Metabolic Dysfunction Biomarkers as Predictors of Early Diabetes
Abstract
:1. Introduction
2. Materials and Methods
Search Strategy and Selection Criteria
3. Results
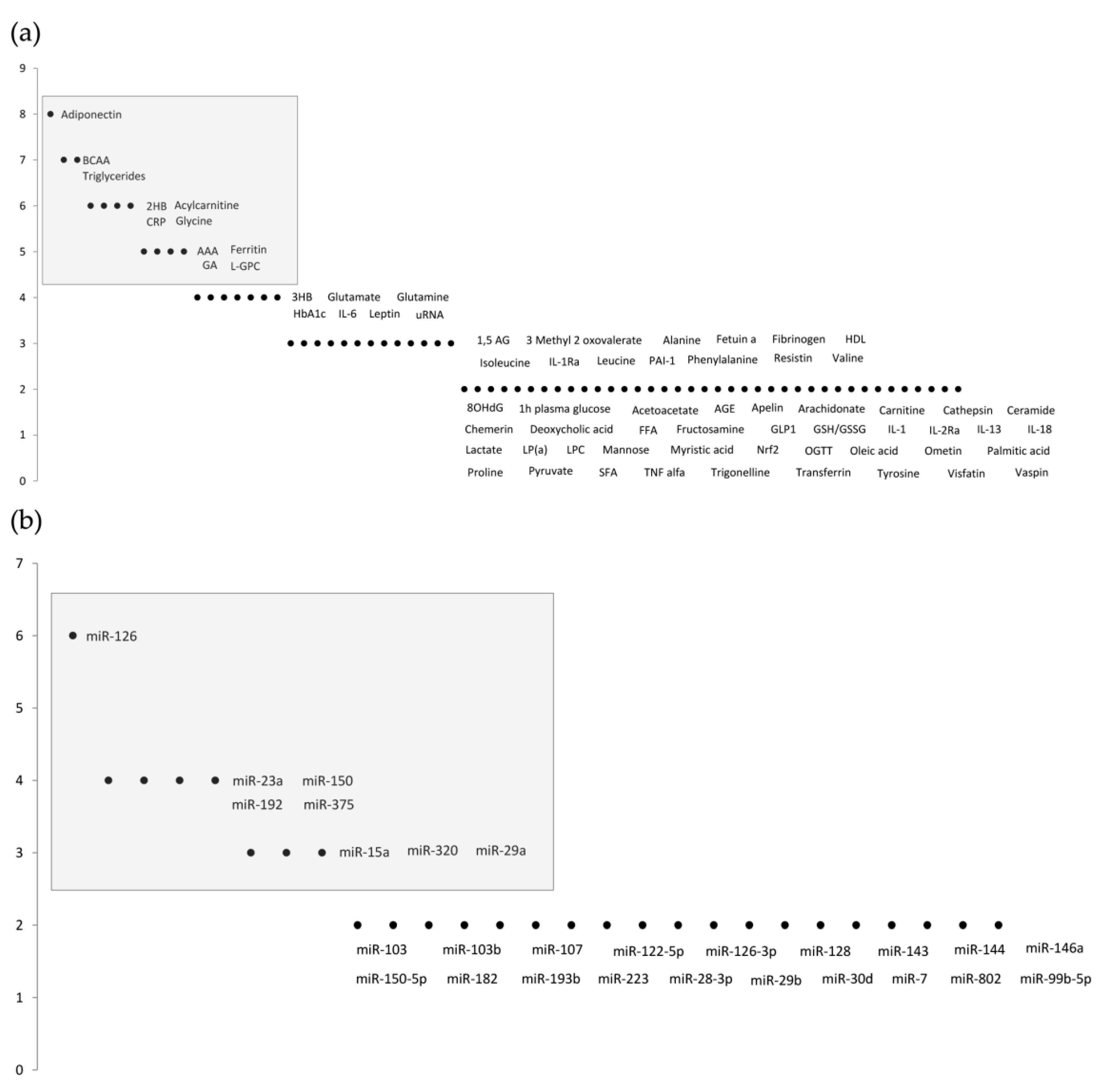
3.1. Metabolomics Studies
3.2. MicroRNA Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF. Diabetes Atlas. Int. Diabetes Fed. 2019, 1, 10–15. [Google Scholar]
- Shah, A.D.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Rodriguez, M.P.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015, 3, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Tolman, K.G.; Fonseca, V.; Dalpiaz, A.; Tan, M.H. Spectrum of Liver Disease in Type 2 Diabetes and Management of Patients with Diabetes and Liver Disease. Diabetes Care 2007, 30, 734–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, K.K. The Diabetes-Cancer Link. Diabetes Spectr. 2014, 27, 276–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buysschaert, M.; Bergman, M. Definition of Prediabetes. Med. Clin. N. Am. 2011, 95, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Keck, J.W.; Thomas, A.R.; Hieronymus, L.; Roper, K.L. Prediabetes Knowledge, Attitudes, and Practices at an Academic Family Medicine Practice. J. Am. Board Fam. Med. 2019, 32, 505–512. [Google Scholar] [CrossRef]
- Alderman, M.H. Prediabetes: An unexplored cardiovascular disease risk factor. J. Hypertens. 2021, 39, 42–43. [Google Scholar] [CrossRef]
- Long, J.; Yang, Z.; Wang, L.; Han, Y.; Peng, C.; Yan, C.; Yan, D. Metabolite biomarkers of type 2 diabetes mellitus and pre-diabetes: A systematic review and meta-analysis. BMC Endocr. Disord. 2020, 20, 1–17. [Google Scholar] [CrossRef]
- Bergman, M.; Abdul-Ghani, M.; DeFronzo, R.A.; Manco, M.; Sesti, G.; Fiorentino, T.V.; Ceriello, A.; Rhee, M.; Phillips, L.S.; Chung, S.; et al. Review of methods for detecting glycemic disorders. Diabetes Res. Clin. Pract. 2020, 165, 108233. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharm. 2019, 70, 809–824. [Google Scholar]
- Thorens, B.; Rodriguez, A.; Cruciani-Guglielmacci, C.; Wigger, L.; Ibberson, M.; Magnan, C. Use of preclinical models to identify markers of type 2 diabetes susceptibility and novel regulators of insulin secretion—A step towards precision medicine. Mol. Metab. 2019, 27, S147–S154. [Google Scholar] [CrossRef]
- Arneth, B.; Arneth, R.; Shams, M. Metabolomics of Type 1 and Type 2 Diabetes. Int. J. Mol. Sci. 2019, 20, 2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigagli, E.; Lodovici, M. Circulating Oxidative Stress Biomarkers in Clinical Studies on Type 2 Diabetes and Its Complications. Oxidative Med. Cell. Longev. 2019, 2019, 1–17. [Google Scholar] [CrossRef]
- Diwaker, A.; Kishore, D.; Singh, V.; Mahapatra, S.P. The Novel Biomarkers in Diabetes. J. Assoc. Physicians India 2019, 67, 65–69. [Google Scholar] [PubMed]
- Jagannathan, R.; Buysschaert, M.; Medina, J.L.; Katz, K.; Musleh, S.; Dorcely, B.; Bergman, M. The 1-h post-load plasma glucose as a novel biomarker for diagnosing dysglycemia. Acta Diabetol. 2018, 55, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Gar, C.; Rottenkolber, M.; Prehn, C.; Adamski, J.; Seissler, J.; Lechner, A. Serum and plasma amino acids as markers of prediabetes, insulin resistance, and incident diabetes. Crit. Rev. Clin. Lab. Sci. 2017, 55, 21–32. [Google Scholar] [CrossRef]
- Maghsoudi, A.S.; Vakhshiteh, F.; Torabi, R.; Hassani, S.; Ganjali, M.R.; Norouzi, P.; Hosseini, M.; Abdollahi, M. Recent advances in biosensor technology in assessment of early diabetes biomarkers. Biosens. Bioelectron. 2018, 99, 122–135. [Google Scholar] [CrossRef]
- Liggi, S.; Griffin, J.L. Metabolomics applied to diabetes—Lessons from human population studies. Int. J. Biochem. Cell Biol. 2017, 93, 136–147. [Google Scholar] [CrossRef]
- Dorcely, B.; Katz, K.; Jagannathan, R.; Chiang, S.S.; Oluwadare, B.; Goldberg, I.J.; Bergman, M. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2017, 10, 345–361. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.; Jagadeeshaprasad, M.G.; Venkatasubramani, V.; Kulkarni, M.J. Abundance matters: Role of albumin in diabetes, a proteomics perspective. Expert Rev. Proteom. 2017, 14, 677–689. [Google Scholar] [CrossRef]
- Yan-Do, R.; MacDonald, P. Impaired “Glycine”-mia in Type 2 Diabetes and Potential Mechanisms Contributing to Glucose Homeostasis. Endocrinology 2017, 158, 1064–1073. [Google Scholar] [CrossRef]
