Cardiorenal Syndrome: New Pathways and Novel Biomarkers
Abstract
1. Introduction
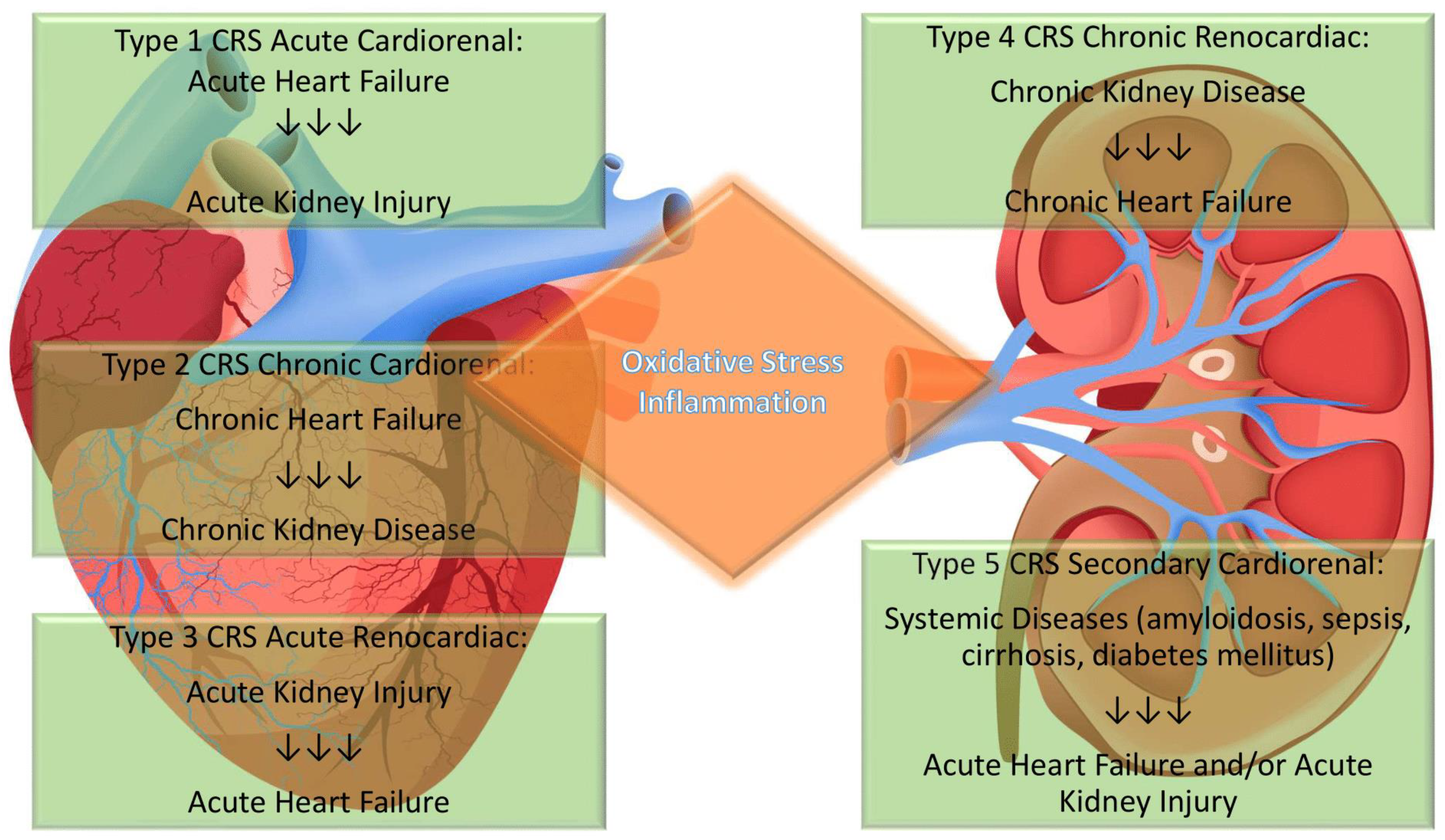
Definition of Cardiorenal Syndrome
2. Pathophysiology of Cardiorenal Syndrome
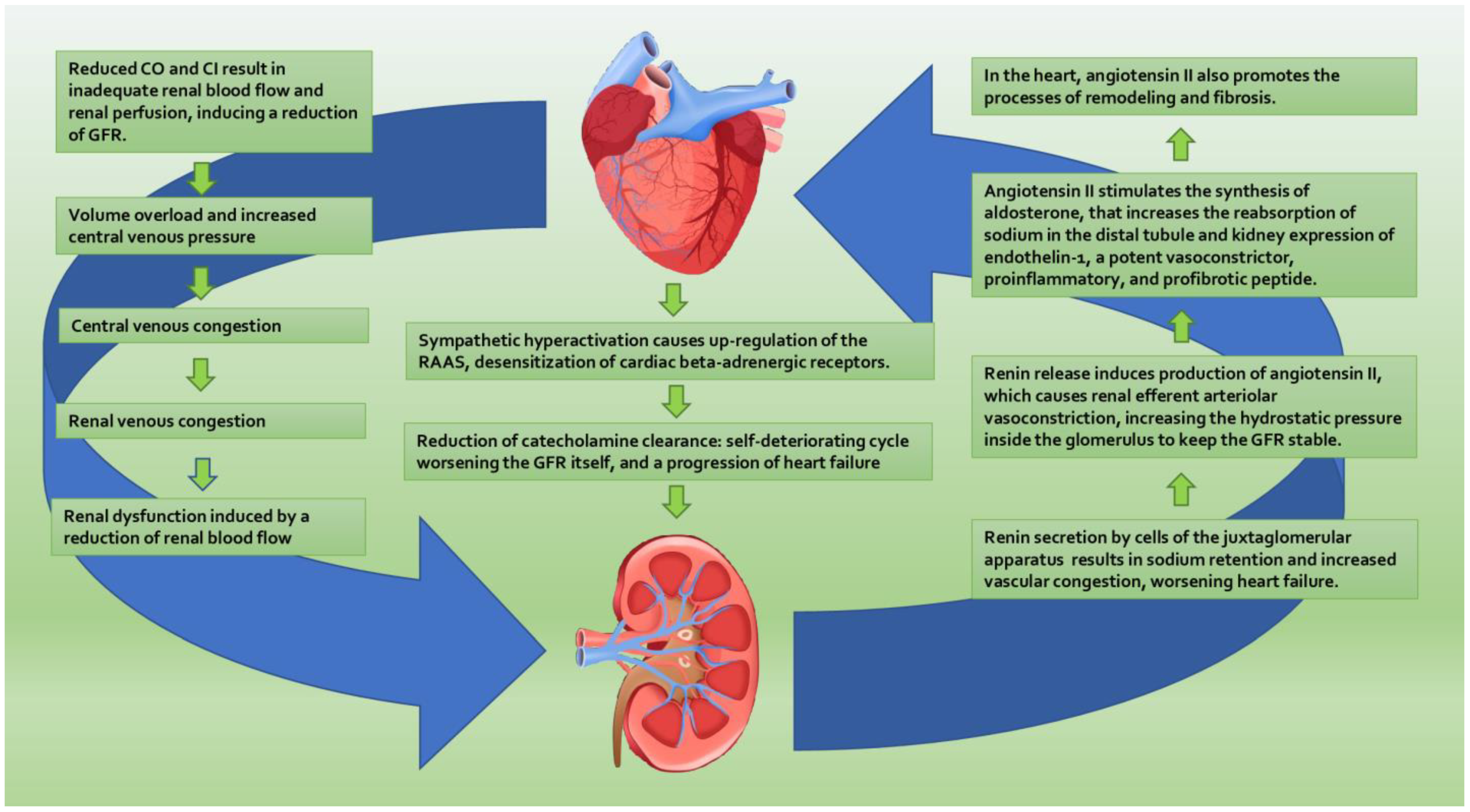
2.1. Hemodynamic Mechanism
2.2. Current AKI Biomarkers and New Standpoints
2.3. Neurohormonal Dysregulation
2.4. Endothelial Dysfunction and Atherosclerosis
2.5. Oxidative Stress and Inflammation
3. Biomarkers Connecting the Heart and Kidney
3.1. A New Look at an “Old” Biomarker: NT-proBNP
3.2. Biomarkers of CKD-Related Bone and Mineral Metabolism and CRS
3.3. Promising Biomarkers for Monitoring and Identifying Cardiorenal Syndrome
3.4. The Future Is Now: The Use of microRNAs for Cardiorenal Syndrome
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santoro, D.; Gembillo, G.; Andò, G. Glomerular Filtration Rate as a Predictor of Outcome in Acute Coronary Syndrome Complicated by Atrial Fibrillation. J. Clin. Med. 2020, 9, 1466. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef]
- Lorin, J.; Guilland, J.-C.; Stamboul, K.; Guenancia, C.; Cottin, Y.; Rochette, L.; Vergely, C.; Zeller, M. Increased Symmetric Dimethylarginine Level Is Associated with Worse Hospital Outcomes through Altered Left Ventricular Ejection Fraction in Patients with Acute Myocardial Infarction. PLoS ONE 2017, 12, e0169979. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.E.; Butler, J.; Wang, Y.; Abraham, W.T.; O’Connor, C.M.; Gottlieb, S.S.; Loh, E.; Massie, B.M.; Rich, M.W.; Stevenson, L.W.; et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J. Am. Coll. Cardiol. 2004, 43, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Bright, R. Cases and observations illustrative of renal disease accompanied by the secretion of albuminous urine. Guys Hosp. Rep. 1836, 10, 338–400. [Google Scholar]
- National Heart, Lung, and Blood Institute. NHLBI Working Group: Cardiorenal Connections in Heart Failure and Cardiovascular Disease. 2004. Available online: https://www.nhlbi.nih.gov/events/2004/cardio-renal-connections-heartfailure-and-cardiovascular-disease (accessed on 15 May 2021).
