Diversity in Sensing and Signaling of Bacterial Sensor Histidine Kinases
Abstract
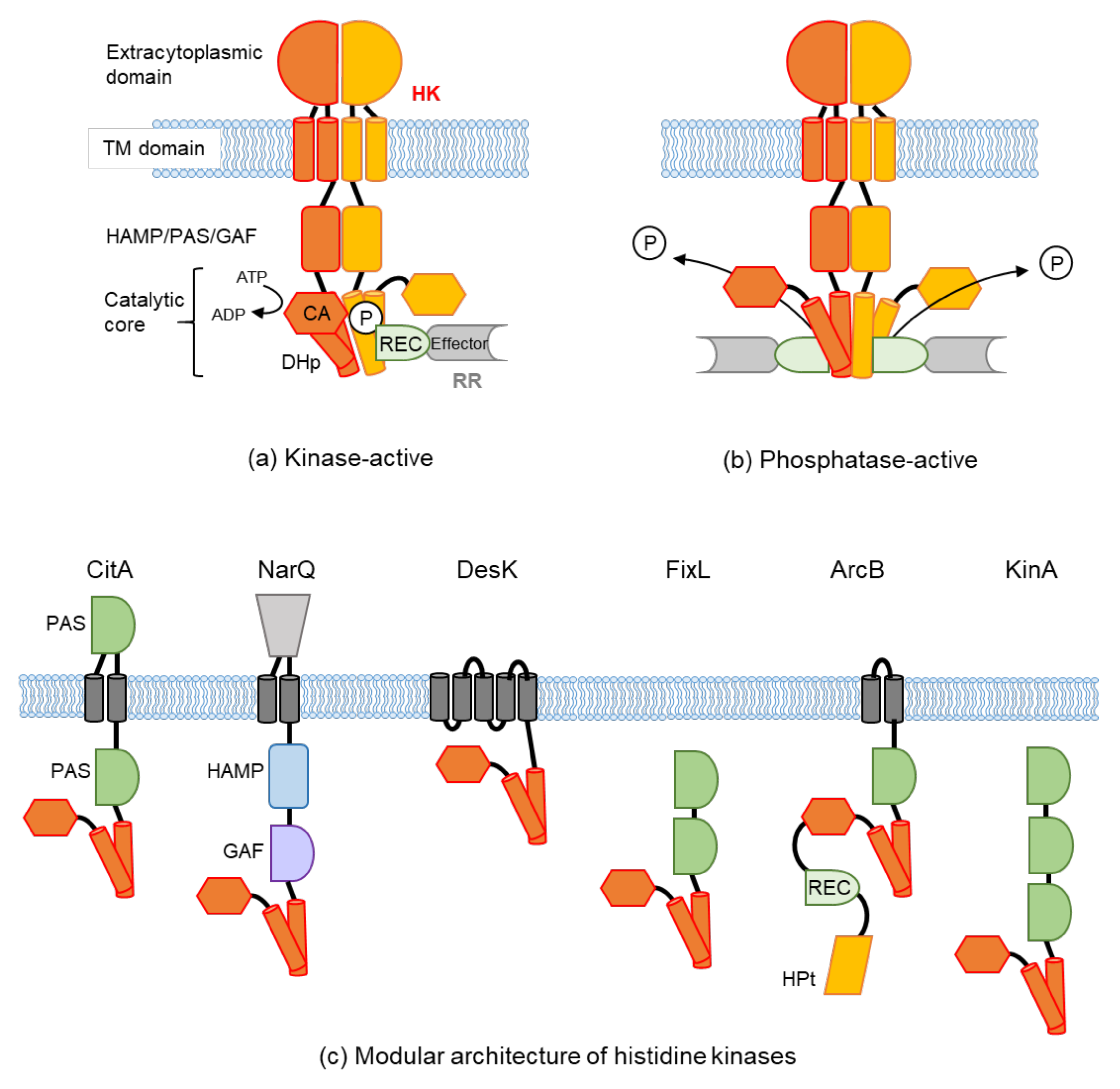
1. Introduction
2. Extracellular Sensing
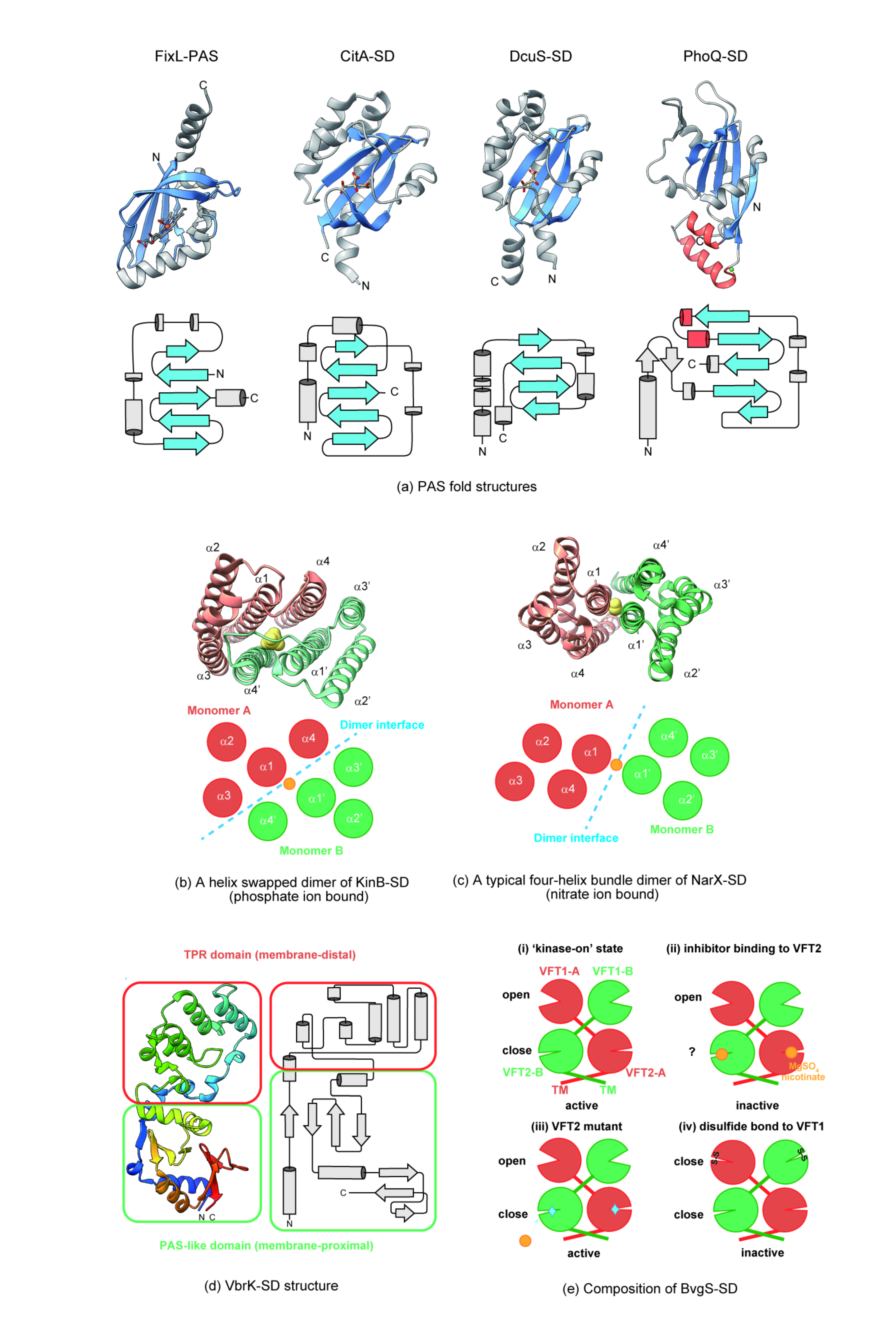
2.1. Structural Features of Periplasm Sensors and Ligand Binding
2.1.1. Sensing by a PAS-Like Domain
| Sensor | PDB | Fold Type | Ligand * | Organism | Ref. |
|---|---|---|---|---|---|
| PDC structures | |||||
| CitA | 2V9A, 5FQ1, 1P0Z, 2J80 | PDC | citrate | K. pneumoniae Geobacillus thermodenitrificans | [22,32,33] |
| DcuS | 1OJG | PDC | malate, oxygen | E. coli | [29] |
| PhoQ | 3BQ8, 1YAX, 6A8V | PDC | Ni2+, Mg2+, Ca2+, SafA | E. coli Salmonella typhimurium | [27,28,34] |
| PhoR | 3CWF | PDC | Bacillus subtilis | [35] | |
| EnvZ | 5XGA | PDC | CHAPS, MzrA | E. coli | [36,37] |
| CusS | 5KU5 | PDC | Ag(I) | E. coli | [38] |
| BaeS | 5WVN 5WVM | PDC | indole | E. coli | [39] |
| DctB | 3E4O, 3E4Q, 3E4P, 3BY9 | double PDC | succinate, Ca2+, oxygen | Sinorhizobium meliloti V. cholerae | [29,40] |
| mmHK1S-Z2 | 3LI9, 3LIA, 3LI8 | double PDC | bistris | Methanosarcina mazei | [41] |
| mmHK1S-Z3 | 3LIB | double PDC | M. mazei | [41] | |
| soHK1S-Z6 | 3LIC | double PDC | ethylene glycol | Shewanella oneidensis | [41] |
| vpHK1S-Z8 | 3LIE 3LID | double PDC | phosphate | V. parahaemolyticus | [41] |
| rpHK1S-Z16 | 3LIF | double PDC | 2 methyl-2,4- pentanediolMPD | Rhodopseudomonas palustris | [41] |
| HptSA | 6LKG, 6LKI | double PDC | G6P | S. aureus | [42] |
| LuxQP | 2HJE, 2HJ9 | double PDC | AI-2 | Vibrio harveyi | [32,43] |
| KinD | 4JGP, 4JGO, 4JGQ,4JGR | double PDC | pyruvate | B. subtilis | [31] |
| All helical structures | |||||
| NarX | 3EZH | all α-helix | nitrate ion | E. coli | [44] |
| NarQ | 5IJI, 5JGP, 5JEF, 5JEQ, 6XYN,6YUE | all α-helix | nitrate ion | E. coli | [17,45,46,47] |
| TorS | 3I9W, 3I9Y, 3O1J, 3O1I, 3OIH | all α-helix | TorT, TMAO | E. coli | [48,49] |
| KinB | 3KKB, 3L34, 4LLE, 4LLC | all α-helix | phosphate ion | P. aeruginosa | [50] |
| Adeh_2942 | 4K0D | all α-helix | acetate | Anaeromyxobacter dehalogenans | [51] |
| Other structures | |||||
| BvgS | 4Q0C, 3MPL, 3MPK | double VFT | nicotine | B. pertussis | [52,53] |
| BT4663 | 4A2M, 4A2L | Y_Y_Y domain | Bacteroides thetaiotaomicron | [54] | |
| VbrK | 7CUS, 7CJR | PAS and TPR-like | β-lactam antibiotics | V. parahaemolyticus | [55,56] |
| VxrA | 7LA6, 7KB7, 7KB3, 7KB9 | PAS and TPR-like | β-lactam antibiotics | V. cholerae | [57] |
2.1.2. Sensing by an All α-Helix Type Structure
2.1.3. Sensing by Other Structures
2.2. How the Signals Are Transduced from Periplasmic Sensors to the Cytoplasmic Domain
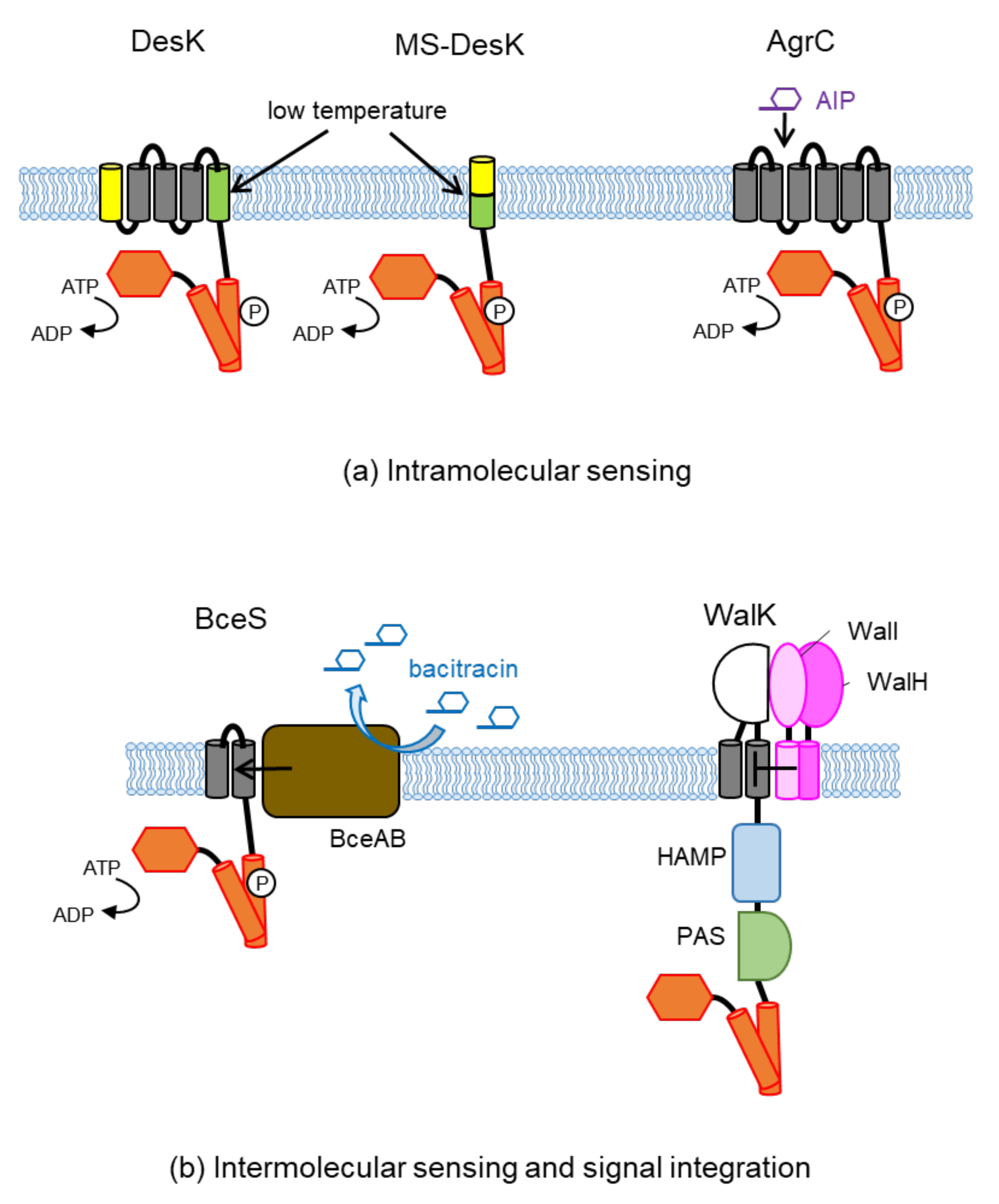
3. Transmembrane Sensing and Control
3.1. Intramolecular Sensing
3.2. Intermolecular Signal Transfer
3.3. Intramolecular Signal Integration
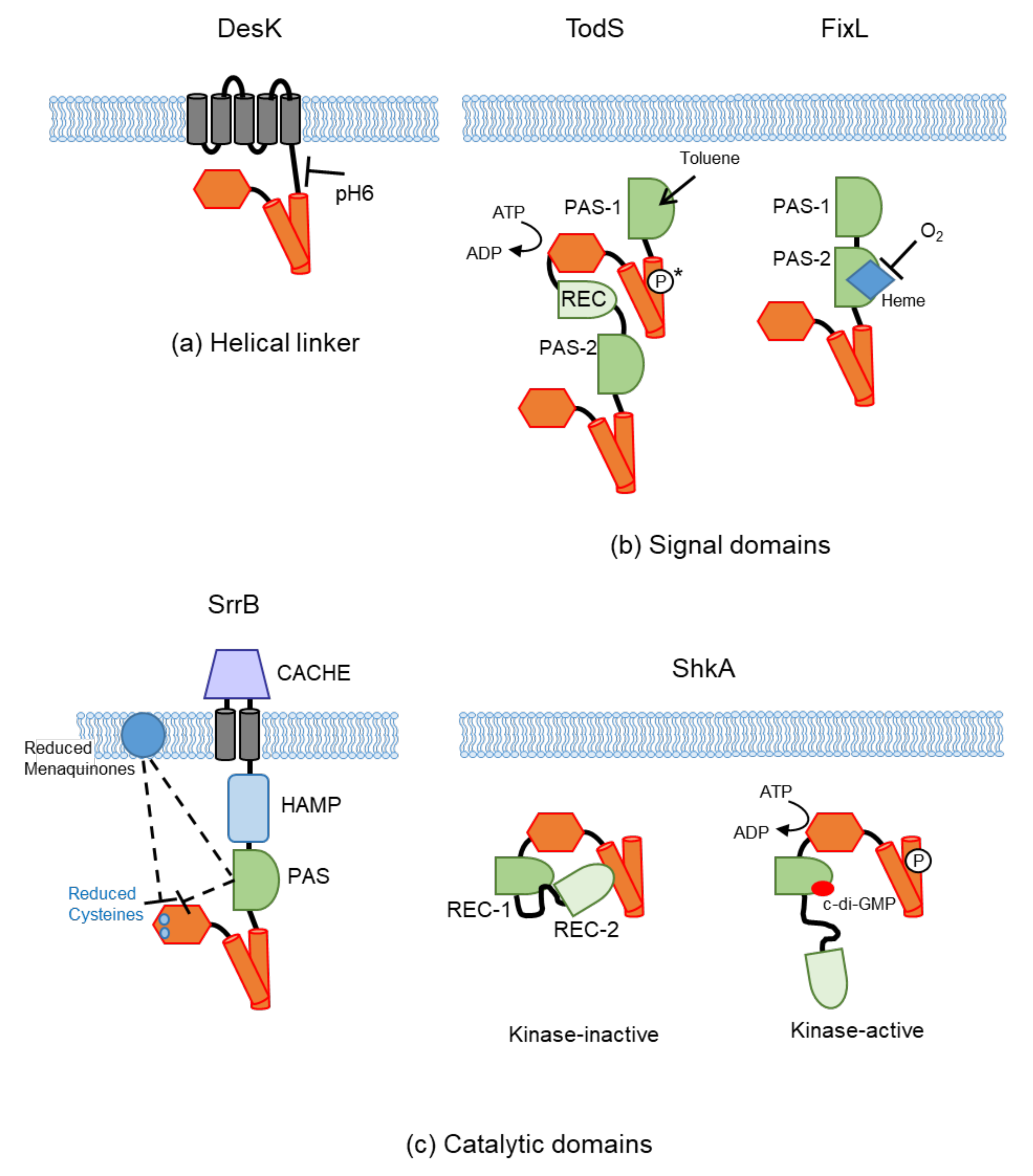
4. Cytoplasmic Sensing
4.1. Sensing at the Helical Linker
4.2. Sensing at the Intracellular Signal Domains (PAS and GAF)
4.2.1. Ligand Binding
4.2.2. Cofactor Binding
4.2.3. Others
4.3. Sensing at the Catalytic Domain
4.3.1. Protein Binding
4.3.2. Second Messengers
4.3.3. Others
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-Component Signal Transduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Phyletic Distribution and Lineage-Specific Domain Architectures of Archaeal Two-Component Signal Transduction Systems. J. Bacteriol. 2018, 200, e00681-17. [Google Scholar] [CrossRef]
- Stephenson, K.; Hoch, J.A. Two-Component and Phosphorelay Signal-Transduction Systems as Therapeutic Targets. Curr. Opin. Pharmacol. 2002, 2, 507–512. [Google Scholar] [CrossRef]
- Gotoh, Y.; Eguchi, Y.; Watanabe, T.; Okamoto, S.; Doi, A.; Utsumi, R. Two-Component Signal Transduction as Potential Drug Targets in Pathogenic Bacteria. Curr. Opin. Microbiol. 2010, 13, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Jamal, S.B.; Hassan, S.S.; Carvalho, P.V.S.D.; Almeida, S.; Barh, D.; Ghosh, P.; Silva, A.; Castro, T.L.P.; Azevedo, V. Two-Component Signal Transduction Systems of Pathogenic Bacteria As Targets for Antimicrobial Therapy: An Overview. Front. Microbiol. 2017, 8, 1878. [Google Scholar] [CrossRef] [PubMed]
- Bem, A.E.; Velikova, N.; Pellicer, M.T.; van Baarlen, P.; Marina, A.; Wells, J.M. Bacterial Histidine Kinases as Novel Antibacterial Drug Targets. ACS Chem. Biol. 2015, 10, 213–224. [Google Scholar] [CrossRef]
- Ortet, P.; Whitworth, D.E.; Santaella, C.; Achouak, W.; Barakat, M. P2CS: Updates of the Prokaryotic Two-Component Systems Database. Nucleic. Acids Res. 2015, 43, D536–D541. [Google Scholar] [CrossRef]
- Gumerov, V.M.; Ortega, D.R.; Adebali, O.; Ulrich, L.E.; Zhulin, I.B. MiST 3.0: An Updated Microbial Signal Transduction Database with an Emphasis on Chemosensory Systems. Nucleic. Acids Res. 2020, 48, D459–D464. [Google Scholar] [CrossRef]
- Cock, P.J.A.; Whitworth, D.E. Evolution of Prokaryotic Two-Component System Signaling Pathways: Gene Fusions and Fissions. Mol. Biol. Evol. 2007, 24, 2355–2357. [Google Scholar] [CrossRef]