- Larsen, M.P.; Torekov, S.S. Glucagon-Like Peptide 1: A Predictor of Type 2 Diabetes? J. Diabetes Res. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, R.T.; Macedo, M.P.; Raposo, J.F. HbA1c, Fructosamine, and Glycated Albumin in the Detection of Dysglycaemic Conditions. Curr. Diabetes Rev. 2015, 12, 14–19. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Almanza-Aguilera, E.; Tulipani, S.; Tinahones, F.J.; Salas-Salvadó, J.; Andres-Lacueva, C. Metabolomics for Biomarkers of Type 2 Diabetes Mellitus: Advances and Nutritional Intervention Trends. Curr. Cardiovasc. Risk Rep. 2015, 9, 1–12. [Google Scholar] [CrossRef]
- Dunmore, S.J.; Brown, J.E.P. The role of adipokines in β-cell failure of type 2 diabetes. J. Endocrinol. 2012, 216, T37–T45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, T.J.; Basu, A. Biomarkers in diabetes: Hemoglobin A1c, vascular and tissue markers. Transl. Res. 2012, 159, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjesing, A.P.; Pedersen, O. ‘Omics’-driven discoveries in prevention and treatment of type 2 diabetes. Eur. J. Clin. Investig. 2012, 42, 579–588. [Google Scholar] [CrossRef] [PubMed]
- González-Sánchez, L.E.; Ortega-Camarillo, C.; Contreras-Ramos, A.; Barajas-Nava, L.A. miRNAs as biomarkers for diagnosis of type 2 diabetes: A systematic review. J. Diabetes 2021, 13, 792–816. [Google Scholar] [CrossRef] [PubMed]
- Athira, S.; Misra, P.; Bhatia, K.; Sibin, M. Identification of circulatory miRNAs as candidate biomarkers in prediabetes—A systematic review and bioinformatics analysis. Gene Rep. 2020, 21, 100954. [Google Scholar] [CrossRef]
- Pielok, A.; Marycz, K. Non-Coding RNAs as Potential Novel Biomarkers for Early Diagnosis of Hepatic Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 4182. [Google Scholar] [CrossRef] [PubMed]
- Vasu, S.; Kumano, K.; Darden, C.M.; Rahman, I.; Lawrence, M.C.; Naziruddin, B. MicroRNA Signatures as Future Biomarkers for Diagnosis of Diabetes States. Cells 2019, 8, 1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zheng, J.; Hu, X.; Chen, L. Dysregulated expression of long noncoding RNAs serves as diagnostic biomarkers of type 2 diabetes mellitus. Endocrine 2019, 65, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, S.; Sarwade, R.D.; Seshadri, V. MicroRNA, Proteins, and Metabolites as Novel Biomarkers for Prediabetes, Diabetes, and Related Complications. Front. Endocrinol. 2018, 9, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashoori, M.R.; Rahmati-Yamchi, M.; Ostadrahimi, A.; Aval, S.F.; Zarghami, N. MicroRNAs and adipocytokines: Promising biomarkers for pharmacological targets in diabetes mellitus and its complications. Biomed. Pharmacother. 2017, 93, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Raffort, J.; Hinault, C.; Dumortier, O.; Van Obberghen, E. Circulating microRNAs and diabetes: Potential applications in medical practice. Diabetologia 2015, 58, 1978–1992. [Google Scholar] [CrossRef] [Green Version]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.-P.; Mitchell, M.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. α-Hydroxybutyrate Is an Early Biomarker of Insulin Resistance and Glucose Intolerance in a Nondiabetic Population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landaas, S. The formation of 2-hydroxybutyric acid in experimental animals. Clin. Chim. Acta 1975, 58, 23–32. [Google Scholar] [CrossRef]
- Cobb, J.; Eckhart, A.; Perichon, R.; Wulff, J.; Mitchell, M.; Adam, K.-P.; Wolfert, R.; Button, E.; Lawton, K.; Elverson, R.; et al. A Novel Test for IGT Utilizing Metabolite Markers of Glucose Tolerance. J. Diabetes Sci. Technol. 2015, 9, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tricò, D.; Prinsen, H.; Giannini, C.; De Graaf, R.; Juchem, C.; Li, F.; Caprio, S.; Santoro, N.; Herzog, R.I. Elevated α-Hydroxybutyrate and Branched-Chain Amino Acid Levels Predict Deterioration of Glycemic Control in Adolescents. J. Clin. Endocrinol. Metab. 2017, 102, 2473–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owei, I.; Umekwe, N.; Stentz, F.; Wan, J.; Dagogo-Jack, S. Amino acid signature predictive of incident prediabetes: A case-control study nested within the longitudinal pathobiology of prediabetes in a biracial cohort. Metabolism 2019, 98, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Walford, G.A.; Davis, J.; Warner, A.S.; Ackerman, R.J.; Billings, L.K.; Chamarthi, B.; Fanelli, R.R.; Hernandez, A.M.; Huang, C.; Khan, S.Q.; et al. Branched chain and aromatic amino acids change acutely following two medical therapies for type 2 diabetes mellitus. Metabolism 2013, 62, 1772–1778. [Google Scholar] [CrossRef] [Green Version]
