Decoding Stem Cells: An Overview on Planarian Stem Cell Heterogeneity and Lineage Progression
Abstract
1. Introduction
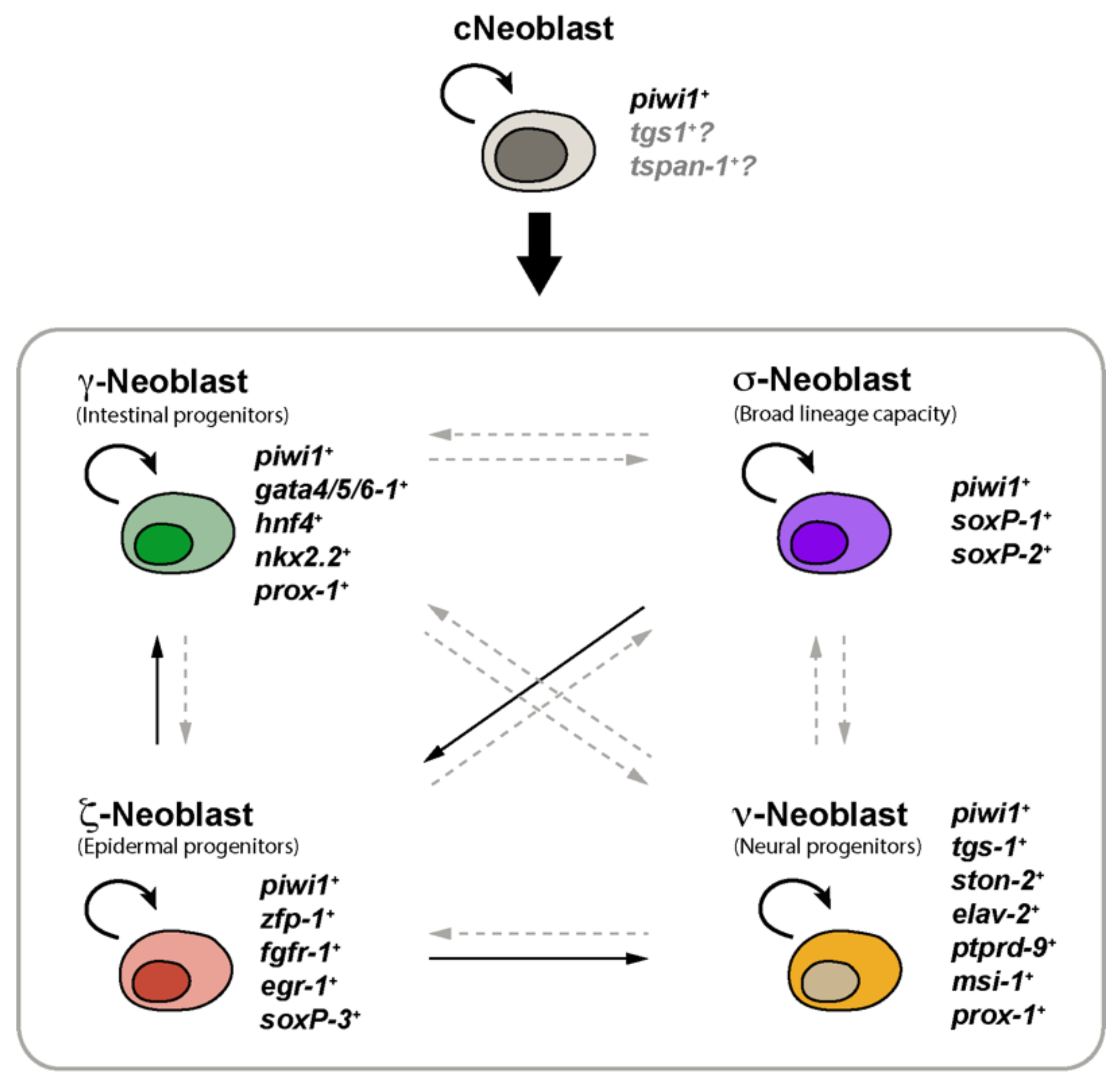
2. Planarian Neoblasts Are a Heterogeneous Population of Stem Cells
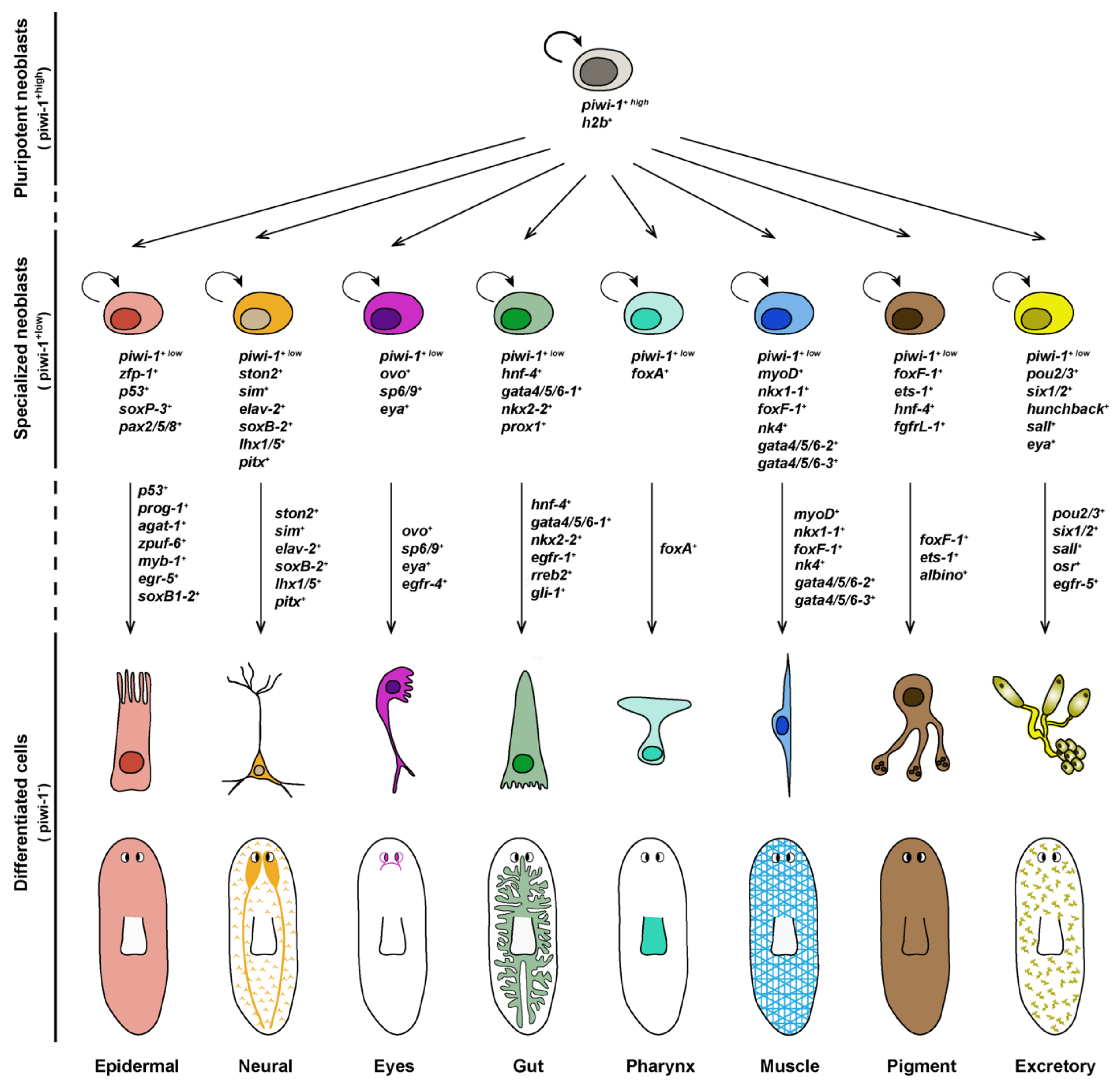
3. From Pluripotent to Lineage-Specialized Stem Cells: Stem Cell Specification
3.1. Distinct Responses of Stem Cells to Amputation and Loss of Specific Organs Occur in Planarians
3.2. The Body-Wall Musculature Serves as a Source of Positional Information in Planarians
3.3. Planarian Muscle Cells Are the Primary Source of Extracellular Matrix
3.4. Neoblasts Cell Fate Specification Occurs through the Cell Cycle: Asymmetric vs. Symmetric Cell Divisions
4. Differentiation of Lineage-Committed Stem Cells into Tissue Specific Cell Types
5. Post-Translational Modifications and Epigenetic Regulation in Neoblasts
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ivankovic, M.; Haneckova, R.; Thommen, A.; Grohme, M.A.; Vila-Farré, M.; Werner, S.; Rink, J.C. Model Systems for Regeneration: Planarians. Development 2019, 146, dev167684. [Google Scholar] [CrossRef] [PubMed]
- Reddien, P.W. The Cellular and Molecular Basis for Planarian Regeneration. Cell 2018, 175, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Cebrià, F.; Adell, T.; Saló, E. Rebuilding a Planarian: From Early Signaling to Final Shape. Int. J. Dev. Biol. 2018, 62, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Asami, M.; Higuchi, S.; Shibata, N.; Agata, K. Isolation of Planarian X-Ray-Sensitive Stem Cells by Fluorescence-Activated Cell Sorting. Dev. Growth Differ. 2006, 48, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.E.; Wang, I.E.; Reddien, P.W. Clonogenic Neoblasts Are Pluripotent Adult Stem Cells That Underlie Planarian Regeneration. Science 2011, 332. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; McKinney, S.A.; Ross, E.J.; Lee, H.C.; Alvarado, A.S. Cultured Pluripotent Planarian Stem Cells Retain Potency and Express Proteins from Exogenously Introduced MRNAs. bioRxiv 2019. [Google Scholar] [CrossRef]
- Newmark, P.A.; Sánchez Alvarado, A. Bromodeoxyuridine Specifically Labels the Regenerative Stem Cells of Planarians. Dev. Biol. 2000, 220, 142–153. [Google Scholar] [CrossRef]
- Zhu, S.J.; Hallows, S.E.; Currie, K.W.; Xu, C.; Pearson, B.J. A Mex3 Homolog Is Required for Differentiation during Planarian Stem Cell Lineage Development. eLife 2015, 4, e07025. [Google Scholar] [CrossRef]
- Fincher, C.T.; Wurtzel, O.; de Hoog, T.; Kravarik, K.M.; Reddien, P.W. Cell Type Transcriptome Atlas for the Planarian Schmidtea Mediterranea. Science 2018, 360, 1–12. [Google Scholar] [CrossRef]
- Plass, M.; Solana, J.; Alexander Wolf, F.; Ayoub, S.; Misios, A.; Glažar, P.; Obermayer, B.; Theis, F.J.; Kocks, C.; Rajewsky, N. Cell Type Atlas and Lineage Tree of a Whole Complex Animal by Single-Cell Transcriptomics. Science 2018, 360, 1–10. [Google Scholar] [CrossRef]
- Swapna, L.S.; Molinaro, A.M.; Lindsay-Mosher, N.; Pearson, B.J.; Parkinson, J. Comparative Transcriptomic Analyses and Single-Cell RNA Sequencing of the Freshwater Planarian Schmidtea Mediterranea Identify Major Cell Types and Pathway Conservation. Genome Biol. 2018, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- van Wolfswinkel, J.C.; Wagner, D.E.; Reddien, P.W. Single-Cell Analysis Reveals Functionally Distinct Classes within the Planarian Stem Cell Compartment. Cell Stem Cell 2014, 15, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.M.; Pearson, B.J. In Silico Lineage Tracing through Single Cell Transcriptomics Identifies a Neural Stem Cell Population in Planarians. Genome Biol. 2016, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wurtzel, O.; Cote, L.E.; Poirier, A.; Satija, R.; Regev, A.; Reddien, P.W. A Generic and Cell-Type-Specific Wound Response Precedes Regeneration in Planarians. Dev. Cell 2015, 35, 632–645. [Google Scholar] [CrossRef]
- Benham-Pyle, B.W.; Brewster, C.E.; Kent, A.M.; Mann, F.G.; Chen, S.; Scott, A.R.; Box, A.C.; Sánchez Alvarado, A. Identification of Rare, Transient Post-Mitotic Cell States That Are Induced by Injury and Required for Whole-Body Regeneration in Schmidtea Mediterranea. Nat. Cell Biol. 2021, 23, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Li, H.; Guo, L.; Gao, X.; McKinney, S.; Wang, Y.; Yu, Z.; Park, J.; Semerad, C.; Ross, E.; et al. Prospectively Isolated Tetraspanin+ Neoblasts Are Adult Pluripotent Stem Cells Underlying Planaria Regeneration. Cell 2018, 173, 1593–1608.e20. [Google Scholar] [CrossRef]
- Resch, A.M.; Palakodeti, D.; Lu, Y.C.; Horowitz, M.; Graveley, B.R. Transcriptome Analysis Reveals Strain-Specific and Conserved Stemness Genes in Schmidtea Mediterranea. PLoS ONE 2012, 7, e34447. [Google Scholar] [CrossRef]
- Solana, J.; Kao, D.; Mihaylova, Y.; Jaber-Hijazi, F.; Malla, S.; Wilson, R.; Aboobaker, A. Defining the Molecular Profile of Planarian Pluripotent Stem Cells Using a Combinatorial RNAseq, RNA Interference and Irradiation Approach. Genome Biol. 2012, 13, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Önal, P.; Grün, D.; Adamidi, C.; Rybak, A.; Solana, J.; Mastrobuoni, G.; Wang, Y.; Rahn, H.P.; Chen, W.; Kempa, S.; et al. Gene Expression of Pluripotency Determinants Is Conserved between Mammalian and Planarian Stem Cells. EMBO J. 2012, 31, 2755–2769. [Google Scholar] [CrossRef] [PubMed]
