The Essential Role of Stathmin in Myoblast C2C12 for Vertical Vibration-Induced Myotube Formation
Abstract
:1. Introduction
2. Results
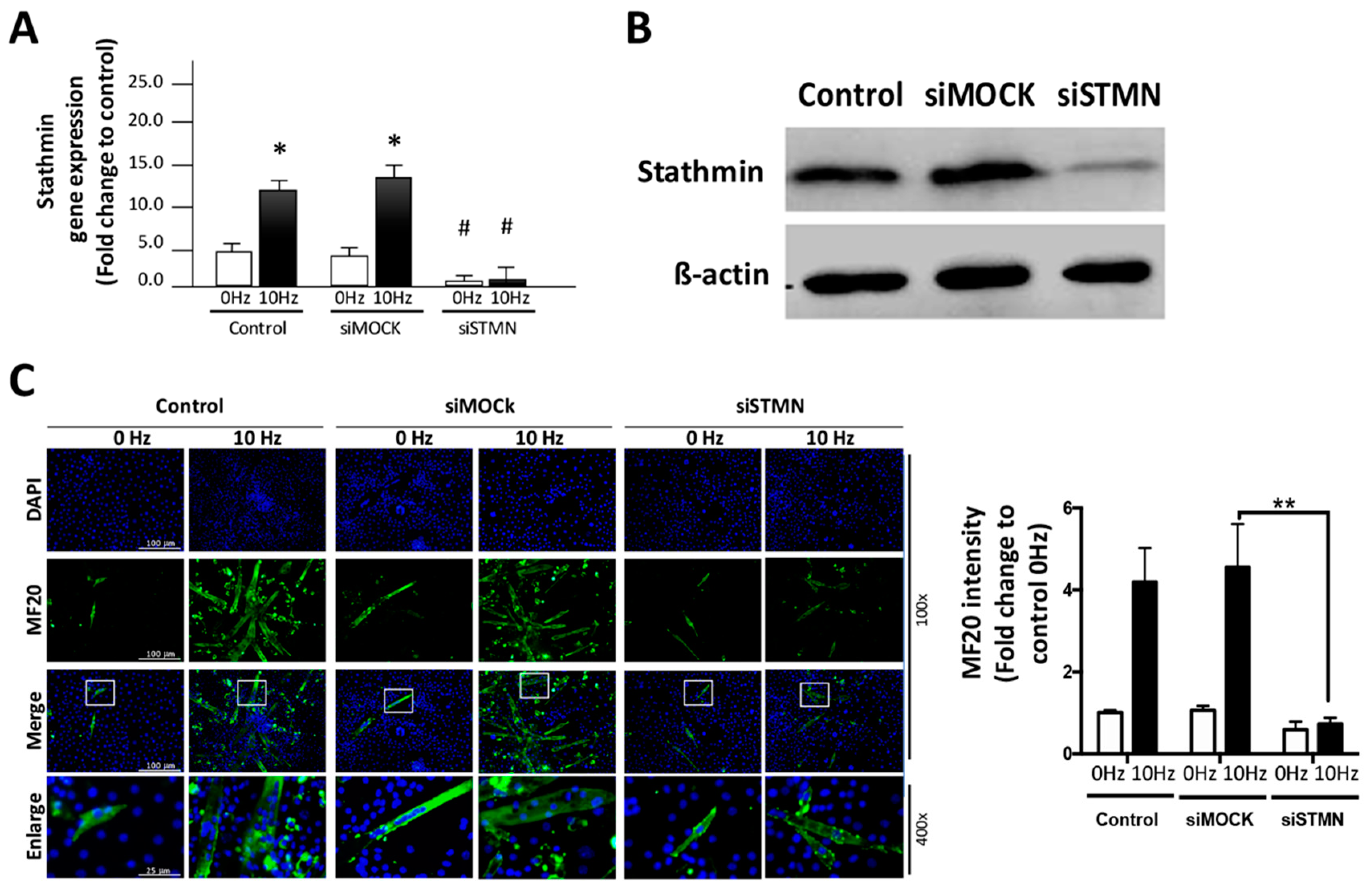
2.1. Stathmin Expression Increases in VV-Stimulated C2C12 Cells
2.2. Stathmin Regulates VV-Stimulated Myotube Formation of C2C12 Cells
2.3. Stathmin siRNA Significantly Reduces VV-Stimulated MRF Expression
2.4. PI3K/Akt Pathway Are Correlated Regulators of Stathmin in VV-Stimulated C2C12 cElls
2.5. TGFBr1, FGF7 and PAK3 Are Involved in VV-Induced Myotube Formation and Are Affected by Stathmin Absence
3. Discussion
4. Materials and Methods
4.1. Culture
4.2. Vertical Vibration (VV) and Inhibitor Treatment
4.3. Two-Dimensional Gel Electrophoresis (2-DE)
4.4. Myotube Formation Immunofluorescence
4.5. Stathmin Knockdown Using Specific Stathmin siRNA in C2C12 Myoblasts
4.6. Immunofluorescence
4.7. Western Blot Analysis
4.8. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cardinale, M.; Wakeling, J. Whole body vibration exercise: Are vibrations good for you? Br. J. Sports Med. 2005, 39, 585–589, discussion 589. [Google Scholar] [CrossRef]
- Goudarzian, M.; Ghavi, S.; Shariat, A.; Shirvani, H.; Rahimi, M. Effects of whole body vibration training and mental training on mobility, neuromuscular performance, and muscle strength in older men. J. Exerc. Rehabil. 2017, 13, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawanabe, K.; Kawashima, A.; Sashimoto, I.; Takeda, T.; Sato, Y.; Iwamoto, J. Effect of whole-body vibration exercise and muscle strengthening, balance, and walking exercises on walking ability in the elderly. Keio J. Med. 2007, 56, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollersheim, T.; Haas, K.; Wolf, S.; Mai, K.; Spies, C.; Steinhagen-Thiessen, E.; Wernecke, K.D.; Spranger, J.; Weber-Carstens, S. Whole-body vibration to prevent intensive care unit-acquired weakness: Safety, feasibility, and metabolic response. Crit. Care 2017, 21, 9. [Google Scholar] [CrossRef] [Green Version]
- Roelants, M.; Delecluse, C.; Goris, M.; Verschueren, S. Effects of 24 weeks of whole body vibration training on body composition and muscle strength in untrained females. Int. J. Sports Med. 2004, 25, 1–5. [Google Scholar] [PubMed] [Green Version]
- Fagnani, F.; Giombini, A.; Di Cesare, A.; Pigozzi, F.; Di Salvo, V. The effects of a whole-body vibration program on muscle performance and flexibility in female athletes. Am. J. Phys. Med. Rehabil. 2006, 85, 956–962. [Google Scholar] [CrossRef] [Green Version]
