Aspirin-Based Organoiron Dendrimers as Promising Anti-Inflammatory, Anticancer, and Antimicrobial Drugs
Abstract
:1. Introduction
2. Methods and Materials
2.1. Instrumentation
2.2. Biological Measurements
2.2.1. Antimicrobial Assay
2.2.2. Method of Testing
2.2.3. MTT Assay
2.2.4. Animals
2.3. Anti-Inflammatory Activity
2.3.1. In Vitro COX-1 and COX-2 Inhibition Assay
2.3.2. Assessment of In Vivo Anti-Inflammatory Activity
In Vivo Rat Paw Edema Assay
Determination of Rat Serum PGE2
Evaluation of Gastrointestinal Toxicity of the Tested Dendrimers
Experimental Design
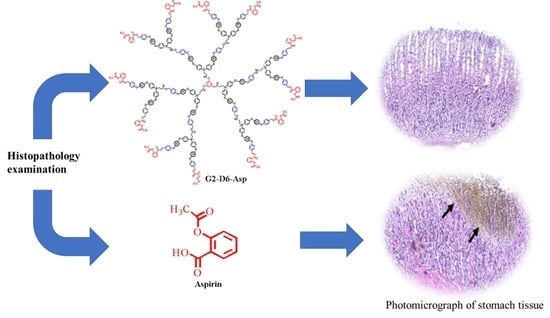
Histopathology Examination
Statistical Analysis
3. Synthesis and Characterization
3.1. General Procedure
3.2. Aspirin-Terminated G1-D3-Asp Dendrimer
3.3. Aspirin-Terminated G2-D6-Asp Dendrimer
3.4. Aspirin-Terminated G3-D9-ASP Dendrimer
3.5. Aspirin-Terminated G4-D12-Asp Dendrimer
4. Results and Discussion
4.1. Syntheses and Characterization of the Aspirin-Based Dendrimers
4.2. Morphological Characterization
4.3. Thermal Analysis of Synthesized Dendrimers
4.4. Electrochemical Properties
4.5. Antimicrobial Activity
4.6. Anticancer Activity
4.7. Aspirin-Based Dendrimer Inhibits Proliferation of MCF-7 and Hep-G2 Cells
4.8. In Vitro COX-1 and COX-2 Inhibition Assay
4.9. Assessment of In Vivo Anti-Inflammatory Activity
4.9.1. In Vivo Rat Paw Edema Assay
4.9.2. Determination of Rat Serum PGE2
4.9.3. Evaluation of Gastrointestinal Toxicity of the Tested Dendrimers
4.9.4. Histopathology Examination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Pedziwiatr-Werbicka, E.; Milowska, K.; Dzmitruk, V.; Ionov, M.; Shcharbin, D.; Bryszewska, M. Dendrimers and hyperbranched structures for biomedical applications. Eur. Polym. J. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Han, L.; Huang, R.; Li, J.; Liu, S.; Huang, S.; Jiang, C. Plasmid pORF-hTRAIL and doxorubicin co-delivery targeting to tumor using peptide-conjugated polyamidoamine dendrimer. Biomaterials 2011, 32, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Wrońska, N.; Majoral, J.P.; Appelhans, D.; Bryszewska, M.; Lisowska, K. Synergistic effects of anionic/cationic dendrimers and levofloxacin on antibacterial activities. Molecules 2019, 24, 2894. [Google Scholar] [CrossRef] [Green Version]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, V.; Palanirajan, V.K.; Tekade, R.K.; Jain, N.K. Dendrimers as therapeutic agents: A systematic review. J. Pharm. Pharmacol. 2009, 61, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.S.; Abdelghani, A.A.; El-Ghezlani, E.G.; Abou El-Ezz, D.; Abdel-Rahman, L.H. Pharmacological evaluation of novel organoiron dendrimers as antimicrobial and anti-inflammatory agents. Macromol. Biosci. 2021, 21, 2000242. [Google Scholar] [CrossRef] [PubMed]
- García-Gallego, S.; Franci, G.; Falanga, A.; Gómez, R.; Folliero, V.; Galdiero, S.; De la Mata Francisco Javier Galdiero, M. Function oriented molecular design: Dendrimers as novel antimicrobials. Molecules 2017, 22, 1581. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.S.; Abdelghani, A.A.; Mishra, A.K. Optical and biological properties of metal-containing macromolecules. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3–41. [Google Scholar] [CrossRef]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Caminade, A.; Laurent, R.; Shi, X.; Majoral, J. Recent therapeutic applications of the theranostic principle with dendrimers in oncology. Sci. China Mater. 2018, 61, 1367–1386. [Google Scholar] [CrossRef] [Green Version]
