion-Dependent β-Thalassemia. N. Engl. J. Med. 2018, 378, 1479–1493. [Google Scholar] [CrossRef]
- Marktel, S.; Scaramuzza, S.; Cicalese, M.P.; Giglio, F.; Galimberti, S.; Lidonnici, M.R.; Calbi, V.; Assanelli, A.; Bernardo, M.E.; Rossi, C.; et al. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent ß-thalassemia. Nat. Med. 2019, 25, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; De La Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kremastinos, D.T.; Farmakis, D.; Aessopos, A.; Hahalis, G.; Hamodraka, E.; Tsiapras, D.; Keren, A. β-thalassemia cardiomyopathy: History, present considerations, and future perspectives. Circ. Heart Fail. 2010, 3, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballas, S.K.; Zeidan, A.M.; Duong, V.H.; Deveaux, M.; Heeney, M.M. The effect of iron chelation therapy on overall survival in sickle cell disease and β-thalassemia: A systematic review. Am. J. Hematol. 2018, 93, 943–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centro Nazionale Sangue. Registro nazionale Talassemie ed Emoglobinopatie. Available online: https://www.centronazionalesangue.it/registro-nazionale-talassemie-ed-emoglobinopatie/ (accessed on 13 September 2019).
- Bonomo, P.; Carta, M.; Forni, G. Raccomandazioni per le strategie trasfusionali nelle emoglobinopatie. Collana Sci. SITE 2014, 3, 1–64. [Google Scholar]
- Sut, C.; Tariket, S.; Chou, M.L.; Garraud, O.; Laradi, S.; Hamzeh-Cognasse, H.; Seghatchian, J.; Burnouf, T.; Cognasse, F. Duration of red blood cell storage and inflammatory marker generation. High Speed Blood Transfus. Equip. 2017, 15, 145–152. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Nemkov, T.; Reisz, J.; Dzieciatkowska, M.; Wither, M.J.; Hansen, K.C. Omics markers of the red cell storage lesion and metabolic linkage. High Speed Blood Transfus. Equip. 2017, 15, 137–144. [Google Scholar] [CrossRef]
- Pertinhez, T.A.; Casali, E.; Lindner, L.; Spisni, A.; Baricchi, R.; Berni, P. Biochemical assessment of red blood cells during storage by 1H nuclear magnetic resonance spectroscopy. Identification of a biomarker of their level of protection against oxidative stress. Blood Transfus. 2014, 12, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Orlov, D.; Karkouti, K. The pathophysiology and consequences of red blood cell storage. Anaesthesia 2015, 70, 29-e12. [Google Scholar] [CrossRef]
- Seghatchian, J. Universal leucodepletion: An overview of some unresolved issues and the highlights of lessons learned. Transfus. Apher. Sci. 2003, 29, 105–117. [Google Scholar] [CrossRef]
- Pertinhez, T.A.; Casali, E.; Baroni, F.; Berni, P.; Baricchi, R.; Spisni, A. A Comparative Study of the Effect of Leukoreduction and Pre-storage Leukodepletion on Red Blood Cells during Storage. Front. Mol. Biosci. 2016, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Gamberini, M.R.; Fortini, M.; Stievano, A.; Calori, E.; Riontino, M.V.; Ceccherelli, G.; Venturelli, D.; Chicchi, R.; Biguzzi, R.; Fagnoni, F.; et al. Impact of the preparation method of red cell concentrates on transfusion indices in thalassemia patients: A randomized crossover clinical trial. Transfusion 2021, 61, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Decreto del Ministero della Salute. Disposizioni Relative ai Requisiti di Qualità e Sicurezza del Sangue e degli Emocomponenti. Publicly. Available online: https://www.gazzettaufficiale.it/eli/id/2015/12/28/15A09709/sg (accessed on 2 November 2015). (In Italian).
- Baroni, F.; Marraccini, C.; Merolle, L.; Piccagli, V.; Lambertini, D.; Iori, M.; Fasano, T.; Casali, E.; Spisni, A.; Baricchi, R.; et al. Red blood cells metabolome changes upon treatment with different X-ray irradiation doses. Ann. Hematol. 2018, 97, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Han, V.; Serrano, K.; Devine, D.V. A comparative study of common techniques used to measure haemolysis in stored red cell concentrates. Vox Sang. 2010, 98, 116–123. [Google Scholar] [CrossRef]
- DeTraglia, M.; Cook, F.; Stasiw, D.; Cerny, L. Erythrocyte fragility in aging. Biochim. Biophys. Acta (BBA)-Biomembr. 1974, 345, 213–219. [Google Scholar] [CrossRef]
- Cappellini, M.-D.; Cohen, A.; Eleftheriou, A.; Piga, A.; Porter, J.; Taher, A. Guidelines for the Clinical Management of Thalassaemia [Internet]. 2nd Revised Edition; Thalassaemia International Federation: Nicosia, Cyprus. Available online: https://www.ncbi.nlm.nih.gov/books/NBK173968/ (accessed on 1 January 2008).