- Zschiedrich, C.P.; Keidel, V.; Szurmant, H. Molecular Mechanisms of Two-Component Signal Transduction. J. Mol. Biol. 2016, 428, 3752–3775. [Google Scholar] [CrossRef]
- Bhate, M.P.; Molnar, K.S.; Goulian, M.; DeGrado, W.F. Signal Transduction in Histidine Kinases: Insights from New Structures. Structure 2015, 23, 981–994. [Google Scholar] [CrossRef]
- Abriata, L.A.; Albanesi, D.; Dal Peraro, M.; de Mendoza, D. Signal Sensing and Transduction by Histidine Kinases as Unveiled through Studies on a Temperature Sensor. Acc. Chem. Res. 2017, 50, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Gushchin, I.; Gordeliy, V. Transmembrane Signal Transduction in Two-Component Systems: Piston, Scissoring, or Helical Rotation? BioEssays 2018, 40, 1700197. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Dubuisson, F.; Mechaly, A.; Betton, J.-M.; Antoine, R. Structural Insights into the Signalling Mechanisms of Two-Component Systems. Nat. Rev. Microbiol. 2018, 16, 585–593. [Google Scholar] [CrossRef]
- Buschiazzo, A.; Trajtenberg, F. Two-Component Sensing and Regulation: How Do Histidine Kinases Talk with Response Regulators at the Molecular Level? Annu. Rev. Microbiol. 2019, 73, 507–528. [Google Scholar] [CrossRef]
- Möglich, A.; Ayers, R.A.; Moffat, K. Structure and Signaling Mechanism of Per-ARNT-Sim Domains. Structure 2009, 17, 1282–1294. [Google Scholar] [CrossRef]
- Gushchin, I.; Melnikov, I.; Polovinkin, V.; Ishchenko, A.; Yuzhakova, A.; Buslaev, P.; Bourenkov, G.; Grudinin, S.; Round, E.; Balandin, T.; et al. Mechanism of Transmembrane Signaling by Sensor Histidine Kinases. Science 2017, 356. [Google Scholar] [CrossRef]
- Mascher, T.; Helmann, J.D.; Unden, G. Stimulus Perception in Bacterial Signal-Transducing Histidine Kinases. Microbiol. Mol. Biol. Rev. 2006, 70, 910–938. [Google Scholar] [CrossRef] [PubMed]
- Krell, T.; Lacal, J.; Busch, A.; Silva-Jiménez, H.; Guazzaroni, M.-E.; Ramos, J.L. Bacterial Sensor Kinases: Diversity in the Recognition of Environmental Signals. Annu. Rev. Microbiol. 2010, 64, 539–559. [Google Scholar] [CrossRef]
- Szurmant, H.; Hoch, J.A. Interaction Fidelity in Two-Component Signaling. Curr. Opin. Microbiol. 2010, 13, 190–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheung, J.; Hendrickson, W.A. Sensor Domains of Two-Component Regulatory Systems. Curr. Opin. Microbiol. 2010, 13, 116–123. [Google Scholar] [CrossRef]
- Reinelt, S.; Hofmann, E.; Gerharz, T.; Bott, M.; Madden, D.R. The Structure of the Periplasmic Ligand-Binding Domain of the Sensor Kinase CitA Reveals the First Extracellular PAS Domain*. J. Biol. Chem. 2003, 278, 39189–39196. [Google Scholar] [CrossRef]
- Pappalardo, L.; Janausch, I.G.; Vijayan, V.; Zientz, E.; Junker, J.; Peti, W.; Zweckstetter, M.; Unden, G.; Griesinger, C. The NMR Structure of the Sensory Domain of the Membranous Two-Component Fumarate Sensor (Histidine Protein Kinase) DcuS of Escherichia Coli *. J. Biol. Chem. 2003, 278, 39185–39188. [Google Scholar] [CrossRef]
- Gong, W.; Hao, B.; Chan, M.K. New Mechanistic Insights from Structural Studies of the Oxygen-Sensing Domain of Bradyrhizobium Japonicum FixL. Biochemistry 2000, 39, 3955–3962. [Google Scholar] [CrossRef]
- Taylor, B.L.; Zhulin, I.B. PAS Domains: Internal Sensors of Oxygen, Redox Potential, and Light. Microbiol. Mol. Biol. Rev. 1999, 63, 479–506. [Google Scholar] [CrossRef]
- Khorchid, A.; Ikura, M. Bacterial Histidine Kinase as Signal Sensor and Transducer. Int. J. Biochem. Cell Biol. 2006, 38, 307–312. [Google Scholar] [CrossRef]
- Cho, U.S.; Bader, M.W.; Amaya, M.F.; Daley, M.E.; Klevit, R.E.; Miller, S.I.; Xu, W. Metal Bridges between the PhoQ Sensor Domain and the Membrane Regulate Transmembrane Signaling. J. Mol. Biol. 2006, 356, 1193–1206. [Google Scholar] [CrossRef]
- Cheung, J.; Bingman, C.A.; Reyngold, M.; Hendrickson, W.A.; Waldburger, C.D. Crystal Structure of a Functional Dimer of the PhoQ Sensor Domain. J. Biol. Chem. 2008, 283, 13762–13770. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Hendrickson, W.A. Crystal Structures of C4-Dicarboxylate Ligand Complexes with Sensor Domains of Histidine Kinases DcuS and DctB *. J. Biol. Chem. 2008, 283, 30256–30265. [Google Scholar] [CrossRef]
- Sevvana, M.; Vijayan, V.; Zweckstetter, M.; Reinelt, S.; Madden, D.R.; Herbst-Irmer, R.; Sheldrick, G.M.; Bott, M.; Griesinger, C.; Becker, S. A Ligand-Induced Switch in the Periplasmic Domain of Sensor Histidine Kinase CitA. J. Mol. Biol. 2008, 377, 512–523. [Google Scholar] [CrossRef]
- Wu, R.; Gu, M.; Wilton, R.; Babnigg, G.; Kim, Y.; Pokkuluri, P.R.; Szurmant, H.; Joachimiak, A.; Schiffer, M. Insight into the Sporulation Phosphorelay: Crystal Structure of the Sensor Domain of Bacillus Subtilis Histidine Kinase, KinD. Protein Sci. 2013, 22, 564–576. [Google Scholar] [CrossRef]