- Ronco, C.; Haapio, M.; House, A.A.; Anavekar, N.; Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 2008, 52, 1527–1539. [Google Scholar] [CrossRef]
- Damman, K.; Van Deursen, V.M.; Navis, G.; Voors, A.A.; Van Veldhuisen, D.J.; Hillege, H.L. Increased Central Venous Pressure Is Associated with Impaired Renal Function and Mortality in a Broad Spectrum of Patients with Cardiovascular Disease. J. Am. Coll. Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Zhou, Y.; Wang, P.; Qi, E.-Y.; Gu, W.-J. Elevated central venous pressure is associated with increased mortality and acute kidney injury in critically ill patients: A meta-analysis. Crit. Care 2020, 24, 1–8. [Google Scholar] [CrossRef]
- Binanay, C.; Califf, R.M.; Hasselblad, V.; O’Connor, C.M.; Shah, M.R.; Sopko, G.; Stevenson, L.W.; Francis, G.S.; Leier, C.V.; Miller, L.W. ESCAPE Investigators and ESCAPE Study Coordinators. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA 2005, 294, 1625–1633. [Google Scholar]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.H.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef]
- Tarvasmäki, T.; Haapio, M.; Mebazaa, A.; Sionis, A.; Silva-Cardoso, J.; Tolppanen, H.; Lindholm, M.G.; Pulkki, K.; Parissis, J.; Harjola, V.-P.; et al. Acute kidney injury in cardiogenic shock: Definitions, incidence, haemodynamic alterations, and mortality. Eur. J. Hear. Fail. 2018, 20, 572–581. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Lei, Y.; Zha, Y.; Liu, H.; Ma, C.; Tian, J.; Chen, P.; Yang, T.; Hou, F.F. Urinary Biomarkers at the Time of AKI Diagnosis as Predictors of Progression of AKI among Patients with Acute Cardiorenal Syndrome. Clin. J. Am. Soc. Nephrol. 2016, 11, 1536–1544. [Google Scholar] [CrossRef]
- Wagener, G.; Minhaz, M.; Mattis, F.A.; Kim, M.; Emond, J.C.; Lee, H.T. Urinary neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury after orthotopic liver transplantation. Nephrol. Dial. Transplant. 2011, 26, 1717–1723. [Google Scholar] [CrossRef]
- Cernaro, V.; Bolignano, D.; Donato, V.; Lacquaniti, A.; Buemi, A.; Crascì, E.; Lucisano, S.; Buemi, M. NGAL is a Precocious Marker of Therapeutic Response. Curr. Pharm. Des. 2011, 17, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Gala-Błądzińska, A.; Romanek, J.; Mazur, D.; Stepek, T.; Braun, M.; Szafarz, P.; Chlebuś, M.; Przybylski, A. Reduced Albuminuria and Potassemia Indicate Early Renal Repair Processes after Resynchronization Therapy in Cardiorenal Syndrome Type 2. Cardiol. Res. Pract. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tasić, D.; Radenkovic, S.; Stojanovic, D.; Milojkovic, M.; Stojanovic, M.; Ilic, M.D.; Kocic, G. Crosstalk of Various Biomarkers That Might Provide Prompt Identification of Acute or Chronic Cardiorenal Syndromes. Cardiorenal Med. 2015, 6, 99–107. [Google Scholar] [CrossRef]
- Conti, G.; Caccamo, D.; Siligato, R.; Gembillo, G.; Satta, E.; Pazzano, D.; Carucci, N.; Carella, A.; Del Campo, G.; Salvo, A.; et al. Association of Higher Advanced Oxidation Protein Products (AOPPs) Levels in Patients with Diabetic and Hypertensive Nephropathy. Medicina 2019, 55, 675. [Google Scholar] [CrossRef] [PubMed]
- Kohler, H.P.; Grant, P.J. Plasminogen-Activator Inhibitor Type 1 and Coronary Artery Disease. N. Engl. J. Med. 2000, 342, 1792–1801. [Google Scholar] [CrossRef]
- Atici, A.; Emet, S.; Cakmak, R.; Yuruyen, G.; Alibeyoglu, A.; Akarsu, M.; Arman, Y.; Kose, M.; Ozgun, E.; Ozcan, M.; et al. Type I cardiorenal syndrome in patients with acutely decompensated heart failure: The importance of new renal biomarkers. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3534–3543. [Google Scholar]
- Vianello, A.; Caponi, L.; Galetta, F.; Franzoni, F.; Taddei, M.; Rossi, M.; Pietrini, P.; Santoro, G. β2-Microglobulin and TIMP1 Are Linked Together in Cardiorenal Remodeling and Failure. Cardiorenal Med. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Gunnerson, K.J.; Shaw, A.D.; Chawla, L.S.; Bihorac, A.; Al-Khafaji, A.; Kashani, K.; Lissauer, M.; Shi, J.; Walker, M.G.; Kellum, J.A. Sapphire Topaz investigators. TIMP2•IGFBP7 biomarker panel accurately predicts acute kidney injury in high-risk surgical patients. J. Trauma Acute Care Surg. 2016, 80, 243–249. [Google Scholar] [CrossRef]
- Hishikari, K.; Hikita, H.; Nakamura, S.; Nakagama, S.; Mizusawa, M.; Yamamoto, T. Urinary Liver-Type Fatty Acid-Binding Protein Level as a Predictive Biomarker of Acute Kidney Injury in Patients with Acute Decompensated Heart Failure. CardioRenal. Med. 2017, 7, 267–275. [Google Scholar] [CrossRef]
- Li, J.; Sheng, X.; Cheng, D.; Wang, F.; Jian, G.; Li, Y.; Xu, T.; Wang, X.; Fan, Y.; Wang, N. Is the mean platelet volume a predictive marker of a high in-hospital mortality of acute cardiorenal syndrome patients receiving continuous renal replacement therapy? Medicina 2018, 97, e11180. [Google Scholar] [CrossRef]