- Labbé, R.M.; Irimia, M.; Currie, K.W.; Lin, A.; Zhu, S.J.; Brown, D.D.R.; Ross, E.J.; Voisin, V.; Bader, G.D.; Blencowe, B.J.; et al. A Comparative Transcriptomic Analysis Reveals Conserved Features of Stem Cell Pluripotency in Planarians and Mammals. Stem Cells 2012, 30, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Scimone, M.L.; Kravarik, K.M.; Lapan, S.W.; Reddien, P.W. Neoblast Specialization in Regeneration of the Planarian Schmidtea Mediterranea. Stem Cell Rep. 2014, 3, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Scimone, M.L.; Srivastava, M.; Bell, G.W.; Reddien, P.W. A Regulatory Program for Excretory System Regeneration in Planarians. Development 2011, 138, 4387–4398. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.E.; Seidel, C.W.; McKinney, S.A.; Sánchez Alvarado, A. Selective Amputation of the Pharynx Identifies a FoxA-Dependent Regeneration Program in Planaria. eLife 2014, 3, e02238. [Google Scholar] [CrossRef]
- Scimone, M.L.; Wurtzel, O.; Malecek, K.; Fincher, C.T.; Oderberg, I.M.; Kravarik, K.M.; Reddien, P.W. FoxF-1 Controls Specification of Non-Body Wall Muscle and Phagocytic Cells in Planarians. Curr. Biol. 2018, 28, 3787–3801.e6. [Google Scholar] [CrossRef]
- Scimone, L.M.; Cote, L.E.; Reddien, P.W. Orthogonal Muscle Fibres Have Different Instructive Roles in Planarian Regeneration. Nature 2017, 551, 623–628. [Google Scholar] [CrossRef]
- Lapan, S.W.; Reddien, P.W. Transcriptome Analysis of the Planarian Eye Identifies Ovo as a Specific Regulator of Eye Regeneration. Cell Rep. 2012, 2, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Lapan, S.W.; Reddien, P.W. Dlx and Sp6-9 Control Optic Cup Regeneration in a Prototypic Eye. PLoS Genet. 2011, 7, e1002226. [Google Scholar] [CrossRef]
- He, X.; Lindsay-Mosher, N.; Li, Y.; Molinaro, A.M.; Pellettieri, J.; Pearson, B.J. FOX and ETS Family Transcription Factors Regulate the Pigment Cell Lineage in Planarians. Development 2017, 144, 4550–4551. [Google Scholar] [CrossRef]
- Raz, A.A.; Wurtzel, O.; Reddien, P.W. Planarian Stem Cells Specify Fate yet Retain Potency during the Cell Cycle. Cell Stem Cell 2021, 28, 1307–1322.e5. [Google Scholar] [CrossRef]
- Baguñà, J. The Planarian Neoblast: The Rambling History of Its Origin and Some Current Black Boxes. Int. J. Dev. Biol. 2012, 56, 19–37. [Google Scholar] [CrossRef]
- Reddien, P.W.; Oviedo, N.J.; Jennings, J.R.; Jenkin, J.C.; Sánchez Alvarado, A. SMEDWI-2 Is a PIWI-like Protein That Regulates Planarian Stem Cells. Science 2005, 310, 1327–1330. [Google Scholar] [CrossRef]
- Salvetti, A.; Rossi, L. Planarian Stem Cell Heterogeneity. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1123. [Google Scholar]
- Molinaro, A.M.; Lindsay-Mosher, N.; Pearson, B.J. Identification of TOR-responsive Slow-cycling Neoblasts in Planarians. EMBO Rep. 2021, 22, e50292. [Google Scholar] [CrossRef]
- Mohamed Haroon, M.; Lakshmanan, V.; Sarkar, S.R.; Lei, K.; Vemula, P.K.; Palakodeti, D. Mitochondrial State Determines Functionally Divergent Stem Cell Population in Planaria. Stem Cell Rep. 2021, 16, 1302–1316. [Google Scholar] [CrossRef]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Pirotte, N.; Stevens, A.S.; Fraguas, S.; Plusquin, M.; van Roten, A.; van Belleghem, F.; Paesen, R.; Ameloot, M.; Cebrià, F.; Artois, T.; et al. Reactive Oxygen Species in Planarian Regeneration: An Upstream Necessity for Correct Patterning and Brain Formation. Oxidative Med. Cell. Longev. 2015, 2015, 1–19. [Google Scholar] [CrossRef]
- Wagner, D.E.; Ho, J.J.; Reddien, P.W. Genetic Regulators of a Pluripotent Adult Stem Cell System in Planarians Identified by RNAi and Clonal Analysis. Cell Stem Cell 2012, 10, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Esteban, G.; González-Sastre, A.; Rojo-Laguna, J.I.; Saló, E.; Abril, J.F. Digital Gene Expression Approach over Multiple RNA-Seq Data Sets to Detect Neoblast Transcriptional Changes in Schmidtea Mediterranea. BMC Genom. 2015, 16, 361. [Google Scholar] [CrossRef]
- Eisenhoffer, G.T.; Kang, H.; Alvarado, A.S. Molecular Analysis of Stem Cells and Their Descendants during Cell Turnover and Regeneration in the Planarian Schmidtea Mediterranea. Cell Stem Cell 2008, 3, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Owlarn, S.; Klenner, F.; Schmidt, D.; Rabert, F.; Tomasso, A.; Reuter, H.; Mulaw, M.A.; Moritz, S.; Gentile, L.; Weidinger, G.; et al. Generic Wound Signals Initiate Regeneration in Missing-Tissue Contexts. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jaenen, V.; Fraguas, S.; Bijnens, K.; Heleven, M.; Artois, T.; Romero, R.; Smeets, K.; Cebrià, F. Reactive Oxygen Species Rescue Regeneration after Silencing the MAPK–ERK Signaling Pathway in Schmidtea Mediterranea. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wenemoser, D.; Lapan, S.W.; Wilkinson, A.W.; Bell, G.W.; Reddien, P.W. A Molecular Wound Response Program Associated with Regeneration Initiation in Planarians. Genes Dev. 2012, 26, 988–1002. [Google Scholar] [CrossRef]
- Wenemoser, D.; Reddien, P.W. Planarian Regeneration Involves Distinct Stem Cell Responses to Wounds and Tissue Absence. Dev. Biol. 2010, 344, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Saló, E.; Baguñà, J. Regeneration and Pattern Formation in Planarians. I. The Pattern of Mitosis in Anterior and Posterior Regeneration in Dugesia (G) Tigrina, and a New Proposal for Blastema Formation. J. Embryol. Exp. Morph. 1984, 83, 63–80. [Google Scholar] [PubMed]