- Bodewein, L.; Schmelter, F.; Di Fiore, S.; Hollert, H.; Fischer, R.; Fenske, M. Differences in toxicity of anionic and cationic PAMAM and PPI dendrimers in zebrafish embryos and cancer cell lines. Toxicol. Appl. Pharmacol. 2016, 305, 83–92. [Google Scholar] [CrossRef]
- Tripathy, S.; Das, M.K. Dendrimers and their applications as novel drug delivery carriers. J. Appl. Pharm. Sci. 2013, 3, 142–149. [Google Scholar]
- Nikzamir, M.; Hanifehpour, Y.; Akbarzadeh, A.; Panahi, Y. Applications of dendrimers in nanomedicine and drug delivery: A review. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2246–2261. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef] [Green Version]
- Abd-El-Aziz, A.S.; Carruthers, S.A.; Aguiar, P.M.; Kroeker, S. Hyperbranched polymers containing cyclopentadienyliron complexes. J. Inorg. Organomet. Polym. Mater. 2005, 15, 349–359. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Todd, E.K.; Okasha, R.M.; Shipman, P.O.; Wood, T.E. Macromolecules containing redox-active neutral and cationic iron complexes. Macromolecules 2005, 38, 9411–9419. [Google Scholar] [CrossRef]
- Cuadrado, I.; Morán, M.; Casado, C.M.; Alonso, B.; Losada, J. Organometallic dendrimers with transition metals. Coord. Chem. Rev. 1999, 395, 193–195. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Abdelghani, A.A.; Wagner, B.D.; Pearson, J.K.; Awad, M.K. Design of blue fluorescence emitter star-shaped macromolecules based on pyrene and anthracene. Polymer 2016, 98, 210–228. [Google Scholar] [CrossRef]
- Astruc, D.; Hamon, J.R.; Althoff, G.; Roman, E.; Batail, P.; Michaud, P.; Mariot, J.P.; Varret, F.; Cozak, D. Design, stabilization, and efficiency of organometallic “electron reservoirs”. 19-Electron sandwiches. eta. 5-C5R5FeI-. eta. 6-C6R’6, a key class active in redox catalysis. J. Am. Chem. Soc. 1979, 101, 5445–5447. [Google Scholar] [CrossRef]
- Astruc, D. Electron-reservoir complexes and other redox-robust reagents: Functions and applications. New J. Chem. 2009, 33, 1191–1206. [Google Scholar] [CrossRef]
- Astruc, D. Electron-transfer processes in dendrimers and their implication in biology, catalysis, sensing and nanotechnology. Nat. Chem. 2012, 4, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.S.; Agatemor, C.; Etkin, N.; Bissessur, R. Tunable room-temperature soft ferromagnetism in magnetoceramics of organometallic dendrimers. J. Mater. Chem. C. 2017, 5, 2268–2281. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Agatemor, C.; Etkin, N.; Overy, D.P.; Lanteigne, M.; McQuillan, K.; Kerr, R.G. Antimicrobial organometallic dendrimers with tunable activity against multidrug-resistant bacteria. Biomacromolecules 2015, 16, 3694–3703. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.S.; Abdelghani, A.A.; El-Sadany, S.K.; Overy, D.P.; Kerr, R.G. Antimicrobial and anticancer activities of organoiron melamine dendrimers capped with piperazine moieties. Eur. Polym. J. 2016, 82, 307–323. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Agatemor, C.; Etkin, N.; Bissessur, R.; Overy, D.; Lanteigne, M.; McQuillan, K.; Kerr, R.G. Quaternized and thiazole-functionalized free radical-generating organometallic dendrimers as antimicrobial platform against multidrug-resistant microorganisms. Macromol. Biosci. 2017, 17, 1700020. [Google Scholar] [CrossRef]