- Slavka, G.; Perkmann, T.; Haslacher, H.; Greisenegger, S.; Marsik, C.; Wagner, O.F.; Endler, G. Mean Platelet Volume May Represent a Predictive Parameter for Overall Vascular Mortality and Ischemic Heart Disease. Arter. Thromb. Vasc. Biol. 2011, 31, 1215–1218. [Google Scholar] [CrossRef]
- Di Lullo, L.; Reeves, P.B.; Bellasi, A.; Ronco, C. Cardiorenal Syndrome in Acute Kidney Injury. Semin. Nephrol. 2019, 39, 31–40. [Google Scholar] [CrossRef]
- Hatamizadeh, P.; Fonarow, G.C.; Budoff, M.J.; Darabian, S.; Kovesdy, C.P.; Kalantar-Zadeh, K. Cardiorenal syndrome: Pathophysiology and potential targets for clinical management. Nat. Rev. Nephrol. 2013, 9, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 42, 1050–1065. [Google Scholar] [CrossRef]
- Yogasundaram, H.; Chappell, M.C.; Braam, B.; Oudit, G.Y. Cardiorenal Syndrome and Heart Failure—Challenges and Opportunities. Can. J. Cardiol. 2019, 35, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Bongartz, L.G.; Cramer, M.J.; Doevendans, P.A.; Joles, J.A.; Braam, B. The severe cardiorenal syndrome: “Guyton revisited”. Eur. Heart J. 2005, 26, 11–17. [Google Scholar] [CrossRef]
- Voors, A.A.; Dittrich, H.C.; Massie, B.M.; DeLucca, P.; Mansoor, G.A.; Metra, M.; Cotter, G.; Weatherley, B.D.; Ponikowski, P.; Teerlink, J.R.; et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: Results from PROTECT (Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function). J. Am. Coll. Cardiol. 2011, 57, 1899–1907. [Google Scholar]
- Konstam, M.A.; Gheorghiade, M.; Burnett JCJr Grinfeld, L.; Maggioni, A.P.; Swedberg, K.; Udelson, J.E.; Zannad, F.; Cook, T.; Ouyang, J.; Zimmer, C.; et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA 2007, 297, 1319–1331. [Google Scholar] [CrossRef]
- Yalta, K.; Yalta, T.; Sivri, N.; Yetkin, E. Copeptin and cardiovascular disease: A review of a novel neurohormone. Int. J. Cardiol. 2013, 167, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Kanai, A.J.; Strauss, H.C.; Truskey, G.; Crews, A.L.; Grunfeld, S.; Malinski, T. Shear Stress Induces ATP-Independent Transient Nitric Oxide Release from Vascular Endothelial Cells, Measured Directly with a Porphyrinic Microsensor. Circ. Res. 1995, 77, 284–293. [Google Scholar] [CrossRef]
- Mendes Ribeiro, A.C.; Brunini, T.M.; Ellory, J.C.; Mann, G.E. Abnormalities in L- arginine transport and nitric oxide biosynthesis in chronic renal and heart failure. Cardiovasc. Res. 2001, 49, 697–712. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney Disease as a Risk Factor for Development of Cardiovascular Disease. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef]
- Manjunath, G.; Tighiouart, H.; Ibrahim, H.; MacLeod, B.; Salem, D.N.; Griffith, J.L.; Coresh, J.; Levey, A.S.; Sarnak, M.J. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J. Am. Coll. Cardiol. 2003, 41, 47–55. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Ruperez, M.; Lorenzo, O.; Esteban, V.; Blanco, J.; Mezzano, S.; Egido, J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int. 2002, 62, S12–S22. [Google Scholar] [CrossRef]
- Minami, Y.; Kajimoto, K.; Sato, N.; Hagiwara, N. Effect of Elevated C-Reactive Protein Level at Discharge on Long-Term Outcome in Patients Hospitalized for Acute Heart Failure. Am. J. Cardiol. 2018, 121, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, J.A.; McAlister, F.A.; Armstrong, P.W. Anemia is common in heart failure and is associated with poor outcomes: Insights from a cohort of 12 065 patients with new-onset heart failure. Circulation 2003, 107, 223–225. [Google Scholar] [CrossRef]
- Adams, K.F., Jr.; Patterson, J.H.; Oren, R.M.; Mehra, M.R.; O’Connor, C.M.; Piña, I.L.; Miller, A.B.; Chiong, J.R.; Dunlap, S.H.; Cotts, W.G.; et al. STAMINA-HFP Registry Investigators. Prospective assessment of the occurrence of anemia in patients with heart failure: Results from the Study of Anemia in a Heart Failure Population (STAMINA-HFP) Registry. Am. Heart J. 2009, 157, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Silverberg, D.S.; Iovine, F.; Calabrò, A.; Campagna, M.S.; Gallotta, M.; Nuti, R. Effects of β-erythropoietin treatment on left ventricular remodeling, systolic function, and B-type natriuretic peptide levels in patients with the cardiorenal anemia syndrome. Am. Hear. J. 2007, 154, 645.e9–645.e15. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Szczech, L.; Tang, K.L.; Barnhart, H.; Sapp, S.; Wolfson, M.; Reddan, D. Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease. N. Engl. J. Med. 2006, 355, 2085–2098. [Google Scholar] [CrossRef]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef] [PubMed]