- Pel, J.J.; Bagheri, J.; van Dam, L.M.; van den Berg-Emons, H.J.; Horemans, H.L.; Stam, H.J.; van der Steen, J. Platform accelerations of three different whole-body vibration devices and the transmission of vertical vibrations to the lower limbs. Med. Eng. Phys. 2009, 31, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Hernandez, J.M.; Garcia-Gonzalez, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Choi, J.; Costa, M.L.; Mermelstein, C.S.; Chagas, C.; Holtzer, S.; Holtzer, H. Myod converts primary dermal fibroblasts, chondroblasts, smooth-muscle, and retinal pigmented epithelial-cells into striated mononucleated myoblasts and multinucleated myotubes. Proc. Natl. Acad. Sci. USA 1990, 87, 7988–7992. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Z.; Wang, G.J.; Ho, M.L.; Wang, Y.H.; Yeh, M.L.; Chen, C.H. Low-magnitude vertical vibration enhances myotube formation in c2c12 myoblasts. J. Appl. Physiol. 2010, 109, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Biaoxue, R.; Xiguang, C.; Hua, L.; Shuanying, Y. Stathmin-dependent molecular targeting therapy for malignant tumor: The latest 5 years’ discoveries and developments. J. Transl. Med. 2016, 14, 279. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.C.; Cassimeris, L. Stathmin and microtubules regulate mitotic entry in hela cells by controlling activation of both aurora kinase a and plk1. Mol. Biol. Cell 2013, 24, 3819–3831. [Google Scholar] [CrossRef] [Green Version]
- Baldassarre, G.; Belletti, B.; Nicoloso, M.S.; Schiappacassi, M.; Vecchione, A.; Spessotto, P.; Morrione, A.; Canzonieri, V.; Colombatti, A. P27(kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell 2005, 7, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ren, J.H.; Lin, F.; Wei, J.X.; Long, M.; Yan, L.; Zhang, H.Z. Stathmin is involved in arsenic trioxide-induced apoptosis in human cervical cancer cell lines via pi3k linked signal pathway. Cancer Biol. Ther. 2010, 10, 632–643. [Google Scholar] [CrossRef]
- Lu, N.T.; Liu, N.M.; Patel, D.; Vu, J.Q.; Liu, L.; Kim, C.Y.; Cho, P.; Khachatoorian, R.; Patel, N.; Magyar, C.E. Oncoprotein stathmin modulates sensitivity to apoptosis in hepatocellular carcinoma cells during hepatitis c viral replication. J. Cell Death 2018, 11, 1179066018785141. [Google Scholar] [CrossRef] [Green Version]
- Machado-Neto, J.A.; Alves, A.P.N.R.; Fernandes, J.C.; Coelho-Silva, J.L.; Scopim-Ribeiro, R.; Fenerich, B.A.; da Silva, F.B.; Scheucher, P.S.; Simões, B.P.; Rego, E.M. Paclitaxel induces stathmin 1 phosphorylation, microtubule stability and apoptosis in acute lymphoblastic leukemia cells. Heliyon 2017, 3, e00405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, C.I.; Atweh, G.F. The role of stathmin in the regulation of the cell cycle. J. Cell Biochem. 2004, 93, 242–250. [Google Scholar] [CrossRef]
- Casadei, L.; Vallorani, L.; Gioacchini, A.M.; Guescini, M.; Burattini, S.; D’Emilio, A.; Biagiotti, L.; Falcieri, E.; Stocchi, V. Proteomics-based investigation in c2c12 myoblast differentiation. Eur. J. Histochem. 2009, 53, e31. [Google Scholar] [CrossRef] [Green Version]