- Reddien, P.W. Specialized Progenitors and Regeneration. Developement 2013, 140, 951–957. [Google Scholar] [CrossRef]
- Rossi, L.; Salvetti, A. Planarian Stem Cell Niche, the Challenge for Understanding Tissue Regeneration. Semin. Cell Dev. Biol. 2019, 87, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Forsthoefel, D.J.; James, N.P.; Escobar, D.J.; Stary, J.M.; Vieira, A.P.; Waters, F.A.; Newmark, P.A. An RNAi Screen Reveals Intestinal Regulators of Branching Morphogenesis, Differentiation, and Stem Cell Proliferation in Planarians. Dev. Cell 2012, 23, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Flores, N.M.; Oviedo, N.J.; Sage, J. Essential Role for the Planarian Intestinal GATA Transcription Factor in Stem Cells and Regeneration. Dev. Biol. 2016, 418, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.M.; Nisperos, S.V.; Weeks, J.; Ghulam, M.; Marín, I.; Zayas, R.M. Identification of HECT E3 Ubiquitin Ligase Family Genes Involved in Stem Cell Regulation and Regeneration in Planarians. Dev. Biol. 2015, 404, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Barberán, S.; Fraguas, S.; Cebrià, F. The EGFR Signaling Pathway Controls Gut Progenitor Differentiation during Planarian Regeneration and Homeostasis. Developement 2016, 143, 2089–2102. [Google Scholar] [CrossRef] [PubMed]
- LoCascio, S.A.; Lapan, S.W.; Reddien, P.W. Eye Absence Does Not Regulate Planarian Stem Cells during Eye Regeneration. Dev. Cell 2017, 40, 381–391.e3. [Google Scholar] [CrossRef]
- Atabay, K.D.; LoCascio, S.A.; de Hoog, T.; Reddien, P.W. Self-Organization and Progenitor Targeting Generate Stable Patterns in Planarian Regeneration. Science 2018, 360, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.M.; Petersen, C.P. Positional Information Specifies the Site of Organ Regeneration and Not Tissue Maintenance in Planarians. eLife 2018, 7, e33680. [Google Scholar] [CrossRef]
- Reddien, P.W. Principles of Regeneration Revealed by the Planarian Eye. Curr. Opin. Cell Biol. 2021, 73, 19–25. [Google Scholar] [CrossRef]
- Bohr, T.E.; Shiroor, D.A.; Adler, C.E. Planarian Stem Cells Sense the Identity of the Missing Pharynx to Launch Its Targeted Regeneration. eLife 2021, 10, e68830. [Google Scholar] [CrossRef]
- Adler, C.E.; Sánchez Alvarado, A. PHRED-1 Is a Divergent Neurexin-1 Homolog That Organizes Muscle Fibers and Patterns Organs during Regeneration. Dev. Biol. 2017, 427, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Cebrià, F.; Vispo, M.; Newmark, P.; Bueno, D.; Romero, R. Myocyte Differentiation and Body Wall Muscle Regeneration in the Planarian Giardia Tigrina. Dev. Genes Evol. 1997, 207, 306–316. [Google Scholar] [PubMed]
- Cebrià, F.; Bueno, D.; Reigada, S.; Romero, R. Intercalary Muscle Cell Renewal in Planarian Pharynx. Dev Genes Evol. 1999, 209, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, C.; Watanabe, K.; Agata, K. The Process of Pharynx Regeneration in Planarians. Dev. Biol. 1999, 211, 27–38. [Google Scholar] [CrossRef] [PubMed]
- González-Sastre, A.; de Sousa, N.; Adell, T.; Saló, E. The Pioneer Factor Smed-Gata456-1 Is Required for Gut Cell Differentiation and Maintenance in Planarians. Int. J. Dev. Biol. 2017, 61, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Witchley, J.N.; Mayer, M.; Wagner, D.E.; Owen, J.H.; Reddien, P.W. Muscle Cells Provide Instructions for Planarian Regeneration. Cell Rep. 2013, 4, 633–641. [Google Scholar] [CrossRef]
- Scimone, L.M.; Cote, L.E.; Rogers, T.; Reddien, P.W. Two FGFRL-Wnt Circuits Organize the Planarian Anteroposterior Axis. eLife 2016, 5, e12845. [Google Scholar] [CrossRef]
- Cebrià, F. Planarian Body-Wall Muscle: Regeneration and Function beyond a Simple Skeletal Support. Front. Cell Dev. Biol. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Hill, E.M.; Petersen, C.P. Wnt/Notum Spatial Feedback Inhibition Controls Neoblast Differentiation to Regulate Reversible Growth of the Planarian Brain. Developement 2015, 142, 4217–4229. [Google Scholar] [CrossRef] [PubMed]
- Adell, T.; Salò, E.; Boutos, M.; Bartscherer, K. Smed-Evi/Wntless Is Required for β-Catenin-Dependent and -Independent Processes during Planarian Regeneration. Development 2009, 136, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Cebrià, F.; Kobayashi, C.; Umesono, Y.; Nakazawa, M.; Mineta, K.; Ikeo, K.; Gojobori, T.; Itohk, M.; Tairak, M.; Sánchez Alvarado, A.; et al. FGFR-Related Gene Nou-Darake Restricts Brain Tissues to the Head Region of Planarians. Nature 2002, 419, 620–624. [Google Scholar] [CrossRef]
- Kobayashi, C.; Saito, Y.; Ogawa, K.; Agata, K. Wnt Signaling Is Required for Antero-Posterior Patterning of the Planarian Brain. Dev. Biol. 2007, 306, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Wurtzel, O.; Oderberg, I.M.; Reddien, P.W. Planarian Epidermal Stem Cells Respond to Positional Cues to Promote Cell-Type Diversity. Dev. Cell 2017, 40, 491–504.e5. [Google Scholar] [CrossRef] [PubMed]