- Kociol, R.D.; Horton, J.R.; Fonarow, G.C.; Reyes, E.M.; Shaw, L.K.; O’Connor, C.M.; Felker, G.M.; Hernandez, A.F. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: Data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ. Heart Fail 2011, 4, 628–636. [Google Scholar] [CrossRef]
- Takahama, H.; Nishikimi, T.; Takashio, S.; Hayashi, T.; Nagai-Okatani, C.; Asada, T.; Fujiwara, A.; Nakagawa, Y.; Amano, M.; Hamatani, Y.; et al. Change in the NT-proBNP/Mature BNP Molar Ratio Precedes Worsening Renal Function in. J. Am. Heart Assoc. 2019, 8, e011468. [Google Scholar] [CrossRef] [PubMed]
- McCallum, W.; Tighiouart, H.; Kiernan, M.S.; Huggins, G.S.; Sarnak, M.J. Relation of Kidney Function Decline and NT-proBNP With Risk of Mortality and Readmission in Acute Decompensated Heart Failure. Am. J. Med. 2019, 133, 115–122.e2. [Google Scholar] [CrossRef]
- Rezk, T.; Lachmann, H.J.; Fontana, M.; Naharro, A.M.; Sachchithanantham, S.; Mahmood, S.; Petrie, A.; Whelan, C.J.; Pinney, J.H.; Foard, D.; et al. Cardiorenal AL amyloidosis: Risk stratification and outcomes based upon cardiac and renal biomarkers. Br. J. Haematol. 2019, 186, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Truong, Q.A.; Szymonifka, J.; Januzzi, J.L.; Contractor, J.H.; Deaño, R.C.; Chatterjee, N.A.; Singh, J.P. Cardiorenal status using amino-terminal pro–brain natriuretic peptide and cystatin C on cardiac resynchronization therapy outcomes: From the BIOCRT Study. Hear. Rhythm. 2019, 16, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Quarles, L.D. The Role of Fibroblast Growth Factor-23 in Cardiorenal Syndrome. Nephron 2013, 123, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Goldsmith, D.; Akcay, A.; Covic, A. Phosphate—the silent stealthy cardiorenal culprit in all stages of chronic kidney disease: A systematic review. Blood Purif. 2009, 27, 220–230. [Google Scholar] [CrossRef]
- Jean, G.; Terrat, J.-C.; Vanel, T.; Hurot, J.-M.; Lorriaux, C.; Mayor, B.; Chazot, C. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol. Dial. Transplant. 2009, 24, 2792–2796. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.D.; Schurgers, L.J.; Brandenburg, V.M.; Christenson, R.H.; Vermeer, C.; Ketteler, M.; Michael, G.; Whooley, M.A. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul Study. Ann. Intern. Med. 2010, 152, 640–648. [Google Scholar] [CrossRef]
- Milovanova, L.I.; Milovanov, I.S.; Kozlovskaia, L.V.; Mukhin, N.A. [New markers of cardio-renal links in chronic kidney disease]. Ter Arkh. 2013, 85, 17–24. (In Russian) [Google Scholar] [PubMed]
- Scialla, J.J.; Xie, H.; Rahman, M.; Anderson, A.H.; Isakova, T.; Ojo, A.; Zhang, X.; Nessel, L.; Hamano, T.; Grunwald, J.E.; et al. Fibroblast Growth Factor-23 and Cardiovascular Events in CKD. J. Am. Soc. Nephrol. 2014, 25, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Kohab, N.; Fujimori, T.; Nishiguchib, S.; Tamorib, A.; Shiomib, S.; Nakatanic, T.; Sugimurac, K.; Kishimotoc, T.; Kinoshitaad, S.; Kurokib, T.; et al. Severely Reduced Production of Klotho in Human Chronic Renal Failure Kidney. Biochem. Biophys. Res. Commun. 2001, 280, 1015–1020. [Google Scholar] [CrossRef]
- Liu, S.; Tang, W.; Zhou, J.; Stubbs, J.R.; Luo, Q.; Pi, M.; Quarles, L.D. Fibroblast Growth Factor 23 Is a Counter-Regulatory Phosphaturic Hormone for Vitamin D. J. Am. Soc. Nephrol. 2006, 17, 1305–1315. [Google Scholar] [CrossRef]
- Gembillo, G.; Cernaro, V.; Salvo, A.; Siligato, R.; Laudani, A.; Buemi, M.; Santoro, D. Role of Vitamin D Status in Diabetic Patients with Renal Disease. Medicina 2019, 55, 273. [Google Scholar] [CrossRef] [PubMed]
- Gembillo, G.; Cernaro, V.; Siligato, R.; Curreri, F.; Catalano, A.; Santoro, D. Protective Role of Vitamin D in Renal Tubulopathies. Metabolism 2020, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Gembillo, G.; Siligato, R.; Amatruda, M.; Conti, G.; Santoro, D. Vitamin D and Glomerulonephritis. Medicina 2021, 57, 186. [Google Scholar] [CrossRef]
- Dobnig, H.; Pilz, S.; Scharnagl, H.; Renner, W.; Seelhorst, U.; Wellnitz, B.; Kinkeldei, J.; Boehm, B.O.; Weihrauch, G.; Maerz, W. Independent Association of Low Serum 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D Levels with All-Cause and Cardiovascular Mortality. Arch. Intern. Med. 2008, 168, 1340–1349. [Google Scholar] [CrossRef]
- Mann, M.C.; Exner, D.V.; Hemmelgarn, B.R.; Sola, D.Y.; Turin, T.C.; Ellis, L.; Ahmed, S.B. Vitamin D Levels Are Associated with Cardiac Autonomic Activity in Healthy Humans. Nutrients 2013, 5, 2114–2127. [Google Scholar] [CrossRef]