- Neiditch, M.B.; Federle, M.J.; Pompeani, A.J.; Kelly, R.C.; Swem, D.L.; Jeffrey, P.D.; Bassler, B.L.; Hughson, F.M. Ligand-Induced Asymmetry in Histidine Sensor Kinase Complex Regulates Quorum Sensing. Cell 2006, 126, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Weisenburger, S.; Boening, D.; Schomburg, B.; Giller, K.; Becker, S.; Griesinger, C.; Sandoghdar, V. Cryogenic Optical Localization Provides 3D Protein Structure Data with Angstrom Resolution. Nat. Methods. 2017, 14, 141–144. [Google Scholar] [CrossRef]
- Yoshitani, K.; Ishii, E.; Taniguchi, K.; Sugimoto, H.; Shiro, Y.; Akiyama, Y.; Kato, A.; Utsumi, R.; Eguchi, Y. Identification of an Internal Cavity in the PhoQ Sensor Domain for PhoQ Activity and SafA-Mediated Control. Biosci. Biotechnol. Biochem. 2019, 83, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Tesar, C.; Gu, M.; Babnigg, G.; Joachimiak, A.; Pokkuluri, P.R.; Szurmant, H.; Schiffer, M. Extracytoplasmic PAS-Like Domains Are Common in Signal Transduction Proteins. J. Bacteriol. 2010, 192, 1156–1159. [Google Scholar] [CrossRef]
- Hwang, E.; Cheong, H.-K.; Kim, S.-Y.; Kwon, O.; Blain, K.Y.; Choe, S.; Yeo, K.J.; Jung, Y.W.; Jeon, Y.H.; Cheong, C. Crystal Structure of the EnvZ Periplasmic Domain with CHAPS. FEBS Lett. 2017, 591, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Gerken, H.; Misra, R. MzrA-EnvZ Interactions in the Periplasm Influence the EnvZ/OmpR Two-Component Regulon. J. Bacteriol. 2010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Affandi, T.; Issaian, A.V.; McEvoy, M.M. The Structure of the Periplasmic Sensor Domain of the Histidine Kinase CusS Shows Unusual Metal Ion Coordination at the Dimeric Interface. Biochemistry 2016, 55, 5296–5306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiu, S.; Jia, S.; Xu, D.; Ran, T.; Wang, W. Crystal Structure of the Sensor Domain of BaeS from Serratia Marcescens FS14. Proteins Struct. Funct. Bioinform. 2017, 85, 1784–1790. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Nan, B.; Nan, J.; Ma, Q.; Panjikar, S.; Liang, Y.-H.; Wang, Y.; Su, X.-D. C4-Dicarboxylates Sensing Mechanism Revealed by the Crystal Structures of DctB Sensor Domain. J. Mol. Biol. 2008, 383, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hendrickson, W.A. Structural Characterization of the Predominant Family of Histidine Kinase Sensor Domains. J. Mol. Biol. 2010, 400, 335–353. [Google Scholar] [CrossRef]
- Wang, M.; Guo, Q.; Zhu, K.; Fang, B.; Yang, Y.; Teng, M.; Li, X.; Tao, Y. Interface Switch Mediates Signal Transmission in a Two-Component System. Proc. Natl. Acad. Sci. USA 2020, 117, 30433–30440. [Google Scholar] [CrossRef] [PubMed]
- Neiditch, M.B.; Federle, M.J.; Miller, S.T.; Bassler, B.L.; Hughson, F.M. Regulation of LuxPQ Receptor Activity by the Quorum-Sensing Signal Autoinducer-2. Mol. Cell 2005, 18, 507–518. [Google Scholar] [CrossRef]
- Cheung, J.; Hendrickson, W.A. Structural Analysis of Ligand Stimulation of the Histidine Kinase NarX. Structure 2009, 17, 190–201. [Google Scholar] [CrossRef]
- Gushchin, I.; Orekhov, P.; Melnikov, I.; Polovinkin, V.; Yuzhakova, A.; Gordeliy, V. Sensor Histidine Kinase NarQ Activates via Helical Rotation, Diagonal Scissoring, and Eventually Piston-Like Shifts. Int. J. Mol. Sci. 2020, 21, 3110. [Google Scholar] [CrossRef]
- Gushchin, I.; Melnikov, I.; Polovinkin, V.; Ishchenko, A.; Gordeliy, V. Crystal Structure of a Proteolytic Fragment of the Sensor Histidine Kinase NarQ. Crystals 2020, 10, 149. [Google Scholar] [CrossRef]
- Melnikov, I.; Polovinkin, V.; Kovalev, K.; Gushchin, I.; Shevtsov, M.; Shevchenko, V.; Mishin, A.; Alekseev, A.; Rodriguez-Valera, F.; Borshchevskiy, V.; et al. Fast Iodide-SAD Phasing for High-Throughput Membrane Protein Structure Determination. Sci. Adv. 2017. [Google Scholar] [CrossRef]
- Moore, J.O.; Hendrickson, W.A. Structural Analysis of Sensor Domains from the TMAO-Responsive Histidine Kinase Receptor TorS. Structure 2009, 17, 1195–1204. [Google Scholar] [CrossRef]
- Moore, J.O.; Hendrickson, W.A. An Asymmetry-to-Symmetry Switch in Signal Transmission by the Histidine Kinase Receptor for TMAO. Structure 2012, 20, 729–741. [Google Scholar] [CrossRef]
- Tan, K.; Chhor, G.; Binkowski, T.A.; Jedrzejczak, R.P.; Makowska-Grzyska, M.; Joachimiak, A. Sensor Domain of Histidine Kinase KinB of Pseudomonas: A HELIX-SWAPPED DIMER*. J. Biol. Chem. 2014, 289, 12232–12244. [Google Scholar] [CrossRef]
- Pokkuluri, P.R.; Dwulit-Smith, J.; Duke, N.E.; Wilton, R.; Mack, J.C.; Bearden, J.; Rakowski, E.; Babnigg, G.; Szurmant, H.; Joachimiak, A.; et al. Analysis of Periplasmic Sensor Domains from Anaeromyxobacter Dehalogenans 2CP-C: Structure of One Sensor Domain from a Histidine Kinase and Another from a Chemotaxis Protein. MicrobiologyOpen 2013, 2, 766–777. [Google Scholar] [CrossRef]
- Herrou, J.; Bompard, C.; Wintjens, R.; Dupré, E.; Willery, E.; Villeret, V.; Locht, C.; Antoine, R.; Jacob-Dubuisson, F. Periplasmic Domain of the Sensor-Kinase BvgS Reveals a New Paradigm for the Venus Flytrap Mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 17351–17355. [Google Scholar] [CrossRef] [PubMed]