- Saltevo, J.; Kautiainen, H.; Vanhala, M. Gender differences in adiponectin and low-grade inflammation among individuals with normal glucose tolerance, prediabetes, and type 2 diabetes. Gend. Med. 2009, 6, 463–470. [Google Scholar] [CrossRef]
- Jiang, Y.; Owei, I.; Wan, J.; Ebenibo, S.; Dagogo-Jack, S. Adiponectin levels predict prediabetes risk: The Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study. BMJ Open Diabetes Res. Care 2016, 4, e000194. [Google Scholar] [CrossRef] [Green Version]
- Stefan, N.; Sun, Q.; Fritsche, A.; Machann, J.; Schick, F.; Gerst, F.; Jeppesen, C.; Joost, H.-G.; Hu, F.B.; Boeing, H.; et al. Impact of the Adipokine Adiponectin and the Hepatokine Fetuin-A on the Development of Type 2 Diabetes: Prospective Cohort- and Cross-Sectional Phenotyping Studies. PLoS ONE 2014, 9, e92238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, H.; Lin, N.; Xing, Z.; Weng, H.; Zhang, H. Association between the level of circulating adiponectin and prediabetes: A meta-analysis. J. Diabetes Investig. 2015, 6, 416–429. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Chen, L.; Han, X.; Ji, L. Human serum acylcarnitine profiles in different glucose tolerance states. Diabetes Res. Clin. Pract. 2014, 104, 376–382. [Google Scholar] [CrossRef]
- Mai, M.; Tönjes, A.; Kovacs, P.; Stumvoll, M.; Fiedler, G.M.; Leichtle, A.B. Serum Levels of Acylcarnitines Are Altered in Prediabetic Conditions. PLoS ONE 2013, 8, e82459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.; Garvey, W.T. Plasma Acylcarnitine Profiles Suggest Incomplete Long-Chain Fatty Acid β-Oxidation and Altered Tricarboxylic Acid Cycle Activity in Type 2 Diabetic African-American Women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef]
- Sun, L.; Liang, L.; Gao, X.; Zhang, H.; Yao, P.; Hu, Y.; Ma, Y.; Wang, F.; Jin, Q.; Li, H.; et al. Early Prediction of Developing Type 2 Diabetes by Plasma Acylcarnitines: A Population-Based Study. Diabetes Care 2016, 39, 1563–1570. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Canela, M.; Guasch-Ferré, M.; Toledo, E.; Clish, C.B.; Razquin, C.; Liang, L.; Wang, D.D.; Corella, D.; Estruch, R.; Hernáez, Á.; et al. Plasma branched chain/aromatic amino acids, enriched Mediterranean diet and risk of type 2 diabetes: Case-cohort study within the PREDIMED Trial. Diabetologia 2018, 61, 1560–1571. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yang, W. Branched-chain amino acids and the association with type 2 diabetes. J. Diabetes Investig. 2015, 6, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Yemelyanov, A. Branched Chain Amino Acids and Risk of Type 2 Diabetes Mellitus: A Literature Review. Master’s Thesis, Georgia State University, Atlanta, GA, USA, 2021. [Google Scholar]
- Festa, A.; Hanley, A.J.; Tracy, R.P.; D’Agostino, R.; Haffner, S.M. Inflammation in the Prediabetic State Is Related to Increased Insulin Resistance Rather Than Decreased Insulin Secretion. Circulation 2003, 108, 1822–1830. [Google Scholar] [CrossRef]
- Van Woudenbergh, G.J.; Kuijsten, A.; Sijbrands, E.J.G.; Hofman, A.; Witteman, J.C.M.; Feskens, E.J.M. Glycemic Index and Glycemic Load and Their Association with C-Reactive Protein and Incident Type 2 Diabetes. J. Nutr. Metab. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sabanayagam, C.; Shankar, A.; Lim, S.C.; Lee, J.; Tai, E.S.; Wong, T.Y. Serum C-reactive protein level and prediabetes in two Asian populations. Diabetologia 2011, 54, 767–775. [Google Scholar] [CrossRef]
- Grossmann, V.; Schmitt, V.H.; Zeller, T.; Panova-Noeva, M.; Schulz, A.; Laubert-Reh, D.; Juenger, C.; Schnabel, R.B.; Abt, T.G.; Laskowski, R.; et al. Profile of the Immune and Inflammatory Response in Individuals with Prediabetes and Type 2 Diabetes. Diabetes Care 2015, 38, 1356–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, F.; Nasab, N.M.; Zadeh, H.J. Elevated serum ferritin concentrations in prediabetic subjects. Diabetes Vasc. Dis. Res. 2008, 5, 15–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Jones, D.; Luo, B.; Sanderson, M.; Soto, J.; Abel, E.D.; Cooksey, R.C.; McClain, N.A. Iron Overload and Diabetes Risk: A Shift from Glucose to Fatty Acid Oxidation and Increased Hepatic Glucose Production in a Mouse Model of Hereditary Hemochromatosis. Diabetes 