- Parker, J.; Hashmi, O.; Dutton, D.; Mavrodaris, A.; Stranges, S.; Kandala, N.-B.; Clarke, A.; Franco, O. Levels of vitamin D and cardiometabolic disorders: Systematic review and meta-analysis. Maturitas 2010, 65, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Giandalia, A.; Giuffrida, A.; Gembillo, G.; Cucinotta, D.; Squadrito, G.; Santoro, D.; Russo, G. Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors. Int. J. Mol. Sci. 2021, 22, 5808. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-H. Hepcidin as a Biomarker of Cardiorenal Syndrome. J. Korean Med. Sci. 2020, 35, e20. [Google Scholar] [CrossRef]
- Nikorowitsch, J.; Borchardt, T.; Appelbaum, S.; Ojeda, F.; Lackner, K.J.; Schnabel, R.B.; Blankenberg, S.; Zeller, T.; Karakas, M. Cardio-Renal Biomarker Soluble Urokinase-Type Plasminogen Activator Receptor Is Associated with Cardiovascular Death and Myocardial Infarction in Patients with Coronary Artery Disease Independent of Troponin, C-Reactive Protein, and Renal Function. J. Am. Hear. Assoc. 2020, 9, e015452. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Naranjo, M.; Kanjanahattakij, N.; Rangaswami, J.; Gupta, S. Cardiorenal syndrome in heart failure with preserved ejection fraction—an under-recognized clinical entity. Hear. Fail. Rev. 2019, 24, 421–437. [Google Scholar] [CrossRef]
- Iacoviello, M.; Di Serio, F.; Rizzo, C.; Leone, M.; Grande, D.; Guida, P.; Gioia, M.I.; Parisi, G.; Leopizzi, T.; Caldarola, P.; et al. Association between high Gal-3 serum levels and worsening of renal function in chronic heart failure outpatients. Biomarkers Med. 2019, 13, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Nakada, Y.; Kawakami, R.; Matsui, M.; Ueda, T.; Nakano, T.; Nakagawa, H.; Nishida, T.; Onoue, K.; Soeda, T.; Okayama, S.; et al. Value of Placental Growth Factor as a Predictor of Adverse Events During the Acute Phase of Acute Decompensated Heart Failure. Circ. J. 2019, 83, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Szczepankiewicz, B.; Paslawska, U.; Paslawski, R.; Gebarowski, T.; Zasada, W.; Michalek, M.; Noszczyk-Nowak, A. The urine podocin/creatinine ratio as a novel biomarker of cardiorenal syndrome in dogs due to degenerative mitral valve disease. J. Phys. Pharm. 2019, 2. [Google Scholar] [CrossRef]
- Rastaldi, M.P.; Ferrario, F.; Giardino, L.; Dell’Antonio, G.; Grillo, C.; Grillo, P.; Strutz, F.; Müller, G.A.; Colasanti, G.; D’Amico, G. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002, 62, 137–146. [Google Scholar] [CrossRef]
- Huntley, B.K.; Heublein, D.M.; Sandberg, S.M.; Burnett, J.C. Cofilin-1: A Possible New Biomarker for Human Heart Failure. J. Card. Fail. 2007, 13, S101. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chou, C.; Chang, C.-H.; Lee, N.-G.; Yu, P.-C.; Chen, Y.-C.; Pan, H.-C.; Fan, P.-C.; Yang, C.-W.; Cherng, W.-J.; et al. Urine Cofilin-1 Detection for Predicting Type 1 Cardiorenal Syndrome in the Coronary Care Unit: A Gold Nanoparticle- and Laser-Based Approach. Cardiorenal Med. 2018, 8, 302–310. [Google Scholar] [CrossRef]
- Martínez, P.J.; Baldán-Martín, M.; López, J.A.; Martín-Lorenzo, M.; Santiago-Hernández, A.; Agudiez, M.; Cabrera, M.; Calvo, E.; Vázquez, J.; Ruiz-Hurtado, G.; et al. Identification of six cardiovascular risk biomarkers in the young population: A promising tool for early prevention. Atherosclerosis 2019, 282, 67–74. [Google Scholar] [CrossRef]
- Gembillo, G.; Siligato, R.; Cernaro, V.; Satta, E.; Conti, G.; Salvo, A.; Romeo, A.; Calabrese, V.; Sposito, G.; Ferlazzo, G.; et al. Monocyte to HDL ratio: A novel marker of resistant hypertension in CKD patients. Int. Urol. Nephrol. 2021, 1–9. [Google Scholar] [CrossRef][Green Version]
- Papageorgiou, N.; Tousoulis, D.; Androulakis, E.; Siasos, G.; Briasoulis, A.; Vogiatzi, G.; Kampoli, A.-M.; Tsiamis, E.; Tentolouris, C.; Stefanadis, C. The role of microRNAs in cardiovascular disease. Curr. Med. Chem. 2012, 19, 2605–2610. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna J. Med. Biotechnol. 2010, 2, 161–179. [Google Scholar]
- Yan, H.; Ma, F.; Zhang, Y.; Wang, C.; Qiu, D.; Zhou, K.; Hua, Y.; Li, Y. miRNAs as biomarkers for diagnosis of heart failure: A systematic review and metaanalysis. Medicine 2017, 96, e6825. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Volkmann, I.; Jazbutyte, V.; Dangwal, S.; Park, D.-H.; Thum, T. Transforming growth factor-beta-induced endothelial-to- mesenchymal transition is partly mediated by microRNA-21. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 361–369. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nat. Cell Biol. 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, J.M.; Schauerte, C.; Hübner, A.; Kölling, M.; Martino, F.; Scherf, K.; Batkai, S.; Zimmer, K.; Foinquinos, A.; Kaucsar, T.; et al. Osteopontin is indispensible for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis. Eur. Hear. J. 2015, 36, 2184–2196. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Volkmann, I.; Thum, T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011, 8, 706–713. [Google Scholar] [CrossRef]