- Amann, R.; Peskar, B.A. Anti-inflammatory effects of aspirin and sodium salicylate. Eur. J. Pharmacol. 2002, 447, 1–9. [Google Scholar] [CrossRef]
- Gilligan, M.M.; Gartung, A.; Sulciner, M.L.; Norris, P.C.; Sukhatme, V.P.; Bielenberg, D.R.; Huang, S.; Kieran, M.W.; Serhan, C.N.; Panigrahy, D. Aspirin-triggered proresolving mediators stimulate resolution in cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 6292–6297. [Google Scholar] [CrossRef] [Green Version]
- Warner, T.D.; Nylander, S.; Whatling, C. Anti-platelet therapy: Cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br. J. Clin. Pharmacol. 2011, 72, 619–633. [Google Scholar] [CrossRef] [Green Version]
- Becattini, C.; Agnelli, G.; Schenone, A.; Eichinger, S.; Bucherini, E.; Silingardi, M.; Bianchi, M.; Moia, M.; Ageno, W.; Vandelli, M.R. Aspirin for preventing the recurrence of venous thromboembolism. N. Engl. J. Med. 2012, 366, 1959–1967. [Google Scholar] [CrossRef] [Green Version]
- Nordin, N.A.; Chai, T.W.; Tan, B.L.; Choi, C.L.; Abd Halim, A.N.; Hussain, H.; Ngaini, Z. Novel synthetic monothiourea aspirin derivatives bearing alkylated amines as potential antimicrobial agents. J. Chem. 2017, 2017, 2378186. [Google Scholar] [CrossRef]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. J. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yan, B.; Xu, W.; Guo, L.; Wang, Z.; Li, G.; Hou, N.; Zhang, J.; Ling, R. Compound C enhances the anticancer effect of aspirin in HER-2-positive breast cancer by regulating lipid metabolism in an AMPK-independent pathway. Int. J. Biol. Sci. 2020, 16, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, G.J.; Stanford, N.; Majerus, P.W. Acetylation of prostaglandin synthase by aspirin. Proc. Natl. Acad. Sci. USA 1975, 72, 3073–3076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and cancer: Insight into tumor progression and immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Thorat, M.A.; Cuzick, J. Role of aspirin in cancer prevention. Curr. Oncol. Rep. 2013, 15, 533–540. [Google Scholar] [CrossRef]
- Lapi, F.; Levi, M.; Simonetti, M.; Cancian, M.; Parretti, D.; Cricelli, I.; Sobrero, A.; Cricelli, C. Risk of prostate cancer in low-dose aspirin users: A retrospective cohort study. Int. J. Cancer 2016, 139, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Hui, K.M. Emergence of aspirin as a promising chemopreventive and chemotherapeutic agent for liver cancer. Cell Death Dis. 2017, 8, e3112. [Google Scholar] [CrossRef]
- Zhong, S.; Chen, L.; Zhang, X.; Yu, D.; Tang, J.; Zhao, J. Aspirin use and risk of breast cancer: Systematic review and meta-analysis of observational studies. Cancer Epidemiol. Prev. Biomark. 2015, 24, 1645–1655. [Google Scholar] [CrossRef] [Green Version]
- Cicala, C.; Ianaro, A.; Fiorucci, S.; Calignano, A.; Bucci, M.; Gerli, R.; Santucci, L.; Wallace, J.L.; Cirino, G. NO-naproxen modulates inflammation, nociception and downregulates T cell response in rat Freund’s adjuvant arthritis. Br. J. Pharmacol. 2000, 130, 1399–1405. [Google Scholar] [CrossRef]