- Dupré, E.; Herrou, J.; Lensink, M.F.; Wintjens, R.; Vagin, A.; Lebedev, A.; Crosson, S.; Villeret, V.; Locht, C.; Antoine, R.; et al. Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS. PLOS Pathog. 2015, 11, e1004700. [Google Scholar] [CrossRef] [PubMed]
- Lowe, E.C.; Baslé, A.; Czjzek, M.; Firbank, S.J.; Bolam, D.N. A Scissor Blade-like Closing Mechanism Implicated in Transmembrane Signaling in a Bacteroides Hybrid Two-Component System. Proc. Natl. Acad. Sci. USA 2012, 109, 7298–7303. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yoon, S. Structural Analysis of the Sensor Domain of the β-Lactam Antibiotic Receptor VbrK from Vibrio Parahaemolyticus. Biochem. Biophys. Res. Commun. 2020, 533, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.C.; Chua, Y.K.; Qian, X.; Lin, J.; Savko, M.; Dedon, P.C.; Lescar, J. Crystal Structure of the Periplasmic Sensor Domain of Histidine Kinase VbrK Suggests Indirect Sensing of β-Lactam Antibiotics. J. Struct. Biol. 2020, 212, 107610. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Teschler, J.K.; Wu, R.; Jedrzejczak, R.P.; Zhou, M.; Shuvalova, L.A.; Endres, M.J.; Welk, L.F.; Kwon, K.; Anderson, W.F.; et al. Sensor Domain of Histidine Kinase VxrA of Vibrio Cholerae: Hairpin-Swapped Dimer and Its Conformational Change. J. Bacteriol. 2021, 203, e00643-20. [Google Scholar] [CrossRef] [PubMed]
- Groisman, E.A.; Duprey, A.; Choi, J. How the PhoP/PhoQ System Controls Virulence and Mg2+ Homeostasis: Lessons in Signal Transduction, Pathogenesis, Physiology, and Evolution. Microbiol. Mol. Biol. Rev. 2021, 85, e00176-20. [Google Scholar] [CrossRef]
- Bader, M.W.; Sanowar, S.; Daley, M.E.; Schneider, A.R.; Cho, U.; Xu, W.; Klevit, R.E.; Le Moual, H.; Miller, S.I. Recognition of Antimicrobial Peptides by a Bacterial Sensor Kinase. Cell 2005, 122, 461–472. [Google Scholar] [CrossRef]
- Molnar, K.S.; Bonomi, M.; Pellarin, R.; Clinthorne, G.D.; Gonzalez, G.; Goldberg, S.D.; Goulian, M.; Sali, A.; DeGrado, W.F. Cys-Scanning Disulfide Crosslinking and Bayesian Modeling Probe the Transmembrane Signaling Mechanism of the Histidine Kinase, PhoQ. Structure 2014, 22, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Itou, J.; Yamane, M.; Demizu, R.; Yamato, F.; Okada, A.; Mori, H.; Kato, A.; Utsumi, R. B1500, a Small Membrane Protein, Connects the Two-Component Systems EvgS/EvgA and PhoQ/PhoP in Escherichia Coli. Proc. Natl. Acad. Sci. USA 2007, 104, 18712–18717. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Ishii, E.; Yamane, M.; Utsumi, R. The Connector SafA Interacts with the Multi-Sensing Domain of PhoQ in Escherichia Coli. Mol. Microbiol. 2012, 85, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Ishii, E.; Eguchi, Y.; Utsumi, R. Mechanism of Activation of PhoQ/PhoP Two-Component Signal Transduction by SafA, an Auxiliary Protein of PhoQ Histidine Kinase in Escherichia Coli. Biosci. Biotechnol. Biochem. 2013, 77, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Waldburger, C.D.; Sauer, R.T. Signal Detection by the PhoQ Sensor-Transmitter: CHARACTERIZATION OF THE SENSOR DOMAIN AND A RESPONSE-IMPAIRED MUTANT THAT IDENTIFIES LIGAND-BINDING DETERMINANTS*. J. Biol. Chem. 1996, 271, 26630–26636. [Google Scholar] [CrossRef]
- Choi, J.; Groisman, E.A. Activation of Master Virulence Regulator PhoP in Acidic PH Requires the Salmonella-Specific Protein UgtL. Sci. Signal. 2017, 10, eaan6284. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Groisman, E.A. Horizontally Acquired Regulatory Gene Activates Ancestral Regulatory System to Promote Salmonella Virulence. Nucleic. Acids Res. 2020, 48, 10832–10847. [Google Scholar] [CrossRef]
- Lippa, A.M.; Goulian, M. Feedback Inhibition in the PhoQ/PhoP Signaling System by a Membrane Peptide. PLoS Genet. 2009, 5, e1000788. [Google Scholar] [CrossRef]
- Salazar, M.E.; Podgornaia, A.I.; Laub, M.T. The Small Membrane Protein MgrB Regulates PhoQ Bifunctionality to Control PhoP Target Gene Expression Dynamics. Mol. Microbiol. 2016, 102, 430–445. [Google Scholar] [CrossRef]
- Yadavalli, S.S.; Goh, T.; Carey, J.N.; Malengo, G.; Vellappan, S.; Nickels, B.E.; Sourjik, V.; Goulian, M.; Yuan, J. Functional Determinants of a Small Protein Controlling a Broadly Conserved Bacterial Sensor Kinase. J. Bacteriol. 2020, 202. [Google Scholar] [CrossRef]
- Lippa, A.M.; Goulian, M. Perturbation of the Oxidizing Environment of the Periplasm Stimulates the PhoQ/PhoP System in Escherichia Coli. J. Bacteriol. 2012, 194, 1457–1463. [Google Scholar] [CrossRef]