- Scimone, M.L.; Atabay, K.D.; Fincher, C.T.; Bonneau, A.R.; Li, D.J.; Reddien, P.W. Muscle and Neuronal Guidepost-like Cells Facilitate Planarian Visual System Regeneration. Science 2020, 368, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Barberán, S.; Martín-Durán, J.M.; Cebrià, F. Evolution of the EGFR Pathway in Metazoa and Its Diversification in the Planarian Schmidtea Mediterranea. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Cote, L.E.; Simental, E.; Reddien, P.W. Muscle Functions as a Connective Tissue and Source of Extracellular Matrix in Planarians. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lindsay-Mosher, N.; Chan, A.; Pearson, B.J. Planarian EGF Repeat-Containing Genes Megf6 and Hemicentin Are Required to Restrict the Stem Cell Compartment. PLoS Genet. 2020, 16, e1008613. [Google Scholar] [CrossRef]
- Dingwall, C.B.; King, R.S. Muscle-Derived Matrix Metalloproteinase Regulates Stem Cell Proliferation in Planarians. Dev. Dyn. 2016, 245, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Abnave, P.; Aboukhatwa, E.; Kosaka, N.; Thompson, J.; Hill, M.A.; Aboobaker, A.A. Epithelial-Mesenchymal Transition Transcription Factors Control Pluripotent Adult Stem Cell Migration in Vivo in Planarians. Developement 2017, 144, 3440–3453. [Google Scholar] [CrossRef]
- Isolani, M.E.; Abril, J.F.; Saló, E.; Deri, P.; Bianucci, A.M.; Batistoni, R. Planarians as a Model to Assess In Vivo the Role of Matrix Metalloproteinase Genes during Homeostasis and Regeneration. PLoS ONE 2013, 8, e55649. [Google Scholar] [CrossRef]
- Van Roten, A.; Barakat, A.Z.; Wouters, A.; Tran, T.A.; Mouton, S.; Noben, J.P.; Gentile, L.; Smeets, K. A Carcinogenic Trigger to Study the Function of Tumor Suppressor Genes in Schmidtea Mediterranea. DMM Dis. Models Mech. 2018, 11, dmm032573. [Google Scholar] [CrossRef]
- Chan, A.; Ma, S.; Pearson, B.J.; Chan, D. Collagen IV Differentially Regulates Planarian Stem Cell Potency and Lineage Progression. Proc. Natl. Acad. Sci. USA 2021, 118, e2021251118. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Shibata, N.; Okumura, R.; Kudome, T.; Nishimura, O.; Tarui, H.; Agata, K. Single-Cell Gene Profiling of Planarian Stem Cells Using Fluorescent Activated Cell Sorting and Its “Index Sorting” Function for Stem Cell Research. Dev. Growth Differ. 2010, 52, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.C.; Cheng, L.-C.; Tk Vu, H.; Lange, J.J.; Mckinney, S.A.; Seidel, C.W.; Sá Nchez Alvarado, A. Egr-5 Is a Post-Mitotic Regulator of Planarian Epidermal Differentiation. eLife 2015, 4, e10501. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.C.; Tu, K.C.; Seidel, C.W.; Robb, S.M.C.; Guo, F.; Sánchez Alvarado, A. Cellular, Ultrastructural and Molecular Analyses of Epidermal Cell Development in the Planarian Schmidtea Mediterranea. Dev. Biol. 2018, 433, 357–373. [Google Scholar] [CrossRef]
- Tran, T.A.; Gentile, L. A Lineage CLOUD for Neoblasts. Semin. Cell Dev. Biol. 2019, 87, 22–29. [Google Scholar] [CrossRef]
- Lei, K.; Thi-Kim Vu, H.; Mohan, R.D.; McKinney, S.A.; Seidel, C.W.; Alexander, R.; Gotting, K.; Workman, J.L.; Sánchez Alvarado, A. Egf Signaling Directs Neoblast Repopulation by Regulating Asymmetric Cell Division in Planarians. Dev. Cell 2016, 38, 413–429. [Google Scholar] [CrossRef]
- Krishna, S.; Palakodeti, D.; Solana, J. Post-Transcriptional Regulation in Planarian Stem Cells. Semin. Cell Dev. Biol. 2019, 87, 69–78. [Google Scholar] [CrossRef]
- Solana, J.; Irimia, M.; Ayoub, S.; Orejuela, M.R.; Zywitza, V.; Jens, M.; Tapial, J.; Ray, D.; Morris, Q.; Hughes, T.R.; et al. Conserved Functional Antagonism of CELF and MBNL Proteins Controls Stem Cell-Specific Alternative Splicing in Planarians. eLife 2016, 5, e16797. [Google Scholar] [CrossRef]
- Schmidt, D.; Reuter, H.; Hüttner, K.; Ruhe, L.; Rabert, F.; Seebeck, F.; Irimia, M.; Solana, J.; Bartscherer, K. The Integrator Complex Regulates Differential SnRNA Processing and Fate of Adult Stem Cells in the Highly Regenerative Planarian Schmidtea Mediterranea. PLoS Genet. 2018, 14, e1007828. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.G.; Currie, K.W.; Pearson, B.J.; Zayas, R.M. Nervous System Development and Regeneration in Freshwater Planarians. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e266. [Google Scholar] [CrossRef] [PubMed]
- Pearson, B.J.; Alvarado, A.S. A Planarian P53 Homolog Regulates Proliferation and Self-Renewal in Adult Stem Cell Lineages. Development 2010, 137, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.J.; Pearson, B.J. Smed-Myb-1 Specifies Early Temporal Identity during Planarian Epidermal Differentiation. Cell Rep. 2018, 25, 38–46.e3. [Google Scholar] [CrossRef]
- Ross, K.G.; Molinaro, A.M.; Romero, C.; Dockter, B.; Cable, K.L.; Gonzalez, K.; Zhang, S.; Collins, E.M.S.; Pearson, B.J.; Zayas, R.M. SoxB1 Activity Regulates Sensory Neuron Regeneration, Maintenance, and Function in Planarians. Dev. Cell 2018, 47, 331–347.e5. [Google Scholar] [CrossRef]
- Cowles, M.W.; Brown, D.D.R.; Nisperos, S.V.; Stanley, B.N.; Pearson, B.J.; Zayas, R.M. Genome-Wide Analysis of the BHLH Gene Family in Planarians Identifies Factors Required for Adult Neurogenesis and Neuronal Regeneration. Developement 2013, 140, 4691–4702. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Galbraith, R.H.; Brubacher, J.L.; Newmark, P.A. A Functional Genomics Screen in Planarians Reveals Regulators of Whole-Brain Regeneration. eLife 2016, 5, e17002. [Google Scholar] [CrossRef] [PubMed]