- Ngaini, Z.; Mortadza, N.A. Synthesis of halogenated azo-aspirin analogues from natural product derivatives as the potential antibacterial agents. Nat. Prod. Res. 2019, 33, 3507–3514. [Google Scholar] [CrossRef]
- Chavan, B.B.; Chitte, P.D.; Choudhary, N.P.; Albhar, K.G.; Hukkeri, V.I. Synthesis and biological evaluation of novel benzimidazole derivative with aspirin as potent antimicrobial and antifungal agents. Int. J. Sci. Res. Rev. 2012, 1, 22–30. [Google Scholar]
- Lawal, A.; Obaleye, J.A. Synthesis, characterization and antibacterial activity of aspirin and paracetamolmetal complexes. Biokemistri 2007, 19, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Mackenzie, G.G.; Ouyang, N.; Sun, Y.; Xie, G.; Johnson, F.; Komninou, D.; Rigas, B. The novel phospho-non-steroidal anti-inflammatory drugs, OXT-328, MDC-22 and MDC-917, inhibit adjuvant-induced arthritis in rats. Br. J. Pharmacol. 2011, 162, 1521–1533. [Google Scholar] [CrossRef] [Green Version]
- Al-Bakri, A.G.; Othman, G.; Bustanji, Y. The assessment of the antibacterial and antifungal activities of aspirin, EDTA and aspirin–EDTA combination and their effectiveness as antibiofilm agents. J. Appl. Microbiol. 2009, 107, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Nie, T.; Huang, L.; Zhou, H.; Atmakur, K.; Gupta, R.C.; Johnson, F.; Rigas, B. Structure-activity relationship study of novel anticancer aspirin-based compounds. Mol. Med. Rep. 2011, 4, 891–899. [Google Scholar]
- Gallardo, A.; Parejo, C.; San Román, J. NSAIDs bound to methacrylic carriers: Microstructural characterization and in vitro release analysis. J. Control. Release 2001, 71, 127–140. [Google Scholar] [CrossRef]
- Ahmad, S.; Tester, R.F.; Corbett, A.; Karkalas, J. Dextran and 5-aminosalicylic acid (5-ASA) conjugates: Synthesis, characterisation and enzymic hydrolysis. Carbohydr. Res. 2006, 341, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Wiwattanapatapee, R.; Lomlim, L.; Saramunee, K. Dendrimers conjugates for colonic delivery of 5-aminosalicylic acid. J. Control. Release 2003, 88, 1–9. [Google Scholar] [CrossRef]
- Juzwa, M.; Rusin, A.; Zawidlak-Węgrzyńska, B.; Krawczyk, Z.; Obara, I.; Jedliński, Z. Oligo (3-hydroxybutanoate) conjugates with acetylsalicylic acid and their antitumour activity. Eur. J. Med. Chem. 2008, 43, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xu, T. The effect of dendrimers on the pharmacodynamic and pharmacokinetic behaviors of non-covalently or covalently attached drugs. Eur. J. Med. Chem. 2008, 43, 2291–2297. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Carruthers, S.A.; Todd, E.K.; Afifi, T.H.; Gavina, J.M. Cationic organoiron polyelectrolyte three-arm star. Polym. Chem. 2005, 43, 1382–1396. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Abdelghani, A.A.; Wagner, B.D.; Abdelrehim, E.M. Aggregation enhanced excimer emission (AEEE) with efficient blue emission based on pyrene dendrimers. Polym. Chem. 2016, 7, 3277–3299. [Google Scholar] [CrossRef]