- Balogh, A.; Mege, R.M.; Sobel, A. Growth and cell density-dependent expression of stathmin in c2 myoblasts in culture. Exp. Cell Res. 1996, 224, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mtor pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Salvesen, H.B.; Carter, S.L.; Mannelqvist, M.; Dutt, A.; Getz, G.; Stefansson, I.M.; Raeder, M.B.; Sos, M.L.; Engelsen, I.B.; Trovik, J.; et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of pi3 kinase activation. Proc. Natl. Acad. Sci. USA 2009, 106, 4834–4839. [Google Scholar] [CrossRef] [Green Version]
- Trovik, J.; Wik, E.; Stefansson, I.; Carter, S.L.; Beroukhim, R.; Oyan, A.M.; Kalland, K.H.; Akslen, L.A.; Salvesen, H.B. Stathmin is superior to akt and phospho-akt staining for the detection of phosphoinositide 3-kinase activation and aggressive endometrial cancer. Histopathology 2010, 57, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, B.; Zheng, W.; Kang, M.; Chen, Q.; Qin, W.; Li, C.; Zhang, Y.; Shao, Y.; Wu, Y. Exosomes derived from pancreatic cancer cells induce insulin resistance in c2c12 myotube cells through the pi3k/akt/foxo1 pathway. Sci. Rep. 2017, 7, 5384. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Lin, Y.H.; Chen, C.H.; Wang, Y.H.; Yeh, M.L.; Cheng, T.L.; Wang, C.Z. Transforming growth factor beta 1 mediates the low-frequency vertical vibration enhanced production of tenomodulin and type i collagen in rat achilles tendon. PLoS ONE 2018, 13, e0205258. [Google Scholar]
- Kiiski, J.; Heinonen, A.; Jarvinen, T.L.; Kannus, P.; Sievanen, H. Transmission of vertical whole body vibration to the human body. J. Bone Min. Res. 2008, 23, 1318–1325. [Google Scholar] [CrossRef] [Green Version]
- Leger, B.; Cartoni, R.; Praz, M.; Lamon, S.; Deriaz, O.; Crettenand, A.; Gobelet, C.; Rohmer, P.; Konzelmann, M.; Luthi, F.; et al. Akt signalling through gsk-3beta, mtor and foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J. Physiol. 2006, 576, 923–933. [Google Scholar] [CrossRef]
- Luo, J.; McMullen, J.R.; Sobkiw, C.L.; Zhang, L.; Dorfman, A.L.; Sherwood, M.C.; Logsdon, M.N.; Horner, J.W.; DePinho, R.A.; Izumo, S.; et al. Class ia phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol. Cell Biol. 2005, 25, 9491–9502. [Google Scholar] [CrossRef] [Green Version]
- Cefalu, S.; Lena, A.M.; Vojtesek, B.; Musaro, A.; Rossi, A.; Melino, G.; Candi, E. Tap63gamma is required for the late stages of myogenesis. Cell Cycle 2015, 14, 894–901. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, Q.; Wang, B.; Wu, W.; Wei, J.; Li, P.; Huang, R. Mir-22 regulates c2c12 myoblast proliferation and differentiation by targeting tgfbr1. Eur. J. Cell Biol. 2018, 97, 257–268. [Google Scholar] [CrossRef]