- Currie, K.W.; Molinaro, A.M.; Pearson, B.J. Neuronal Sources of Hedgehog Modulate Neurogenesis in the Adult Planarian Brain. eLife 2016, 5, e19735. [Google Scholar] [CrossRef] [PubMed]
- März, M.; Seebeck, F.; Bartscherer, K. A Pitx Transcription Factor Controls the Establishment and Maintenance of the Serotonergic Lineage in Planarians. Developement 2013, 140, 4499–4509. [Google Scholar] [CrossRef]
- Currie, K.W.; Pearson, B.J. Transcription Factors Lhx1/5-1 and Pitx Are Required for the Maintenance and Regeneration of Serotonergic Neurons in Planarians. Developement 2013, 140, 3577–3588. [Google Scholar] [CrossRef] [PubMed]
- Cowles, M.W.; Omuro, K.C.; Stanley, B.N.; Quintanilla, C.G.; Zayas, R.M. COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians. PLoS Genet. 2014, 10, e1004746. [Google Scholar] [CrossRef]
- Pineda, D.; Gonzalez, J.; Callaerts, P.; Ikeo, K.; Gehring, W.J.; Salo, E. Searching for the Prototypic Eye Genetic Network: Sine Oculis Is Essential for Eye Regeneration in Planarians. Proc. Natl. Acad. Sci. USA 2000, 97, 4525–4529. [Google Scholar] [CrossRef] [PubMed]
- Mannini, L.; Rossi, L.; Deri, P.; Gremigni, V.; Salvetti, A.; Saló, E.; Batistoni, R. Djeyes Absent (Djeya) Controls Prototypic Planarian Eye Regeneration by Cooperating with the Transcription Factor Djsix-1. Dev. Biol. 2004, 269, 346–359. [Google Scholar] [CrossRef]
- Fraguas, S.; Barberán, S.; Cebrià, F. EGFR Signaling Regulates Cell Proliferation, Differentiation and Morphogenesis during Planarian Regeneration and Homeostasis. Dev. Biol. 2011, 354, 87–101. [Google Scholar] [CrossRef]
- Emili, E.; Pallarès, M.E.; Romero, R.; Cebrià, F. Smed-Egfr-4 Is Required for Planarian Eye Regeneration. Int. J. Dev. Biol. 2019, 63, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Forsthoefel, D.J.; Cejda, N.I.; Khan, U.W.; Newmark, P.A. Cell-Type Diversity and Regionalized Gene Expression in the Planarian Intestine. eLife 2020, 9, e52613. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Han, X.S.; Li, F.F.; Huang, S.; Qin, Y.W.; Zhao, X.X.; Jing, Q. Forkhead Containing Transcription Factor Albino Controls Tetrapyrrole-Based Body Pigmentation in Planarian. Cell Discov. 2016, 2, 1–17. [Google Scholar] [CrossRef]
- Thi-Kim Vu, H.; Rink, J.C.; McKinney, S.A.; McClain, M.; Lakshmanaperumal, N.; Alexander, R.; Sanchez Alvarado, A. Stem Cells and Fluid Flow Drive Cyst Formation in an Invertebrate Excretory Organ. eLife 2015, 4, e07405. [Google Scholar] [CrossRef] [PubMed]
- Rink, J.C.; Vu, H.T.K.; Alvarado, A.S. The Maintenance and Regeneration of the Planarian Excretory System Are Regulated by EGFR Signaling. Development 2011, 138, 3769–3780. [Google Scholar] [CrossRef] [PubMed]
- Forsthoefel, D.J.; Park, A.E.; Newmark, P.A. Stem Cell-Based Growth, Regeneration, and Remodeling of the Planarian Intestine. Dev. Biol. 2011, 356, 445–459. [Google Scholar] [CrossRef]
- Lindsay-Mosher, N.; Pearson, B.J. The True Colours of the Flatworm: Mechanisms of Pigment Biosynthesis and Pigment Cell Lineage Development in Planarians. Semin. Cell Dev. Biol. 2019, 87, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Umesono, Y.; Kuroki, Y.; Agata, K.; Hashimoto, C. Proliferation Maintains the Undifferentiated Status of Stem Cells: The Role of the Planarian Cell Cycle Regulator Cdh1. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wong, L.L.; Bruxvoort, C.G.; Cejda, N.I.; Otero, J.R.; Forsthoefel, D.J. Intestine-Enriched Apolipoprotein b Orthologs Regulate Stem Cell 1 Differentiation and Regeneration in Planarians 2 3. bioRxiv 2021. [Google Scholar] [CrossRef]
- Solana, J.; Gamberi, C.; Mihaylova, Y.; Grosswendt, S.; Chen, C.; Lasko, P.; Rajewsky, N.; Aboobaker, A.A. The CCR4-NOT Complex Mediates Deadenylation and Degradation of Stem Cell MRNAs and Promotes Planarian Stem Cell Differentiation. PLoS Genet. 2013, 9, e1004003. [Google Scholar] [CrossRef]
- Dagan, Y.; Yesharim, Y.; Bonneau, A.R.; Schwartz, S.; Wurtzel, O. M6A Is Required for Resolving Progenitor Identity during 1 Planarian Stem Cell Differentiation. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kim, I.V.; Duncan, E.M.; Ross, E.J.; Gorbovytska, V.; Nowotarski, S.H.; Elliott, S.A.; Alvarado, A.S.; Kuhn, C.-D. Planarians Recruit PiRNAs for MRNA Turnover in Adult Stem Cells. Genes Dev. 2019, 33, 1575–1590. [Google Scholar] [CrossRef]
- Rouhana, L.; Weiss, J.A.; King, R.S.; Newmark, P.A. PIWI Homologs Mediate Histone H4 MRNA Localization to Planarian Chromatoid Bodies. Developement 2014, 141, 2592–2601. [Google Scholar] [CrossRef]
- Kashima, M.; Agata, K.; Shibata, N. Searching for Non-Transposable Targets of Planarian Nuclear PIWI in Pluripotent Stem Cells and Differentiated Cells. Dev. Growth Differ. 2018, 60, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Kashima, M.; Ishiko, T.; Nishimura, O.; Rouhana, L.; Misaki, K.; Yonemura, S.; Saito, K.; Siomi, H.; Siomi, M.C.; et al. Inheritance of a Nuclear PIWI from Pluripotent Stem Cells by Somatic Descendants Ensures Differentiation by Silencing Transposons in Planarian. Dev. Cell 2016, 37, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Kashima, M.; Agata, K.; Shibata, N. What Is the Role of PIWI Family Proteins in Adult Pluripotent Stem Cells? Insights from Asexually Reproducing Animals, Planarians. Dev. Growth Differ. 2020, 62, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Barberán, S.; Cebrià, F. The Role of the EGFR Signaling Pathway in Stem Cell Differentiation during Planarian Regeneration and Homeostasis. Semin. Cell Dev. Biol. 2019, 87, 45–57. [Google Scholar] [CrossRef]