2010, 60, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunutsor, S.K.; Apekey, T.A.; Walley, J.; Kain, K. Ferritin levels and risk of type 2 diabetes mellitus: An updated systematic review and meta-analysis of prospective evidence. Diabetes/Metab. Res. Rev. 2013, 29, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Harding, A.H.; Allison, M.; Sandhu, M.S.; Welch, A.; Luben, R.; Bingham, S.; Khaw, K.T.; Wareham, N.J. Elevated serum ferritin levels predict new-onset type 2 diabetes: Results from the EPIC-Norfolk prospective study. Diabetology 2007, 50, 949–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-E. Alternative biomarkers for assessing glycemic control in diabetes: Fructosamine, glycated albumin, and 1,5-anhydroglucitol. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Danese, E.; Montagnana, M.; Nouvenne, A.; Lippi, G. Advantages and Pitfalls of Fructosamine and Glycated Albumin in the Diagnosis and Treatment of Diabetes. J. Diabetes Sci. Technol. 2015, 9, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrinello, C.M.; Selvin, E. Beyond HbA1c and Glucose: The Role of Nontraditional Glycemic Markers in Diabetes Diagnosis, Prognosis, and Management. Curr. Diabetes Rep. 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Selvin, E.; Rawlings, A.; Grams, M.; Klein, R.; Sharrett, A.R.; Steffes, M.; Coresh, J. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: A prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014, 2, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef]
- Pérez-Matos, M.C.; Morales, M.; Toloza, F.J.K.; Ricardo-Silgado, M.L.; Mantilla-Rivas, J.O.; Pinzón-Cortes, J.A.; Perez-Mayorga, M.; Jiménez, E.; Guevara, E.; Mendivil, C.O. The Phospholipid Linoleoylglycerophosphocholine as a Biomarker of Directly Measured Insulin Resistance. Diabetes Metab. J. 2017, 41, 466–473. [Google Scholar] [CrossRef]
- Ahn, N.; Baumeister, S.E.; Amann, U.; Rathmann, W.; Peters, A.; Huth, C.; Thorand, B.; Meisinger, C. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamasaki, H.; Noda, M.; Moriyama, S.; Yoshikawa, R.; Katsuyama, H.; Sako, A.; Mishima, S.; Kakei, M.; Ezaki, O.; Yanai, H. Daily Physical Activity Assessed by a Triaxial Accelerometer Is Beneficially Associated with Waist Circumference, Serum Triglycerides, and Insulin Resistance in Japanese Patients with Prediabetes or Untreated Early Type 2 Diabetes. J. Diabetes Res. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Shimodaira, M.; Niwa, T.; Nakajima, K.; Kobayashi, M.; Hanyu, N.; Nakayama, T. Serum Triglyceride Levels Correlated with the Rate of Change in Insulin Secretion Over Two Years in Prediabetic Subjects. Ann. Nutr. Metab. 2014, 64, 38–43. [Google Scholar] [CrossRef]
- Al-Kafaji, G.; Al-Mahroos, G.; Alsayed, N.A.; Hasan, Z.A.; Nawaz, S.; Bakhiet, M. Peripheral blood microRNA-15a is a potential biomarker for type 2 diabetes mellitus and pre-diabetes. Mol. Med. Rep. 2015, 12, 7485–7490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawal, S.; Munasinghe, P.E.; Nagesh, P.T.; Lew, J.K.S.; Jones, G.T.; Williams, M.; Davis, P.; Bunton, D.; Galvin, I.F.; Manning, P.; et al. Down-regulation of miR-15a/b accelerates fibrotic remodelling in the Type 2 diabetic human and mouse heart. Clin. Sci. 2017, 131, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Chang, H.; Cheng, T.; Lee, G.D.; Chen, X.; Qi, K. Micro-ribonucleic acid-23a-3p prevents the onset of type 2 diabetes mellitus by suppressing the activation of nucleotide-binding oligomerization-like receptor family pyrin domain containing 3 inflammatory bodies-caused pyroptosis through negatively regulating NIMA-related kinase 7. J. Diabetes Investig. 2021, 12, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, H.; Si, H.; Li, X.; Ding, X.; Sheng, Q.; Chen, P.; Zhang, H. Serum miR-23a, a potential biomarker for diagnosis of pre-diabetes and type 2 diabetes. Acta Diabetol. 2014, 51, 823–831. [Google Scholar] [CrossRef]