- Gerken, H.; Charlson, E.S.; Cicirelli, E.M.; Kenney, L.J.; Misra, R. MzrA: A Novel Modulator of the EnvZ/OmpR Two-Component Regulon. Mol. Microbiol. 2009, 72, 1408–1422. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.I.; Biemann, H.-P.; Privé, G.G.; Pandit, J.; Koshland Jr, D.E.; Kim, S.-H. High-Resolution Structures of the Ligand Binding Domain of the Wild-Type Bacterial Aspartate Receptor. J. Mol. Biol. 1996, 262, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R.; Chiang, R.C.; Kalman, L.V.; Gunsalus, R.P. Role of the Periplasmic Domain of the Escherichia Coli NarX Sensor-Transmitter Protein in Nitrate-Dependent Signal Transduction and Gene Regulation. Mol. Microbiol. 1996, 21, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Tajima, H.; Imada, K.; Sakuma, M.; Hattori, F.; Nara, T.; Kamo, N.; Homma, M.; Kawagishi, I. Ligand Specificity Determined by Differentially Arranged Common Ligand-Binding Residues in Bacterial Amino Acid Chemoreceptors Tsr and Tar*. J. Biol. Chem. 2011, 286, 42200–42210. [Google Scholar] [CrossRef]
- Ansaldi, M.; Bordi, C.; Lepelletier, M.; Méjean, V. TorC Apocytochrome Negatively Autoregulates the Trimethylamine N-Oxide (TMAO) Reductase Operon in Escherichia Coli. Mol. Microbiol. 1999, 33, 284–295. [Google Scholar] [CrossRef]
- Cotter, P.A.; Jones, A.M. Phosphorelay Control of Virulence Gene Expression in Bordetella. Trends Microbiol. 2003, 11, 367–373. [Google Scholar] [CrossRef]
- Li, L.; Wang, Q.; Zhang, H.; Yang, M.; Khan, M.I.; Zhou, X. Sensor Histidine Kinase Is a β-Lactam Receptor and Induces Resistance to β-Lactam Antibiotics. Proc. Natl. Acad. Sci. USA 2016, 113, 1648–1653. [Google Scholar] [CrossRef]
- Falke, J.J.; Erbse, A.H. The Piston Rises Again. Structure 2009, 17, 1149–1151. [Google Scholar] [CrossRef]
- Salvi, M.; Schomburg, B.; Giller, K.; Graf, S.; Unden, G.; Becker, S.; Lange, A.; Griesinger, C. Sensory Domain Contraction in Histidine Kinase CitA Triggers Transmembrane Signaling in the Membrane-Bound Sensor. Proc. Natl. Acad. Sci. USA 2017, 114, 3115–3120. [Google Scholar] [CrossRef]
- Monzel, C.; Unden, G. Transmembrane Signaling in the Sensor Kinase DcuS of Escherichia Coli: A Long-Range Piston-Type Displacement of Transmembrane Helix 2. Proc. Natl. Acad. Sci. USA 2015, 112, 11042–11047. [Google Scholar] [CrossRef]
- Mascher, T. Bacterial (Intramembrane-Sensing) Histidine Kinases: Signal Transfer Rather than Stimulus Perception. Trends Microbiol. 2014, 22, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.S.; Hernandez-Arriaga, A.M.; Cybulski, L.E.; Erazo, A.C.; de Mendoza, D. Molecular Basis of Thermosensing: A Two-Component Signal Transduction Thermometer in Bacillus Subtilis. EMBO J. 2001, 20, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Altabe, S.G.; Aguilar, P.; Caballero, G.M.; de Mendoza, D. The Bacillus Subtilis Acyl Lipid Desaturase Is a Delta5 Desaturase. J. Bacteriol. 2003, 185, 3228–3231. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, L.E.; Martín, M.; Mansilla, M.C.; Fernández, A.; de Mendoza, D. Membrane Thickness Cue for Cold Sensing in a Bacterium. Curr. Biol. 2010, 20, 1539–1544. [Google Scholar] [CrossRef]
- Almada, J.C.; Bortolotti, A.; Ruysschaert, J.M.; de Mendoza, D.; Inda, M.E.; Cybulski, L.E. Interhelical H-Bonds Modulate the Activity of a Polytopic Transmembrane Kinase. Biomolecules 2021, 11, 938. [Google Scholar] [CrossRef]
- Inda, M.E.; Vazquez, D.B.; Fernández, A.; Cybulski, L.E. Reverse Engineering of a Thermosensing Regulator Switch. J. Mol. Biol. 2019, 431, 1016–1024. [Google Scholar] [CrossRef]
- Novick, R.P.; Geisinger, E. Quorum Sensing in Staphylococci. Annu. Rev. Genet. 2008, 42, 541–564. [Google Scholar] [CrossRef]
- Fritz, G.; Dintner, S.; Treichel, N.S.; Radeck, J.; Gerland, U.; Mascher, T.; Gebhard, S. A New Way of Sensing: Need-Based Activation of Antibiotic Resistance by a Flux-Sensing Mechanism. mBio 2015, 6, e00975. [Google Scholar] [CrossRef]
- Szurmant, H.; Mohan, M.A.; Imus, P.M.; Hoch, J.A. YycH and YycI Interact To Regulate the Essential YycFG Two-Component System in Bacillus Subtilis. J. Bacteriol. 2007, 189, 3280–3289. [Google Scholar] [CrossRef]
- Poupel, O.; Moyat, M.; Groizeleau, J.; Antunes, L.C.S.; Gribaldo, S.; Msadek, T.; Dubrac, S. Transcriptional Analysis and Subcellular Protein Localization Reveal Specific Features of the Essential WalKR System in Staphylococcus Aureus. PLoS ONE 2016, 11, e0151449. [Google Scholar] [CrossRef]
- Cameron, D.R.; Jiang, J.-H.; Kostoulias, X.; Foxwell, D.J.; Peleg, A.Y. Vancomycin Susceptibility in Methicillin-Resistant Staphylococcus Aureus Is Mediated by YycHI Activation of the WalRK Essential Two-Component Regulatory System. Sci. Rep. 2016, 6, 30823. [Google Scholar] [CrossRef]
- Albanesi, D.; Martín, M.; Trajtenberg, F.; Mansilla, M.C.; Haouz, A.; Alzari, P.M.; de Mendoza, D.; Buschiazzo, A. Structural Plasticity and Catalysis Regulation of a Thermosensor Histidine Kinase. Proc. Natl. Acad. Sci. USA 2009, 106, 16185–16190. [Google Scholar] [CrossRef]
- Inda, M.E.; Oliveira, R.G.; de Mendoza, D.; Cybulski, L.E. The Single Transmembrane Segment of Minimal Sensor DesK Senses Temperature via a Membrane-Thickness Caliper. J. Bacteriol. 2016, 198, 2945–2954. [Google Scholar] [CrossRef]