- Hillege, M.M.G.; Galli Caro, R.A.; Offringa, C.; de Wit, G.M.J.; Jaspers, R.T.; Hoogaars, W.M.H. Tgf-beta regulates collagen type i expression in myoblasts and myotubes via transient ctgf and fgf-2 expression. Cells 2020, 9, 375. [Google Scholar] [CrossRef] [Green Version]
- Sieiro, D.; Melendez, J.; Morin, V.; Salgado, D.; Marcelle, C. Auto-inhibition of myoblast fusion by cyclic receptor signalling. bioRxiv 2019, 553420. [Google Scholar]
- Hsu, R.Y.; Lin, Y.-C.; Redon, C.; Sun, Q.; Singh, D.K.; Wang, Y.; Aggarwal, V.; Mitra, J.; Matur, A.; Moriarity, B. Orca/lrwd1 regulates homologous recombination at alt-telomeres by modulating heterochromatin organization. iScience 2020, 23, 101038. [Google Scholar] [CrossRef]
- Yan, X.-M.; Zhang, Z.; Liu, J.-B.; Li, N.; Yang, G.-W.; Luo, D.; Zhang, Y.; Yuan, B.; Jiang, H.; Zhang, J.-B. Genome-wide identification and analysis of long noncoding rnas in longissimus muscle tissue from kazakh cattle and xinjiang brown cattle. Asian-Australas. J. Anim. Sci. 2020. [Google Scholar] [CrossRef]
- Hannon, K.; Kudla, A.J.; McAvoy, M.J.; Clase, K.L.; Olwin, B.B. Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J. Cell Biol. 1996, 132, 1151–1159. [Google Scholar] [CrossRef] [Green Version]
- Neuhaus, P.; Oustanina, S.; Loch, T.; Kruger, M.; Bober, E.; Dono, R.; Zeller, R.; Braun, T. Reduced mobility of fibroblast growth factor (fgf)-deficient myoblasts might contribute to dystrophic changes in the musculature of fgf2/fgf6/mdx triple-mutant mice. Mol. Cell Biol. 2003, 23, 6037–6048. [Google Scholar] [CrossRef] [Green Version]
- Piccand, J.; Meunier, A.; Merle, C.; Jia, Z.; Barnier, J.V.; Gradwohl, G. Pak3 promotes cell cycle exit and differentiation of beta-cells in the embryonic pancreas and is necessary to maintain glucose homeostasis in adult mice. Diabetes 2014, 63, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Maglorius Renkilaraj, M.R.L.; Baudouin, L.; Wells, C.M.; Doulazmi, M.; Wehrle, R.; Cannaya, V.; Bachelin, C.; Barnier, J.V.; Jia, Z.; Nait Oumesmar, B.; et al. The intellectual disability protein pak3 regulates oligodendrocyte precursor cell differentiation. Neurobiol. Dis. 2017, 98, 137–148. [Google Scholar] [CrossRef]
- Joseph, G.A.; Lu, M.; Radu, M.; Lee, J.K.; Burden, S.J.; Chernoff, J.; Krauss, R.S. Group i paks promote skeletal myoblast differentiation in vivo and in vitro. Mol. Cell Biol. 2017, 37, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Iness, A.; Yoon, J.; Grider, J.R.; Murthy, K.S.; Kellum, J.M.; Kuemmerle, J.F. Noncanonical stat3 activation regulates excess tgf-beta1 and collagen i expression in muscle of stricturing crohn’s disease. J. Immunol. 2015, 194, 3422–3431. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liu, T.; Wu, J.C.; Luo, S.Z.; Chen, R.; Lu, L.G.; Xu, M.Y. Stat3 aggravates tgf-beta1-induced hepatic epithelial-to-mesenchymal transition and migration. Biomed. Pharm. 2018, 98, 214–221. [Google Scholar] [CrossRef]