- Bagge, A.; Clausen, T.R.; Larsen, S.; Ladefoged, M.; Rosenstierne, M.W.; Larsen, L.; Vang, O.; Nielsen, J.H.; Dalgaard, L. MicroRNA-29a is up-regulated in beta-cells by glucose and decreases glucose-stimulated insulin secretion. Biochem. Biophys. Res. Commun. 2012, 426, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.-Z.; Dong, J.; Zhang, J.; Wang, S.; He, Y.; Yan, Y.-X. Identification of Neuroendocrine Stress Response-Related Circulating MicroRNAs as Biomarkers for Type 2 Diabetes Mellitus and Insulin Resistance. Front. Endocrinol. 2018, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, G.; Yang, C.; Zhou, K.; Shen, B.; Liang, H.; Jiang, X. The Role of Circulating MicroRNA-126 (miR-126): A Novel Biomarker for Screening Prediabetes and Newly Diagnosed Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2014, 15, 10567–10577. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Lv, C.; Li, L.; Chen, S.; Liu, S.; Wang, C.; Su, B. Plasma miR-126 Is a Potential Biomarker for Early Prediction of Type 2 Diabetes Mellitus in Susceptible Individuals. BioMed Res. Int. 2013, 2013, 761617. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Lucena, R.; Camargo, A.; Alcala-Diaz, J.F.; Romero-Baldonado, C.; Luque, R.M.; Van Ommen, B.; Delgado-Lista, J.; Ordovás, J.M.; Pérez-Martínez, P.; Rangel-Zuñiga, O.A.; et al. A plasma circulating miRNAs profile predicts type 2 diabetes mellitus and prediabetes: From the CORDIOPREV study. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ying, W.; Tseng, A.; Chang, R.C.-A.; Wang, H.; Lin, Y.-L.; Kanameni, S.; Brehm, T.; Morin, A.; Jones, B.; Splawn, T.; et al. miR-150 regulates obesity-associated insulin resistance by controlling B cell functions. Sci. Rep. 2016, 6, 20176. [Google Scholar] [CrossRef]
- Parrizas, M.; Brugnara, L.; Esteban, Y.; Gonzalez-Franquesa, A.; Canivell, S.; Murillo, S.; Gordillo-Bastidas, E.; Cussó, R.; Cadefau, J.A.; Garcia-Roves, P.M.; et al. Circulating miR-192 and miR-193b Are Markers of Prediabetes and Are Modulated by an Exercise Intervention. J. Clin. Endocrinol. Metab. 2015, 100, E407–E415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, Y.N.; Pittas, A.G.; Pratley, R.E.; Seyhan, A.A. Circulating levels of miR-7, miR-152 and miR-192 respond to vitamin D supplementation in adults with prediabetes and correlate with improvements in glycemic control. J. Nutr. Biochem. 2017, 49, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhang, Y.; Zeng, C.; Xu, F.; Yan, J.; Weng, J. miR-192 is upregulated in T1DM, regulates pancreatic β-cell development and inhibits insulin secretion through suppressing GLP-1 expression. Exp. Ther. Med. 2018, 16, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Yi, S.; Yong, D.; Shaozhuang, L.; Guangyong, Z.; Sanyuan, H. miR-320 mediates diabetes amelioration after duodenal-jejunal bypass via targeting adipoR1. Surg. Obes. Relat. Dis. 2018, 14, 960–971. [Google Scholar] [CrossRef]
- Gao, J.; Ailifeire, M.; Wang, C.; Luo, L.; Zhang, J.; Yuan, L.; Zhang, L.; Li, X.; Wang, M. miR-320/VEGFA axis affects high glucose-induced metabolic memory during human umbilical vein endothelial cell dysfunction in diabetes pathology. Microvasc. Res. 2019, 127, 103913. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhao, Y.; Yin, Z.; Wang, D.W.; Chen, C. The role of miR-320 in glucose and lipid metabolism disorder-associated diseases. Int. J. Biol. Sci. 2021, 17, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Poy, M.N.; Hausser, J.; Trajkovski, M.; Braun, M.; Collins, S.; Rorsman, P.; Zavolan, M.; Stoffel, M. miR-375 maintains normal pancreatic-and-cell mass. Proc. Natl. Acad. Sci. USA 2009, 106, 5813–5818. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, X.; Liang, Y.; Liu, S.; Xiao, H.; Li, F.; Cheng, H.; Fu, Z. miR-375 enhances palmitate-induced lipoapoptosis in insulin-secreting NIT-1 cells by repressing myotrophin (V1) protein expression. Int. J. Clin. Exp. Pathol. 2010, 3, 254–264. [Google Scholar]
- Al-Muhtaresh, H.A.; Al-Kafaji, G. Evaluation of Two-Diabetes Related microRNAs Suitability as Earlier Blood Biomarkers for Detecting Prediabetes and type 2 Diabetes Mellitus. J. Clin. Med. 2018, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Zhu, J.; Han, W.; Jiang, X.; Xu, M.; Zhao, Y.; Dong, Q.; Pang, Z.; Guan, Q.; Gao, L.; et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A clinical study. Acta Diabetol. 2010, 48, 61–69. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum MicroRNAs Are Promising Novel Biomarkers. PLoS ONE 2008, 3, e3148. [Google Scholar] [CrossRef] [Green Version]
- Bonora, E.; Tuomilehto, J. The Pros and Cons of Diagnosing Diabetes with A1C. Diabetes Care 2011, 34, S184–S190. [Google Scholar] [CrossRef] [Green Version]