- Cybulski, L.E.; Ballering, J.; Moussatova, A.; Inda, M.E.; Vazquez, D.B.; Wassenaar, T.A.; de Mendoza, D.; Tieleman, D.P.; Killian, J.A. Activation of the Bacterial Thermosensor DesK Involves a Serine Zipper Dimerization Motif That Is Modulated by Bilayer Thickness. Proc. Natl. Acad. Sci. USA 2015, 112, 6353–6358. [Google Scholar] [CrossRef]
- Inda, M.E.; Vandenbranden, M.; Fernández, A.; de Mendoza, D.; Ruysschaert, J.-M.; Cybulski, L.E. A Lipid-Mediated Conformational Switch Modulates the Thermosensing Activity of DesK. Proc. Natl. Acad. Sci. USA 2014, 111, 3579–3584. [Google Scholar] [CrossRef]
- Yuan, J.; Jin, F.; Glatter, T.; Sourjik, V. Osmosensing by the Bacterial PhoQ/PhoP Two-Component System. Proc. Natl. Acad. Sci. USA 2017, 114, E10792–E10798. [Google Scholar] [CrossRef] [PubMed]
- Geisinger, E.; George, E.A.; Chen, J.; Muir, T.W.; Novick, R.P. Identification of Ligand Specificity Determinants in AgrC, the Staphylococcus Aureus Quorum-Sensing Receptor. J. Biol. Chem. 2008, 283, 8930–8938. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.O.; Winzer, K.; Clarke, S.R.; Chan, W.C.; Williams, P. Differential Recognition of Staphylococcus Aureus Quorum-Sensing Signals Depends on Both Extracellular Loops 1 and 2 of the Transmembrane Sensor AgrC. J. Mol. Biol. 2008, 381, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Muir, T.W. Regulation of Virulence in Staphylococcus Aureus: Molecular Mechanisms and Remaining Puzzles. Cell. Chem. Biol. 2016, 23, 214–224. [Google Scholar] [CrossRef]
- Vasquez, J.K.; West, K.H.J.; Yang, T.; Polaske, T.J.; Cornilescu, G.; Tonelli, M.; Blackwell, H.E. Conformational Switch to a β-Turn in a Staphylococcal Quorum Sensing Signal Peptide Causes a Dramatic Increase in Potency. J. Am. Chem. Soc. 2020, 142, 750–761. [Google Scholar] [CrossRef]
- Nakayama, J.; Cao, Y.; Horii, T.; Sakuda, S.; Akkermans, A.D.; de Vos, W.M.; Nagasawa, H. Gelatinase Biosynthesis-Activating Pheromone: A Peptide Lactone That Mediates a Quorum Sensing in Enterococcus Faecalis. Mol. Microbiol. 2001, 41, 145–154. [Google Scholar] [CrossRef]
- Patching, S.G.; Edara, S.; Ma, P.; Nakayama, J.; Hussain, R.; Siligardi, G.; Phillips-Jones, M.K. Interactions of the Intact FsrC Membrane Histidine Kinase with Its Pheromone Ligand GBAP Revealed through Synchrotron Radiation Circular Dichroism. Biochim. Biophys. Acta 2012, 1818, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Gebhard, S.; Mascher, T. Antimicrobial Peptide Sensing and Detoxification Modules: Unravelling the Regulatory Circuitry of Staphylococcus Aureus. Mol. Microbiol. 2011, 81, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Gibbon, M.J.; Van der Kamp, M.W.; Pudney, C.R.; Gebhard, S. Conformation Control of the Histidine Kinase BceS of Bacillus Subtilis by Its Cognate ABC-Transporter Facilitates Need-Based Activation of Antibiotic Resistance. Mol. Microbiol. 2021, 115, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Bhate, M.P.; Lemmin, T.; Kuenze, G.; Mensa, B.; Ganguly, S.; Peters, J.M.; Schmidt, N.; Pelton, J.G.; Gross, C.A.; Meiler, J.; et al. Structure and Function of the Transmembrane Domain of NsaS, an Antibiotic Sensing Histidine Kinase in Staphylococcus Aureus. J. Am. Chem. Soc. 2018, 140, 7471–7485. [Google Scholar] [CrossRef]
- Pflüger, T.; Hernández, C.F.; Lewe, P.; Frank, F.; Mertens, H.; Svergun, D.; Baumstark, M.W.; Lunin, V.Y.; Jetten, M.S.M.; Andrade, S.L.A. Signaling Ammonium across Membranes through an Ammonium Sensor Histidine Kinase. Nat. Commun. 2018, 9, 164. [Google Scholar] [CrossRef]
- Szurmant, H.; Bu, L.; Brooks, C.L.; Hoch, J.A. An Essential Sensor Histidine Kinase Controlled by Transmembrane Helix Interactions with Its Auxiliary Proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 5891–5896. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic. Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Poupel, O.; Proux, C.; Jagla, B.; Msadek, T.; Dubrac, S. SpdC, a Novel Virulence Factor, Controls Histidine Kinase Activity in Staphylococcus Aureus. PLoS Pathog. 2018, 14, e1006917. [Google Scholar] [CrossRef]
- Firon, A.; Tazi, A.; Da Cunha, V.; Brinster, S.; Sauvage, E.; Dramsi, S.; Golenbock, D.T.; Glaser, P.; Poyart, C.; Trieu-Cuot, P. The Abi-Domain Protein Abx1 Interacts with the CovS Histidine Kinase to Control Virulence Gene Expression in Group B Streptococcus. PLoS Pathog. 2013, 9, e1003179. [Google Scholar] [CrossRef]
- Storz, G.; Wolf, Y.I.; Ramamurthi, K.S. Small Proteins Can No Longer Be Ignored. Annu. Rev. Biochem. 2014, 83, 753–777. [Google Scholar] [CrossRef]
- Garai, P.; Blanc-Potard, A. Uncovering Small Membrane Proteins in Pathogenic Bacteria: Regulatory Functions and Therapeutic Potential. Mol. Microbiol. 2020, 114, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Ishii, E.; Utsumi, R. Molecular Mechanism of Bacterial Two-Component Signal Transduction Networks via Connectors. Available online: https://www.caister.com/hsp/abstracts/twocomponentsystems/08.html (accessed on 8 September 2021).