- Edwards, E.R.; Antunes, E.F.; Botelho, E.C.; Baldan, M.R.; Corat, E.J. Evaluation of residual iron in carbon nanotubes purified by acid treatments. Appl. Surf. Sci. 2011, 258, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Abd-El-Aziz, A.S.; Todd, E.K.; Epp, K.M. Synthesis and characterization of novel organoiron polymers. J. Inorg. Organomet. Polym. 1998, 8, 127–133. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; May, L.J.; Hurd, J.A.; Okasha, R.M. First ring-opening metathesis polymerization of norbornenes containing cationic iron moieties. J. Polym. Sci. A Polym. Chem. 2001, 39, 2716–2722. [Google Scholar] [CrossRef]
- Neises, B.; Steglich, W. Simple method for the esterification of carboxylic acids. Angew. Chem. Int. Ed. Engl. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; El-Ghezlani, E.G.; Abdelghani, A.A. Design of organoiron dendrimers containing paracetamol for enhanced antibacterial efficacy. Molecules 2020, 25, 4514. [Google Scholar] [CrossRef]
- Raghavendran, H.R.B.; Srinivasan, P.; Rekha, S. Immunomodulatory activity of fucoidan against aspirin-induced gastric mucosal damage in rats. Int. Immunopharmacol. 2011, 11, 157–163. [Google Scholar] [CrossRef]
- Yamamoto, O.; Okada, Y.; Okabe, S. Effects of a proton pump inhibitor, omeprazole, on gastric secretion and gastric and duodenal ulcers or erosions in rats. Dig. Dis. Sci. 1984, 29, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.K. A method for quantitative assessment of experimentally produced ulcers in stomach of rats. Experientia 1969, 25, 1224. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- El-Shiekh, R.A.; Salama, A.; Al-Mokaddem, A.K.; Bader, A.; Abdel-Sattar, E.A.; Russelioside, B. A pregnane glycoside for treatment of gastric ulcer via modulation of heat shock protein-70 and vascular endothelial growth factor. Steroids 2021, 165, 108759. [Google Scholar] [CrossRef]
- Lee, C.; Su, L.; Liu, J.; Chang, C.; Chen, R.; Yang, K. Aspirin enhances opsonophagocytosis and is associated to a lower risk for klebsiella pneumoniae invasive syndrome. BMC Infect. Dis. 2014, 14, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.H.; Wong, W.M.; Dailidiene, D.; Berg, D.E.; Gu, Q.; Lai, K.C.; Lam, S.K.; Wong, B. Aspirin inhibits the growth of Helicobacter pylori and enhances its susceptibility to antimicrobial agents. Gut 2003, 52, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Obad, J.; Šušković, J.; Kos, B. Antimicrobial activity of ibuprofen: New perspectives on an “Old” non-antibiotic drug. Eur. J. Pharm. Sci. 2015, 71, 93–98. [Google Scholar] [CrossRef]
- Ngaini, Z.; Kui, H.B. Synthesis and antibacterial activity of azo and aspirin-azo derivatives. Malays. J. Anal. Sci. 2017, 21, 1183–1194. [Google Scholar]
- Abd-El-Aziz, A.S.; Agatemor, C.; Etkin, N.; Overy, D.P.; Kerr, R.G. Redox-active cationic organoiron complex: A promising lead structure for developing antimicrobial agents with activity against Gram-positive pathogens including methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. RSC Adv. 2015, 5, 86421–86427. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef]
- Chan, A.K.Y.; Tsang, Y.C.; Chu, C.H.; Tsang, C.S.P. Aspirin as an antifungal-lock agent in inhibition of candidal biofilm formation in surgical catheters. Infect. Drug Resist. 2021, 14, 1427. [Google Scholar] [CrossRef]