- Verma, K.D.M.; Dahiya, K.; Malik, D.; Pk, S.; Devi, R.; Soni, A.; Ghalaut, V.S. Effect of Blood Storage on Complete Biochemistry. J. Blood Disord. Transfus. 2015, 6, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Paglia, G.; D’Alessandro, A.; Rolfsson, Ó.; Sigurjónsson, Ó.E.; Bordbar, A.; Palsson, S.; Nemkov, T.; Hansen, K.C.; Gudmundsson, S.; Palsson, B.O. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood 2016, 128, e43–e50. [Google Scholar] [CrossRef]
- Taher, A.; Cappellini, M.-D.; Cohen, A. Guidelines for the Management of Transfusion Dependent Thalassaemia (4th Edition—2021). Available online: https://issuu.com/internationalthalassaemiafederation/docs/final_guideline_4th (accessed on 13 September 2021).
- Cabibbo, S.; Fidone, C.; Antolino, A.; Manenti, O.; Garozzo, G.; Travali, S.; Bennardello, F.; Di Stefano, R.; Bonomo, P. Clinical effects of different types of red cell concentrates in patients with thalassemia and sickle cell disease. Transfus. Clin. Biol. 2007, 14, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Cohle, S.D.; Saleem, A.; Makkaoui, D.E. Effects of storage of blood on stability of hematologic parameters. Am. J. Clin. Pathol. 1981, 76, 67–69. [Google Scholar] [CrossRef] [Green Version]
- Van de Watering, L.M.; Brand, A. Effects of storage of red cells. Transfus. Med. Hemothe. 2008, 35, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, M.; Seghatchian, M.; Dilger, P.; Contreras, M.; Thorpe, R. Cytokine accumulation in stored red cell concentrates: Effect of buffy-coat removal and leucoreduction. Transfus. Sci. 2000, 23, 7–16. [Google Scholar] [CrossRef]
- Benson, D.D.; Beck, A.; Burdine, M.S.; Brekken, R.; Silliman, C.C.; Barnett, C.C. Accumulation of Pro-Cancer Cytokines in the Plasma Fraction of Stored Packed Red Cells. J. Gastrointest. Surg. 2012, 16, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Te Velde, A.A.; Huijbens, R.J.; Heije, K.; de Vries, J.E.; Figdor, C.G. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood 1990, 76, 1392–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapur, R.; Kim, M.; Aslam, R.; McVey, M.J.; Tabuchi, A.; Luo, A.; Liu, J.; Li, Y.; Shanmugabhavananthan, S.; Speck, E.R.; et al. T regulatory cells and dendritic cells protect against transfusion-related acute lung injury via IL-10. Blood 2017, 129, 2557–2569. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Kriebardis, A.; Rinalducci, S.; Antonelou, M.; Hansen, K.C.; Papassideri, I.S.; Zolla, L. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfussion 2015, 55, 205–219. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Nemkov, T.; Kelher, M.; West, F.B.; Schwindt, R.K.; Banerjee, A.; Moore, E.E.; Silliman, C.C.; Hansen, K.C. Routine storage of red blood cell (RBC) units in additive solution-3: A comprehensive investigation of the RBC metabolome. Transfussion 2015, 55, 1155–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett-Guerrero, E.; Veldman, T.H.; Doctor, A.; Telen, M.J.; Ortel, T.L.; Reid, T.S.; Mulherin, M.A.; Zhu, H.; Buck, R.D.; Califf, R.M.; et al. Evolution of adverse changes in stored RBCs. Proc. Natl. Acad. Sci. USA 2007, 104, 17063–17068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Shevkoplyas, S.S. Anaerobic storage of red blood cells. High Speed Blood Transfus. Equip. 2010, 8, 220–236. [Google Scholar] [CrossRef]