- Deb, S.; Felix, D.A.; Koch, P.; Deb, M.K.; Szafranski, K.; Buder, K.; Sannai, M.; Groth, M.; Kirkpatrick, J.; Pietsch, S.; et al. Tnfaip2/Exoc3-driven Lipid Metabolism Is Essential for Stem Cell Differentiation and Organ Homeostasis. EMBO Rep. 2021, 22, e49328. [Google Scholar] [CrossRef] [PubMed]
- Scimone, M.L.; Meisel, J.; Reddien, P.W. The Mi-2-like Smed-CHD4 Gene Is Required for Stem Cell Differentiation in the Planarian Schmidtea Mediterranea. Development 2010, 137, 1231–1241. [Google Scholar] [CrossRef]
- Strand, N.S.; Allen, J.M.; Zayas, R.M. Post-Translational Regulation of Planarian Regeneration. Semin. Cell Dev. Biol. 2019, 87, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Dattani, A.; Sridhar, D.; Aziz Aboobaker, A. Planarian Flatworms as a New Model System for Understanding the Epigenetic Regulation of Stem Cell Pluripotency and Differentiation. Semin. Cell Dev. Biol. 2019, 87, 79–94. [Google Scholar] [CrossRef]
- Wang, Y.C.; Peterson, S.E.; Loring, J.F. Protein Post-Translational Modifications and Regulation of Pluripotency in Human Stem Cells. Cell Res. 2014, 24, 143–160. [Google Scholar] [CrossRef]
- Avgustinova, A.; Benitah, S.A. Epigenetic Control of Adult Stem Cell Function. Nat. Rev. Mol. Cell Biol. 2016, 17, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Godini, R.; Lafta, H.Y.; Fallahi, H. Epigenetic Modifications in the Embryonic and Induced Pluripotent Stem Cells. Gene Expr. Patterns 2018, 29, 1–9. [Google Scholar] [CrossRef]
- Bannister, A.J.; Falcão, A.M.; Castelo-Branco, G. Histone Modifications and Histone Variants in Pluripotency and Differentiation. In Chromatin Regulation and Dynamics; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Bernstein, B.E.; Mikkelsen, T.S.; Xie, X.; Kamal, M.; Huebert, D.J.; Cuff, J.; Fry, B.; Meissner, A.; Wernig, M.; Plath, K.; et al. A Bivalent Chromatin Structure Marks Key Developmental Genes in Embryonic Stem Cells. Cell 2006, 125, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, A.; Meshorer, E. Chromatin Remodeling and Bivalent Histone Modifications in Embryonic Stem Cells. EMBO Rep. 2015, 16, 1609–1619. [Google Scholar] [CrossRef]
- Stelman, C.R.; Smith, B.M.; Chandra, B.; Roberts-Galbraith, R.H. CBP/P300 Homologs CBP2 and CBP3 Play Distinct Roles in Planarian Stem Cell Function. Dev. Biol. 2021, 473, 130–143. [Google Scholar] [CrossRef]
- Almuedo-Castillo, M.; Crespo, X.; Seebeck, F.; Bartscherer, K.; Salò, E.; Adell, T. JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling. PLoS Genet. 2014, 10, e1004400. [Google Scholar] [CrossRef]
- Tasaki, J.; Shibata, N.; Nishimura, O.; Itomi, K.; Tabata, Y.; Son, F.; Suzuki, N.; Araki, R.; Abe, M.; Agata, K.; et al. ERK Signaling Controls Blastema Cell Differentiation during Planarian Regeneration. Development 2011, 138, 2417–2427. [Google Scholar] [CrossRef]
- Peiris, T.H.; Ramirez, D.; Barghouth, P.G.; Oviedo, N.J. The Akt Signaling Pathway Is Required for Tissue Maintenance and Regeneration in Planarians. BMC Dev. Biol. 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Peiris, T.H.; Weckerle, F.; Ozamoto, E.; Ramirez, D.; Davidian, D.; García-Ojeda, M.E.; Oviedo, N.J. TOR Signaling Regulates Planarian Stem Cells and Controls Localized and Organismal Growth. J. Cell Sci. 2012, 125, 1657–1665. [Google Scholar] [CrossRef]
- González-Estévez, C.; Felix, D.A.; Smith, M.D.; Paps, J.; Morley, S.J.; James, V.; Sharp, T.V.; Aboobaker, A.A. SMG-1 and MTORC1 Act Antagonistically to Regulate Response to Injury and Growth in Planarians. PLoS Genet. 2012, 8, e1002619. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, N.; Rodríguez-Esteban, G.; Rojo-Laguna, J.I.; Saló, E.; Adell, T. Hippo Signaling Controls Cell Cycle and Restricts Cell Plasticity in Planarians. PLoS Biol. 2018, 16, e2002399. [Google Scholar] [CrossRef] [PubMed]
- Thiruvalluvan, M.; Barghouth, P.G.; Tsur, A.; Broday, L.; Oviedo, N.J. SUMOylation Controls Stem Cell Proliferation and Regional Cell Death through Hedgehog Signaling in Planarians. Cell. Mol. Life Sci. 2018, 75, 1285–1301. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Li, Y.Q.; Wang, C.; Han, X.S.; Li, G.; Wang, J.Y.; Li, D.S.; Qin, Y.W.; Shi, Y.; Brewer, G.; et al. Heterochromatin Protein 1 Promotes Self-Renewal and Triggers Regenerative Proliferation in Adult Stem Cells. J. Cell Biol. 2013, 201, 409–425. [Google Scholar] [CrossRef]
- Trost, T.; Haines, J.; Dillon, A.; Mersman, B.; Robbins, M.; Thomas, P.; Hubert, A. Characterizing the Role of SWI/SNF-Related Chromatin Remodeling Complexes in Planarian Regeneration and Stem Cell Function. Stem Cell Res. 2018, 32, 91–103. [Google Scholar] [CrossRef]
- Mihaylova, Y.; Abnave, P.; Kao, D.; Hughes, S.; Lai, A.; Jaber-Hijazi, F.; Kosaka, N.; Aboobaker, A.A. Conservation of Epigenetic Regulation by the MLL3/4 Tumour Suppressor in Planarian Pluripotent Stem Cells. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Duncan, E.M.; Chitsazan, A.D.; Seidel, C.W.; Alvarado, A.S. Set1 and MLL1/2 Target Distinct Sets of Functionally Different Genomic Loci in Vivo. Cell Rep. 2015, 13, 2741–2755. [Google Scholar] [CrossRef] [PubMed]