- Gao, R.; Stock, A.M. Molecular Strategies for Phosphorylation-Mediated Regulation of Response Regulator Activity. Curr. Opin. Microbiol. 2010, 13, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, J.S. Signaling Mechanisms of HAMP Domains in Chemoreceptors and Sensor Kinases. Annu. Rev. Microbiol. 2010, 64, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Dunin-Horkawicz, S.; Lupas, A.N. Comprehensive Analysis of HAMP Domains: Implications for Transmembrane Signal Transduction. J. Mol. Biol. 2010, 397, 1156–1174. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.U.; Dunin-Horkawicz, S.; Mondéjar, L.G.; Hulko, M.; Hantke, K.; Martin, J.; Schultz, J.E.; Zeth, K.; Lupas, A.N.; Coles, M. The Mechanisms of HAMP-Mediated Signaling in Transmembrane Receptors. Structure 2011, 19, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Gushchin, I.; Aleksenko, V.A.; Orekhov, P.; Goncharov, I.M.; Nazarenko, V.V.; Semenov, O.; Remeeva, A.; Gordeliy, V. Nitrate- and Nitrite-Sensing Histidine Kinases: Function, Structure, and Natural Diversity. Int. J. Mol. Sci. 2021, 22, 5933. [Google Scholar] [CrossRef]
- Anantharaman, V.; Balaji, S.; Aravind, L. The Signaling Helix: A Common Functional Theme in Diverse Signaling Proteins. Biol. Direct 2006, 1, 25. [Google Scholar] [CrossRef]
- Saita, E.; Abriata, L.A.; Tsai, Y.T.; Trajtenberg, F.; Lemmin, T.; Buschiazzo, A.; Dal Peraro, M.; de Mendoza, D.; Albanesi, D. A Coiled Coil Switch Mediates Cold Sensing by the Thermosensory Protein DesK. Mol. Microbiol. 2015, 98, 258–271. [Google Scholar] [CrossRef]
- Bortolotti, A.; Vazquez, D.B.; Almada, J.C.; Inda, M.E.; Drusin, S.I.; Villalba, J.M.; Moreno, D.M.; Ruysschaert, J.M.; Cybulski, L.E. A Transmembrane Histidine Kinase Functions as a PH Sensor. Biomolecules 2020, 10, 1183. [Google Scholar] [CrossRef]
- Busch, A.; Lacal, J.; Martos, A.; Ramos, J.L.; Krell, T. Bacterial Sensor Kinase TodS Interacts with Agonistic and Antagonistic Signals. Proc. Natl. Acad. Sci. USA 2007, 104, 13774–13779. [Google Scholar] [CrossRef]
- Gong, W.; Hao, B.; Mansy, S.S.; Gonzalez, G.; Gilles-Gonzalez, M.A.; Chan, M.K. Structure of a Biological Oxygen Sensor: A New Mechanism for Heme-Driven Signal Transduction. Proc. Natl. Acad. Sci. USA 1998, 95, 15177–15182. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; López-Redondo, M.; Miguel-Romero, L.; Kulhankova, K.; Cahill, M.P.; Tran, P.M.; Kinney, K.J.; Kilgore, S.H.; Al-Tameemi, H.; Herfst, C.A.; et al. The SrrAB Two-Component System Regulates Staphylococcus Aureus Pathogenicity through Redox Sensitive Cysteines. Proc. Natl. Acad. Sci. USA 2020, 117, 10989–10999. [Google Scholar] [CrossRef] [PubMed]
- Dubey, B.N.; Agustoni, E.; Böhm, R.; Kaczmarczyk, A.; Mangia, F.; von Arx, C.; Jenal, U.; Hiller, S.; Plaza-Menacho, I.; Schirmer, T. Hybrid Histidine Kinase Activation by Cyclic Di-GMP–Mediated Domain Liberation. Proc. Natl. Acad. Sci. USA 2020, 117, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Burden, L.M.; Hurley, J.H. Structure of the GAF Domain, a Ubiquitous Signaling Motif and a New Class of Cyclic GMP Receptor. EMBO J. 2000, 19, 5288–5299. [Google Scholar] [CrossRef] [PubMed]
- Monk, I.R.; Shaikh, N.; Begg, S.L.; Gajdiss, M.; Sharkey, L.K.R.; Lee, J.Y.H.; Pidot, S.J.; Seemann, T.; Kuiper, M.; Winnen, B.; et al. Zinc-Binding to the Cytoplasmic PAS Domain Regulates the Essential WalK Histidine Kinase of Staphylococcus Aureus. Nat. Commun. 2019, 10, 3067. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Moffat, K. Crystal Structures of Deoxy and CO-Bound BjFixLH Reveal Details of Ligand Recognition and Signaling. Biochemistry 2005, 44, 4627–4635. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.S.A.; Saeki, A.; Hikima, T.; Nishizono, Y.; Hisano, T.; Kamaya, M.; Nukina, K.; Nishitani, H.; Nakamura, H.; Yamamoto, M.; et al. Architecture of the Complete Oxygen-Sensing FixL-FixJ Two-Component Signal Transduction System. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef]
- Ukaegbu, U.E.; Rosenzweig, A.C. Structure of the Redox Sensor Domain of Methylococcus Capsulatus (Bath) MmoS. Biochemistry 2009, 48, 2207–2215. [Google Scholar] [CrossRef]
- Müllner, M.; Hammel, O.; Mienert, B.; Schlag, S.; Bill, E.; Unden, G. A PAS Domain with an Oxygen Labile [4Fe-4S](2+) Cluster in the Oxygen Sensor Kinase NreB of Staphylococcus Carnosus. Biochemistry 2008, 47, 13921–13932. [Google Scholar] [CrossRef]
- Podust, L.M.; Ioanoviciu, A.; Ortiz de Montellano, P.R. 2.3 Å X-Ray Structure of the Heme-Bound GAF Domain of Sensory Histidine Kinase DosT of Mycobacterium Tuberculosis. Biochemistry 2008, 47, 12523–12531. [Google Scholar] [CrossRef] [PubMed]
- Georgellis, D.; Kwon, O.; Lin, E.C. Quinones as the Redox Signal for the Arc Two-Component System of Bacteria. Science 2001, 292, 2314–2316. [Google Scholar] [CrossRef] [PubMed]
- Inada, S.; Okajima, T.; Utsumi, R.; Eguchi, Y. Acid-Sensing Histidine Kinase With a Redox Switch. Front. Microbiol. 2021, 12, 652546. [Google Scholar] [CrossRef]
- Mann, T.H.; Seth Childers, W.; Blair, J.A.; Eckart, M.R.; Shapiro, L. A Cell Cycle Kinase with Tandem Sensory PAS Domains Integrates Cell Fate Cues. Nat. Commun. 2016, 7, 11454. [Google Scholar] [CrossRef]
- Moscoso, J.A.; Schramke, H.; Zhang, Y.; Tosi, T.; Dehbi, A.; Jung, K.; Gründling, A. Binding of Cyclic Di-AMP to the Staphylococcus Aureus Sensor Kinase KdpD Occurs via the Universal Stress Protein Domain and Downregulates the Expression of the Kdp Potassium Transporter. J. Bacteriol. 2016, 198, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Jacques, D.A.; Langley, D.B.; Jeffries, C.M.; Cunningham, K.A.; Burkholder, W.F.; Guss, J.M.; Trewhella, J. Histidine Kinase Regulation by a Cyclophilin-like Inhibitor. J. Mol. Biol. 2008, 384, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Whitten, A.E.; Jacques, D.A.; Hammouda, B.; Hanley, T.; King, G.F.; Guss, J.M.; Trewhella, J.; Langley, D.B. The Structure of the KinA-Sda Complex Suggests an Allosteric Mechanism of Histidine Kinase Inhibition. J. Mol. Biol. 2007, 368, 407–420. [Google Scholar] [CrossRef]
- Bick, M.J.; Lamour, V.; Rajashankar, K.R.; Gordiyenko, Y.; Robinson, C.V.; Darst, S.A. How to Switch off a Histidine Kinase: Crystal Structure of Geobacillus Stearothermophilus KinB with the Inhibitor Sda. J. Mol. Biol. 2009, 386, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Lüttmann, D.; Heermann, R.; Zimmer, B.; Hillmann, A.; Rampp, I.S.; Jung, K.; Görke, B. Stimulation of the Potassium Sensor KdpD Kinase Activity by Interaction with the Phosphotransferase Protein IIA(Ntr) in Escherichia Coli. Mol. Microbiol. 2009, 72, 978–994. [Google Scholar] [CrossRef]
- Mörk-Mörkenstein, M.; Heermann, R.; Göpel, Y.; Jung, K.; Görke, B. Non-Canonical Activation of Histidine Kinase KdpD by Phosphotransferase Protein PtsN through Interaction with the Transmitter Domain. Mol. Microbiol. 2017, 106, 54–73. [Google Scholar] [CrossRef]
- Wang, L.C.; Morgan, L.K.; Godakumbura, P.; Kenney, L.J.; Anand, G.S. The Inner Membrane Histidine Kinase EnvZ Senses Osmolality via Helix-Coil Transitions in the Cytoplasm. EMBO J. 2012, 31, 2648–2659. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mizusaki, H.; Kenney, L.J. A FRET-Based DNA Biosensor Tracks OmpR-Dependent Acidification of Salmonella during Macrophage Infection. PLoS Biol. 2015, 13, e1002116. [Google Scholar] [CrossRef]
- Liu, Y.; Rose, J.; Huang, S.; Hu, Y.; Wu, Q.; Wang, D.; Li, C.; Liu, M.; Zhou, P.; Jiang, L. A PH-Gated Conformational Switch Regulates the Phosphatase Activity of Bifunctional HisKA-Family Histidine Kinases. Nat. Commun. 2017, 8, 2104. [Google Scholar] [CrossRef] [PubMed]
- Mideros-Mora, C.; Miguel-Romero, L.; Felipe-Ruiz, A.; Casino, P.; Marina, A. Revisiting the PH-Gated Conformational Switch on the Activities of HisKA-Family Histidine Kinases. Nat. Commun. 2020, 11, 769. [Google Scholar] [CrossRef]
- Dubey, B.N.; Lori, C.; Ozaki, S.; Fucile, G.; Plaza-Menacho, I.; Jenal, U.; Schirmer, T. Cyclic Di-GMP Mediates a Histidine Kinase/Phosphatase Switch by Noncovalent Domain Cross-Linking. Sci. Adv. 2016, 2, e1600823. [Google Scholar] [CrossRef]