- Elfering, A.; Thomann, J.; Schade, V.; Radlinger, L. Stochastic resonance whole body vibration reduces musculoskeletal pain: A randomized controlled trial. World J. Orthop. 2011, 2, 116. [Google Scholar] [CrossRef] [PubMed]
- van Nes, I.J.; Geurts, A.C.; Hendricks, H.T.; Duysens, J. Short-term effects of whole-body vibration on postural control in unilateral chronic stroke patients: Preliminary evidence. Am. J. Phys. Med. Rehabil. 2004, 83, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Bosco, C.; Colli, R.; Introini, E.; Cardinale, M.; Tsarpela, O.; Madella, A.; Tihanyi, J.; Viru, A. Adaptive respsonses of human skeletal muscle to vibration exposure. Clin. Physiol. 1999, 19, 183. [Google Scholar] [CrossRef] [PubMed]
- Runge, M.; Rehfeld, G.; Resnicek, E. Balance training and exercise in geriatric patients. J. Musculoskelet Neuronal Interact. 2000, 1, 61–65. [Google Scholar] [PubMed]





| Gene Symbol | Forward | Reverse |
|---|---|---|
| Stathinin | 5′-CCAGGCTTTTGAGCTGATTC-3′ | 5′-GCGTCTTTCTTCTGCAGCTT-3′ |
| MyoD | 5′-GCTTCTATCGCCGCCACTCC-3′ | 5′-CGCACATGCTCATCCTCACG-3′ |
| Decorin | 5′-ACAGCATCACCGTTATGGAGAATG-3′ | 5′-TCACAGCCGAGTAGGAAGCC-3′ |
| Collagen type I | 3′-TCAGAGGCGAAGGCAACAGTC-3′ | 3′-GCAGGCGGGAGGTCTTGG-3′ |
| Myogenin | 5′-GCATGCAAGGTGTGTAAGAG-3′ | 5′-GCGCAGGATCTCCACTTTAG-3′ |
| p53 | 5′- GATGACTGCCATGGAGGAGT -3′ | 5′- CTCGGGTGGCTCATAAGGTA -3′ |
| GAPDH | 5′-ATTGTGGAAGGGCTCATGACC-3′ | 5′-ATGCAGGGATGATGTTCTGGG-3′ |
| FGF7 | 5′-GACAAACGAGGCAAAGTGAAAGG-3′ | 5′-TGCCACAGTCCTGATTTCCA-3′ |
| TGFBrl | 5′-CCGCAACAACGCCATCTATG-3′ | 5′-CCCGAATGTCTGACGTATTGAAG-3′ |
| PAK3 | 5′-AAATTGGTCAAGGGGCATCAG-3′ | 5′-ACCCATAGTTCATCACCCACC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Chou, L.-Y.; Chou, H.-C.; Chen, C.-H.; Kang, L.; Cheng, T.-L.; Wang, C.-Z. The Essential Role of Stathmin in Myoblast C2C12 for Vertical Vibration-Induced Myotube Formation. Biomolecules 2021, 11, 1583. https://doi.org/10.3390/biom11111583
Lin Y-H, Chou L-Y, Chou H-C, Chen C-H, Kang L, Cheng T-L, Wang C-Z. The Essential Role of Stathmin in Myoblast C2C12 for Vertical Vibration-Induced Myotube Formation. Biomolecules. 2021; 11(11):1583. https://doi.org/10.3390/biom11111583
Chicago/Turabian StyleLin, Yi-Hsiung, Liang-Yin Chou, Hsin-Chiao Chou, Chung-Hwan Chen, Lin Kang, Tsung-Lin Cheng, and Chau-Zen Wang. 2021. "The Essential Role of Stathmin in Myoblast C2C12 for Vertical Vibration-Induced Myotube Formation" Biomolecules 11, no. 11: 1583. https://doi.org/10.3390/biom11111583
APA StyleLin, Y.-H., Chou, L.-Y., Chou, H.-C., Chen, C.-H., Kang, L., Cheng, T.-L., & Wang, C.-Z. (2021). The Essential Role of Stathmin in Myoblast C2C12 for Vertical Vibration-Induced Myotube Formation. Biomolecules, 11(11), 1583. https://doi.org/10.3390/biom11111583