- Alem, M.A.; Douglas, L.J. Effects of aspirin and other nonsteroidal anti-inflammatory drugs on biofilms and planktonic cells of candida albicans. Antimicrob. Agents Chemother. 2004, 48, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kock, J.L.; Sebolai, O.M.; Pohl, C.H.; Van Wyk, P.W.; Lodolo, E.J. Oxylipin studies expose aspirin as antifungal. FEMS Yeast Res. 2007, 7, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Patra, M.; Senges, C.H.R.; Ott, I.; Stepanek, J.J.; Pinto, A.; Prochnow, P.; Vuong, C.; Langklotz, S.; Metzler-Nolte, N. Analysis of the mechanism of action of potent antibacterial hetero-tri-organometallic compounds: A structurally new class of antibiotics. ACS Chem. Biol. 2013, 8, 1442–1450. [Google Scholar] [CrossRef]
- Deffert, C.; Cachat, J.; Krause, K. Phagocyte NADPH oxidase, chronic granulomatous disease and mycobacterial infections. Cell Microbiol. 2014, 16, 1168–1178. [Google Scholar] [CrossRef]
- Rada, B.; Leto, T.L. Oxidative innate immune defenses by nox/duox family NADPH oxidases. Trends Innate Immun. 2008, 15, 164–187. [Google Scholar]
- Rohwer, N.; Kühl, A.A.; Ostermann, A.I.; Hartung, N.M.; Schebb, N.H.; Zopf, D.; McDonald, F.M.; Weylandt, K. Effects of chronic low-dose aspirin treatment on tumor prevention in three mouse models of intestinal tumorigenesis. Cancer Med. 2020, 9, 2535–2550. [Google Scholar] [CrossRef]
- Recio Despaigne, A.A.; Da Silva, J.G.; Da Costa, P.R.; Dos Santos, R.G.; Beraldo, H. ROS-mediated cytotoxic effect of copper (II) hydrazone complexes against human glioma cells. Molecules 2014, 19, 17202–17220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-El-Aziz, A.S.; Agatemor, C. Emerging opportunities in the biomedical applications of dendrimers. J. Inorg. Organomet. Polym. Mater. 2018, 28, 369–382. [Google Scholar] [CrossRef]
- Parsekar, S.U.; Haldar, P.; Antharjanam, P.S.; Kumar, M.; Koley, A.P. Synthesis, characterization, crystal structure, DNA and human serum albumin interactions, as well as antiproliferative activity of a cu (II) complex containing a Schiff base ligand formed in situ from the cu (II)-induced cyclization of 1, 5-bis (salicylidene) thiocarbohydrazide. Appl. Organomet. Chem. 2021, 35, e6152. [Google Scholar]
- Van de Loosdrecht, A.A.; Beelen, R.; Ossenkoppele, G.J.; Broekhoven, M.G.; Langenhuijsen, M. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J. Immunol. Methods 1994, 174, 311–320. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Ryan, P.M.; Dang, M.; Clark, C.; Kontogiannis, V.; Rahmani, J.; Varkaneh, H.K.; Salehisahlabadi, A.; Day, A.S. Association of aspirin therapy with risk of hepatocellular carcinoma: A systematic review and dose-response analysis of cohort studies with 2.5 million participants. Pharmacol. Res. 2020, 151, 104585. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; John, A.; Benedict, S. Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. Eur. J. Pharmacol. 2011, 668, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Fujita, K.; Gong, J.; Nakahara, M.; Iwama, H.; Liu, S.; Yoneyama, H.; Morishita, A.; Nomura, T.; Tani, J. Aspirin inhibits hepatocellular carcinoma cell proliferation in vitro and in vivo via inducing cell cycle arrest and apoptosis. Oncol. Rep. 2020, 44, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Bhatia, P.; Alam, O.; Naim, M.J.; Nawaz, F.; Sheikh, A.A.; Jha, M. Recent advancement in the discovery and development of COX-2 inhibitors: Insight into biological activities and SAR studies (2008–2019). Bioorg. Chem. 2019, 89, 103007. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.B.; Fayez, A.M.; EL-Shaymaa, E.; Awadallah, M.; Halim, P.A. Novel tetrazole-based selective COX-2 inhibitors: Design, synthesis, anti-inflammatory activity, evaluation of PGE2, TNF-α, IL-6 and histopathological study. Bioorg. Chem. 2020, 104, 104308. [Google Scholar] [CrossRef]