- Hubert, A.; Henderson, J.M.; Ross, K.G.; Cowles, M.W.; Torres, J.; Zayas, R.M. Epigenetic Regulation of Planarian Stem Cells by the SET1/MLL Family of Histone Methyltransferases. Epigenetics 2013, 8, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Dattani, A.; Kao, D.; Mihaylova, Y.; Abnave, P.; Hughes, S.; Lai, A.; Sahu, S.; Aboobaker, A.A. Epigenetic Analyses of Planarian Stem Cells Demonstrate Conservation of Bivalent Histone Modifications in Animal Stem Cells. Genome Res. 2018, 28, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Fraguas, S.; Cárcel, S.; Vivancos, C.; Molina, M.D.; Ginés, J.; Mazariegos, J.; Sekaran, T.; Bartscherer, K.; Romero, R.; Cebrià, F. CREB-Binding Protein (CBP) Gene Family Regulates Planarian Survival and Stem Cell Differentiation. Dev. Biol. 2021, 476, 53–67. [Google Scholar] [CrossRef]
- Voss, A.K.; Thomas, T. Histone Lysine and Genomic Targets of Histone Acetyltransferases in Mammals. BioEssays 2018, 40. [Google Scholar] [CrossRef]
- Dutto, I.; Scalera, C.; Prosperi, E. CREBBP and P300 Lysine Acetyl Transferases in the DNA Damage Response. Cell. Mol. Life Sci. 2018, 75, 1325–1338. [Google Scholar] [CrossRef]
- Yuan, L.W.; Giordano, A. Acetyltransferase Machinery Conserved in P300/CBP-Family Proteins. Oncogene 2002, 21, 2253–2260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, P.D.; Kahn, M. Kat3 Coactivators in Somatic Stem Cells and Cancer Stem Cells: Biological Roles, Evolution, and Pharmacologic Manipulation. Cell Biol. Toxicol. 2016, 32, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.-L.; Ma, H.; Nguyen, C.; Lam, C.; Kahn, M. Specific Inhibition of CBP-Catenin Interaction Rescues Defects in Neuronal Differentiation Caused by a Presenilin-1 Mutation. Proc. Natl. Acad. Sci. USA 2005, 102, 12171–12176. [Google Scholar] [CrossRef]
- Rebel, V.I.; Kung, A.L.; Tanner, E.A.; Yang, H.; Bronson, R.T.; Livingston, D.M. Distinct Roles for CREB-Binding Protein and P300 in Hematopoietic Stem Cell Self-Renewal. Proc. Natl. Acad. Sci. USA 2002, 99, 14789–14794. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M. Wnt Signaling in Stem Cells and Cancer Stem Cells: A Tale of Two Coactivators. In Progress in Molecular Biology and Translational Science; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 153, pp. 209–244. [Google Scholar]
- Kato, Y.; Shi, Y.; He, X. Neuralization of the Xenopus Embryo by Inhibition of P300/ CREB-Binding Protein Function. J. Neurosci. 1999, 19, 9364–9373. [Google Scholar] [CrossRef]
- Niu, K.; Xu, H.; Xiong, Y.Z.; Zhao, Y.; Gao, C.; Seidel, C.W.; Pan, X.; Ying, Y.; Lei, K. Canonical and Early Lineage-Specific Stem Cell Types Identified in Planarian SirNeoblasts. Cell Regen. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Hall, R.N.; Weill, U.; Khariton, M.; Leal-Ortiz, S.; Drees, L.; Chai, C.; Xue, Y.; Rosental, B.; Quake, S.R.; Alvarado, A.S.; et al. Heterologous Reporter Expression in the Planarian Schmidtea Mediterranea through Somatic MRNA Transfection. bioRxiv 2021. [Google Scholar] [CrossRef]
- García-Castro, H.; Kenny, N.J.; Iglesias, M.; Álvarez-Campos, P.; Mason, V.; Elek, A.; Schönauer, A.; Sleight, V.A.; Neiro, J.; Aboobaker, A.; et al. ACME Dissociation: A Versatile Cell Fixation-Dissociation Method for Single-Cell Transcriptomics. Genome Biol. 2021, 22, 1–34. [Google Scholar] [CrossRef] [PubMed]


| Lineage | Gene Name | In Situ Expression | piwi-1+ or PIWI-1+ Coexpression | RNAi Phenotype | Reference |
|---|---|---|---|---|---|
| Epidermal | zfp-1 | Progenitors | yes | Depletion of epidermal progenitors | [12,37] |
| soxP-3 | Progenitors | n.d. | Reduced early progeny markers | [12,80] | |
| egr-1 | Progenitors | yes | n.d. | [12,37] | |
| p53 | Progenitors and early progeny | yes | Reduced epidermal progeny | [12,80,87] | |
| prog-1 | Early progeny | yes | n.d. | [8,39] | |
| myb-1 | Early and late progeny | yes | Absent early progeny fate | [88] | |
| agat-1 | Late progeny | no | n.d. | [39] | |
| Pax2/5/8 | Late progeny and mature epidermis | n.d. | Reduced early progeny markers | [80] | |
| zpuf-6 | Late progeny and mature epidermis | n.d. | n.d. | [79] | |
| egr-5 | Late progeny | no | Impaired epidermal differentiation | [79] | |
| SoxB1-2 | Neuroectodermal progenitors | yes | Abnormal behavior and movement | [89] | |
| Neural | ston-2 | Progenitors and different neurons | yes | n.d. | [13] |
| elav-2 | Progenitors and differentiated neurons | yes | n.d. | [13] | |
| klf | Progenitors and differentiated neurons | yes | Absent cintillo sensory neurons | [21] | |
| pax3/7 | Progenitors and differentiated neurons | yes | Absent dbh+ neurons | [21] | |
| neuroD-1 | Progenitors and differentiated neurons | yes | n.d. | [21,90] | |
| soxB-2 | Progenitors and differentiated neurons | yes | Reduced neural progenitor expression | [21,91] | |
| arrowhead | Differentiated neurons | n.d. | Defects at the brain commissure | [91] | |
| mblk | Progeny and different neurons | n.d. | Small brain regeneration | [91] | |
| tcf/lef-1 | Progenitors and differentiated neurons | yes | Reduced dorsolateral GABA neurons | [21] | |
| nkx2.2 | Progenitors and differentiated neurons | yes | Reduced ventromedial neurons | [21] | |