- Radin, M.S. Pitfalls in Hemoglobin A1c Measurement: When Results may be Misleading. J. Gen. Intern. Med. 2013, 29, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Mamas, M.; Dunn, W.; Neyses, L.; Goodacre, R. The role of metabolites and metabolomics in clinically applicable biomarkers of disease. Arch. Toxicol. 2011, 85, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W. Circulating MicroRNA Biomarker Studies: Pitfalls and Potential Solutions. Clin. Chem. 2015, 61, 56–63. [Google Scholar] [CrossRef]
- Fatica, A.; Bozzoni, I. Long non-coding RNAs: New players in cell differentiation and development. Nat. Rev. Genet. 2014, 15, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Lederberg, J.; McCray, A. ’Ome Sweet’ Omics—A Genealogical Treasury of Words. Scientist 2001, 15, 8–10. [Google Scholar]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Krämer, M.; Gummesson, A.; Perkins, R.; Bergström, G.; Bäckhed, F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390.e3. [Google Scholar] [CrossRef] [PubMed]


| Biomarker | Description/Outcomes | Advantages/Disadvantages | References |
|---|---|---|---|
| 2-Hydroxybutyrate (2HB) | 2HB is a metabolite of alpha-ketobutyrate synthesis produced in the threonine and methionine catabolism and glutathione anabolism; it is a predictive marker of hyperglycemia and beta-cell dysfunction; Elevated levels of 2HB are associated with insulin resistance, oxidative stress, lipid oxidation, and diabetic state aggravation. Decreased levels of 2HB were observed 6 months after bariatric surgery as a representative improvement of the pathology. | 2HB has proven to be a biomarker independent of sex, age, BMI, and collection site; however, it is still in a premature investigation stage. | [39,40,41,42] |
| Aromatic Amino Acids (AAAs) | AAAs, tyrosine, and phenylalanine are amino acids with an integrated aromatic ring. Phenylalanine is a precursor of tyrosine, and tyrosine is a precursor of catecholamines. Both tyrosine and phenylalanine are glucogenic and ketogenic amino acids. Increased levels of tyrosine and phenylalanine were observed in obesity-related insulin resistance, and predicted the development of T2D. After diabetic treatment with glipizide and metformin, AAA levels changed in accordance with the patient’s insulin resistance status. | Different expression patterns of amino acids can be predictive of prediabetes in various cohorts. Additionally, significance can be altered after variable adjustment of body mass index (BMI), age, sex, race/ethnicity, and FPG levels. | [18,43,44] |
| Adiponectin | Adiponectin is a hormone secreted from the adipose tissue with insulin sensitivity, antidiabetic, anti-inflammatory, and anti-atherogenic properties. Adiponectin stimulates a broad spectrum of metabolic actions via ceramidase activation; it is directly correlated with insulin sensitivity, and inversely correlated with T2D development risk. Lower adiponectin levels were observed 10 years prior to T2D diagnosis. | A biomarker independent of ethnic differences, it can be affected by sex-specific mechanisms nevertheless. Certain studies do not corroborate the lower adiponectin levels in prediabetics compared with healthy individuals. | [45,46,47,48] |
| Acylcarnitine | Acylcarnitines result from the conjugations of acyl-coenzyme A with carnitine conjugation for the transport of fatty acids through the inner mitochondrial membrane for beta-oxidation. They are associated with the NF-κB pathway, and can promote insulin resistance and inflammation. Acylcarnitine has shown to be higher in prediabetes due to the dysregulation of mitochondrial fatty acid oxidation. A panel of acylcarnitines was observed to be associated with T2D development in a 6-year follow-up. | Some acylcarnitines did not show any association with body fat or waist–hip ratio, fat mass, or fat distribution. Overall, they are independent biomarkers of traditional risk factors. | [49,50,51,52] |
| Branched-Chain Amino Acids (BCAAs) | BCAAs such as leucine, isoleucine, and valine are the most abundant and essential amino acids present in a regular diet. Accumulation of BCAAs activates via mTOR and, consequently, S6 kinase, which leads to serine phosphorylation of the substrate-1 (IRS–1) insulin receptor, causing insulin resistance. High levels of BCAAs are associated with obesity, insulin resistance, impaired glucose tolerance, and T2D. BCAA levels normalize after bariatric surgery. | Phenotypic and genetic factors can influence BCAA levels, which can reveal associations with both sex and BMI. There is still some debate on whether BCAAs are the cause or the effect and, as such, whether they should be considered a biomarker. | [53,54,55] |