- Cheng, S.-T.; Wang, F.-F.; Qian, W. Cyclic-Di-GMP Binds to Histidine Kinase RavS to Control RavS-RavR Phosphotransfer and Regulates the Bacterial Lifestyle Transition between Virulence and Swimming. PLoS Pathog. 2019, 15, e1007952. [Google Scholar] [CrossRef] [PubMed]
- Ukaegbu, U.E.; Henery, S.; Rosenzweig, A.C. Biochemical Characterization of MmoS, a Sensor Protein Involved in Copper-Dependent Regulation of Soluble Methane Monooxygenase. Biochemistry 2006, 45, 10191–10198. [Google Scholar] [CrossRef] [PubMed]
- Malpica, R.; Franco, B.; Rodriguez, C.; Kwon, O.; Georgellis, D. Identification of a Quinone-Sensitive Redox Switch in the ArcB Sensor Kinase. Proc. Natl. Acad. Sci. USA 2004, 101, 13318–13323. [Google Scholar] [CrossRef]
- Bekker, M.; Alexeeva, S.; Laan, W.; Sawers, G.; Teixeira de Mattos, J.; Hellingwerf, K. The ArcBA Two-Component System of Escherichia Coli Is Regulated by the Redox State of Both the Ubiquinone and the Menaquinone Pool. J. Bacteriol. 2010, 192, 746–754. [Google Scholar] [CrossRef]
- Alvarez, A.F.; Rodriguez, C.; Georgellis, D. Ubiquinone and Menaquinone Electron Carriers Represent the Yin and Yang in the Redox Regulation of the ArcB Sensor Kinase. J. Bacteriol. 2013, 195, 3054–3061. [Google Scholar] [CrossRef]
- Van Beilen, J.W.A.; Hellingwerf, K.J. All Three Endogenous Quinone Species of Escherichia Coli Are Involved in Controlling the Activity of the Aerobic/Anaerobic Response Regulator ArcA. Front. Microbiol. 2016, 7, 1339. [Google Scholar] [CrossRef]
- Eguchi, Y.; Utsumi, R. Alkali Metals in Addition to Acidic PH Activate the EvgS Histidine Kinase Sensor in Escherichia Coli. J. Bacteriol. 2014, 196, 3140–3149. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Bell, J.; Clarke, K.; Chandler, R.; Pathak, P.; Xia, Y.; Marshall, R.L.; Weinstock, G.M.; Loman, N.J.; Winn, P.J.; et al. Characterization of Mutations in the PAS Domain of the EvgS Sensor Kinase Selected by Laboratory Evolution for Acid Resistance in Escherichia Coli. Mol. Microbiol. 2014, 93, 911–927. [Google Scholar] [CrossRef]
- Bock, A.; Gross, R. The Unorthodox Histidine Kinases BvgS and EvgS Are Responsive to the Oxidation Status of a Quinone Electron Carrier. Eur. J. Biochem. 2002, 269, 3479–3484. [Google Scholar] [CrossRef]
- Ma, P.; Phillips-Jones, M.K. Membrane Sensor Histidine Kinases: Insights from Structural, Ligand and Inhibitor Studies of Full-Length Proteins and Signalling Domains for Antibiotic Discovery. Molecules 2021, 26, 5110. [Google Scholar] [CrossRef]
- Dupré, E.; Wohlkonig, A.; Herrou, J.; Locht, C.; Jacob-Dubuisson, F.; Antoine, R. Characterization of the PAS Domain in the Sensor-Kinase BvgS: Mechanical Role in Signal Transmission. BMC Microbiol. 2013, 13, 172. [Google Scholar] [CrossRef]
- Sobran, M.A.; Cotter, P.A. The BvgS PAS Domain, an Independent Sensory Perception Module in the Bordetella Bronchiseptica BvgAS Phosphorelay. J. Bacteriol. 2019, 201, e00286-19. [Google Scholar] [CrossRef] [PubMed]
- Heermann, R.; Jung, K. The Complexity of the “simple” Two-Component System KdpD/KdpE in Escherichia Coli. FEMS Microbiol. Lett. 2010, 304, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Lüttmann, D.; Göpel, Y.; Görke, B. The Phosphotransferase Protein EIIA(Ntr) Modulates the Phosphate Starvation Response through Interaction with Histidine Kinase PhoR in Escherichia Coli. Mol. Microbiol. 2012, 86, 96–110. [Google Scholar] [CrossRef]
- Jenal, U.; Reinders, A.; Lori, C. Cyclic Di-GMP: Second Messenger Extraordinaire. Nat. Rev. Microbiol. 2017, 15, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Lori, C.; Ozaki, S.; Steiner, S.; Böhm, R.; Abel, S.; Dubey, B.N.; Schirmer, T.; Hiller, S.; Jenal, U. Cyclic Di-GMP Acts as a Cell Cycle Oscillator to Drive Chromosome Replication. Nature 2015, 523, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Biondi, E.G.; Skerker, J.M.; Arif, M.; Prasol, M.S.; Perchuk, B.S.; Laub, M.T. A Phosphorelay System Controls Stalk Biogenesis during Cell Cycle Progression in Caulobacter Crescentus. Mol. Microbiol. 2006, 59, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Kinkel, T.L.; Roux, C.M.; Dunman, P.M.; Fang, F.C. The Staphylococcus Aureus SrrAB Two-Component System Promotes Resistance to Nitrosative Stress and Hypoxia. mBio 2013, 4, e00696-13. [Google Scholar] [CrossRef]
- Mashruwala, A.A.; van de Guchte, A.; Boyd, J.M. Impaired Respiration Elicits SrrAB-Dependent Programmed Cell Lysis and Biofilm Formation in Staphylococcus Aureus. eLife 2017, 6, e23845. [Google Scholar] [CrossRef]
- Foo, Y.H.; Gao, Y.; Zhang, H.; Kenney, L.J. Cytoplasmic Sensing by the Inner Membrane Histidine Kinase EnvZ. Prog. Biophys. Mol. Biol. 2015, 118, 119–129. [Google Scholar] [CrossRef]
- Alphen, W.V.; Lugtenberg, B. Influence of Osmolarity of the Growth Medium on the Outer Membrane Protein Pattern of Escherichia Coli. J. Bacteriol. 1977, 131, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Winardhi, R.S.; Morgan, L.K.; Yan, J.; Kenney, L.J. Non-Canonical Activation of OmpR Drives Acid and Osmotic Stress Responses in Single Bacterial Cells. Nat. Commun. 2017, 8, 1587. [Google Scholar] [CrossRef]
- Choi, J.; Groisman, E.A. Acidic PH Sensing in the Bacterial Cytoplasm Is Required for Salmonella Virulence. Mol. Microbiol. 2016, 101, 1024–1038. [Google Scholar] [CrossRef]
- Ghosh, M.; Wang, L.C.; Huber, R.G.; Gao, Y.; Morgan, L.K.; Tulsian, N.K.; Bond, P.J.; Kenney, L.J.; Anand, G.S. Engineering an Osmosensor by Pivotal Histidine Positioning within Disordered Helices. Structure 2019, 27, 302–314.e4. [Google Scholar] [CrossRef]
- Willett, J.W.; Crosson, S. Atypical Modes of Bacterial Histidine Kinase Signaling. Mol. Microbiol. 2017, 103, 197–202. [Google Scholar] [CrossRef]
- Schramke, H.; Tostevin, F.; Heermann, R.; Gerland, U.; Jung, K. A Dual-Sensing Receptor Confers Robust Cellular Homeostasis. Cell Rep. 2016, 16, 213–221. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Sensor | PDB | Sensing Domain | Signal/Ligand | Organism | Ref. |
|---|---|---|---|---|---|
| DesK | helical linker | pH | B. subtilis | [121] | |
| TodS | PAS | toluene | Pseudomonas putida | [122] | |
| WalK | 4MN5, 6 | PAS | Zn2+ | S. aureus | [127] |
| FixL | 1BV5, 6 1XJ2, 3, 4, 6 | PAS + heme | O2 | B. japonicum | [24,128,129] |
| MmoS | PAS + FAD | O2 | Methylococcus capsulatus | [130] | |
| NreB | PAS + [4Fe-4S]2+cluster | O2 | S. carnosus | [131] | |
| DosT | 2VZW | GAF + heme | O2 | M. tuberculosis | [132] |
| ArcB | PAS | redox | E. coli | [133] | |
| EvgS | PAS | redox | E. coli | [134] | |
| CckA | PAS | c-di-GMP | Caulobacter crescetus | [135] | |
| ShkA | 6QRJ, 6QRL | REC | c-di-GMP | C. crescetus | [125] |
| KdpD | USP | c-di-AMP | S. aureus | [136] | |
| KinA | 2ZP2 | DHp | KipI | B. subtilis | [137] |
| KinA, KinB | DHp | Sda | B. subtilis | [138,139] | |
| KdpD | DHp | PtsN | E. coli | [140,141] | |
| EnvZ | DHp | Osmolality, pH | Salmonella, E. coli | [142,143] | |
| HK853 | 5UHT, 6AZR 6RGY, 6RFV, 6RGZ, 6RH0 | DHp | pH | T. maritima | [144,145] |
| SrrB | 6PAJ | CA | redox | S. aureus | [124] |
| CckA | 5IDM | CA | c-di-GMP | C. crescetus | [146] |
| RavS | CA | c-di-GMP | X. capestris | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, E.; Eguchi, Y. Diversity in Sensing and Signaling of Bacterial Sensor Histidine Kinases. Biomolecules 2021, 11, 1524. https://doi.org/10.3390/biom11101524
Ishii E, Eguchi Y. Diversity in Sensing and Signaling of Bacterial Sensor Histidine Kinases. Biomolecules. 2021; 11(10):1524. https://doi.org/10.3390/biom11101524
Chicago/Turabian StyleIshii, Eiji, and Yoko Eguchi. 2021. "Diversity in Sensing and Signaling of Bacterial Sensor Histidine Kinases" Biomolecules 11, no. 10: 1524. https://doi.org/10.3390/biom11101524
APA StyleIshii, E., & Eguchi, Y. (2021). Diversity in Sensing and Signaling of Bacterial Sensor Histidine Kinases. Biomolecules, 11(10), 1524. https://doi.org/10.3390/biom11101524