- Parajapati, S.K.; Maurya, S.D.; Das, M.K.; Tilak, V.K.; Verma, K.K.; Dhakar, R.C. Potential application of dendrimers in drug delivery: A concise review and update. J. Drug Deliv. Ther. 2016, 6, 71–88. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, Z.; Ma, M.; Xu, T. Dendrimers as drug carriers: Applications in different routes of drug administration. J. Pharm. Sci. 2008, 97, 123–143. [Google Scholar] [CrossRef]
- Liu, Y.; Tee, J.K.; Chiu, G.N.C. Dendrimers in oral drug delivery application: Current explorations, toxicity issues and strategies for improvement. Curr. Pharm. Des. 2015, 21, 2629–2642. [Google Scholar] [CrossRef]
- Sadekar, S.; Ghandehari, H. Transepithelial transport and toxicity of PAMAM dendrimers: Implications for oral drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 571–588. [Google Scholar] [CrossRef] [Green Version]
- Kolhatkar, R.B.; Swaan, P.; Ghandehari, H. Potential oral delivery of 7-ethyl-10-hydroxy-camptothecin (SN-38) using poly (amidoamine) dendrimers. Pharm. Res. 2008, 25, 1723–1729. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, D.S.; Vijayalakshmi, N.; Swaan, P.W.; Ghandehari, H. G3. 5 PAMAM dendrimers enhance transepithelial transport of SN38 while minimizing gastrointestinal toxicity. J. Control. Release 2011, 150, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.M.; Abd-Elgwwad, S.K.; Hasan, R.A.A.; Abd-Elgalil, M.M. Trio-clar versus Portulaca oleracea and/or Foeniculum vulgare extracts on induced gastric ulcer in adult female albino rats. A histological study. Egypt. J. Histol. 2020, 43, 1008–1033. [Google Scholar] [CrossRef]


























| G1-D3-Asp | G2-D6-Asp | G3-D9-Asp | G4-D12-Asp | Asp | Gentamicin | Ampicillin | Nystatin | |
|---|---|---|---|---|---|---|---|---|
| B. subtilis | 14 ± 0.11 | 16 ± 0.10 | 16 ± 0.09 | 20 ± 0.12 | 23 ± 0.08 | - | 25 ± 0.06 | - |
| S. aureus | 19 ± 0.09 | 21 ± 0.11 | 24 ± 0.10 | 28 ± 0.10 | 30 ± 0.07 | - | 15 ± 0.05 | - |
| E. coli | 10 ± 0.10 | 11 ± 0.09 | 13 ± 0.11 | 17 ± 0.11 | 18 ± 0.06 | 17 ± 0.04 | - | - |
| K. pneumoniae | 15 ± 0.08 | 19 ± 0.10 | 22 ± 0.10 | 23 ± 0.09 | 26 ± 0.11 | 22 ± 0.02 | - | - |
| C. albicans | 15 ± 0.09 | 19 ± 0.11 | 21 ± 0.07 | 21 ± 0.12 | 29 ± 0.09 | - | - | 21 ± 0.11 |
| A. niger | 18 ± 0.11 | 20 ± 0.09 | 21 ± 0.11 | 25 ± 0.11 | 30 ± 0.10 | - | - | 15 ± 0.08 |
| G1-D3-Asp | G2-D6-Asp | G3-D9-Asp | G4-D12-Asp | Aspirin | Doxorubicin | |
|---|---|---|---|---|---|---|
| Hep-G2 | 372.21 ± 0.09 | 324.95 ± 0.10 | 105.05 ± 0.05 | 111.77 ± 0.11 | 192.40 ± 0.04 | 4.80 ± 0.09 |
| MCF-7 | 323.18 ± 0.07 | 320.59 ± 0.12 | 193.46 ± 0.04 | 191.66 ± 0.09 | 208.96 ± 0.05 | 5.70 ± 0.07 |
| HEK-293 | 342.01 ± 0.06 | 320.48 ± 0.09 | 224.31 ± 0.07 | 234.11 ± 0.10 | 240.18 ± 0.03 | 205.07 ± 0.05 |
| Code | COX-1 µm IC50 | COX-2 µm IC50 | SI * |