- Liu, X.-J.; Hong, Q.; Wang, Z.; Yu, Y.-Y.; Zou, X.; Xu, L.-H. MicroRNA21 promotes interstitial fibrosis via targeting DDAH1: A potential role in renal fibrosis. Mol. Cell. Biochem. 2015, 411, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Y.; Zhao, W.; Fu, G.; Li, Q.; Min, X.; Guo, Y.-F. Circulating miRNA-21 as a diagnostic biomarker in elderly patients with type 2 cardiorenal syndrome. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.; Boeckel, J.-N.; Groß, S.; Klotsche, J.; Palapies, L.; Leistner, D.; Pieper, L.; Stalla, G.K.; Lehnert, H.; Silber, S.; et al. Improved risk stratification in prevention by use of a panel of selected circulating microRNAs. Sci. Rep. 2017, 7, 4511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xing, Q.; Zhou, X.; Li, J.; Li, Y.; Zhang, L.; Zhou, Q.; Tang, B. Circulating miRNA-21 is a promising biomarker for heart failure. Mol. Med. Rep. 2017, 16, 7766–7774. [Google Scholar] [CrossRef]
- Ishrat, R.; Ahmed, M.M.; Tazyeen, S.; Alam, A.; Farooqui, A.; Ali, R.; Imam, N.; Tamkeen, N.; Ali, S.; Malik, Z.; et al. In Silico Integrative Approach Revealed Key MicroRNAs and Associated Target Genes in Cardiorenal Syndrome. Bioinform. Biol. Insights 2021, 15. [Google Scholar] [CrossRef]
- Siasos, G.; Bletsa, E.; Stampouloglou, P.K.; Oikonomou, E.; Tsigkou, V.; Paschou, S.A.; Vlasis, K.; Marinos, G.; Vavuranakis, M.; Stefanadis, C.; et al. MicroRNAs in cardiovascular disease. Hell. J. Cardiol. 2020, 61, 165–173. [Google Scholar] [CrossRef]
- Gembillo, G.; Ingrasciotta, Y.; Crisafulli, S.; Luxi, N.; Siligato, R.; Santoro, D.; Trifirò, G. Kidney Disease in Diabetic Patients: From Pathophysiology to Pharmacological Aspects with a Focus on Therapeutic Inertia. Int. J. Mol. Sci. 2021, 22, 4824. [Google Scholar] [CrossRef]
- Fu, S.; Zhao, S.; Ye, P.; Luo, L. Biomarkers in Cardiorenal Syndromes. BioMed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, G.; Barone, R.; Di Terlizzi, V.; Correale, M.; Brunetti, N.; Iacoviello, M. Biomarkers in Cardiorenal Syndrome. J. Clin. Med. 2021, 10, 3433. [Google Scholar] [CrossRef] [PubMed]
- Seliger, S. The Cardiorenal Syndrome: Mechanistic Insights and Prognostication with Soluble Biomarkers. Curr. Cardiol. Rep. 2020, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Description | References |
|---|---|---|
| Renal Biomarkers | ||
| Serum creatinine (SCr), albuminuria, cystatin C (CysC), potassium, eGFR | Classical biomarkers for the assessment of kidney function. Evidence for several indicators in CRS, especially when combined, has increased in recent years. For example, reduced albuminuria and potassemia indicate early renal repair processes after resynchronization therapy in CRS2. | Gala-Błądzińska et al. [16] |
| TIMP2•IGFBP7 | Urinary tissue inhibitor of metalloproteinase-2 • Insulin-like growth factor–binding protein 7 (TIMP2•IGFBP7). This is a combined marker of acute kidney damage. It can predict the onset of moderate to severe AKI, and is elevated in CRS patients. | Gunnerson et al. [22] |
| Urinary angiotensinogen | A marker of acute kidney damage and a strong predictor of worsening of AKI, with death in acute decompensated heart failure. The urinary angiotensinogen measured at the time of CRS diagnosis shows improved risk stratification. | Chen et al. [13] |
| NAG | Urinary enzyme N-acetyl-β-d-glucosaminidase (NAG). Measured at the time of CRS diagnosis, it shows improved risk stratification in determining which patients will experience adverse outcomes. | Chen et al. [13] |
| KIM-1 | Urinary kidney injury molecule 1 (KIM-1). Measured at the time of CRS diagnosis, it shows improved risk stratification in determining which patients will experience adverse outcomes. | Chen et al. [13] |
| NGAL | Urinary and serum neutrophil gelatinase associated lipocalin (NGAL). It is primarily used (especially its urinary determination) as early marker of renal damage. | Chen et al. [13] Cernaro et al. [15] |
| L-FABP | Liver fatty acid-binding protein (L-FABP). This is a marker of acute kidney damage in acute decompensated heart failure. | Hishikari et al. [23] |
| b2M and TIMP1 | β2-microglobulin (b2M) and tissue inhibitor of metalloproteinases 1 (TIMP1). Their values have been demonstrated to be potentially linked to severity of CRS. | Vianello et al. [21] |
| UP/Cr | Urine podocin/creatinine ratio (UP/Cr) is an emerging biomarker of CRS. In a study conducted in dogs, the UP/Cr in the CVD and CKD groups was significantly higher than in the control group. | Szczepankiewicz et al. [89] |
| Cardiac Biomarkers | ||
| hs-cTnI | High sensitivity troponin I (hs-cTnI). Marker of myocardial injury, which increases with declining eGFR. Sustained elevation is associated with a higher mortality risk. | Chen et al. [13] |