| C-Reactive Protein (CRP) | CRP is an inflammatory biomarker of hepatic origin associated with the acute phase response; it responds to transcription factors released by macrophages and adipocytes. Higher CRP levels were found in patients with prediabetes and insulin resistance, rendering it a sensitive biomarker for early T2D diagnosis. These results may be a consequence of the low state of chronic inflammation grade found before the onset of type 2 diabetes. | Association between CRP and prediabetes is independent of age, sex, ethnicity, alcohol consumption, smoking, hypertension, BMI, and total cholesterol. It is still in an early investigation stage for prediabetes signaling. | [56,57,58,59] |
| Ferritin | Ferritin is a protein (acute phase reactant) involved in iron storage, which is able to release iron in a controlled manner. Iron contributes to insulin resistance via many pathways, such as β-cell oxidative stress and β-cell apoptosis through ROS formation. Iron metabolism seems to be correlated with T2D status: uncontrolled T2D is associated with iron deficiency. High ferritin levels translate to an increased risk of developing T2D. Dietary restriction and chelation may prevent T2D progression. | The threshold level is still uncertain, and may vary according to age and sex. Ferritin levels are predictive of diabetes progression independently of a comprehensive range of risk factors, such as physical activity, smoking, and family history. | [60,61,62,63] |
| Glycated Albumin (GA) | Albumin is the most commonly studied soluble protein, and is highly susceptible to post-translational modifications (PTMs). One frequent modification is glycation, resulting in GA. GA plays a vital role in diabetic pathophysiology; it is inversely correlated with obesity and positively correlated with diabetes. The increase observed in diabetes is associated with secondary comorbidities. GA can act as an antigen, elicit the immune response, and form complexes that can accumulate in the arteries and kidneys, leading to nephropathy and atherosclerosis. | Accurate assessment for short-term glycemic control. The enzymatic method is sensitive, fast, and less susceptible to pre-analytical variables. Values of GA are not reliable in individuals with abnormal albumin metabolism. | [22,64,65,66,67] |
| Glycine | Glycine is a nonessential stable amino acid, able to be synthesized by the body from serine. Glycine is a precursor of protein metabolism, and can act as a neurotransmitter and as a co-ligand for N-methyl-D-aspartate glutamate receptors to control insulin secretion and liver glucose output, functioning on both the pancreas and the brain. Lower glycine levels are associated with an increased risk of prediabetes, type 2 diabetes, and obesity, and are also correlated with insulin resistance and glucose intolerance. | Glycine levels are not dependent exclusively on glycemic status, and may vary in individuals with abnormal amino acid metabolisms or metabolic syndrome. | [10,18,23,68] |
| Linoleoyl-glycerophosphocholine (LGPC) | Linoleoyl-glycerophosphocholine (LGPC) is a metabolite of the phospholipase A2 hepatic enzyme and lecithin-cholesterol acyltransferase. Known for its anti-inflammatory properties, it acts as a non-competitive enzyme inhibitor of phospholipase A2, usually increasing during the inflammatory state. This metabolite’s plasma concentration is associated with an increased risk of developing insulin resistance, impaired glucose tolerance, and diabetes. | Independent of age, sex, body mass index, familial diabetes, fasting glucose, waist circumference, blood pressure, glycosylated hemoglobin, triglycerides, and high-density lipoprotein cholesterol. | [21,69] |
| Triglycerides | Triglycerides are the most common lipids present in the body, and are composed of three fatty acids and a glycerol molecule. They are often an indication of conditions such as obesity and metabolic dysfunction. High levels of triglycerides are associated with diabetic progression, beta-cell dysfunction, and impaired insulin secretion. Studies have demonstrated that the product of triglycerides and glucose is able to discriminate prediabetes and diabetes, and triglyceride levels can be improved with physical activity and, therefore, improve glycemic status. | Triglycerides have already been implemented in clinical practice. In prediabetic individuals, high levels of triglycerides are a predictive factor for T2D progression. Studies found variations between different ethnicities. | [70,71,72] |