|---|---|---|---|
| Celecoxib | 14.7 | 0.045 | >300 |
| Rofecoxib | 14.5 | 0.025 | >500 |
| Indomethacin | 0.1 | 0.080 | >500 |
| Diclofenac sodium | 3.8 | 0.84 | >4 |
| Aspirin | 1.3 | 5.9 | 0.22 |
| G1-D3-Asp | 1.5 | 6.8 | 0.22 |
| G2-D6-Asp | 1.3 | 6.5 | 0.20 |
| G3-D9-Asp | 1.3 | 6.4 | 0.20 |
| G4-D12-Asp | 1.2 | 6.3 | 0.19 |
| Groups | Paw Thickness (mm) | % Inhibition | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 4 h | 6 h | 1 h | 2 h | 4 h | 6 h | |
| Normal | 3.11 b ± 0.10 | 3.19 b ± 0.16 | 3.19 b ± 0.22 | 3.14 b ± 0.20 | - | - | - | - |
| Control | 5.10 ± 0.20 | 6.92 ± 0.37 | 7.64 ± 0.42 | 9.65 ± 1.8 | - | - | - | - |
| Aspirin | 3.32 b ± 0.23 | 4.00 b ± 0.36 | 4.96 b ± 0.48 | 5.08 b ± 0.56 | 34.82 | 42.23 | 35.08 | 47.38 |
| G1-D3-Asp | 4.54 b ± 0.34 | 5.23 b ± 0.59 | 6.44 b ± 0.44 | 7.50 b ± 0.76 | 10.91 | 24.37 | 15.78 | 22.31 |
| G2-D6-Asp | 3.45 b ± 0.34 | 3.99 b ± 0.79 | 4.32 b ± 0.59 | 4.97 b ± 0.80 | 32.29 | 42.34 | 43.53 | 48.51 |
| G3-D9-Asp | 4.27 b ± 0.30 | 4.71 b ± 0.32 | 5.58 b ± 0.55 | 5.93 b ± 0.38 | 16.15 | 31.95 | 26.94 | 38.60 |
| G4-D12-Asp | 3.96 b ± 0.23 | 4.84 b ± 0.40 | 6.21 b ± 0.51 | 7.00 b ± 0.73 | 22.20 | 30.01 | 18.76 | 27.52 |
| Compound No. | PGE2 | |
|---|---|---|
| Mean Serum Concentration (Pg/mL) | Inhibition% | |
| Normal | 122.72 b ± 5.53 | - |
| Control | 293.68 ± 13.78 | - |
| Aspirin | 81.051 b ± 14.39 | 72.40 |
| G1-D3-Asp | 190.22 b ± 20.44 | 35.23 |
| G2-D6-Asp | 103.30 b ± 22.76 | 64.83 |
| G3-D9-Asp | 139.51 b ± 18.82 | 52.50 |
| G4-D12-Asp | 184.05 b ± 29.67 | 37.33 |
| Compound No. | Gastric Mucosal Lesions | ||
|---|---|---|---|
| Ulcer Area (mm) | Ulcer Score | Ulcer Index | |
| Normal | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Aspirin | 86.91 ± 9.51 | 5.00 ± 0.00 | 1.45 a ± 0.16 |
| G1-D3-Asp | 5.17 ± 1.13 | 1.17 ± 0.41 | 0.09 b ± 0.02 |
| G2-D6-Asp | 16.78 ± 2.71 | 3.17 ± 0.41 | 0.28 a,b ± 0.04 |
| G3-D9-Asp | 17.98 ± 0.93 | 3.00 ± 0.00 | 0.30 a,b ± 0.02 |
| G4-D12-Asp | 7.58 ± 1.00 | 2.00 ± 0.00 | 0.13 a,b ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-El-Aziz, A.S.; Benaaisha, M.R.; Abdelghani, A.A.; Bissessur, R.; Abdel-Rahman, L.H.; Fayez, A.M.; El-ezz, D.A. Aspirin-Based Organoiron Dendrimers as Promising Anti-Inflammatory, Anticancer, and Antimicrobial Drugs. Biomolecules 2021, 11, 1568. https://doi.org/10.3390/biom11111568
Abd-El-Aziz AS, Benaaisha MR, Abdelghani AA, Bissessur R, Abdel-Rahman LH, Fayez AM, El-ezz DA. Aspirin-Based Organoiron Dendrimers as Promising Anti-Inflammatory, Anticancer, and Antimicrobial Drugs. Biomolecules. 2021; 11(11):1568. https://doi.org/10.3390/biom11111568
Chicago/Turabian StyleAbd-El-Aziz, Alaa S., Maysun R. Benaaisha, Amani A. Abdelghani, Rabin Bissessur, Laila H. Abdel-Rahman, Ahmed M. Fayez, and Doaa Abou El-ezz. 2021. "Aspirin-Based Organoiron Dendrimers as Promising Anti-Inflammatory, Anticancer, and Antimicrobial Drugs" Biomolecules 11, no. 11: 1568. https://doi.org/10.3390/biom11111568
APA StyleAbd-El-Aziz, A. S., Benaaisha, M. R., Abdelghani, A. A., Bissessur, R., Abdel-Rahman, L. H., Fayez, A. M., & El-ezz, D. A. (2021). Aspirin-Based Organoiron Dendrimers as Promising Anti-Inflammatory, Anticancer, and Antimicrobial Drugs. Biomolecules, 11(11), 1568. https://doi.org/10.3390/biom11111568