| arx | Differentiated neurons | yes | Reduced ventromedial neurons | [92] | |
| Pitx | Serotonergic and other neurons | yes | Absence of serotonergic neurons | [93,94] | |
| lhx1/5 | Serotonergic and other neurons | yes | Absence of serotonergic neurons | [21,93,94] | |
| hesl-3 | Progenitors and differentiated neurons | yes | Defects in CNS pattern and organization | [90] | |
| sim | Progenitors and differentiated neurons | yes | Defects in CNS regeneration | [21,90] | |
| coe | Progenitors and differentiated neurons | yes | Defects in CNS size and organization | [90,95] | |
| ap-2 | Progenitors and differentiated neurons | yes | Reduced TrpA-expressing neurons | [21,42] | |
| runt-1 | Neoblasts at wounds | yes | Perturbed ap-2+; sp6-9+ expression and neural differentiation | [42] | |
| Eyes | ovo | Progenitor and differentiated eye cells | yes | Lack of eye cells | [26] |
| six1/2 | Progenitor and differentiated eye cells | n.d. | Lack of eye cells | [27,42,96,97] | |
| eya | Progenitor and differentiated eye cells | yes | Lack of eye cells | [26,27,42,97] | |
| sp6/9 | Progenitor and differentiated PC cells | yes | Lack of PC cells | [27,42] | |
| dlx | Progenitor and differentiated PC cells | yes | Lack of PC cells | [27,42] | |
| otxA | Progenitor and differentiated PH cells | yes | Lack of PH cells | [21,26,27] | |
| meis | Progenitor and differentiated PH cells | yes | Disorganized eye regeneration | [21,26] | |
| klf | Progenitor and differentiated PH cells | yes | Disorganized eye regeneration | [21,26] | |
| foxQ2 | Progenitor and differentiated PH cells | yes | Disorganized eye regeneration | [21,26] | |
| soxB | Progenitor and differentiated PH cells | yes | Small eyes and lack anterior PH cells | [21,26] | |
| egfr-1 | Differentiated PC cells | n.d. | Reduced number PC cells | [98] | |
| egr-4 | Differentiated PH cells | n.d. | Less differentiated and more eye progenitors | [99] | |
| Intestinal | gata4/5/6-1 | Progenitors and differentiated gut cells | yes | Impaired differentiated gut progenitors | [5,12,48,60] |
| nkx2-2 | Progenitors and differentiated gut cells | n.d. | Impaired gut regeneration and lysis | [12,47] | |
| hnf4 | Progenitors and differentiated gut cells | yes | Reduced expression gut markers | [5,12,21] | |
| egfr1/nrg-1 | Gut cells | yes | Impaired gut progenitor differentiation | [50] | |
| rreb2 | Several gut cell types | n.d. | Impaired goblet cell regeneration | [100] | |
| gli-1 | Several gut cell types | n.d. | Impaired goblet cell regeneration | [100] | |
| Pharyngeal | egfr-1 | Pharynx and others | n.d. | Aberrant pharynx regeneration | [98] |
| FoxA | Progenit. and differentiated pharynx cells | yes | Impaired pharynx regeneration | [21,23] | |
| Muscular | nkx1-1 | Progenitors and BWM | yes | Lack of circular fibers and bifurcated AP axis and fused heads | [24,25] |
| myoD | Progenitors and BWM | yes | Lack of longitudinal fibers and defects in regeneration initiation | [24,25] | |
| foxF-1 | Progenitors and non-BWM(DVM, DVL, IM, PM) | yes | Depigmentation, lack of non-BWM and ML defects | [24] | |
| nk4 | Progenitors and DVL cells | yes | Reduced DVL markers and ML defects | [24] | |
| gata4/5/6-2 | Progenitors and DVM cells | yes | Reduced DVM number and ML defects | [24] | |
| gata4/5/6-3 | Progenitors and IM and PM cells | yes | Reduced number of IM cells | [24] | |
| Pigment | ets-1 | Progenitors and differentiated cells | yes | Depigmentation and reduce markers | [9,28] |
| foxF-1 | Progenitors and differentiated cells | yes | Depigmentation and lack of markers | [9,28] | |
| fgfrL-1 | Differentiated pigment cells | n.d. | Depigmentation and reduced punctated marker | [28] | |
| albino | Progenitors and differentiated cells | yes | Depigmentation and lack markers | [28,101] | |
| Excretory | Six1/2 | Progenitors and tubule cells | yes | Impaired protonephridia regeneration | [22] |
| Pou2/3 | Progenitors and tubule-related cells | yes | Impaired protonephridia regeneration | [22,102] | |
| hunchback | n.d. | n.d. | Impaired protonephridia regeneration | [22] | |
| eya | Progenitors and differentiated cells | yes | Impaired protonephridia regeneration | [22] | |
| osr | Tubule cells | yes | n.d. | [22] | |
| SalI | Progenitors and tubule cells | yes | Edemas and decreased tubule cell number | [22] | |
| Egfr-5 | Flame cells | n.d | Absence of flame cells and protonephridia disorganization | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, M.D.; Cebrià, F. Decoding Stem Cells: An Overview on Planarian Stem Cell Heterogeneity and Lineage Progression. Biomolecules 2021, 11, 1532. https://doi.org/10.3390/biom11101532
Molina MD, Cebrià F. Decoding Stem Cells: An Overview on Planarian Stem Cell Heterogeneity and Lineage Progression. Biomolecules. 2021; 11(10):1532. https://doi.org/10.3390/biom11101532
Chicago/Turabian StyleMolina, M. Dolores, and Francesc Cebrià. 2021. "Decoding Stem Cells: An Overview on Planarian Stem Cell Heterogeneity and Lineage Progression" Biomolecules 11, no. 10: 1532. https://doi.org/10.3390/biom11101532
APA StyleMolina, M. D., & Cebrià, F. (2021). Decoding Stem Cells: An Overview on Planarian Stem Cell Heterogeneity and Lineage Progression. Biomolecules, 11(10), 1532. https://doi.org/10.3390/biom11101532