| miRNAs | Description/Outcomes | References |
|---|---|---|
| miRNA-15a | miRNA-15a is associated with several biological processes, such as angiogenesis and insulin production; it is also involved in the activation of TGFβR1, CTGF, and p53 proteins. Lower miRNA-15a levels were found in individuals who developed T2D in a 10-year follow-up. The association between miRNA-15a and diabetic progression was still significant after variable adjustment for age, sex, BMI, and hypertension status. | [73,74] |
| miRNA-23a | miRNA-23a indirectly targets SMAD4—a critical pathway in the regulation of insulin-dependent glucose transport activity. NEK7 is also a target of miRNA-23a and, in animal models, a low level of NLRP3 induced pyroptosis, mitigating the hepatic and renal complications of T2D. The levels of miRNA-23a are lower in prediabetic and T2D patients compared with healthy individuals. Levels of miRNA-23a can also distinguish prediabetic and T2D patients. | [75,76] |
| miRNA-29a | miRNA-29a was observed to improve pancreatic beta-cell function in in vitro studies. Likewise, upregulation of miRNA-29a is implicated in diabetic progression by IGT and decreased insulin secretion. Higher expression of miRNA-29a is an independent predictor of T2D, IFG, and IR. Additionally, it is significantly correlated with stress hormone levels. | [77,78] |
| miRNA-126 | One of the most studied miRNAs in prediabetic conditions, it is highly correlated with VEGF, and with the promotion of angiogenesis. Anti-miRNA-126 targets SPRED-1 via Ras/ERK/VEGF and PI3K/Akt/eNOS, inhibiting the proliferation and migration of endothelial progenitor cells and promoting apoptosis. Low levels of miRNA-126 are strongly correlated with the progression of the disease. | [79,80] |
| miRNA-150 | Previous miRNA-150 studies demonstrated its regulatory function in beta-cell formation, hematopoietic stem cell differentiation, and obesity-induced inflammation and insulin resistance by controlling adipose tissue and beta-cell function. In the CORDIOPREV study, prediabetic progressors were evaluated in a 5-year follow-up; miRNA-150 levels were higher in plasma several years before the diagnosis of T2D. | [81,82] |
| miRNA-192 | miRNA-192 is involved in IFG and IGT, triglyceride levels, and the fatty liver index. Moreover, miRNA-192 inhibited the proliferation of pancreatic beta-cell lines and insulin secretion. Levels of miRNA-192 are found to be higher in diabetic subjects. Interestingly, vitamin D supplementation modulates miRNA-192 levels, improving the hyperglycemic status in prediabetic patients. | [83,84,85] |
| miRNA-320 | Expression of miRNA-320 is associated with VEGF, IGF1, and FGF. The VEGFa/miRNA-320 axis modulates proliferation, apoptosis, and angiogenesis of endothelial cells, and has been reported to be an active player in diabetic progression. miRNA-320 is positively correlated with prediabetic incidence, and improves diabetic progression via adipoR1 after duodenal–jejunal bypass. | [86,87,88] |
| miRNA-375 | miRNA-375 is a pancreatic-islet-specific miRNA involved in regulating insulin secretion and maintaining average pancreatic alpha and beta-cell mass. miRNA-375 levels are higher and independently associated in prediabetic and diabetic individuals. Deregulation of miRNA-375 was observed years before the onset of T2D in the CORDIOPREV trial. | [89,90,91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luís, C.; Baylina, P.; Soares, R.; Fernandes, R. Metabolic Dysfunction Biomarkers as Predictors of Early Diabetes. Biomolecules 2021, 11, 1589. https://doi.org/10.3390/biom11111589
Luís C, Baylina P, Soares R, Fernandes R. Metabolic Dysfunction Biomarkers as Predictors of Early Diabetes. Biomolecules. 2021; 11(11):1589. https://doi.org/10.3390/biom11111589
Chicago/Turabian StyleLuís, Carla, Pilar Baylina, Raquel Soares, and Rúben Fernandes. 2021. "Metabolic Dysfunction Biomarkers as Predictors of Early Diabetes" Biomolecules 11, no. 11: 1589. https://doi.org/10.3390/biom11111589
APA StyleLuís, C., Baylina, P., Soares, R., & Fernandes, R. (2021). Metabolic Dysfunction Biomarkers as Predictors of Early Diabetes. Biomolecules, 11(11), 1589. https://doi.org/10.3390/biom11111589