- Marraccini, C.; Merolle, L.; Casali, E.; Baricchi, R.; Pertinhez, T.A. Proof of concept: Hypoxanthine from stored red blood cells induces neutrophil activation. Blood Transfus. Trasfus. Sangue 2020. [Google Scholar] [CrossRef]
- Reilly, M.; Bruno, C.D.; Prudencio, T.M.; Ciccarelli, N.; Guerrelli, D.; Nair, R.; Ramadan, M.; Luban, N.L.C.; Posnack, N.G. Potential Consequences of the Red Blood Cell Storage Lesion on Cardiac Electrophysiology. J. Am. Heart Assoc. 2020, 9, e017748. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.G.; Spahn, D.R.; Carson, J.L. Red blood cell transfusion in clinical practice. Lancet 2007, 370, 415–426. [Google Scholar] [CrossRef]
- Hod, E.A.; Brittenham, G.M.; Billote, G.B.; Francis, R.O.; Ginzburg, Y.Z.; Hendrickson, J.E.; Jhang, J.; Schwartz, J.; Sharma, S.; Sheth, S.; et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non–transferrin-bound iron. Blood 2011, 118, 6675–6682. [Google Scholar] [CrossRef]
| Method 1 | Method 2 | p-Value | |
|---|---|---|---|
| Total volume (mL/unit) | 303 ± 12 | 258 ± 14 | <0.01 |
| Total protein (g/unit) | 12.3 ± 2.6 | 7.2 ± 1.9 | <0.05 |
| Supernatant volume (mL/unit) | |||
| 2 days | 114 ± 10 | 109 ± 13 | n.s. |
| 7 days | 108 ± 22 | 110 ± 29 | n.s. |
| 14 days | 118 ± 18 | 115 ± 21 | n.s. |
| Storage | Method | IFN-γ | IL-1β | IL-2 | IL-4 | IL-5 | IL-6 | IL-10 | TNF-α |
|---|---|---|---|---|---|---|---|---|---|
| 2 days | 1 | 502 ± 308 | 176 ± 51 | 143 ± 100 | 120 ± 103 | 187 ± 49 | 303 ± 97 | 6.8 ± 2.4 | 155 ± 130 |
| 2 | 447 ± 202 | 97 ± 36 | 98 ± 32 | 166 ± 106 | 232 ± 12 | 545 ± 19 | 3.5 ± 1.8 | 68 ± 48 | |
| p < 0.05 | |||||||||
| 7 days | 1 | 314 ± 127 | 96 ± 36 | 61 ± 36 | 148 ± 106 | 154 ± 12 | 204 ± 19 | 4.4 ± 1.8 | 74 ± 48 |
| 2 | 448 ± 215 | 90 ± 44 | 91 ± 42 | 76 ± 48 | 145 ± 18 | 224 ± 40 | 4.8 ± 2.6 | 81 ± 54 | |
| 14 days | 1 | 296 ± 202 | 92 ± 160 | 90 ± 150 | 136 ± 60 | 193 ± 86 | 298 ± 146 | 3.3 ± 1.3 | 96 ± 53 |
| 2 | 422 ± 252 | 133 ± 11 | 85 ± 42 | 62 ± 31 | 145 ± 7 | 218 ± 22 | 3.5 ± 2.8 | 52 ± 24 | |
| p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiroli, D.; Merolle, L.; Quartieri, E.; Chicchi, R.; Fasano, T.; De Luca, T.; Molinari, G.; Pulcini, S.; Pertinhez, T.A.; Di Bartolomeo, E.; et al. Comparison of Two Alternative Procedures to Obtain Packed Red Blood Cells for β-Thalassemia Major Transfusion Therapy. Biomolecules 2021, 11, 1638. https://doi.org/10.3390/biom11111638
Schiroli D, Merolle L, Quartieri E, Chicchi R, Fasano T, De Luca T, Molinari G, Pulcini S, Pertinhez TA, Di Bartolomeo E, et al. Comparison of Two Alternative Procedures to Obtain Packed Red Blood Cells for β-Thalassemia Major Transfusion Therapy. Biomolecules. 2021; 11(11):1638. https://doi.org/10.3390/biom11111638
Chicago/Turabian StyleSchiroli, Davide, Lucia Merolle, Eleonora Quartieri, Roberta Chicchi, Tommaso Fasano, Tiziana De Luca, Giuseppe Molinari, Stefano Pulcini, Thelma A. Pertinhez, Erminia Di Bartolomeo, and et al. 2021. "Comparison of Two Alternative Procedures to Obtain Packed Red Blood Cells for β-Thalassemia Major Transfusion Therapy" Biomolecules 11, no. 11: 1638. https://doi.org/10.3390/biom11111638
APA StyleSchiroli, D., Merolle, L., Quartieri, E., Chicchi, R., Fasano, T., De Luca, T., Molinari, G., Pulcini, S., Pertinhez, T. A., Di Bartolomeo, E., Biguzzi, R., Baricchi, R., & Marraccini, C. (2021). Comparison of Two Alternative Procedures to Obtain Packed Red Blood Cells for β-Thalassemia Major Transfusion Therapy. Biomolecules, 11(11), 1638. https://doi.org/10.3390/biom11111638