| Galectin-3 | A marker of cardiac fibrosis, stress and remodeling. It is associated with the progression of renal dysfunction in patients with heart disease. | Iacoviello et al. [90] |
| Neurohormones and hormones | ||
| BNP; NT-proBNP; emBNP | B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) are the gold standard biomarkers for heart failure. The elevation of the NT-proBNP/estimated mature BNP (emBNP) ratio precedes the worsening of renal function in patients with acute HF, and has been strongly associated with decreases in eGRF. | Kociol et al. [45] |
| AVP and copeptin | Copeptin is the C-terminal part of the arginine vasopressin (AVP), and is an easily measurable and stable molecule in plasma. Copeptin could be correlated with CVD in patients with CKD or ERSD. | Yalta et al. [33] |
| Oxidative stress and inflammatory biomarkers | ||
| XOA | The increased xanthine oxidoreductase activity (XOA), the elevated CysC value and reduced eGFR are significantly related to risk of acute CRS. | Tasić et al. [17] |
| IL-18 | Interleukin 18 (IL-18) is a cytokin used as a marker in acute kidney damage. Measured at the time of CRS diagnosis, it shows improved risk stratification in determining which patients will experience adverse outcomes. | Chen et al. [13] |
| Mineral and bone disorder biomarkers | ||
| FGF-23 | Fibroblast growth factor 23 (FGF-23) is involved in phosphate homeostasis. It could be used as marker of renal and heart failure. | Jean et al. [71] Scialla et al. [74] |
| Vitamin D | Vitamin D has pleiotropic effects and, as a marker, has a significant impact on the cardiovascular, nervous, endocrine and immune systems. However, its role in CRS patients is not clear. | Mann et al. [81] Binanay et al. [10] |
| MicroRNAs | ||
| MiR-21; miR-122-5p, miR-222-3p, miR-21-5p, miR-146a-5p, miR-29b-3p | MicroRNAs (miR) in clinical practice also involves CRS. MiR-122-5p, miR-222-3p, miR-21-5p, miR-146a-5p, and miR-29b-3p, correlated with CRS. MiR-21 has been shown to be an independent influencing factor for CRS. | Yan et al. [78] Keller et al. [85] Wang et al. [84] |
| Other biomarkers | ||
| MPV | Mean platelet volume (MPV). This might be a useful predictor of acute CRS prognosis. | Li et al. [67] |
| Hepcidin | There is a link between elevated serum hepcidin (high in anemia of inflammation that is hyporesponsive to erythropoiesis-stimulating agents) and cardiac geometry (relative wall thickness and left ventricular mass index). It is correlated with prognosis in patients with heart failure. | Kim et al. [88] |
| suPAR | Soluble urokinase-type plasminogen activator receptor (suPAR) might represent a valuable biomarker for risk estimation in coronary artery disease and HF. Its prognostic value remains valid also after adjusting for eGFR. | Nikorowitsch et al. [91] |
| PlGF | Placental growth factor (PIGF) is a key molecule in CRS and a predictor of adverse events in chronic kidney disease patients. | Nakada et al. [92] |
| Urinary cofilin-1 | Acts as a modulator of epithelial-mesenchymal transition or de-differentiated renal tubular cells, which are essential for AKI and renal function recovery, and is related to severity of HF. It is a highly potential biomarker for predicting CRS among coronary care unit patients. | Rastaldi et al. [91] Huntley et al. [72] Chen et al. [73] |
| ADX, ECP, FETUB, GDF15, GUAD, NOTCH1 | Urinary adrenodoxin (ADX), eosinophil cationic protein (ECP), fetuin B (FETUB), growth differentiation factor 15 (GDF15), guanine deaminase (GUAD) and neurogenic locus notch homolog protein 1 (NOTCH1) are a panel of urinary proteins, already used for CV risk, that might be useful in foreseeing and preventing CRS features. | Martinez et al. [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gembillo, G.; Visconti, L.; Giusti, M.A.; Siligato, R.; Gallo, A.; Santoro, D.; Mattina, A. Cardiorenal Syndrome: New Pathways and Novel Biomarkers. Biomolecules 2021, 11, 1581. https://doi.org/10.3390/biom11111581
Gembillo G, Visconti L, Giusti MA, Siligato R, Gallo A, Santoro D, Mattina A. Cardiorenal Syndrome: New Pathways and Novel Biomarkers. Biomolecules. 2021; 11(11):1581. https://doi.org/10.3390/biom11111581
Chicago/Turabian StyleGembillo, Guido, Luca Visconti, Maria Ausilia Giusti, Rossella Siligato, Alessia Gallo, Domenico Santoro, and Alessandro Mattina. 2021. "Cardiorenal Syndrome: New Pathways and Novel Biomarkers" Biomolecules 11, no. 11: 1581. https://doi.org/10.3390/biom11111581
APA StyleGembillo, G., Visconti, L., Giusti, M. A., Siligato, R., Gallo, A., Santoro, D., & Mattina, A. (2021). Cardiorenal Syndrome: New Pathways and Novel Biomarkers. Biomolecules, 11(11), 1581. https://doi.org/10.3390/biom11111581










